Abstract
The heart is an insulin-dependent and energy consuming organ in which insulin and nutritional signaling integrates to the regulation of cardiac metabolism, growth, and survival. Heart failure is highly associated with insulin resistance and heart failure patients suffer from the cardiac energy deficiency, structural and functional dysfunction. Chronic pathological conditions, such as obesity and type 2 diabetes mellitus, involve various mechanisms in promoting heart failure by remodeling metabolic pathways, modulating cardiac energetics, and impairing cardiac contractility. Recent studies demonstrated that insulin receptor substrate-1, −2 (IRS-1, −2) are major mediators of both insulin and insulin-like growth factor-1 (IGF-1) signaling responsible for myocardial energetics, structure, function, and organismal survival. Importantly, the insulin receptor substrates (IRS) play an important role in activation of the phosphatidylinositide-3 dependent kinase (PI-3K) that controls Akt and Foxo1 signaling cascade, regulating the mitochondrial function, cardiac energy metabolism, and the renin-angiotensin system. Dysregulation of this branch in signaling cascades by insulin resistance in the heart through the endocrine system promotes heart failure, providing a novel mechanism for diabetic cardiomyopathy. Therefore, targeting this branch of IRS→PI-3K→Foxo1 signaling cascade and associated pathways may provide a fundamental strategy for the therapeutic and nutritional development in control of metabolic and cardiovascular diseases. In this review, we focus on insulin signaling and resistance in the heart and the role energetics play in cardiac metabolism, structure and function.
Introduction
Insulin resistance is the major underlying mechanism for metabolic and cardiovascular dysfunction in obesity and type 2 diabetes mellitus (T2D) (Qi 2016). T2D is a high-risk factor in promoting heart failure and its prevalence has risen at an alarming rate over the past few decades and two-thirds of patients with T2D have died from heart failure (Roger, et al. 2011). Multiple factors implicate the pathogenesis of diabetes-associated cardiac dysfunction, including hyperglycemia, insulin resistance, hyperinsulinemia, hyperlipidemia, oxidative stresses, and inflammation (Battiprolu, et al. 2010). These factors can cause energy metabolism alteration, calcium mishandling, mitochondrial dysfunction, apoptosis, and myocardial structural damage and functional loss (Battiprolu et al. 2010). All of the factors often interact each other therefore making T2D a complex disease to treat. In the early 1970’s, the concept of diabetic cardiomyopathy was recognized on the basis of hyperglycemia-induced cardiac dysfunction, independent of hypertension and coronary artery diseases (Rubler, et al. 1972). Over the past decades, Insulin resistance is believed to be a primary contributor to metabolic and cardiovascular dysfunction, and serves as a high risk factor for organ damage in promotion of heart failure and death in patients with diabetes (Roger et al. 2011). Thus, exploring the mechanism of insulin action and resistance opens a new chapter to expand our knowledge of diabetic cardiomyopathy. In this review, we discuss the mechanisms of insulin in governing cardiac energetics, and update on how insulin resistance promotes cellular dysfunction at the molecular level with interacting signaling cascade involving in IRS-1, −2, and Foxo1 genes.
Cardiac Insulin Action and Metabolic Flexibility
Upon food intake of carbohydrates, fatty acids, or proteins, pancreatic β-cells secret insulin, the most important endocrine hormone in promoting anabolic metabolism in target tissues and maintaining a balance of glucose and lipids in the blood circulation (Bertrand, et al. 2008; Riehle and Abel 2016). In addition to facilitating glucose uptake and energy storage as macromolecules, such as lipids in the adipose tissue, and glycogen and protein in the skeletal muscle and liver, insulin also targets the heart to control energy metabolism in support of cardiac growth and function.
In comparison with other tissues, the heart is unique in that it consumes about 10% of whole body energy expenditure for cardiac contraction, and is capable of utilizing all classes of substrates as energy resources, including carbohydrate, lipids, amino acids, and ketone bodies (Kolwicz, et al. 2013). The cardiomyocytes generate ATP via the substrate phosphorylation in the cytoplasm, which plays a key role in the heart under ischemia. However, the cardiomyocytes generate the major amount of ATP via the oxidative phosphorylation in the mitochondria that maintain cardiac function in the presence of oxygen. A human heart produces and consumes 3.5~6 kg of ATP every day with a myocardial process so dependent on oxidative phosphorylation that the mitochondria occupy one-third of the cell volume in each cardiomyocyte (Kolwicz et al. 2013).
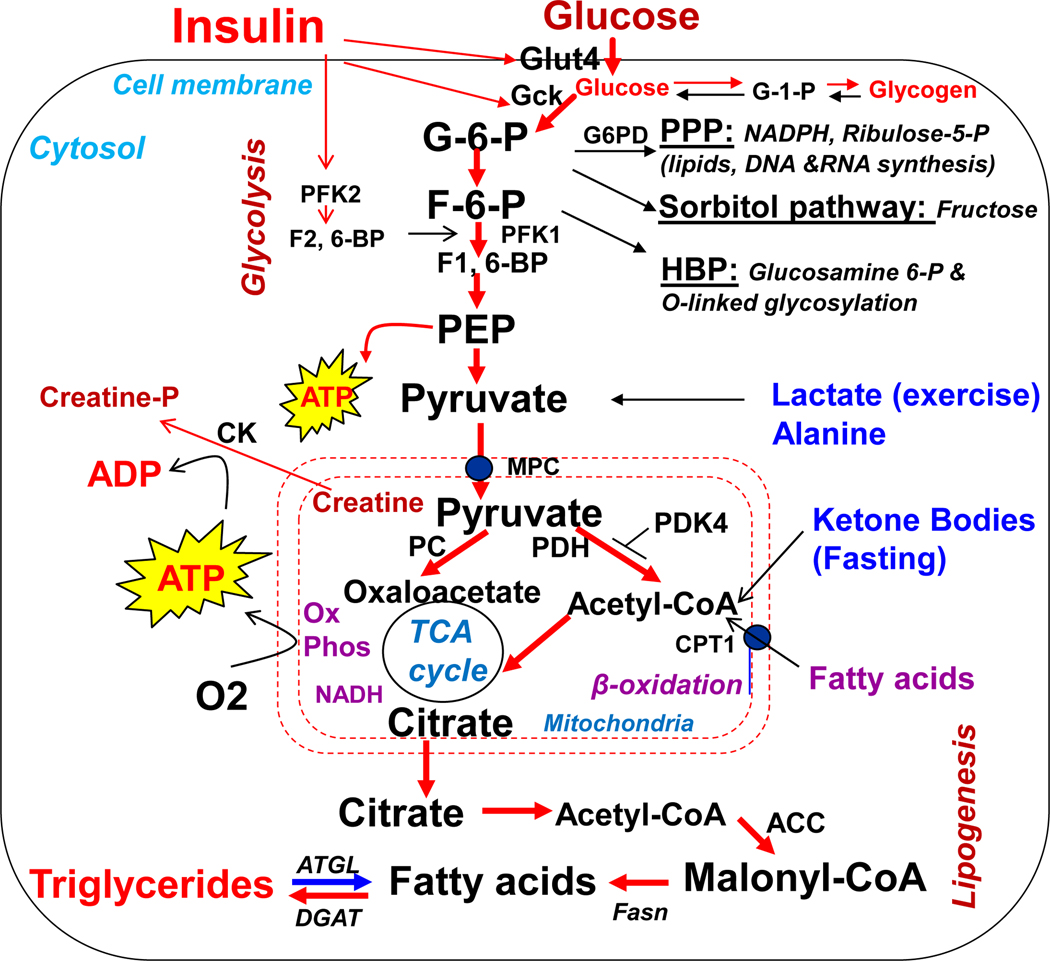
Insulin is a major regulator in cellular energetics and metabolism in cardiomyocytes (Fig.1). During the postprandial state, insulin secretion stimulates glucose uptake and utilization and synthesis of macromolecules in the heart. Glucose provides a favored oxidized substrate and generates 60~70% of ATP, while fatty acid oxidation provides only 20% of ATP in the heart (Bertrand et al. 2008). During fasting states when insulin level decreases, counter energy hormones such as glucagon increase, activating the catabolic metabolism, including enhanced lipolysis in the adipose tissue and increased fatty acid influx to the heart. Under these conditions, fatty acid oxidation acts as a major substrate for ATP production in generating 60~70% ATP in the heart, and is favored over glucose (20%) and lactate (10%). Under prolonged fasting conditions, ketone bodies and amino acids contribute to ATP generation by converting to the tricarboxylic acid cycle intermediates and acetyl-CoA (Bertrand et al. 2008; Kolwicz et al. 2013). The substrate preferences for ATP production during the transition between the postprandial and fasting state are tightly controlled by the availability of nutrients and hormones. According to Randle, glucose utilization suppresses lipid utilization and vice versa, a process mediated by the presence of insulin and its sensitivity to enhance glucose utilization and suppress fatty acid oxidation (Randle, et al. 1963). Thus, fatty acid oxidation rate increases in the fasting condition when insulin levels are low. However, this metabolic flexibility or adaptive transition under normal conditions is largely impaired in the heart when exposed to metabolic and mechanic stresses, particularly under conditions such as insulin resistance and T2D.
Figure 1.
Metabolic flexibility of cardiomyocyte in use of glucose, fatty acids, lactate, amino acids, ketone bodies for generation of ATP to support cardiac contractile function. Insulin stimulates anabolic metabolism, including glucose uptake, glycolysis, and synthesis of glycogen, ribonucleotide, and lipid synthesis, while insulin inhibits the β-oxidation of fatty acids. An excess amount of ATP can be stored in creatine phosphate, and activated pentose phosphate pathway promotes synthesis of macromolecules and cardiac hypertrophy. Hexosamine biosynthetic pathway promotes glycosylation of many cellular proteins and bioactivity of target proteins and biological responses, particularly under hyperglycemia or insulin resistance. G-6-P-glucose-6-phosphate, G-1-P-gluocse-1-phosphate, F-6-P-fructose-6-phosphate, F-1,6-BP-fructose-1.2,-biphosphate, F2,6-BP-fructoase-2,6-biphosphate, PFK1-phosphofructokinase-1, PFK2-phosphofructokinase-2, NADPH-nicotinamide adenine dinucleotide phosphate, AR-aldose reductase, GFA-glutamine fructose-6-phosphate aminotransferase, PEP-phosphoenolpyruvate, MPC-mitochondrial pyruvate carrier, TCA-tricarboxylic acid, PPP-pentose phosphate pathway, G6PD-glucose-6-phosphate dehydrogenase, Gck-glucokinase, Glut- glucose transporter, HBP-hexosamine biosynthetic pathway, CTP1-carnitine-palmitoyltransferase-1, PDK4-pyruvate dehydrogenase kinase-4, PDH-pyruvate dehydrogenase, Ox phos-oxidative phosphorylation, β-oxidation-fatty acid beta-oxidation, NADH-nicotinamide adenine dinucleotide, DGAT-diacylglycerol O-acyltransferase, ATGL-adipose triglyceride lipase, Fasn-fatty acid synthase, TAG-triglycerides. ACC- Acetyl-CoA carboxylase, PC-pyruvate carboxylase, CK-Creatine kinase, Creatine-P- Creatine phosphate.
Control of Cardiac Homeostasis by IRS-1 and IRS-2
The metabolic control by insulin is tightly coupled to the insulin signaling cascade (Fig. 2). Over the past a few years, creation of insulin receptor substrates (IRS) in genetically-engineered mouse models has provided major breakthroughs in our understanding of insulin-dependent control in cardiac energy metabolism and homeostasis, particularly for the role of protein kinase Akt (Guo 2014). Through previous experimentations and evidence, mice lacking the insulin receptor in the heart were born with reduced heart size and mitochondrial dysfunction, reflecting the key role of insulin receptor signaling in the regulation of postnatal cardiac size and substrate utilization (Belke, et al. 2002). To investigate the role of cardiac insulin signaling, we generated H-DKO mice with heart-specific deletion of both IRS-1 and IRS-2 genes using the Cre/loxP genetic approach. The mice all developed dilated cardiomyopathy and males died of heart failure at the age of 6~9 weeks with cardiac energetic deficiency, mitochondrial dysfunction, myocardial structural damage, and contractile functional loss (Qi, et al. 2013; Riehle, et al. 2013). These studies revealed novel findings of insulin action in control of cardiac growth, homeostasis, and survival. Thus, we proposed that suppression of IRS-1 and IRS-2 may serve as a fundamental mechanism in cardiac insulin resistance and associated heart failure (Qi et al. 2013).
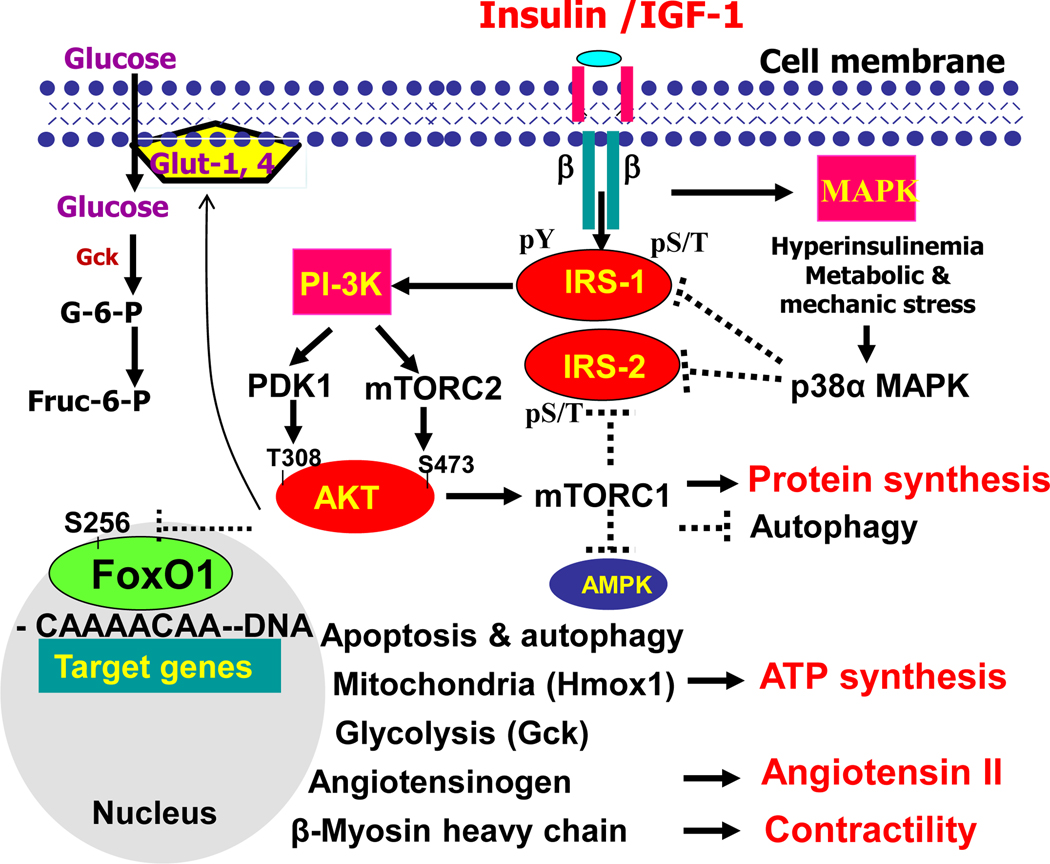
Figure 2.
Insulin/IGF-1 signaling via IRS1,2-coupled PI-3K→AKT→Foxo1 and mTOR activation in control of energy metabolism, protein synthesis, mitochondrial function, autophagy, apoptosis, and gene expression of angiotensinogen and β-myosin heavy chain in cardiomyocytes. Insulin or IGF-1 binding to the insulin receptor or IGF-1 receptor, stimulates the receptor tyrosine kinases, recruiting IRS-1,−2 and activating PI-3K to generate second messenger PIP3, which then activates PDK1 and mTORC2 for the activation of downstream effectors, including Akt which promotes mTORC1 activity and protein synthesis and inactivation of AMPK and autophagy. In addition to promoting glut-1, −4 gene expression and their cellular membrane association, PI-3K and Akt also suppress transcription factor Foxo1, which promotes apoptosis and autophagy, gene expression of angiotensinogen and β-myosin heavy chain, heme oxygenase −1, while suppressing glucokinase gene expression for glucose oxidation and unitization. Hyperinsulinemia or other metabolic and mechanic stress activates intracellular protein kinases, such as p38α, promotes serine and threonine phosphorylation of IRS, inhibits tyrosine phosphorylation of IRS, and promotes IRS ubiquitination or degradation, and desensitizes insulin/IGF-1 signaling propagation for Akt activation. Abbreviation: IR- insulin receptor; IRS- insulin receptor substrate; pY-phosphorylated tyrosine; pS/T- phosphorylated serine or threonine. PI-3K-phosphatidylinositol (PI)-3-kinase; PDK1- phosphoinositide-dependent protein kinase-1; Foxo1-Forkhead/winged helix transcription factor O-class member 1; mTORC1-mammalian target of rapamycin complex-1; mTORC2-mammalian target of rapamycin complex-2; Hmoxo-1-heme oxygenase-1; MAPK-mitogen-activated protein kinase.
Insulin is known to activate both Raf→MAP kinase and PI-3K→Akt kinase signaling cascades, similar to that of IGF-1 and its receptor (IGF-1R) which shares high homology with the insulin receptor (IR) (White 2003). Activated IR or IGF-1R directly phosphorylates tyrosine residues on several substrates including IRS-1, −2, −3, −4, Shc, Grb-2-assocated protein (Gab1), Dock1, Cbl, and APS adaptor proteins, subsequently providing specific docking sites for other signaling proteins in activating downstream protein kinases (White 2003). Mice lacking both IR and IGF-1-R in the heart displayed similar cardiac phenotype to that of H-DKO mice, in which elimination of PI-3K and Akt activity in cardiomyocytes contributed to cardiac failure and animal death (Laustsen, et al. 2007; Qi et al. 2013). These studies revealed a fundamental mechanism for endogenous kinase activation of PI-3K and Akt that is entirely dependent on IRS-1 and −2, or insulin and IGF-1 in the heart.
Many other growth factors, such as VEGF and PDGF, are capable of activating PI-3K and Akt in cells (Sussman, et al. 2011). For example, PDGF activates Akt in an IRS-independent manner in mouse embryonic fibroblasts, as we previously demonstrated (Guo, et al. 2006). We expect that the release of growth factors in the heart under different metabolic and mechanic conditions may provide additional mechanisms for activation of endogenous PI-3K and Akt and their compartmentation in cells. Understanding these factors involved in the complex pathophysiology may shed important insights in the dynamic changes of cardiac metabolism and adaptation. Evidently, a tightly controlled PI-3K and Akt by insulin and IGF-1 via IRS-1, −2 play central roles in governing myocardial metabolism and homeostasis, at least in the heart of male H-DKO mice, as we previously reported (Qi et al. 2013). In addition, similar to H-DKO mice, liver-specific IRS-1 and IRS-2 double genes knockout mice (L-DKO mice) also displayed mitochondrial dysfunction and liver damage, indicative as induction of mitochondrial fusion protein Mfn1 and reduction in mitochondrial electron transport chain complex III/IV and albumin gene expression; however the mice survived over 12 months, rather than experiencing early death (Cheng, et al. 2009; Guo, et al. 2009). Taken together, these data suggest that different types of cells may tolerate the loss of Akt, therefore insulin resistance may promote metabolic dysfunction and organ failure originating from various tissues. In this case, cardiomyocytes are more IRS-1, −2-dependent for survival than hepatocytes in mitochondrial function, insulin-stimulated glucose uptake and utilization, and IRS-dependent cardiac structural maintenance for cardiac homeostasis.
Mora et al. demonstrated similar cardiac phenotype results with mice lacking cardiac phosphoinositide-dependent protein kinase-1 (PDK1) downstream from PI-3K, including dilated cardiomyopathy and death from heart failure (Mora, et al. 2003). Thus, PDK1 is a critical upstream kinase for Akt activation by inducing phosphorylation at T308, while phosphoinositide-dependent protein kinase-2 (PDK2), a candidate which is not well known but likely to be mTOR complex 2 (mTORC2 or rictor-mTOR), partially activates Akt by inducing phosphorylation at S473 (Guo 2014; Sarbassov, et al. 2005).
Critical roles of Akt in the heart
Protein kinase Akt has several downstream effectors, including mTOR complex 1 (mTORC1 or raptor mTOR) for protein synthesis and suppression of autophagy (Shirakabe, et al. 2016), glucose transporter type 4 (Glu-4) for glucose uptake, and forkhead box protein Foxo1 for control of gene transcription (Guo, et al. 1999; Rena, et al. 1999) (Fig. 2). Mice lacking the mTORC1 target p70S6K or Glut-4 were not significantly affected in cardiac growth and contractility (Abel, et al. 1999; McMullen, et al. 2004). However, recent studies demonstrated that mice lacking cardiac TORC1 displayed failure in embryonic heart development and growth (Shende, et al. 2011; Zhang, et al. 2010; Zhu, et al. 2013). These animal models and data provide mechanistic insights on the role of endogenous Akt→TORC1 pathway in governing cardiac growth, metabolism and survival.
Akt activation not only stimulates protein synthesis and growth, but also integrates intracellular signaling into the nutrient metabolism, such as glucose oxidation and fatty acid synthesis, by promoting cell membrane translocation of Glut-4 and gene expression of glucokinase (Gck) and fatty acid synthase (Fasn). The levels of gene expression responsible for glucose utilization and lipid synthesis are significantly reduced in the absence of Akt activity in the cardiomyocytes lacking both IRS-1 and IRS-2 genes (Qi et al. 2013). In cardiomyocytes, IGF-1 enhances contractile function in a PI3K/Akt-dependent pathway that promotes Serca2a gene expression and Ca2+ handling (Kim, et al. 2008).
Akt is known to be a survival player in promoting anabolic metabolism and suppressing autophagy and apoptosis (DeBosch, et al. 2006; Shiojima, et al. 2002). However, a constitutively prolonged activation of Akt in the heart is detrimental for cardiac remodeling (Matsui, et al. 2002). We expect that catabolic metabolism and its counter regulation for insulin and Akt are required for maintenance of cardiac function and homeostasis, such as autophagy. At the physiological level, the anabolic and catabolic metabolism are controlled by feeding and fasting conditions, respectively, by nutritional and hormonal regulation. An imbalance between anabolism and catabolism promotes disease, such as cardiac hypertrophy or cachexia.
Cardiac Foxo1 signaling
The metabolic and survival regulation of Akt may partially depend on the Foxo1-mediated gene transcriptional programming. Foxo1 serves as an important regulator in suppressing glucokinase gene expression, limiting glucose oxidation and utilization in the heart and increasing autophagy (Battiprolu, et al. 2012; Liu, et al. 2009; Qi et al. 2013). Foxo1 stimulates cellular apoptosis by promoting gene expression of Bcl2 family members, such as Bim1 for caspase activation (Papanicolaou, et al. 2008). Overexpression of Foxo1 in the heart dose result in premature death in animals and aberrant expression is associated with cardiac failure in humans (Hannenhalli, et al. 2006).
Cardiac protection is achieved by Foxo1 inactivation in rodents. Mice lacking Foxo1 in the heart displayed almost normal cardiac growth and function (Qi, et al. 2015), and Foxo1 inactivation partially rescued cardiac dysfunction and death in mice lacking cardiac IRS-1 and IRS-2 (Qi et al. 2015), with similar results observed in db/db mice (Battiprolu et al. 2012). In particular, we demonstrated the key role of Foxo1 in stimulating cardiac dysfunction, through promoting β-myosin heavy chain (β-MHC) gene expression, a myocardial structural gene responsible for reduction in cardiac contractility (Qi et al. 2015). Although diminished activity of Akt in animal hearts with T2D may enhance Foxo1-dependent gene expression responsible for atrophy, autophagy, and apoptosis in many cells, which contribute to organopathy (Guo 2014), appropriate expression of Foxo1 promotes autophagy and anti-oxidative gene expression that is beneficial to the heart (Sengupta, et al. 2011; Sengupta, et al. 2009). We expect, however, hyper activation of Foxo1 under pathological conditions is detrimental to health due to stimulation of cellular apoptosis. The complex interplay of various factors as well as the underlying mechanism involved in Foxo1 activation and heart failure require further investigation.
IRS-1 and IRS-2 are critical for adult cardiac homeostasis and function, via a suppression of Foxo1 or activation of mTORC1 downstream from PDK1 and Akt, evidenced by that suppression of IRS-1 and IRS-2 in adult heart caused Foxo1 activation, reduction in mTORC1, and cardiac dysfunction (Qi et al. 2015). During early embryogenesis, however, PDK1 activity is not required for the cardiac development (Mora et al. 2003). Similarly, Foxo1 is important for angiogenesis during vascular developmental stage but not necessary for the cardiac development (Hosaka, et al. 2004; Qi et al. 2015). Overexpression of cardiac Foxo1 in mouse embryos prevents cell proliferation and heart development (Evans-Anderson, et al. 2008). Taken together, these studies provide solid evidence that IRS and Foxo1 signaling are significant components for energy metabolism and organ survival in adult hearts, and are mainly regulated by nutrients and hormones, such as insulin and IGF-1. We propose that IRS and Foxo1 serve as key mechanisms for maintaining cardiac energy homeostasis and survival by nutrients and endocrine systems, which are disrupted upon metabolic and mechanic stresses that occur under unhealthy conditions, such as diabetes, ischemia, and mechanical overload.
Cardiac insulin resistance, remodeling, p38α MAPK, and heart failure
Cardiac muscle requires either IRS-1 or IRS-2 for maintenance of endogenous Akt activation and Foxo1 inactivation, thus promoting cardiac function and survival (Qi et al. 2013; Riehle, et al. 2014). Both metabolic and mechanic stresses activate intracellular protein kinases that trigger serine or threonine phosphorylation of IRS proteins, which couple with IRS ubiquitination and degradation (Copps and White 2012). For example, insulin-induced mTORC1 activation triggers and promotes IRS-2 ubiquitination and degradation in fibroblasts (Guo et al. 2006). p38α MAPK (p38α) is another important protein kinase that is able to promote IRS-1 degradation and IRS-2 ubiquitination in cardiac myocytes (Qi et al. 2013).
Multiple protein kinase activation under metabolic stress and mechanic stretch shut down insulin action, thus restricting the anabolic metabolism. The heart employs protein kinase activation, which results in IRS degradation to deal with stresses so that anabolic metabolism is limited. Upon the hyperinsulinemia-induced metabolic stress, activation of p38α is necessary and sufficient for induction of insulin resistance through suppression of IRS-1 and IRS-2 in cardiomyocytes (Qi et al. 2013) (Fig. 2).
p38α is an important enzyme responsible for both pro-inflammatory factor TNFα and mechanic stretch signaling (Hannenhalli et al. 2006; Li, et al. 2005). Activation of p38α promotes IRS-1 and IRS-2 degradation in cardiomyocytes upon metabolic stresses, such as hyperinsulinemia (Qi et al. 2013), thus we propose that p38α may serve as a strong molecular link between insulin resistance and inflammation, and/or mechanic stretch, providing a powerful therapeutic target for improving myocardial dysfunction. The critical role of p38α in control of IRS-1 and IRS-2 stability in cardiomyocytes highlights the importance of future studies in assessing the regulatory system in humans. Furthermore, identifying the mechanisms of degradation of IRS-1 via Ser-636 and IRS-2 ubiquitination by p38 will enhance our understanding the molecular basis of insulin resistance (Qi et al. 2013). Nonetheless, targeting the endogenous protein kinases, such as inhibitors of p38α, as blockers of IRS-1 and IRS-2 degradation, may eventually provide us effective interventions nutritionally and pharmaceutically for the treatment of diabetes and associated cardiovascular failure.
Fetal gene programming, energy metabolism and heart failure
Activation of fetal gene profiling is characteristic of cardiac remodeling and heart failure (Dirkx, et al. 2013), including β-MHC, a Foxo1 target gene we have recently identified (Qi et al. 2015). Reprogramming of fetal gene expression was found in adverse cardiac remodeling and biomechanical stresses in failing or aging hearts (Koitabashi and Kass 2012; Marber, et al. 2010). The transcriptional reprogramming in the cardiac remodeling may relate to a diminished cardiac insulin action followed by Foxo1 activation, as insulin resistance develops upon aging processes.
During the fetal development, nutrients from maternal supply are essential for growth and survival. In this stage, fetal gene expression is highly expressed in the presence of very low insulin levels; however in the postnatal stage, insulin and IGF-1 become highly expressed and secreted from the pancreas and liver, respectively. They then play important roles in suppressing expression of cardiac fetal gene programming, through activation of IRS-1, −2, and inhibition of cardiac Foxo1.
As aging progresses, insulin resistance gradually develops, the catabolic metabolism enhances, and cardiac remodeling occurs. The Foxo1 inhibitory mechanism by insulin and IGF-1 becomes lost to some extent. Thus we propose that the fetal gene programming is reactivated partly due to insulin resistance and Foxo1 activation (Qi et al. 2015). During early development of insulin resistance and T2D, when hyperinsulinemia from pancreas compensates for inefficient glucose uptake and utilization, it also suppresses fatty acid oxidation in myocardial mitochondria. Subsequently, when the intermediate of saturated fatty acids accumulates, such as ceramide derived from palmitate being a potent activator for p38α, it results in IRS degradation and Foxo1 activation. This activation stimulates cellular apoptosis or expression of genes, including heme oxygenase-1 (Hmox-1), a new target gene of Foxo1 degrading heme, an essential component for the electron transport chain of mitochondria, thus limiting mitochondrial biogenesis and function (Cheng et al. 2009).
When the mitochondrial function is reduced, incomplete glucose and lipid oxidation then activates other synthetic pathways, including pentose phosphate pathway (PPP), sorbitol, and hexosamine biosynthetic pathways (HBP), which promote macromolecule synthesis and protein glycosylation that further stimulate cardiac hypertrophy followed by heart failure, often seen in rodents such as L-DKO and db/db mice, or patients with T2D (Qi et al. 2013). In the failing heart, energy deficiency is often indicated by reduction in ATP synthesis and activation of AMP-dependent protein kinase (AMPK) (Ji, et al. 2013), an energy deficient sensor that promotes beneficial glucose uptake and fatty acid oxidation for energy production (Zaha and Young 2012). All the processes are driven by mitochondrial dysfunction that controls ATP production, reactive oxygen species (ROS) generation, and induction of cellular apoptosis. Taken together, we believe that targeting mitochondrial biogenesis and metabolism would provide a powerful strategy to prevent or treat heart failure in the future.
Crosstalk between insulin signaling and renin-angiotensin system via Foxo1
Currently, the first line of clinical treatment for the patients with heart failure is the blocker for the renin-angiotensin system or the β-adrenergic receptors (Koitabashi and Kass 2012). Insulin protects the heart and reduces blood pressure (Anderson, et al. 1991). In recent years, we have established a potential molecular link between insulin and angiotensin signaling (Qi, et al. 2014). By identifying that Foxo1 targets and stimulates gene expression of angiotensinogen, a precursor of peptide hormone angiotensin II (Ang II), which promotes cardiovascular constriction, we demonstrated that insulin reduces blood pressure through suppression of Foxo1-mediated angiotensinogen gene expression and production of Ang II (Qi et al. 2014).
Angiotensinogen is largely expressed in the liver and in the adipose tissue particularly in obese populations (Yiannikouris, et al. 2012). The components of renin-angiotensin signaling are also minimally expressed in the heart and enhanced by hyperglycemia, which promotes myocardial death and diabetic cardiomyopathy (Singh, et al. 2008). Targeting the intracellular renin-angiotensin system achieved beneficial effect on cardiac protection in type1 diabetes mellitus and hyperglycemia-induced cardiac dysfunction (Kumar, et al. 2012). Taken together, with the elevated gene expression of angiotensinogen by hyperglycemia, existence of intracellular angiotensin system in various tissues, expansion of adipose tissue, and elevated blood pressure of animals with obesity and T2D (Su, et al. 2008; Yiannikouris et al. 2012), we expect that failure of suppression of angiotensinogen for production of Ang II by insulin resistance may have a significant impact on the development of hypertension and associated cardiovascular dysfunction in humans.
Crosstalk between insulin signaling and β-adrenergic receptor signaling via IRS
Increased catecholamine release, including epinephrine and norepinephrine, stimulates the sympathetic nervous system (SNS) and promotes heart failure (Bristow 2011). Binding of catecholamine to the cardiac β1-adrenergic receptor (β1-AR, mainly) and β2-adregnergic receptor (β2-AR, to a lesser extent), two family members of G-protein-coupled receptors (GPCRs), stimulates cardiac rate and contractility by largely activating Gs proteins (stimulatory proteins) when bound to guanosine diphosphate (GTP). Gs-proteins stimulate the effector adenylate cyclase, which converts ATP to the second messenger adenosine 3’,5’-monophosphate (cAMP) and activates the cAMP-dependent protein kinase A (PKA). PKA is the major effector of cAMP and phosphorylates a variety of substrates in control of myocardial Ca2+ handling and contractility under physiological conditions (Fig. 3). In heart failure pathogenesis, elevated SNS activation and subsequent catecholamine overdrive via β-AR stimulation was initially an adaptive mechanism to compensate for decreased heart rate and cardiac contractility and to maintain mean arterial pressure, however it eventually maladapted to promote disease progression, including myocardial ischemia, pathologic hypertrophy, arrhythmogenicity, myocardial fibrosis, necrosis and apoptosis (Lymperopoulos, et al. 2013; Zhang, et al. 2013). This maladaptive response results partially from down-regulation and desensitization of cardiac β-ARs due to chronic catecholamine stimulation. In the failing hearts, excess catecholamine on β-AR stimulation induces selective down-regulation of β1-AR and alters the ratio of β1-AR to β2-AR from an 80:20 distribution to a ratio of 60:40 and both β1-AR and β2-AR in failing hearts prevail in a desensitized condition (Rudomanova and Blaxall 2017). Recent studies demonstrated that β-AR desensitization is largely mediated by the G-protein-coupled receptor kinase-2 (GRK-2) that is highly expressed in the failing heart. Phosphorylation of serine or threonine residues of β-AR in turn recruits β-arrestin 2, leading to the uncoupling Gα with Gβγ and arresting signaling propagation for cardiac contractile function. This also serves as a new and important mechanism by which β-AR signaling is suppressed or desensitized and its role in promoting heart failure (Rudomanova and Blaxall 2017).
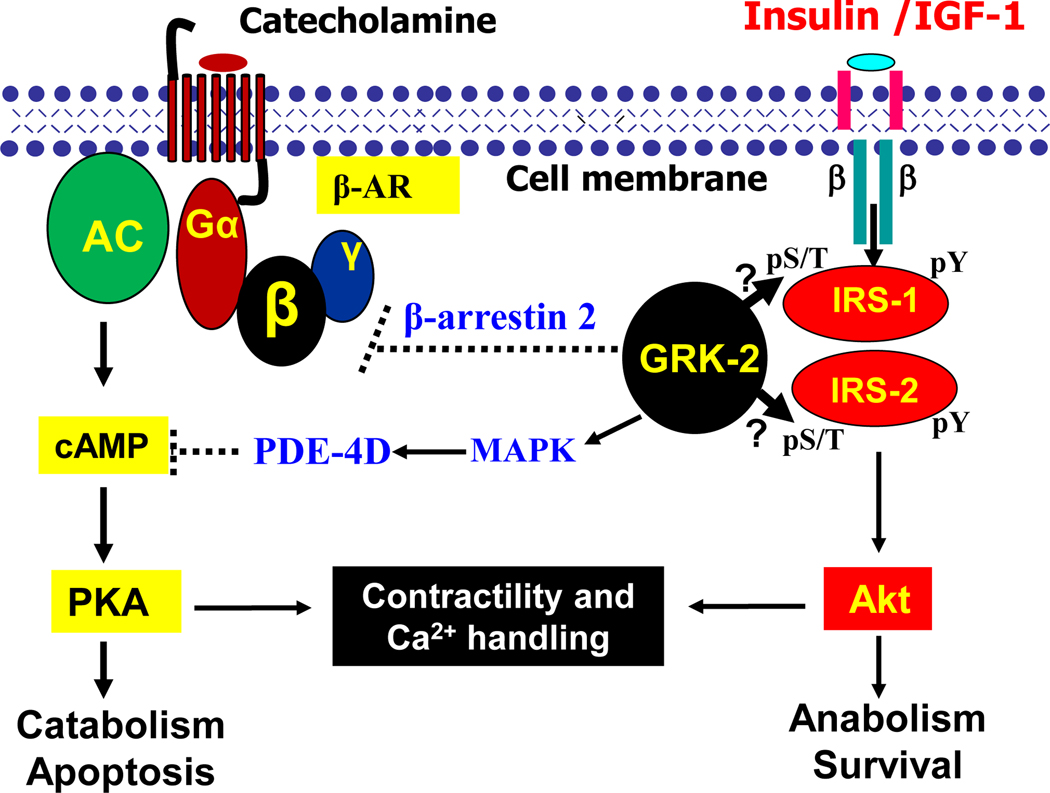
Figure 3.
Crosstalk between insulin/IGF-1 signaling and β-adrenergic receptor signaling in control of cardiac catabolism and anabolism via the GRK2. Binding of catecholamine agonists to the G-protein coupled receptor (GCPR) that has seven transmembrane domains produces a conformational change in the GPCR, which promotes the binding of G-protein to the intracellular binding site on the receptor. The G-protein is heterotrimeric and activated Gα and Gβγ subunits are responsible for the activation of specific effectors, which produce different second messengers that generate a wide range of cellular responses, particularly for Ca2+ handling, cardiac contractility, catabolism, and apoptosis. The desensitization of GPCR is triggered by interaction with GRK-2 which phosphorylates the β-AR and enhances its interaction with β-arrestin. GRK-2 also activates PDE-4D gene expression by activating MAPK, thus suppressing cAMP production and PKA activity. An increase in receptor affinity toward β-arrestin for Gα protein uncoupling to Gβγ subunit which arrests the signal propagation. It is expected that GRK-2 may interact and phosphorylate IRS-1 and IRS-2, desensitizing insulin/IGF-1 signaling in the activation of PI-3K and Akt for control of anabolism and survival. Abbreviation: AC- adenylate cyclase; β-AR- beta-adrenergic receptors; Gα- G-protein α subunit; β- G-protein β subunit; γ- G-protein γ subunit; GRK-2- G-protein coupled receptor kinase-2; cAMP- cyclic 3’,5’-adenosine monophosphate; PDE-4D- phosphodiesterase 4D.
Fu et al. reported that insulin suppresses catecholamine-induced cAMP elevation and PKA activation in cardiomyocytes in an IRS-1 or IRS-2 -dependent manner, and insulin inhibits cardiac contractility by inducing Gi (inhibitory proteins)-based β2-AR (Fu, et al. 2014). Moreover, insulin promotes cAMP degradation by inducing expression of gene encoding phosphodiesterase-4D (PDE-4D), a process that requires MAPK activation involving the stimulation of GRK-2 and interaction of β2-AR and arrestin 2, which is active in high-fat diet-induced insulin resistant heart. However, inhibiting GRK-2 by small compounds, such as paroxetine, significantly suppresses insulin-mediated MAPK activation and PDE-4D induction, restoring the β-AR action and preventing diabetes-related cardiac contractile dysfunction (Wang, et al. 2017).
Considering the enhanced GRK-2 in insulin resistant or failing heart, we predict that GRK-2 may serve as a protein kinase to target IRS-1 and IRS-2 serine/threonine phosphorylation in promoting insulin resistance, even though the mechanism has not yet been established and potential phosphorylation residues of IRS-1, −2 by GRK-2 still need further identification. Indeed, deletion of half amount of GRK-2 gene expression systemically in mice protected against high-fat diet (HFD)-induced insulin resistance and obesity (Garcia-Guerra, et al. 2010), as well as enhanced cardiac insulin-stimulated Akt phosphorylation and glut-4 membrane translocation, and reduced cardiac steatosis and fibrosis (Lucas, et al. 2014; Lucas, et al. 2016). By contrast, over expression of GRK2 in cardiomyocytes attenuated insulin-induced Akt phosphorylation and glut-4 membrane translocation (Ciccarelli, et al. 2011). In 3T3-L1 adipocytes, it was shown that the regulator of G-protein signaling (RGS) domain that interacts with Gαq/11 in GRK2 is required for insulin inhibition on glucose transport (Usui, et al. 2004). Thus, GRK-2 is a negative regulator of insulin signaling. A unified pathway that merges IRS signaling to β-AR signaling via GRK-2 was proposed in Fig. 3. Recent studies showed that insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure (Smooke, et al. 2005), in which hyperinsulinemia and its induction of Gi-based β2-AR can promotes cardiac dysfunction and failure (Zhu, et al. 2012). Our recent study in cardiomyocytes demonstrated that chronic excess insulin stimulation desensitizes insulin signaling for Akt activation by suppressing IRS-1 and IRS-2, which likely occurs in HFD-induced insulin resistant heart via p38α activation. Alternatively, the cardiac suppression of IRS-1 and IRS-2 in HFD-treated mice may also relate to GRK-2 upregulation that promotes IRS serine/threonine phosphorylation and protein degradation, desensitizing both insulin signaling for Akt activation and β-AR signaling for PKA activation, which results in cardiac contractile dysfunction under conditions of hyperinsulinemia and excess catecholamine stimulation during diabetes and heart failure development.
Obesity paradox in heart failure
Studies in humans and animal models have revealed that heart failure is highly associated with generalized insulin resistance (Riehle and Abel 2016; Velez, et al. 2014). However, recent studies have reported a significant increase in survival rates of obese individuals in the context of heart failure, leading to the unexpected phenomenon known as the “obesity paradox” (Lavie, et al. 2013). An underlying reason involves cardiac cachexia with a poor heart failure prognosis and in the promotion of catabolic metabolism. This condition is counteracted by the effect of obesity that would adversely affect cachexia and promote the anabolic metabolism and survival despite the role of the heart in metabolic stresses. Furthermore, hormones and cytokines secreted from expansion of adipose tissues in obese individuals may offer positive cardio-protective actions although such factors have yet to be identified. Under insulin resistant conditions, such as in the case of HFD-fed mice, hyperinsulinemia activates endogenous MAPK pathway that suppresses catecholamine-induced cAMP and PKA signaling in promoting a dual role in control of cardiac contractility and apoptosis, to some extent, depending on the cellular context, therefore explaining the paradox of hyperinsulinemia and obesity on the cardiac function (Fig. 3). Overall, we expect that heart failure occurs through the gradual suppression of Akt signaling and increase of catecholamine-induced cAMP production and highly activated PKA for induction of apoptosis during heart failure development. Eventually, cAMP-induced PKA activation can be largely suppressed at a later phase of heart failure, indicative as an induction of cardiac contractile dysfunction, GRK2 activation, and desensitization of the β-AR function (Fig. 3 & 4).
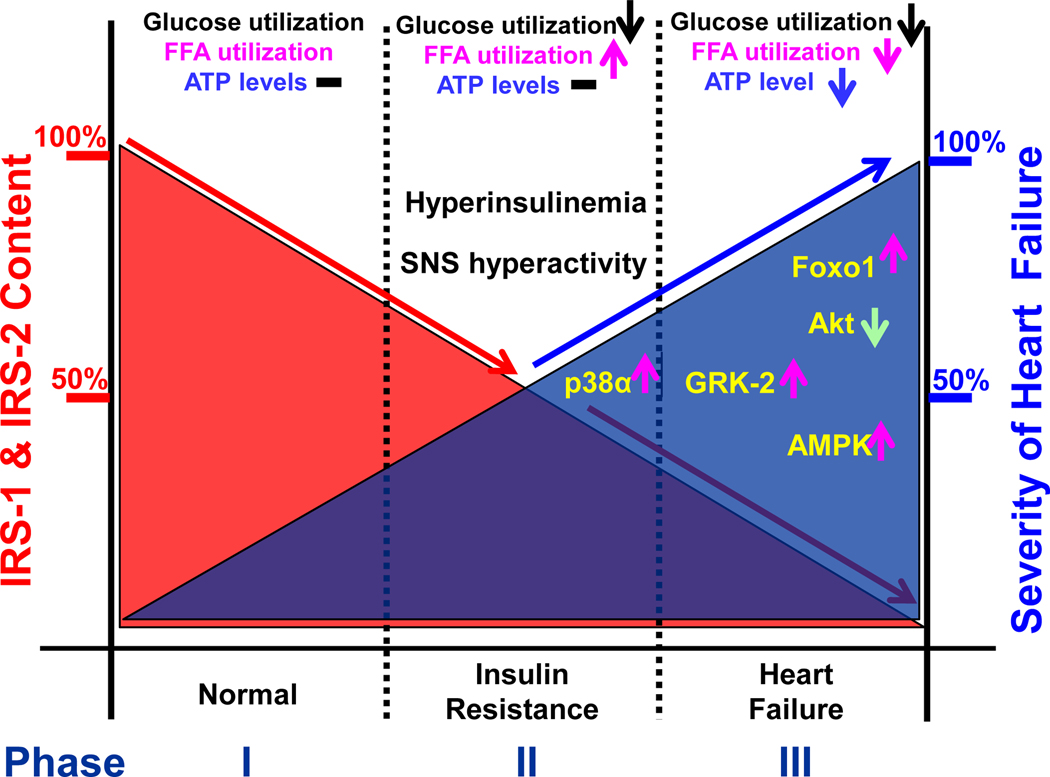
Figure 4.
A proposed model for the dynamic changes of cardiac IRS-1 and IRS-2 synthesis in control of metabolic adaptation, cardiac insulin resistance, remodeling, and heart failure. The cardiac IRS-1 and IRS-2 protein levels are tightly correlated to the severity of heart failure and associated with myocardial intracellular protein kinases and Foxo1 activation. Under feeding conditions, IRS proteins slightly decreased to less than 25% and ATP homeostasis is maintained by glucose and free fatty acid (FFA) oxidation. Under insulin resistant state, further down regulation of IRS protein by less than 50% desensitizes glucose uptake and utilization with enhanced FFA oxidation, thus ATP homeostasis can be well maintained even when obesity occurs. However, down regulation of IRS proteins by more than 50% will result in cardiac dysfunction and 100% loss of IRS proteins will cause severe heart failure and death, in which a number of factors including activation of p38α, GRK-2, AMPK, and Foxo1, as well as inactivation of Akt are shown.
Conclusion and Prospect
Akt inactivation and Foxo1 activation following suppression of IRS-1 and IRS-2 provide a fundamental mechanism for cardiac insulin resistance, activation of MAPK, and elevation of the renin-angiotensin signaling cascade, which are present in many pathological conditions. The regulatory mechanisms of IRS→Akt→Foxo1 cascades and their interactions with other signaling cascades, such as β-AR signaling, should be further explored under different cellular and environmental contexts, including ischemia, hypertrophy, and malnutrition. Targeting IRS-1 and IRS-2 by activating the Akt→Foxo1 signaling cascade and associated protein kinases and target genes will be critical for the prevention and treatment of diabetes and associated cardiac dysfunction in the future.
Funding
Dr. Shaodong Guo’s research is supported by grants from the American Diabetes Association Career Development Award (1-15-CD-09) and Minority Undergraduate Internship Award (1-17-MUI-008), the American Heart Association (BGIA-7880040), the National Institutes of Health (RO1 DK095118), Faculty Start-up from the Texas A&M University AgriLife Research, and the USDA National Institute of Food and Agriculture (Hatch 1010958). Dr. Shaodong Guo is recipient of the year 2015 American Diabetes Association Research Excellence Thomas R. Lee Award.
Footnotes
Declaration of Interest
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review reported.
References
- Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy JT, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, et al. 1999. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 104 1703–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EA, Hoffman RP, Balon TW, Sinkey CA & Mark AL 1991. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87 2246–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiprolu PK, Gillette TG, Wang ZV, Lavandero S & Hill JA 2010. Diabetic Cardiomyopathy: Mechanisms and Therapeutic Targets. Drug Discov Today Dis Mech 7 e135–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, et al. 2012. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest 122 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, et al. 2002. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest 109 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand L, Horman S, Beauloye C & Vanoverschelde JL 2008. Insulin signalling in the heart. Cardiovasc Res 79 238–248. [DOI] [PubMed] [Google Scholar]
- Bristow MR 2011. Treatment of chronic heart failure with beta-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res 109 1176–1194. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P & White MF 2009. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med 15 1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli M, Chuprun JK, Rengo G, Gao E, Wei Z, Peroutka RJ, Gold JI, Gumpert A, Chen M, Otis NJ, et al. 2011. G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation 123 1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copps KD & White MF 2012. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55 2565–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M & Muslin AJ 2006. Akt1 is required for physiological cardiac growth. Circulation 113 2097–2104. [DOI] [PubMed] [Google Scholar]
- Dirkx E, da Costa Martins PA & De Windt LJ 2013. Regulation of fetal gene expression in heart failure. Biochim Biophys Acta 1832 2414–2424. [DOI] [PubMed] [Google Scholar]
- Evans-Anderson HJ, Alfieri CM & Yutzey KE 2008. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res 102 686–694. [DOI] [PubMed] [Google Scholar]
- Fu Q, Xu B, Liu Y, Parikh D, Li J, Li Y, Zhang Y, Riehle C, Zhu Y, Rawlings T, et al. 2014. Insulin inhibits cardiac contractility by inducing a Gi-biased beta2-adrenergic signaling in hearts. Diabetes 63 2676–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Jurado-Pueyo M, Zalba G, Diez J, Murga C, Fernandez-Veledo S, Mayor F Jr. & Lorenzo M 2010. G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes 59 2407–2417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guo S 2014. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol 220 T1–T23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A, Rossetti L, Sajan M, Farese RV & White MF 2009. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol 29 5070–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Dunn SL & White MF 2006. The reciprocal stability of FOXO1 and IRS2 creates a regulatory circuit that controls insulin signaling. Mol Endocrinol 20 3389–3399. [DOI] [PubMed] [Google Scholar]
- Guo S, Rena G, Cichy S, He X, Cohen P & Unterman T 1999. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem 274 17184–17192. [DOI] [PubMed] [Google Scholar]
- Hannenhalli S, Putt ME, Gilmore JM, Wang J, Parmacek MS, Epstein JA, Morrisey EE, Margulies KB & Cappola TP 2006. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation 114 1269–1276. [DOI] [PubMed] [Google Scholar]
- Hosaka T, Biggs WH 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK & Arden KC 2004. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A 101 2975–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Zhang X, Liu W, Huang Q, Yang W, Fu F, Ma H, Su H, Wang H, Wang J, et al. 2013. AMPK-regulated and Akt-dependent enhancement of glucose uptake is essential in ischemic preconditioning-alleviated reperfusion injury. PLoS One 8 e69910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Abdellatif M, Koul S & Crystal GJ 2008. Chronic treatment with insulin-like growth factor I enhances myocyte contraction by upregulation of Akt-SERCA2a signaling pathway. Am J Physiol Heart Circ Physiol 295 H130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koitabashi N & Kass DA 2012. Reverse remodeling in heart failure--mechanisms and therapeutic opportunities. Nat Rev Cardiol 9 147–157. [DOI] [PubMed] [Google Scholar]
- Kolwicz SC Jr., Purohit S & Tian R 2013. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res 113 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Thomas CM, Yong QC, Chen W & Baker KM 2012. The intracrine renin-angiotensin system. Clin Sci (Lond) 123 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen PG, Russell SJ, Cui L, Entingh-Pearsall A, Holzenberger M, Liao R & Kahn CR 2007. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol Cell Biol 27 1649–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV & Ventura HO 2013. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 1 93–102. [DOI] [PubMed] [Google Scholar]
- Li M, Georgakopoulos D, Lu G, Hester L, Kass DA, Hasday J & Wang Y 2005. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation 111 2494–2502. [DOI] [PubMed] [Google Scholar]
- Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi J, Liu Z & Cao W 2009. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem 284 31484–31492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas E, Jurado-Pueyo M, Fortuno MA, Fernandez-Veledo S, Vila-Bedmar R, Jimenez-Borreguero LJ, Lazcano JJ, Gao E, Gomez-Ambrosi J, Fruhbeck G, et al. 2014. Downregulation of G protein-coupled receptor kinase 2 levels enhances cardiac insulin sensitivity and switches on cardioprotective gene expression patterns. Biochim Biophys Acta 1842 2448–2456. [DOI] [PubMed] [Google Scholar]
- Lucas E, Vila-Bedmar R, Arcones AC, Cruces-Sande M, Cachofeiro V, Mayor F Jr. & Murga C 2016. Obesity-induced cardiac lipid accumulation in adult mice is modulated by G protein-coupled receptor kinase 2 levels. Cardiovasc Diabetol 15 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G & Koch WJ 2013. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res 113 739–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marber MS, Molkentin JD & Force T 2010. Developing small molecules to inhibit kinases unkind to the heart: p38 MAPK as a case in point. Drug Discov Today Dis Mech 7 e123–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R & Rosenzweig A 2002. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem 277 22896–22901. [DOI] [PubMed] [Google Scholar]
- McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Dorfman AL, Longnus S, Pende M, Martin KA, Blenis J, et al. 2004. Deletion of ribosomal S6 kinases does not attenuate pathological, physiological, or insulin-like growth factor 1 receptor-phosphoinositide 3-kinase-induced cardiac hypertrophy. Mol Cell Biol 24 6231–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora A, Davies AM, Bertrand L, Sharif I, Budas GR, Jovanovic S, Mouton V, Kahn CR, Lucocq JM, Gray GA, et al. 2003. Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. EMBO J 22 4666–4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou KN, Izumiya Y & Walsh K 2008. Forkhead transcription factors and cardiovascular biology. Circ Res 102 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Guo X, Guo S. 2016. Insulin Resistance in Obesity. Metabolic Syndrome: A Comphensive Textbook, Ed. Ahima R.1st Edition, Springer; Reference 479–504. [Google Scholar]
- Qi Y, Xu Z, Zhu Q, Thomas C, Kumar R, Feng H, Dostal DE, White MF, Baker KM & Guo S 2013. Myocardial Loss of IRS1 and IRS2 Causes Heart Failure and Is Controlled by p38alpha MAPK During Insulin Resistance. Diabetes 62 3887–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Zhang K, Wu Y, Xu Z, Yong QC, Kumar R, Baker KM, Zhu Q, Chen S & Guo S 2014. Novel mechanism of blood pressure regulation by forkhead box class O1-mediated transcriptional control of hepatic angiotensinogen. Hypertension 64 1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Zhu Q, Zhang K, Thomas C, Wu Y, Kumar R, Baker KM, Xu Z, Chen S & Guo S 2015. Activation of Foxo1 by Insulin Resistance Promotes Cardiac Dysfunction and beta-Myosin Heavy Chain Gene Expression. Circ Heart Fail 8 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN & Newsholme EA 1963. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1 785–789. [DOI] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG & Cohen P 1999. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem 274 17179–17183. [DOI] [PubMed] [Google Scholar]
- Riehle C & Abel ED 2016. Insulin Signaling and Heart Failure. Circ Res 118 1151–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle C, Wende AR, Sena S, Pires KM, Pereira RO, Zhu Y, Bugger H, Frank D, Bevins J, Chen D, et al. 2013. Insulin receptor substrate signaling suppresses neonatal autophagy in the heart. J Clin Invest 123 5319–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle C, Wende AR, Zhu Y, Oliveira KJ, Pereira RO, Jaishy BP, Bevins J, Valdez S, Noh J, Kim BJ, et al. 2014. Insulin receptor substrates are essential for the bioenergetic and hypertrophic response of the heart to exercise training. Mol Cell Biol 34 3450–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. 2011. Heart disease and stroke statistics−−2011 update: a report from the American Heart Association. Circulation 123 e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW & Grishman A 1972. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30 595–602. [DOI] [PubMed] [Google Scholar]
- Rudomanova V & Blaxall BC 2017. Targeting GPCR-Gbetagamma-GRK2 signaling as a novel strategy for treating cardiorenal pathologies. Biochim Biophys Acta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM & Sabatini DM 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307 1098–1101. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Molkentin JD, Paik JH, DePinho RA & Yutzey KE 2011. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem 286 7468–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Molkentin JD & Yutzey KE 2009. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem 284 28319–28331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, Krishnan J, Lerch R, Hall MN, Ruegg MA, et al. 2011. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation 123 1073–1082. [DOI] [PubMed] [Google Scholar]
- Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, Rosenzweig A, Kahn CR, Abel ED & Walsh K 2002. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem 277 37670–37677. [DOI] [PubMed] [Google Scholar]
- Shirakabe A, Ikeda Y, Sciarretta S, Zablocki DK & Sadoshima J 2016. Aging and Autophagy in the Heart. Circ Res 118 1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP, Le B, Khode R, Baker KM & Kumar R 2008. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 57 3297–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smooke S, Horwich TB & Fonarow GC 2005. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 149 168–174. [DOI] [PubMed] [Google Scholar]
- Su W, Guo Z, Randall DC, Cassis L, Brown DR & Gong MC 2008. Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol 295 H1634–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, Gude N, Avitabile D, Alvarez R, Sundararaman B, et al. 2011. Myocardial AKT: the omnipresent nexus. Physiol Rev 91 1023–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui I, Imamura T, Satoh H, Huang J, Babendure JL, Hupfeld CJ & Olefsky JM 2004. GRK2 is an endogenous protein inhibitor of the insulin signaling pathway for glucose transport stimulation. EMBO J 23 2821–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez M, Kohli S & Sabbah HN 2014. Animal models of insulin resistance and heart failure. Heart Fail Rev 19 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu Y, Fu Q, Xu B, Zhang Y, Kim S, Tan R, Barbagallo F, West T, Anderson E, et al. 2017. Inhibiting Insulin-Mediated beta2-Adrenergic Receptor Activation Prevents Diabetes-Associated Cardiac Dysfunction. Circulation 135 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MF 2003. Insulin signaling in health and disease. Science 302 1710–1711. [DOI] [PubMed] [Google Scholar]
- Yiannikouris F, Karounos M, Charnigo R, English VL, Rateri DL, Daugherty A & Cassis LA 2012. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. Am J Physiol Regul Integr Comp Physiol 302 R244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaha VG & Young LH 2012. AMP-activated protein kinase regulation and biological actions in the heart. Circ Res 111 800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, et al. 2010. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest 120 2805–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Szeto C, Gao E, Tang M, Jin J, Fu Q, Makarewich C, Ai X, Li Y, Tang A, et al. 2013. Cardiotoxic and cardioprotective features of chronic beta-adrenergic signaling. Circ Res 112 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Petrashevskaya N, Ren S, Zhao A, Chakir K, Gao E, Chuprun JK, Wang Y, Talan M, Dorn GW 2nd, et al. 2012. Gi-biased beta2AR signaling links GRK2 upregulation to heart failure. Circ Res 110 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Pires KM, Whitehead KJ, Olsen CD, Wayment B, Zhang YC, Bugger H, Ilkun O, Litwin SE, Thomas G, et al. 2013. Mechanistic target of rapamycin (Mtor) is essential for murine embryonic heart development and growth. PLoS One 8 e54221. [DOI] [PMC free article] [PubMed] [Google Scholar]