Abstract
Schistosomiasis is the cause of a chronic debilitating disease which accounts for significant mortality and morbidity every year, especially in tropical and subtropical areas. An epitope derived from the protective surface protein 9B-Ag of Schistosoma mansoni, designated 9B peptide-1, was previously showed to be protective in mice when conjugated to bovine serum albumin and administered subcutaneously in complete Freund’s adjuvant. In this work, this protective peptide was expressed in the flagellin of a Salmonella vaccine strain, and the isolated recombinant flagella were used for immunization of mice. Since during the invasion of the parasite into the host the schistosomula migrate first to the lungs, the intranasal route of administration was employed in order to halt the parasite at an early stage of the infection. Such intranasal immunization with this peptide expressed in flagellin, without the addition of adjuvants, resulted in a significant humoral response and also led to protection against challenge infection, manifested as a reduction of the worm burden by an average of 42%.
Infection by several strains of Schistosoma, mainly S. mansoni, S. hematobium, and S. japonicum strains, constitutes a major parasitic disease which afflicts around 200 million people, mostly in developing countries (6). These species are also important pathogens for several domestic animal species and cause economic losses in areas of endemicity. The disease is associated with daily production of eggs by the adult worm. The eggs that fail to escape the body are deposited into the liver, intestine, and genitourinary tract, where they stimulate a strong inflammatory reaction and granuloma formation that eventually leads to death (38).
In many cases drug therapy is ineffective in areas of endemicity, and thus the development of a vaccine is apparently the only practical measure for disease control. The use of irradiated cercariae for vaccination is the best animal model described hitherto, leading to up to 90% protection against challenge infection (25). However, culturing of the parasitic pathogen in large amounts for the purpose of vaccine preparation is completely impractical. Hence, the identification of relevant immunogens and their preparation by synthetic or recombinant DNA technologies are imperative for the development of an antischistosome vaccine (4).
A variety of immunodominant molecules have been described as candidate vaccines against schistosomiasis, since they induce a substantial degree of protection (reviewed by Bergquist [6]). Among these partially protecting schistosome antigens are the following: glutathione S-transferase (GST) (Sm28 GST) (7, 8, 21), the muscle protein paramyosin (Sm97) (22), the irradiation-associated vaccine antigen (IrV-5) (27, 28), triose-phosphate isomerase (9, 23), the membrane antigen Sm23 (24), and fatty acid binding protein 14 (Sm14) (35). These candidate vaccine antigens are either ubiquitous enzymes usually involved in metabolic pathways, muscle proteins, or surface antigens, and they induced 30 to 60% protection against challenge infection. Recently, intranasal (i.n.) immunization of mice with a recombinant BCG strain expressing GST was reported to induce humoral responses as well as antibodies which neutralized the enzyme activity (12). The presence of similar antibodies has been shown to correlate with protection against schistosomiasis in humans (39). Furthermore, efforts are being made to induce specific B-cell and T-helper-cell responses by identifying different antigenic determinants in protective antigens (23, 24). Thus, a cytotoxic T-cell C-terminal lipopeptide has been derived from the vaccine candidate Sm28 GST (21).
Our group has described a protective surface antigen designated 9B-Ag, which comprises two subunits of 45 and 30 kDa (16, 32). It is an abundant protein in the cercariae and schistosomula and is very scarce in the adult worm. 9B-Ag, unique for the parasite, is a highly protective antigen, leading to 45% protection when administered in complete Freund’s adjuvant (CFA) (32) and to 65% protection when delivered within proteosome vesicles (34). We have identified a protective epitope of this antigen, designated 9B peptide-1, and showed that immunization with this epitope conjugated to bovine serum albumin (BSA) led to greater than 40% protection of mice when administered subcutaneously in CFA (33).
An alternative approach to express an epitope is by using recombinant DNA technology. In this approach, synthetic oligonucleotides coding for amino acid sequence of epitopes from various antigens are inserted into an appropriate vector for expression of the respective epitopes (2). The flagellin of a Salmonella vaccine strain was found to be an adequate carrier of epitopes for vaccination against viral and bacterial agents (31). The flagella exhibited built-in adjuvanticity (13) and did not have a carrier suppression effect on the immune response against the inserted epitope (5). Indeed, immunization with the recombinant flagella expressing epitopes of various viral and bacterial pathogens was shown to evoke humoral as well as cellular immune responses against the inserted epitope, which resulted in protection against a challenge infection (13, 18, 19, 30, 37). This approach was used here for designing a vaccine against schistosomiasis.
In the present study we immunized mice with hybrid flagella that express the 9B peptide-1 epitope, administered via the i.n. route, in order to induce a local mucosal immune response in the lungs. We report that such vaccination led to a significant local and systemic humoral response, as well as to protection against challenge infection.
MATERIALS AND METHODS
Mice.
Inbred C57BL/6J mice (2 to 4 months old) from Harlan Laboratories (Rehovot, Israel), as well as outbred CD1 mice (4 to 6 weeks old, for maintenance of the parasite life cycle), were obtained from the Weizmann Institute Animal Breeding Center (Rehovot, Israel). Mice were maintained under specific-pathogen-free conditions, with Purina chow and autoclaved water given ad libitum, at constant room temperature and humidity. Routine health control of the mice included examination for the presence of ecto- and endoparasites, microbiological tests, and serological surveys for viruses.
Parasite.
A Puerto Rican strain of S. mansoni was maintained in outbred CD1 mice and Biomphalaria glabrata snails. S. mansoni cercariae were artificially transformed into schistosomula, and the bodies were separated from the tails in a 60% Percoll gradient (14). These schistosomula were incubated for 3 h at 37°C in defined synthetic medium (DSM) in an atmosphere of 5% CO2. DSM is a mixture of RPMI 1640 and nutrient mixture F12 (1:1) and was obtained from Gibco (Grand Island, N.Y.). Before use, DSM was supplemented with 100 U of penicillin per ml and 100 μg of streptomycin per ml (Kibutz Beit Haemek, Israel), 2 mM l-glutamine (Gibco), and 200 mM HEPES buffer (pH 7.2) (Pharmacia). Adult worms were obtained by liver perfusion from chronically infected mice at 6 to 7 weeks postinfection as described previously (26). Cercariae and schistosomula were homogenized in 20 mM Tris HCl (pH 7.5)–3 mM MgCl2–100 mM NaCl–2 mM CaCl2–10% glycerol containing the protease inhibitors soybean trypsin inhibitor (100 mg/ml), aprotonin (0.2 U/ml), phenylmethylsulfonyl fluoride (2 mM), pepstatin (1 μg/ml), leupeptin (2 μg/ml), and benzamidine (1 mM). The homogenates were then sonicated and centrifuged in a Microfuge (12,800 × g) for 15 min, and the samples were frozen at −70°C. Supernatant fractions were used for enzyme-linked immunosorbent assays (ELISAs).
Infection and determination of disease severity.
Infection with cercariae (larval form of the schistosomiasis parasite) was performed by challenging mice with 70 cercariae by the ring infection method. At 6 to 8 weeks after infection, mice were sacrificed, livers were perfused, and adult worms were counted. Liver perfusion was performed in phosphate-buffered saline (PBS) supplemented with 500 U of heparin (Sigma Chemical Co., St. Louis, Mo.) per ml in order to release adult worms, and the total parasite count was recorded (26). In most cases, comparable numbers of male and female worms were observed.
Preparation of peptide.
9B peptide-1 (GFTTNEERYNVFAE) was synthesized by M. Fridkin (Department of Organic Chemistry, The Weizmann Institute) by the solid-phase method with a multipeptide synthesizer (Abimed AMS 422) and purified by high-pressure liquid chromatography. The peptides were coupled to BSA or hemocyanin by the carbodiimide conjugation method with 1-ethyl-3(3-dimethylaminopropyl)carbodiimide (EDAC) (29). A 1:38 molar ratio of carrier to peptide was used.
Preparation of recombinant flagellin.
The construction of the recombinant bacteria was performed as described by Newton et al. (20). The synthetic oligonucleotide GGT TTC ACT ACT AAC GAA GAA AGA TAT AAC GTT TTC GCT GAA was inserted into plasmid pLS408, which was kindly provided by B. A. Stocker, and eventually transformed into Salmonella dublin SL5928 as described elsewhere (15). The transformed S. dublin was selected for ampicillin resistance and motility under the light microscope. Selected clones were grown overnight in Luria-Bertani medium containing ampicillin, and the flagella were purified by acidic cleavage by the technique described by Ibrahim et al. (11).
Radioactive labeling of protein.
The proteins (recombinant flagella or purified goat antirabbit antibodies) were radioiodinated with 125I by the chloramine-T method as described previously (10).
Immunization and protection studies.
Groups of 8 to 10 mice (C57BL/6J) were immunized i.n. with the recombinant flagella containing 9B peptide-1 or with native flagella (50 μg/50 μl three times with 2- to 3-week intervals). During the immunization, the mice were under light ether anesthesia. Sera were collected 3 weeks after the second immunization and tested by ELISA. At 3 to 4 weeks after the last booster, mice were exposed to challenge infection. The level of protection was calculated from the decrease in worm burden in the immunized mice compared to that in the control group.
ELISA.
Whole parasite sonicate (10 μg/well) or 9B peptide-1 in 2% gluteraldehyde–PBS solution or, alternatively, peptide-protein conjugates (5 μg/ml) in sodium carbonate buffer (pH 9.6) were allowed to adsorb to ELISA plates (Immunoplate; Nunc, Roskilde, Denmark) for 2 h at 25°C or for 18 h at 4°C. The plates were washed three times with PBS containing 0.1% Tween 20 (PBS-Tween). The wells were then blocked for 90 min at 25°C with PBS containing 1% BSA. After three washes with PBS-Tween, serial dilutions of the different sera from the immunized mice, as specified, were added and incubated for 2 h at 37°C or for 18 h at 4°C. Excess antibody was washed off with PBS-Tween, and a second antibody, goat anti-mouse immunoglobulin (Ig) conjugated to peroxidase, was added and incubated for 2 h at 37°C. Finally, 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt (ABTS) was added as a substrate. The color caused by the hydrolysis of the substrate was determined at 414 nm with an ELISA reader (Multiskan MCC/340 MK II; Labsystems, Helsinki, Finland). As a negative control we used normal mouse sera (NMS) obtained from C57BL/6J mice, and as a positive control we used sera from acutely infected mice taken 9 weeks after exposure to 300 cercariae.
Western blotting.
Western blotting of electrophoresed proteins (7 to 10 μg/well) was performed as described by Towbin et al. (36). Proteins were electroblotted from sodium dodecyl sulfate-polyacrylamide gels to nitrocellulose in a 50 mM Tris-glycine buffer (pH 8.3) for 1 h at 200 mA. After blocking with PBS–0.05% Tween 20 containing 10% low-fat (1%) milk, the filter was incubated overnight at 4°C with the antibody samples diluted in PBS. The samples included sera from rabbits immunized subcutaneously with 9B peptide-1 coupled to BSA (1 mg/injection in CFA three times with 2-week intervals). The filter was then washed with blocking solution three times at room temperature for 15 min and twice with PBS–0.05% Tween 20, followed by incubation for 2 h at room temperature with the second antibody (purified goat anti-rabbit Ig) labeled with 125I, added in the blocking solution. Antibody binding was detected by autoradiography.
Complement-dependent cytotoxicity assay.
The susceptibility of schistosomula to antibodies and complement was determined as described previously (32). Briefly, 200 schistosomula in 65 ml of DSM were incubated for 20 min with an equal volume of specific antiserum or control serum diluted 1:2 at 37°C in a 5% CO2 atmosphere. The complement (either fresh or heat-inactivated guinea pig serum at a 1:9 final dilution) was added, and the cultures were incubated for additional 18 h. Live and dead parasites were counted under an inverted microscope.
Statistical analysis.
Statistical analysis was performed with the Stat View II program (Abacus Concepts Inc., Berkley, Calif.) on a Macintosh II Ci. The F test was utilized to calculate probability (P) values. Results are presented as means ± standard errors.
RESULTS
Preparation of recombinant flagellin.
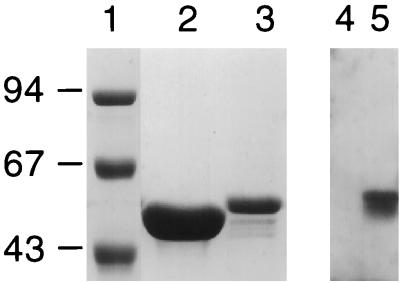
Recombinant flagellin expressing 9B peptide-1 was produced, and the flagella were purified, as described in Materials and Methods. Figure 1 (lanes 1 to 3) shows Coomassie blue staining of the recombinant 9B peptide-1 flagellin and the native flagellin. A molecular weight shift between the recombinant and native proteins is seen. To test the antigenicity of the recombinant protein, sera from rabbits immunized with 9B peptide-1 conjugated to BSA were reacted with the protein by Western blotting (Fig. 1, lanes 4 and 5). A sharp band is visible where the serum against 9B peptide-1 reacts with the recombinant flagellin, while it did not react at all with the native flagellin.
FIG. 1.
Expression of recombinant 9B peptide-1 flagellin protein and its reaction with anti-9B peptide-1 antibodies. Lanes 1 to 3, Coomassie blue staining of purified proteins on sodium dodecyl sulfate–10% polyacrylamide gels. A shift in molecular weight of the recombinant flagellin (lane 3) compared to the native flagellin (lane 2) is seen. Molecular weight markers (in thousands) are shown in lane 1. Lanes 4 and 5, Western blot developed with anti-9B peptide-1–BSA antibodies. No reaction is observed with native flagella (lane 4), while a strong specific reaction is observed with the recombinant 9B-expressing flagella (lane 5).
Biodistribution of flagella expressing 9B peptide-1 after i.n. administration.
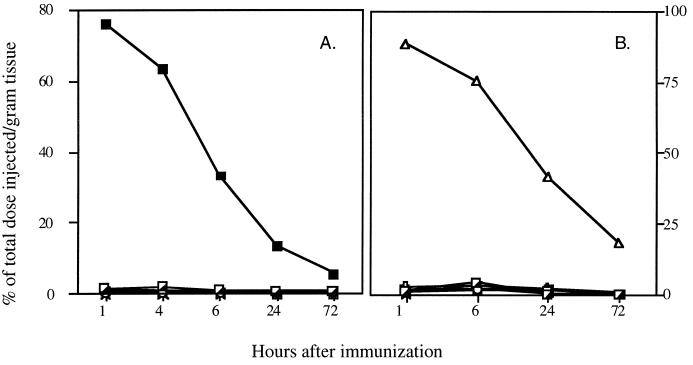
In order to assess the feasibility of using the i.n. route of immunization with the recombinant 9B flagella, it was necessary to determine how long the immunogen stays in the lungs. For that purpose, the flagella were radiolabeled by the chloramine-T method, and their biodistribution after i.n. administration was determined. As shown in Fig. 2, the lungs retained a very high percentage of radioactivity, while almost no radioactivity was observed in the internal organs (e.g., intestine, liver, kidney, heart, gut, and spleen). Thus, at 6 h following the injection, 43% of the total radioactivity per gram of tissue was still present in the lungs (Fig. 2A). In contrast, only marginal amounts were detected either in the lungs or in any of the other internal organs when the radiolabeled vaccine was administered to the footpad (f.p. immunization). In this case, most of the labeled construct was concentrated in the injected leg (Fig. 2B), so that at 6 h after injection, 75% of the total radioactivity was present at this site. This distribution indicates that the flagella do not have affinity to a specific organ and are retained at the administration site until degraded by proteolysis. The prolonged presence in the lungs after i.n. administration enables the elicitation of a local immune response.
FIG. 2.
Pharmacokinetics of 125I-labeled recombinant 9B peptide-1-expressing flagella in mice. Mice were immunized i.n. (A) or f.p. (B) with the labeled protein. The distribution of radioactive protein in the lungs (■), intestine (┌), liver (○), kidney (▭), spleen (Y=), gut (×), heart (+), and leg (▵) was determined at the specified times. A high percentage of the total radioactivity was found at the site of immunization up to 24 h postimmunization.
Humoral response against 9B peptide-1.
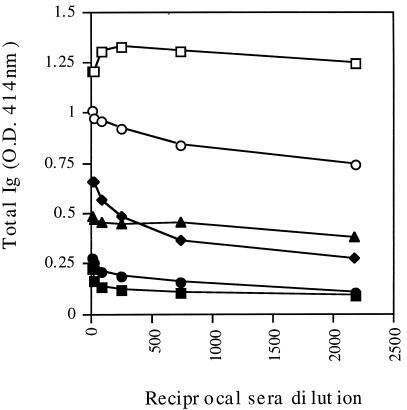
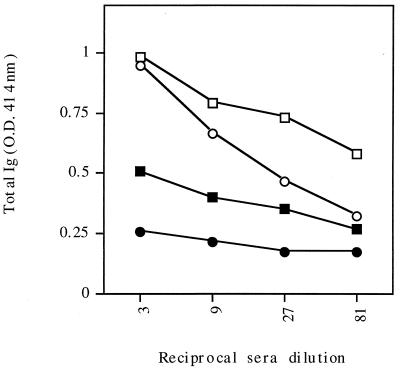
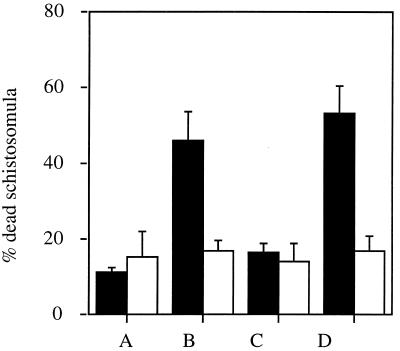
C57BL/6J mice were immunized three times at 3-week intervals either i.n. or f.p. with the recombinant flagella expressing 9B peptide-1. Serum samples obtained 2 to 3 weeks after the last immunization were assayed for the presence of anti-9B peptide-1 antibodies by using ELISA plates coated with 9B peptide-1 conjugated to BSA. Figure 3 shows that both routes of administration led to a marked antibody production compared to that in control groups. A significant antibody titer (total Ig) was also detected in these sera when they were reacted with lysate of either a 2-h schistosomulum extract (10 μg/well) or cercariae (Fig. 4). The antibodies generated by i.n. immunization were tested for their activity in complement-mediated lysis of 3.5-h schistosomula. As seen in Fig. 5, serum antibodies against 9B peptide-1-expressing-flagella were effective in mediating 46% lysis of the parasite, while serum antibodies against native flagella caused only 16% lysis and NMS led to 11% lysis. Sera from infected C57BL/6J mice (2 months postinfection), used as a positive control, induced 53% lysis. Controls of heat-inactivated complement produced a maximum of 17% lysis, emphasizing the specificity of the anti-9B peptide-1 complement-mediated lysis.
FIG. 3.
Recognition of 9B peptide-1 by serum antibodies. C57BL/6J mice were immunized twice with recombinant 9B peptide-1-expressing flagella i.n. (□) or f.p. (○), native flagella i.n. (⧫) or f.p. (▴), or serum from a CFA-injected mouse f.p. (■) and NMS (●) as controls. The plate was coated with 5 μg of 9B peptide-1 conjugated to BSA per ml. O.D., optical density.
FIG. 4.
Recognition of 2-h schistosomula and cercariae by serum antibodies of immunized mice. C57BL/6J mice were immunized i.n. twice with recombinant 9B peptide-1-expressing flagella (empty symbols) or native flagella (filled symbols). ELISA plates were coated with a 10-μg/ml concentration of lysate of either 2-h schistosomula (squares) or cercariae (circles). O.D., optical density.
FIG. 5.
Complement-mediated lysis of 3.5-h schistosomula by serum antibodies elicited against recombinant 9B peptide-1-expressing flagella. Lysis of schistosomula in the presence of sera from normal C57BL/6J mice (bars A), sera from mice immunized i.n. with recombinant (bars B) or native (bars C) flagella, and sera from infected animals (positive control) (bars D) is shown. Guinea pig serum was used as a source of complement (■), and lysis was compared to that in the presence of heat-inactivated complement (□). Results are means and standard errors from three experiments.
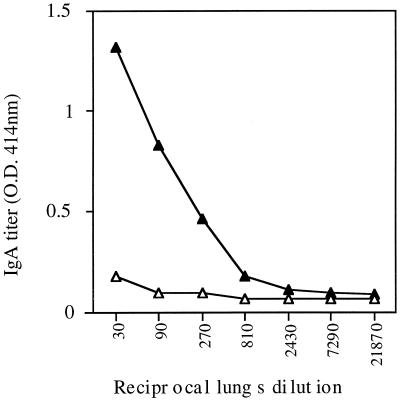
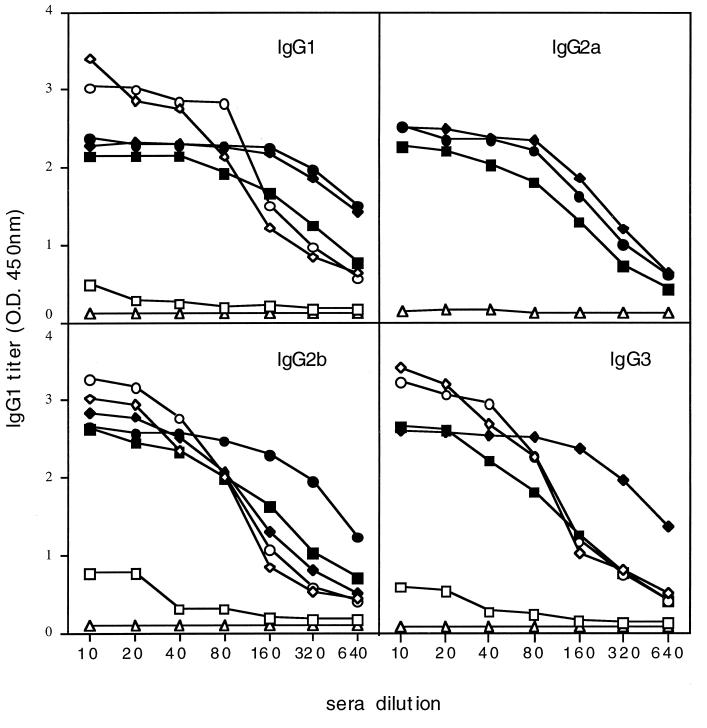
Further examination of the antibody titer as a result of i.n. administration of 9B peptide-1-expressing flagella revealed a strong local humoral response, manifested as high titers of lung IgA antibodies, specific to 9B peptide-1, compared to a negligible response towards native flagella (Fig. 6). The i.n. immunization also leads to high levels of circulating IgA in the serum. It should be mentioned that both the i.n. and f.p. routes of immunization led to an IgG response in the serum, and the isotype profile indicates the involvement of both Th1 and Th2 responses. The levels of IgG1 (which is presumed to be involved with the Th2 response), as well as IgG2a, IgG2b, and IgG3 (Th1), reactive with the 9B peptide-1 flagellin construct and the free 9B peptide-1 are shown in Fig. 7. The results demonstrate that the two routes of administration led to comparable responses.
FIG. 6.
Local humoral response against 9B peptide-1 in the lungs of immunized mice. C57BL/6J mice were immunized twice i.n. with recombinant 9B-expressing flagella (▴) or with native flagella (▵). Their lungs were removed, homogenized in PBS, and tested by ELISA for the IgA response against 9B peptide-1 conjugated to BSA. O.D., optical density.
FIG. 7.
Isotype profile of serum antibodies following i.n. or f.p. immunization. Serum samples were obtained 2 to 3 weeks after the last immunization of mice. The mice were immunized i.n. either with the native flagellin (squares) or with recombinant flagellin (diamonds) or f.p. with recombinant flagellin (circles). Results with preimmune serum are indicated by triangles. The coating antigen for the ELISA microplate was either the recombinant 9B peptide-1 flagellin (empty symbols) or 9B peptide-1 (filled symbols). The second antibody reacts specifically with different isotypes of the serum antibodies (IgG1, IgG2a, IgG2b, and IgG3) as indicated.
We were not able to detect T-cell proliferation towards 9B peptide-1 upon immunization of either C57BL/6J or BALB/c mice. Although there was a very high proliferative response to the flagellum carrier after either i.n. or f.p. immunization with the recombinant flagella, many attempts to elicit a cellular response against the parasite or 9B peptide-1 failed. Thus, no specific T-cell proliferation in response to the peptide was detected in either spleens or lymph nodes of the mice, whether they were immunized with the recombinant 9B peptide-1-expressing flagella or with a conjugate of 9B peptide-1 and either BSA or ovalbumin (OVA), by either route of immunization. This indicates that most probably 9B peptide-1 is not a T-cell epitope.
Protection against cercaria infection.
The ability of the flagella expressing 9B peptide-1, administered i.n., to elicit protection from infection by the parasite in the immunized mice was examined by challenge infection with cercariae and determination of the worm burden 6 to 8 weeks later by liver perfusion. In these experiments, which are summarized in Table 1, i.n. immunization resulted in significant protection, ranging from 30 to 53%, as determined by the reduction in worm burden.
TABLE 1.
Protection of mice from infection by S. mansoni after i.n. vaccination with recombinant 9B peptide-1-expressing flagella, in comparison to f.p. immunizationa
| Expt no. | Route | Antigen | No. of worms (mean ± SE) | % Protectionb |
|---|---|---|---|---|
| 1 | i.n. | Fla-9B | 25.88 ± 2.33 | 30* |
| Native Fla | 35.85 ± 2.31 | 2 | ||
| None | 36.57 ± 4.70 | 0 | ||
| 2 | i.n. | Fla-9B | 6.25 ± 2.06 | 53* |
| Native Fla | 18.37 ± 2.87 | 0 | ||
| None | 13.50 ± 1.33 | 0 | ||
| 3 | i.n. | Fla-9B | 27.40 ± 3.22 | 42* |
| Native Fla | 36.33 ± 3.00 | 4 | ||
| None | 38.00 ± 4.00 | 0 | ||
| 4 | f.p. | Fla-9B | 24.40 ± 1.59 | 32* |
| Native Fla | 35.00 ± 2.87 | 0 | ||
| None | 34.00 ± 1.00 | 0 | ||
| 5–8 | f.p. | BSA-9B | 17.75 ± 2.75 | 39* |
| BSA | 32.00 ± 3.55 | 0 | ||
| None | 29.33 ± 1.15 | 0 |
In three replicate experiments, 8 to 10 C57BL/6J mice per group were immunized i.n. three times with either recombinant (Fla-9B) or native (Native Fla) flagella, and 4 weeks later they were infected with cercariae. At 7 to 8 weeks after infection, liver perfusion was performed and the number of adult worms was counted.
Calculated from the number of worms in the immunized mice compared to that in the untreated mice. *, P < 0.05.
DISCUSSION
The fact that 9B peptide-1 conjugated to BSA elicited protective immunity in mice (33) prompted us to investigate whether it would also be effective via i.n. immunization. The results presented above demonstrate that a synthetic recombinant construct in which the 9B peptide-1 epitope of S. mansoni was expressed in the flagellin of an S. dublin vaccine strain led to protection of mice against schistosomiasis. Furthermore, i.n. vaccination with this construct was effective, leading to significant protection, as manifested by an average 42% reduction of the adult worm burden.
One of the current goals in the field of vaccines is the development of noninvasive and practical routes of administration via mucosal surfaces. The rationale for i.n. vaccination against schistosomiasis is based on the fact that shortly after skin penetration of the mammalian host, the schistosomula migrate to the lungs and reside there for several days before they reach the liver, where they develop into sexually mature worms that can lay eggs. Hence, effective immunity in the lungs could help in eliminating the infection at the earliest stage after parasite invasion. Biodistribution experiments demonstrated that following i.n. administration of the recombinant 9B peptide-1-expressing flagella, it persisted for more than 6 h in the lungs. This indicates that although a short peptide by itself is usually nonimmunogenic and undergoes proteolytic degradation within 1 h (17), its expression as a part of a fusion protein with flagella enables its prolonged (>6-h) exposure to the host immune system. The prolonged retention in the lungs, taken together with our previous observation that the flagellin serves both as a carrier and as an adjuvant for the epitope it expresses (13), renders this recombinant antigen suitable for the induction of an efficient immune response, both systemic and local, in the lung mucosal tissue.
Indeed, high levels of anti-9B peptide-1 serum antibodies, as well as high titers of IgA in the lungs, were obtained after i.n. immunization with the recombinant flagella, which correlated with a protective effect against challenge infection. This is in accord with previous reports that IgA antibodies contribute to the protective immunity against schistosomiasis both in humans and in animal models (39). It has thus been shown that in humans there is a close association between the production of IgA antibodies to Sm28 GST and a decrease in egg production as well as viability (8). It has also been reported that the resistant population among S. mansoni-infected human subjects had high titers of IgA antibodies to a particular peptide derived from GST (3). The importance of both Th1 and Th2 responses for protection against schistosome infection was shown by Anderson et al. (1). Our data shows that the isotype profile of the serum antibodies towards the recombinant flagellum vaccine evokes both Th1 and Th2 immunity.
The humoral immune response evoked by the 9B peptide-1-expressing flagella also seems to be adequate. The serum antibodies that it elicited are reactive with the whole parasite extract, both cercarial and schistosomular antigens, while antibodies generated in response to immunization with the native flagella did not show such a reaction. Furthermore, the functional role of these serum antibodies in the mice immunized i.n. with the 9B peptide-1-expressing flagella was demonstrated by their effectivity in complement-mediated lysis, the level of which was comparable to that elicited by sera from infected mice. It is noteworthy that similarly active antibodies were previously induced by f.p. immunization of mice with 9B peptide-1 coupled to BSA (33).
The level of protection obtained by the i.n. immunization with 9B peptide-1 (30 to 50%) is comparable to that obtained by f.p. immunization with this peptide coupled to a carrier protein like BSA (33). The extent of protection is within the range of protection obtained with other vaccine candidates, which is between 30 and 60% (6). However, the advantage of the approach employed in the present study is that the immunizing agent was a defined peptide, and immunization was via the convenient, noninvasive i.n. route, without the need for any adjuvants. Elimination of the parasite from the host at an early stage of the infection decreases the worm burden and therefore reduces both morbidity and pathology (26).
ACKNOWLEDGMENT
This study was supported in part by a grant B104-CT98-0294 (DG12 SSM1) from Fourth Framework Programme of the EU.
REFERENCES
- 1.Anderson S, Shires V L, Wilson R A, Mountford A P. In the absence of IL-2, the induction of Th1-mediated protective immunity by the attenuated schistosome vaccine is impaired, revealing an alternative pathway with Th2-type characteristics. Eur J Immunol. 1998;28:2827–2838. doi: 10.1002/(SICI)1521-4141(199809)28:09<2827::AID-IMMU2827>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 2.Arnon A, Levi R. Synthetic recombinant vaccines against viral agents. Int Arch Allergy Immunol. 1995;108:321–326. doi: 10.1159/000237175. [DOI] [PubMed] [Google Scholar]
- 3.Auriault C, Gras M H, Pierce R J, Butterworth A E, Wolowczuk I, Capron M, Ouma J H, Balloul J M, Khalife J, Neyrinck J L, et al. Antibody response of Schistosoma mansoni-infected human subjects to the recombinant P28 glutathione-S-transferase and to synthetic peptides. J Clin Microbiol. 1990;28:1918–1924. doi: 10.1128/jcm.28.9.1918-1924.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Yedidia T, Arnon R. Design of peptide and polypeptide vaccines. Current Opin Biotechnol. 1997;8:442–448. doi: 10.1016/s0958-1669(97)80066-3. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Yedidia T, Arnon R. Effect of pre-existing carrier immunity on the efficacy of synthetic influenza vaccine. Immunol Lett. 1998;64:9–15. doi: 10.1016/s0165-2478(98)00073-x. [DOI] [PubMed] [Google Scholar]
- 6.Bergquist N R. Controlling schistosomiasis by vaccination: a realistic opinion? Parasitol Today. 1995;11:191–194. [Google Scholar]
- 7.Capron A, Dessaint J P, Capron M, Pierce R J. Vaccine strategies against schistosomiasis. Mem Inst Oswaldo Cruz. 1992;4:19–27. doi: 10.1590/s0074-02761992000800003. [DOI] [PubMed] [Google Scholar]
- 8.Capron A, Riveau G, Grzych J M, Boulanger D, Capron M, Pierce R. Development of a vaccine strategy against human and bovine schistosomiasis. Background and update. Trop Geogr Med. 1994;46:242–246. [PubMed] [Google Scholar]
- 9.Harn D A, Gu W, Oligino L D, Mitsuyama M, Gebremichael A, Richter D. A protective monoclonal antibody specifically recognizes and alters the catalytic activity of schistosome triose-phosphate isomerase. J Immunol. 1992;148:562–567. [PubMed] [Google Scholar]
- 10.Hunter W M, Greenwood F C. Preparation of iodine 131 labeled growth hormone of high specific activity. Nature. 1962;194:595–596. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim G F, Fleet G H, Lyons M L, Walter R A. Method for the isolation of highly purified Salmonella flagellin. J Clin Microbiol. 1985;22:1040–1044. doi: 10.1128/jcm.22.6.1040-1044.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kremer L, Riveau G, Baulard A, Capron A, Locht C. Neutralizing antibody responses elicited in mice immunized with recombinant bacillus Calmette-Guerin producing the Schistosoma mansoni glutathione S-transferase. J Immunol. 1996;156:4309–4317. [PubMed] [Google Scholar]
- 13.Levi R, Arnon R. Synthetic recombinant influenza vaccine induces efficient long-term immunity and cross-strain protection. Vaccine. 1996;14:85–92. doi: 10.1016/0264-410x(95)00088-i. [DOI] [PubMed] [Google Scholar]
- 14.Levi-Schaffer F, Schryer M D, Smolarsky M. The resistance of schistosomula of Schistosoma mansoni to antibodies and complement in-vitro does not correlate with the binding of antibodies to the surface of the parasite. J Immunol. 1982;129:2744–2751. [PubMed] [Google Scholar]
- 15.McEwen J, Levi R, Horwitz R J, Arnon R. Synthetic recombinant vaccine expressing influenza haemagglutinin epitope in Salmonella flagellin leads to partial protection in mice. Vaccine. 1992;10:405–411. doi: 10.1016/0264-410x(92)90071-q. [DOI] [PubMed] [Google Scholar]
- 16.Mendlovic F, Arnon R, Tarrab-Hazdai R, Puri J. Genetic control of immune response to a purified Schistosoma mansoni antigen. II. Establishment and characterization of specific I-A and I-E restricted T-cell clones. Parasite Immunol. 1989;11:683–694. doi: 10.1111/j.1365-3024.1989.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 17.Muller S, Benkirane N, Guichard G, Van Regenmortel M, Brown F. The potential of retro-inverso peptides as synthetic vaccines. Exp Opin Invest Drugs. 1998;7:1429–1438. doi: 10.1517/13543784.7.9.1429. [DOI] [PubMed] [Google Scholar]
- 18.Newton S M, Joys T M, Anderson S A, Kennedy R C, Hovi M E, Stocker B A. Expression and immunogenicity of an 18-residue epitope of HIV1 gp41 inserted in the flagellar protein of a Salmonella live vaccine. Res Microbiol. 1995;146:203–216. doi: 10.1016/0923-2508(96)80276-2. [DOI] [PubMed] [Google Scholar]
- 19.Newton S M, Kotb M, Poirier T P, Stocker B A, Beachey E H. Expression and immunogenicity of a streptococcal M protein epitope inserted in Salmonella flagellin. Infect Immun. 1991;59:2158–2165. doi: 10.1128/iai.59.6.2158-2165.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton S M, Jacob C O, Stocker B A. Immune response to cholera toxin epitope inserted in Salmonella flagellin expression and live-vaccine potential. Science. 1989;244:70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- 21.Pancre V, Gras-Masse H, Delanoye A, Herno J, Capron A, Auriault C. Induction of cytotoxic T-cell activity by the protective antigen of Schistosoma mansoni Sm28GST or its derived C-terminal lipopeptide. Scand J Immunol. 1996;44:485–492. doi: 10.1046/j.1365-3083.1996.d01-340.x. [DOI] [PubMed] [Google Scholar]
- 22.Pearce E J, James S L, Hieny S, Lanar D E, Sher A. Induction of protective immunity against Schistosoma mansoni by vaccination with schistosome paramyosin (Sm97), a nonsurface parasite antigen. Proc Natl Acad Sci USA. 1988;85:5678–5682. doi: 10.1073/pnas.85.15.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds S R, Dahl C E, Harn D A. T and B epitope determination and analysis of multiple antigenic peptides for Schistosoma mansoni experimental vaccine triose-phosphate isomerase. J Immunol. 1994;152:193–200. [PubMed] [Google Scholar]
- 24.Reynolds S R, Schoemaker C B, Harn D A. T and B cell epitope mapping of SM 23, an integral membrane protein of Schistosoma mansoni. J Immunol. 1992;149:3995–4001. [PubMed] [Google Scholar]
- 25.Richter D, Incani R N, Harn D A. Isotype responses to candidate vaccine antigens in protective sera obtained from mice vaccinated with irradiated cercariae of Schistosoma mansoni. Infect Immun. 1993;61:3003–3011. doi: 10.1128/iai.61.7.3003-3011.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smithers S R, Terry R J. The infection of laboratory hosts with cercariae of S. mansoni and the recovery of adults worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- 27.Soisson L A, Reid G D R, Farah I O, Nyindo M, Strand M. Protective immunity in baboons vaccinated with a recombinant antigen or radiation-attenuated cercariae of Schistosoma mansoni is antibody-dependent. J Immunol. 1993;151:4782–4789. [PubMed] [Google Scholar]
- 28.Soisson L M A, Masterson C P, Tom T D, McNally M T, Lowell G H, Strand M. Induction of protective immunity in mice using a 62-kDa recombinant fragment of a Schistosoma mansoni surface antigen. J Immunol. 1992;149:3612–3620. [PubMed] [Google Scholar]
- 29.Staros J V, Wright R W, Swingle D M. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986;156:220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- 30.Stocker B A. Aromatic-dependent Salmonella as live vaccine presenters of foreign epitopes as inserts in flagellin. Res Microbiol. 1990;141:787–796. doi: 10.1016/0923-2508(90)90112-4. [DOI] [PubMed] [Google Scholar]
- 31.Stocker B A, Newton S M. Immune responses to epitopes inserted in Salmonella flagellin. Int Rev Immunol. 1994;11:167–178. doi: 10.3109/08830189409061724. [DOI] [PubMed] [Google Scholar]
- 32.Tarrab-Hazdai R, Levi-schaffer F, Brenner V, Horowitz S, Eshhar Z, Arnon R. Protective monoclonal antibodies against Schistosoma mansoni. Antigen isolation, characterization and suitability for active immunization. J Immunol. 1985;135:2772–2779. [PubMed] [Google Scholar]
- 33.Tarrab-Hazdai R, Schechtman D, Arnon R. Synthesis and characterization of a protective peptide-based vaccine against Schistosoma mansoni. Infect Immun. 1998;66:4526–4530. doi: 10.1128/iai.66.9.4526-4530.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarrab-Hazdai R, Schechtman D, Lowell G, Pirak E, Arnon R. Proteosome delivery of a protective 9B-antigen against Schistosoma mansoni. Int J Immunopharmacol. 1999;21:205–218. doi: 10.1016/s0192-0561(98)00083-6. [DOI] [PubMed] [Google Scholar]
- 35.Tendler M, Brito C A, Vilar M M, Serra-Freire N, Diogo C M, Almeida M S, Delbem A C B, Silva J F DA, Savino W, Garratt R C, et al. A Schistosoma mansoni fatty acid-binding protein, Sm14, is the potential basis of a dual-purpose anti-helminth vaccine. Proc Natl Acad Sci USA. 1996;93:269–273. doi: 10.1073/pnas.93.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Towbin H, Stahelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma N K, Ziegler H K, Stocker B A, Schoolnik G K. Induction of a cellular immune response to a defined T-cell epitope as an insert in the flagellin of a live vaccine strain of Salmonella. Vaccine. 1995;13:235–244. doi: 10.1016/0264-410x(95)93308-v. [DOI] [PubMed] [Google Scholar]
- 38.Warren K S, Domingo E Q, Cowan R T. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967;51:735–756. [PMC free article] [PubMed] [Google Scholar]
- 39.Yang W, Gobert G N, McManus D P. Oral vaccination of mice with recombinant Schistosoma japonicum proteins induces specific anti-parasite antibodies and damage to adult worms after a challenge infection. Int J Parasitol. 1997;27:843–853. doi: 10.1016/s0020-7519(97)00053-2. [DOI] [PubMed] [Google Scholar]