Abstract
During organismal development, organs and systems are built following a genetic blueprint that produces structures capable of performing specific physiological functions. Interestingly, we have learned that the physiological activities of developing tissues also contribute to their own morphogenesis. Specifically, physiological activities such as fluid secretion and cell contractility generate hydrostatic pressure that can act as a morphogenetic force. Here, we first review the role of hydrostatic pressure in tube formation during animal development and discuss mathematical models of lumen formation. We then illustrate specific roles of the notochord as a hydrostatic scaffold in anterior-posterior axis development in chordates. Finally, we cover some examples of how fluid flows influence morphogenetic processes in other developmental contexts. Understanding how fluid forces act during development will be key for uncovering the self-organizing principles that control morphogenesis.
Keywords: hydrostatic pressure, tube formation, lumen, morphogenesis, notochord, axis elongation
1. INTRODUCTION
More than 100 years ago, D’Arcy Thompson (1917) approached the problem of morphogenesis from a physical perspective and attempted to establish mathematical relationships akin to laws of organismal development. That concept has slowly been gaining momentum, leading in recent years to a strong interest in understanding the biophysical principles of morphogenesis. One of the most active areas has been tissue mechanics, which, propelled by advances in quantitative biology, has yielded important findings and concepts that help understand the connections between growth, mechanical stress, and morphogenesis (Irvine & Shraiman 2017). While this field has made considerable progress in understanding how contractile systems and the cell cortex define the mechanical properties of tissues, much less is known about the role of fluid mechanics in animal morphogenesis. This is somewhat surprising, as the applicability of hydraulics to animal physiology was already established by the work of Ernest Starling (1896) at the end of the nineteenth century and has been an area of sophisticated research in plant biology for decades. However, in recent years, genetic and biophysical studies have uncovered a broad potential for fluid mechanics as a driving force in morphogenetic processes. In this context, the potential of hydrostatic pressure as a morphogenetic force is particularly interesting, as it can act at a long range and exert considerable force. Importantly, hydrostatic pressure can be regulated rapidly through osmotic mechanisms by the output of tissue physiology, thus providing a mechanism for dynamically controlling morphogenetic events during development.
The effects of hydrostatic pressure on morphogenesis are tightly linked to the material properties of biological tissues and how these respond to forces acting upon them. Biological tubes are made of soft materials and, therefore, for their lumen to be open, they must be filled by a substance that can generate internal pressure and/or act as a scaffold (Navis & Bagnat 2015). Typically, in biological tubes, the lumen contains fluid, mostly liquid, or gas in the case of airways. Sometimes, most commonly in invertebrates, gels or solid matrix can also be found as a luminal scaffold. Interestingly, these luminal contents are produced and secreted by the epithelial cells surrounding the lumen and generally are the product of or are substances related to the physiological function of the organ in which they reside. Thus, the function of the organ contributes to its morphogenesis. Here, we review major findings linking hydrostatic pressure generation to lumen and tube morphogenesis during animal development. We also discuss recent mathematical models of lumen formation that feature prominent roles for hydrostatic pressure. Then, we review recent findings on the role of hydrostatic pressure in the notochord in axial development. Finally, we cover some specific examples in which other aspects of fluid mechanics have been implicated in developmental processes.
2. ROLES OF HYDROSTATIC PRESSURE IN TUBE MORPHOGENESIS
In this section, we examine how hydrostatic pressure contributes to tube formation in metazoans. We first discuss the basic physical principles governing lumen formation. Next, we review the biological processes and tissue products involved in lumen expansion and coalescence and consider some ways in which the environment can physically contribute to tube formation. Then, we consider the role of hydrostatic pressure in tube patterning, cell fate specification, and signaling.
2.1. Basic Physical and Physiological Principles of Lumen Formation in Tubes
To introduce the basic physical principles governing lumen expansion, we consider an ideal scenario in which a lumen can be approximated as a spherical, fluid-filled cavity with a boundary consisting of a uniform layer of epithelial cells (see Figure 1a). Note that in biological systems, water always follows the distribution of solutes (e.g., ions and small molecules), and it is the transport of these solutes at the expense of energy that defines how water is distributed between compartments. When the concentration of solutes inside the lumen (cin) is larger than that outside (cout), an osmotic imbalance, i.e., osmotic pressure (ΔΠ) is created (see Figure 1b). This leads to fluid influx (Jf), which generates internal fluid pressure (Pin). Methods for measuring or estimating luminal pressure are compiled in a recent review (Chan & Hiiragi 2020). The lumen inflates only if Pin is greater than the fluid pressure outside (Pout) the lumen boundary. The resulting hydrostatic pressure (ΔP = Pin − Pout) due to lumen expansion obeys the Laplace law (see Figure 1c). As the lumen size grows, Pin increases while Pout remains the same, resulting in increased hydrostatic pressure. This increased internal pressure generates a larger stress (σ) on the lumen walls, which in turn opposes further influx of fluid. Therefore, lumen growth is controlled by a balance of two opposing mechanisms: the osmotic pressure that supports expansion by fluid influx and generates the internal pressure and the counterbalancing hydrostatic pressure that generates wall stress and opposes the influx. The fluid flux (Jf) is therefore given by Figure 1d. For a lumen with steady size, Jf = 0, whereas for an expanding lumen, Jf > 0, and for a contracting lumen, Jf < 0. Here, we have not added the equation for ion flux nor the cross-coupling of ions and water, and we instead refer the reader to an excellent recent theoretical review (Torres-Sánchez et al. 2021). Further, because ions are charged, their motion is also influenced by the electric field across the lumen boundary. Moreover, due to hydrostatic pressure, the lumen wall is under stress, and the lumen shape and size also depend upon whether the tissue response to the stress is elastic or viscoelastic (see Section 3).
Figure 1.
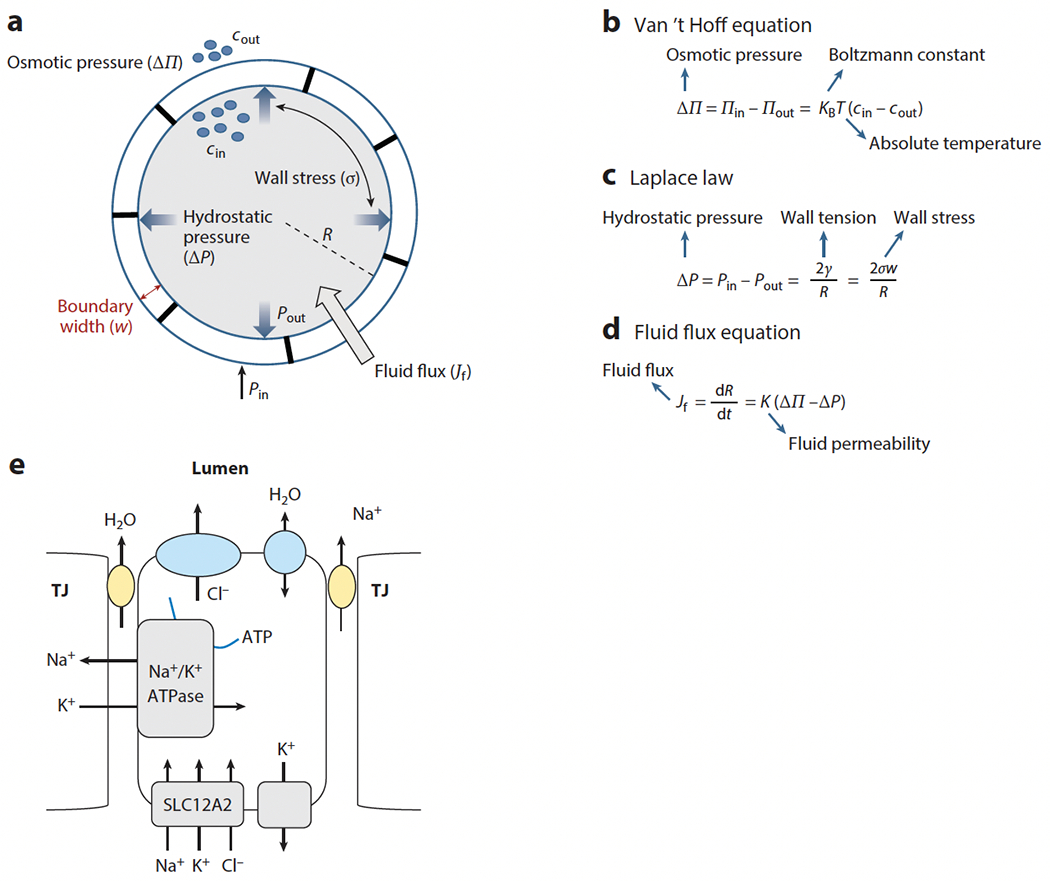
Physical and physiological principles of fluid transport in epithelial tubes. (a) Schematic of basic parameters ruling fluid flux in epithelial tubes. The symbols cin/cout and Pin/Pout are the solute concentrations and pressures inside and outside the lumen, respectively. R is the lumen radius, and w is the width of the epithelial wall. (b–d) Basic parameters and laws ruling fluid flux. (e) Schematic of water and ion transport across an epithelial layer. Water may be transported via the paracellular or transcellular route. TJ stands for tight junction.
The generation of luminal pressure is crucial for lumen expansion (Mosaliganti et al. 2019, Navis et al. 2013), and this occurs via the accumulation of fluid and/or secreted cargos ruled by osmotic forces. Typically, the transport of ions and other solutes occurs downstream of the electrochemical gradient generated by the Na+/K+-ATPase, which generates a gradient of K+ that promotes the influx of Cl− into cells via channels of the Na+/K+/Cl− cotransporter (NKCC) family, such as SLC12A2. Cl− may then be transported across the apical membrane via the cystic fibrosis transmembrane conductance regulator (CFTR) or ANO1 channels. While the electrochemical mechanisms driving Na+ and water transport across epithelia show tissue specificity and are still intensively debated (Larsen & Sorensen 2020), it has become clear that tight junctions (TJs) play a key role. TJs have been shown to form ion-selective pores (Colegio et al. 2002, Furuse et al. 2001) and are likely also the main route for transepithelial water transport (Tanaka et al. 2017). However, in some epithelia, aquaporins also provide a significant transcellular route for water (MacAulay 2021). Generally, water transport into the lumen occurs via paracellular (i.e., through TJs) transport of Na+ and water as well as transcellular transport of Cl− (Figure 1c). Thus, via regulating the expression, localization, and activity of the Na+/K+-ATPase, ion channels and TJs of epithelial cells control fluid flow and lumen expansion in tissues (Navis & Bagnat 2015). In turn, these processes also interact with activities such as cell contractility and physical boundaries provided by surrounding tissues to shape lumen formation and other morphogenetic processes such as budding. Understanding how these activities are cross-regulated and how they are balanced by mechanical feedback is therefore key to understanding organ formation and physiology.
2.2. Lumen Expansion via Fluid Accumulation and Cargo Secretion
Early work using embryological manipulations in chick embryos showed that cerebrospinal fluid pressure is required for brain ventricle expansion (Desmond & Jacobson 1977). Similarly, surgical perturbations and physiological measurements in the developing sheep lung suggested that fluid secreted by the epithelium is important for lung morphogenesis (Harding & Hooper 1996). However, it was the advent of forward genetic screens in Drosophila that opened the way to understanding the role of luminal contents in tube formation. Genetic data implicated secreted proteins and chitin synthesis and deposition in tracheal tube morphogenesis and size control (Devine et al. 2005, Jazwinska et al. 2003, Tonning et al. 2005). This phenomenon was clearly illustrated by a remarkable live microscopy study (Tsarouhas et al. 2007) revealing that the Drosophila tracheal system lumen is initially filled with solid chitin, which is then hydrolyzed, cleared, and ultimately replaced by gas. Around the same time, studies in zebrafish (Lowery & Sive 2005) implicated the Na+/K+-ATPase in brain ventricle inflation and provided clear genetic evidence supporting a role for fluid accumulation in lumen expansion. These initial studies implicated luminal pressure and fluid or cargo secretion in lumen formation and highlighted a major role for the Na+/K+-ATPase. However, tissues differ in how they generate hydrostatic pressure, which can be done via fluid or cargo secretion, and in the mechanisms controlling fluid secretion. The main difference stems from whether secretion occurs mainly via the paracellular route (i.e., through TJs) or the transcellular route, which also requires the activity of apical ion channels.
2.2.1. Transcellular and paracellular control of fluid secretion.
Studies in the zebrafish gut, ear, and Kupffer’s vesicle (KV), and from mammalian systems including the salivary glands, lungs, and the blastocyst, have shown that lumen formation in vivo is subject to genetic and physiological regulation. Work in these systems identified key ion channels and regulatory mechanisms controlling fluid secretion and lumen formation. In the zebrafish gut, the transcription factor Hnf1β regulates single lumen formation by controlling the expression of the Na+/K+-ATPase and the TJ protein claudin 15 (Bagnat et al. 2007). Paracellular transport via TJs promotes fluid accumulation into small nascent lumina and their coalescence into a single lumen (Figure 2a). However, single lumen formation in the zebrafish gut involves a biphasic process in which multiple small lumina initially expand and coalesce to yield intermediates with large lumina that are then resolved into one continuous lumen via the Rab11-dependent remodeling of adhesions (Alvers et al. 2014) (Figure 2a). Processes akin to the first phase of lumen formation in the zebrafish gut were then described in the zebrafish KV (Navis et al. 2013), otic vesicle (Abbas & Whitfield 2009, Hoijman et al. 2015, Mosaliganti et al. 2019, Swinburne et al. 2018), and brain (Zhang et al. 2010), and in the mouse blastocyst (Chan et al. 2019, Dumortier et al. 2019, Ryan et al. 2019). Inhibition of the Na+/K+-ATPase, quantitative microscopy, and pressure measurements in the zebrafish otic vesicle (Mosaliganti et al. 2019) and the mouse blastocyst (Chan et al. 2019, Ruiz-Herrero et al. 2017) provided direct evidence consistent with the general physical principles described above (Figure 1) and served as the basis for mathematical models of lumen formation (see Section 3).
Figure 2.
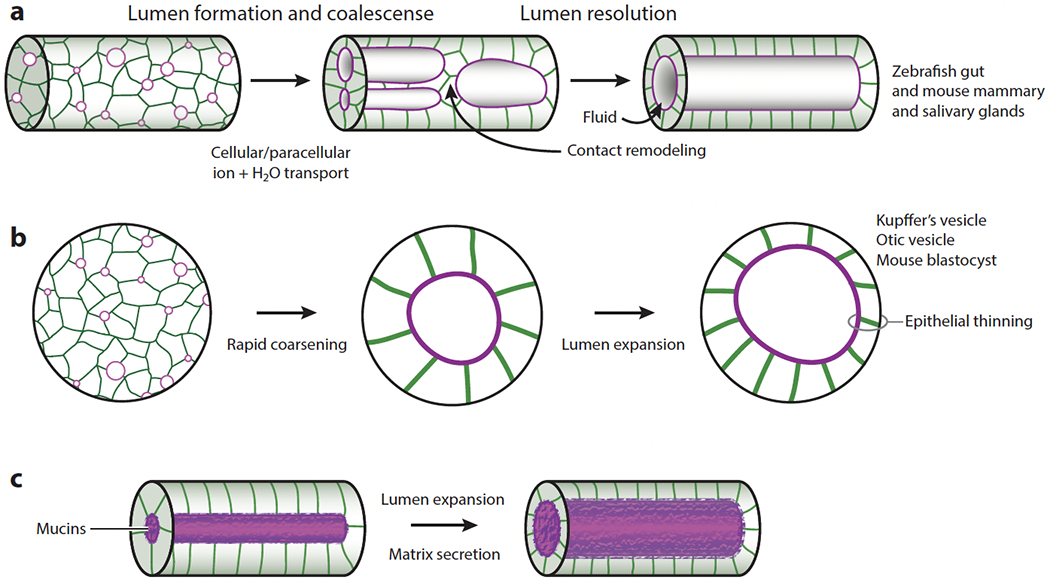
Expansion and coalescence of multiple lumina. (a) In the zebrafish gut, multiple lumina form, expand via the paracellular transport of ions and water, and coalesce into an intermediate with a double lumen or large lumina. Lumen resolution requires contact remodeling. Similar processes occur in the mammalian mammary and salivary glands (Nedvetsky et al. 2014). Panel a adapted from Alvers et al. (2014). (b) Rapid coarsening of multiple lumina and lumen expansion via transcellular transport in the zebrafish Kupffer’s vesicle (KV) and otic vesicle and in the mouse blastocyst. Epithelial thinning occurs in the zebrafish KV (A. Dasgupta et al. 2018) and otic vesicle (Hoijman et al. 2015, Mosaliganti et al. 2019). (c) Lumen expansion via mucin secretion in the Drosophila hindgut (Syed et al. 2012) and ommatidium (Husain et al. 2006).
In other organs, lumen formation is driven by transcellular transport. For example, KV morphogenesis relies on transcellular transport via the apical Cftr channel (Navis et al. 2013), which allows rapid coalescence of the small nascent lumina (Figure 2b) and is enhanced by cyclic AMP (cAMP) and facilitated by cytokinetic bridge formation (Rathbun et al. 2020). Transcellular transport also seems to underlie excretory canal formation in Caenorhabditis elegans (Khan et al. 2013, Kolotuev et al. 2013) and in the ascidian notochord, which relies on the Slc26 chloride channel (Deng et al. 2013). Lumen formation in the mouse salivary gland is similar to that in the zebrafish gut but instead relies on transcellular transport and is controlled by innervation. Vasoactive intestinal peptide secretion from parasympathetic ganglia was shown to promote ductal growth and lumen coalescence via cAMP-dependent CFTR activation (Nedvetsky et al. 2014). Control of secretion by innervation offers the possibility of interorgan and systemic regulation and may underlie development and physiological regulation of secretion in other organs such as the pancreas (Yang et al. 2022). Themes common to all these mechanisms are the requirement of Na+/K+-ATPase activity as the driving force for fluid secretion and the stimulatory role of cAMP, which acts on apical channels such as CFTR and is thus a major regulator of hydrostatic pressure.
2.2.2. Secreted cargos in lumen opening.
While fluid secretion is the major driver of lumen expansion in most systems, secretion of cargos has also been implicated in tube morphogenesis in the Drosophila trachea (Dong et al. 2013, 2014; Forster et al. 2010; Grieder et al. 2008; Jazwinska et al. 2003; Rosa et al. 2018) and salivary glands (Fox et al. 2010, Rousso et al. 2016). In an elegant study in the Drosophila hindgut (Syed et al. 2012), secretion of the mucin Tenectin was shown to expand the lumen in a dose-dependent manner (Figure 2c). Similarly, in the Drosophila eye, secretion of the glycosaminoglycan (GAG) and Agrin-like protein encoded by the eyes shut gene opens the ommatidial lumen, the interrhabdomeral space (Husain et al. 2006). Mucin secretion has also been implicated in lumen formation in the mouse aorta (Strilic et al. 2010). In that study, secretion of Podocalyxin was proposed to function in lumen expansion as an anti-adhesive factor acting via electrostatic repulsion. However, calculations by Torres-Sánchez et al. (2021) support only an anti-adhesive role, as repulsion is expected to be of short range and insufficient to drive lumen expansion. Finally, recent studies (Caviglia et al. 2016, Ryan et al. 2019) point to an emerging role for secretory lysosomes in lumen formation. The implication of mucins in lumen formation is particularly interesting, as these molecules confer a high swelling pressure (Kovach 1995) but due to their large size and material properties are not equilibrated by transport down their concentration gradient as easily as small ions. Thus, while secretion of mucins involves complex and energetically costly biosynthetic processes, once deployed in the lumen, these molecules can expand and act as luminal scaffolds without the need for constant energy-dependent transport processes. Regulation of lumen formation by secreted cargos can be exerted at the transcriptional level that defines the secretory capacity of the tissue (Fox et al. 2010) and by controlling the rate or timing of exocytosis (Rousso et al. 2016).
2.2.3. Cell contractility and external boundaries shaping lumen formation.
The control of lumen formation and organ morphogenesis through the regulation of the basic physical processes described in Figure 1 is illustrated by several interesting studies in zebrafish. For example, in the zebrafish brain, loss of the myosin phosphatase regulator Mypt1 results in a small ventricle due to increased phosphorylation and activation of the myosin regulatory light chain, suggesting that epithelial relaxation is needed for expansion of the brain ventricle lumen to occur (Gutzman & Sive 2010). Accordingly, epithelial relaxation would reduce wall strain and thus ΔP (Figure 1b), leading to increased fluid flow and lumen expansion. This interplay between epithelial tension and lumen expansion is also the basis for the processes that control organ size in the zebrafish otic vesicle. Initial work (Hoijman et al. 2015) revealed epithelial thinning and mitotic rounding as being key to lumen formation, leading the authors to suggest that fluid was transferred from the epithelium to the lumen. However, subsequent work (Mosaliganti et al. 2019) combining direct measurements of internal pressure and mechanical manipulations with quantitative tissue contractility measurements and modeling (see Section 3) led to different conclusions. Consistent with the basic fluid flow equation (Figure 1d) and their theoretical model (see Section 3), the authors concluded that a negative feedback loop between hydrostatic pressure and fluid transport controls lumen size (Mosaliganti et al. 2019). Epithelial thinning results from the stretch caused by increased luminal pressure and not from the transfer of fluid from cells into the lumen. In this scenario, cell divisions could effectively relax tensions, leading to a reduced ΔP and increased fluid influx, thus causing epithelial thinning (see also Section 3.1). Differences in tissue material properties may also underlie the asymmetrical shape of the zebrafish KV (Dasgupta et al. 2018a). Thus, the material properties of the tissue may cause differential strain that accounts for the geometry of the lumen.
2.3. Hydrostatic Pressure in Tube Patterning and Cell Fate Specification
In addition to its direct role in lumen formation, hydrostatic pressure has also been implicated in tube budding and branching, and in cell fate specification. These can be considered emerging properties of tissue physiology that may play a significant role in behaviors that can be characterized as self-organizing. In this section, we examine examples of processes regulated or influenced by hydrostatic pressure which likely have broad implications and are likely to impact other organs and systems.
2.3.1. Role of hydrostatic pressure in tube patterning.
Work in mouse lung explants (Nogawa & Hasegawa 2002) showed that hypertonic media promotes budding. More recently, an in vitro study (Yang et al. 2021) aided by modeling suggested that actomyosin contraction and volume reduction promote the formation of crypt-like structures in gut organoids. However, the proposed volume reduction mechanism involving the glucose transporter Sglt1 seems implausible, as it would require that glucose is first secreted into the cyst lumen and then removed. Alternatively, activation of basolateral K+ and or Cl− channels may drive fluid absorption and lumen shrinking. Nevertheless, the mathematical model and conceptual framework from Yang et al. (2021) provide a convincing explanation for the phenomenon observed previously by Nogawa & Hasegawa (2002) and may also be applicable to other branching organs such as the kidney and mammary gland. However, several studies (Jaslove & Nelson 2018, Nelson et al. 2017) using lung explants have shown that transmural (hydrostatic) pressure patterns the surrounding smooth muscle and defines the sites and timing of branching. This phenomenon is illustrated most dramatically by a recent comprehensive study (Palmer et al. 2021) showing how luminal pressure and muscle contraction determine the formation of the highly lobed epithelium of the lizard lung. Using modeling and experimental manipulations, the researchers showed that the internal pressure acts on the surrounding smooth muscle layer to pattern a honeycomb-like network. Then, as the luminal pressure increases, it forces the epithelial wall through the gaps in the network, leading to the formation of a structure similar to squeezed stress balls. Importantly, hydrostatic pressure provides a robust mechanism for synchronizing branching throughout the organ. While the molecular mechanisms linking hydrostatic pressure and muscle contraction are not fully known, recent work (Jaslove et al. 2022, Stanton et al. 2021) implicates retinoic acid and Fgf10. Other potential links between luminal pressure and signaling can be found in the zebrafish lateral line and in the mouse blastocyst. In both cases, formation and expansion of a luminal cavity facilitates Fgf signaling (Durdu et al. 2014, Ryan et al. 2019).
So far, we have considered luminal pressure as a morphogenetic regulator. However, several studies have shown that secreted GAGs can also shape tissues from the abluminal surface. For example, the secretion of hyaluronic acid on the basal side and actin-rich cytocinches shape the budding and morphogenesis of semicircular canals in the zebrafish otic vesicle (Munjal et al. 2021). Additionally, the GAG-rich cardiac jelly has been shown to shape signaling, cell migration, and cell volume in the heart (Kiefer et al. 1988, Mellman et al. 2012, Paolini et al. 2021, Peal et al. 2009, Vignes et al. 2022).
2.3.2. Hydrostatic pressure in cell fate determination.
Other emerging roles of hydrostatic pressure include cell fate determination and embryonic layering (Chan & Hiiragi 2020). While the cellular mechanisms are unclear, increased lung expansion alters the proportions of type I and type II alveolar epithelial cells in fetal sheep (Flecknoe et al. 2000) and proliferation (Nardo et al. 2000). Consistently, transient in utero disruption of CFTR in rats causes phenotypic changes in alveolar type II cells in adult rats (Gad et al. 2009). More recently, comprehensive studies in the mouse blastocyst showed that hydrostatic pressure functions in cell fate determination via influencing cell division and lineage allocation, as loss of luminal expansion (e.g., hypertonic medium or mechanical puncturing) causes inner cell mass expansion at the expense of the trophoectoderm (Chan et al. 2019). Luminal pressure was also shown to facilitate embryonic layering by promoting the spatial segregation of epiblast and primitive endoderm lineages (Ryan et al. 2019). It has also been suggested that hydrostatic pressure can affect chondrocyte differentiation through chromatin remodeling (Maki et al. 2021), raising the possibility that cell differentiation in the blastocyst is also partly mediated by a similar mechanism.
3. MATHEMATICAL MODELS OF LUMEN FORMATION
In this section, we discuss some recent theoretical models exploring physical mechanisms controlling lumen growth. In the preceding sections, we considered how osmotic and hydrostatic pressures are generated and how their balance controls fluid flow into the lumen. Here, we first consider how the tissue’s response to luminal pressure shapes lumen formation, how electric effects influence lumen growth, and how external tissues help shape lumen formation in in vivo contexts. Then, we consider basic physical aspects of single lumen formation.
3.1. Dynamics of Lumen Growth: Role of Pressure, Tissue Material Properties, and Electric Effects
In Section 2.1, we introduced the basic factors ruling lumen expansion in epithelial tubes. However, other factors, such as rupture of the multicellular wall or TJ opening, can also regulate lumen growth dynamics and size (Ruiz-Herrero et al. 2017) (Figure 3a). Due to rupture or opening of TJs, the lumen can leak fluid, which reduces both Pin and the hydrostatic pressure (ΔP), resulting in reduced wall tension (γ). Consequently, the influx of fluid is increased and, if the rupture is followed by healing, the lumen may then regain its original size. Thus, rupture-healing cycles can generate size oscillations in multicellular cysts (Ruiz-Herrero et al. 2017). This oscillatory behavior was shown to depend on the response of the tissue to the stress developed on the lumen wall due to growth. When the response of the tissue is elastic (Figure 3a, subpanel i), the model predicts that the cyst oscillates between maximum and minimum sizes. By contrast, if the response is viscoelastic (Figure 3a, subpanel ii), oscillations occur with the mean radius growing over time. This latter mode of lumen expansion in the absence of leaks has been proposed to underlie growth control in the zebrafish otic vesicle (Mosaliganti et al. 2019). In this system, the buildup of internal pressure was shown to increase lumen size at a constant rate during its growth phase. The hydrostatic pressure provides size control by restricting the inward fluid flux to a constant magnitude, and the solute concentration is regulated accordingly to maintain this uniform fluid flux. As expected, because fluid is not lost due to leakage, the internal pressure acts on the lumen wall continuously, causing tissue-wide deformations such as epithelial thinning. If the lumen is punctured, however, the vesicle growth rate increases due to loss of Pin and ΔP. This phenomenon, called catch-up growth, illustrates how hydrostatic pressure can function as a size regulator in tubular structures (Mosaliganti et al. 2019).
Figure 3.
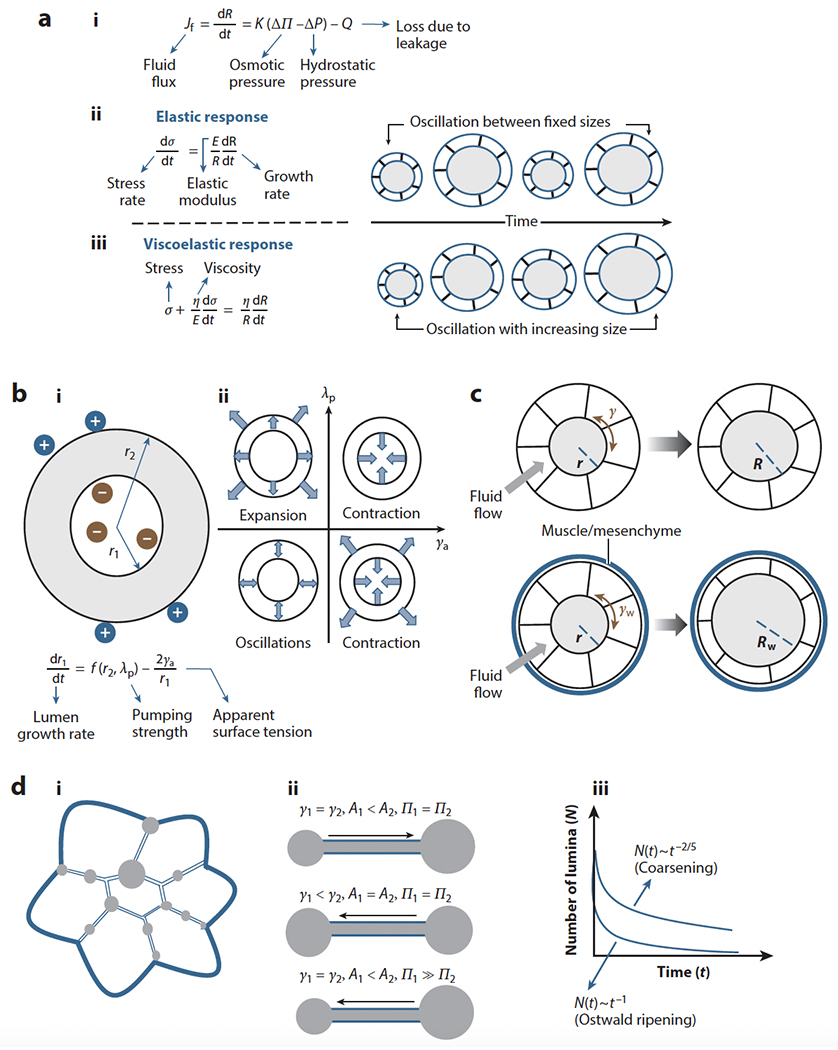
Mathematical models of lumen formation. (a) Models of oscillatory lumen expansion behaviors. (i) Fluid flux equation. (ii) An elastic response in the mouse blastocyst produces oscillations between fixed sizes (Ruiz-Herrero et al. 2017), whereas (iii) a viscoelastic response in the zebrafish otic vesicle (Mosaliganti et al. 2019) produces oscillations with lumen size increase. (b) Flexoelectricity model. (i) Schematic of electric and surface curvature effects and mathematical expression of lumen growth. (ii) State diagram of lumen growth at different pumping and surface tension conditions showing expansion, contraction, and oscillatory behaviors. Panel b adapted from Duclut et al. (2019, 2021). (c) Schematic of the potential effect of external layers on lumen formation. If confinement (Rodriguez-Fraticelli et al. 2012) or muscle interactions (Alvers et al. 2014) reduce the stress on the epithelium, lumen formation is more efficient. Eventually it may lead to epithelial thinning (Mosaliganti et al. 2019). (d) Single lumen formation via coarsening. (i) Schematic of a multi-lumen structure. (ii) Schematic of fluid flow between microlumina of different areas (A) and tensions (γ) under isosmotic conditions (Π1 = Π2) or with a large osmotic gradient (Π1 >> Π2). (iii) Schematic of expected lumen number decay as a function of time following coarsening or Ostwald ripening. Panel d adapted from Le Verge-Serandour & Turlier (2021).
Another interesting lumen geometry is the case of bicellular cysts such as those found in hepatocytes, in which fluid loss due to paracellular leaks via TJs may become significant (S. Dasgupta et al. 2018). In this work, the coupling of ion flux with fluid flux was explicitly considered, and both hydrostatic and osmotic pressures are regulated to determine the lumen size. To compensate for leakage and maintain an osmotic gradient, solutes (primarily salt ions) must be pumped into the lumen. Therefore, a critical ion transport efficiency exists above which the lumen grows and below which the lumen contracts. S. Dasgupta et al. (2018) also predict the existence of screening lengths, ξf for water and ξi for ions, that are a measure of the length beyond which the lumen gets decoupled from the paracellular edge effects. Small screening lengths imply weak coupling, allowing transcellular transport to dominate and to support larger lumens. Conversely, larger screening length would imply strong coupling with the leaking edges and would confine the lumens to smaller sizes.
Most studies of lumen dynamics assume that although ions are charged, the electric field across the lumen boundary is negligible. However, it has been proposed recently that interesting scenarios occur when the effect of charged ions on curved surfaces, named flexoelectricity, is considered (Duclut et al. 2019). Due to flexoelectricity, the surface tension of the lumen boundary is modified, and an effective pumping accounting for both the active pumping and electric effects must be considered. Lumen growth is then determined by the competition between the effective pumping strength (λp) and the apparent surface tension (γa). For a positive apparent surface tension (γa > 0), the lumen shrinks in most of the cases. However, for a negative apparent surface tension (γa < 0), a lumen is always formed, irrespective of positive or negative effective pumping (see Figure 3b). Interesting perturbations to this model, such as those imposing external fluid flow and electric currents, were subsequently explored and suggested a potential therapeutic mechanism to suppress cancerous cysts (Duclut et al. 2021).
Another recent study (Vasquez et al. 2021) considered the potential role of cell geometry in determining the lumen shape of small cysts such as Madin-Darby canine kidney (MDCK) cell spheroids. A 2D vertex model was used to illustrate how membrane size changes could lead to lumen expansion under isotonic conditions and very small internal pressure. However, such conditions would only apply to small diameter tubes, and whether the postulated conditions occur in vivo is unclear.
The theoretical models that we have discussed so far are inspired by cyst-like structures and do not consider the role of external tissues such as muscle and mesenchyme that surround most tubes, nor do they consider how these factors affect lumen dynamics. The presence of a relatively rigid and permeable wall aids lumen formation and inflation (Rodriguez-Fraticelli et al. 2012), promotes single lumen formation (Alvers et al. 2014), and may also explain epithelial thinning observed in some tissues during lumen expansion (A. Dasgupta et al. 2018, Hoijman et al. 2015, Mosaliganti et al. 2019). One possible explanation is that the lumen boundary tension (γw) in the presence of an external wall is reduced (γw < γ). Therefore, under the same osmotic pressure, a lumen boundary with a rigid external wall may experience significantly reduced stress, leading to increased fluid flux and faster lumen growth (Rw > R) (Figure 3c). However, the lumen boundary can expand only up to a certain limit, eventually causing epithelium thinning and cell division due to increased stress.
3.2. Single Lumen Formation: Coarsening and Coalescence
Until now, we have considered the processes of expansion and contraction at the level of a single lumen. However, as described in Section 2, lumen formation in vivo typically proceeds via the formation, expansion, and coalescence of multiple lumina. While in large tubes, the final step of lumen resolution requires the remodeling of adhesions (Alvers et al. 2014), earlier steps are clearly dominated by fluid transport (Bagnat et al. 2007) and dynamic interactions between the nascent lumina (Dumortier et al. 2019). This concept was explored to explain the dynamics of connected microlumina in the mouse blastocyst (Le Verge-Serandour & Turlier 2021, Dumortier et al. 2019), where it was shown that microlumina would be hydraulically coupled and exchange fluid. The model considered two fluid-filled, spherical lumina of areas A1 and A2 (with A1 < A2) that were connected by a channel. If the lumen surface tension (γ) and the osmotic pressure is the same for both, then the Laplace law dictates that due to the hydrostatic pressure difference, fluid will flow from A1 to A2. Because the ionic concentration in different lumina may differ, osmotic pressure differences may also exist among them. In such cases, the competition between hydrostatic and osmotic pressures determines the direction of fluid flow between them. Thus, the fluid flow from A1 to A2 due to Laplace’s law could be suppressed by an osmotic flow from A2 to A1 if the ion concentration in A1 is much larger than that in A2. In a connected network of such microlumina, all the fluid is expected to get discharged into one dominant lumen, a phenomenon called lumen coarsening. Such a coarsening mechanism has been compared with Ostwald ripening (Dumortier et al. 2019). However, as is pointed out in the subsequent theoretical paper (Le Verge-Serandour & Turlier 2021), the number of lumina (N) decays with time as N ~ t−2/5, a behavior that differs from Ostwald ripening-like decay, in which N ~ t−1 (Baldan 2002, Voorhees 1985) (Figure 3d). Overall, the dynamics of multiple connected lumina seem to undergo coarsening in the earlier stages and coalescence in the later stages. In the earlier stages, the distance between the lumina being small, the pressure difference cannot be screened entirely. Therefore, they would discharge fluid into the dominant lumen. In the later stages, two large lumina separated by a large distance would appear, and these two lumina would now be able to transfer fluid due to pressure gradients, if the screening length is large. Additionally, these lumina may increase their size due to the activity of apical channels such as CFTR (Navis et al. 2013, Nedvetsky et al. 2014). As the lumina grow further in size, the interlumen distance decreases, finally coming into contact and coalescing into a single lumen (Alvers et al. 2014). Another mechanism leading to single lumen formation is anastomosis, which requires complex rearrangement of TJs. This process has been described in a variety of organs including the avian lung (Palmer & Nelson 2020), the zebrafish vasculature (Herwig et al. 2011), and the ascidian notochord (Dong et al. 2011) (Figure 4a).
Figure 4.
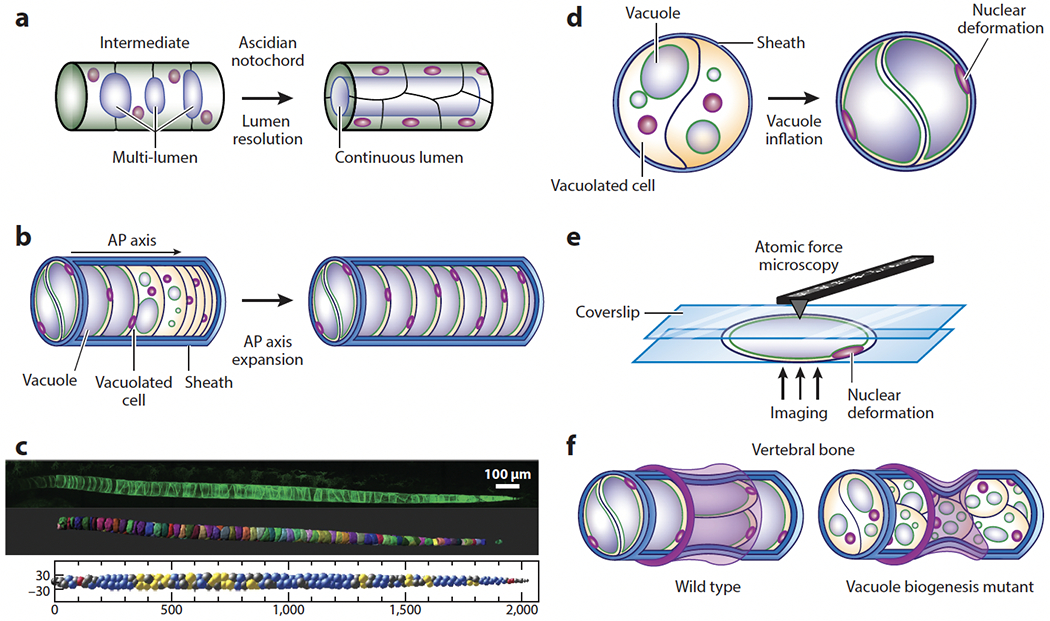
Hydrostatic pressure in the anterior-posterior (AP) axis of chordates. (a) Schematic of continuous lumen formation in the ascidian notochord. Lumen inflation in the notochord provides a hydrostatic scaffold for the free-swimming larva. Note that the multi-lumen intermediate resembles the organization of the vertebrate notochord. (b) Formation and inflation of fluid-filled vacuoles in the vertebrate notochord facilitates AP axis elongation. (c, top) Confocal image, (middle) 3D rendering, and (bottom) 3D arrangement of vacuolated cells in the zebrafish notochord. Vacuolated cells arrange in a stereotypical pattern (see middle panel) dictated by the relative sizes of the cells and the notochord and the aspect ratio of the tube (Norman et al. 2018). Different 3D patterns are color coded. Images in panel c provided by James Norman. (d) Inflation of vacuoles inside vacuolated cells leads to hydrostatic pressure generation inside the semirigid notochord sheath. Nuclear deformation can be used to estimate relative pressure (Bagwell et al 2020). (e) Compression of isolated vacuolated cells using atomic force microscopy and calibration of nuclear deformation may allow the determination of pressure values in vivo via noninvasive imaging. (f, top) Hydrostatic pressure in the notochord resists the compressive force of vertebral bone growth, leading to the formation of symmetrical, hourglass-shaped vertebrae. (Bottom) Loss or fragmentation of vacuoles reduces internal notochord pressure, making the structure more easily deformable and causing vertebral malformations.
4. ROLE OF HYDROSTATIC PRESSURE IN CHORDATE AXIAL DEVELOPMENT
Hydrostatic pressure has also been shown to act as a morphogenetic force in anterior-posterior (AP) axis development in chordates (Bagnat & Gray 2020). This potential was first illustrated by an ex vivo study (Adams et al. 1990) exploring the osmotic behavior and mechanical properties of notochords dissected from Xenopus tadpoles. In that influential study, the researchers showed that osmotic swelling of the notochord provides flexural stiffness to the AP axis and suggested a possible role in axis elongation. Similarly, ascidian larvae hold an extended AP axis and are able to swim due to the flexural stiffness conferred by the notochord (Jiang & Smith 2007). The ascidian notochord is a closed tube that forms via a complex TJ remodeling process (Dong et al. 2011) and inflates via the activity of the Slc26 chloride channel (Deng et al. 2013) (Figure 4a). By contrast, in the vertebrate notochord, large vacuolated cells encased by a thick extracellular matrix called the notochord sheath are found instead of a continuous lumen (Ellis et al. 2013b, Yasuoka 2020). This architecture changes the structure of the notochord from uniform to a granular system (Behringer & Chakraborty 2019) and imbues it with new properties that are key for spine formation. How the transition from the hollow ascidian notochord to the compartmentalized structure of vertebrates occurred is not known. However, we speculate that it might have originated from the multi-lumen intermediate observed in ascidians (Dong et al. 2011) (Figure 4a). Considering this scenario, it would be interesting to explore if lumen formation in the ascidian notochord involves secretory lysosomes.
Work in zebrafish has shown that, following convergence and extension, the chordamesoderm forms a well-aligned structure at the midline that resembles a stack of coins (Dale & Topczewski 2011) from which vacuolated cells differentiate via a Notch-dependent process (Yamamoto et al. 2010). Each notochord vacuolated cell then forms a single fluid-filled vacuole, in fact a lysosome-related organelle (LRO), that expands via fluid accumulation powered by the activity of the vacuolar H+-ATPase (Ellis et al. 2013a). Because the circumference of the notochord sheath is relatively inelastic, vacuole inflation leads to expansion of the embryonic AP axis (Figure 4b), whereas loss or fragmentation of vacuoles impairs axis elongation and causes kinking of the spine in the late larva (Ellis et al. 2013a). Remarkably, as vacuoles inflate, they arrange in a stereotypical pattern following a simple packing principle (Norman et al. 2018, Sorrell & Lubkin 2021) (Figure 4c). The contribution of vacuolated cells to posterior elongation of the AP axis is due to the generation of hydrostatic pressure, which is apparent by the extrusion of cells when the caudal end of the notochord is severed (Norman et al. 2018) and by the deformation of the vacuolated cell nuclei (Bagwell et al. 2020). Axis elongation is also aided by the addition of cells at the posterior end of the notochord (McLaren & Steventon 2021) and requires mechanical coupling between the notochord and the surrounding paraxial mesoderm (Dray et al. 2013).
As vacuoles inflate, their cell nuclei are deformed without changing their volume. Thus, nuclear shape can be used to estimate the relative internal pressure of the notochord using noninvasive microscopy (Bagwell et al. 2020) (Figure 4d). Absolute pressure values could be obtained from isolated cells by measuring nuclear deformation, the internal pressure of vacuolated cells, and the cell surface tension using the experimental setup of parallel plate compression (Caille et al. 2002, Peeters et al. 2005) (Figure 4e). Upon compression, the cell is expected to undergo viscoelastic relaxation. Then, by applying Laplace’s law, one can determine the surface tension and internal pressure using the known magnitude of the compressive force (Forgacs et al. 1998, Norotte et al. 2008). Nuclear deformation at different pressure values from isolated cells may then be used to construct a calibration curve to estimate pressure in vivo using nuclear deformation data.
The notochord’s hydrostatic pressure generated by vacuole inflation and optimal packing also facilitates proper vertebral morphogenesis. As vertebral bodies grow concentrically around the notochord, the internal pressure resists compression, leading to the formation of symmetrical hourglass-shaped vertebrae (Bagwell et al. 2020) (Figure 4f). Then, as vertebral bodies grow further, selective vacuole fragmentation and cell rearrangements allow the notochord to absorb compression locally at each vertebra, thus preventing the formation and accumulation of deformations along the axis. However, when vacuoles fragment prematurely or are lost, the notochord loses internal pressure and is deformed asymmetrically, leading to kinking of the spine axis (Bagwell et al. 2020) (Figure 4f). Vacuole fragmentation occurs in LRO biogenesis mutants (Ellis et al. 2013a) and due to loss of the regulatory kinase Dstyk (Bagwell et al. 2020, Sun et al. 2020) and may underlie a fraction of congenital scoliosis cases. Whether the developmental regulation of vacuole fragmentation occurs via modulation of those pathways or if it is controlled by mechanical signals is unclear. One possible mechanism linking notochord compression to the regulation of cellular processes acting on vacuoles is nuclear deformation, as recent studies (Lomakin et al. 2020, Venturini et al. 2020) have linked nuclear deformation to cellular processes via calcium signaling.
5. PRESSURE GRADIENTS AND CYTOPLASMIC FLOWS
Pressure gradients can generate tissue-wide cytoplasmic flows in several developmental contexts. In the Drosophila egg chamber, the oocyte develops within a 16-cell cyst (Pepling et al. 1999). Prior to fertilization, the cytoplasmic content of these nurse cells is transferred to the oocyte, which grows significantly in size while the nurse cells shrink and ultimately die (Jenkins et al. 2013, Pepling et al. 1999). This transfer of cytoplasm by the nurse cells is controlled by fluid flows across the ring canals, which are cytoplasmic bridges that form from arrested cleavage furrows (Theurkauf & Hazelrigg 1998). Recent experiments (Imran Alsous et al. 2021) have shown that these flows can be understood in terms of simple hydraulic concepts and an effective Young-Laplace law, in which pressure gradients generate flows of material from the smaller cells into the larger ones. Such flows can explain why the oocyte grows while the nurse cells shrink. A similar physical mechanism has been shown for the selection of which germ cells become oocytes in C. elegans (Chartier et al. 2021). This model was supported by experiments in which fluid flows are induced by laser-controlled temperature gradients (Mittasch et al. 2018). These experiments strongly support hydraulic instability as a driver of oocyte growth. Experiments in Drosophila oogenesis (Lu et al. 2022) have shown that active cortical dynein activity is required for cytoplasmic flows at earlier stages, supporting an interplay between cytoskeletal forces and hydrodynamics.
Cytoskeletal forces can also generate bulk cytoplasmic flows during the early cleavage stages of embryogenesis (Shamipour et al. 2021). In Drosophila embryos, embryo-wide cytoplasmic flows are observed during nuclear cycles 4-6 that play a crucial role in spreading the nuclei along the AP axis (Deneke et al. 2019, Royou et al. 2002, von Dassow & Schubiger 1994). These flows are instructed by the cell cycle oscillator. Specifically, the activity of cell cycle phosphatase PP1 was shown to spread from the nuclei to the cortex at mitotic exit, thus coupling nuclear and cortical dynamics (Deneke et al. 2019). Localized increase in PP1 activity generates gradients of actomyosin-dependent contractility at the cortex. Such gradients drive cortical flows, which in turn generate bulk flows by hydrodynamic coupling. Importantly, these flows have the correct geometry to facilitate the spreading of nuclei across the AP axis. When nuclei are not uniformly positioned across the AP axis, cell cycle oscillations are local; such local oscillations can generate gradients of contractility, which in turn generate bulk flows that facilitate nuclear spreading. However, once nuclei are uniformly positioned across the AP axis, cell cycle oscillations are global and uniform and, consequently, no significant gradients of actomyosin contractility are generated, resulting in little to no bulk flow in the embryo. Thus, the coupling of biochemical and mechanical signals can generate self-organized nuclear positioning by exploiting hydrodynamic forces (Deneke et al. 2019). A similar process in which the coupling of the cell cycle and cytoskeleton can drive bulk flows has been shown to drive ooplasmic segregation in zebrafish early embryos (Shamipour et al. 2019). Ooplasmic segregation describes the process by which yolk and cytoplasm become physically separated in early embryos (Leung et al. 2000). In zebrafish, this process was recently shown to be controlled not by cortical actomyosin contractility but rather by contractility in the bulk cytoplasm (Shamipour et al. 2019). Such contractility is also entrained with the cell cycle oscillations and is positively, rather than negatively, regulated by Cdk1 activity. From a physical standpoint, these flows can be described by multifluid models, similar to the ones of poroelasticity (Mitchison et al. 2008). Specifically, the actomyosin network can be described as an active contractile gel and the soluble part of the cytoplasm (cytosol) as a passive viscous fluid. The coupling between the gel and the cytosol is usually described by a simple friction term. Determining how well such theoretical frameworks can describe the observed flows will reveal important insights into how cytoplasmic flows are generated in embryos.
6. CONCLUSIONS
In recent years, there has been considerable progress in our knowledge of how physiological processes act during morphogenesis. This has brought to our attention the need to understand the underlying physical principles ruling morphogenesis, leading to the generation of mathematical models of lumen and tube formation based on experimental data. Purely theoretical models have also been generated, and these provide the framework for the development of increasingly more comprehensive theories. However, the generation of more accurate and realistic models will require better quantitative data, particularly for the more complex structures, as well as consideration of the influence of the environment and surrounding tissues (Karzbrun et al. 2021, Mitchell et al. 2021). Of particular relevance is the influence of muscle layers (Alvers et al. 2014, Palmer et al. 2021, Varner et al. 2015) and the extracellular matrix and other external constraints acting upon tissues (Munjal et al. 2021, Rodriguez-Fraticelli et al. 2012, Vignes et al. 2022). Our current understanding of the physiological and physical processes acting on morphogenesis could also guide future genetic screens to identify new genetic regulators and cellular effectors of key processes. Eventually, the integration of experimental observations and mathematical models may allow us to define the self-organizing logic of biological systems.
ACKNOWLEDGMENTS
We thank Daniel Levic for critical reading of the manuscript and Briana Peskin for drawing figures. M.B. and B.D. were supported by a National Institutes of Health (NIH) grant (DK121007) and S.D.T. by a different NIH grant (R01-GM136763). M.B. is a Howard Hughes Medical Institute Faculty Scholar.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abbas L, Whitfield TT. 2009. Nkcc1 (Slc12a2) is required for the regulation of endolymph volume in the otic vesicle and swim bladder volume in the zebrafish larva. Development 136:2837–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Keller R, Koehl MA. 1990. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development 110:115–30 [DOI] [PubMed] [Google Scholar]
- Alvers AL, Ryan S, Scherz PJ, Huisken J, Bagnat M. 2014. Single continuous lumen formation in the zebrafish gut is mediated by smoothened-dependent tissue remodeling. Development 141:1110–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnat M, Cheung ID, Mostov KE, Stainier DY. 2007. Genetic control of single lumen formation in the zebrafish gut. Nat. Cell Biol 9:954–60 [DOI] [PubMed] [Google Scholar]
- Bagnat M, Gray RS. 2020. Development of a straight vertebrate body axis. Development 147:dev175794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagwell J, Norman J, Ellis K, Peskin B, Hwang J, et al. 2020. Notochord vacuoles absorb compressive bone growth during zebrafish spine formation. eLife 9:e51221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldan A 2002. Progress in Ostwald ripening theories and their applications to nickel-base superalloys Part I: Ostwald ripening theories. J. Mater. Sci 37:2171–202 [Google Scholar]
- Behringer RP, Chakraborty B. 2019. The physics of jamming for granular materials: a review. Rep. Prog. Phys 82:012601. [DOI] [PubMed] [Google Scholar]
- Caille N, Thoumine O, Tardy Y, Meister JJ. 2002. Contribution of the nucleus to the mechanical properties of endothelial cells. J. Biomech 35:177–87 [DOI] [PubMed] [Google Scholar]
- Caviglia S, Brankatschk M, Fischer EJ, Eaton S, Luschnig S. 2016. Staccato/Unc-13–4 controls secretory lysosome-mediated lumen fusion during epithelial tube anastomosis. Nat. Cell Biol 18:727–39 [DOI] [PubMed] [Google Scholar]
- Chan CJ, Costanzo M, Ruiz-Herrero T, Monke G, Petrie RJ, et al. 2019. Hydraulic control of mammalian embryo size and cell fate. Nature 571:112–16 [DOI] [PubMed] [Google Scholar]
- Chan CJ, Hiiragi T. 2020. Integration of luminal pressure and signalling in tissue self-organization. Development 147:dev181297. [DOI] [PubMed] [Google Scholar]
- Chartier NT, Mukherjee A, Pfanzelter J, Furthauer S, Larson BT, et al. 2021. A hydraulic instability drives the cell death decision in the nematode germ line. Nat. Phys 17:920–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. 2002. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am. J. Physiol. Cell Physiol 283:C142–47 [DOI] [PubMed] [Google Scholar]
- Dale RM, Topczewski J. 2011. Identification of an evolutionarily conserved regulatory element of the zebrafish col2a1a gene. Dev. Biol 357:518–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A, Merkel M, Clark MJ, Jacob AE, Dawson JE, et al. 2018. Cell volume changes contribute to epithelial morphogenesis in zebrafish Kupffer’s vesicle. eLife 7:e30963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Gupta K, Zhang Y, Viasnoff V, Prost J. 2018. Physics of lumen growth. PNAS 115: E4751–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke VE, Puliafito A, Krueger D, Narla AV, De Simone A, et al. 2019. Self-organized nuclear positioning synchronizes the cell cycle in Drosophila embryos. Cell 177:925–41.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Nies F, Feuer A, Bocina I, Oliver D, Jiang D. 2013. Anion translocation through an Slc26 transporter mediates lumen expansion during tubulogenesis. PNAS 110:14972–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond ME, Jacobson AG. 1977. Embryonic brain enlargement requires cerebrospinal fluid pressure. Dev. Biol 57:188–98 [DOI] [PubMed] [Google Scholar]
- Devine WP, Lubarsky B, Shaw K, Luschnig S, Messina L, Krasnow MA. 2005. Requirement for chitin biosynthesis in epithelial tube morphogenesis. PNAS 102:17014–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Deng W, Jiang D. 2011. Distinct cytoskeleton populations and extensive crosstalk control Ciona notochord tubulogenesis. Development 138:1631–41 [DOI] [PubMed] [Google Scholar]
- Dong B, Kakihara K, Otani T, Wada H, Hayashi S. 2013. Rab9 and retromer regulate retrograde trafficking of luminal protein required for epithelial tube length control. Nat. Commun 4:1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Miao G, Hayashi S. 2014. A fat body-derived apical extracellular matrix enzyme is transported to the tracheal lumen and is required for tube morphogenesis in Drosophila. Development 141:4104–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray N, Lawton A, Nandi A, Julich D, Emonet T, Holley SA. 2013. Cell-fibronectin interactions propel vertebrate trunk elongation via tissue mechanics. Curr. Biol 23:1335–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclut C, Prost J, Jülicher F. 2021. Hydraulic and electric control of cell spheroids. PNAS 118:e2021972118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclut C, Sarkar N, Prost J, Jülicher F. 2019. Fluid pumping and active flexoelectricity can promote lumen nucleation in cell assemblies. PNAS 116:19264–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier JG, Le Verge-Serandour M, Tortorelli AF, Mielke A, de Plater L, et al. 2019. Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst. Science 365:465–68 [DOI] [PubMed] [Google Scholar]
- Durdu S, Iskar M, Revenu C, Schieber N, Kunze A, et al. 2014. Luminal signalling links cell communication to tissue architecture during organogenesis. Nature 515:120–24 [DOI] [PubMed] [Google Scholar]
- Ellis K, Bagwell J, Bagnat M. 2013a. Notochord vacuoles are lysosome-related organelles that function in axis and spine morphogenesis. J. Cell Biol 200:667–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis K, Hoffman BD, Bagnat M. 2013b. The vacuole within: how cellular organization dictates notochord function. Bioarchitecture 3:64–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flecknoe S, Harding R, Maritz G, Hooper SB. 2000. Increased lung expansion alters the proportions of type I and type II alveolar epithelial cells in fetal sheep. Am. J. Physiol. Lung Cell. Mol. Physiol 278:L1180–85 [DOI] [PubMed] [Google Scholar]
- Forgacs G, Foty RA, Shafrir Y, Steinberg MS. 1998. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys. J 74:2227–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster D, Armbruster K, Luschnig S. 2010. Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Curr. Biol 20:62–68 [DOI] [PubMed] [Google Scholar]
- Fox RM, Hanlon CD, Andrew DJ. 2010. The CrebA/Creb3-like transcription factors are major and direct regulators of secretory capacity. J. Cell Biol 191:479–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Furuse K, Sasaki H, Tsukita S. 2001. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol 153:263–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad A, Callender DL, Killeen E, Hudak J, Dlugosz MA, et al. 2009. Transient in utero disruption of cystic fibrosis transmembrane conductance regulator causes phenotypic changes in alveolar type II cells in adult rats. BMC Cell Biol. 10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieder NC, Caussinus E, Parker DS, Cadigan K, Affolter M, Luschnig S. 2008. γCOP is required for apical protein secretion and epithelial morphogenesis in Drosophila melanogaster. PLOS ONE 3:e3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzman JH, Sive H. 2010. Epithelial relaxation mediated by the myosin phosphatase regulator Mypt1 is required for brain ventricle lumen expansion and hindbrain morphogenesis. Development 137:795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R, Hooper SB. 1996. Regulation of lung expansion and lung growth before birth. J. Appl. Physiol. 1985 81:209–24 [DOI] [PubMed] [Google Scholar]
- Herwig L, Blum Y, Krudewig A, Ellertsdottir E, Lenard A, et al. 2011. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr. Biol 21:1942–48 [DOI] [PubMed] [Google Scholar]
- Hoijman E, Rubbini D, Colombelli J, Alsina B. 2015. Mitotic cell rounding and epithelial thinning regulate lumen growth and shape. Nat. Commun 6:7355. [DOI] [PubMed] [Google Scholar]
- Husain N, Pellikka M, Hong H, Klimentova T, Choe K-M, et al. 2006. The agrin/perlecan-related protein eyes shut is essential for epithelial lumen formation in the Drosophila retina. Dev. Cell 11:483–93 [DOI] [PubMed] [Google Scholar]
- Imran Alsous J, Romeo N, Jackson JA, Mason FM, Dunkel J, Martin AC. 2021. Dynamics of hydraulic and contractile wave-mediated fluid transport during Drosophila oogenesis. PNAS 118:e2019749118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine KD, Shraiman BI. 2017. Mechanical control of growth: ideas, facts and challenges. Development 144:4238–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaslove JM, Goodwin K, Sundarakrishnan A, Spurlin JW III, Mao S, et al. 2022. Transmural pressure signals through retinoic acid to regulate lung branching. Development 149:dev199726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaslove JM, Nelson CM. 2018. Smooth muscle: a stiff sculptor of epithelial shapes. Philos. Trans. R. Soc. B 373:20170318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinska A, Ribeiro C, Affolter M. 2003. Epithelial tube morphogenesis during Drosophila tracheal development requires Piopio, a luminal ZP protein. Nat. Cell Biol 5:895–901 [DOI] [PubMed] [Google Scholar]
- Jenkins VK, Timmons AK, McCall K. 2013. Diversity of cell death pathways: insight from the fly ovary. Trends Cell Biol. 23:567–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Smith WC. 2007. Ascidian notochord morphogenesis. Dev. Dyn 236:1748–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karzbrun E, Khankhel AH, Megale HC, Glasauer SMK, Wyle Y, et al. 2021. Human neural tube morphogenesis in vitro by geometric constraints. Nature 599:268–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan LA, Zhang H, Abraham N, Sun L, Fleming JT, et al. 2013. Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat. Cell Biol 15:143–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer SW, Morrow NS, Metzler CW. 1988. Alcohol aversion generalization in rats: specific disruption of taste and odor cues with gustatory neocortex or olfactory bulb ablations. Behav. Neurosci 102:733–39 [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim YJ, Adams ME. 2018. Endocrine regulation of airway clearance in Drosophila. PNAS 115:1535–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolotuev I, Hyenne V, Schwab Y, Rodriguez D, Labouesse M. 2013. A pathway for unicellular tube extension depending on the lymphatic vessel determinant Prox1 and on osmoregulation. Nat. Cell Biol 15:157–68 [DOI] [PubMed] [Google Scholar]
- Kovach IS. 1995. The importance of polysaccharide configurational entropy in determining the osmotic swelling pressure of concentrated proteoglycan solution and the bulk compressive modulus of articular cartilage. Biophys. Chem 53:181–87 [DOI] [PubMed] [Google Scholar]
- Larsen EH, Sorensen JN. 2020. Stationary and nonstationary ion and water flux interactions in kidney proximal tubule: mathematical analysis of isosmotic transport by a minimalistic model. Rev. Physiol. Biochem. Pharmacol 177:101–47 [DOI] [PubMed] [Google Scholar]
- Le Verge-Serandour M, Turlier H. 2021. A hydro-osmotic coarsening theory of biological cavity formation. PLOS Comput. Biol 17:e1009333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CF, Webb SE, Miller AL. 2000. On the mechanism of ooplasmic segregation in single-cell zebrafish embryos. Dev. Growth Differ 42:29–40 [DOI] [PubMed] [Google Scholar]
- Lomakin AJ, Cattin CJ, Cuvelier D, Alraies Z, Molina M, et al. 2020. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 370:eaba2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Sive H. 2005. Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development 132:2057–67 [DOI] [PubMed] [Google Scholar]
- Lu W, Lakonishok M, Serpinskaya AS, Gelfand VI. 2022. A novel mechanism of bulk cytoplasmic transport by cortical dynein in Drosophila ovary. eLife 11:e75538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAulay N 2021. Molecular mechanisms of brain water transport. Nat. Rev. Neurosci 22:326–44 [DOI] [PubMed] [Google Scholar]
- Maki K, Nava MM, Villeneuve C, Chang M, Furukawa KS, et al. 2021. Hydrostatic pressure prevents chondrocyte differentiation through heterochromatin remodeling. J. Cell Sci 134:jcs247643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren SBP, Steventon BJ. 2021. Anterior expansion and posterior addition to the notochord mechanically coordinate zebrafish embryo axis elongation. Development 148:dev199459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman K, Huisken J, Dinsmore C, Hoppe C, Stainier DY. 2012. Fibrillin-2b regulates endocardial morphogenesis in zebrafish. Dev. Biol 372:111–19 [DOI] [PubMed] [Google Scholar]
- Mitchell NP, Cislo D, Shankar S, Lin Y, Shraiman BI, Streichan SJ. 2021. Visceral organ morphogenesis via calcium-patterned muscle contractions. bioRxiv 2021.11.07.467658. 10.1101/2021.11.07.467658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Charras GT, Mahadevan L. 2008. Implications of a poroelastic cytoplasm for the dynamics of animal cell shape. Semin. Cell Dev. Biol 19:215–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittasch M, Gross P, Nestler M, Fritsch AW, Iserman C, et al. 2018. Non-invasive perturbations of intracellular flow reveal physical principles of cell organization. Nat. Cell Biol 20:344–51 [DOI] [PubMed] [Google Scholar]
- Mosaliganti KR, Swinburne IA, Chan CU, Obholzer ND, Green AA, et al. 2019. Size control of the inner ear via hydraulic feedback. eLife 8:e39596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munjal A, Hannezo E, Tsai TY, Mitchison TJ, Megason SG. 2021. Extracellular hyaluronate pressure shaped by cellular tethers drives tissue morphogenesis. Cell 184:6313–25.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardo L, Maritz G, Harding R, Hooper SB. 2000. Changes in lung structure and cellular division induced by tracheal obstruction in fetal sheep. Exp. Lung Res 26:105–19 [DOI] [PubMed] [Google Scholar]
- Navis A, Bagnat M. 2015. Developing pressures: fluid forces driving morphogenesis. Curr. Opin. Genet. Dev 32:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navis A, Marjoram L, Bagnat M. 2013. Cftr controls lumen expansion and function of Kupffer’s vesicle in zebrafish. Development 140:1703–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedvetsky PI, Emmerson E, Finley JK, Ettinger A, Cruz-Pacheco N, et al. 2014. Parasympathetic innervation regulates tubulogenesis in the developing salivary gland. Dev. Cell 30:449–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Gleghorn JP, Pang MF, Jaslove JM, Goodwin K, et al. 2017. Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development 144:4328–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa H, Hasegawa Y. 2002. Sucrose stimulates branching morphogenesis of embryonic mouse lung in vitro: a problem of osmotic balance between lumen fluid and culture medium. Dev. Growth Differ 44:383–90 [DOI] [PubMed] [Google Scholar]
- Norman J, Sorrell EL, Hu Y, Siripurapu V, Garcia J, et al. 2018. Tissue self-organization underlies morphogenesis of the notochord. Philos. Trans. R. Soc. B 373:20170320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norotte C, Marga F, Neagu A, Kosztin I, Forgacs G. 2008. Experimental evaluation of apparent tissue surface tension based on the exact solution of the Laplace equation. Europhys. Lett 81:46003 [Google Scholar]
- Palmer MA, Nelson CM. 2020. Fusion of airways during avian lung development constitutes a novel mechanism for the formation of continuous lumena in multicellular epithelia. Dev. Dyn 249:1318–33 [DOI] [PubMed] [Google Scholar]
- Palmer MA, Nerger BA, Goodwin K, Sudhakar A, Lemke SB, et al. 2021. Stress ball morphogenesis: how the lizard builds its lung. Sci. Adv 7:eabk0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini A, Fontana F, Pham VC, Rodel CJ, Abdelilah-Seyfried S. 2021. Mechanosensitive Notch-Dll4 and Klf2-Wnt9 signaling pathways intersect in guiding valvulogenesis in zebrafish. Cell Rep. 37:109782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peal DS, Burns CG, Macrae CA, Milan D. 2009. Chondroitin sulfate expression is required for cardiac atrioventricular canal formation. Dev. Dyn 238:3103–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters EA, Oomens CW, Bouten CV, Bader DL, Baaijens FP. 2005. Viscoelastic properties of single attached cells under compression. J. Biomech. Eng 127:237–43 [DOI] [PubMed] [Google Scholar]
- Pepling ME, de Cuevas M, Spradling AC. 1999. Germline cysts: a conserved phase of germ cell development? Trends Cell Biol. 9:257–62 [DOI] [PubMed] [Google Scholar]
- Rathbun LI, Colicino EG, Manikas J, O’Connell J, Krishnan N, et al. 2020. Cytokinetic bridge triggers de novo lumen formation in vivo. Nat. Commun 11:1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli AE, Auzan M, Alonso MA, Bornens M, Martin-Belmonte F. 2012. Cell confinement controls centrosome positioning and lumen initiation during epithelial morphogenesis. J. Cell Biol 198:1011–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa JB, Metzstein MM, Ghabrial AS. 2018. An Ichor-dependent apical extracellular matrix regulates seamless tube shape and integrity. PLOS Genet. 14: e1007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousso T, Schejter ED, Shilo BZ. 2016. Orchestrated content release from Drosophila glue-protein vesicles by a contractile actomyosin network. Nat. Cell Biol 18:181–90 [DOI] [PubMed] [Google Scholar]
- Royou A, Sullivan W, Karess R. 2002. Cortical recruitment of nonmuscle myosin II in early syncytial Drosophila embryos: its role in nuclear axial expansion and its regulation by Cdc2 activity. J. Cell Biol 158:127–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrero T, Alessandri K, Gurchenkov BV, Nassoy P, Mahadevan L. 2017. Organ size control via hydraulically gated oscillations. Development 144:4422–27 [DOI] [PubMed] [Google Scholar]
- Ryan AQ, Chan CJ, Graner F, Hiiragi T. 2019. Lumen expansion facilitates epiblast-primitive endoderm fate specification during mouse blastocyst formation. Dev. Cell 51:684–97.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamipour S, Caballero-Mancebo S, Heisenberg CP. 2021. Cytoplasm’s got moves. Dev. Cell 56:213–26 [DOI] [PubMed] [Google Scholar]
- Shamipour S, Kardos R, Xue SL, Hof B, Hannezo E, Heisenberg CP. 2019. Bulk actin dynamics drive phase segregation in zebrafish oocytes. Cell 177:1463–79.e18 [DOI] [PubMed] [Google Scholar]
- Sorrell EL, Lubkin SR. 2021. Bubble packing, eccentricity, and notochord development. Cells Dev. 169:203753. [DOI] [PubMed] [Google Scholar]
- Stanton AE, Goodwin K, Sundarakrishnan A, Jaslove JM, Gleghorn JP, et al. 2021. Negative transpulmonary pressure disrupts airway morphogenesis by suppressing Fgf10. Front. Cell Dev. Biol 9:725785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling EH. 1896. On the absorption of fluids from the connective tissue spaces. J. Physiol 19:312–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilic B, Eglinger J, Krieg M, Zeeb M, Axnick J, et al. 2010. Electrostatic cell-surface repulsion initiates lumen formation in developing blood vessels. Curr. Biol 20:2003–9 [DOI] [PubMed] [Google Scholar]
- Sun X, Zhou Y, Zhang R, Wang Z, Xu M, et al. 2020. Dstyk mutation leads to congenital scoliosis-like vertebral malformations in zebrafish via dysregulated mTORC1/TFEB pathway. Nat. Commun 11:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburne IA, Mosaliganti KR, Upadhyayula S, Liu T-L, Hildebrand DGC, et al. 2018. Lamellar projections in the endolymphatic sac act as a relief valve to regulate inner ear pressure. eLife 7:e37131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed ZA, Bouge AL, Byri S, Chavoshi TM, Tang E, et al. 2012. A luminal glycoprotein drives dose-dependent diameter expansion of the Drosophila melanogaster hindgut tube. PLOS Genet. 8:e1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Tamura A, Suzuki K, Tsukita S. 2017. Site-specific distribution of claudin-based paracellular channels with roles in biological fluid flow and metabolism. Ann. N.Y. Acad. Sci 1405:44–52 [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Hazelrigg TI. 1998. In vivo analyses of cytoplasmic transport and cytoskeletal organization during Drosophila oogenesis: characterization of a multi-step anterior localization pathway. Development 125:3655–66 [DOI] [PubMed] [Google Scholar]
- Thompson D 1917. On Growth and Form. Cambridge, UK: Cambridge Univ. Press. 1st ed. [Google Scholar]
- Tonning A, Hemphala J, Tang E, Nannmark U, Samakovlis C, Uv A. 2005. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev. Cell 9:423–30 [DOI] [PubMed] [Google Scholar]
- Torres-Sánchez A, Kerr Winter M, Salbreux G. 2021. Tissue hydraulics: physics of lumen formation and interaction. Cells Dev July 30:203724. In press. [DOI] [PubMed] [Google Scholar]
- Tsarouhas V, Senti KA, Jayaram SA, Tiklova K, Hemphala J, et al. 2007. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev. Cell 13:214–25 [DOI] [PubMed] [Google Scholar]
- Varner VD, Gleghorn JP, Miller E, Radisky DC, Nelson CM. 2015. Mechanically patterning the embryonic airway epithelium. PNAS 112:9230–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez CG, Vachharajani VT, Garzon-Coral C, Dunn AR. 2021. Physical basis for the determination of lumen shape in a simple epithelium. Nat. Commun 12:5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini V, Pezzano F, Català Castro F, Häkkinen HM, Jiménez-Delgado S, et al. 2020. The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science 370:eaba2644. [DOI] [PubMed] [Google Scholar]
- Vignes H, Vagena-Pantoula C, Prakash M, Fukui H, Norden C, et al. 2022. Extracellular mechanical forces drive endocardial cell volume decrease during zebrafish cardiac valve morphogenesis. Dev. Cell 57:598–609.e5 [DOI] [PubMed] [Google Scholar]
- von Dassow G, Schubiger G. 1994. How an actin network might cause fountain streaming and nuclear migration in the syncytial Drosophila embryo. J. Cell Biol 127:1637–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees PW. 1985. The theory of Ostwald ripening. J. Stat. Phys 38:231 [Google Scholar]
- Yamamoto M, Morita R, Mizoguchi T, Matsuo H, Isoda M, et al. 2010. Mib-Jag1-Notch signalling regulates patterning and structural roles of the notochord by controlling cell-fate decisions. Development 137:2527–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Xue S-L, Chan CJ, Rempfler M, Vischi D, et al. 2021. Cell fate coordinates mechano-osmotic forces in intestinal crypt formation. Nat. Cell Biol 23:733–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YHC, Briant LJB, Raab CA, Mullapudi ST, Maischein HM, et al. 2022. Innervation modulates the functional connectivity between pancreatic endocrine cells. eLife 11:e64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka Y 2020. Morphogenetic mechanisms forming the notochord rod: the turgor pressure-sheath strength model. Dev. Growth Differ 62:379–90 [DOI] [PubMed] [Google Scholar]
- Zhang J, Piontek J, Wolburg H, Piehl C, Liss M, et al. 2010. Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion. PNAS 107:1425–30 [DOI] [PMC free article] [PubMed] [Google Scholar]


