Abstract
Esophageal cancer (EC) is a common malignancy with a poor prognosis worldwide. There are two core pathways that repair double‐strand breaks, homologous recombination (HR) and non‐homologous end joining (NHEJ) and numerous proteins are recognized that affect the occurrence of HR and NHEJ. Altered DNA damage response (DDR) pathways are associated with cancer susceptibility and affect therapeutic response and resistance in cancers. DDR pathway alterations in EC are still poorly understood. Therefore, the identification of alterations in specific genes in DDR pathways may potentially result in novel treatments for resistant cancers, especially EC. In this review, we aimed to focus on different aspects of DNA damage and repair processes in EC. Also, we reviewed new therapeutic strategies via targeting DNA repair machinery components.
Keywords: DNA repair pathways, esophageal cancer, therapeutic strategies
1. INTRODUCTION
Esophageal cancer (EC) is the eighth most common cancer in the world. 1 , 2 It is characterized by a poor prognosis, high mortality, and variation in geographical location. 3 It is estimated that approximately 17 000 people are diagnosed with EC each year in the United States, of which approximately 16 000 are expected to die. 4 Esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) are the most common types of EC. 5 , 6 The 5‐year survival rate for breast and colon cancers is 90%, while it is about 18% for EC, indicating the need to identify more effective treatments. 7
Esophageal cancer risk factors can be divided into genetic and non‐genetic factors. 8 Genetic ones include mutations in regenerative genes regulating the cell cycle and differentiation, epidermal growth factor receptor, tyrosine kinase receptor, and factors in the vascular endothelial growth factor signal pathway. 9 Environmental factors include smoking, age over 50 years, obesity, gastroesophageal reflux disease (GERD), drinking alcohol, and having precancerous cells in esophageal and biliary reflux. 10
The most current therapeutic strategies for EC include radiotherapy, chemotherapy, and surgery. Besides that, chemo‐radiotherapy or other combinations of such treatments are also used in the treating patients with EC. 11 , 12 Among these surgeries are the most commonly accepted method. 13 Defects in the genome can lead to DNA damage response (DDR) and cell cycle arrest at the checkpoint, followed by DNA repair mechanisms maintaining the stability of the genome. 14 One type of DNA damage is the double‐strand break (DSB), which can increase the risk of cancer if the repair process is poorly carried out. 15
Two main DNA repair mechanisms are mostly involved in DSB–namely, non‐homologous end joining (NHEJ) and homologous recombination (HR). 16 HR repair (HRR), a more perfect and precise one, is carried out through the homologous chromosome so that a healthy strand is used as a pattern for the damaged strand. However, NHEJ only binds to two damaged ends by removing a few nucleotides, which is susceptible to mistakes. 17 Although the extent of changes in the gene expression of DDR is not thoroughly understood, mutations and loss of function of genes in the DDR system led to susceptibility to cancer. Thus, the gene expression regulation in the DNA repair system can have a great impact on cancer progression and open a new window in cancer treatment. 18
In this review, we aimed to focus on different aspects of DNA damage and repair processes in EC. Also, we introduced a new therapeutic strategy via targeting DNA repair machinery components. By targeting proteins involved in DNA repair, cancer cells become more sensitive to radiotherapy, which increases the effectiveness of radiotherapy and treatment of cancer patients. 19
2. ESOPHAGEAL CANCER CLASSIFICATION
Two of the most common types of EC are EAC and ESCC. EAC is more common in developed countries, while ESCC highly occurs in developing countries; also, the third type is called small cell carcinoma, which is very rare. 20 , 21 Regarding the stages, it should be noted that the first stage is the primary tumor, the second stage is lymph node involvement, and the third stage is metastasis. 22 EC cancer has no symptoms in the early stages and usually shows symptoms over the age of 50; in other words, the disease has no symptoms until metastasis, so that the death rate is high. Given these reasons, regular check‐ups for those people with high risk and early detection are crucial. 23
Both types of cancer cells originate from the epithelium lining of the esophagus. 24 , 25 There are multiple treatment options for EAC and ESCC, including surgery (i.e., the most common treatment), radiation therapy, chemotherapy, chemoradiation therapy, laser therapy, and electrocoagulation. 26 Various signs and symptoms of EC, including difficulty in swallowing, weight loss, chest pain, and cough, have been reported. EC usually occurs in old age and is rare in young people. 27 x‐ray chest, chest computed tomography (CT), esophagoscopy, and positron emission tomography–computed tomography (PET‐CT) is the most widely used methods for the detection of EC. 28
Esophageal adenocarcinoma originates from the lower third of the esophagus mucus‐secreting glands. Barrett's disease results from the constant exposure of esophageal mucus cells to gastric acid, which is a major risk factor associated with this type of cancer. 29 Thus, GERD and Barrett's esophagus are the most significant risk factors associated with adenocarcinoma cells. Due to the protective effects of female sex hormones, the incidence of EAC in women is less common than in men. 30
Esophageal squamous cell carcinoma originates from squamous cells and involves the upper two‐thirds of esophageal tissues. 31 This type of cancer mostly depends on the lifestyle and results from smoking and alcohol consumption. Approximately half of the cases are due to smoking, and less than half of the cases are due to alcohol consumption. Also, hot drinks, trauma, and nutritional deficiencies can be other causes of this type of cancer. 32
It should be noted that damage to the DNA repair system is one of the causes of cancer, but the presence of this system in cancer cells causes cancer cells to survive because in radiotherapy and chemotherapy they work through damage to the cell genome That's why sensitizing cancer cells to radiation and chemotherapy drugs can help to eliminate cancer cells. 33
3. DNA REPAIR SYSTEM IN ESOPHAGEAL CANCER
3.1. HR Pathway
Homologous recombination is a type of recombination in which fragments are exchanged between identical DNA molecules inherited from parents. 34 HR is carried out for a variety of purposes, such as recombination during meiosis and repairing of DSBs. When DSBs occur, another homologous strand is used to accurately repair the genome, called the HRR process. 35
More precisely, the 5'end strand is removed by exonucleases during the resection process. Then, the RAD5 protein causes a 3′ end attack on the same region of the homologous chromosome. 36 Moreover, the DNA polymerase extends the 3′ end strands of the invading strand, and the other 3 strands are extended by a DNA polymerase on the displaced DNA. Finally, the newly synthesized strand is attached to the 5 exonuclease‐derived strands, a Holliday structure is formed, and the two strands are separated. 37
The RAD51 gene is a major component of the HR process and plays a key role in maintaining genome integrity. Interestingly, moderate reductions in the RAD51 gene have been shown to reduce or inhibit HR levels in EC cells while significantly decrease the expression of this gene is associated with a maximum 50% increase in HR activity. The reason is that moderate silence of this pathway reduces the amount of HR, but complete silence activates other DNA repair pathways; to increase the effect of radiotherapy, it is better to silence this gene moderately. 38
The RAD51C gene is one of the paralogs of the RAD51 gene. 38 The RAD51C gene is highly expressed in ESCC cells, causing cancer progression, low survival, and increased tumor size via the p38/Akt pathway due to the activation of the RAD51C pathway through the single‐strand annealing (SSA) mechanism. 4
3.1.1. Targeting the HR pathway in cancer cells
Regarding the significant roles of HR in cancer cell survival, monotherapy against HR or in combination with other therapies targeting other biological pathways may efficiently reduce cancer cell survival and induce apoptosis in these cells. 39
The treatment of EC by targeting the regulation of HR and telomerase in EC has interesting results, where telomerase increases HR through the RAD51 pathway and maintains genome stability. Inhibition of telomerase prevents telomere elongation and also increases RAD51. Hybrid inhibition of HR and telomerase induces apoptosis and cell cycle arrest in G2‐M and prevents the growth of cancer cells. 40
The protein encoded by the XRCC3 gene is a RAD51‐related protein involved in double‐stranded DNA repair and maintains the chromosome structure. The XRCC3 protein has a higher expression rate in ESCC cells than in normal esophageal cells, reducing the effect of chemoradiotherapy; hence, it is possible to increase the efficacy of chemotherapy and radiotherapy by silencing this gene. 41
Moreover, the NRAGE gene, a member of the melanoma‐related antigen family, is significantly expressed in the nucleus of radiotherapy‐resistant esophageal cells. The decreased expression of this gene leads to a fall in the HRR level and so makes cancer cells more sensitive to radiotherapy. This protein forms a ternary structure with RNF8 and BARD1 proteins that are involved in HR regulation. 42 ATM is another major protein involved in DNA repair, maintaining chromosome stability by increasing HRR. Further, miR‐101 downregulates the expression of ATM and so improves the toxic effects of radiotherapy in ESCC resistant cells. 43 Another miRNA that can be considered in the diagnosis and treatment of EC is miR‐127‐3p. Also, it can compromise HRR by reducing the expression of the XRCC3 gene by targeting miRNA 3′ UTR. 44
In order to increase the effect of proton beam therapy (PBT), chemical agents targeting HR can be used to increase the sensitivity of cancer cells. The combination of Olaparib with PBT can efficiently reduce the resistance of cancer cells to radiation by increasing DSB, thus inducing apoptosis in cancer cells. Poly (ADP‐ribose) polymerase (PARP) is a cellular protein involved in DNA repair converting the single‐stranded DNA break into DSB, which therefore induces HR. 45 To date, based on our research in this literature review article, there is no clinical trial study about targeting genes that are involved in DNA repair. But two studies about the PARP gene, that is one of the genes involved in DNA repair, will be examined in the future. In the first study, Rucaparib would be tested on 220 participants (ClinicalTrials.gov Identifier: NCT04171700). In the second study, a combination of Olaparib, Pembrolizumab, and Paclitaxel will be tested on 71 cancer patients (ClinicalTrials.gov Identifier: NCT04592211).
Bortezomib is an anticancer drug that activates caspase by reducing the expression of HIF‐1 and VEGF genes; this compound delays DNA repair and induces apoptosis; accordingly, it increases sensitivity to radiotherapy. 46
The PRA1 gene is a subset of RPA, which is involved in HR processes and DNA replication. The increased expression of this gene in EC, colon cancer, etc. causes cell proliferation; also, it induces resistance to radiotherapy. The RPA1 gene promotes cell proliferation from G1 to the S cell cycle by upregulating CDK‐4/cyclin‐D levels. 47
Also, a bioinformatics study showed that Mirin and NU7441 (i.e., HR and NHEJ inhibitors, respectively) improved the radiotherapy effect and increased DSB. One of the most well‐known instances of HR inhibitors, mirin was developed against nuclease motion of MRE11 and used to impressively inhibit several cancers. Likewise, NU7441, a selective inhibitor for DNA‐PK, blocked the NHEJ pathway and induced DSB and led to increased radio‐sensitivity. 18
Also, studies have shown that mitochondrial superoxide dismutase (MnSOD) has a high potential for genome stability and cancer cell survival. Mice exposed to the plasmid with the MnSOD gene had a 7‐fold increase in HR, and when HR occurred, it showed a yellow fluorescent signal. 48 YM155 is a small molecule that inhibits survivin. Survivin plays an outstanding role in preventing apoptosis and resistance in all therapy types against cancer cells. YM155 also increases radiation sensitivity by reducing HRR, see Figure 1. 49
FIGURE 1.
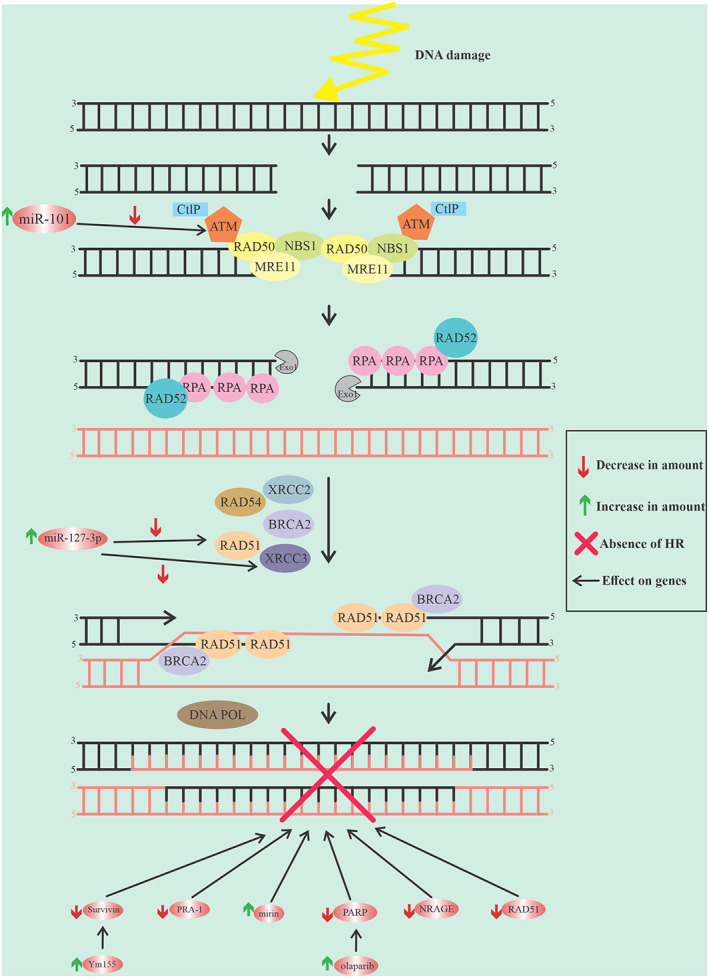
The graphic represents the details of the HR pathway and the factors that involved in interfering this path
3.2. NHEJ Pathway
Non‐homologous end joining is another type of repair in which the ends of the two strands are connected without the need for another homologous strand. 50 Non‐homologous end joining initiates the binding of the ku70/80 protein to the damaged end of the chromosome and provides a basis for the binding of subsequent factors, including DNA‐dependent protein kinase catalytic subunit (DNA‐PKcs), PAXX, DNA polymerase, and Artemis. 51 DNA‐PKcs has a strong affinity for the ku70/80 protein and so forms complexes. Then, the Artemis nuclease processes the end of the strand by removing a number of nucleotides and facilitates the connection of the ends of the two strands. 52
Afterward, μ and λ polymerases add a number of nucleotides, and, finally, x‐ray repair cross‐complementing protein 4 (XRCC4), XLF, and PAXX (which together form DNA ligase IV) bind the two broken strands. 53 The assessment of XRCC4 gene expression in 92 patients with ESCC showed that in more than 30% of patients, XRCC4 gene expression decreased. Patients with an increased expression level of this gene have a higher mortality rate than those with decreased expression levels. 54
3.2.1. Targeting the NHEJ pathway in EC cancer therapy
A therapeutic strategy to increase the efficiency of radiotherapy is the application of histone deacetylases (HDACs), such as valproic acid (VPA). The formation of γH2AX by phosphorylation of serine 139 of histones (H2AX) is a rapid way to induce DDR through NHEJ. VPA has been shown to decrease DDR levels in EC cancer cells. Also, VPA increases KU70 acetylation and promotes cell apoptosis. 55
The expression of HOXC10 has been shown to be higher in EC tumors than in normal cells. The product of this gene associates with cell proliferation by binding to the promoter of the ERB‐B2 gene and promoting the proliferation of cancer cells. HOXC10 also accelerates NHEJ by binding to the KU70 protein. Thus, targeting and silencing of this gene can decrease NHEJ and increase radio‐sensitivity. 56
A transcription co‐activator with the PDZ binding domain (TAZ) has a big impact on the proliferation and survival of cancer cells after radiotherapy. This transcription factor plays a role in DDR by regulating the expression of NHEJ genes, which is controlled by thymine DNA glycosylase (TDG). The inhibition of TDG increases the efficiency of radiotherapy and reduces cell growth in EC, see Fig. 2. 57
FIGURE 2.
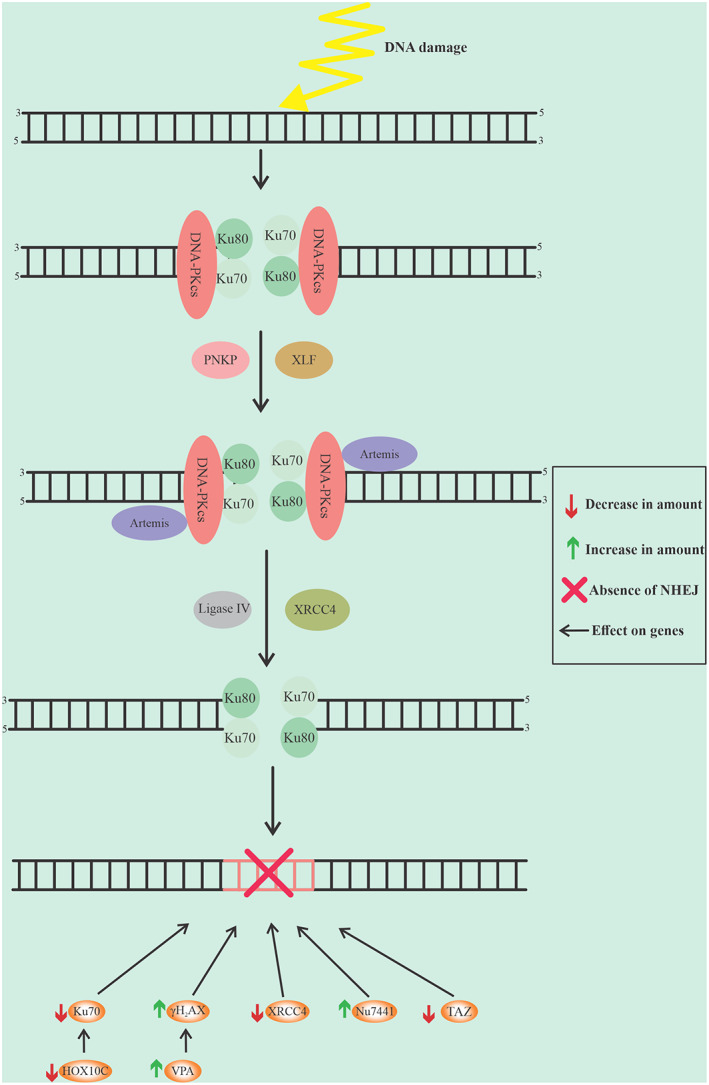
The graphic represents the details of the NHEJ pathway and the factors that involved in reducing this path
4. CONCLUSION
Currently, radiotherapy, chemotherapy and chemo‐radiotherapy are treatment options for EC. Also, radiotherapy is one of the most effective ways to treat cancer; in this regard, one of the challenges is the resistance of cancer cells to radiotherapy. Accordingly, we showed that silencing repair genes is necessary and more effective to complete radiotherapy. Indeed, DNA repair machinery can moderate radiotherapy resistance. In this regard, the main targeting components of DNA repair machinery such as RAD51, XRCC3, RPA‐1 in HR and XRCC4, KU70, KU80 in NHEJ can open a new window for treatment of the EC by sensitizing tumor cells to radiotherapy. However, further studies are needed to investigate the development of novel DDR components inhibitors in the EC.
AUTHOR CONTRIBUTIONS
Mohammad Reza Kheirandish: Writing – original draft (equal). Seyed Mostafa Mir: Data curation (equal); investigation (equal). Mehdi Sheikh Arabi: Supervision (lead); validation (lead); writing – review and editing (lead).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICS STATEMENT
This study was approved by the Ethics Committee of Golestan University of Medical Sciences (IR.GOUMS. REC.1400.072).
ACKNOWLEDGMENTS
The author is grateful to Golestan University of Medical Sciences, Gorgan, Iran, for providing all kinds of facilities to prepare this manuscript.
Kheyrandish MR, Mir SM, Sheikh Arabi M. DNA repair pathways as a novel therapeutic strategy in esophageal cancer: A review study. Cancer Reports. 2022;5(11):e1716. doi: 10.1002/cnr2.1716
Funding information Golestan University of Medical Sciences
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Then EO, Michell Lopez SS, Gayam V, Sunkara T, Culliford A, Gaduputi V. Esophageal cancer: an updated surveillance epidemiology and end results database analysis. World J Oncol. 2020;11(2):55‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akagündüz DD, Türker PF. Nutritional support in older patients with esophageal cancer undergoing chemoradiotherapy. Nutrit Cancer. 2022;74(10):3634‐3639. doi: 10.1080/01635581.2022.2096245 [DOI] [PubMed] [Google Scholar]
- 3. Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13(6):1010‐1021. [DOI] [PubMed] [Google Scholar]
- 4. Chiu WC, Fang PT, Lee YC, et al. DNA repair protein Rad51 induces tumor growth and metastasis in esophageal squamous cell carcinoma via a p38/Akt‐dependent pathway. Ann Surg Oncol. 2020;27(6):2090‐2101. [DOI] [PubMed] [Google Scholar]
- 5. Keles M, Sahin I, Kurt A, et al. Mitochondrial DNA deletions in patients with esophagitis, Barrett's esophagus, esophageal adenocarcinoma and squamous cell carcinoma. Afr Health Sci. 2019;19(1):1671‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Expression profile, clinical significance and biological functions of IGF2BP2 in esophageal squamous cell carcinoma [Internet]. [cited 2022 Jul 30]. Available from: https://www.spandidos-publications.com/10.3892/etm.2022.11177 [DOI] [PMC free article] [PubMed]
- 7. Duggan SP, Garry C, Behan FM, et al. siRNA library screening identifies a druggable immune‐signature driving esophageal adenocarcinoma cell growth. Cell Mol Gastroenterol Hepatol. 2018;5(4):569‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong Y, Sun D, Li J, Ye S, Zhu Y. Association of Tumor Protein 53 polymorphism with esophageal cancer cases: a systematic review and meta‐analysis. Indian J Pharm Sci. 2022;2(14):136‐143. [Google Scholar]
- 9. Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. 2018;41(3):210‐215. [DOI] [PubMed] [Google Scholar]
- 10. Nordenstedt H, Lagergren J. Environmental factors in the etiology of gastroesophageal reflux disease. Expert Rev Gastroenterol Hepatol. 2008;2(1):93‐103. [DOI] [PubMed] [Google Scholar]
- 11. Mokhtari RB, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022‐33843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Chen L, Luan S, Zhou J, Xiao X, Yang Y, et al. The development and progress of nanomedicine for esophageal cancer diagnosis and treatment. Seminars in Cancer Biology [Internet] . 2022. [cited 2022 Jul 30]; https://www.sciencedirect.com/science/article/pii/S1044579X2200013X [DOI] [PubMed]
- 13. Yang YM, Hong P, Xu WW, He QY, Li B. Advances in targeted therapy for esophageal cancer. Signal Transduct Target Ther. 2020;5(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdulrahman GO, Curtin NJ. Targeting DNA damage response pathways in cancer. J. Med. Chem. III. 2017;5:104‐133. [Google Scholar]
- 15. Rother MB, Pellegrino S, Smith R, et al. CHD7 and 53BP1 regulate distinct pathways for the re‐ligation of DNA double‐strand breaks. Nat Commun. 2020;11(1):1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fei Z, Gu W, Xie R, Su H, Jiang Y. Artesunate enhances radiosensitivity of esophageal cancer cells by inhibiting the repair of DNA damage. J Pharmacol Sci. 2018;138(2):131‐137. [DOI] [PubMed] [Google Scholar]
- 17. Nandi B, Talluri S, Kumar S, et al. The roles of homologous recombination and the immune system in the genomic evolution of cancer. J Transl Sci. 2019;5(2):10‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang G, Guo S, Zhang W, et al. A comprehensive analysis of alterations in DNA damage repair pathways reveals a potential way to enhance the radio‐sensitivity of esophageal squamous cell cancer. Front Oncol. 2020;10(1):575711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang RX, Zhou PK. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020;5(1):1‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Esophageal Cancer: Priorities for Prevention | SpringerLink [Internet] . [cited 2022. Apr 24]. Available from: https://www.springer.com/article/10.1007/s40471-014-0015-3
- 21. Kim N. Sex difference of esophageal cancer: esophageal squamous cell carcinoma vs. esophageal adenocarcinoma. In: Kim N, ed. Sex/Gender‐Specific Medicine in the Gastrointestinal Diseases. Springer Nature; 2022:69‐92. [Google Scholar]
- 22. Cancers | Free Full‐Text | Distribution of Lymph Node Metastases in Esophageal Carcinoma Patients Undergoing Upfront Surgery: A Systematic Review [Internet] . [cited 2022 Apr 24]. Available from: https://www.mdpi.com/2072-6694/12/6/1592 [DOI] [PMC free article] [PubMed]
- 23. Li Y, Du L, Wang Y, et al. Modeling the cost‐effectiveness of esophageal cancer screening in China. Cost Eff Resour Alloc. 2020;18(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Testa U, Castelli G, Pelosi E. Esophageal cancer: genomic and molecular characterization, stem cell compartment and clonal evolution. Medicines. 2017;4(3):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sahm V, Maurer C, Baumeister T, et al. Telomere shortening accelerates tumor initiation in the L2‐IL1B mouse model of Barrett esophagus and emerges as a possible biomarker. Oncotarget. 2022. Feb;14(13):347‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jabbour SK, Thomas CR Jr. Radiation therapy in the postoperative management of esophageal cancer. J GastrointestOncol. 2010;1(2):102‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wikman A, Johar A, Lagergren P. Presence of symptom clusters in surgically treated patients with esophageal cancer: implications for survival. Cancer. 2014;120(2):286‐293. [DOI] [PubMed] [Google Scholar]
- 28. Nakaminato S, Toriihara A, Makino T, Kawano T, Kishimoto S, Shibuya H. Prevalence of esophageal cancer during the pretreatment of hypopharyngeal cancer patients: routinely performed esophagogastroduodenoscopy and FDG‐PET/CT findings. Acta Oncol. 2012;51(5):645‐652. [DOI] [PubMed] [Google Scholar]
- 29. Que J, Garman KS, Souza RF, Spechler SJ. Pathogenesis and cells of origin of Barrett's esophagus. Gastroenterology. 2019;157(2):349‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ng CK, Ma K, Cheng Y, Miyashita T, Harmon JW, Meltzer SJ. Krüppel‐like factor 5 promotes sonic hedgehog signaling and neoplasia in Barrett's esophagus and esophageal adenocarcinoma. Transl Oncol. 2019;12(11):1432‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bealy MABI. Frequency of Human Papilloma Virus (16 and 18) and Epstein Bar Virus among Sudanese Patients with Esophageal Cancer using Immunohistochemistry and Polymerase Chain Reaction [PhD Thesis]. Sudan University of Science & Technology; 2017.
- 32. Oze I, Charvat H, Matsuo K, et al. Revisit of an unanswered question by pooled analysis of eight cohort studies in Japan: does cigarette smoking and alcohol drinking have interaction for the risk of esophageal cancer? Cancer Med. 2019;8(14):6414‐6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hosoya N, Miyagawa K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014;105(4):370‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Principe DR, Narbutis M, Koch R, Rana A. Frequency and prognostic value of mutations associated with the homologous recombination DNA repair pathway in a large pan cancer cohort. Sci Rep. 2020;10(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ranjha L, Howard SM, Cejka P. Main steps in DNA double‐strand break repair: an introduction to homologous recombination and related processes. Chromosoma. 2018;127(2):187‐214. [DOI] [PubMed] [Google Scholar]
- 36. Bryant E. Systems Genetics of DNA Damage Tolerance: Cisplatin, RAD5 & CRISPR‐Mediated Nonsense. Columbia University; 2019. [Google Scholar]
- 37. Zhang H, Hua Y, Jiang Z, et al. Cancer‐associated fibroblast–promoted lncRNA DNM3OS confers radioresistance by regulating DNA damage response in esophageal squamous cell carcinoma. Clin Cancer Res. 2019;25(6):1989‐2000. [DOI] [PubMed] [Google Scholar]
- 38. Pal J, Nanjappa P, Kumar S, et al. Impact of RAD51C‐mediated homologous recombination on genomic integrity in Barrett's adenocarcinoma cells. J Gastroenterol Hepatol Res. 2017;6(1):2286‐2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Catara G, Colanzi A, Spano D. Combinatorial strategies to target molecular and signaling pathways to disarm cancer stem cells. Front Oncol. 2021;26(11):689131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu R, Pal J, Buon L, et al. Targeting homologous recombination and telomerase in Barrett's adenocarcinoma: impact on telomere maintenance, genomic instability and tumor growth. Oncogene. 2014;33(12):1495‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cheng J, Liu W, Zeng X, et al. XRCC 3 is a promising target to improve the radiotherapy effect of esophageal squamous cell carcinoma. Cancer Sci. 2015;106(12):1678‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang Q, Pan Q, Li C, Xu Y, Wen C, Sun F. NRAGE is involved in homologous recombination repair to resist the DNA‐damaging chemotherapy and composes a ternary complex with RNF8–BARD1 to promote cell survival in squamous esophageal tumorigenesis. Cell Death Differ. 2016;23(8):1406‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen M, Liu P, Chen Y, et al. Long noncoding RNA FAM201A mediates the radiosensitivity of esophageal squamous cell cancer by regulating ATM and mTOR expression via miR‐101. Front Genet. 2018;5(9):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhen N, Yang Q, Zheng K, et al. MiroRNA‐127‐3p targets XRCC3 to enhance the chemosensitivity of esophageal cancer cells to a novel phenanthroline‐dione derivative. Int J Biochem Cell Biol. 2016;79:158‐167. [DOI] [PubMed] [Google Scholar]
- 45. Kageyama S, Ichiro JD, Hojo H, et al. PARP inhibitor olaparib sensitizes esophageal carcinoma cells to fractionated proton irradiation. J Radiat Res. 2020;61(2):177‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang D, Qin Q, Jiang QJ, Wang DF. Bortezomib sensitizes esophageal squamous cancer cells to radiotherapy by suppressing the expression of HIF‐1α and apoptosis proteins. J Xray Sci Technol. 2016;24(4):639‐646. [DOI] [PubMed] [Google Scholar]
- 47. Wang J, Yang T, Chen H, Li H, Zheng S. Oncogene RPA1 promotes proliferation of hepatocellular carcinoma via CDK4/cyclin‐D pathway. Biochem Biophys Res Commun. 2018;498(3):424‐430. [DOI] [PubMed] [Google Scholar]
- 48. Niu Y, Wang H, Wiktor‐Brown D, et al. Irradiated esophageal cells are protected from radiation‐induced recombination by MnSOD gene therapy. Radiat Res. 2010;173(4):453‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Small‐molecule survivin inhibitor YM155 enhances radiosensitization in esophageal squamous cell carcinoma by the abrogation of G2 checkpoint and suppression of homologous recombination repair | Journal of Hematology & Oncology [Internet]. [cited 2022 Apr 24]. Available from: https://jhoonline.biomedcentral.com/articles/10.1186/s13045-014-0062-8 [DOI] [PMC free article] [PubMed]
- 50. Non‐homologous DNA end joining and alternative pathways to double‐strand break repair | Nature Reviews Molecular Cell Biology [Internet]. [cited 2022 Apr 24]. Available from: https://www.nature.com/articles/nrm.2017.48 [DOI] [PMC free article] [PubMed]
- 51. Repair of G1 induced DNA double‐strand breaks in S‐G2/M by alternative NHEJ | Nature Communications [Internet]. [cited 2022 Apr 24]. Available from: https://www.nature.com/articles/s41467-020-19060-w [DOI] [PMC free article] [PubMed]
- 52. Role for Artemis nuclease in the repair of radiation‐induced DNA double strand breaks by alternative end joining ‐ PubMed [Internet]. [cited 2022 Apr 24]. Available from: https://pubmed.ncbi.nlm.nih.gov/25973742/ [DOI] [PubMed]
- 53. Pannunzio NR, Watanabe G, Lieber MR. Nonhomologous DNA end‐joining for repair of DNA double‐strand breaks. J Biol Chem. 2018;293(27):10512‐10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hori M, Someya M, Matsumoto Y, et al. Influence of XRCC4 expression in esophageal cancer cells on the response to radiotherapy. Med Mol Morphol. 2017. Mar;50(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 55. Inhibitory effects of valproic acid in DNA double‐strand break repair after irradiation in esophageal squamous carcinoma cells [Internet]. [cited 2022. Apr 24]. Available from: https://www.spandidos-publications.com/10.3892/or.2015.4089 [DOI] [PubMed]
- 56. HOXC10 upregulation confers resistance to chemoradiotherapy in ESCC tumor cells and predicts poor prognosis | Oncogene [Internet] . [cited 2022. Apr 24]. Available from: https://www.nature.com/articles/s41388-020-1375-4 [DOI] [PubMed]
- 57. Zhou W, Zhang L, Chen P, Li S, Cheng Y. Thymine DNA glycosylase‐regulated TAZ promotes radioresistance by targeting nonhomologous end joining and tumor progression in esophageal cancer. Cancer Sci. 2020;111(10):3613‐3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


