Abstract
We report a 51‐year‐old woman with thyroid eye disease and biopsy‐proven pretibial myxedema that was subsequently treated with teprotumumab with improvement.
Keywords: Graves' disease, pretibial myxedema, teprotumumab, thyroid eye disease
It is important to monitor patients with Graves' disease after thyroidectomy for thyroid eye disease (TED) and pretibial myxedema. Our patient demonstrated a successful treatment of both TED and pretibial myxedema with teprotumumab.

1. INTRODUCTION
Approximately 60–80% of all cases of hyperthyroidism are attributed to Graves' disease. Thyroid eye disease (TED) occurs in 30% of patients with Graves' disease and pretibial myxedema in up to 4%. 1 , 2 , 3 Graves' disease complicated by both TED and pretibial myxedema is uncommon, occurring in less than 5% of all Graves' disease cases, with a prevalence of 0.15/10,000. 1 , 2 Treatment of thyroid eye disease and pretibial myxedema are varied and often target each specific condition. 4 , 5 We report a case of Graves' disease complicated by concurrent TED and pretibial myxedema presenting post‐thyroidectomy that was successfully treated with teprotumumab.
2. CASE REPORT
A 51‐year‐old African American female with no significant past medical history presented with tachycardia, palpitations, heat intolerance, weight loss, anxiety, and panic attacks. She was found to have an undetectable TSH, elevated free T4 of 6.05 ng/dl (reference 0.93–1.7), elevated total T3 of 332.4 ng/dl (reference 80–200), elevated thyroid‐stimulating immunoglobulin (TSI) level of 19.50 IU/L (reference 0.00–0.55), and elevated thyrotropin receptor antibody (TRAb) level of 27 U/L (reference <1.0), confirming the diagnosis of Graves' disease. Her initial ophthalmic examination revealed no exophthalmos, chemosis, lid retraction, lid lag, or reduced extraocular motility. She did not use tobacco products. She was found to have thyromegaly and bedside ultrasound revealed an enlarged, heterogeneous, and hypervascular thyroid with no evidence of nodules. The patient was subsequently started on atenolol 12.5 mg twice daily and methimazole 20 mg daily for 7 months until thyroid surgery. At the time of her thyroidectomy, she exhibited no ophthalmologic or dermatologic changes.
Three months following thyroidectomy, she developed hyperpigmentation of the bilateral anterior lower extremities with firm, compressible papules and scattered coalescent plaques (Figure 1). Initial workup was negative for tinea pedis, deep vein thrombosis, and venous insufficiency, and the rash was not responsive to typical treatments. Ultimately, a skin punch biopsy revealed a pallorous dermal layer with widely spaced collagen bundles. Colloidal iron stain demonstrated increased mucin in the papillary and reticular dermis and the subcutaneous soft tissue. Elastin fibers were fragmented and reduced with an elastin stain (Figure 2A,B). Given her clinical history, these changes were consistent with a diagnosis of pretibial myxedema.
FIGURE 1.

Showing pretibial myxedema before and after teprotumumab.
FIGURE 2.

(A, B) A punch biopsy (H&E stain) which revealed a pallor dermal layer had pallor with widely spaced collagen bundles (A). Colloidal iron stain demonstrated increased mucin in the papillary and reticular dermis and the subcutaneous soft tissue (B).
Around this time, the patient noticed periorbital swelling and tearing. She was noted to have TED with proptosis, spontaneous orbital pain, and edema and erythema of her eyelids and conjunctiva without evidence of binocular diplopia or compressive optic neuropathy (Figure 3). She was initiated on intravenous teprotumumab infusions (10 mg/kg/dose for the 1st infusion, and then 20 mg/kg/dose every 3 weeks for the next 7 doses) with significant improvement of TED and moderate subjective improvement of pretibial myxedema.
FIGURE 3.
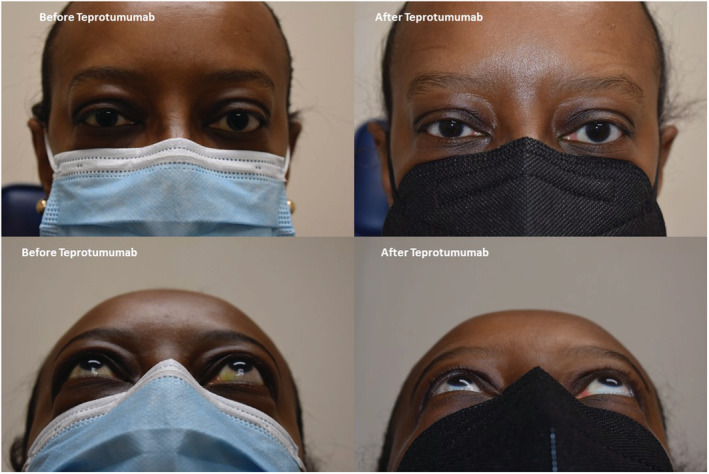
Showing thyroid eye disease before and after teprotumumab.
3. DISCUSSION
Pretibial myxedema occurs as a result of the deposition of glycosaminoglycans (GAG) secreted by fibroblasts as a result of cytokines from lymphocytic infiltration. These fibroblasts have been found to express thyroid‐stimulating hormone receptor. The resulting pathologic changes are mucinous edema with deposition of mucin in the papillary and reticular dermis as well as fragmentation of collagen fibers. 6 The expansion itself is also variable amongst patients. In a similar manner, orbital accumulation of GAG and subsequent expansion of retrobulbar tissue leads to the clinical manifestation of exophthalmos. 2 , 7 Despite total or partial thyroid gland removal, circulating thyroid antibodies continue to contribute to the accumulation of GAG. 8 , 9
Pretibial myxedema management depends on the symptomatology of presentation. Although many asymptomatic cases do not require treatment, patients may request it for aesthetic purposes. Other patients may experience symptoms such as pruritis or irritation, or have excessive tissue expansion. In these situations, topical or intralesional glucocorticoids can be used, though there is a 30% chance of recurrence after about 3.5 years. 6 Intralesional octreotide has demonstrated a beneficial effect on regression of refractory pretibial myxedema, with the likely mechanism of action via the suppression of hyaluronic acid secretion by fibroblasts through IGF‐1 inhibition. 10 Currently, there is a paucity of data regarding treatment for refractory pretibial myxedema. 11 , 12 However, there are recent case reports of teprotumumab leading to improvement in pretibial myxedema. Additional studies are needed to determine the exact efficacy of teprotumumab for this condition. 11 In this case, our patient reported moderate improvement in hyperpigmentation and regression of the pretibial lesions.
Management of TED includes hyperthyroidism reversal and, if moderate to severe, treatment of orbital inflammation with systemic glucocorticoid or teprotumumab administration. Patients with TED should not be treated with radioiodine ablation as it can lead to worsening of the disease due to the spillover effect of autoantibodies following ablation. Other possible treatments include surgical interventions to include orbital decompression, strabismus surgery, and lid retraction repair, all of which are indicated only in the stable, inactive phase of TED. In cases of vision‐threatening optic neuropathy due to TED, urgent orbital decompression is the treatment of choice. 13 Teprotumumab is the first FDA‐approved treatment for active TED. 14 It is a human monoclonal IgG1 antibody that selectively binds to insulin‐like growth factor 1 receptor (IGF‐1R). Teprotumumab was shown to reduce clinical activity score and proptosis similarly to surgery, 15 , 16 with the secondary effect of decreasing diplopia in some patients. In this patient, treatment with teprotumumab decreased proptosis by two millimeters (Figure 3) and improved eyelid retraction and lagophthalmos. This is the first reported case of a patient presenting with both TED and pretibial myxedema following thyroidectomy who was successfully treated with teprotumumab. IGF‐1 receptors are upregulated in fibroblasts of patients with Graves' disease. This leads to hypersecretion of hyaluronic acid and GAGs and predisposition of patients to develop TED, and likely, pretibial myxedema. 17 The IGF‐1R blocking mechanism of teprotumumab downregulates the action of IGF‐1 and TSH in fibroblasts, decreasing the tissue changes caused by proinflammatory cytokines. 18 The long‐term durability of benefit and cost‐effectiveness of teprotumumab for TED and pretibial myxedema remain to be determined.
4. CONCLUSION
This case report demonstrates the first presentation of a patient who developed both TED and pretibial myxedema following total thyroidectomy with clinically significant improvement of both conditions following treatment with teprotumumab. This case illustrates the importance of continued monitoring for both TED and pretibial myxedema following thyroidectomy, as well as the consideration of treatment with teprotumumab to target both conditions. While further research is needed at this time to determine the exact efficacy of teprotumumab for the treatment of pretibial myxedema, there is building evidence in the literature for its use.
AUTHOR CONTRIBUTIONS
BKB – author. EC – reviewer. DPL‐ reviewer. JRH‐ reviewer. PV‐ reviewer, pathologist. TCD ‐ reviewer. MKMS‐ reviewer. TDH‐ reviewer, mentor.
CONFLICT OF INTEREST
None to declare.
ETHICS STATEMENT
The manuscript has been reviewed and approved by the IRB and Public Affairs Office.
CONSENT
The authors have confirmed that patient consent has been signed and collected in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or the U.S. Government.
Bowens BK, Chou E, LaChance DP, et al. Graves' disease complicated by concurrent thyroid eye disease and pretibial myxedema successfully treated with teprotumumab. Clin Case Rep. 2022;10:e06621. doi: 10.1002/ccr3.6621
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Anderson CK, Miller OF 3rd. Triad of exophthalmos, pretibial myxedema, and acropachy in a patient with Graves' disease. J Am Acad Dermatol. 2003;48(6):970‐972. [DOI] [PubMed] [Google Scholar]
- 2. Pokhrel B, Bhusal K. Graves disease. [updated 2021 Jul 21]. StatPearls [Internet]. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK448195/ [PubMed] [Google Scholar]
- 3. Perros P, Hegedüs L, Bartalena L, et al. Graves' orbitopathy as a rare disease in Europe: a European group on Graves' orbitopathy (EUGOGO) position statement. Orphanet J Rare Dis. 2017;12(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoang TD, Stocker DJ, Chou EL, Burch HB. 2022 update on clinical management of Graves' disease and thyroid eye disease. Endocrinol Metab Clin North Am. 2022;51(2):287‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6(5):295‐309. [DOI] [PubMed] [Google Scholar]
- 6. Doshi DN, Blyumin ML, Kimball AB. Cutaneous manifestations of thyroid disease. Clin Dermatol. 2008;26(3):283‐287. [DOI] [PubMed] [Google Scholar]
- 7. Kahaly G, Förster G, Hansen C. Glycosaminoglycans in thyroid eye disease. Thyroid. 1998;8(5):429‐432. [DOI] [PubMed] [Google Scholar]
- 8. Khoo TK, Bahn RS. Pathogenesis of thyroid eye disease: the role of autoantibodies. Thyroid. 2007;17(10):1013‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marcocci C, Bruno‐Bossio G, Manetti L, et al. The course of thyroid eye disease is not influenced by near total thyroidectomy: a case‐control study. Clin Endocrinol (Oxf). 1999;51(4):503‐508. [DOI] [PubMed] [Google Scholar]
- 10. Shinohara M, Hamasaki Y, Katayama I. Refractory pretibial myxoedema with response to intralesional insulin‐like growth factor 1 antagonist (octreotide): downregulation of hyaluronic acid production by the lesional fibroblasts. Br J Dermatol. 2000;143(5):1083‐1086. [DOI] [PubMed] [Google Scholar]
- 11. Varma A, Rheeman C, Levitt J. Resolution of pretibial myxedema with teprotumumab in a patient with graves disease. JAAD Case Rep. 2020;6(12):1281‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jimenez A, Hull C, Zone J. Rituximab therapy for a severe case of pretibial myxedema. JAAD Case Rep. 2021;3(10):28‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kauh CY, Gupta S, Douglas RS, et al. Compressive optic neuropathy and repeat orbital decompression: a case series. Ophthalmic Plast Reconstr Surg. 2015;31(5):385‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. FDA approves first treatment for thyroid eye disease.FDA approves first treatment for thyroid eye disease. Caution‐https://www.fda.gov/news‐events/press‐announcements/fda‐approves‐first‐treatment‐thyroid‐eye‐disease
- 15. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid‐associated ophthalmopathy. N Engl J Med. 2017;376(18):1748‐1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341‐352. [DOI] [PubMed] [Google Scholar]
- 17. Davies TF, Andersen S, Latif R, et al. Graves’ disease. Nat Rev Dis Primers. 2020;6(1):52. [DOI] [PubMed] [Google Scholar]
- 18. Chen H, Mester T, Raychaudhuri N, et al. Teprotumumab, an IGF‐1R blocking monoclonal antibody inhibits TSH and IGF‐1 action in fibrocytes. J Clin Endocrinol Metab. 2014;99(9):E1635‐E1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


