Abstract
Background
Three‐dimensional image‐guided brachytherapy is the standard of care in cervical cancer radiotherapy. In addition, the usefulness of the so‐called “hybrid brachytherapy (HBT)” has been reported, which involves the addition of needle applicators to conventional intracavitary brachytherapy for interstitial irradiation.
Aim
To evaluate the clinical outcomes of CT‐based HBT consisting of transvaginal insertion of needle applicators (CT‐based transvaginal HBT) and only intravenous sedation without general or saddle block anesthesia.
Methods and results
This is a retrospective chart review of patients who received definitive radiotherapy, including CT‐based transvaginal HBT, between February 2012 and July 2019. The inclusion criteria were as follows: (i) histologically diagnosed disease, (ii) untreated cervical cancer, (iii) International Federation of Gynecology and Obstetrics (FIGO) stage IB1–IVA disease in the 2008 FIGO staging system, and (iv) patients who underwent CT‐based transvaginal HBT at least once in a series of intracavitary brachytherapy. Overall, 54 patients fulfilled the eligibility criteria in the present study. The median follow‐up period was 32 (IQR, 19–44) months. No patient complained of symptoms such as persistent bleeding or abdominal pain after the treatment. The 3‐year local control (LC), disease‐free survival, and overall survival rates for all 54 patients were 86.6%, 60.3%, and 90.7% (95% CI [81.3%–100.0%]), respectively. The 3‐year LC rate was 87.7% in patients with FIGO III–IVA and 90.4% in tumor size >6.0 cm. The incidence rate of late adverse events, grade ≥3, in the rectum and bladder was 0% and 1.8%, respectively. In the dose‐volume histogram analyses, transvaginal HBT increased the dose of HR‐CTVD90 by ~7.5% without significantly increasing the dose of organs at risk.
Conclusion
Considering the favorable clinical outcomes, CT‐based transvaginal HBT may be a good option for treating cervical cancer.
Keywords: brachytherapy, cervical cancer, hybrid brachytherapy, interstitial brachytherapy, intracavitary brachytherapy, radiotherapy
1. INTRODUCTION
Uterine cervical cancer is one of the most common cancers in women worldwide. 1 Radiotherapy (RT) plays a crucial role as a definitive treatment for patients with stage IB–IVA cervical cancer. Several randomized phase III studies and meta‐analyses have established the use of platinum‐based concurrent chemoradiotherapy (CCRT) as the standard of care for patients with stage IB–IVA cervical cancer. 2 , 3 , 4
Intracavitary brachytherapy is a pivotal treatment for all patients with cervical cancer receiving RT or CCRT. 5 In 2005, the Groupe Européen de Curiethérapie and the European Society for Radiotherapy & Oncology Gynecology proposed the concept of a three‐dimensional image‐guided brachytherapy (3D‐IGBT). 6 , 7 These studies provided the core concepts and defined the terms that would be used in 3D‐IGBT. 6 , 7 Many studies have demonstrated the relationship between local control (LC) probability and dose to the high‐risk clinical target volume (HR‐CTV) since these concepts were first introduced. 8 , 9 , 10 The transition from traditional brachytherapy to 3D‐IGBT has led to improved LC rates and reduced late toxicity. 8 , 9 , 10 More recently, EMBRACE‐I, a prospective multicenter study that employed magnetic resonance imaging (MRI)‐guided 3D‐IGBT for cervical cancer, has been published. 11 In this study, the actuarial overall 5‐year LC rate was 92%, with limited severe toxicity in normal organs. 11
MRI is undoubtedly the ideal imaging modality for 3D‐IGBT, owing to its superior visualization in soft tissue compared to computed tomography (CT). 12 However, its availability for 3D‐IGBT is still limited in clinical settings. A recent nationwide survey of 3D‐IGBT in Japan showed that only 4% of 3D‐IGBT was performed using MRI. 13 Similar surveys conducted in the US and Canada report limited use of MRI‐based 3D‐IGBT (34%–57%). 14 , 15 Notably, ~90% of newly diagnosed cervical cancer occur in low‐ to middle‐income countries, where access to MRI‐based 3D‐IGBT is difficult. 16 Establishing a beneficial and easily accessible treatment strategy that replaces MRI‐based 3D‐IGBT is, therefore, crucial to improving treatment outcomes for a larger number of patients with cervical cancer. CT‐based 3D‐IGBT presents as a viable alternative. Several guidelines or protocols on HR‐CTV contouring for CT‐based IGBT have been reported hitherto, 17 , 18 and an international recommendation for CT‐based 3D‐IGBT has been recently published. 19 With careful consideration of target volume contouring, the LC rates of these studies consisting of CT‐based 3D‐IGBT are comparable to those of MRI‐based IGBT. 10 , 20 , 21
For irregularly shaped and/or bulky cervical cancer, the interstitial approach may be an effective treatment method. However, recent National Comprehensive Cancer Network guidelines mention that such interstitial brachytherapy should only be performed by individuals and institutions with appropriate experience and expertise. 22 As a simplified approach, the usefulness of the so‐called “hybrid brachytherapy (HBT),” in which needle applicators for interstitial irradiation are added to conventional intracavitary brachytherapy, has been reported. 23 , 24 Recently, Murakami et al. reported the initial outcomes of CT‐based HBT for locally advanced cervical cancer. 24 In this study, additional interstitial needle catheters were inserted perineally or vaginally under transrectal ultrasound guidance using saddle block anesthesia or local anesthesia and intravenous sedation. 25 Although this method is simpler and easier to use than the interstitial approach, it still requires specialized skills and knowledge about the saddle block anesthesia procedure for perineal needle insertion.
Tan et al. proposed a method of HBT in an environment with limited medical resources, such as outpatient set‐ups. 26 Although they did not report the oncological outcomes, they reported the feasibility of a combination of oxycodone 5 mg capsules, midazolam, and a paracervical block. As a further simplified approach, we have been performing CT‐based HBT consisting of transvaginal insertion of needle applicators (CT‐based transvaginal HBT) and only intravenous sedation without general or saddle block anesthesia. Here, we report the clinical outcomes, including the safety of CT‐based transvaginal HBT, in patients with cervical cancer in our institution.
2. METHODS
2.1. Patient eligibility
We retrospectively reviewed clinical outcomes in consecutive patients with cervical cancer who were treated with definitive RT/CCRT, including CT‐based transvaginal HBT, between February 2012 and July 2019 in our hospital. The inclusion criteria were as follows: (i) histologically diagnosed disease, (ii) untreated cervical cancer, (iii) International Federation of Gynecology and Obstetrics (FIGO) stage IB1–IVA disease in the 2008 FIGO staging system, and (iv) patients who underwent CT‐based transvaginal HBT at least once in a series of intracavitary brachytherapy. The Ethical Review Board committee of our institution approved this study (QST 20‐043, approved on March 11, 2021).
2.2. External beam radiotherapy and chemotherapy
External beam radiotherapy (EBRT) involves a combination of whole pelvic (WP) irradiation, and central shielding (CS), similar to traditional methods in Japan and parts of Asia. 27 , 28 CS has been used as a part of EBRT in anteroposterior/posteroanterior fields to lower the irradiation dose to the bladder and rectum. In brief, up to ~50 Gy radiation was delivered to the WP and pelvis sidewall, with a daily fraction dose of 1.8 or 2.0 Gy using 10 megavolt X‐rays. After 20, 30, or 40 Gy of WP‐EBRT, a 3‐cm wide CS was inserted. Boost EBRT of 6–10 Gy in 3–5 fractions were performed for patients with pelvic nodal metastasis. For patients with para‐aortic lymph node (PAN) metastases, 40 Gy of prophylactic EBRT to the para‐aortic lymph node region was performed, followed by 16–18 Gy in 8–9 fractions of boost EBRT to the metastatic PANs. Weekly cisplatin (40 mg/m2, up to five courses) was concurrently administered with EBRT. Patients older than 70 years or with severe concomitant diseases, including renal dysfunction, ischemic heart disease, or severe diabetes, did not receive chemotherapy.
2.3. Brachytherapy
Intracavitary brachytherapy using a 192Ir remote after loading system (microSelectron, Nucletron, Veenendaal, the Netherlands) was performed weekly. Three to five (an average of four) fractions of brachytherapy were administered after starting CS irradiation. A set of Fletcher‐Suit Asian‐Pacific applicators were used for the majority of patients. A tandem‐vaginal cylinder applicator was used for some patients with tumor infiltration into the lower vagina, or those who had a narrow vagina. Additional interstitial catheters (Trocar Point needles, 1.5‐mm φ, Nucletron; Elekta, Stockholm, Sweden) were inserted transvaginally into the tumor during HBT. Intravenous sedation using flunitrazepam was performed before the applicator insertion. The details of pain relief and sedation in our hospital are shown in Data S1. All applicator insertions were performed freehand under transabdominal ultrasound guidance.
After applicator implantation, CT data were acquired with the patient in the supine position. The CT slice thickness was 3 mm, and CT‐based treatment planning was performed. HR‐CTV contouring was performed with the same delineations as the Japanese Radiation Oncology Study Group recommendations. 17 The findings of the gynecological examinations performed at diagnosis, brachytherapy, and those of MRI examinations performed at diagnosis and just before the first brachytherapy session were used as references.
2.4. Indications for CT‐based transvaginal HBT
The transvaginal HBT in our hospital was implemented in cases with one or more of the following criteria: (i) the tumor extended to the pelvic wall on gynecological examination/MRI findings prior to intracavitary irradiation, (ii) tumor remained unevenly distributed on the bladder or rectum side, (iii) ellipticity (ratio of the shortest distance to the longest distance from the center of the uterine lumen to the edge of the tumor on the MRI axial image) was greater than 2, and (iv) (at second or later brachytherapy) insufficient dose or overdose of HR‐CTV in organs at risk (OARs) in the previous brachytherapy. Ellipticity was assessed using MRI before the first brachytherapy (Data S2). HBT was not performed if the patient took anticoagulants. The same criteria were consistently used to evaluate all patients who were included in the present study.
2.5. Dose‐volume histogram parameters
We estimated the composited dose to HR‐CTVD90 and OARs D2cc (the minimum dose delivered to the highest irradiated 2‐cc region). The cumulative EBRT and brachytherapy doses were summarized and normalized to a biological equivalent dose of 2 Gy per fraction (EQD2) using a linear‐quadratic model with an alpha/beta of 10 Gy for the HR‐CTV and 3 Gy for the OARs. The doses of pelvic irradiation with CS were not added to the EQD2, as was done in previous studies. 10 , 19 , 20
2.6. Dose prescription and optimization of HBT
All treatment plans were formulated by the Oncentra planning system (Nucletron, Veenendaal, the Netherlands). The initial plan for each patient was generated based on Point A prescription. Dose adaptation by changing the dose at Point A was performed so that a dose >6 Gy was delivered to HR‐CTVD90. Thereafter, the dwell time allocation, including interstitial catheters, was modified. Finally, dose distribution was fine‐tuned using “graphical optimization” function. The aiming dose for HR‐CTV and OARs in each brachytherapy session in our institution and an actual session of CT‐based transvaginal HBT are shown in Figure 1. In each brachytherapy session, the doses aimed for HR‐CTVD90, Rectum D2cc, and Bladder D2cc were ≥7.0, <5.5, and <6.5 Gy, respectively. There was no change in the prescription dose for each stage.
FIGURE 1.

Aiming dose for HR‐CTV or OARs in each brachytherapy session and actual case who underwent CT‐based transvaginal hybrid brachytherapy. HR‐CTV, high‐risk clinical target volume; OARs, organs at risk
2.7. Follow‐up and evaluation for clinical outcomes
All patients were carefully monitored after treatment until they awakened and confirmed symptoms such as pain or bleeding after awakening. Patients' follow‐ups were scheduled every 1–3 months for the first 2 years and every 3–6 months thereafter. Gynecological examinations and imaging evaluations, including CT and MRI, were performed regularly. CT was taken once at the end of treatment, and every 6 months thereafter for the first 2 years. MRIs were taken 1 and 3 months after treatment, and every 6 months thereafter for the first 2 years. After which, CT and MRI were each taken annually. A tumor biopsy was performed for confirmation in cases of suspected local recurrence. Late toxicities in the present study were defined as any toxicity occurring 6 months after the initiation of RT. The grades of late toxicities were assessed in accordance with the toxicity criteria of the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer system. 29
2.8. Statistical analysis
The LC, disease‐free survival (DFS), overall survival (OS), and cumulative occurrence rates of late toxicity were evaluated using the Kaplan–Meier method. The LC and survival durations were calculated from the initiation of treatment. The log‐rank test was used for univariate analysis. The clinical factors in the two groups were compared using the Mann–Whitney U test. Statistical significance was set at p < .05, and all statistical tests were two‐sided. Statistical calculations were performed using the IBM SPSS Statistics 27 software (IBM, Armonk, NY, United States).
3. RESULTS
3.1. Patient and treatment characteristics
The patient and treatment characteristics are summarized in Table 1. A total of 54 patients met the eligibility criteria in the present study. The median patient age was 65 (Interquartile range [IQR], 53–74) years, and the median follow‐up period was 32 (IQR, 19–44) months. Regarding histological subtypes, 45 patients had squamous cell carcinoma (Sq), and nine had adenocarcinoma or adenosquamous carcinoma (Ad/Adsq). The median tumor size at diagnosis was 6.0 (IQR, 4.9–6.8) cm, and 39 patients received concurrent chemotherapy. The median value of the ellipticity, which is one of the inclusion criteria of this study, was 2.07 (IRQ, 1.63–2.75). All patients underwent CT‐based IGBT; 51 patients underwent four sessions of CT‐based IGBT, two underwent three sessions of CT‐based IGBT, and one underwent five sessions of CT‐based IGBT. Of the 54 patients, 37 (68.5%) underwent three or more sessions of CT‐based transvaginal HBT.
TABLE 1.
Patients and treatment characteristics
| Characteristics | n = 54 |
|---|---|
| Age, years | 65 (53–74) |
| Follow‐up period, months | 32 (19–44) |
| FIGO stage (2008) | |
| IB | 2 (3.7%) |
| II | 24 (44.4%) |
| III | 21 (38.9%) |
| IVA | 7 (13.0%) |
| Histological subtypes | |
| Squamous cell carcinoma | 45 (83.3%) |
| Adenocarcinoma/adenosquamous carcinoma | 9 (16.7%) |
| Pelvic LN metastasis | |
| No | 23 (42.6%) |
| Yes | 31 (57.4%) |
| Para‐aortic LN metastasis | |
| No | 42 (77.8%) |
| Yes | 12 (22.2%) |
| Tumor size, cm | 6.0 (4.9–6.8) |
| Weekly cisplatin administrations | |
| No | 15 (27.8%) |
| Yes | 39 (72.2%) |
| EBRT, EQD2 | |
| Whole pelvic irradiation (median), Gy | 30 (20–40) |
| Central shielding irradiation (median), Gy | 20 (20–30) |
| Number of HBT sessions | |
| 1 session | 9 (16.7%) |
| 2 sessions | 8 (14.8%) |
| 3 sessions | 15 (27.8%) |
| 4 sessions | 21 (38.9%) |
| 5 sessions | 1 (1.8%) |
Note: Data except EBRT are median (IQR, interquartile range) or n (%), EBRT is median (range).
Abbreviations: EBRT, External beam radiotherapy; EQD2, equivalent dose of 2 Gy per fraction; FIGO, International Federation of Gynecology and Obstetrics; HBT, hybrid brachytherapy; LN, lymph node; NOS, not otherwise specified.
3.2. Clinical outcomes
No patient complained of symptoms such as persistent bleeding or abdominal pain after the treatment. Figure 2 shows the clinical outcomes of the entire cohort of the present study. The 3‐year LC, DFS, and OS rates for all 54 patients were 86.6% (95% confidence interval [CI] 76.5%–96.7%], 60.3% (95% CI [47.0%–73.6%]), and 90.7% (95% CI [81.3%–100.0%]), respectively. Of the 54 patients analyzed, 52 were still alive, and two patients had died at the last follow‐up. Local tumor recurrence was observed in six of the 54 patients by the last follow‐up. Of the six patients with local tumor recurrence, three had Sq, and the other three had Ad/Adsq. All six patients who had local recurrence had pelvic lymph node or distant metastatic recurrence at or shortly after the time of diagnosis of local recurrence. Of the six patients with local tumor recurrence, five received chemotherapy, and the remaining patient received supportive care due to old age and weakened general condition. Four had died of cancer, and two were still alive with the disease at the last follow‐up date.
FIGURE 2.
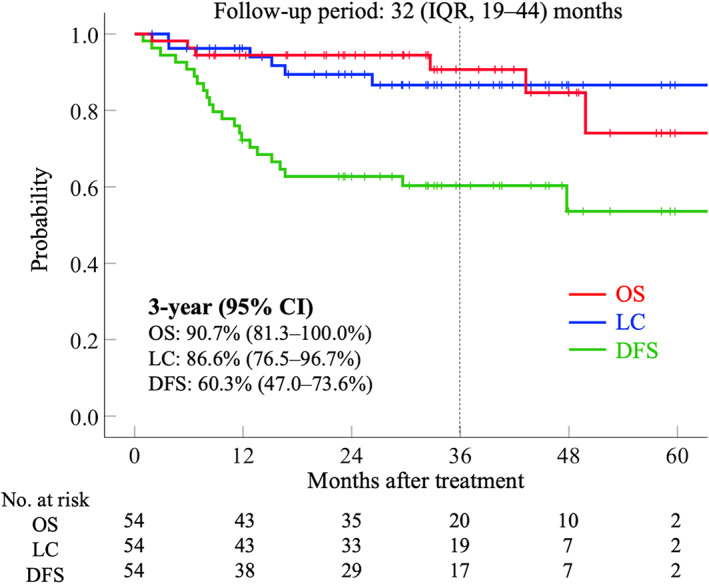
Clinical outcomes calculated by the Kaplan–Meier method. Red line indicates overall survival, blue line indicates local control, and green line indicates disease‐free survival. CI, confidence interval; DFS, disease‐free survival; IQR, interquartile range; LC, local control; No., number; OS, overall survival
Table 2 shows the results of the univariate analyses based on clinical factors. The 3‐year LC rate was 87.7% in patients with FIGO III–IVA and 90.4% in patients with tumor size >6.0 cm. The 3‐year LC rate for Sq was 92.6%, while the 3‐year LC rate for Ad/Adsq was 66.7%, with Ad/Adsq showing a slightly worse LC rate. No statistically significant differences were observed in LC classified by FIGO stage (IB–II vs. III–IVA), histological subtypes, tumor size (≤6.0 cm vs. >6.0 cm), and concurrent use of chemotherapy. No statistically significant differences were observed in DFS or OS according to these clinical factors.
TABLE 2.
Univariate analyses by clinical factors
| Factor | No. of patients | LC | DFS | OS | |||
|---|---|---|---|---|---|---|---|
| 3‐year (%) | p value | 3‐year (%) | p value | 3‐year (%) | p value | ||
| FIGO stage (2008) | .955 | .474 | .356 | ||||
| IB–II | 26 | 86.0 | 65.4 | 89.3 | |||
| III–IVA | 28 | 87.7 | 54.5 | 92.9 | |||
| Histological subtypes | .053 | .551 | .889 | ||||
| Sq | 45 | 92.6 | 58.9 | 93.3 | |||
| Ad/Adsq | 9 | 66.7 | 66.7 | 83.3 | |||
| Tumor size | .600 | .241 | .938 | ||||
| ≤6.0 cm | 31 | 83.6 | 54.4 | 90.7 | |||
| >6.0 cm | 23 | 90.4 | 69.1 | 91.3 | |||
| Concurrent chemotherapy | .257 | .568 | .401 | ||||
| No | 15 | 79.0 | 66.7 | 93.3 | |||
| Yes | 39 | 90.2 | 58.2 | 89.9 | |||
Abbreviations: Ad, adenocarcinoma; Adsq, adenosquamous carcinoma; DFS, disease‐free survival; FIGO, International Federation of Gynecology and Obstetrics; LC, local control; No., number; OS, overall survival; Sq, squamous cell carcinoma.
There were no adverse events related to the CT‐based transvaginal HBT procedure. Regarding late rectal toxicities, five patients developed grade ≥1 rectal toxicity. Two patients developed grade 1 toxicity, and three patients developed grade 2 toxicity requiring blood transfusion, argon plasma coagulation, or short‐term systemic corticosteroid use. The patient who required corticosteroids had been using 0.5 mg of dexamethasone tablet for about 4 weeks. None of the patients developed grade ≥3 rectal toxicity. Regarding late bladder toxicities, six patients developed grade ≥1 bladder toxicity, four patients developed grade 1 toxicity, and one patient developed grade 2 toxicity requiring blood transfusion. One patient developed grade 4 toxicity and a vesicovaginal fistula. The patient who developed grade 4 toxicity originally presented with a bladder‐invading tumor. Thus, the incidence of late adverse events of grade ≥3 in the rectum and bladder was 0% and 1.8%, respectively.
3.3. Relationship between dose‐volume histogram parameters and clinical outcomes
Table 3 shows the relationship between dose‐volume histogram (DVH) parameters and clinical outcomes. Regarding the cumulative dose of HR‐CTVD90, the mean ± SD values of brachytherapy in patients without and with local recurrence were 41.5 ± 5.1 Gy EQD2 and 38.9 ± 7.1 Gy EQD2, respectively, with no statistically significant differences observed (p = .336). The mean dose of WP was significantly higher in patients with local recurrence (p = .022); three of the six patients had received WP up to the equivalent of 40 Gy because the shrinkage of the tumor was slow in these three cases. The combined dose of WP and brachytherapy was not significantly different between the groups (p = .572) (Table 3A).
TABLE 3.
Relationship between dose and local control/late adverse events
| (A) Dose of HR‐CTV and local control/recurrence | |||
|---|---|---|---|
| Factor | HR‐CTVD90 (EQD2): mean ± SD | ||
| BT (Gy) | WP (Gy) | BT + WP (Gy) | |
| Local controlled (n = 48) | 41.5 ± 5.1 | 30.4 ± 3.5 | 71.9 ± 4.8 |
| Local recurrence (n = 6) | 38.9 ± 7.1 | 34.2 ± 4.7 | 73.1 ± 4.6 |
| p value | .277 | .022 | .572 |
| (B) Dose of rectum D2cc and late rectal toxicity | |||
|---|---|---|---|
| Factor | Rectum D2cc (EQD2): mean ± SD | ||
| BT (Gy) | WP (Gy) | BT + WP (Gy) | |
| Rectum grade 0 (n = 49) | 34.0 ± 8.0 | 30.4 ± 3.8 | 64.4 ± 9.2 |
| Rectum grade ≥1 (n = 5) | 37.4 ± 7.2 | 31.5 ± 4.8 | 68.9 ± 6.7 |
| p value | .374 | .558 | .301 |
| (C) Dose of bladder D2cc and late bladder toxicity | |||
|---|---|---|---|
| Factor | Bladder D2cc (EQD2): mean ± SD | ||
| BT (Gy) | WP (Gy) | BT + WP (Gy) | |
| Rectum grade 0 (n = 48) | 43.1 ± 9.8 | 30.6 ± 4.0 | 73.7 ± 11.3 |
| Rectum grade ≥1 (n = 6) | 47.9 ± 6.8 | 29.5 ± 0.3 | 77.4 ± 6.9 |
| p value | .258 | .512 | .445 |
Abbreviations: BT, brachytherapy; EQD2, equivalent dose of 2 Gy per fraction; HR‐CTV, high‐risk clinical target volume; WP, whole pelvic irradiation.
Regarding the cumulative dose of rectum D2cc, the average ± SD values of brachytherapy in patients without and with toxicity were 34.0 ± 8.0 Gy EQD2 and 37.4 ± 7.2 Gy EQD2, respectively. There were no statistically significant differences in the mean dose of rectum D2cc between patients with and without toxicity in any classification, brachytherapy, WP, or brachytherapy plus WP (Table 3B). Regarding the cumulative dose of bladder D2cc, the average ± SD values of brachytherapy in patients without and with toxicity were 43.1 ± 9.8 Gy EQD2 and 47.9 ± 6.8 Gy EQD2, respectively. There were no statistically significant differences in the mean dose of bladder D2cc between patients with and without toxicity in any classification, brachytherapy, WP, or brachytherapy plus WP (Table 3C).
3.4. Timing and significance of HBT
For all 54 patients analyzed in the present study, a total of 215 brachytherapy sessions were performed, including conventional brachytherapy, which did not use interstitial catheters, and transvaginal HBT. Table 4 shows the ratio of conventional brachytherapy to transvaginal HBT in each session. Five sessions of brachytherapy were performed on one patient. Except for the fifth session of brachytherapy, transvaginal HBT was performed most frequently in the second session.
TABLE 4.
Ratio of conventional brachytherapy and hybrid brachytherapy in each session
| First session | Second session | Third session | Fourth session | Fifth session | |
|---|---|---|---|---|---|
| Conventional, n (%) | 17 (31.5) | 12 (22.2) | 16 (29.6) | 15 (28.8) | 0 (0.0) |
| Hybrid, n (%) | 37 (68.5) | 42 (77.8) | 38 (70.4) | 37 (71.2) | 1 (100.0) |
Abbreviation: n, number.
Next, we compared the dose parameters in HR‐CTV and OARs between conventional brachytherapy and transvaginal HBT (Table 5). The mean dose (range) of HR‐CTVD90 in conventional brachytherapy and transvaginal HBT were 6.81 (3.89–8.47) Gy and 7.32 (3.41–9.89) Gy, respectively. The mean dose of HR‐CTVD90 in transvaginal HBT was significantly higher than that in conventional brachytherapy (p < .001). The mean dose (range) of rectum D2cc in conventional brachytherapy and transvaginal HBT were 5.09 (2.18–7.18) Gy and 5.21 (2.39–7.93) Gy, respectively. The mean dose (range) of bladder D2cc in conventional brachytherapy and transvaginal HBT were 5.99 (4.28–8.24) Gy and 5.99 (3.49–10.35) Gy, respectively. There were no statistically significant differences between conventional brachytherapy and transvaginal HBT with respect to rectum D2cc and bladder D2cc.
TABLE 5.
Comparisons of dose parameters in HR‐CTV and OARs between conventional and hybrid brachytherapy
| Conventional (Gy), 60 sessions | Hybrid (Gy), 155 sessions | p value | |
|---|---|---|---|
| HR‐CTVD90, range (mean) | 3.89–8.47 (6.81) | 3.41–9.89 (7.32) | <.001 |
| Rectum D2cc, range (mean) | 2.18–7.18 (5.09) | 2.39–7.93 (5.21) | .416 |
| Bladder D2cc, range (mean) | 4.28–8.24 (5.99) | 3.49–10.35 (5.99) | 1.000 |
Abbreviations: HR‐CTV, high‐risk clinical target volume; OARs, organs at risk.
We further assessed the difference in the incidence of local recurrence among patients who did and did not receive HBT at the first session. Of the 37 patients who received HBT at the first session, four had local recurrence. In contrast, of the 17 who did not receive HBT at the first session, two had local recurrence. There was no statistically significant difference in the frequency of recurrence between the two groups (p = .917). Regarding the total dose to the HR‐CTVD90, the group that received HBT for the first time had a significantly higher total dose (~4.8% [72.0 ± 4.8 Gy EQD2 vs. 68.7 ± 4.2 Gy EQD2; p = .020]) compared to the group that did not receive HBT at the first session. However, there were no statistically significant differences in the mean dose of rectum D2cc or bladder D2cc between the two groups (Table 6).
TABLE 6.
Comparisons of incidence of local recurrence, dose parameters in HR‐CTV and OARs between patients who missed the HBT at the first session and those who received HBT at the first session
| No. of patients with local recurrence | HR‐CTVD90 (EQD2): mean ± SD BT + WP | Rectum D2cc (EQD2): mean ± SD BT + WP | Bladder D2cc (EQD2): mean ± SD BT + WP | |
|---|---|---|---|---|
| Patients who received the HBT at the first session (n = 37) | 4 | 72.0 ± 4.8 | 64.9 ± 8.9 | 74.1 ± 11.0 |
| Patients who missed the HBT at the first session (n = 17) | 2 | 68.7 ± 4.2 | 61.4 ± 9.4 | 72.0 ± 8.2 |
| p value | .917 | .020 | .201 | .494 |
Abbreviations: BT, brachytherapy; HBT, hybrid brachytherapy; high‐risk clinical target volume; No., number; OARs, organs at risk; WP, whole pelvic irradiation.
4. DISCUSSION
First, we found favorable clinical outcomes, especially in LC rate in the present study. Previous studies consisting of non‐IGBT suggested that tumor size and stage were poor prognostic factors for cervical cancer. 30 , 31 Parker et al. reported that the 2‐years LC rate in tumors sized >6 cm was 51.9%. 31 In contrast, the overall 3‐year LC rate was 86.6%. Notably, the 3‐year LC rate in the present study was 90.4% in patients with tumor size >6.0 cm. This improvement in LC is attributed to the effect of IGBTs, and the LC values are comparable to that reported in EMBRACE‐I, which was performed with MRI‐based IGBTs. 11 The incidence of late adverse events of grade ≥3 in the rectum and bladder was 0% and 1.8%, respectively. Therefore, CT‐based hybrid brachytherapy for cervical cancer may be a reasonable treatment strategy for this disease.
In the present study, we did not find any relationship between DVH parameters and clinical outcomes. This was probably due to the small number of local recurrences and late adverse events. Because a combination of WP irradiation and CS was used in the present study, interpreting the values of DVH parameters requires close attention. Among studies that used a similar method, including WP irradiation and CS, Okazaki et al. reported that HR‐CTVD90 and HR‐CTVD98 doses for brachytherapy sessions were significantly associated with LC. 20 In their analysis, it was demonstrated that the aiming dose of HR‐CTVD90 for brachytherapy sessions to achieve favorable LC is 36.0 EQD2. The mean ± SD values of HR‐CTVD90 for brachytherapy sessions was 41.5 ± 5.1 Gy EQD2 in patients with local controlled cancer in the present study. Thus, our results support the findings of Okazaki et al. 20 However, the mean ± SD values of HR‐CTVD90 for brachytherapy sessions were 38.9 ± 7.1 Gy EQD2 in the patients with local recurrence, and the dose to the HR‐CTVD90 itself did not appear to be insufficient even in the recurrent cases. It may be noteworthy that three of the six cases with local recurrence were Ad/Adsq subtypes. In fact, many recent reports indicate that patients with Ad/Adsq of cervical cancer show lower LC rates compared to those with the Sq subtype. 32 , 33 , 34 Further studies are needed to determine whether there is indeed a difference in the dose required for local control of Ad/Adsq and Sq, and if so, the optimal dose to cure Ad/Adsq.
One of the clinical utilities of CT‐based transvaginal HBT is that it does not require a saddle block or general anesthesia. No patient complained of symptoms such as persistent bleeding or abdominal pain after the treatment. Moreover, there were no adverse events related to CT‐based transvaginal HBT with intravenous sedation in the present study. Another convenience of CT‐based transvaginal HBT is the easy switching from conventional brachytherapy to HBT during the procedure. As mentioned above, CT‐based transvaginal HBT does not require general anesthesia or a special applicator. In fact, transvaginal HBT was performed most frequently during the second session in the present study. A similar tendency was also reported in a recent dosimetric survey for CT‐based IGBT, including HBT, in a Japanese prospective multi‐institutional study. 35 This is probably the result of switching to HBT in the second session when an insufficient dose of HR‐CTV or an excessive dose of OARs was observed in the first session using conventional brachytherapy. However, as shown in Table 6, patients who received the HBT at the first session obtained a higher dose of ~4.8% to HR‐CTVD90 than those who did not receive the HBT at the first session. There was no statistically significant difference in the frequency of recurrence between the two groups; this could be due to differences in background factors, particularly tumor size, between the two groups. Although LC advantage was not shown in this study if HBT was applied from the first session, we can obtain better HR‐CTVD90 without increasing doses to OARs; HBT should be performed from the first session for patients who need it.
Importantly, we used multiple analgesics with transvenous sedation in the present study. In addition, we always need to fully explain other treatment options, including anesthesia methods, to patients in any environment. With careful considerations of these points, CT‐based transvaginal HBT without performing general anesthesia or saddle block would be a good treatment option.
The present study also demonstrated the dosimetric advantage of HBT. Transvaginal HBT increased the dose of HR‐CTVD90 by about 7.5% (6.81 Gy for conventional brachytherapy and 7.32 Gy for HBT) without significantly increasing the dose of OARs. Liu et al. previously reported that CT‐based HBT is effective in cases with tumor diameters greater than 5 cm, using DVH analysis. 36 In our study, the median tumor size was 6.0 cm, which is consistent with the results of Liu et al. 36 Considering the favorable clinical results of our study, patients with a larger tumor size are good candidates for HBT. HBT can be applied not only to increase the dose to the HR‐CTV, but also to reduce the dose to the OARs. This perspective may be important, especially for irregularly shaped tumors. As a measure of this irregularity, we used the ellipticity in the present study, which was 2.07 (IRQ, 1.63–2.75). There have been no reports on the validation of appropriate ellipticity as an indication criterion for HBT. Although we have used certain criteria for HBT indications, further studies on these indications are necessary.
To date, many studies have introduced a combination of intracavitary and interstitial brachytherapy. 23 , 25 , 37 , 38 Interstitial ring applicators (Elekta Brachytherapy, Netherlands) and Venezia applicator (Elekta Brachytherapy, Netherlands) are already commercially available, and their clinical use is gradually increasing, especially in Europe and the United States. There is no doubt that these applicators are a logical way to combine intracavitary and interstitial brachytherapy. However, their high cost limits their use in hospitals in regions such as Asia and Africa, where the number of patients with cervical cancer is increasing. 16 The method we have reported here could be implemented with existing applicators and thus can be expected to be implemented in more facilities.
This study has several limitations which include the small cohort of patients and short follow‐up periods. The lack of significant differences in DFS and OS in stage and tumor size is probably due to these limitations. In addition, this was a single‐institution retrospective analysis. A multicenter prospective study is currently ongoing to determine the clinical significance of CT‐based transvaginal HBT, and this study would validate our findings. Since the present study employed CT‐based IGBT and EBRT, including CS, sufficient care should be taken when comparing DVH parameters with studies employing MRI‐based IGBT and EBRT without CS. Furthermore, studies that actually compare cost‐effectiveness in our strategy with other MRI‐based IGBTs are needed.
In conclusion, we reported the clinical advantages of CT‐based transvaginal HBT. With the fact that none of the patients complained of symptoms after the procedure, favorable LC rate, mild toxicity, and possible cost‐effectiveness of the series of procedures, our strategy may be a good option for cervical cancer.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
AUTHOR CONTRIBUTIONS
Noriyuki Okonogi: Conceptualization (lead); data curation (equal); formal analysis (lead); investigation (equal); methodology (equal); visualization (lead); writing – original draft (lead). Kazutoshi Murata: Conceptualization (equal); formal analysis (equal); methodology (equal); writing – original draft (supporting). Toshiaki Matsui: Data curation (equal); investigation (equal). Yuma Iwai: Data curation (equal). Yasumasa Mori: Data curation (equal). Takashi Kaneko: Data curation (equal). Masaru Wakatsuki: Conceptualization (equal); formal analysis (equal); methodology (equal); writing – review and editing (equal). Hiroshi Tsuji: Writing – review and editing (equal).
ETHICS STATEMENT
The study was approved by the Ethical Review Board committee of our institution (QST 20‐043, approved on March 11, 2021). Because of the retrospective nature, the need for written informed consent was waived for this study. Instead, patients who refused to be included in this study were given an opt‐out policy, which was uploaded on the webpage of our institution. The present study complied with the Declaration of Helsinki.
Supporting information
APPENDIX DATA S1 The specific examples for pain relief and sedation for brachytherapy.
APPENDIX DATA S2 One of the criteria for implement of HBT in our hospital; The ellipticity
ACKNOWLEDGMENTS
We would like to thank the radiology technicians and nurses at the QST Hospital for their support and dedication to this study.
Okonogi N, Murata K, Matsui T, et al. Clinical advantage and outcomes of computed tomography‐based transvaginal hybrid brachytherapy performed only by sedation without general or saddle block anesthesia. Cancer Reports. 2022;5(11):e1607. doi: 10.1002/cnr2.1607
DATA AVAILABILITY STATEMENT
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
REFERENCES
- 1. International Agency for Research on Cancer . Accessed September 22, 2021. https://gco.iarc.fr/today/home.
- 2. Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta‐analysis. Lancet. 2001;358(9284):781‐786. doi: 10.1016/S0140-6736(01)05965-7 [DOI] [PubMed] [Google Scholar]
- 3. Green JA, Kirwan JM, Tierney JF, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005;20(3):CD002225. doi: 10.1002/14651858.CD002225.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas GM. Improved treatment for cervical cancer‐‐concurrent chemotherapy and radiotherapy. N Engl J Med. 1999;340(15):1198‐1200. doi: 10.1056/NEJM199904153401509 [DOI] [PubMed] [Google Scholar]
- 5. Nag S, Erickson B, Thomadsen B, Orton C, Demanes JD, Petereit D. The American Brachytherapy Society recommendations for high‐dose‐rate brachytherapy for carcinoma of the cervix. Int J Radiation Biol Phys. 2000;48(1):201‐211. [DOI] [PubMed] [Google Scholar]
- 6. Haie‐Meder C, Pötter R, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC‐ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74(3):235‐245. doi: 10.1016/j.radonc.2004.12.015 [DOI] [PubMed] [Google Scholar]
- 7. Pötter R, Haie‐Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image‐based treatment planning in cervix cancer brachytherapy‐3D dose volume parameters and aspects of 3D image‐based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78(1):67‐77. doi: 10.1016/j.radonc.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 8. Dimopoulos JC, Pötter R, Lang S, et al. Dose‐effect relationship for local control of cervical cancer by magnetic resonance image‐guided brachytherapy. Radiother Oncol. 2009;93(2):311‐315. [DOI] [PubMed] [Google Scholar]
- 9. Sturdza A, Pötter R, Fokdal LU, et al. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120(3):428‐443. [DOI] [PubMed] [Google Scholar]
- 10. Ohno T, Noda SE, Okonogi N, et al. In‐room computed tomography‐based brachytherapy for uterine cervical cancer: results of a 5‐year retrospective study. J Radiat Res. 2017;58(4):543‐551. PMID: 27986859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pötter R, Tanderup K, Schmid MP, et al. MRI‐guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE‐I): a multicentre prospective cohort study. Lancet Oncol. 2021;22(4):538‐547. doi: 10.1016/S1470-2045(20)30753-1 PMID: 33794207. [DOI] [PubMed] [Google Scholar]
- 12. Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Potter R. Computed tomography versus magnetic resonance imaging‐based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68(2):491‐498. doi: 10.1016/j.ijrobp.2006.12.021 [DOI] [PubMed] [Google Scholar]
- 13. Toita T, Ohno T, Ikushima H, et al. National survey of intracavitary brachytherapy for intact uterine cervical cancer in Japan. J Radiat Res. 2018;59(4):469‐476. doi: 10.1093/jrr/rry035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grover S, Harkenrider MM, Cho LP, et al. Image guided cervical brachytherapy: 2014 survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2016;94(3):598‐604. doi: 10.1016/j.ijrobp.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 15. Taggar AS, Phan T, Traptow L, Banerjee R, Doll CM. Cervical cancer brachy‐therapy in Canada: a focus on interstitial brachytherapy utilization. Brachytherapy. 2017;16(1):161‐166. doi: 10.1016/j.brachy.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 16. Hull R, Mbele M, Makhafola T, et al. Cervical cancer in low and middle‐income countries. Oncol Lett. 2020;20(3):2058‐2074. doi: 10.3892/ol.2020.11754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohno T, Wakatsuki M, Toita T, et al. Recommendations for high‐risk clinical target volume definition with computed tomography for three‐dimensional image‐guided brachytherapy in cervical cancer patients. J Radiat Res. 2017;58(3):341‐350. doi: 10.1093/jrr/rrw109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hegazy N, Pötter R, Kirisits C, et al. High‐risk clinical target volume delineation in CT‐guided cervical cancer brachytherapy: impact of information from FIGO stage with or without systematic inclusion of 3D documentation of clinical gynecological examination. Acta Oncol. 2013;52(7):1345‐1352. doi: 10.3109/0284186X.2013.813068 [DOI] [PubMed] [Google Scholar]
- 19. Mahantshetty U, Poetter R, Beriwal S, et al. IBS‐GEC ESTRO‐ABS recommendations for CT based contouring in image guided adaptive brachytherapy for cervical cancer. Radiother Oncol. 2021;160:273‐284. doi: 10.1016/j.radonc.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okazaki S, Murata K, Noda SE, et al. Dose‐volume parameters and local tumor control in cervical cancer treated with central‐shielding external‐beam radiotherapy and CT‐based image‐guided brachytherapy. J Radiat Res. 2019;60(4):490‐500. doi: 10.1093/jrr/rrz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murakami N, Kasamatsu T, Wakita A, et al. CT based three dimensional dose‐volume evaluations for high‐dose rate intracavitary brachytherapy for cervical cancer. BMC Cancer. 2014;14:447. doi: 10.1186/1471-2407-14-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. NCCN Guidelines . Cervical Cancer. Accessed September 22, 2021. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
- 23. Wakatsuki M, Ohno T, Yoshida D, et al. Intracavitary combined with CT‐guided interstitial brachytherapy for locally advanced uterine cervical cancer: introduction of the technique and a case presentation. J Radiat Res. 2011;52(1):54‐58. doi: 10.1269/jrr.10091 [DOI] [PubMed] [Google Scholar]
- 24. Oike T, Ohno T, Noda SE, et al. Can combined intracavitary/interstitial approach be an alternative to interstitial brachytherapy with the Martinez Universal Perineal Interstitial Template (MUPIT) in computed tomography‐guided adaptive brachytherapy for bulky and/or irregularly shaped gynecological tumors? Radiat Oncol. 2014;9:222. doi: 10.1186/s13014-014-0222-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murakami N, Kobayashi K, Shima S, et al. A hybrid technique of intracavitary and interstitial brachytherapy for locally advanced cervical cancer: initial outcomes of a single‐institute experience. BMC Cancer. 2019;19(1):221. doi: 10.1186/s12885-019-5430-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan PW, Koh VY, Tang JI. Outpatient combined intracavitary and interstitial cervical brachytherapy: barriers and solutions to implementation of a successful programme—a single institutional experience. J Contemp Brachytherapy. 2015;7(3):259‐263. doi: 10.5114/jcb.2015.52625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Japan Society of Obstetrics and Gynaecology, the Japanese Society of Pathology, the Japan Radiological Society . General Rules for Clinical and Pathological Study of Uterine Cervical Cancer in Japan. Kanehara and Co. Ltd; 1999. [Google Scholar]
- 28. Kobayashi D, Okonogi N, Wakatsuki M, et al. Impact of CT‐based brachytherapy in elderly patients with cervical cancer. Brachytherapy. 2019;18(6):771‐779. doi: 10.1016/j.brachy.2019.08.002 PMID: 31506225. [DOI] [PubMed] [Google Scholar]
- 29. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341‐1346. doi: 10.1016/0360-3016(95)00060-C [DOI] [PubMed] [Google Scholar]
- 30. Toita T, Kato S, Ishikura S, et al. Radiotherapy quality assurance of the Japanese Gynecologic Oncology Group study (JGOG1066): a cooperative phase II study of concurrent chemoradiotherapy for uterine cervical cancer. Int J Clin Oncol. 2011;16(4):379‐386. doi: 10.1007/s10147-011-0196-4 [DOI] [PubMed] [Google Scholar]
- 31. Parker K, Gallop‐Evans E, Hanna L, Adams M. Five years' experience treating locally advanced cervical cancer with concurrent chemoradiotherapy and high‐dose‐rate brachytherapy: results from a single institution. Int J Radiat Oncol Biol Phys. 2009;74(1):140‐146. doi: 10.1016/j.ijrobp.2008.06.1920 [DOI] [PubMed] [Google Scholar]
- 32. Minkoff D, Gill BS, Kang J, Beriwal S. Cervical cancer outcome prediction to high‐dose rate brachytherapy using quantitative magnetic resonance imaging analysis of tumor response to external beam radiotherapy. Radiother Oncol. 2015;115(1):78‐83. doi: 10.1016/j.radonc.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 33. Niibe Y, Kenjo M, Onishi H, et al. High‐dose‐rate intracavitary brachytherapy combined with external beam radiotherapy for stage IIIb adenocarcinoma of the uterine cervix in Japan: a multi‐institutional study of Japanese Society of Therapeutic Radiology and Oncology 2006‐2007 (study of JASTRO 2006‐2007). Jpn J Clin Oncol. 2010;40(8):795‐799. doi: 10.1093/jjco/hyq053 [DOI] [PubMed] [Google Scholar]
- 34. Huang YT, Wang CC, Tsai CS, et al. Long‐term outcome and prognostic factors for adenocarcinoma/adenosquamous carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80(2):429‐436. doi: 10.1016/j.ijrobp.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 35. Otani Y, Ohno T, Ando K, et al. Dosimetric feasibility of computed tomography‐based image‐guided brachytherapy in locally advanced cervical cancer: a Japanese prospective multi‐institutional study. J Radiat Res. 2021;62(3):502‐510. doi: 10.1093/jrr/rraa138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu ZS, Guo J, Lin X, et al. Clinical feasibility of interstitial brachytherapy using a "hybrid" applicator combining uterine tandem and interstitial metal needles based on CT for locally advanced cervical cancer. Brachytherapy. 2016;15(2):562‐569. doi: 10.1016/j.brachy.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 37. Serban M, Kirisits C, de Leeuw A, et al. Ring versus ovoids and intracavitary versus intracavitary‐interstitial applicators in cervical cancer brachytherapy: results from the EMBRACE I study. Int J Radiat Oncol Biol Phys. 2020;106(5):1052‐1062. doi: 10.1016/j.ijrobp.2019.12.019 [DOI] [PubMed] [Google Scholar]
- 38. Walter F, Maihöfer C, Schüttrumpf L, et al. Combined intracavitary and interstitial brachytherapy of cervical cancer using the novel hybrid applicator Venezia: clinical feasibility and initial results. Brachytherapy. 2018;17(5):775‐781. doi: 10.1016/j.brachy.2018.05.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX DATA S1 The specific examples for pain relief and sedation for brachytherapy.
APPENDIX DATA S2 One of the criteria for implement of HBT in our hospital; The ellipticity
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.


