Abstract
Biologicals have become an integral part of cancer treatment both as therapeutic agents and as supportive care agents. It is important to know that biologics are large, complex molecular entities requiring extensive immunogenicity testing and pharmacovigilance strategies to ensure no immune response is evoked in the body. Oncology's pharmacological market is dominated by biologics; however, their high development and manufacturing costs are burdensome to health care systems. Biologics being the most expensive prescription drugs on the market limit the accessibility for necessary treatment in the case of many patients. As biologics patents expire, the development of biosimilars is underway in an effort to lower costs and enable patients to access new cancer therapies. Regulatory guidelines for biosimilars have now been established and are constantly being revised to address any issues, facilitating their robust development. Moreover, many scientific societies offer guidance to help stakeholders better understand current regulations and biosimilar's safety. Despite the potential cost benefits, lack of knowledge about biosimilars, and the possibility of immunogenicity have created an uncertain environment for healthcare professionals and patients. In this review, we provide an overview of relevant legislation and regulations, pharmacoeconomics, and stakeholder perceptions regarding biosimilars. The article also describes biosimilars in development, as well as the ones currently available on the market.
Keywords: bevacizumab, biosimilars, epoetins, filgrastim, new developments, oncology, pegfilgrastim, rituximab
1. INTRODUCTION
Biologics are generally large complex molecules produced through biotechnology in a living system such as microorganism, plant cell, or animal cell. These products are used to diagnose, prevent, treat, and cure medical conditions. 1 Biosimilars are biological drugs that are designed to be highly similar to the existing marketed biologics. 2 The high level of similarity to the originator biologic is defined in terms of physicochemical characteristics, efficacy, and safety as outlined by the respective regulatory authorities. 3 , 4 A generic drug is a medication that has the same active ingredients and provides the same clinical benefits as that of a brand name drug. It is created to have an identical dosage form, safety, strength, route of administration, quality, performance characteristics, and intended use. 1 However, biosimilars are not a generic version of biologics, as it is not possible to develop an identical biochemical entity. This is mainly due to the inherent complexity of the proteins and their associated manufacturing processes. 5 Inherent variation is common within each lot and between lots during the manufacturing of biologics as well as biosimilars. Both Biosimilars and generics are approved through different abbreviated pathways that do not require extensive clinical studies. 6 So far, 29 biosimilars for various indications have been approved in the United States 7 whereas 64 biosimilars were approved in Europe. 8 The European Medical Agency (EMA) was the first to approve a biosimilar in 2006 and to provide guidance for biosimilar development and approval. 9 However, the pathway for marketing biosimilars in the United States has had several barriers. 10 Even though the patent protection of several originator biologics was close to the expiration date, the market competition that was seen with chemical drugs through generics did not occur with biosimilars. 11 Generics have been able to be marketed in the United States since 1984 due to the established abbreviated pathway through the Hatch‐Waxmann Act. 12 However, with biologics the FDA lacked a clear regulatory pathway for the approval of biosimilars until 2010. This was one of the main reasons for the slow adoption of biosimilars in the US when compared to Europe. 13 Moreover, the marketing launch of biosimilars in the US is delayed by patent infringement lawsuits, exclusionary contracts, and anticompetitive tactics of brand name manufacturers. 14
2. THE NEED FOR BIOSIMILARS IN CANCER
Cancer is among the leading causes of death worldwide. Globally, cancer accounts for about one in every six deaths, which is more than HIV, tuberculosis, and malaria combined. 15 In 2020, there were about 19.3 million estimated new cases and 10 million cancer‐related deaths worldwide. Among these deaths, one‐quarter of the cases occur in low‐ and medium‐Human Development Index countries, which lack resources and medical systems to address the disease burden. 16 By 2040, the global cancer burden is expected to increase to an estimated 27.5 million cases and 16.3 million deaths based on the aging and growth of the population. 17 The National Cancer Institute estimates the direct medical costs related to cancer treatment in the United States were $183 billion in 2015 and are expected to increase to $246 billion by 2030, a 34% raise. 18 However, owing to the advances in personalized treatments and inflation, this increase is likely to be an underestimation. 19 , 20 With the advent of biosimilars, market competition is on the rise which can help in increasing the accessibility and decreasing the cost burden to cancer patients.
2.1. Stake holders' perceptions on biosimilars/barriers to implementation
There are numerous obstacles to the integration of biosimilars into oncology treatment. One major barrier is the patient and prescriber perception of biosimilars. Survey responses collected from 1201 US physicians across specialties by the biosimilars Forum through SERMO (global social media/network organization for physicians) indicated knowledge gaps among physicians. Lack of awareness about biologics, biosimilars, the approval process for biosimilars, safety & immunogenicity, interchangeability, and substitution of biosimilars was observed. 21 Another survey involving 500 US‐based hematologists and oncologists also indicated critical education gaps. Almost 49% of the respondents were not familiar with the concept of extrapolation and 81% of respondents were hesitant to prescribe biosimilars until an average sales price (ASP) was established. However, 77% of respondents were receptive to receiving communications about biosimilars from professional organizations like ASCO. 22 Moreover, there is a growing concern that regulatory guidelines of generics may be applied to biologics, which has led several states to amend older laws to address the complex molecular characteristics of biologic products and biosimilars. 23
Given the novelty of biosimilars and their reduced emphasis on clinical testing, there is a great need for education among prescribers and patients. 24 American Society of Clinical Oncology (ASCO) provides information and guidance to the oncology community on the use of biosimilars, their safety & efficacy, interchangeability, substitution, regulatory considerations, and prescriber & patient education. CancerLinQ, an integrated real‐time data resource also provides valuable information on the use of biosimilars and their effectiveness. 23 The FDA also offers educational webinars and presentations to help clinicians better understand current regulations and biosimilar's safety. 25 A few other scientific societies including National Comprehensive Cancer Network (NCCN), 26 European Society for Medical Oncology (ESMO) 27 also provide guidance on biosimilars. Additionally, European Public Assessment Reports (EPARs) published by the EMA help clinicians in evaluating the appropriate use of biosimilars in Europe. 28 In the case of patients, the primary education source is the treating physician. 29 Several patient advocacy groups including CancerCare, 30 Susan G. Komen, 31 Global Colon Cancer Association, 32 and so forth, also provide a broad range of educational materials tailored for patient use to facilitate their understanding and acceptance of biosimilars.
2.1.1. Overview of biosimilar legislation and regulation
Historically in the United States, biologics were regulated by the Public Health Hygienic Laboratory, a precursor of NIH, which was then transferred to the Bureau of Biologics at the FDA in 1972. 33 After a decade later, the Bureau of Drugs and Bureau of Biologics were merged into a single entity to form National Center for Drugs and Biologics (NCDB). 34 However, in 1987 the Center for Drugs and Biologics was divided back into the Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER). 35 The jurisdictional responsibilities of the two centers were assigned through the Intercenter Agreement issued by the FDA in 1991. 36 Traditional biologics including vaccines, blood, blood products, allergenic extracts, certain devices, and test kits are regulated by CBER. The center also regulates gene & cellular therapy products and tissue transplants from human and non‐human sources. 37 CDER on the other hand regulates prescription, over‐the‐counter & generic drugs, 38 and most therapeutic biologics including monoclonal antibodies, cytokines, growth factors, enzymes, immunomodulators, and so forth. 39
Most of the biological products were approved under the Public Health Service Act (PHSA) while some of them are licensed as drugs under the Federal Food, Drug, and Cosmetic Act (FFDCA). 40 In 2010, Congress established an abbreviated licensure pathway for biological products that demonstrated to be biosimilar or interchangeable to a previously licensed biological product. This new regulatory authority for FDA was accomplished through the Biologics Price Competition and Innovation Act (BPCIA) of 2009, which was enacted as Title VII of the Patient Protection and Affordable Care Act (ACA). 11 As a part of the implementation of BPCIA, three draft guidances on the development of biosimilars were released by the FDA in 2012 41 and the final versions were released in 2015. 42 The BPCIA has also set periods of regulatory exclusivity for brand name biologics and biosimilars as well as laid procedures for resolving patent disputes. 11 Biologics are offered 12 years of exclusivity during which the FDA cannot approve any biosimilar or interchangeable product referencing the brand name biologic. However, a BLA (Biologics License Application) of a biosimilar or interchangeable product can be submitted after 4 years from the date on which the reference product was first licensed. 43 A BLA can be submitted directly by an applicant or through a legal entity involved in the manufacturing, who is responsible for product compliance according to the established standards. Form 356 h is to be submitted along with the BLA, which includes information about the applicant, product, manufacturing process, preclinical & clinical studies, and draft labeling of the product. 44 Also, effective from March 23, 2020, biological products under BPCIA, which were previously approved as drugs under 505 of the FFDCA are transitioned to biological licenses under section 351 of the PHSA. 45
Over the years, the FDA has released additional guidance on a variety of other areas related to biosimilars and all of these documents can be accessed through the FDA website. 42 The agency's database “Purple Book” contains information about all FDA‐licensed biologics regulated by the CDER including their biosimilars and interchangeable products. In‐depth information about the date on which the biological product was licensed, if the biological product has proven to be a biosimilar or interchangeable to an already licensed biological drug, and the expiration dates of applicable exclusivities of the reference biologics can be obtained. Also, the database provides information about licensed products regulated by the CBER. 46
On the other hand, guidelines for the regulation of medicines in the European Union (EU) are very well established. A dedicated pathway for the development and approval of biosimilars was introduced in 2004. 47 General guidelines on biosimilars were issued by EMA to introduce the concept and to provide biosimilar manufacturers with a user guide containing relevant scientific information. 48 In the EU, biologics are offered 8 years of exclusivity during which a biosimilar referencing the brand name biologic cannot be marketed. 4 Biotechnology products including biosimilars are approved by the EMA through a marketing authorization application (MAA) following a centralized procedure. 47 This procedure authorizes the manufacturers to market their products throughout the European Economic Area (EEA) with a single marketing authorization application. EEA includes all EU member states, and three countries of the European Free Trade Association (EFTA)‐Iceland, Liechtenstein, and Norway. 49 The MAA's for biosimilars are evaluated by EMA's scientific committees including Committee for Medical Products for Human Use (CHMP), Pharmacovigilance Risk Assessment Committee (PRAC) as well as EU experts & specialists on biological medicines (Biologics Working Party) and biosimilars (Biosimilar Working Party). 47 The scientific opinion obtained after the EMA's evaluation is recommended to the European Commission, which ultimately decides if an EU‐wide marketing authorization must be granted. Once approved, the decision of the commission is published in the Community Register of medicinal products for human use. In addition, the EMA also publishes a European public assessment report (EPAR) for each application that has been granted/refused a marketing authorization. 49 The complete list of centrally authorized biosimilars approved to date can be accessed from the EMA's website. 8
2.1.2. Biosimilars in oncology
Currently, there are only a few biosimilars approved for cancer treatment and supportive care. Biosimilars are available for monoclonal antibodies (mAb) including Rituximab, Trastuzumab & Bevacizumab, and supportive agents including filgrastim, pegfilgrastim, epoetin α & epoetin ζ. 50
Filgrastim and pegfilgrastim
The first‐ever biosimilar product to be marketed in the United States was Zarxio® (filgrastim‐sndz) and was approved by FDA Y.T.T March 2015. 51 Later in 2018, Nivestym® (filgrastim‐aafi) was approved, both of these biosimilars can be used for the same indications as the reference drug, Neupogen® (Filgrastim). 52 Filgrastim is a recombinant granulocyte colony‐stimulating factor (G‐CSF) that regulates neutrophil production from bone marrow. Filgrastim is used to reduce febrile neutropenia in patients with non‐myeloid malignancies receiving myelosuppressive anticancer agents or myeloablative chemotherapy followed by bone marrow transplantation. It is also used in patients with acute myeloid leukemia receiving induction or consolidation chemotherapy for reducing the time of neutrophil recovery and the duration of fever. 53 In Europe, nine biosimilars of Filgrastim are approved by the EMA including Accofil®, 54 Biograstim® 55 Filgrastim Hexal®, 56 Filgrastim Ratiopharm®, 57 Grastofil®, 58 Nivestim®, 59 Ratiograstim®, 60 Tevegrastim® 61 and Zarzio®. 62 However, the marketing of Biograstim® and Filgrastim Ratiopharm® was withdrawn by the EMA at the request of their respective marketing authorization holders. 55 , 57 Two other G‐CSFs that are commonly used for treating chemotherapy‐induced neutropenia (CIN) include pegfilgrastim and lenograstim. Filgrastim and lenograstim are short‐acting G‐CSFs that are injected daily during chemotherapy while pegfilgrastim is a long‐acting G‐CSF, administered once per chemotherapy cycle. 63 Pegfilgrastim has an additional polyethylene glycol unit, which causes an increase in the size of the molecule, thereby prolonging the half‐life of the drug. 64 Once bound to G‐CSF receptors, filgrastim, pegfilgrastim, lenograstim, and all biosimilars act to increase the proliferation and maturation of neutrophils thereby decreasing the risk for neutropenia as seen in Figure 1. The JAK–STAT signaling pathway is activated and results in the translocation of JAK3 to the nucleus. Once in the nucleus, JAK3 binds to DNA and activates transcription linked to neutrophil proliferation 65 as seen in Figure 2. Eight approved biosimilars for pegfilgrastim are available in Europe including Pelgraz®, 66 Udenyca®, 67 Fulphila®, 68 Pelmeg® 69 Ziextenzo® 70 Grasustek® 71 Cegfila®, 72 and Nyvepria®. 73 Whereas in United States, for Neulasta® (pegfilgrastim) four biosimilars are approved: Fulphila® (pegfilgrastim‐jmdb) 74 Udenyca® (pegfilgrastim‐cbqv), 75 Ziextenzo® (pegfilgrastim‐bmez) 76 and Nyvepria® (pegfilgrastim‐apgf). 77 To, date no biosimilars for lenograstim are available.
FIGURE 1.

A visual representation of the effects filgrastim, pegfilgrastim, and their biosimilars have upon binding to granulocyte colony stimulating factor receptors.
FIGURE 2.
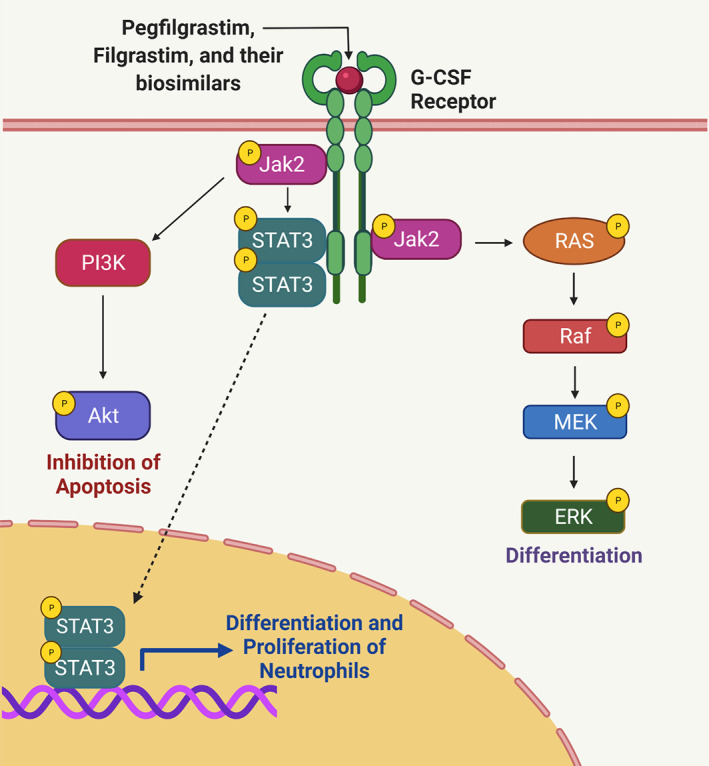
Pegfilgrastim, filgrastim and their biosimilars' mechanism of action. Once bound to the granulocyte colony stimulating factor receptor, the JAK–STAT signaling pathway is activated, leading to neutrophil survival, proliferation, and differentiation.
Epoetins
Epoetins are used for treating chemotherapy‐induced anemia (CIA), reducing the need for blood transfusions thereby improving the quality of life. These are similar to erythropoietin hormone, secreted by the kidneys that stimulate red blood cell production (erythropoiesis) in the bone marrow and are also referred to as erythropoiesis‐stimulating agents (ESAs). 78 , 79 Epoteins and their biosimilars bind to the erythropoietin receptor and activate the JAK–STAT signaling pathway. JAK3 translocates to the nucleus and binds to DNA activating transcription linked to red blood cell proliferation 80 (Figure 3). Five epoetin biosimilars are approved in Europe including three epoetin α (EPO‐ α) biosimilars: Abseamed®, 81 Binocrit®, 82 Epoetin‐ α hexal®, 83 and two epoetin ζ (EPO‐ζ) biosimilars Retacrit® 84 & Silapo®. 85 In the United States only one ESA agent, Retacrit® (epoetin alfa‐epbx) 86 has been approved for the reference drug, Epogen®/Procrit® (EPO‐α). Both EPO‐α and EPO‐ζ have been approved for treating chemotherapy‐induced and symptomatic anemia in patients with solid tumors, malignant lymphoma, or multiple myeloma. 87 , 88 Figure 4 represents the timeline of FDA approval of supportive cancer care biosimilars. Tables 1 and 2 lists the biosimilar drugs approved for supportive cancer care by the FDA and EMA respectively.
FIGURE 3.

Epogen/Procrit and its biosimilars' mechanism of action. Once bound to the erythropoietin receptor, the JAK–STAT signaling pathway is activated, leading to red blood cell survival, proliferation, and differentiation.
FIGURE 4.

Timeline: A timeline about FDA approval of biosimilars of oncogenic biologics.
TABLE 1.
FDA approved supportive care biosimilars in oncology
| Reference Biologic (Active substance) | Reference Biologic manufacturer(s) | Biosimilar (active substance) | Biosimilar manufacturer | Approval date |
|---|---|---|---|---|
| Neupogen® (Filgrastim) |
Amgen Inc |
Zarxio® (filgrastim‐sndz) | Sandoz Inc | 2015 |
| Nivestym® (filgrastim‐aafi) | Hospira Inc | 2018 | ||
| Neulasta® (pegfilgrastim) | Amgen Inc | Fulphila® (pegfilgrastim‐jmdb) | Mylan GmbH | 2018 |
| Udenyca® (pegfilgrastim‐cbqv) | Coherus BioSciences, Inc | 2018 | ||
| Ziextenzo® (pegfilgrastim‐bmez) | Sandoz Inc | 2019 | ||
| Nyvepria® (pegfilgrastim‐apgf) | Hospira Inc | 2020 | ||
| Epogen/Procrit (epoetin α) | Amgen Inc/Janssen Biotech Inc | Retacrit® (epoetin alfa‐epbx) | Hospira Inc | 2018 |
TABLE 2.
EMA approved supportive care biosimilars in oncology
| Reference Biologic (Active substance) | Reference Biologic manufacturer(s) | Biosimilar (active substance) | Biosimilar manufacturer | Approval date |
|---|---|---|---|---|
| Neupogen® (Filgrastim) |
Amgen Inc |
Accofil® | Accord Healthcare S.L.U. | 2014 |
| Filgrastim Hexal® | Hexal AG | 2009 | ||
| Grastofil® | Accord Healthcare, SLU | 2013 | ||
| Nivestim® | Pfizer Europe MA EEIG | 2010 | ||
| Ratiograstim® | Ratiopharm GmbH | 2008 | ||
| Tevegrastim® | Teva GmbH | 2008 | ||
| Zarzio® | Sandoz GmbH | 2009 | ||
| Neulasta® (pegfilgrastim) | Amgen Inc | Pelgraz® | Accord Healthcare S.L.U. | 2018 |
| Fulphila® | Mylan S.A.S | 2018 | ||
| Udenyca® | ERA Consulting GmbH | 2018 | ||
| Pelmeg® | Mundipharma Corporation (Ireland) Limited | 2018 | ||
|
Ziextenzo® |
Sandoz GmbH | 2018 | ||
| Grasustek® | Juta Pharma GmbH | 2019 | ||
| Cegfila® | Mundipharma Corporation (Ireland) Limited | 2019 | ||
| Nyvepria® | Pfizer Europe MA EEIG | 2020 | ||
|
Eprex®/Erypo® (epoetin α) |
Janssen‐Cilag GmbH | Abseamed® | Medice Arzneimittel Pütter GmbH Co. KG | 2007 |
| Binocrit® | Sandoz GmbH | 2007 | ||
| Epoetin‐ α hexal® | Hexal AG | 2007 | ||
| Retacrit® | Pfizer Europe MA EEIG | 2007 | ||
| Silapo® | Stada Arzneimittel AG | 2007 |
2.1.3. Monoclonal antibodies
Bevacizumab
Bevacizumab is a recombinant humanized monoclonal antibody, that targets vascular endothelial growth factor (VEGF‐A) and inhibits the formation of new blood vessels (angiogenesis) and the growth of new tumors (Figure 5). 89 Avastin® (Bevacizumab) is used for various indications including metastatic colorectal cancer (mCRC), non‐squamous non–small cell lung cancer (NSCLC), glioblastoma, metastatic renal cell carcinoma (mRCC), and persistent, recurrent, or metastatic carcinoma of the cervix either as a single agent or in combination with chemotherapy/biologic response modifier. 90 The patent of Avastin® in the United States expired in 2019 whereas in Europe the patent will expire in 2022. 91 Currently, two biosimilars of Avastin® (bevacizumab) are available in the United States including Mvasi® (bevacizumab‐awwb) 92 and Zirabev® (bevacizumab‐bvzr). 93 Both of these biosimilars 94 , 95 along with few others including Aybintio®, 96 Equidacent®, 97 Oyavas®, 98 and Alymsys® 99 are approved in Europe. However, these biosimilars could face a delay in reaching the market until relevant patents and regulatory exclusivities expire. 100
FIGURE 5.
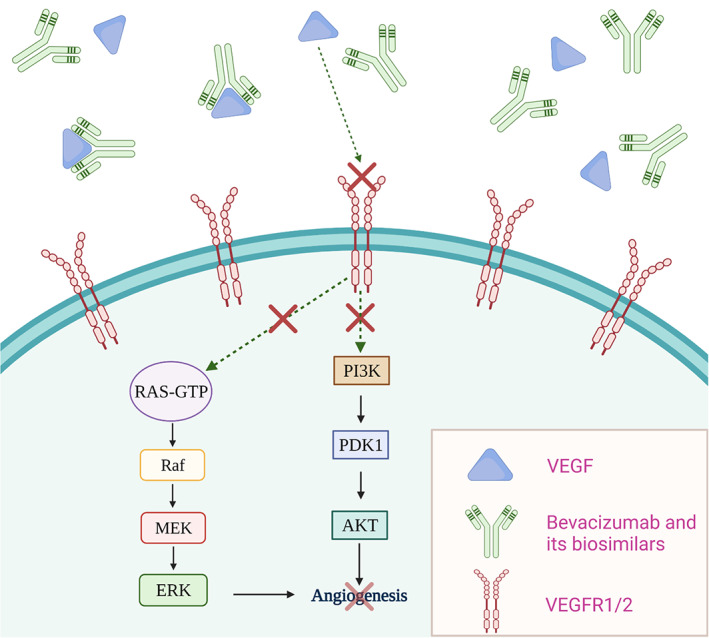
Bevacizumab and its biosimilars' mechanism of action. Upon entry, bevacizumab and its biosimilars bind to VEGF, thereby inhibiting the interaction between VEGF and VEGFR1/2. This in turn blocks angiogenesis signaling pathways.
Rituximab
Rituxan® (Rituximab) is a genetically engineered chimeric human monoclonal antibody that targets the CD20 antigen, found on the surface of B lymphocytes. By binding to the CD20 antigen, rituximab and its biosimilars increase IL‐10 and B‐cell lymphoma‐2 (Bcl‐2) thereby inducing cellular apoptosis 101 (Figure 6). Rituxan® is indicated for treating patients with Non‐Hodgkin's Lymphoma (NHL) and Chronic Lymphocytic Leukemia (CLL). 102 It is indicated for use as a single agent for relapsed/refractory, low‐grade/ follicular CD20‐positive, B‐cell NHL, and in patients with non‐progressing, low‐grade, CD20‐positive, B‐cell NHL after first‐line cyclophosphamide, vincristine, and prednisolone (CVP) chemotherapy. It is also used in combination with chemotherapy in patients with previously untreated follicular & diffuse large B‐cell, CD20 positive B‐cell NHL, and as single‐agent maintenance therapy in patients who achieved a complete/partial response to Rituxan®. In patients with CLL, Rituxan® is used in combination with chemotherapeutics: fludarabine and cyclophosphamide (FC). 103 The patent for Rituxan® (rituximab) in the United States expired in 2016 104 which led to the development of biosimilars Truxima® (rituximab‐abbs), 105 Ruxience® (rituximab‐pvvr), 106 and recently Riabni® (rituximab‐arrx). 107 Even in Europe, the patent for MabThera® (rituximab) expired in 2013, 104 and six biosimilars for rituximab were approved by EMA including Blitzima®, 108 Truxima®, 109 Ruxience®, 110 Riximyo®, 111 Rixathon® 112 and Ritemvia®. 113
FIGURE 6.

Cell death mechanisms involved in rituximab and its biosimilars' b‐cell binding. This antibody and its biosimilars induce an apoptotic control pathway through binding with CD20 receptors. Cellular binding also facilitates two other cell death pathways including phagocytosis and lysis by natural killer cells.
Trastuzumab
Herceptin® (Trastuzumab) is a humanized monoclonal antibody that selectively binds to the extracellular domain of the human epidermal growth factor receptor 2 protein (HER2). 114 The effects of trastuzumab and its biosimilars binding to HER2 receptors are presented in Figure 7. Herceptin® is indicated for patients with (a) metastatic HER2‐overexpressing breast cancer either as a single agent or in combination with paclitaxel (b) metastatic HER2‐overexpressing gastric cancer in combination with cisplatin and capecitabine/5‐fluorouracil and (c) HER2‐overexpressing breast cancer as an adjuvant treatment in combination with chemotherapeutics or as a single agent following multi‐modality anthracycline‐based treatment. 115 The patent of Herceptin® (Trastuzumab) in the United States expired in 2019 whereas in Europe the patent expired in 2014. 116 Ogivri® (trastuzumab‐dkst) was the first biosimilar to Herceptin® (Trastuzumab) to be approved by the FDA. Later in United States four more biosimilars including Herzuma® (trastuzumab‐pkrb), 117 Trazimera® (trastuzumab‐qyyp), 118 Ontruzant® (trastuzumab‐dttb), 119 Kanjinti® (trastuzumab‐anns) 120 were approved. Ontruzant®, 121 Herzuma®, 122 Trazimera® 123 Kanjinti®, 124 and Ogivri® 125 are also approved in Europe. Recently, EMA approved another biosimilar for trastuzumab namely Zercepac®. 126 Tables 3 and 4 lists the biosimilar drugs approved for monoclonal antibodies‐ Bevacizumab, Rituximab & Trastuzumab by FDA and EMA respectively.
FIGURE 7.

Trastuzumab and its biosimilars' mechanisms of action. (1). Upon binding to HER2 receptors, endocytosis and further degradation of the receptor occurs. This antibody and its biosimilars also (2) prevent receptor cleavage and (3) dimerization with other HER receptors. (4) Cell mediated cytotoxicity is also induced through dual binding with HER2 receptors and immune effector cells.
TABLE 3.
FDA approved mAB biosimilars in oncology
| Reference Biologic (Active substance) | Reference Biologic manufacturer(s) | Biosimilar (active substance) | Biosimilar manufacturer | Approval date |
|---|---|---|---|---|
| Avastin® (Bevacizumab) | Genentech, Inc. | Mvasi® (bevacizumab‐awwb) | Amgen Inc | 2017 |
| Zirabev® (bevacizumab‐bvzr) | Pfizer Inc. | 2019 | ||
| Rituxan® (Rituximab) | Genentech, Inc. | Truxima® (rituximab‐abbs) | Celltrion, Inc | 2018 |
| Ruxience® (rituximab‐pvvr) | Pfizer Ireland Pharmaceuticals | 2019 | ||
| Riabni® (rituximab‐arrx) | Amgen, Inc | 2020 | ||
| Herceptin® (Trastuzumab) | Genentech, Inc | Ontruzant® (trastuzumab‐dttb) | Samsung Bioepis Co., Ltd | 2019 |
| Trazimera® (trastuzumab‐qyyp) | Pfizer Inc | 2018 | ||
| Herzuma® (trastuzumab‐pkrb) | Celltrion, Inc | 2018 | ||
| Kanjinti® (trastuzumab‐anns) | Amgen Inc | 2019 | ||
| Ogivri® (trastuzumab‐dkst) | Mylan GmbH | 2017 |
TABLE 4.
EMA approved mAB biosimilars in oncology
| Reference Biologic (Active substance) | Reference Biologic manufacturer(s) | Biosimilar (active substance) | Biosimilar manufacturer | Approval date |
|---|---|---|---|---|
| Avastin® (bevacizumab) | Roche Registration GmbH | Mvasi® | Amgen Technology (Ireland) UC | 2018 |
| Zirabev® | Pfizer Europe MA EEIG | 2019 | ||
| Aybintio® | Samsung Bioepis NL B.V. | 2020 | ||
| Equidacent® | Centus Biotherapeutics Europe Limited | 2020 | ||
| Oyavas® | STADA Arzneimittel AG | 2021 | ||
| Alymsys® | Mabxience Research SL | 2021 | ||
| MabThera® (rituximab) | Roche Registration GmbH | Blitzima® | Celltrion Healthcare Hungary Kft. | 2017 |
| Truxima® | Celltrion Healthcare Hungary Kft. | 2017 | ||
| Ruxience® | Pfizer Europe MA EEIG | 2020 | ||
| Riximyo® | Sandoz GmbH | 2017 | ||
| Rixathon® | Sandoz GmbH | 2017 | ||
| Ritemvia® | Celltrion Healthcare Hungary Kft. | 2017 | ||
| Herceptin® (Trastuzumab) | Roche Registration GmbH | Ontruzant® | Samsung Bioepis NL B.V. | 2017 |
| Trazimera® | Pfizer Europe MA EEIG | 2018 | ||
| Herzuma® | Celltrion Healthcare Hungary Kft. | 2018 | ||
| Kanjinti® | Amgen Europe B.V. | 2018 | ||
| Ogivri® | Mylan S.A.S | 2018 | ||
| Zercepac® | Accord Healthcare S.L.U. | 2020 |
2.1.4. Pharmacoeconomics of biosimilars in oncology
A comparative cost analysis was performed using the average wholesale price (AWP) per unit of biologics and biosimilars. The prices of these drugs in the United States were accessed from Red Book® through the database, Micromedex. The current interpretation is based on the drug prices in June 2021. A comparison of the AWP costs between biosimilars and their reference products is provided in Table 5 and the relative biosimilar prices are shown in Figure 8.
TABLE 5.
Biologic and Biosimilar Average wholesale price per unit (AWP) in US$, June 2021
| Reference Biologic (Active substance) | Biosimilar | Biosimilar, average wholesale price per unit | Reference product, average wholesale price per unit |
|---|---|---|---|
| Avastin® (Bevacizumab) | Mvasi® | 25 mg/ml vial: $203.22 | 25 mg/ml vial $239.08 |
| Zirabev® | 25 mg/ml vial: $184.02 | ||
| Rituxan® (Rituximab) | Truxima® | 10 mg/ml vial: $101.46 | 10 mg/ml vial: $112.74 |
| Ruxience® | 10 mg/ml vial: $86.01 | ||
| Riabni® | 10 mg/ml vial: $86.01 | ||
| Herceptin® (Trastuzumab) | Ontruzant® | 150 mg PDS: $1589.59 | 150 mg PDS: $1870.10 |
| Trazimera® | 150 mg PDS: $1453.32 | ||
| Herzuma® | 150 mg PDS: $1683.00 | ||
| Kanjinti® | 150 mg PDS: $1584.54 | ||
| Ogivri® | 150 mg PDS: $1589.59 | ||
| Neupogen® (Filgrastim) | Zarxio® | 480 mcg/0.8 ml vial: $658.47 | 480 mcg/0.8 ml vial: $797.15 |
| Nivestym® | 480 mcg/0.8 ml vial: $525.60 | ||
| Neulasta® (pegfilgrastim) | Fulphila® | 6 mg/0.6 ml vial: $8350 | 6 mg/0.6 ml vial: $12462.11 |
| Udenyca® | 6 mg/0.6 ml vial: $8350 | ||
| Ziextenzo® | 6 mg/0.6 ml vial: $7851.06 | ||
| Nyvepria® | 6 mg/0.6 ml vial: $7850 | ||
| Epogen/Procrit (epoetin α) | Retacrit® | 2000 u/ml vial: $26.47 | 2000 u/ml vial: $39.79 |
FIGURE 8.

Relative prices of oncology biosimilars and their biosimilars
For bevacizumab, the percentage savings with biosimilars ranged from 15% to 23%. Among bevacizumab biosimilars, the savings were significantly higher with Zirabev® when compared to the originator product, Avastin. In the case of rituximab biosimilars, the percentage savings ranged from 10% to 23.7%. Biosimilars including Ruxience® and Riabni® offered greater savings and are currently the most cost‐effective alternatives to Rituxan®. With Herceptin biosimilars, the savings ranged from 15% to 22.2% with the highest cost savings observed with Trazimera®. In addition to these biosimilars, the biosimilars for supportive cancer care agents also provide significant savings when compared to their reference products. The savings range from 17.3% to 34% with filgrastim biosimilars, 33 to 37% with pegfilgrastim biosimilars, and 33.5% with Epogen biosimilar.
3. BIOSIMILARS IN CLINICAL TRIALS
Several biosimilar candidates are being globally developed, investigated, and are currently in various stages of clinical development & regulatory approval. This section summarizes the studies involving prospective biosimilar candidates in oncology based on their clinical research progress. Figures 9, 10, and 11 provide the clinical trial information of the respective candidates.
-
Candidates awaiting regulatory agency response : Few manufacturers have submitted Biologics License Application (BLA) for their proposed bevacizumab biosimilar candidates (BAT1706, MYL‐1402O, SB8, and FKB238) that are currently under FDA review. Bio‐thera (BAT1706) seeks approval for its candidate use in treating non‐small cell lung cancer, recurrent glioblastoma, metastatic renal cell carcinoma, persistent/recurrent/metastatic cervical cancer, and mCRC in combination with chemotherapy. 127 The application is based on the positive results from preclinical, pharmacokinetic, and international, multicenter phase 3 comparative safety & efficacy studies. 128 Mylan and Biocon Limited (MYL‐1402O) seeks approval for the same indications as to the originator, bevacizumab. The application is supported by phase 3 findings from comparative safety, efficacy, and immunogenicity evaluating global study 129 This company also submitted its marketing authorization application to the EMA. 130 , 131
Luye Pharma has announced that the marketing authorization application for its biosimilar candidate (LY01008) has been accepted by the China Center for Drug Evaluation of the National Medical Products Administration (NMPA). The application was based on data generated from two comparative studies: a pharmacokinetics study in healthy volunteers and a safety & efficacy study in metastatic/recurrent non‐squamous non‐small cell lung cancer patients. Both of these studies compared the biosimilar candidate to its reference drug, Avastin® and have met their pre‐defined primary endpoints. 132 Other biosimilar candidates SB8 (Samsung Bioepsis) and FKB238 (Centus Biotherapeutics) have already received marketed authorization from the EU and are available as Aybintio® 96 and Equidacent® 97 respectively. The BLA of these candidates was accepted by the FDA in 2019 and the manufacturers are looking forward to launching their products in the US. 131 , 133 HD201 from Prestige biopharma, a biosimilar candidate to trastuzumab has completed phase 3 studies in HER2 positive breast cancer patients. The marketing application of biosimilar HD201 has also been accepted by the EMA. 134 Innovent Biologics, Inc and Eli Lilly have announced that NMPA has accepted their New Drug Application (NDA) for IBI301, a biosimilar candidate to rituximab. The application is based on the clinical data obtained from studies including Phase 3‐ safety & efficacy of IBI301 along with chemotherapy and phase 1 pharmacokinetics & safety assessment in patients with untreated CD20‐positive diffuse large B‐cell lymphoma (DLBCL). 135
Candidates that have completed Phase 3 studies : HLX01 by Shanghai Henlius Biotech, completed a dose‐escalation, 136 safety, pharmacokinetic & pharmacodynamic studies 137 in patients with CD20 positive B‐cell lymphomas in comparison to Mabthera®. A phase 3 study evaluating safety and efficacy of the candidate in combination with chemotherapy was also investigated. 138 Currently, a follow‐up study of HLX01 to determine the overall survival (OS) and progression‐free survival (PFS) is underway. 139 RTXM83 (mAbxience S.A) is another biosimilar candidate, to Mabthera® that has completed phase 3 trials in patients with DLBCL. 140 Biosimilar HLX04 from Henlius Biotech has met the primary end point in phase 3 safety, efficacy, and immunogenicity studies in comparison to its reference drug, bevacizumab. This study investigated the candidate in combination with oxaliplatin and fluoropyrimidine‐based chemotherapy (XELOX or mFOLFOX6) as first‐line treatment in patients with mCRC. 141 Also, HLX04 combined with Henlius's anti‐PD‐1 monoclonal antibody (mAb) HLX10 is being investigated for the treatment of different types of cancer including advanced solid tumors (phase 1) 142 and advanced hepatocellular carcinoma (HCC) (phase 2). 143 Another biosimilar candidate of bevacizumab, BI 695502 by Boehringer Ingelheim has completed two comparative phase 3 studies. The candidate was evaluated in combination with chemotherapeutics as a first line treatment and as maintenance therapy in patients with lung cancer & mCRC respectively. 144 , 145 Two biosimilar candidates of R‐Pharm, RPH001 (reference drug: bevacizumab) and RPH‐002 (reference drug cetuximab) have also completed phase 3 studies and the results are yet to be updated by the company. 146
Candidates currently undergoing Phase 3 : TQB2303, is a rituximab biosimilar candidate of Chia Tai Tianqing Pharmaceutical Group Co., Ltd and it is currently being investigated in two clinical studies (Phase 1/2 & Phase 3) involving CD20‐positive DLBCL patients. 147 , 148 SCT400 by Sinocelltech Ltd has completed a phase 1 safety and efficacy study in patients with B‐cell Non‐Hodgkin's lymphoma. 149 The company has initiated phase 2 150 and phase 3 151 studies comparing the candidate to the reference drug rituximab. Few other biosimilar candidates to rituximab that are undergoing phase 3 trials to demonstrate their equivalent efficacy include DRL_RI, 152 by Dr. Reddy's Laboratories Limited, SIBP‐02 153 by Shanghai Institute of Biological Products, and GB241 by Genor Pharma. 154 GB221, another candidate of Genor Biopharma completed a safety & pharmacokinetic study following single‐dose administration in patients with metastatic breast cancer. This study is conducted in comparison to the reference drug, Herceptin®. 155 Phase I/II studies of single/multiple doses of GB221 156 and a phase 3 study to evaluate progression‐free survival (PFS) using combinational therapy are underway. 157 HLX02, (Henlius Biotech) is being investigated in three comparative safety & immunogenicity clinical studies including two phase 1 studies in healthy volunteers 158 , 159 and a phase 3 study in breast cancer patients. 160 This trastuzumab candidate has already been approved in the EU (Zercepac®). 126 The company has also entered into a collaboration with Accord Healthcare, US granting an exclusive right to develop and commercialize in the US and Canada. 161 Another biosimilar candidate of trastuzumab undergoing comparative phase 3 studies in breast cancer patients include SIBP‐01, which is developed by the Shanghai Institute of Biological Products. 162 Quite a few prospective bevacizumab biosimilars have completed phase one studies and are currently undergoing comparative safety & efficacy (phase 3) studies. These include CT‐P16 by Celltrion, 163 HD204 by Prestige biopharma 164 CBT124 by Cipla BioTech 165 and MIL 60 by Beijing Mabworks Biotech. 166 All of these biosimilar candidates are evaluated for their use in treating patients with non‐small cell lung cancer. Two biosimilar candidates for the originator Xgeva® are also undergoing phase 3 studies. These include LY01011 by Luye Pharma 164 and QL 1206 by Qilu Pharmaceuticals. 167
Candidates in Phase 1 and 2 : Trastuzumab biosimilar candidates including CMAB809 by Taizhou Mabtech Pharmaceutical Co., Ltd, 168 ALT02 by Alteogen, Inc, 132 and DMB‐3111 by Meiji Seika Pharma & Dong‐A‐Socio Holdings have completed phase 1 similarity studies. 169 Another trastuzumab biosimilar candidate of Alteogen Inc, ALT‐L2 170 has completed global phase 2 testing and is getting ready for phase 3 studies. 171 BP‐102 by Jiangsu‐Hengrui‐Medicine is currently in phase 2 clinical trials. The proposed bevacizumab biosimilar candidate is evaluated in chemotherapy‐naive patients with non‐squamous NSCLC. 172 Another biosimilar candidate of bevacizumab, GB222 (Genor Pharma) is currently undergoing various phase 1 trials for the treatment of glioblastoma multiforme, non‐squamous non‐small cell lung cancer, and mCRC. 173 Biosimilar candidate for originator drug denosumab, TK006 is developed by Jiangsu T‐Mab Biopharma Co., Ltd. TK006 is currently being evaluated (phase 1) for its safety upon single, multiple doses in patients with breast cancer‐related bone metastases. 174 , 175 Few biosimilar candidates for supportive care agent Neulasta® have completed phase 1 studies. These include INTP5 by Intas Pharmaceuticals, Ltd. 176 PF‐06881894 by Pfizer, 177 and B12019 by Cinfa Biotech. 178 QL0605 by Qilu Pharmaceutical Co., Ltd is another biosimilar for Neulasta®, which is currently undergoing phase 1 studies. 179
Candidates in preclinical development : Biosimilar candidates for rituximab that are in the early stages of development include BXT 2336 by Bioxpress therapeutics 180 & a plant‐based product by iBio & AzarGen Biotechnologies. 181 Few biosimilar candidates from Prestige biopharma are also in early development. These include PBP1602 (reference drug: aflibercept), PBP1701 (reference drug: ipilimumab), and PBP1801 (reference drug: pertuzumab). 182 CMAB810 (Taizhou Mabtech Pharmaceutical Co., Ltd) is another biosimilar candidate for reference drug pertuzumab which is in preclinical studies. 183 Biosimilars for originators Opdivo® and Keytruda® are in active development by NeuClone Ltd. 184 Furthermore, biosimilar candidates Xdivane, (reference drug Opdivo®) and Spherotide, (reference drug, Decapeptyl®) by Xbrane Biopharma are also in their preclinical studies. 185
FIGURE 9.

Prospective biosimilar candidates of Rituximab that are in different phases of clinical trials.
FIGURE 10.

Prospective biosimilar candidates of Bevacizumab that are in different phases of clinical trials.
FIGURE 11.
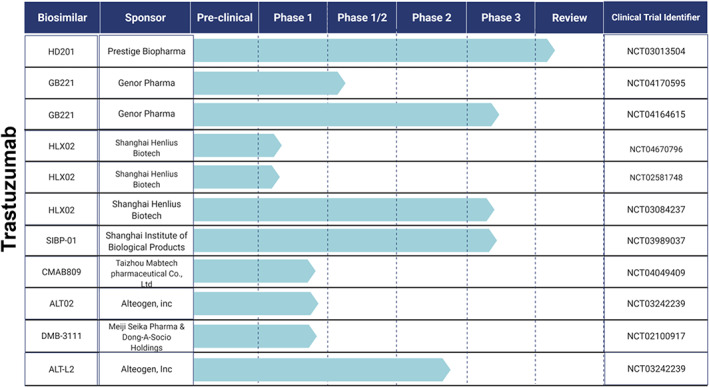
Prospective biosimilar candidates of Trastuzumab that are in different phases of clinical trials.
However, few companies have suspended clinical development of their rituximab biosimilar candidates including BI 695500 (Boehringer Ingelheim Pharmaceuticals), 186 Kikuzubam (Probiomed), 187 SAIT101 (Archigen Biotech Limited), 188 TL011 (Teva Pharmaceutical Industries), 189 JHL1101 (JHL Biotech, Inc) 190 , 191 and GP2013 (Sandoz) 192 either due to the changes in regulatory requirements or marketing decisions. 193 JHL Biotech, Inc also suspended clinical development of its two other biosimilars candidates including JHL1188 (reference drug: trastuzumab) and JHL1149 (reference drug: bevacizumab) due to legal issues. 190 Shanghai Henlius Biotech withdrew its rituximab biosimilar candidate HLX01 from phase 3 studies due to strategic reasons. The study was intended to evaluate the candidate safety and efficacy in patients with low tumor burden follicular lymphoma. 194 For the same reasons, Jiangsu‐Hengrui‐Medicine also withdrew its bevacizumab candidate BP‐102, from a phase 2 evaluation in patients with mCRC. 195
4. CONCLUSIONS AND FUTURE PERSPECTIVES
The use of biosimilars is rapidly evolving and will continue to play an important role in the future care of cancer patients. 196 Many biosimilars are expected to be available in the coming years and their use will largely depend on patient and provider acceptance, which is in turn based on an adequate understanding of the safety and efficacy of these agents in cancer treatment. 23 Therefore, education of patients and providers on various aspects of biosimilars is necessary to increase confidence in biosimilars and for their successful incorporation in oncology practice. Furthermore, rigorous regulatory frameworks and close post‐marketing monitoring of these drugs are required to ensure their safety and efficacy in a real‐world setting. 64
AUTHOR CONTRIBUTIONS
Rinda Devi Bachu: Conceptualization (equal); data curation (equal); methodology (equal); resources (equal); visualization (equal); writing – original draft (equal). Mariam Abou‐Dahech: Formal analysis (equal); methodology (equal); software (equal); validation (equal); visualization (equal). Swapnaa Balaji: Data curation (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); validation (supporting). Sai H.S. Boddu: Formal analysis (supporting); methodology (supporting); visualization (supporting); writing – review and editing (supporting). Samson Amos: Validation (supporting); writing – review and editing (supporting). Vishal Singh: Data curation (supporting); methodology (supporting); visualization (supporting). R. Jayachandra Babu: Conceptualization (supporting); data curation (supporting); project administration (supporting); supervision (supporting); visualization (equal); writing – review and editing (equal). Amit K. Tiwari: Conceptualization (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal).
FUNDING INFORMATION
The manuscript has been supported in part by Susan G. Komen Breast Cancer Foundation (CCR18548498 to Amit K. Tiwari) and Department of Defense (W81XWH210053 to Amit K. Tiwari). The views expressed in this article are those of authors and may not reflect the official policy or position of the Department of the Army, Department of Defense or the US Government or Susan G. Komen Breast Cancer Foundation.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICS STATEMENT
This review did not require any ethical clearance. However, all participants practiced highest ethical standards as mandated by National Institute of Health throughout the preparation of this manuscript.
ACKNOWLEDGMENTS
We thank Dr. Charles Ashby, St. John's University for their critical review of this work. Also, during the preparation of this manuscript, the author acknowledges the Department of Pharmacology and Experimental Therapeutics, University of Toledo for providing resources and assistance for graduate students.
Bachu RD, Abou‐Dahech M, Balaji S, et al. Oncology biosimilars: New developments and future directions. Cancer Reports. 2022;5(11):e1720. doi: 10.1002/cnr2.1720
Funding information Department of Defense, Grant/Award Number: W81XWH210053; Susan G. Komen Breast Cancer Foundation, Grant/Award Number: CCR18548498
Contributor Information
R. Jayachandra Babu, Email: ramapjb@auburn.edu.
Amit K. Tiwari, Email: amit.tiwari@utoledo.edu.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. U.S. Food & Drug Administration (FDA) . Biosimilar and Interchangeable Products. Accessed June 19, 2022 Available from: https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products#generic.
- 2. Gascón P, Tesch H, Verpoort K, et al. Clinical experience with Zarzio® in Europe: what have we learned? Support Care Cancer. 2013;21(10):2925‐2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. U.S. Food & Drug Administration . Guidance Document. Scientific Considerations in Demonstrating Biosimilarity to a Reference product. Accessed April 14, 2021. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/scientific-considerations-demonstrating-biosimilarity-reference-product.
- 4. European Medicines Agency . Biosimilar Medicines: Overview. Accessed April 14, 2021. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview.
- 5. Verrill M, Declerck P, Loibl S, Lee J, Cortes J. The rise of oncology biosimilars: from process to promise. Future Oncol. 2019;15(28):3255‐3265. [DOI] [PubMed] [Google Scholar]
- 6. Biosimilars Info Sheet . Generics and Biosimilars Available from: https://www.fda.gov/media/154912/download.
- 7. U.S. Food & Drug Administration (FDA) . Biosimilar Product Information. Accessed April 19, 2021 Available from: https://www.fda.gov/drugs/biosimilars/biosimilar-product-information.
- 8. European Medicines Agency . Medicines Type‐Biosimilars. Accessed April 26, 2021. Available from: https://www.ema.europa.eu/en/medicines/search_api_aggregation_ema_medicine_types/field_ema_med_biosimilar.
- 9. Gherghescu I, Delgado‐Charro MB. The biosimilar landscape: an overview of regulatory approvals by the EMA and FDA. Pharmaceutics. 2021;13(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nabhan C, Valley A, Feinberg BA. Barriers to oncology biosimilars uptake in the United States. Oncologist. 2018;23(11):1261‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Congressional Research Service . Biologics and Biosimilars: Background and Key Issues. Updated June 6, 2019. Available from: https://crsreports.congress.gov/product/pdf/R/R44620.
- 12. Gupta R, Kesselheim AS, Downing N, Greene J, Ross JS. Generic drug approvals since the 1984 hatch‐Waxman act. JAMA Intern Med. 2016;176(9):1391‐1393. [DOI] [PubMed] [Google Scholar]
- 13. McCamish M, Woollett G, eds. Worldwide Experience with Biosimilar Development. MAbs. Taylor & Francis; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Congressional Research Service . Biologics and Biosimilars: Background and Key Issues. Updated October 27, 2017. Available from: https://crsreports.congress.gov/product/pdf/R/R44620/11.
- 15. Roth GA, Abate D, Abate KH, et al. Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. The Lancet. 2018;392(10159):1736‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 17. American Cancer Society . Global Cancer Facts & Figures. 4th ed. American Cancer Society; 2018. [Google Scholar]
- 18. Ofman JJ, Fendrick AM, Raza A. Novel multicancer early detection technology‐potential value to employers and the workforce. Am J Manag Care. 2020;26: (10 Spec No.):SP363. [DOI] [PubMed] [Google Scholar]
- 19. Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626‐633. [DOI] [PubMed] [Google Scholar]
- 20. Memorial Sloan Kettering Cancer Center . Price and value of cancer drugs. Available from: [mskcc.org/research-areas/programs-centers/health-policy-outcomes/cost-drugs].
- 21. Cohen H, Beydoun D, Chien D, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther. 2016;33(12):2160‐2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Series C, Insights E, Care V‐B, Forums E. Community Oncologists' perception and acceptance of Biosimilars in oncology. J Clin Pathways. 2018;4(2):43‐47. [Google Scholar]
- 23. Lyman GH, Balaban E, Diaz M, et al. American Society of Clinical Oncology statement: biosimilars in oncology. J Clin Oncol. 2018;36(12):1260‐1265. [DOI] [PubMed] [Google Scholar]
- 24. Emily A LB, Edward L, Robert R, Samantha R, Kristen S, Carlos S, Mark S, Kim W. The future of the U.S Biosimilars market: development, education, and utilization. 2016. [Available from: https://www.focr.org/sites/default/files/pdf/The%20Future%20of%20U.S.%20Biosimilars_0.pdf].
- 25. U.S. Food & Drug Administration . Biosimilars. Webinars, Presentations, and Articles. [Available from: https://www.fda.gov/drugs/biosimilars/webinars-presentations-and-articles#webinars].
- 26. Camacho LH, Frost CP, Abella E, Morrow PK, Whittaker S. Biosimilars 101: considerations for US oncologists in clinical practice. Cancer Med. 2014;3(4):889‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim WS, Ogura M, Kwon H‐C, Choi D. Looking to the future and learning lessons from the recent past: changing stakeholder perceptions of biosimilars in cancer. Future Oncol. 2017;13(15s):17‐29. [DOI] [PubMed] [Google Scholar]
- 28. Barosi G, Bosi A, Abbracchio MP, et al. Key concepts and critical issues on epoetin and filgrastim biosimilars. A position paper from the Italian Society of Hematology, Italian Society of Experimental Hematology, and Italian Group for Bone Marrow Transplantation. Haematologica. 2011;96(7):937‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vandenplas Y, Simoens S, Van Wilder P, Vulto AG, Huys I. Informing patients about biosimilar medicines: the role of European patient associations. Pharmaceuticals. 2021;14(2):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Welch AR. Biosimilar Education: Perspectives from 4 Patient Advocacy Groups: Biosimilar Development; 2017. [Available from: https://www.biosimilardevelopment.com/doc/biosimilar-education-perspectives-from-patient-advocacy-groups-0001].
- 31. Welch AR. Breast Cancer Patient Organization Sets Sights on Biosimilars 2019. [Available from: https://www.biosimilardevelopment.com/doc/breast-cancer-patient-organization-sets-sights-on-biosimilars-0001].
- 32. Spiegel A. Global colon cancer association. The Promise of Biosimilars: A Patient Advocate's Perspective 2018. [Available from: https://safebiologics.org/wp-content/uploads/2018/09/GCCA-AndrewSpiegel-BiosimilarsFDA.pdf].
- 33. Gruber MF, Marshall VB. Regulation and testing of vaccines. Plotkin's Vaccines. 2018:1547‐1565. [Google Scholar]
- 34. U.S. Food & Drug Administration . A Brief History of the Center for Drug Evaluation and Research. The Merger of Drug and Biologics. [Available from: https://www.fda.gov/about-fda/fda-history-exhibits/brief-history-center-drug-evaluation-and-research].
- 35. U.S. Food & Drug Administration . A Brief History of the Center for Drug Evaluation and Research. NCDB Divided Back into Drugs and Biologics Centers. [Available from: https://www.fda.gov/about-fda/fda-history-exhibits/brief-history-center-drug-evaluation-and-research].
- 36. U.S. Food & Drug Administration . Intercenter Agreement between the Center for Drug Evaluation and Research and the Center for Biologics Evaluation and Research. [Available from: https://www.fda.gov/combination-products/jurisdictional-information/intercenter-agreement-between-center-drug-evaluation-and-research-and-center-biologics-evaluation].
- 37. U.S. Food & Drug Administration . Regulated Products. CBER Regulated Products. Accessed April 25, 2021. Available from: https://www.fda.gov/industry/regulated-products/cber-regulated-products.
- 38. U.S. Food & Drug Administration . FDA Organization. Center for Drug Evaluation and Research (CDER). Accessed April 25, 2021. Available from: https://www.fda.gov/about-fda/fda-organization/center-drug-evaluation-and-research-cder.
- 39. U.S. Food & Drug Administration . Transfer of Therapeutic Products to the Center for Drug Evaluation and Research (CDER). Accessed April 25, 2021. Available from: https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/transfer-therapeutic-products-center-drug-evaluation-and-research-cder.
- 40. U.S. Food & Drug Administration . Guidance, Compliance & Regulatory Information. "Deemed to be a License" Provision of the BPCI Act. Accessed April 25, 2021. Available from: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/deemed-be-license-provision-bpci-act.
- 41. Federal Register . A Notice by the Food and Drug Administration. Draft Guidances Relating to the Development of Biosimilar Products; Public Hearing; Request for Comments. [Available from: https://www.federalregister.gov/documents/2012/03/02/2012-5070/draft-guidances-relating-to-the-development-of-biosimilar-products-public-hearing-request-for].
- 42. U.S. Food & Drug Administration . Guidance, Compliance & Regulatory Information (Biologics). Biosimilars Guidelines. Accessed April 25, 2021. Available from: https://www.fda.gov/vaccines-blood-biologics/general-biologics-guidances/biosimilars-guidances.
- 43. Competition BP . Title Vii—Improving Access to Innovative Medical Therapies. 2010.
- 44. U.S. Food & Drug Administration . Biologics License Applications (BLA) Process (CBER). [Available from: https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/biologics-license-applications-bla-process-cber].
- 45. U.S. Food & Drug Administration . Guidance, Compliance & Regulatory Information. "Deemed to be a License" Provision of the BPCI Act. Accessed April 26, 2021. Available from: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/deemed-be-license-provision-bpci-act.
- 46. U.S. Food & Drug Administration (FDA) . Purple Book. Accessed April 22, 2021 Available from: https://purplebooksearch.fda.gov/.
- 47. European Medicines Agency . Biosimilars in the EU. Information guide for healthcare professionals. Updated February, 2019. [Available from: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf].
- 48. Wang J, Chow S‐C. On the regulatory approval pathway of biosimilar products. Pharmaceuticals. 2012;5(4):353‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. European Medicines Agency . Human Regulatory. Obtaining an EU marketing authorization, step by step. Accessed April 27, 2021. Available from: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/obtaining-eu-marketing-authorisation-step-step.
- 50. Patel KB, Arantes LH Jr, Tang WY, Fung S. The role of biosimilars in value‐based oncology care. Cancer Manag Res. 2018;10:4591‐4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Awad M, Singh P, Hilas O. Zarxio (filgrastim‐sndz): the first biosimilar approved by the FDA. Pharmacy and Therapeutics. 2017;42(1):19‐23. [PMC free article] [PubMed] [Google Scholar]
- 52. U.S. Food & Drug Administration (FDA) . Biosimilar Product Information. Nivestym (filgrastim‐aafi). Accessed January 30, 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761080s000lbl.pdf.
- 53. Ludwig H, Bokemeyer C, Aapro M, et al. Chemotherapy‐induced neutropenia/febrile neutropenia prophylaxis with biosimilar filgrastim in solid tumors versus hematological malignancies: MONITOR‐GCSF study. Future Oncol. 2019;15(8):897‐907. [DOI] [PubMed] [Google Scholar]
- 54. European Medicines Agency . Human medicine European public assessment report (EPAR):Accofil. Accessed January 31, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/accofil.
- 55. European Medicines Agency . Human medicine European public assessment report (EPAR):Biograstim. Accessed January 31, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/biograstim.
- 56. European Medicines Agency . Human medicine European public assessment report (EPAR):Filgrastim Hexal. Accessed January 31, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/filgrastim-hexal.
- 57. European Medicines Agency . Human medicine European public assessment report (EPAR):Filgrastim ratiopharm. Accessed January 31, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/filgrastim-ratiopharm.
- 58. European Medicines Agency . Human medicine European public assessment report (EPAR):Grastofil. Accessed January 31, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/grastofil.
- 59. European Medicines Agency . Human medicine European public assessment report (EPAR):Nivestim. Accessed January 31, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/nivestim.
- 60. European Medicines Agency . Human medicine European public assessment report (EPAR):Ratiograstim. Accessed January 31, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ratiograstim.
- 61. European Medicines Agency . Human medicine European public assessment report (EPAR):Tevagrastim. Accessed January 31, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/tevagrastim.
- 62. European Medicines Agency . Human medicine European public assessment report (EPAR):Zarzio. Accessed January 31, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/zarzio.
- 63. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony‐stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta‐analysis. BMC Cancer 2011;11(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Konstantinidou S, Papaspiliou A, Kokkotou E. Current and future roles of biosimilars in oncology practice. Oncol Lett. 2020;19(1):45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wright CR, Ward AC, Russell AP. Granulocyte colony‐stimulating factor and its potential application for skeletal muscle repair and regeneration. Mediators Inflamm. 2017;2017:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. European Medicines Agency . Human medicine European public assessment report (EPAR): Pelgraz. Accessed January 30, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/pelgraz.
- 67. European Medicines Agency . Human medicine European public assessment report (EPAR): Udenyca. Accessed January 30, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/udenyca.
- 68. European Medicines Agency . Human medicine European public assessment report (EPAR): Fulphila. Accessed January 30, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/fulphila-0.
- 69. European Medicines Agency . Human medicine European public assessment report (EPAR): Pelmeg. Accessed January 30, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/pelmeg.
- 70. European Medicines Agency . Human medicine European public assessment report (EPAR): Ziextenzo. Accessed January 30, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ziextenzo.
- 71. European Medicines Agency . Human medicine European public assessment report (EPAR): Grasustek. Accessed January 30, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/grasustek.
- 72. European Medicines Agency . Human medicine European public assessment report (EPAR): Cegfila. Accessed January 30, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/cegfila.
- 73. European Medicines Agency . Human medicine European public assessment report (EPAR): Nyvepria. Accessed January 30, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/nyvepria.
- 74. U.S. Food & Drug Administration (FDA) . Press Announcements. FDA approves first biosimilar to Neulasta to help reduce the risk of infection during cancer treatment. June 04, 2018. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-biosimilar-neulasta-help-reduce-risk-infection-during-cancer-treatment.
- 75. U.S. Food & Drug Administration (FDA) . Prescribing Information. Udenyca™ (pegfilgrastim‐cbqv). Accessed January 30, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761039s000lbl.pdf.
- 76. U.S. Food & Drug Administration (FDA) . Prescribing Information. Ziextenzo™ (pegfilgrastim‐bmez). Accessed January 30, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761045lbl.pdf.
- 77. U.S. Food & Drug Administration (FDA) . Prescribing Information. Nyvepria™ (pegfilgrastim‐apgf). Accessed January 30, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761111lbl.pdf.
- 78. Tonelli M, Hemmelgarn B, Reiman T, et al. Benefits and harms of erythropoiesis‐stimulating agents for anemia related to cancer: a meta‐analysis. CMAJ. 2009;180(11):E62‐E71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jelkmann W. Physiology and pharmacology of erythropoietin. Transfusion Medicine and Hemotherapy. 2013;40(5):302‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Won H‐H, Park I, Lee E, Kim J‐W, Lee D. Comparative analysis of the JAK/STAT signaling through erythropoietin receptor and thrombopoietin receptor using a systems approach. BMC Bioinformatics. 2009;10(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. European Medicines Agency . Human medicine European public assessment report (EPAR):Abseamed. Accessed February 8, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/abseamed.
- 82. European Medicines Agency . Human medicine European public assessment report (EPAR): Binocrit. Accessed February 8, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/binocrit.
- 83. European Medicines Agency . Human medicine European public assessment report (EPAR): Epoetin Alfa Hexal. Accessed February 8, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/epoetin-alfa-hexal.
- 84. European Medicines Agency . Human medicine European public assessment report (EPAR): Retacrit. Accessed February 8, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/retacrit.
- 85. European Medicines Agency . Human medicine European public assessment report (EPAR): Silapo. Accessed February 8, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/silapo.
- 86. U.S. Food & Drug Administration (FDA) . Prescribing Information. Retacrit®(epoetin alfa‐epbx). Accessed February 8, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125545s000lbl.pdf.
- 87. Henry DH. The evolving role of epoetin alfa in cancer therapy. Oncologist. 2004;9(1):97‐107. [DOI] [PubMed] [Google Scholar]
- 88. Losem C, Koenigsmann M, Rudolph C. Biosimilar Retacrit®(epoetin zeta) in the treatment of chemotherapy‐induced symptomatic anemia in hematology and oncology in Germany (ORHEO)–non‐interventional study. Onco Targets Ther. 2017;10:1295‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Moen MD. Bevacizumab. Bevacizumab Drugs. 2010;70(2):181‐189. [DOI] [PubMed] [Google Scholar]
- 90. U.S. Food & Drug Administration (FDA) . Prescribing Information. Avastin® (bevacizumab). Accessed February 2, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125085s301lbl.pdf.
- 91. Busse A, Lüftner D. What does the pipeline promise about upcoming biosimilar antibodies in oncology? Breast Care. 2019;14(1):10‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. U.S. Food & Drug Administration (FDA) . Prescribing Information. Mvasi™ (bevacizumab‐awwb). Accessed February 4, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761028s000lbl.pdf.
- 93. U.S. Food & Drug Administration (FDA) . Prescribing Information. Zirabev™ (bevacizumab‐bvzr). Accessed February 4, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761099s000lbl.pdf.
- 94. European Medicines Agency . Human medicine European public assessment report (EPAR): Mvasi. Accessed February 4, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/mvasi.
- 95. European Medicines Agency . Human medicine European public assessment report (EPAR): Zirabev. Accessed February 4, 2021.
- 96. European Medicines Agency . Human medicine European public assessment report (EPAR): Aybintio. Accessed February 4, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/aybintio.
- 97. European Medicines Agency . Human medicine European public assessment report (EPAR): Equidacent. Accessed February 4, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/equidacent.
- 98. European Medicines Agency . Human medicine European public assessment report (EPAR): Oyavas. Accessed February 4, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/oyavas.
- 99. European Medicines Agency . Human medicine European public assessment report (EPAR): Alymsys. Accessed February 4, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/alymsys.
- 100. Moorkens E, Vulto AG, Huys I, eds. An overview of patents on therapeutic monoclonal antibodies in Europe: are they a hurdle to biosimilar market entry? Mabs. Taylor & Francis; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Smith MR. Rituximab (monoclonal anti‐CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22(47):7359‐7368. [DOI] [PubMed] [Google Scholar]
- 102. Wang S, Weiner G. Rituximab: a review of its use in non‐Hodgkin's lymphoma and chronic lymphocytic leukemia. Expert Opin Biol Ther. 2008;8:759‐768.18476787 [Google Scholar]
- 103. U.S. Food & Drug Administration (FDA) . Prescribing Information. Rituxan® (rituximab). Accessed February 2, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103705s5367s5388lbl.pdf.
- 104. Greenwald M, Tesser J, Sewell KL. Biosimilars have arrived: rituximab. Art Ther. 2018;2018:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. U.S. Food & Drug Administration (FDA) . Prescribing Information. Truxima™ (rituximab‐abbs). Accessed February 2, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761088s000lbl.pdf.
- 106. U.S. Food & Drug Administration (FDA) . Prescribing Information. Ruxience™ (rituximab‐pvvr). Accessed February 2, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761103s000lbl.pdf.
- 107. U.S. Food & Drug Administration (FDA) . Prescribing Information. Riabni™ (rituximab‐arrx). Accessed February 2, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761140s000lbl.pdf.
- 108. European Medicines Agency . Human medicine European public assessment report (EPAR):Blitzima. Accessed February 2, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/blitzima.
- 109. European Medicines Agency . Human medicine European public assessment report (EPAR):Truxima. Accessed February 2, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/truxima.
- 110. European Medicines Agency . Human medicine European public assessment report (EPAR):Ruxience. Accessed February 2, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ruxience.
- 111. European Medicines Agency . Human medicine European public assessment report (EPAR):Riximyo. Accessed February 2, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/riximyo.
- 112. European Medicines Agency . Human medicine European public assessment report (EPAR):Rixathon. Accessed February 2, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/rixathon.
- 113. European Medicines Agency . Human medicine European public assessment report (EPAR):Ritemvia. Accessed February 2, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ritemvia.
- 114. Yang J, Yu S, Yang Z, et al. Efficacy and safety of anti‐cancer biosimilars compared to reference biologics in oncology: a systematic review and meta‐analysis of randomized controlled trials. BioDrugs. 2019;33(4):357‐371. [DOI] [PubMed] [Google Scholar]
- 115. U.S. Food & Drug Administration (FDA) . Prescribing Information. Herceptin® (trastuzumab). Accessed February 6, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf.
- 116. Derbyshire M. Patent expiry dates for best‐selling biologicals. Generics and Biosimilars Initiative J. 2015;4(4):178‐180. [Google Scholar]
- 117. U.S. Food & Drug Administration (FDA) . Prescribing Information. Herzuma®(trastuzumab‐pkrb). Accessed February 6, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761091s001s002lbl.pdf.
- 118. U.S. Food & Drug Administration (FDA) . Prescribing Information. Trazimera®(trastuzumab‐qyyp). Accessed February 6, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761081s000lbl.pdf.
- 119. U.S. Food & Drug Administration (FDA) . Prescribing Information. Ontruzant®(trastuzumab‐dttb). Accessed February 6, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761100Orig1s000Lbl.pdf.
- 120. U.S. Food & Drug Administration (FDA) . Prescribing Information. Kanjinti®(trastuzumab‐anns). Accessed February 6, 2021 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761073s000lbl.pdf.
- 121. European Medicines Agency . Human medicine European public assessment report (EPAR): Ontruzant. Accessed February 6, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ontruzant.
- 122. European Medicines Agency . Human medicine European public assessment report (EPAR): Herzuma. Accessed February 6, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/herzuma.
- 123. European Medicines Agency . Human medicine European public assessment report (EPAR): Trazimera. Accessed February 6, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/trazimera.
- 124. European Medicines Agency . Human medicine European public assessment report (EPAR): Kanjinti. Accessed February 6, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/kanjinti.
- 125. European Medicines Agency . Human medicine European public assessment report (EPAR): Ogivri. Accessed February 6, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ogivri.
- 126. European Medicines Agency . Human medicine European public assessment report (EPAR): Zercepac. Accessed February 6, 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/zercepac.
- 127. Business Wire : Bio‐Thera Solutions Announces FDA Accepts Biologics License Application for BAT1706, a Proposed Biosimilar to Avastin® Available from: https://www.businesswire.com/news/home/20210128005703/en/Bio-Thera-Solutions-Announces-FDA-Accepts-Biologics-License-Application-for-BAT1706-a-Proposed-Biosimilar-to-Avastin%C2%AE.
- 128. Generics and Biosimilars Initiative . EMA accepts application for bevacizumab biosimilar BAT1706. Accessed February 27, 2021. Available from: https://www.gabionline.net/Biosimilars/News/EMA-accepts-application-for-bevacizumab-biosimilar-BAT1706.
- 129. Cancer network . FDA Accepts BLA for Proposed Biosimilar to Bevacizumab, MYL‐1402O. Available from: https://www.cancernetwork.com/view/fda-accepts-bla-proposed-biosimilar-bevacizumab-myl-1402o.
- 130. The Centre for Biosimilars . Pandemic Delays FDA Decision on Biocon's Bevacizumab Application. Available from: https://www.centerforbiosimilars.com/view/pandemic-delays-fda-decision-on-biocon-s-bevacizumab-application.
- 131. OncLive . FDA Defers Action on BLA for Bevacizumab Biosimilar MYL‐1402O. Available from: https://www.onclive.com/view/fda-defers-action-on-bla-for-bevacizumab-biosimilar-myl-1402o.
- 132. Luye Pharma . Marketing Authorization Application for Luye Pharma's Bevacizumab Injection Accepted in China Available from: https://www.luye.cn/lvye_en/view.php?id=1819.
- 133. The Centre for Biosimilars . Genentech Files to Block Marketing of Centus' Bevacizumab Biosimilar. Available from: https://www.centerforbiosimilars.com/view/genentech-files-to-block-marketing-of-centus-bevacizumab-biosimilar.
- 134. European Pharmaceutical Review , Proposed biosimilar accepted for review by EMA. Available from: https://www.europeanpharmaceuticalreview.com/news/89339/proposed-biosimilar-accepted-for-review-by-ema/.
- 135. NMPA Accepts New Drug Application for IBI301, a Biosimilar Product Candidate of Rituximab (MabThera/Rituxan) [press release].
- 136. National Library of Medicine (U.S.) . A Study to Evaluate Safety, Tolerability, PK and PD of HLX01 in Patients with CD20‐positive B‐cell Lymphoma. Identifier: NCT03218072 Available from: https://clinicaltrials.gov/ct2/show/NCT03218072.
- 137. National Library of Medicine (U.S.) . A Pharmacokinetic and Pharmacodynamic Study Comparing HLX01 And Rituximab in Patients With CD20‐Positive, B‐cell Lymphoma. Identifier: NCT02584920 Available from: https://clinicaltrials.gov/ct2/show/NCT02584920.
- 138. National Library of Medicine (U.S.) . Clinical Study to Compare the Efficacy and Safety of Rituximab Biosimilar HLX01 and Rituximab in Combination With CHOP, in Previously Untreated Subjects With CD20+ DLBCL. Identifier: NCT02787239 Available from: https://clinicaltrials.gov/ct2/show/NCT02787239.
- 139. National Library of Medicine (U.S.) . Follow‐up the OS and PFS of Rituximab Biosimilar HLX01 and MabThera, in Untreated Subjects With CD20+ DLBCL. Identifier: NCT04491721 Available from: https://clinicaltrials.gov/ct2/show/NCT04491721.
- 140. Candelaria M, González DE, Delamain MT, et al. Rituximab biosimilar RTXM83 versus reference rituximab in combination with CHOP as first‐line treatment for diffuse large B‐cell lymphoma: a randomized, double‐blind study. Leuk Lymphoma. 2019;60:3375‐3385. [DOI] [PubMed] [Google Scholar]
- 141. Biosimilar Development . Phase 3 Trial Of Henlius Bevacizumab Biosimilar HLX04 In Metastatic Colorectal Cancer Met Study Primary Endpoint. Available from: https://www.biosimilardevelopment.com/doc/phase-trial-of-henlius-bevacizumab-biosimilar-hlx-in-metastatic-colorectal-cancer-met-study-primary-endpoint-0001.
- 142. National Library of Medicine (U.S.) . A Phase I Clinical Study to Evaluate the Safety, Tolerability and Pharmacokinetics of Anti‐PD‐1 Antibody (HLX10) in Combination with Avastin Biosimilar (HLX04) in Patients with Advanced Solid Tumors. Identifier: NCT03757936 Available from: https://clinicaltrials.gov/ct2/show/NCT03757936.
- 143. National Library of Medicine (U.S.) . A Clinical Study Evaluating the Use of HLX10 in Combination with HLX04 for the Treatment of Advanced Hepatocellular Carcinoma (HCC) Patients with Disease Progression or Intolerant Toxicity After Standard Treatment. Identifier: NCT03973112 Available from: https://clinicaltrials.gov/ct2/show/NCT03973112.
- 144. National Library of Medicine (U.S.) . Phase III Trial BI 695502 plus Chemotherapy versus Avastin® plus Chemotherapy in Patients with Lung Cancer. Identifier: NCT02272413 Available from: https://clinicaltrials.gov/ct2/show/NCT02272413.
- 145. National Library of Medicine (U.S.) . Open‐label, Single Arm Trial of BI 695502 in Patients with Previously Untreated Metastatic Colorectal Cancer (INVICTAN®‐3). Identifier: NCT02776683 Available from: https://clinicaltrials.gov/ct2/show/NCT02776683.
- 146. R‐Pharm‐US LLC. Research & Development. Pipeline Available from: https://www.rpharm-us.com/pages/research/index.php.
- 147. National Library of Medicine (U.S.) . Study of TQB2303 in Patients with CD20‐Positive Diffuse Large B‐cell Lymphoma (DLBCL). Identifier: NCT03777085 Available from: https://clinicaltrials.gov/ct2/show/NCT03777085.
- 148. National Library of Medicine (U.S.) . Study of TQB2303 in Patients with Aggressive CD20 Positive Non‐Hodgkin's Lymphoma. Identifier: NCT03456466 Available from: https://clinicaltrials.gov/ct2/show/NCT03456466.
- 149. National Library of Medicine (U.S.) . Safety, Pharmacokinetics and Pharmacodynamics of Recombinant Chimeric Anti‐CD20 Monoclonal Antibody in Patients with B‐cell Non‐Hodgkin's Lymphoma. Identifier: NCT02206308 Available from: https://clinicaltrials.gov/ct2/show/NCT02206308.
- 150. National Library of Medicine (U.S.) . A Pharmacokinetic Study Comparing SCT400 and Rituximab in Patients with B‐cell Non‐Hodgkin's Lymphoma. Identifier: NCT02456207 Available from: https://clinicaltrials.gov/ct2/show/NCT02456207.
- 151. National Library of Medicine (U.S.) . A Study Comparing the Efficiency and Safety of S‐CHOP(Cyclophosphamide, Hydroxydaunomycin, Oncovin, and Prednisone) Versus R‐CHOP in Untreated CD20(Cluster of Differentiation Antigen 20)‐Positive DLBCL Patients. Identifier: NCT02772822 Available from: https://clinicaltrials.gov/ct2/show/NCT02772822.
- 152. National Library of Medicine (U.S.) . Compare the Efficacy, Safety, and Immunogenicity of Proposed Rituximab Biosimilar (DRL_RI) With MabThera® in LTB Follicular Lymphoma (FLINTER) (FLINTER). Identifier: NCT03976102 Available from: https://clinicaltrials.gov/ct2/show/NCT03976102.
- 153. National Library of Medicine (U.S.) . A Study Comparing SIBP‐02 and Rituximab Combination with CHOP in Previously Untreated Subjects with CD20+ DLBCL. Identifier: NCT04361279 Available from: https://clinicaltrials.gov/ct2/show/NCT04361279.
- 154. National Library of Medicine (U.S.) . A Study Comparing the Efficacy and Safety of G‐CHOP Versus R‐CHOP in Untreated Diffuse Large B‐cell Lymphoma Patients. Identifier: NCT03650933.
- 155. National Library of Medicine (U.S.) . Clinical Trial of Comparative Study of GB221 Pharmacokinetics. Identifier: NCT04175171 Available from: https://clinicaltrials.gov/ct2/show/NCT04175171.
- 156. National Library of Medicine (U.S.) . Clinical Study of Recombinant Anti‐HER2 Humanized Monoclonal Antibody for Injection. Identifier: NCT04170595 Available from: https://clinicaltrials.gov/ct2/show/NCT04170595.
- 157. National Library of Medicine (U.S.) . Clinical Study of Recombinant Anti‐HER2 Humanized Monoclonal Antibody (GB221) for Injection. Identifier: NCT04164615 Available from: https://www.clinicaltrials.gov/ct2/show/NCT04164615.
- 158. National Library of Medicine (U.S.) . A Safety and Pharmacokinetic Study Between HLX02 and Herceptin®(US‐licensed and EU‐approved) in Healthy Chinese Male Subjects. Identifier: NCT04670796 Available from: https://clinicaltrials.gov/ct2/show/NCT04670796.
- 159. National Library of Medicine (U.S.) . A Safety and Pharmacokinetic Study Between HLX02 and Herceptin®(U.S. and German Sourced) in Healthy Chinese Male Subjects. Identifier: NCT02581748 Available from: https://clinicaltrials.gov/ct2/show/NCT02581748.
- 160. National Library of Medicine (U.S.) . Compare Efficacy, Safety and Immunogenicity of HLX02 and Herceptin in Previously Untreated HER2 +Overexpressing Metastatic Breast Cancer. Identifier: NCT03084237 Available from: https://clinicaltrials.gov/ct2/show/NCT03084237.
- 161. Henlius Press Release . Henlius Granted Exclusive Development and Commercialization Rights of HLX02 in the US and Canada to Accord Available from: https://www.henlius.com/en/NewsDetails-2779-26.html.
- 162. National Library of Medicine (U.S.) . A Study of SIBP‐01 or CN‐Trastuzumab Plus Docetaxel And Carboplatin In HER2 Positive Breast Cancer. Identifier: NCT03989037 Available from: https://clinicaltrials.gov/ct2/show/NCT03989037.
- 163. National Library of Medicine (U.S.) . To Compare Efficacy and Safety of CT‐P16 and EU‐Approved Avastin as First‐Line Treatment for Metastatic or Recurrent Non‐Squamous Non‐Small Cell Lung Cancer. Identifier: NCT03676192 Available from: https://clinicaltrials.gov/ct2/show/NCT03676192.
- 164. National Library of Medicine (U.S.) . A Trial to Compare the Efficacy, Safety, Pharmacokinetics and Immunogenecity of HD204 to Avastin® in Advanced Non‐squamous Non‐small Cell Lung Cancer Patients. Identifier: NCT03390686 Available from: https://clinicaltrials.gov/ct2/show/NCT03390686.
- 165. National Library of Medicine (U.S.) . Susbtance Record: Bevacizumab. PubChem SID:178103377 Available from: https://pubchem.ncbi.nlm.nih.gov/substance/178103377.
- 166. National Library of Medicine (U.S.) . MIL60 versus Bevacizumab in Patients with Treatment‐naïve Non‐squamous Non‐small Cell Lung Cancer. Identifier: NCT03196986 Available from: https://clinicaltrials.gov/ct2/show/NCT03196986.
- 167. National Library of Medicine (U.S.) . To Evaluate the Efficacy and Safety of QL1206 and Xgeva in Patients With Bone Metastases From Solid Tumors. Identifier: NCT04550949 Available from: https://clinicaltrials.gov/ct2/show/NCT04550949.
- 168. National Library of Medicine (U.S.) . Pharmacokinetic, Safety and Immunogenicity Study of CMAB809 and Herceptin in Healthy Volunteers. Identifier: NCT04049409 Available from: https://clinicaltrials.gov/ct2/show/NCT04049409.
- 169. Morita J, Tanaka M, Nomoto M, et al. Pharmacokinetic bioequivalence, safety, and immunogenicity of DMB‐3111, a trastuzumab biosimilar, and trastuzumab in healthy Japanese adult males: results of a randomized trial. BioDrugs. 2016;30(1):17‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Korea Biomedical Review . Alteogen's sales nearly doubled in 2020. Available from: https://www.koreabiomed.com/news/articleView.html?idxno=10529.
- 171. Bioworld . Alteogen licenses ALT‐B4 to a top 10 global pharma in potential $1B+ deal.
- 172. National Library of Medicine (U.S.) . A Study of SHR‐1210 in Combination with BP102 in Subjects with Non‐squamous NSCLC. Identifier: NCT03666728 Available from: https://clinicaltrials.gov/ct2/show/NCT03666728.
- 173. Genor Biopharma , Research & Development: Pipeline Available from: https://www.genorbio.com/en/research-development/pipeline/.
- 174. National Library of Medicine (U.S.) . Safety Assessment of Repeated Administration of TK006 in Patients with Breast Cancer‐related Bone Metastases. Identifier: NCT03487055 Available from: https://clinicaltrials.gov/ct2/show/NCT03487055.
- 175. National Library of Medicine (U.S.) . Assessment of TK006 in Patients with Breast Cancer‐related Bone Metastases. Identifier: NCT03239756 Available from: https://clinicaltrials.gov/ct2/show/NCT03239756.
- 176. National Library of Medicine (U.S.) . Comparative Immunogenicity Study of Multiple Doses of Proposed Pegfilgrastim Biosimilar, INTP5 of Intas Pharmaceuticals Ltd., India against Neulasta of Amgen Inc., USA in Healthy, Adult Human Subjects. Identifier: NCT04015232 Available from: https://clinicaltrials.gov/ct2/show/NCT04015232.
- 177. National Library of Medicine (U.S.) . A Study to Assess Immune Response to Multiple Doses of PF‐06881894 or US‐Approved Neulasta in Healthy Volunteers. Identifier: NCT03273842 Available from: https://clinicaltrials.gov/ct2/show/NCT03273842.
- 178. National Library of Medicine (U.S.) . Immunogenicity and Pharmacodynamic of B12019 and Neulasta® in Healthy Subjects. Identifier: NCT02912377 Available from: https://clinicaltrials.gov/ct2/show/NCT02912377.
- 179. National Library of Medicine (U.S.) . A Phase 1 Study to Assess the Immunogenicity of QL0605 Compared to US Neulasta in Healthy Subjects. Identifier: NCT04651036 Available from: https://clinicaltrials.gov/ct2/show/NCT04651036.
- 180. Bioxpress Therapeutics , mAb Biosimilar pipeline. Available from: https://bioxpressbiosimilars.com/biosimilar-pipeline/.
- 181. iBio's Collaboration with South Africa's AzarGen Biotechnologies Advances to Next Stage Available from: http://www.ibioinc.com/ibios-collaboration-with-south-africas-azargen-biotechnologies-advances-to-next-stage/.
- 182. Prestige Biopharma Development Pipelines Available from: https://www.prestigebiopharma.com/development-pipelines/.
- 183. Mabpharm Limited . Research and Development. Product Centre Available from: http://www.mabpharm.cn/en/research_product.php.
- 184. Neuclone Products Pipeline. Available from: https://neuclone.com/pipeline/.
- 185. Xbrane Biopharma Biosimilars. Available from: https://www.xbrane.com/en/products/.
- 186. Generics and Biosimilars Initiative . Boehringer Ingelheim stops biosimilar rituximab development. Accessed February 20, 2021. Available from: https://www.gabionline.net/Biosimilars/News/Boehringer-Ingelheim-stops-biosimilar-rituximab-development.
- 187. Generics and Biosimilars Initiative . Biosimilars of rituximab. Accessed February 19, 2021. Updated 8 January 2021. Available from: https://www.gabionline.net/Biosimilars/General/Biosimilars-of-rituximab.
- 188. Biosimilars Review & Report . Archigen Closes Its Doors After Failing to Introduce Rituximab Biosimilar. Accessed February 19, 2021. Available from: https://biosimilarsrr.com/2020/11/20/archigen-closes-its-doors-after-failing-to-introduce-rituximab-biosimilar/.
- 189. Generics and Biosimilars Initiative . Teva halts phase III biosimilar rituximab trial. Accessed February 19, 2021. Available from: https://www.gabionline.net/Biosimilars/News/Teva-halts-phase-III-biosimilar-rituximab-trial.
- 190. Big Molecule Watch . Genentech, Inc. and JHL Biotech, Inc. File a Stipulation for Entry of a Proposed Consent Judgment and Permanent Injunction; Court Order in Response Asks Parties to Answer Questions. Accesssed February 19, 2021.
- 191. National Library of Medicine (U.S.) . A Study to Compare the Efficacy and Safety of JHL1101 versus Rituximab in Patients with Previously Untreated Diffuse Large B‐Cell Lymphoma (DLBCL). Identifier: NCT03670901 Available from: https://clinicaltrials.gov/ct2/show/NCT03670901.
- 192. Center for biosimilars , Sandoz abandons its US biosimilar rituximab plans Available from: https://www.centerforbiosimilars.com/view/sandoz-abandons-its-us-biosimilar-rituximab-plans.
- 193. Pierpont TM, Limper CB, Richards KL. Past, present, and future of rituximab—the world's first oncology monoclonal antibody therapy. Front Oncol. 2018;8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. National Library of Medicine (U.S.) . Evaluate the Efficacy and Safety of HLX01 versus Mabthera in Patients with Low Tumour Burden Follicular Lymphoma. Identifier: NCT04671420 Available from: https://clinicaltrials.gov/ct2/show/NCT04671420.
- 195. National Library of Medicine (U.S.) . SHR‐1210 in Combination with BP102 and XELOX in Patient with Metastatic Colorectal Cancer. Identifier: NCT03645876 Available from: https://clinicaltrials.gov/ct2/show/NCT03645876.
- 196. Nixon N, Hannouf M, Verma S. The evolution of biosimilars in oncology, with a focus on trastuzumab. Current Oncol. 2018;25(1):S171‐S179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


