Abstract
A 5‐year‐old Pomeranian was diagnosed with anterior uveitis, hyphema, and secondary glaucoma OD. Concurrent retinal hemorrhage, perivascular sheathing, and papilledema were identified OS. Work‐up identified small cell lymphocytosis (>900 × 109/L), anemia, and thrombocytopenia. The patient was diagnosed with B‐cell chronic lymphocytic leukemia as a cause of the ocular findings.
Keywords: canine, oncology, ophthalmology, veterinary
Ocular disease may be the initial manifestation of systemic disorders such as neoplasia, infection, or immune‐mediated disease. Patients may develop multiple abnormalities such as hyphema, uveitis, retinal lesions, and secondary glaucoma. Although CLL is a rare disorder in dogs, early diagnosis and treatment can lead to a long‐term clinical improvement.

1. CASE REPORT
1.1. Presentation and physical examination
A 6‐kg, 5‐yr‐old spayed female Pomeranian was referred to LSU on emergency for suspected glaucoma due to a two‐day history of blepharospasm, cloudiness, and redness of the right eye. The patient was inappetent 1 day prior but had been otherwise clinically well until presentation. There was no significant medical history other than suspected arthritis that was being managed with orally administered carprofen (Rimadyl, Zoetis, Parsippany). On physical examination, she was bright and alert, and in good body condition. She was mildly febrile (39.4°C) and hypertensive (170 mmHg) as measured by Doppler ultrasonography. Mucous membranes were pale. Cardiopulmonary auscultation was unremarkable. Abdominal palpation revealed significant cranial abdominal organomegaly. Peripheral lymph nodes palpated within normal limits.
1.2. Ophthalmic examination findings
Ocular examination demonstrated an absent menace response in the right eye. Direct pupillary light reflex (PLR) in the right eye (oculus dexter, OD) could not be assessed due to diffuse hyphema; consensual PLR to the left eye (oculus sinister, OS) was absent. Direct PLR OS was present and complete; a consensual PLR (OS to OD) was unable to be visualized due to hyphema. Intraocular pressure (Tono‐Pen, Reichert Technologies, Depew) was elevated OD at 46 mmHg and low‐normal OS at 8 mmHg following application of one drop of proparacaine (Proparacaine, Akorn Pharmaceuticals) per eye; neither eye retained fluorescein stain. There was severe episcleral injection OD with moderate yellow mucoid discharge. Diffuse corneal edema was evident with concurrent 4+ aqueous flare and hyphema OD (Figure 1). The anterior segment changes present precluded further evaluation of the intraocular structures OD. Fundic examination OS following dilation with topical tropicamide (Tropicamide, Akorn Pharmaceuticals) identified multifocal intraretinal and subretinal hemorrhages of varying sizes ranging from punctate to approximately 1 disc diameter. Perivascular sheathing of the retinal vessels and mild “box‐carring” (a term used to describe immediately adjacent segmental vascular diameter variability) of the retinal arterioles was present. Additionally, mild papilledema of the optic nerve head and a large focal chorioretinal scar was identified in the non‐tapetal fundus OS.
FIGURE 1.

Clinical photograph of the right eye on presentation. The patient was blepharospastic with moderate yellow mucoid discharge present. Significant episcleral injection and diffuse corneal edema were present with concurrent aqueous flare and hyphema OD.
1.3. Diagnostic investigation
A complete blood count (CBC, Advia 120; Siemens) demonstrated marked anemia (PCV 12%; reference interval: 37%–55%), thrombocytopenia (68 × 109/L; reference interval: 220–600 × 109/L), and an extreme leukocytosis (~980 × 109/L; reference interval: 8.0–14.5 × 109/L; Table 1, Day 0). However, the leukocytosis exceeded the upper linearity limit set by Siemens for non‐human blood samples for this instrument (100 × 109/L), so this concentration was considered an approximation. On a Wright's Giemsa stained blood smear, the marked leukocytosis was confirmed and characterized predominantly (~95%) as small lymphocytes with round, eccentric nuclei that measured mostly 6–8 to rarely10 μm in diameter. The chromatin was coarse to condensed, and nucleoli were inapparent. These lymphocytes had small amounts of basophilic cytoplasm. Several lysed nuclei and cytoplasmic fragments were present throughout the blood smear, and no platelet clumps were observed. The interpretation was chronic lymphocytic leukemia. Based on the absence of polychromasia on the smear, the anemia was presumed non‐regenerative. However, the Advia analyzer indicated a reticulocytosis (495 × 109/L), likely due to spurious enumeration of cytoplasmic fragments from leukemic cells as reticulocytes. 1 Significant abnormalities noted on serum biochemical panel included only hypoalbuminemia (total protein 53 g/L; reference interval: 54–70 g/L; albumin 23 g/L; and reference interval: 26–34 g/L). A urinalysis was not performed.
TABLE 1.
Selected sequential hematologic findings in a dog with chronic lymphocytic leukemia before and during chemotherapy
| Days since initial presentation | 0 | 8 | 16 | 50 | 121 | 824 | 977 | 984 | Reference interval |
|---|---|---|---|---|---|---|---|---|---|
| PCV a (%) | 12 | 15 | 23 | 33 | 39 | 42 | 41 | 39 | 37–55 |
| Platelets (×109/L) | 68 | 57 | 232 | 407 | 494 | 236 | 104 | 74 | 220–600 |
| WBC (×109/L) | 981.8± | 522.3 b | 280.2 b | 96.3 | 12.3 | 7.1 | 11.4 | 11.5 | 8.0–14.5 |
| Abs Seg Neutrophils (×109/L) | 34.4 | 20.9 | 8.4 | 10.1 | 10.6 | 5.4 | 4.4 | 3.5 | 3.0–11.5 |
| Abs Band Neutrophils (×109/L) | 0.0 | 2.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0–0.3 |
| Abs Lymphocytes (×109/L) | 942.5 | 491.0 | 263.4 | 85.2 | 0.5 | 1.14 | 6.27 | 7.94 | 1.0–4.8 |
| Abs Monocytes (×109/L) | 4.9 | 7.8 | 8.4 | 1.0 | 0.1 | 0.6 | 0.5 | 0.1 | 0.1–1.4 |
Packed cell volume.
Approximation; result above linearity set by Siemens for non‐human blood sample analyzed by Advia 120.
To further immunophenotype the neoplastic lymphocytes, peripheral blood was submitted for flow cytometric analysis (Clinical Immunology Laboratory, College of Veterinary Medicine & Biomedical Sciences, Colorado State University, Fort Collins, USA). Immunophenotypic analysis revealed a homogeneous expansion of small CD21+ lymphocytes, diagnostic for B‐cell chronic lymphocytic leukemia (B‐CLL).
No abnormalities were detected on thoracic radiographs. Abdominal radiographs showed severe hepatosplenomegaly and decreased serosal detail, but no other clinically significant abnormalities. Due to the inability to evaluate the intraocular structures and concern for retinal detachment, an ocular ultrasound was performed. Ultrasound of the right eye confirmed the presence of a complete bullous retinal detachment; no evidence of detachment was identified OS.
1.4. Diagnosis and treatment
Based on clinical and laboratory findings, a diagnosis of chronic lymphocytic leukemia resulting in anterior uveitis and secondary glaucoma was made. The patient was administered l‐asparaginase (400 IU/kg, L‐asparaginase, LGM Pharma) subcutaneously, and prednisone (2 mg/kg/day, Prednisone, West‐Ward Pharmaceuticals) was dispensed to be orally administered by the owner beginning 3 days later. Anterior uveitis and secondary glaucoma were treated with a ¼′′ strip of neo‐poly‐dex ointment (Neomycin and polymyxin B sulfates and dexamethasone ophthalmic ointment, Bausch & Lomb) OD Q 8 h and one drop of dorzolamide ophthalmic solution (Dorzolamide hydrochloride 2%, Hi‐tech Pharmaceuticals) OD Q 8 h, respectively; tramadol (4 mg/kg PO Q 8 hr) was dispensed for pain relief (Tramadol hydrochloride, Caraco Pharmaceutical Laboratories). Enucleation of the right eye was recommended once the patient was stable enough for anesthesia. Repeated blood pressure evaluation identified no persistence of systemic hypertension, and systemic therapy was not indicated.
One week following discharge the owner reported that the patient was eating well and behaving normally. Repeat ophthalmic examination showed a large, central superficial corneal ulcer OD. Intraocular pressure OD had decreased to 7 mmHg while hyphema and uveitis were still present. Given the improvement in intraocular pressure OD and concurrent intraocular inflammation, it was recommended to continue treatment with dorzolamide ophthalmic solution. Examination OS identified several new retinal hemorrhages; however, the papilledema and perivascular sheathing had subjectively reduced. The neo‐poly‐dex ointment was discontinued due to the corneal ulcer. Neo‐poly‐bac ointment (Neomycin and polymyxin B sulfates and bacitracin ophthalmic ointment, Bausch & Lomb) and diclofenac ophthalmic solution (Diclofenac sodium 0.1% ophthalmic solution, Akorn Pharmaceuticals) were initiated. CBC demonstrated a significant decrease in absolute lymphocytosis (491 × 109/L), mild neutrophilia (20.9 × 109/L), and moderate polychromasia and few giant platelets (indicative of regeneration), but other hematologic values remained relatively stable (Table 1, Day 8). The patient was started on a COP‐based protocol consisting of cyclophosphamide (200 mg/m2 PO), vincristine (Vincristine sulfate, Pfizer Inc., 0.5 mg/m2 IV), and prednisone (10 mg PO Q24 h). The patient was discharged and instructed to continue oral prednisone and tramadol at home.
Two weeks following initial presentation, the absolute lymphocytosis had further halved (263.4 × 109/L) from initial CBC, and the anemia and thrombocytopenia were improving (Table 1, Day 16). Ocular examination OD identified buphthalmos with resultant lagophthalmos. Corresponding with the area of lagophthalmos, the central ulcer had progressed and was now deep with associated cellular infiltrate. Corneal cytology revealed a population of neutrophils and mixed bacteria (cocci and bacilli). Retinal examination OS showed resolution of papilledema and no new retinal hemorrhages (Figure 2). Enucleation OD was recommended and performed without complication.
FIGURE 2.
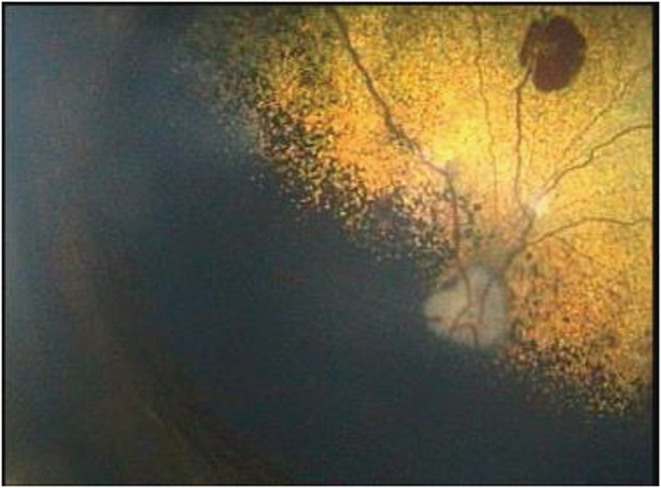
Fundic photograph of the left eye taken two‐weeks after instituting medical therapy. Mild box‐carring of the retinal vessels with a large focal retinal hemorrhage in the tapetal fundus are evident.
Histopathology of the enucleated eye confirmed ulcerative keratitis with suppurative inflammation. Panuveal and retinal infiltration by a monomorphic population of small lymphocytes with widespread accumulation within blood vessels was identified, compatible with intraocular chronic lymphocytic leukemia (Figure 3). Secondary inflammation had resulted in massive exudation, fibrovascular membrane formation, and collapse of the iridocorneal angle resulting in secondary glaucoma. Extensive retinal degeneration and optic nerve degeneration were evident; complete pathologic retinal detachment was also confirmed. Immunohistochemical staining of the enucleated eye revealed that the lymphoid infiltrate exhibited strong diffuse cytoplasmic labeling against CD20 (CD3; A0452; 1:300; Dako) (Figure 3) with only scattered CD3 (CD20; RB‐9013‐P; 1:400; Thermo Scientific)‐positive cells.
FIGURE 3.

Iris stroma was extensively infiltrated with a monomorphic population of small hyperchromatic lymphocytes (H&E stain, bar = 100 μm). Inset: These lymphocytes have strong diffuse cytoplasmic immunoreactivity against CD20 (IHC, bar = 100 μm).
Once the systemic lymphocyte count decreased below 100 × 109/L, approximately 6 weeks post‐diagnosis (Table 1, Day 50), the patient was switched to orally administered Chlorambucil (Chlorambucil, Wedgewood Pharmacy, Scottsdale) chemotherapy (1.7 mg PO Q24 h). Follow‐up ophthalmic examination identified a healed enucleation surgical site with no evidence of anterior uveitis or new retinal hemorrhages noted OS. Further reduction in the degree of perivascular sheathing was reported, and baseline diagnostic parameters (Schirmer tear testing, intraocular pressure, fluorescein stain) were within normal limits.
The patient was maintained on 5 mg oral prednisone daily with a slow taper in the frequency of oral chlorambucil treatment over time (3.7 mg/m2 Q 48–Q 72 h). Ocular examination 3 months following diagnosis identified primarily age‐related changes OS including nuclear sclerosis, a punctate nuclear cataract and peripheral cystic retinal degeneration. On fundic examination, perivascular sheathing had resolved, and focal chorioretinal scars were noted in locations of previously noted retinal hemorrhage. No new hemorrhages were noted, and there was no evidence of uveitis. Recommendations were made to continue treatment through the oncology service and recheck with ophthalmology as needed.
Monitored lymphocyte counts remained within normal limits (Table 1, Days 121 and 824), and the patient lacked systemic or ocular clinical signs for approximately 32 months. At this time, thrombocytopenia and lymphocytosis were documented on survey bloodwork (Table 1, Day 977). Chlorambucil was discontinued, due to a presumed lack of response, and the patient was changed to therapy with cyclophosphamide (Cyclophosphamide, Roxane Laboratories, Inc, 232 mg/m2). One week later, significant peripheral lymphadenomegaly involving the mandibular, prescapular and popliteal lymph nodes developed, and the owner reported occasions of discomfort at home (Table 1, Day 984). Abdominal organomegaly was not identified on physical examination. Oral prednisone therapy was increased to 10 mg PO Q24 h, and the owner declined further diagnostics and therapeutic options. The patient was subsequently euthanized approximately 1 month later due to progressive discomfort reported by the owner; a necropsy was declined.
2. DISCUSSION
Chronic lymphocytic leukemia (CLL) is a neoplastic clonal proliferation of small lymphocytes characterized by a persistent peripheral lymphocytosis. CLL is most commonly diagnosed in middle age to older animals, with a median age of 10–11 years (range 1.5–15). 2 , 3 In one study, CLL represented 8% of canine samples submitted for flow cytometry for suspected lymphoproliferative disease. 3
Ninety‐five percent of reported cases of CLL in humans are of B‐cell (B lymphocyte) lineage, in contrast to dogs where a T‐cell immunophenotype is more common. 4 The patient in this report was classified as having B‐CLL based on homogeneous expansion of small CD21+ lymphocytes identified by flow cytometry, and further supported by the diffuse, strong cytoplasmic labeling of neoplastic cells against CD20 on immunohistochemistry. In a study of B‐CLL in dogs, small breed dogs were overrepresented. 3 , 5 One of the small breed dogs reported to have an increased odds of developing leukemia‐CLL was the Pomeranian. 3
B‐CLL is considered by many to be an indolent disease, characterized by a slow clinical progression. 6 In one report, 62% of dogs were asymptomatic at the time of diagnosis. 6 For those patients symptomatic at the time of diagnosis, clinical signs were generally variable and nonspecific, including lethargy, inappetance, weight loss, vomiting, polyuria/polydipsia, lymphadenopathy, intermittent lameness, fever, diarrhea, and labored breathing. 5 , 6 , 7
Although ocular abnormalities are reported in patients with B‐CLL, the current report presents an unusual case in that the patient presented with ocular disease as the presenting complaint. 3 , 6 Ocular signs are reported in hematopoietic neoplasms in both human and veterinary literature, but the overall incidence is relatively unknown. 2 , 7 , 8 , 9 , 10 , 11 A large prospective study of 94 dogs with multicentric lymphoma found that 37% of cases had ocular involvement, with the most common findings being anterior uveitis, intraocular hemorrhage, and retinal hemorrhage. 8 Seventy‐eight percent of the cases with leukemia had ocular disease. 8 Secondary glaucoma has been reported infrequently as a presenting sign of lymphoma. 9 , 10 In one retrospective study evaluating B‐CLL in dogs, 6 cases or 5% of patients, were reported to have ocular lesions, unfortunately, a description of the clinical findings associated with these changes was not specified in this report. 6
The prevalence of ocular disease in humans with leukemia varies from 12% to 90% depending on the type of leukemia and the study design. 11 , 12 The incidence of sight‐threatening ocular complications in humans with CLL appears to be low, and up to 60% of patients may be completely asymptomatic (no ocular signs) at the time of diagnosis. 11 Ocular disease can develop as the result of a direct leukemic infiltrate or secondary to hyperviscosity, thrombocytopenia, anemia, or a combination of these factors. 11 , 13 One common ocular manifestation of leukemia in humans is the presence of intraretinal hemorrhages. Neoplasia has been implicated in disrupting normal hemostatic mechanisms that can increase the risk for retinal hemorrhage according to one report. 14 Dogs with neoplasia frequently have hemostatic conditions including thrombocytopenia and hypercoaguability. 14 Direct infiltration of leukemic cells can manifest as retinal hemorrhage and may also be represented by gray‐white nodules, perivascular sheathing and rarely swelling of the optic nerve. 13 , 15
Retinal detachment is a rare complication of acute lymphoid leukemia and has been postulated to develop secondary to choroidal ischemia and retinal pigmented epithelial dysfunction leading to exudative detachment. 16 Choroidal ischemia is thought to occur secondary to occlusion of choriocapillaries and delayed choroidal circulation. 16 , 17 The choroid was the most frequently involved site in one report of ocular involvement in human patients with leukemia. 17 In dogs with ocular lymphoma, particularly of high‐grade subtypes, histopathology identified involvement of the choroid in 45% of cases evaluated. 18 The choroid was similarly involved in this patient and may have contributed to the development of retinal detachment. However, in contrast to the patient in this report, most of the patients in the study by Lanza et. al had either T‐cell lymphoma or diffuse large B‐cell lymphoma. 18
Hyperviscosity syndrome is a clinical syndrome typically resulting from reduced blood flow and may occur secondary to excessive levels of immunoglobulins from plasma cells or lymphocytes or a pathological cell count increase. Hyperviscosity was first identified in dogs with lymphoid leukemia in 1977. 19 One of the three patients in this original study was noted to have funduscopic abnormalities. 19 Vascular compression and hyperviscosity have also been proposed as a cause of papilledema in humans with CLL. 20 Microvascular changes associated with hyperviscosity have been associated with thrombosis, hemorrhage, box‐carring of retinal vessels, papilledema, hemorrhage, and exudation. 21
The ocular lesions documented in cases of canine B‐CLL were found to be consistent with lymphoproliferative disease or hyperglobulinemia and hyperviscosity syndrome. 6 Hyperviscosity is suspected to be a factor in the development of the ocular changes noted in our patient. However, serum viscosity was not performed in this case to confirm our clinical suspicion.
While cytopenias are considered uncommon in dogs with CLL, anemia and thrombocytopenia were reported in 26% and 7% of dogs with B‐CLL, respectively, and sometimes concurrently (bicytopenia). 3 In one report, mild normocytic, normochromic non‐regenerative anemia was often identified in patients with CLL. 2 Myelophthisis was considered the likely cause for the bicytopenia in this case and was reinforced by the improvement of cytopenias with chemotherapy.
A wide range in survival times has been reported for B‐CLL with a median survival time of 300 days in one retrospective review. 6 The total survival time in this report of 1004 days is within the range of reported survival of 1–1644 reported. 6 Survival times for B‐CLL are significantly shorter in the Boxer breed and those patients in which CD21+ B cells express higher levels of Ki67 (an indicator of cellular proliferation). 6
3. CONCLUSION
This case represents a unique ocular manifestation of a systemic disease. Ocular changes included hyphema, secondary glaucoma, retinal detachment, retinal hemorrhage, perivascular sheathing, and “box‐carring” as well as papilledema. It is considered likely that the ocular complications in this patient were multifactorial and related to a combination of direct neoplastic infiltration, hyperviscosity effects, anemia, hypertension, and thrombocytopenia. To the authors' knowledge, this is the first report of secondary glaucoma as a presenting complaint for chronic lymphocytic leukemia in a dog.
AUTHOR CONTRIBUTIONS
Shannon D. Dehghanpir involved in evaluation of clinical samples, interpretation of clinical pathology findings, table development, manuscript preparation, and review and editing. Colleen Sheridan involved in patient evaluation and case management, and initial manuscript preparation and review. Dawn Evans involved in collection and interpretation of diagnostic pathology samples and image collection, and manuscript review. Andrew C. Lewin involved in case review, image review, and manuscript preparation and review. Bonnie Boudreaux involved in patient evaluation and case management, and manuscript preparation, review, and editing. Renee T. Carter involved in patient evaluation and case management, image collection, and manuscript preparation, review, and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
None.
ETHICAL APPROVAL
This case report, and the patient care described, conforms to institutional ethical guidelines. Informed consent was obtained from the owner for all diagnostics, procedures, and treatment utilized in the management of this case.
CONSENT
Informed consent was obtained from the client to publish the material contained within this report.
ACKNOWLEDGMENT
None.
Dehghanpir SD, Sheridan C, Evans D, Lewin AC, Boudreaux B, Carter RT. Hyphema and secondary glaucoma as a presenting complaint in a dog with chronic lymphocytic leukemia. Clin Case Rep. 2022;10:e06575. doi: 10.1002/ccr3.6575
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Zandecki M, Genevieve F, Gerard J, Godon A. Spurious counts and spurious results on haematology analysers: a review. Part II: white blood cells, red blood cells, haemoglobin, red cell indices and reticulocytes. Int J Lab Hematol. 2007;29:21‐41. [DOI] [PubMed] [Google Scholar]
- 2. Workman HC, Vernau W. Chronic lymphocytic leukemia in dogs and cats: the veterinary perspective. Vet Clin Small Anim. 2003;33:1379‐1399. [DOI] [PubMed] [Google Scholar]
- 3. Bromberek JL, Rout ED, Agnew MR, Yoshimoto J, Morley PS, Avery AC. Breed distribution and clinical characteristics of B cell chronic lymphocytic leukemia in dogs. J Vet Intern Med. 2016;30:215‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vernau W, Moore PF. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet Immunol Immunopathol. 1999;69:145‐164. [DOI] [PubMed] [Google Scholar]
- 5. Calvert J. B‐cell chronic lymphocytic leukemia in a miniature American Eskimo dog. Can Vet J. 2019;60:1353‐1355. [PMC free article] [PubMed] [Google Scholar]
- 6. Rout ED, Labadie JD, Yoshimoto JA, Avery PR, Curran KM, Avery AC. Clinical outcome and prognostic factors in dogs with B‐cell chronic lymphocytic leukemia: a retrospective study. J Vet Intern Med. 2021;35:1918‐1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leifer CE, Matus RE. Lymphoid leukemia in the dog. Vet Clin North Am Small Anim Pract. 1985;15:723‐739. [DOI] [PubMed] [Google Scholar]
- 8. Krohne S, Henderson N, Richardson R. Prevalence of ocular involvement in dogs with multicentric lymphoma: prospective evaluation of 94 cases. Vet Comp Ophthalmol. 1994;4:127‐135. [Google Scholar]
- 9. Barron CN, Saunderls LZ, Jubb KV. Intraocular tumors in animals: III. Secondary intraocular tumors. Am J Vet Res. 1963;24:835‐853. [PubMed] [Google Scholar]
- 10. Cello RM, Hutcherson B. Ocular changes in malignant lymphoma of dogs. Cornell Vet. 1962;52:492‐523. [PubMed] [Google Scholar]
- 11. Buchan J, McKibbin M, Burton T. The prevalence of ocular disease in chronic lymphocytic leukaemia. Eye. 2003;17:27‐30. [DOI] [PubMed] [Google Scholar]
- 12. Kincaid MC, Green WR. Ocular and orbital involvement in leukemia. Surv Ophthalmol. 1983;27:211‐232. [DOI] [PubMed] [Google Scholar]
- 13. Carter KL, Hassan M, Do DV, et al. Acute lymphocytic leukemia masquerading as acute retinal necrosis. Am J Ophthalmol. 2020;18:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Violette NP, Ledbetter EC. Punctate retinal hemorrhage and its relation to ocular and systemic disease in dogs: 83 cases. Vet Ophthalmol. 2018;21(3):233‐239. [DOI] [PubMed] [Google Scholar]
- 15. Nagpal MP, Mehrotra NS, Mehta RC, Shukla CK. Leukemic optic nerve infiltration in a patient with acute lymphoblastic leukemia. Retin Cases Brief Rep. 2016;10(2):127‐130. [DOI] [PubMed] [Google Scholar]
- 16. Chinta S, Ranj PK, Manusani U. Bilateral exudative retinal detachment as a presenting sign of acute lymphoblastic leukemia. Middle East Afr J Ophthalmol. 2012;19(4):401‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leonardy NJ, Rupani M, Dent G, Klintworth GK. Analysis of 135 autopsy eyes for ocular involvement in leukemia. Am J Ophthalmol. 1990;109(4):436‐444. [DOI] [PubMed] [Google Scholar]
- 18. Lanza MR, Musciano AR, Dubielzig RD, Durham AC. Clinical and pathological classification of canine intraocular lymphoma. Vet Ophthalmol. 2018;21(2):167‐173. [DOI] [PubMed] [Google Scholar]
- 19. MacEwen EG, Hurvitz AI. Diagnosis and management of monoclonal gammopathies. Vet Clin North Am. 1977;7(1):119‐132. [DOI] [PubMed] [Google Scholar]
- 20. Berryman J, Moshiri A, Chang M. Chronic lymphocytic leukaemia presenting as branch retinal artery occlusion and optic disc infiltration. BMJ Case Rep. 2018;2018:bcr‐2018‐227691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers AP, Estes M. Hyperviscosity syndrome. StatPearls.NCBI Bookshelf. StatPearls Publishing; 2020:1‐5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


