Abstract
Background
Therapeutic strategies for unresectable hepatocellular carcinoma (u‐HCC) in geriatric patients are important for real‐world practice. However, there remain no established biomarkers or therapeutic strategies regarding the best second‐line agent after atezolizumab plus bevacizumab therapy.
Aim
In this study, we investigated the usefulness of modified Geriatric 8 (mG8) score in examining elderly patients (≥75 years old) with unresectable hepatocellular carcinoma (u‐HCC) using sorafenib or lenvatinib as first‐line therapy.
Methods and results
This study assessed 101 elderly patients with u‐HCC for their mG8 score (excluding elements of age from 8 items) and classified them into 2 groups according to their mG8 score: ≥11 as the high‐score group and ≤ 10 as the low‐score group. Among those taking sorafenib, no significant differences were noted in overall survival (OS) and progression free survival (PFS) between low and high mG8 score groups. Only modified albumin–bilirubin (ALBI) grade (2b/3 vs. 1/2a: HR 0.34; 95% CI, 0.17–0.69; p = .0029) was significantly associated with OS. Among those taking lenvatinib, patients with a high mG8 score (n = 26) had longer survival than those with a low mG8 score (n = 10) (20.0 months vs. 7.7 months: HR 0.31, 95% CI 0.11–0.89; p = .029). Intrahepatic tumor volume (<50% vs. ≥50%: HR 16.7; 95% CI, 1.71–163; p = .016) and α‐fetoprotein (AFP) (<400 vs. ≥400: HR 3.38; 95% CI 0.84–19.7; p = .031) remained significant factors independently associated with OS.
Conclusions
The mG8 score may contribute to making a decision when considering either sorafenib or lenvatinib as a treatment option for u‐HCC in elderly patients.
Keywords: 1, Geriatric 8 score, hepatocellular carcinoma 2, lenvatinib 4, sorafenib 3
1. BACKGROUND
Hepatocellular carcinoma (HCC) has been identified as the most common cancer in the liver and one of the leading causes of cancer‐related deaths worldwide. 1 HCC affects a heterogeneous population, but recently, the number of elderly cases has been increasing. Thus, therapeutic strategies for unresectable hepatocellular carcinoma (u‐HCC) in geriatric patients are important for real‐world practice. The International Society of Geriatric Oncology published a recommendation that elderly cancer patients should be evaluated via a geriatric assessment (GA). Included in the GA is the Geriatric 8 (G8) 2 screening tool consisting of eight items dealing with age, food intake, weight loss, mobility, neuropsychological problems, body mass index, prescription drugs, and a self‐perception of health using Mini‐Nutritional Assessment questionnaire. The G8 score ranges from 0 to 17, with a higher score suggestive of a better health status. G8 has already shown prognostic value regarding survival outcomes in elderly patients with various cancers and hematological malignancies. 3 , 4 , 5 , 6
For the treatment of u‐HCC, tyrosine kinase inhibitors (TKIs) such as sorafenib, 7 , 8 and regorafenib, 9 lenvatinib, 10 and cabozantinib 11 have been approved in many countries. Ramucirumab, 12 a vascular endothelial growth factor‐2 (VEGFR‐2) antibody, has also been administered in u‐HCC patients with high AFP levels (≥400 ng/mL) as second‐line treatment. Furthermore, combination therapy of atezolizumab plus bevacizumab 13 was approved in the United States and Japan in 2020 and has since become the first‐line treatment for u‐HCC. However, there remain no established biomarkers or therapeutic strategies regarding the best second‐line agent after atezolizumab plus bevacizumab therapy. In most cases, sorafenib or lenvatinib is chosen because these were the previous first‐line agents before combination therapy was recommended. Thus, in this study, we investigated the usefulness of mG8 in elderly u‐HCC patients who received sorafenib or lenvatinib as first‐line treatment and proposed a therapeutic strategy for such patients treated with TKI.
2. MATERIALS AND METHODS
2.1. Patients
From January 2014 to February 2021, 189 patients with u‐HCC received sorafenib or lenvatinib as first‐line therapy at Musashino Red Cross Hospital. Among these, a total of 101 elderly patients (aged ≥ 75 years) were included. The Japan Gerontological Society proposed the age classification in 2017. 14 The proposal included the definition that aged from 65 to 74 years was pre‐old age and aged over 75 years was old age. According to the proposal, we introduced a cutoff age of 75 years in this study. The diagnosis of HCC was based on the guidelines proposed by either the Liver Cancer Study Group of Japan, 15 the European Association for the Study of the Liver, 16 or the American Association for the Study of Liver Diseases. 17 The treatment for HCC was discussed by a tumor board. The study was conducted according to the guide‐lines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Musashino Red Cross Hospital (protocol code: 1099, June 17, 2020). Informed consent was obtained from all subjects involved in the study.
2.2. Treatment protocol
Sorafenib was administered from July 2009 until March 2018, and after April 2018, when lenvatinib became available, the first‐line agent was decided after discussing with the attending physician. As regards the standard doses of chemotherapy, sorafenib was given at 800 mg/day, while lenvatinib was given at 12 and 8 mg/day for patients ≥60 and <60 kg, respectively. We then reduced the drug's initial dose considering patients’ condition and modified this according to the presence of vascular endothelial growth factor‐2 (VEGFR‐2). Antitumor responses were determined according to the modified Response Evaluation Criteria in Solid Tumor (mRESIST), using dynamic computed tomography (CT) or magnetic resonance imaging (MRI) within 4–8 weeks and every 8 weeks thereafter. AEs were then graded at every visit based on Common Terminology Criteria for Adverse Events(CTCAE) version 5.0. Informed consent was obtained from all subjects involved in the study.
2.3. Evaluation of geriatric patients with the functional score
The G8 score 2 is normally used to assess elderly people, specifically regarding their functional decline, in the form of a multi‐item test. The G8 screening tool consists of eight items as follows: food intake, weight loss, mobility, neuropsychological problems, body mass index, medications, self‐impression about health, and age. In this study, we used a modified G8 (mG8) score, which consists of seven items dealing with food intake, weight loss, mobility, neuropsychological problem, body mass index, prescription drugs, and self‐perception of health. Age was excluded from our mG8 score, because age alone does not reflect functional status in the aging process. In this group, the median mG8 score was 11, so a score of ≥11 was defined as a high score, while ≤10 was a low score.
2.4. Statistical analysis
Statistical analyses were then performed using the EZR version 2.23 (Saitama Medical Centre, Jichi Medical University, Shimotsuke, Japan). 18 The primary endpoint was the duration of overall survival (OS). OS was measured as the time from starting treatment with sorafenib or lenvatinib until the date of death from any cause or censored at the last follow‐up date. OS was analyzed using the Kaplan–Meier method via the log‐rank test and Cox proportional hazards regression analysis. Outcome variables were analyzed using the χ 2 test or Fisher's exact test for categorical variables, while the Mann–Whitney U test was used for continuous variables. A Cox proportional regression hazard model was used to examine predictors of OS. A p‐value of <.05 was considered statistically significant.
3. RESULTS
3.1. Baseline patient characteristics
In total, 101 patients were enrolled in this study. The baseline characteristics of these patients were shown in Table 1; these were similar between sorafenib and lenvatinib patients, except for extrahepatic spread. There was no difference in the mG8 score between the two groups. The baseline characteristics according to mG8 score in each therapy group are shown in Tables S1 and S2.
TABLE 1.
Baseline characteristics
| Factor | Group | Sorafenib (n = 65) | Lenvatinib (n = 36) | p‐Value |
| BCLC stage, n (%) | A | 1 (1.5) | 0 (0.0) | 0.165 |
| B | 32 (49.2) | 24 (66.7) | ||
| C | 32 (49.2) | 12 (33.3) | ||
| 0 | 44 (67.7) | 21 (58.3) | 0.21 | |
| ECOG‐PS, n (%) | 1 | 18 (27.7) | 15 (41.7) | |
| 2 | 3 (4.6) | 0 (0.0) | ||
| Gender, n (%) | F | 20 (30.8) | 9 (25.0) | 0.648 |
| M | 45 (69.2) | 27 (75.0) | ||
| Age, years median (range) | 80 (75, 98) | 81 (75, 93) | 0.217 | |
| Etiology, n (%) | ALD | 8 (12.3) | 8 (22.2) | 0.487 |
| HBV | 3 (4.6) | 1 (2.8) | ||
| HBV + HCV | 1 (1.5) | 0 (0.0) | ||
| HCV | 38 (58.5) | 16 (44.4) | ||
| Others | 15 (23.1) | 11 (30.6) | ||
| Major vascular invasion (%) | No | 56 (86.2) | 32 (88.9) | 0.767 |
| Yes | 9 (13.8) | 4 (11.1) | ||
| Extrahepatic spread (%) | Yes | 27 (41.5) | 7 (19.4) | 0.029 |
| No | 38 (58.5) | 29 (80.6) | ||
| Tumor volume ≥ 50% (%) | No | 57 (89.1) | 33 (91.7) | 1 |
| Yes | 7 (10.9) | 3 (8.3) | ||
| Child‐Pugh score (%) | 5 | 30 (46.2) | 21 (60.0) | 0.464 |
| 6 | 27 (41.5) | 10 (28.6) | ||
| 7 | 7 (10.8) | 3 (8.6) | ||
| 8 | 1 (1.5) | 1 (2.9) | ||
| ALBI score, median (range) | −2.14 (−2.93, −1.33) | −2.36 (−3.21, −1.35) | 0.105 | |
| Baseline DCP, median (range) | 267 (8.90, 54 104) | 606 (10.2, 427 125) | 0.442 | |
| Baseline AFP median (range) | 36.6 (1.80, 69 100) | 30.4 (1.6, 97 455) | 0.633 | |
| mG8 score | 11 (8, 15) | 12 (8, 14) | 0.09 |
Abbreviations: BCLS stage: Barcelona Clinic Liver Cancer stage; ECOG‐PS: the Eastern Cooperative Oncology Group Scale of Performance Status; ALBI score: albumin–bilirubin score; DCP: des‐gamma‐carboxy prothrombin; AFP: α‐fetoprotein;mG8 score: modified Geriatric 8 score.
The median OS and PFS in all patients were 15.0 and 3.0 months, respectively. The median treatment duration of sorafenib and lenvatinib were 2.5 and 3.5 months, respectively. The median OS was 14.7 months (95% CI, 9.4–20.3) in the sorafenib group, while this was 20.0 months (95% CI, 9.8 to not reached) in the lenvatinib group, but this was not significantly different between the two (HR 0.69, 95% CI 0.38–1.26; p = .23) (Figure 1A). However, a significant difference was noted in PFS between the two groups (5.6 vs. 2.3 months, HR 0.57, 95% CI 0.34–0.95, p = .03) (Figure 1B). In the sorafenib group, the objective response rate (ORR) was 12.7%, and the disease control rate (DCR) was 65.5%. On the other hand, in the lenvatinib group, the ORR was 56.5%, while the DCR was 73.9%. The ORR was significantly better in the lenvatinib group (56.5% vs. 12.7%, p < .001), but there was no significant difference in DCR between the two groups (73.9% vs. 65.5%, p = .80).
FIGURE 1.
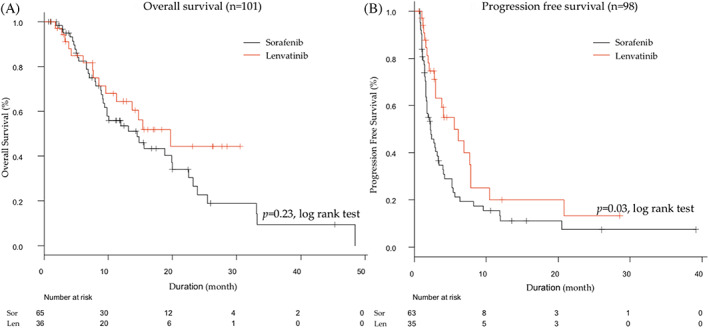
Comparison between sorafenib and lenvatinib patients in overall (A) and progression‐free survival (B)
3.2. AEs during the therapy
The AEs noted during sorafenib and lenvatinib treatment are shown in Table 2. The major AEs (≥40%) were hand‐foot skin reactions (HFSRs) (44.6%) and hypertension (43.1%) in sorafenib group, while these were hypertension (63.9%), general fatigue (58.3%), and appetite loss (52.8%) in the lenvatinib group. The discontinuation rate related to AEs was 30.8% for the sorafenib group and 44.4% for the lenvatinib group. We further classified sorafenib and lenvatinib into two groups according to their mG8 score, that is, the low‐score (mG8 ≤ 10) group and the high‐score (mG8 ≥ 11) group. In both sorafenib and lenvatnib groups, the total number of HFSRs was higher among patients with high mG8 scores (p = .003 and p = .016, respectively).
TABLE 2.
Adverse events
| Sorafenib | Lenvatinib | ||||||
|---|---|---|---|---|---|---|---|
| Total | mG8 low | mG8 high | Total | mG8 low | mG8 high | ||
| Adverse events | n = 65 | n = 22 | n = 43 | n = 36 | n = 10 | n = 26 | |
| Hypertension, n (%) | Any grade | 28 (43.1) | 7 (31.8) | 21 (48.8) | 23 (63.9) | 7 (70.0) | 16 (61.5) |
| Grades 3–4 | 1 (1.5) | 0 (0) | 1 (2.3) | 3 (8.3) | 0 (0) | 3 (11.5) | |
| Hand‐foot skin reactions, n (%) | Any grade | 29 (44.6) | 4 (18.2) a | 25 (58.1) a | 11 (30.6) | 0 (0) b | 11 (42.3) b |
| Grades 3–4 | 3 (4.6) | 0 (0) | 3 (7) | 0 (0) | 0 (0) | 0(0) | |
| Diarrhea, n (%) | Any grade | 12 (18.5) | 4 (18.2) | 8 (18.6) | 9 (25.0) | 2 (20.0) | 7 (26.9) |
| Grades 3–4 | 3 (4.6) | 2 (9.1) | 1 (2.3) | 2 (5.6) | 1 (10.0) | 1 (3.8) | |
| Liver dysfunction, n (%) | Any grade | 10 (15.4) | 3 (13.6) | 7 (16.3) | 8 (22.2) | 4 (40.0) | 4 (15.4) |
| Grades 3–4 | 2 (3.1) | 1 (4.5) | 1 (2.3) | 1 (2.8) | 0 (0) | 1 (3.8) | |
| Appetite loss, n (%) | Any grade | 23 (35.4) | 5 (22.7) | 18 (41.9) | 19 (52.8) | 6 (60.0) | 13 (50.0) |
| Grades 3–4 | 2 (3.1) | 1 (4.5) | 1 (2.3) | 1 (2.8) | 1(10.0) | 0 (0) | |
| Fatigue, n (%) | Any grade | 22 (33.8) | 10 (45.5) | 12 (27.9) | 21 (58.3) | 7 (70) | 14 (53.8) |
| Grades 3–4 | 1 (1.5) | 0 (0) | 1 (2.3) | 0(0) | 1 (3.58) | 0 (0) | |
The total number of hand‐foot skin reactions (HFSRs) was higher among patients in the high mG8 group (p = .003).
The total number of hand‐foot skin reactions (HFSRs) was higher among patients in the high mG8 group (p = .016).
3.3. The impact of modified G8 score in sorafenib and lenvatinib therapy
We classified all patients into two groups based on their mG8 scores: the low‐score (mG8 ≤ 10) group (n = 32) and the high‐score (mG8 ≥ 11) group (n = 69). There were no significant differences in OS and PFS between low‐ and high‐score groups (Figure 2A,B). We further analyzed OS and PFS according to the mG8 score in each therapy separately. In the sorafenib group, the median OS and PFS were 15.8 and 1.6 months in the low‐score group, respectively, while in the high‐score group, these were 15.0 and 2.9 months, respectively (Figure 3A,B). There were no significant differences in OS and PFS between high‐ and low‐score groups. In the lenvatinib group, patients with high mG8 scores (n = 26) had longer survival than those patients with a low mG8 score (n = 10) (20.0 months vs. 7.7 months, HR 0.31, 95% CI 0.11–0.89; p = .029) (Figure 3C). However, there were no significant differences in PFS between low‐ and high‐score groups (6.2 months vs. 5.6 months, p = .61) (Figure 3D).
FIGURE 2.
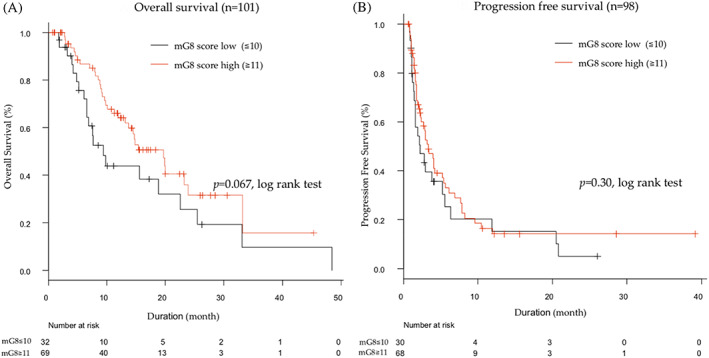
Overall survival and progression‐free survival outcomes in all patients. (A) Kaplan–Meier estimates of overall survival by mG8 scores. (B) Kaplan–Meier estimates of progression‐free survival by mG8 scores
FIGURE 3.
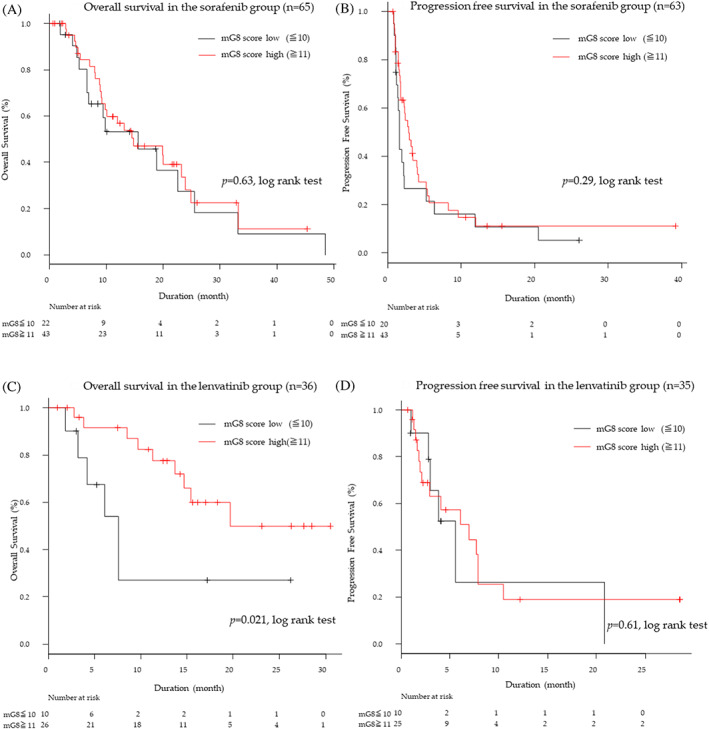
Overall survival and progression‐free survival outcomes in the sorafenib and lenvatinib group. (A) Kaplan–Meier estimates of overall survival in the sorafenib by mG8 scores. (B) Kaplan–Meier estimates of progression‐free survival in the sorafenib by mG8 scores. (C) Kaplan–Meier estimates of overall survival in the lenvatinib by mG8 scores. (D) Kaplan–Meier estimates of progression‐free survival in the lenvatinib by mG8 scores
We classified mG8 high score (mG8 ≥ 11) patients into two groups based on their therapeutic agents: sorafenib group (n = 43) and lenvatinib group (n = 26). There were no significant differences in OS and PFS between sorafenib and lenvatinib groups (Figure S1A,B). We further classified mG8 low score (mG8 ≤ 10) patients into two groups based on their therapeutic agents: sorafenib group (n = 22) and lenvatinib group (n = 10). There were no significant differences in OS and PFS between sorafenib and lenvatinib groups (Figure S1A,B).
3.4. Factors associated with PFS
In the univariate analysis of the sorafenib group, only the modified ALBI grade (2b/3 vs. 1/2a: HR 0.48; 95% CI 0.27–0.88; p = .017) was associated with PFS. Among the patients treated with lenvatinib, in the univariate analysis, intrahepatic tumor volume (<50% vs. ≥50%: HR 44.1; 95% CI 3.77–515; p = .0025), and AFP(<400 vs. ≥400: HR 2.95; 95% CI 1.16–7.51; p = .023) were associated with PFS. In the multivariate analysis, intrahepatic tumor volume (<50% vs. ≥50%: HR 24.8; 95% CI 2.04–301; p = .012) was only a significant factor (Table S3).
3.5. Factors associated with OS
In the univariate analysis of all patients, modified albumin–bilirubin (ALBI) grade 19 , 20 (2b/3 vs. 1/2a: HR 0.45; 95% CI, 0.26–0.78; p = .0047), intrahepatic tumor volume (<50% vs. ≥50%: HR 3.31; 95% CI 1.47–7.45; p = .0038), DCP (<400 vs. ≥400: HR 2.23; 95% CI 1.28–3.89; p = .0047), and AFP (<400 vs. ≥400: HR 1.90; 95% CI 1.02–3.53; p = .042) were all associated with OS. In the multivariate analysis, modified ALBI grade (2b/3 vs. 1/2a: HR 0.38; 95% CI 0.21–0.68; p = .0011), intrahepatic tumor volume (<50% vs. ≥50%: HR 2.80; 95% CI 1.16–6.76; p = 0.022), and pretreatment DCP (<400 vs. ≥400: HR 2.08; 95% CI 1.15–3.75; p = 0.016) remained independent prognostic factors for OS (Table 3).
TABLE 3.
Factors associated with OS
| All patients | sorafenib | lenvatinib | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
| Factor | HR(95% CI) | p‐Value | HR(95% CI) | p‐Value | HR(95% CI) | p‐Value | HR(95% CI) | p‐Value | HR(95% CI) | p‐Value | HR(95% CI) | p‐Value |
| Gender | ||||||||||||
| Female | 1 | 1 | 1 | |||||||||
| Male | 1.25(0.68–2.28) | 0.47 | 1.24(0.6–2.42) | 0.53 | 1.32(0.37–4.74) | 0.67 | ||||||
| Age | ||||||||||||
| 75–79 | 1 | 1 | 1 | |||||||||
| ≥80 | 1.26(0.72–2.2) | 0.43 | 1.22(0.64–2.33) | 0.55 | 1.11(0.38–3.27) | 0.84 | ||||||
| ECOG‐PS | ||||||||||||
| 1/2 | 1 | 1 | 1 | |||||||||
| 0 | 1.2(0.68–2.11) | 0.54 | 0.98(0.49–1.97) | 0.96 | 1.36(0.49–3.78) | 0.56 | ||||||
| Major vascular invasion | ||||||||||||
| VP0/1/2 | 1 | 1 | 1 | |||||||||
| VP3/4 | 1.88(0.83–4.22) | 0.13 | 2.01(0.77–5.28) | 0.16 | 1.81(0.4–8.18) | 0.44 | ||||||
| Extrahepatic spread | ||||||||||||
| Yes | 1 | 1 | 1 | |||||||||
| No | 0.97(0.55–1.71) | 0.9 | 1.03(0.54–1.98) | 0.92 | 1.16(0.33–4.12) | 0.82 | ||||||
| Modified ALBI grade | ||||||||||||
| 2b/3 | 1 | 1 | 1 | 1 | ||||||||
| 1/2a | 0.45(0.26–0.78) | 0.0047 | 0.38(0.21–0.68) | 0.0011 | 0.34(0.17–0.69) | 0.0029 | 0.78(0.25–2.46) | 0.67 | ||||
| mG8 score | ||||||||||||
| ≤10 | 1 | 1 | 1 | |||||||||
| ≥11 | 0.60(0.34–1.05) | 0.07 | 0.85(0.44–1.64) | 0.63 | 0.31(0.11–0.89) | 0.029 | 0.42(0.13–1.38) | 0.15 | ||||
| Intrahepatic tumor volume | ||||||||||||
| <50% | 1 | 1 | 1 | 1 | ||||||||
| ≥50% | 3.31(1.47–7.45) | 0.0038 | 2.80(1.16–6.76) | 0.022 | 2.44(0.94–6.35) | 0.067 | 13.6(2.19–84.3) | 0.0051 | 16.7(1.71–163) | 0.016 | ||
| DCP | ||||||||||||
| <400 mAU/mL | 1 | 1 | 1 | 1 | ||||||||
| ≥400 mAU/mL | 2.23(1.28–3.89) | 0.0047 | 2.08(1.15–3.75) | 0.016 | 1.64(0.87–3.1) | 0.13 | 4.99(1.36–18.3) | 0.015 | 3.92(0.96–16) | 0.057 | ||
| AFP | ||||||||||||
| <400 mAU/mL | 1 | 1 | 1 | 1 | 1 | |||||||
| ≥400 mAU/mL | 1.90(1.02–3.53) | 0.042 | 1.12(0.58–2.19) | 0.73 | 1.21(0.57–2.55) | 0.63 | 4.61(1.37–15.5) | 0.013 | 3.38(0.84–19.7) | 0.031 | ||
| TKI | ||||||||||||
| Sorafenib | 1 | |||||||||||
| Lenvatinib | 0.69(0.38–1.26) | 0.23 | ||||||||||
Abbreviations: ECOG‐PS: the Eastern Cooperative Oncology Group Scale of Performance Status; modified ALBI score: modified albumin–bilirubin score; mG8 score: modified Geriatric 8 score,DCP: des‐gamma‐carboxy prothrombin; AFP: α‐fetoprotein; TKI: tyrosine kinase inhibitor.
In the univariate analysis of the sorafenib group, only the modified ALBI grade (2b/3 vs. 1/2a: HR 0.34; 95% CI 0.17–0.69; p = .0029) was associated with OS, whereas there were no significant differences regarding gender, ECOG‐PS, age, major portal invasion, extrahepatic spread, extrahepatic tumor volume, AFP level, DCP level, and mG8 score (Table 3).
Among the patients treated with lenvatinib, in the univariate analysis, modified G8 score (≤10 vs. ≥11: HR0.31; 95% CI 0.11–0.89; p = .029), intrahepatic tumor volume (<50% vs. ≥50%: HR 13.6; 95% CI, 2.19–84.3; p = .0051), DCP (<400 vs. ≥400: HR 4.99; 95% CI 1.36–18.3; p = .015), and AFP(<400 vs. ≥400: HR 4.61; 95% CI 1.37–15.5; p = .013) were associated with OS. In the multivariate analysis, intrahepatic tumor volume (<50% vs. ≥50%: HR 16.7; 95% CI 1.71–163; p = 0.016), and AFP (<400 vs. ≥400: HR 3.38; 95% CI 0.84–19.7; p = 0.031) remained independent prognostic factors for OS (Table 3). In lenvatinib patients, patients with a high mG8 score survived significantly longer than patients with a low mG8 score. In lenvatinib group, there was no significant difference in the duration of medication, discontinuation rate due to AEs, and relative dose intensity during the initial 4 weeks of therapy, but transition rate to post‐treatment was higher in mG8 high‐score group than low‐score groups (Table 4).
TABLE 4.
Factors for treatment in lenvanitib group
| Factor | Low mG8 (n = 10) | High mG8 (n = 26) | p‐Value |
|---|---|---|---|
| Transition rate to posttreatment, n (%) | 4 (40.0) | 18 (78.3) | 0.049 |
| Discontinuation rate due to AEs, n (%) | 6 (60.0) | 10 (38.5) | 0.285 |
| 4W‐RDI, median (range) | 0.49 (0.07, 1.00) | 0.79 (0.02, 1.00) | 0.119 |
| Duration of medication, median (range) | 93.50 (4.00, 643) | 106.00 (1.00, 868) | 0.48 |
Abbreviation: 4W‐RDI: relative dose intensity during the initial 4 weeks of therapy.
4. DISCUSSION
To the best of our knowledge, this is the first study to show the usefulness of GA in elderly patients with u‐HCC treated with systemic sorafenib or lenvatinib as first‐line therapy. The median age of HCC patients has been rising because the number of patients with nonalcoholic steatohepatitis (NASH) has been increasing in many countries, 21 , 22 and such patients develop HCC at older ages compared to those with hepatitis B or C infection. The individual variations among these elderly patients were associated with the difficulty of deciding the most recommended therapy, because judging performance status is often more complicated than in nonelderly patients.
The usefulness of the G8 score in patients with cancer has already been reported in previous studies. Agemi et al. 23 reported that an impaired G8 score (≤14) was an independent prognostic factor for OS in lung cancer patients. Yamada et al. 24 demonstrated that a lower G8 score (<9.5) was associated with poorer self‐reliance rates in the 438 patients who had oral squamous cell carcinoma and were aged 75 years and older. In this study, we used the mG8 score, which consisted of seven variables, eliminating the effect of age, because in real‐world practice, there are super‐agers who maintain physical and mental ability in their 80s to 90s. Martinez‐Tapia et al. 25 already proposed a different mG8 score consisting of six items, including weight loss, cognition/mood, performance status, self‐rated health status, polypharmacy, and history of heart failure/coronary heart disease. Their mG8 score also did not include age. Recently, Kaibori, et al 26 reported perioperative maintenance of G8 score was an independent prognostic indicator for both RFS and OS in elderly HCC patients who received hepatectomy.
Our study revealed that in all patients, there was no significant difference in OS between groups with high and low mG8 scores. However, in the lenvatinib group, patients with low mG8 scores (≤10) had poorer OS than those with high mG8 scores (≥11). In the lenvatinib group, there was no significant difference in the duration of medication, discontinuation rate due to AEs, and relative dose intensity, but transition rate to posttreatment was higher in the high‐score group than in the low‐score group. Interestingly, our mG8 score was not a significant factor associated with OS in patients treated with sorafenib.
One of the speculations for the reason is inhibition of the fibroblast growth factor 19 (FGF19). It was already reported that FGF19 increased skeletal muscle mass and strength. 27 Lenvatinib inhibits fibroblast growth factor (FGF) receptors 1–4, and FGF19 is a tumor biomarker of lenvatinib‐susceptible HCC. 28 Using lenvatinib in patients with a high mG8 score would worsen general conditions, including muscle mass and strength, and may be associated with shorter OS.
In this study, the OS did not differ between patients treated with lenvatinib and sorafenib, although the PFS was significantly better in lenvaintib group than the sorafenib group. Similar results were already reported in the Phase 3 study of lenvatinib (REFLECT study) 10 and also in the study in which the OS and PFS were compared between sorafenib and lenvatinib after first‐line atezolizumab plus bevacizumab in advanced HCC patient. 29 There is still no understandable reason for the discrepancy, and prospective large cohort studies have been required.
In the treatment of advanced HCC, atezolizumab plus bevacizumab has become the first‐line therapy. However, there are no established biomarkers used as a basis for choosing the second‐line agent. According to a Phase 3 trial (REFLECT trial), 10 lenvatinib showed noninferiority in OS compared to sorafenib. Our mG8 score can aid in determining a recommended therapeutic strategy in elderly patients with u‐HCC.
The cut‐off value of AFP was defined as 400 ng/mL in most recent studies for advanced HCC, according to the REACH 30 and REACH‐2 study, 12 while there was no established cut‐off value of DCP in patients with unresectable HCC. In this study, we adapted the cut‐off value of DCP as 400 mAU/mL according to the previous studies, which had revealed the association between DCP 400 mAU/ml and prognosis after living‐donor liver transplantation 31 or liver resection. 32 These studies also showed the relationship between DCP 400mAU/ml and biological malignant potential including microvascular invasion and poorly differentiated HCC.
There are certain limitations to our study. The study was a retrospective, single‐center study, and the number of patients was small. All therapeutic decisions, including the initial dose, dose modification, interruption duration, and post‐sorafenib or lenvatinib therapies, were made by each individual investigator. Moreover, our modified G8 score was not validated in any other cohort, and the subgroup analysis should be carefully evaluated because of a minimal number.
In advanced HCC era, immuno‐combination therapy is recommended as the first line. Most advanced HCC patients were a candidate for sequential therapy with multiple molecular targeted agents. Modified G8 score would be a valuable tool to make a therapeutic strategy for the sequence. It was already reported that 64% of the geriatric patients took more than four kinds of drugs in Tokyo, Japan, while 32.3% of the 80–89 years patients was prescribed within 1–4 drug. 33 The G8 score was proposed in 2012, and the diversity in geriatric patients has been promoted. Even though our study had a small number of patients, the mG8 score would be meaningful in real‐world practice, especially in a super‐aging society. It is the first study about modified G8 score, and we used the median value of mG8 score as a cut‐off value. It is necessary to evaluate the cut‐off value of mG8 in a large cohort study.
EASL recommended considering the several factors, including patient characteristics and comorbidities, when we selected a second‐line agent. 34 We think the mG8 score is one of the patient characteristics, which may contribute to making a therapeutic strategy in geriatric patients with unresectable HCC. Further studies with a large cohort should be conducted to provide the clinical value of mG8 score. This study was the first report to reveal the usefulness of the GA in elderly patients with unresectable HCC who received systemic therapy. These results can help inform therapeutic strategies based on an individual patient's status. Further investigation in a larger cohort is thus necessary to establish the recommended therapies in each patient. Our results suggest that the grouping according to mG8 score is useful for selecting a TKI for elderly patients with HCC. In the manuscript about updated BCLC staging system (BCLC 2022), a multiparametric evaluation at multidisciplinary tumor boards is recommended, 35 and a modified G8 score would become a useful parameter, especially in aged or super‐aged societies.
5. CONCLUSIONS
The mG8 score may contribute to making a decision when considering either sorafenib or lenvatinib as a treatment option for u‐HCC in elderly patients.
AUTHOR CONTRIBUTIONS
Kaoru Tsuchiya: Conceptualization (equal); data curation (equal); methodology (equal); project administration (equal); resources (equal); writing – original draft (equal). Yutaka Yasui: Resources (equal). Kento Inada: Resources (equal). Sakura Kirino: Resources (equal). Koji Yamashita: Resources (equal). Yuka Hayakawa: Resources (equal). Leona Osawa: Resources (equal). Mayu Higuchi: Resources (equal). Kenta Takaura: Resources (equal). Chiaki Maeyashiki: Resources (equal). Shun Kaneko: Resources (equal). Nobuharu Tamaki: Resources (equal). Hiroyuki Nakanishi: Resources (equal). Jun Itakura: Resources (equal). Yuka Takahashi: Resources (equal). Yasuhiro Asahina: Supervision (equal). Ryuichi Okamoto: Supervision (equal). Masayuki Kurosaki: Funding acquisition (equal); supervision (equal); validation (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
Kaoru Tsuchiya, Masayuki Kurosaki, and Namiki Izumi received advisory board fees and honoraria for speakers' bureau from Bayer, Eli Lilly Japan, Chugai Pharmaceutical Company, and Eisai. The Japanese Ministry of Health, Labour and Welfare had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
INFORMED CONSENT STATEMENT
Informed consent was obtained from all subjects involved in the study.
ETHICS STATEMENT
The study was conducted according to the guide‐lines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Musashino Red Cross Hospital (protocol code: 1099, June 17, 2020).
Supporting information
Table S1 Baseline sorafenib patient characteristics
Table S2. Baseline lenvatinib patient characteristics
Table S3. Factors associated with PFS
Figure S1 Overall survival and progression‐free survival outcomes in the high mG8 score group. (A) Kaplan–Meier estimates of overall survival by TKI. (B) Kaplan–Meier estimates of progression‐free survival by TKI.
Figure S2. Overall survival and progression‐free survival outcomes in the low mG8 score group. (A) Kaplan–Meier estimates of overall survival by TKI. (B) Kaplan–Meier estimates of progression‐free survival by TKI.
ACKNOWLEDGMENTS
The authors extend our gratitude to Shinobu Kobori for assistance.
Sekiguchi S, Tsuchiya K, Yasui Y, et al. Clinical usefulness of geriatric assessment in elderly patients with unresectable hepatocellular carcinoma receiving sorafenib or lenvatinib therapy. Cancer Reports. 2022;5(11):e1613. doi: 10.1002/cnr2.1613
Shuhei Sekiguchi and Kaoru Tsuchiya contributed equally to this work.
Funding information Ministry of Health, Labour and Welfare, Grant/Award Numbers: JP19fk0210025h0003, JP21fk0210067h0002
DATA AVAILABILITY STATEMENT
Data Availability Statment: The data presented in this study are available upon request from the corresponding author.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Bellera CA, Rainfray M, Mathoulin‐Pelissier S, et al. Screening older cancer patients: first evaluation of the G‐8 geriatric screening tool. Ann Oncol. 2012;23(8):2166‐2172. [DOI] [PubMed] [Google Scholar]
- 3. Ishii R, Ogawa T, Ohkoshi A, Nakanome A, Takahashi M, Katori Y. Use of the Geriatric‐8 screening tool to predict prognosis and complications in older adults with head and neck cancer: a prospective, observational study. J Geriatr Oncol. 2021;12(7):1039‐1043. [DOI] [PubMed] [Google Scholar]
- 4. Middelburg JG, Middelburg RA, van Zwienen M, et al. Impaired geriatric 8 score is associated with worse survival after radiotherapy in older patients with cancer. Clin Oncol (R Coll Radiol). 2021;33(4):e203–e10. [DOI] [PubMed] [Google Scholar]
- 5. van Walree IC, Scheepers E, van Huis‐Tanja L, et al. A systematic review on the association of the G8 with geriatric assessment, prognosis and course of treatment in older patients with cancer. J Geriatr Oncol. 2019;10(6):847‐858. [DOI] [PubMed] [Google Scholar]
- 6. Sakurai M, Karigane D, Kasahara H, et al. Geriatric screening tools predict survival outcomes in older patients with diffuse large B cell lymphoma. Ann Hematol. 2019;98(3):669‐678. [DOI] [PubMed] [Google Scholar]
- 7. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378‐390. [DOI] [PubMed] [Google Scholar]
- 8. Cheng A‐L, Kang Y‐K, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia‐Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double‐blind, placebo‐controlled trial. Lancet Oncol. 2009;10(1):25‐34. [DOI] [PubMed] [Google Scholar]
- 9. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double‐blind, placebo‐controlled, phase 3 trial. The Lancet. 2017;389(10064):56‐66. [DOI] [PubMed] [Google Scholar]
- 10. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. The Lancet. 2018;391(10126):1163‐1173. [DOI] [PubMed] [Google Scholar]
- 11. Abou‐Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu AX, Kang Y‐K, Yen C‐J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α‐fetoprotein concentrations (REACH‐2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282‐296. [DOI] [PubMed] [Google Scholar]
- 13. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894‐1905. [DOI] [PubMed] [Google Scholar]
- 14. Ouchi Y, Rakugi H, Arai H, et al. Redefining the elderly as aged 75 years and older: proposal from the joint Committee of Japan Gerontological Society and the Japan geriatrics society. Geriatr Gerontol Int. 2017;17(7):1045‐1047. [DOI] [PubMed] [Google Scholar]
- 15. Kokudo N, Hasegawa K, Akahane M, et al. Evidence‐based clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2013 update (3rd JSH‐HCC guidelines). Hepatol Res. 2015;45(2):n/a. [DOI] [PubMed] [Google Scholar]
- 16. European Association for the Study of the Liver , Electronic address eee, European Association for the Study of the L . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182‐236. [DOI] [PubMed] [Google Scholar]
- 17. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358‐380. [DOI] [PubMed] [Google Scholar]
- 18. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence‐based approach‐the ALBI grade. J Clin Oncol. 2015;33(6):550‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hiraoka A, Michitaka K, Kumada T, et al. Validation and potential of albumin‐bilirubin grade and prognostication in a Nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6(4):325‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagaoki Y, Hyogo H, Aikata H, et al. Recent trend of clinical features in patients with hepatocellular carcinoma. Hepatol Res. 2012;42(4):368‐375. [DOI] [PubMed] [Google Scholar]
- 22. Sagnelli E, Macera M, Russo A, Coppola N, Sagnelli C. Epidemiological and etiological variations in hepatocellular carcinoma. Infection. 2020;48(1):7‐17. [DOI] [PubMed] [Google Scholar]
- 23. Agemi Y, Shimokawa T, Sasaki J, et al. Prospective evaluation of the G8 screening tool for prognostication of survival in elderly patients with lung cancer: a single‐institution study. PLoS One. 2019;14(1):e0210499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamada SI, Hasegawa T, Okuyama K, et al. Clinical significance of the G8 screening tool in elderly patients with oral squamous cell carcinoma. Clin Oral Investig. 2020;24(6):1953‐1961. [DOI] [PubMed] [Google Scholar]
- 25. Martinez‐Tapia C, Canoui‐Poitrine F, Bastuji‐Garin S, et al. Optimizing the G8 screening tool for older patients with cancer: diagnostic performance and validation of a six‐item version. Oncologist. 2016;21(2):188‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaibori M, Matsushima H, Ishizaki M. Perioperative geriatric assessment as a predictor of long‐term hepatectomy outcomes in elderly patients with hepatocellular carcinoma. Cancers. 2021;13(4):842. doi: 10.3390/cancers13040842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benoit B, Meugnier E, Castelli M, et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med. 2017;23(8):990‐996. [DOI] [PubMed] [Google Scholar]
- 28. Myojin Y, Kodama T, Maesaka K, et al. ST6GAL1 is a novel serum biomarker for Lenvatinib‐susceptible FGF19‐driven hepatocellular carcinoma. Clin Cancer Res. 2021;27(4):1150‐1161. [DOI] [PubMed] [Google Scholar]
- 29. Yoo C, Kim JH, Ryu MH, et al. Clinical outcomes with multikinase inhibitors after progression on first‐line Atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: a multinational multicenter retrospective study. Liver Cancer. 2021;10(2):107‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu AX, Park JO, Ryoo B‐Y, et al. Ramucirumab versus placebo as second‐line treatment in patients with advanced hepatocellular carcinoma following first‐line therapy with sorafenib (REACH): a randomised, double‐blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859‐870. [DOI] [PubMed] [Google Scholar]
- 31. Fujiki M, Takada Y, Ogura Y, et al. Significance of des‐gamma‐carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant. 2009;9(10):2362‐2371. [DOI] [PubMed] [Google Scholar]
- 32. Matsukuma S, Sakamoto K, Tokuhisa Y, et al. Outcomes following liver resection for multinodular Barcelona clinic liver cancer‐B hepatocellular carcinoma. Oncol Lett. 2018;16(5):6383‐6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ishizaki T, Mitsutake S, Hamada S, et al. Drug prescription patterns and factors associated with polypharmacy in >1 million older adults in Tokyo. Geriatr Gerontol Int. 2020;20(4):304‐311. [DOI] [PubMed] [Google Scholar]
- 34. Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol. 2021;75(4):960‐974. [DOI] [PubMed] [Google Scholar]
- 35.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76(3):681‐693. doi: 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Baseline sorafenib patient characteristics
Table S2. Baseline lenvatinib patient characteristics
Table S3. Factors associated with PFS
Figure S1 Overall survival and progression‐free survival outcomes in the high mG8 score group. (A) Kaplan–Meier estimates of overall survival by TKI. (B) Kaplan–Meier estimates of progression‐free survival by TKI.
Figure S2. Overall survival and progression‐free survival outcomes in the low mG8 score group. (A) Kaplan–Meier estimates of overall survival by TKI. (B) Kaplan–Meier estimates of progression‐free survival by TKI.
Data Availability Statement
Data Availability Statment: The data presented in this study are available upon request from the corresponding author.


