Abstract
BACKGROUND & AIMS:
Improved therapy has substantially increased survival of persons with cystic fibrosis (CF). But the risk of colorectal cancer (CRC) in adults with CF is 5–10 times greater compared to the general population, and 25–30 times greater in CF patients after an organ transplantation. To address this risk, the CF Foundation convened a multistakeholder task force to develop CRC screening recommendations.
METHODS:
The 18-member task force consisted of experts including pulmonologists, gastroenterologists, a social worker, nurse coordinator, surgeon, epidemiologist, statistician, CF adult, and a parent. The committee comprised 3 workgroups: Cancer Risk, Transplant, and Procedure and Preparation. A guidelines specialist at the CF Foundation conducted an evidence synthesis February–March 2016 based on PubMed literature searches. Task force members conducted additional independent searches. A total of 1159 articles were retrieved. After initial screening, the committee read 198 articles in full and analyzed 123 articles to develop recommendation statements. An independent decision analysis evaluating the benefits of screening relative to harms and resources required was conducted by the Department of Public Health at Erasmus Medical Center, Netherlands using the Microsimulation Screening Analysis model from the Cancer Innervation and Surveillance Modeling Network. The task force included recommendation statements in the final guideline only if they reached an 80% acceptance threshold.
RESULTS:
The task force makes 10 CRC screening recommendations that emphasize shared, individualized decision-making and familiarity with CF-specific gastrointestinal challenges. We recommend colonoscopy as the preferred screening method, initiation of screening at age 40 years, 5-year re-screening and 3-year surveillance intervals (unless shorter interval is indicated by individual findings), and a CF-specific intensive bowel preparation. Organ transplant recipients with CF should initiate CRC screening at age 30 years within 2 years of the transplantation because of the additional risk for colon cancer associated with immunosuppression.
CONCLUSIONS:
These recommendations aim to help CF adults, families, primary care physicians, gastroenterologists, and CF and transplantation centers address the issue of CRC screening. They differ from guidelines developed for the general population with respect to the recommended age of screening initiation, screening method, preparation, and the interval for repeat screening and surveillance.
Keywords: Cystic Fibrosis, CFTR, Colon, Rectum, Large Bowel, Intestine, Cancer, Screening, Colonoscopy, Recommendations, Cost-Effectiveness Analysis
Continued therapeutic advances have impressively increased longevity in cystic fibrosis (CF). More than half of the individuals with CF captured in the CF Foundation patient registry are over the age of 18 years. The current median predicted survival is 41 years, and persons born in 2015 have an estimated average life expectancy of 45 years.1 The increasing longevity of adults with CF puts them at risk for other diseases, such as gastrointestinal cancer.
Recent reports show that individuals with CF have an increased risk of colorectal cancer (CRC) compared to age-matched individuals without CF.2-5 The rising risk of CRC with age and the increasing lifespan of individuals with CF imply that the number of cases of CRC in this population will climb.
In 2013, Maisonneuve et al3 published a cohort study based on data from the CF Foundation patient registry covering the years 1990–2009. Based on incidence rates of cancer estimated using data on the general population from the Surveillance, Epidemiology and End Results Program of the National Cancer Institute, this study revealed a standardized incidence ratio of 6.2 for colon cancer (95% confidence interval, 4.2–9.0) in non-transplanted CF persons based on 26 cases. Our literature review disclosed multiple anecdotal published reports (single cases or case series) of CF patients with colon or rectal cancer. All publications found that nearly all CRC develop in persons less than age 50 years, implying that initiating screening at age 50 years, the current recommendation for the average risk non-CF population, may not be appropriate for the CF population. Limited data showed that the majority of tumors arose in the right colon.6-17
Only sparse data exist on systematic colonoscopic screening of individuals with CF.18,19 Colonoscopy screening of CF adults aged 40 years or older at the University of Minnesota found that approximately 25% of individuals had one or more advanced adenomas, as defined by size ≥1 cm and histologic findings.19 The majority of these individuals had more than 3 adenomatous polyps on their index screening colonoscopy. Furthermore, either rescreening or surveillance colonoscopies at 1- to 3-year intervals continued to show high incidence of adenomatous polyps, including those with advanced features. These results suggest earlier development and accelerated progression of adenomatous polyps in CF.
The mechanisms responsible for increased risk of CRC in CF are unclear. However, the CFTR gene is a tumor suppressor gene in the intestinal tract in mice, where loss of CFTR is associated with intestinal tumor formation.20,21 CFTR promotes secretion of chloride and bicarbonate, and plays critical roles in epithelial homeostasis in the gastrointestinal tract.22,23 Decreased level of hydration of the mucus layer likely contributes to bacterial overgrowth at the mucosal surface, which might result in greater tonic stimulation of epithelia.24-26 Absence of CFTR is associated with dysregulation of the immune response, intestinal stem cells, and growth signaling regulators.20 Notably, loss of CFTR expression in early stage human CRC in non-CF patients is associated with markedly decreased disease-free survival.20
Based on available evidence, the average age of onset of CRC in CF persons is approximately 40 years, or about 20 to 30 years younger than in the non-CF population.2 Furthermore, when compared to US data from Surveillance, Epidemiology and End Results Program studies, the incidence of CRC in the CF population at age 40–49 years is similar to the incidence of CRC in 65- to 69-year-old persons in the non-CF population.27
Recognizing the urgency and importance that the combination of increased life expectancy and a large age-related increased risk of CRC creates, the CF Foundation created a task force to develop CRC screening recommendations for adults with CF, including both the transplant and non-transplant population.
Methodology
The CRC screening task force convened in April 2015 at the CF Foundation Headquarters. The 18-member task force consisted of pulmonologists, gastroenterologists, a social worker, nurse coordinator, surgeon, epidemiologist, statistician, adult with CF, and a parent of a child with CF. The committee was divided into 3 workgroups: Cancer Risk, Transplant, and Procedure and Preparation.
At the initial meeting, the task force determined the scope of the document; developed (PICO) Population, Intervention, Comparison, and Outcome questions; and determined relevant search terms. The American Gastroenterological Association Governing Board reviewed and approved the PICO questions.
PubMed was searched for relevant, published, articles in February–March 2016. The search terms used can be found in the Supplementary Material. Task force members also conducted their own independent searches.
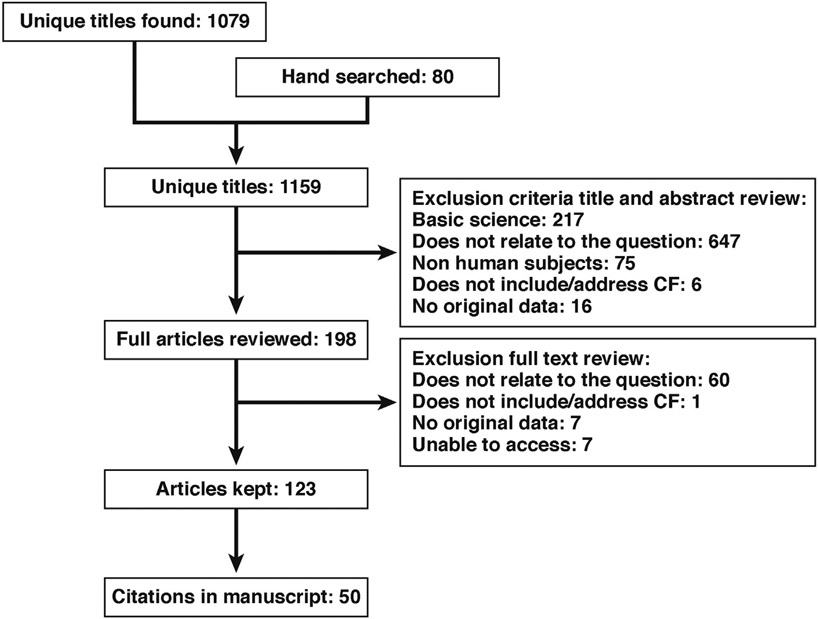
A total of 1159 articles were retrieved. Of these articles, 198 articles were read at the full-text level. In total, 50 articles were included in the final manuscript (Figure 1).
Figure 1.
Search strategy diagram.
The initial review of the literature at the title level was conducted by a guidelines specialist at the CF Foundation and task force members. Task force members then reviewed the relevant abstracts and full articles. After a thorough review of the records, each workgroup drafted recommendation statements based on their PICO questions. Ten recommendation statements were considered and voted on, and an 80% agreement threshold was agreed on before the meeting.
Modeling
The CF Foundation collaborated with the Department of Public Health of Erasmus Medical Center, University Medical Center Rotterdam, Netherlands, to conduct modeling on CRC screening strategies for adults with CF. The goal of the modeling was to provide estimates of the benefits and costs of screening in the CF population given that life expectancy and CRC risk differ from the general population. Data including age distribution and life expectancy used for this modeling came were calculated using 2010–2014 CF Foundation Patient Registry data.28 Most of the excess mortality risk in CF is not due to CRC. The modeling was conducted using the MISCAN-Colon model, which is part of the Cancer Intervention and Surveillance Modeling Network.29
In addition to the 10 listed recommendations drawn up by the task force, the modeling group developed a unique program to determine the most effective strategy for screening this high-risk population. A brief description of the methodology and main findings of the modeling procedures are presented here, more detailed information is available in a separate report in this journal.30
Microsimulation Screening Analysis-Colon Model
The existing Microsimulation Screening Analysis-Colon model for the general US population was adjusted to reflect the increased CRC risk and the elevated all-cause mortality in CF patients. Two versions of this model were developed: one for CF patients with an organ transplant and the other for CF patients without an organ transplant. Subsequently, we used these models to predict the benefits, harms, and resources associated with implementation of 101 different screening strategies that varied by test (colonoscopy or fecal immunochemical test [FIT]), age to begin and end screening, and screening interval. We used incremental cost-effectiveness analysis to determine the impact of various screening strategies for the CF population with and without organ transplant.
External Review
The manuscript and recommendation statements were distributed to the CF Community, Lung Transplant Community, and Gastrointestinal Community through CF Foundation, International Society of Heart and Lung Transplantation and American Gastroenterological Association listservs reviewers were given 2 weeks to submit comments and feedback using an online survey tool, Survey Monkey.31 Committee chairs reviewed and responded to comments and updated the manuscript.
Results
The task force found limited information on CRC screening in adults with CF, and available reports consisted mostly of retrospective reviews of patient records and case–control studies. The task force identified no randomized clinical trials comparing results in screened vs nonscreened patients or reports comparing results of colonoscopy with results of less-invasive screening procedures.
The task force developed ten recommendations (Table 1). Based on the quality and limited number of studies, the recommendations represent the consensus opinion of the task force. All but 2 statements reached 100% agreement; recommendations 4 and 7 reached 94% agreement. The American Gastroenterological Association reviewed and agrees with the recommendations in this statement.
Table 1.
Recommendation Statements
| Consensus Recommendation Statement | Votes | % Agreement | |
|---|---|---|---|
| 1 | The CF Foundation recommends that all decisions on colorectal cancer screening and surveillance in individuals with CF be based on shared decisions between the provider and individual with CF about: treatment, comorbidities, safety, and quality of life. | 18/18 | 100 |
| 2 | The CF Foundation recommends that all colorectal cancer screening and surveillance for individuals with CF are jointly managed by CF health care professionals and an endoscopist. | 18/18 | 100 |
| 3 | The CF Foundation recommends colonoscopy as the screening examination for CRC in individuals with CF. | 18/18 | 100 |
| 4 | The CF Foundation concludes that the evidence is insufficient to recommend the use of computed tomography colonography, stool-based tests, or flexible sigmoidoscopy in individuals with CF for the purpose of CRC screening. | 17/18 | 94 |
| 5 | The CF Foundation recommends that CRC screening begin at age 40 y in individuals with CF with continued rescreening every 5 y. | 18/18 | 100 |
| 6 | The CF Foundation recommends that individuals with CF who have undergone a colonoscopy that had any adenomatous polyps have surveillance colonoscopy in 3 y, unless a shorter interval is indicated by individual findings, with subsequent intervals based on the most recent endoscopic examination. | 18/18 | 100 |
| 7 | The CF Foundation recommends that individuals with CF who are 30 years of age and older and have adequately recovered after receiving a solid organ transplantation begin CRC screening within 2 years of transplantation, except when they have had a negative colonoscopy within the past 5 y. | 17/18 | 94 |
| 8 | The CF Foundation recommends continued CRC rescreening every 5 y in individuals with CF who have received a solid organ transplant. | 18/18 | 100 |
| 9 | The CF Foundation recommends that individuals with CF who have undergone a solid organ transplantation and had colonoscopy that had any adenomatous polyps have surveillance colonoscopy in 3 years, unless a shorter interval is indicated by individual findings, with subsequent intervals based on the most recent endoscopic examination. | 18/18 | 100 |
| 10 | The CF Foundation recommends that adults with CF undergoing a colonoscopy receive intensive regimens for bowel preparation to allow for optimal examination. The intensive regimen should include: 3–4 washes (minimum of 1 L purgative per wash) with the last wash occurring within 4–6 h before the examination. | 18/18 | 100 |
Results of Microsimulation Screening Analysis–Colon Cancer Model for Colonoscopy
The Microsimulation Screening Analysis-Colon model predicted that in non-transplant CF patients, the optimal colonoscopy strategy was colonoscopy every 5 years between age 40 and 75 years. This strategy resulted in 44 extra years of life per 1000 CF patients screened at a net cost of $0.7 million compared to no screening. The incremental costs per life-year gained (LYG) compared to a similar strategy that ended screening at age 70 were $84,000 per LYG.30
For CF patients with an organ transplant, the optimal colonoscopy strategy was colonoscopy every 3 years from age 35 to 55 years. Age 55 years was utilized because of the small population of individuals over the age of 55 years. The recommendations below have no upper age limit. This strategy resulted in 56 extra life-years per 1000 patients screened at a net cost of $1.3 million compared to no screening. The incremental costs per LYG compared to colonoscopy every 5 years in the same age range were $71,000. However, the optimal colonoscopy interval was sensitive to the age of transplant (optimal interval of 5 and 10 years for organ transplant ages of 25 and 20 years, respectively, and beginning screening at 30 years). The task force additionally considered the modeling projection that 64 extra life-years were gained compared to no screening if colonoscopy was started at age 30 years. This more than doubled cost to $166,000 per LYG. Because transplantation is already an expensive and high-risk procedure, the committee judged that improving the likelihood of achieving the potentially high benefit by reducing deaths from CRC was consistent with the original intent to undergo transplantation. In both cohorts (organ transplant and non-transplant), the upper age limit was a result of a small number of individuals in these age groups rather than lack of efficacy based on modeling.30
Recommendations
1. The Cystic Fibrosis Foundation recommends that all decisions on colorectal cancer screening and surveillance in individuals with cystic fibrosis be based on shared decisions between the provider and individual with cystic fibrosis about treatment, comorbidities, safety, and quality of life.
The decision to proceed with screening is more complicated in the CF population than in the non-CF population. The complexity arises from several issues: (1) although individuals with CF are at considerably higher risk of developing CRC than non-CF persons, all other factors being equal, the overall survival may be limited in certain subgroups of people with CF due to serious concurrent comorbidities32; (2) preparation for colonoscopy (see below) is more time-consuming in individuals with CF than in other populations18; (3) the risk associated with colonoscopy in the general population is low but may be increased in individuals with CF33; and (4) other modalities, such as FIT, which may be suitable for the general population, have not been evaluated in the CF population. Given these complexities, the task force members unanimously agreed that individuals with CF and their providers should engage in a shared decision-making process to carefully assess the risks and benefits of CRC screening and its impact on the health and quality of life for the adult with CF. Importantly, screening and surveillance recommendations presented in this manuscript were developed for asymptomatic CF patients. Physicians should recognize that CF is a colon cancer syndrome and consider diagnostic evaluation when patients present with new, suggestive symptoms or laboratory abnormalities.
2. The Cystic Fibrosis Foundation recommends that all colorectal cancer screening and surveillance for individuals with cystic fibrosis are jointly managed by cystic fibrosis health care professionals and an endoscopist.
Ideally, colonoscopic screening is performed by a CF-trained endoscopist familiar with the multiple gastrointestinal challenges in the CF patient population. However, many CF Foundation–accredited care centers do not have a dedicated adult gastroenterologist. Individuals with CF are often referred to an endoscopist who may have limited experience in CF and may be unfamiliar with the unique preparation required for CRC screening. Further management may be provided by a gastroenterologist, endoscopist, and other care providers. To ensure CF-specific CRC screening issues are addressed, the task force recommends that close cooperation between the CF care provider and an endoscopist must precede and continue after completion of the CRC screening.
3. The Cystic Fibrosis Foundation recommends colonoscopy as the screening examination for colorectal cancer in individuals with cystic fibrosis.
Recommendations for the method of screening differ depending upon the risk category of the person or group selected for screening34 Colonoscopy, unlike other screening procedures, can detect as well as remove polyps. This is one of the main reasons why colonoscopy is the screening procedure of choice for other high-risk groups, for example, Lynch syndrome.35 Similar reasoning applies to CF, which is another genetically determined high-risk group. In addition, there are no published data of utilization of other modalities in individuals with CF.
4. The Cystic Fibrosis Foundation concludes that the evidence is insufficient to recommend the use of computed tomography colonography, stool-based tests, or flexible sigmoidoscopy in individuals with cystic fibrosis for the purpose of colorectal cancer screening.
The task force found no published evidence on the utility of any of these procedures in CRC screening for individuals with CF. Given the high prevalence of polyps in the CF population, the pretest probability of requiring a colonoscopy after any other type of screening procedure is high. Stool-based tests, such as FIT, or perhaps multitargeted stool DNA testing, might be valuable as a first-line test, as suggested by the results obtained by modeling, but they have never been evaluated in the CF population. Until further data are available, the task force cannot recommend for or against FIT testing. If FIT test characteristics (ie, specificity and sensitivity) are similar to the general population, these tests could be useful for the CF population. Sigmoidoscopy alone examines only the descending colon and is unsuitable in the CF population because of the increased frequency of polyps and cancers beyond the reach of this instrument.3,36 Similarly, computed tomography colonography cannot be recommended.
5. The Cystic Fibrosis Foundation recommends that colorectal cancer screening begin at age 40 years in individuals with cystic fibrosis with continued re-screening every 5 years.
Colonoscopic screening starting at age 40 years has demonstrated high frequency of adenomatous polyps, including advanced adenomas.19 Although starting screening at age 30 years may uncover polyps and even some tumors, screening has to balance the risk of screening with the benefits of early detection. With respect to the screening interval, approximately half of the patients with negative index screening colonoscopies developed adenomatous polyps including advanced adenomas, within 5 years.19 Given the high recurrence rate of adenomas and advanced adenomas, an interval of 5 years for re-screening, compared to 10 years in the general population, is recommended for individuals with CF. The recommendation to initiate screening with colonoscopy at age 40 years is consistent with and supported by the modeling estimates.
6. The Cystic Fibrosis Foundation recommends that individuals with cystic fibrosis who have undergone a colonoscopy that had any adenomatous polyps have surveillance colonoscopy in 3 years, unless a shorter interval is indicated by individual findings, with subsequent intervals based on the most recent endoscopic examination.
The shorter intervals (3 years) for most surveillance examinations recommended for individuals with CF vs 5 years in the general population are similar to recommendations for patients with other genetic colon cancer syndromes.35 However, the CF Foundation recommendation for a 3-year interval for surveillance of patients with multiple or advanced polyps does not differ from the general population with the same findings.37 The recommendation takes into consideration the burden of a more intensive bowel preparation regimen that is necessary for colonoscopic examinations in CF patients and additional commonly present comorbidities. The endoscopist should also consider quality of the colon preparation and level of confidence that all polyp tissue has been resected in providing individualized recommendations for surveillance interval and utilize a shorter interval when appropriate.
7. The Cystic Fibrosis Foundation recommends that individuals with cystic fibrosis who are 30 years of age and older and have adequately recovered after receiving a solid organ transplant begin colorectal cancer screening within 2 years of transplantation except when they have had a negative colonoscopy within the past 5 years.
Although the absolute risk of CRC in individuals with CF is extremely low for patients younger than 30 years, the risk of CRC in individuals with CF greatly increases after lung transplantation, with the risk being 25–30 times the age-adjusted baseline.2-4,19,36 Median survival of transplant patients, especially those patients that survive the first postoperative year, has now increased beyond 10 years.38 Increased post-transplantation survival means that many transplant patients will enter older age groups where there is an increased risk of cancer. Screening should be performed after recovery from the operation and within 2 years after the procedure, unless they have had a negative colonoscopy within the previous 5 years.
8. The Cystic Fibrosis Foundation recommends continued colorectal cancer rescreening every 5 years in individuals with cystic fibrosis who have received a solid organ transplant.
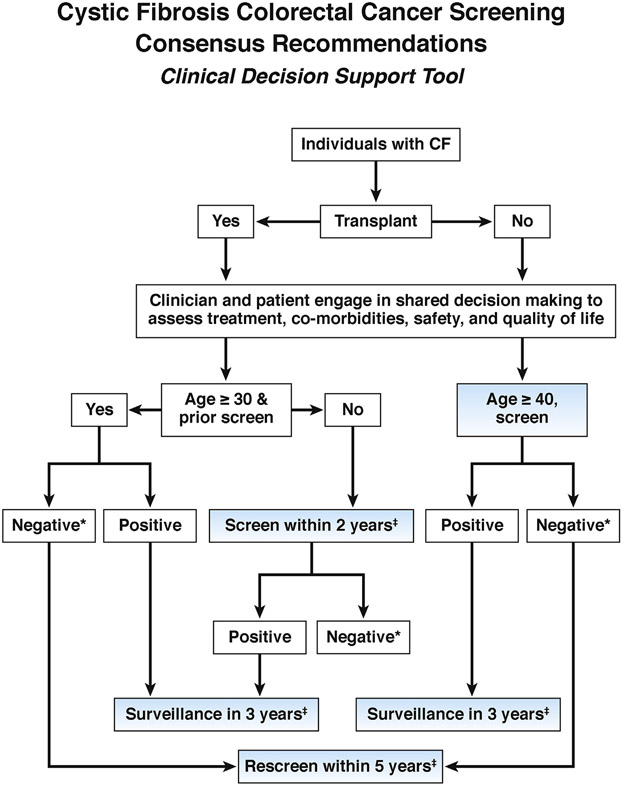
There is a paucity of data on the re-screening of individuals with CF after lung transplant and an initial screen that discovers no adenomas or CRC, except for one single center study.19 Based on generally more-intensive screening recommendations for other high-risk populations,34 the task force voted to recommend rescreening every 5 years (see Figure 2).
Figure 2.
Clinical decision support tool for CRC screening with colonoscopy in adults with CF. *Negative = no adenomatous polyps on examination with good to excellent bowel preparation (score ≥6 on the Boston Bowel Preparation Scale). Testing should be repeated if the bowel preparation is not considered good. ‡Screen = screening colonoscopy.
9. The Cystic Fibrosis Foundation recommends that individuals with cystic fibrosis who have undergone a solid organ transplant and had colonoscopy that had any adenomatous polyps have surveillance colonoscopy in 3 years, unless a shorter interval is indicated by individual findings, with subsequent intervals based on the most recent endoscopic examination.
Limited data9,19 suggest that there is an increased risk of recurrent high-grade polyps in individuals with CF, therefore surveillance should be performed every 3 years. However, in cases with extremely high-risk findings, such as very large polyps or multiple polyps, the standard surveillance interval of 3 years may have to be shortened, potentially requiring retesting within 1 year.39
10. The Cystic Fibrosis Foundation recommends that adults with cystic fibrosis undergoing a colonoscopy receive intensive regimens for bowel preparation to allow for optimal examination. The intensive regimen should include: three to four washes (minimum of 1-liter purgative per wash) with the last wash occurring within 4–6 hours before the examination.
An adequate bowel preparation is necessary for a quality colonoscopy and optimal detection of colon polyps40,41; however, bowel preparation is difficult in CF patients. Groups that reported systematic results of colonoscopies in CF patients used intensive CF-specific bowel preparation regimens.9,18,19 The main elements to a better preparation include split prep regimens, that is, several smaller volume washes are superior to a single larger-volume wash; performance of the examination soon after the last wash; and (3) patient education to emphasize the importance of bowel preparation for the examination and different elements involved. A sample CF-specific preparation from the Minnesota Cystic Fibrosis Center is included in Supplementary Material.
Conclusions
The CF CRC screening task force recommends screening with colonoscopy beginning at age 40 years for non-transplanted patients with CF and age 30 years for persons who have undergone and successfully recovered from a transplantation procedure. All decisions around CRC screening should be made in concert with the adult with CF. These discussions should include the consideration of comorbidities, safety, treatments, and quality of life. These recommendations are similar to the guidelines for screening in the non-CF population, where the recommended age for initial screening is about 10 years earlier than the average age of onset of cancer. The task force recommendations assume selection of patients whose anticipated life expectancy is sufficient to benefit from a screening procedure. We do not recommend a specific lung function below which colonoscopy is not recommended, as survival depends on many more factors, best assessed by the CF health care team. At present, we have insufficient information to assess the utility of screening procedures other than colonoscopy; such modalities could be extremely valuable, especially for individuals with CF with reduced lung function. In addition, current age recommendations are based on best-available evidence and efforts to balance the burden of frequent potentially unnecessary screening vs the benefit of early identification of malignant polyps. These recommendations will need to be updated when additional information becomes available about the potential benefit of alternative screening procedures, and the role of other risk factors, such as sex, mutational status, and family history.
Discussion and Areas for Future Research
Raising Awareness of Colorectal Cancer Risk and Acceptability of Screening
Because of the absence of any information on other screening procedures, the task force recommends colonoscopy screening as the current best screening procedure. Thus, a key issue is acceptability of this procedure by the CF community. One member of the task force contacted several CF care centers and found a high degree of compliance when colonoscopy was recommended, with level of patient education on bowel preparations being a key factor for acceptance. Because many patients and center directors appear to be unaware of the increased risk of CRC in CF patients, an educational component will be required to increase compliance with the listed recommendations.
Importance of Other Potential Risk Factors
There are many other risk factors within the CF population for which there is insufficient evidence to determine the impact on overall CRC risk. These factors include family history of CRC, sex, age of CF diagnosis, presence or absence of meconium ileus, diabetes, distal intestinal obstruction syndrome, and pancreas function status. Further information will be required to determine the potential importance of all these potential modifying factors.
With respect to mutational status, a recent cohort study found patients with severe genotypes had a somewhat higher risk of CRC than patients with milder mutations.3 However, milder mutations are present in a small minority of patients with CF (<10%) and therefore too few data exist to make any different recommendations for that group at this time. In any case, such patients have longer survival, thus possibly longer time to develop CRC. In an Australian screening study,9 all 4 CRCs and 1 ileal tumor developed in CF persons who were Delta F508 homozygotes. However, additional studies must be conducted to determine the cancer risk in older patients with milder CF mutations.
Role of Fecal Immunochemical Test Testing and Other Screening Modalities
The task force did not recommend FIT testing, but the modeling results suggest that screening with FIT, assuming that the method is both sensitive and specific, may be even more cost-effective in individuals with CF than screening with colonoscopy.30
Considering both screening modalities, the model found that the optimal screening strategy for non-transplant CF patients is annual FIT from age 35 to 75 years. This strategy was both more effective (46 vs 44 LYG) and less costly ($2.5 vs $2.6 million) than the optimal colonoscopy strategy. For post-transplantation patients with CF, the optimal strategy of annual FIT was slightly less effective than the optimal colonoscopy strategy (54 vs 56 LYG), but the task force decided the incremental resources required for the colonoscopy strategy ($1.3 million with colonoscopy vs $1.0 million for FIT) was acceptable.
Although FIT screening remained the optimal strategy in most sensitivity analyses, evidence for the performance of FIT in CF patients is currently lacking. The model findings of performance of FIT should therefore be confirmed in clinical studies before recommendations for FIT screening can be made in CF patients. Important information about the suitability of FIT testing and other screening modalities in the CF population could be obtained from a study combining and comparing synchronous screening using the testing modality and colonoscopy screening. Another test of potential value would be a stool-based test for tumor DNA. Although noninvasive procedures would have several potential advantages, any positive test would require a confirmatory colonoscopy.
Comparison of Modeling Results and Task Force Recommendations
The recommendations of the task force were consistent with the model recommended strategies for non-transplant CF patients to begin colonoscopy screening at age 40 years with repeat colonoscopy every 5 years assuming negative screenings. The modeling provided insights into how to balance the LYG with screening to the burden of screening for non-transplant and transplant CF patients.30 The model considered age to begin screening, intervals of repeat screening, and surveillance intervals for those with adenomas or cancers. For non-transplant patients, screening beginning at age 40 years with 5-year interval provided the best balance between LYG and burden of screening for the non-transplant subjects with incremental cost-effectiveness ratios below the commonly used threshold of $100,000. For transplant patients, Microsimulation Screening Analysis modeling suggested starting colonoscopy screening at age 35 years, given that transplant had occurred at age 30 years if the incremental cost-effectiveness ratio level of $100,000 was used. If transplant occurred before age 30 years, the model suggested that patients should receive colonoscopy starting at age 30 years. The task force elected to also recommend colonoscopy screening fairly soon after recovery from transplantation for patients transplanted at age 30 years to increase the efficacy of transplantation itself (Figure 2).
Generally, modeling strategies consider age to begin and age to end screening; however, we did not designate an age to end screening for individuals with CF because there were few differences in effectiveness by end age. (See modeling paper and its appendices).30 As with the non-CF population, a prudent approach would be to stop screening in adults with CF who have less than a 10-year life expectancy.
Discussion of Special Considerations In The Transplant Population
All data on organ transplants relate to lung transplant, as it accounts for >90% of transplantation procedures in CF patients. In addition, no data exist on liver transplant recipients (second most common), and occasional other solid organ transplants for individuals with CF. Liver transplant recipients use lower immunosuppression and likely have lower CRC risk; however, they have improved survival and thus a longer time to develop CRC.42 Based on this, the recommendations are for all solid organ transplant recipients with CF1,43 (As of September 30th, 2016, 5039 organ transplants have been performed for CF: 4622 lung transplants, 358 liver transplants, and 59 heart-lung transplants; https://optn.transplant.hrsa.gov/data/). Re-transplant patients should follow the same recommendations as first transplants.
Careful evaluation of the patient’s CRC risk and survival likelihood should be taken into account each time point that screening is considered. In cases where expected survival is limited (ie, <10 years), screening should not be performed. For adults appropriately selected, lung transplantation usually increases survival probability. Therefore, a lung transplantation candidate with a short life expectancy is likely to become a screening candidate before and after transplantation at the appropriate ages described here because the potential survival then increases to approximately 10 years.32,38
A particularly difficult situation will arise in patients with severe disease who cannot undergo screening before lung transplantation. In this case, careful consideration should be given to factors like age, risk factors, and risk of colonoscopy. The CF and lung transplantation team should discuss any other screening options acknowledging their limitations, including the option of delaying screening until after lung transplantation.
Discussion About Colonoscopy: Preparation Details, Poor Preparation Repeat Procedures, Right-Side Lesions, and Rapid Regrowth
A high-quality bowel preparation is essential for optimal detection of colon polyps.44-46 The physicochemical characteristics of stool and intestinal mucus complicate bowel preparation. A number of Food and Drug Administration–approved preparations exist, but given the increased-intensity regimens needed in CF patients, different preparations need to be tested for results and patient acceptability. In addition, non-Food and Drug Administration–approved preparations, such as over-the-counter polyethylene glycol combined with sport drinks, are commonly used in the general population.47-49 These have the advantage of greater patient tolerability and may have a role in CF patients, but careful attention needs to be paid to potential electrolyte shifts if such preparations are intensified in volume.50
Surveillance and Rescreening
At this time, we recommend a 3-year surveillance interval for those with adenomas detected and a 5-year rescreening interval for adults with CF who have prior negative findings. This ensures simplicity for implementation and following of the recommendations. Decisions should always be based on last colonoscopy. As more data become available, it is possible that different sub-populations will need more or less frequent schedules for rescreening and surveillance. Our recommendations are making an effort to balance the risk of missing advanced CRC and minimizing the burden and risk of too frequent examinations.
Prevention of Cancer in the Cystic Fibrosis Population
At present, screening appears to offer the best way to lower the risk of developing CRC in CF persons. Lifestyle modification by avoiding known CRC risk factors, such as smoking and obesity are unlikely to be effective because these factors are usually absent in individuals with CF. There is the possibility that chemopreventive agents, such as cyclooxygenase inhibitors (eg, aspirin) and 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) could be helpful.51 Finally, CFTR modulators, which increase the expression of the CFTR protein, might also decrease the likelihood of CRC.
Supplementary Material
Acknowledgments
Cystic Fibrosis Colorectal Cancer Screening Task Force: Amy Leigh Braid, Community Advisor to the Cystic Fibrosis Foundation; Joanne Cullina, MSN, APN, ACNS-B, Northwestern University; Anne Daggett, MSW, LCSW, St. Luke’s Cystic Fibrosis Center of Idaho; Aliza Fink, DSc, Medical Department, Cystic Fibrosis Foundation; Andrea Gini, MSc, Department of Public Health Erasmus MC, Rotterdam, The Netherlands; Denis Hadjiliadis, MD, MHS, Paul F Harron Jr Associate Professor of Medicine Perelman School of Medicine, University of Pennsylvania; Sarah Hempstead, MS, Medical Department, Cystic Fibrosis Foundation; Alexander Khoruts, MD, Professor of Medicine, Division of Gastroenterology, Hepatology, and Nutrition, University of Minnesota; Iris Lansdorp-Vogelaar, PhD, Assistant Professor, Department of Public Health, Erasmus MC, Rotterdam, The Netherlands; David Lieberman, MD, Professor of Medicine Chief, Division of Gastroenterology and Hepatology Oregon Health and Science University; Theodore Liou, MD, Adult Cystic Fibrosis Center Division of Respiratory, Critical Care and Occupational Pulmonary Medicine Department of Internal Medicine University of Utah Salt Lake City; Paula Lomas, MAS, RN, CCRP, Medical Department, Cystic Fibrosis Foundation, Bethesda, Maryland; Albert Lowenfels, MD, Professor of Community and Family Medicine Emeritus Professor of Surgery New York Medical College; Patrick Maisonneuve, Dipl. Eng., Director, Unit of Clinical Epidemiology, Division of Epidemiology and Biostatistics, European Institute of Oncology, Milan, Italy; Bruce Marshall, MD, Medical Department, Cystic Fibrosis Foundation; Keith Meyer, MD, MS, Section of Pulmonary and Critical Care Medicine, University of Wisconsin School of Medicine and Public Health; Anil Rustgi, MD, Chief of Gastroenterology, T. Grier Miller Professor of Medicine University of Pennsylvania Perelman School of Medicine; Kathy Sabadosa, MPH, The Dartmouth Institute for Health Policy and Clinical Practice; Geisel School of Medicine at Dartmouth; Aasma Shaukat, MD, MPH, GI Section Chief, Minneapolis VAHCS, Associate Professor University of Minnesota; Ann Zauber, PhD, Member and Attending, Department of Epidemiology and Biostatistics Memorial Sloan Kettering Cancer Center New York, NY.
Abbreviations used in this paper:
- CF
cystic fibrosis
- CRC
colorectal cancer
- FIT
fecal immunochemical test
- LYG
life-years gained
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2017.12.012.
References
- 1.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2015 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation, 2016. [Google Scholar]
- 2.Maisonneuve P, FitzSimmons SC, Neglia JP, Campbell PW 3rd, Lowenfels AB. Cancer risk in non-transplanted and transplanted cystic fibrosis patients: a 10-year study. J Natl Cancer Inst 2003;95:381–387. [DOI] [PubMed] [Google Scholar]
- 3.Maisonneuve P, Marshall BC, Knapp EA, Lowenfels AB. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst 2013;105:122–129. [DOI] [PubMed] [Google Scholar]
- 4.Fink AK, Yanik EL, Marshall BC, et al. Cancer risk among lung transplant recipients with cystic fibrosis. J Cyst Fibros 2016;16:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neglia JP, FitzSimmons SC, Maisonneuve P, et al. The risk of cancer among patients with cystic fibrosis. Cystic Fibrosis and Cancer Study Group. N Engl J Med 1995;332:494–499. [DOI] [PubMed] [Google Scholar]
- 6.Alexander CL, Urbanski SJ, Hilsden R, Rabin H, MacNaughton WK, Beck PL. The risk of gastrointestinal malignancies in cystic fibrosis: case report of a patient with a near obstructing villous adenoma found on colon cancer screening and Barrett’s esophagus. J Cyst Fibros 2008;7:1–6. [DOI] [PubMed] [Google Scholar]
- 7.Brown JA, Chaun H, Mason AC. Delayed diagnosis of colon carcinoma in cystic fibrosis. Clin Radiol 2000;55:240–242. [DOI] [PubMed] [Google Scholar]
- 8.Gilchrist FJ, Jones AM, Bright-Thomas RJ. Intussusception and metastatic colon cancer in an adult with cystic fibrosis. J R Soc Med 2012;105(Suppl 2):S40–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gory I, Brown G, Wilson J, et al. Increased risk of colorectal neoplasia in adult patients with cystic fibrosis: a matched case-control study. Scand J Gastroenterol 2014;49:1230–1236. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Jimenez I, Fischman D, Cheriyath P. Colon cancer in cystic fibrosis patients: is this a growing problem? J Cyst Fibros 2008;7:343–346. [DOI] [PubMed] [Google Scholar]
- 11.Ibele AR, Koplin SA, Slaughenhoupt BL, et al. Colonic adenocarcinoma in a 13-year-old with cystic fibrosis. J Pediatr Surg 2007;42(10):E1–E3. [DOI] [PubMed] [Google Scholar]
- 12.Lees AN, Reid DW. Management dilemma; a woman with cystic fibrosis and severe lung disease presenting with colonic carcinoma: a case report. J Med Case Rep 2008;2:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenna PB, Mulcahy E, Waldron D. Early onset of colonic adenocarcinoma associated with cystic fibrosis—a case report. Ir Med J 2006;99:310–311. [PubMed] [Google Scholar]
- 14.Redington AN, Spring R, Batten JC. Adenocarcinoma of the ileum presenting as non-traumatic clostridial myonecrosis in cystic fibrosis. Br Med J (Clin Res Ed) 1985;290(6485):1871–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuels LE, Amrom G, Kerstein MD. Adenocarcinoma of the ileum in a patient with cystic fibrosis. Dig Dis Sci 1995;40:107–108. [DOI] [PubMed] [Google Scholar]
- 16.Siraganian PA, Miller RW, Swender PT. Cystic fibrosis and ileal carcinoma. Lancet 1987;2(8568):1158. [DOI] [PubMed] [Google Scholar]
- 17.Soriano E, Fischman D, Cheriyath P. Membranoproliferative glomerulonephritis in patients with cystic fibrosis: coincidence or comorbidity? A case series. South Med J 2008;101:641–645. [DOI] [PubMed] [Google Scholar]
- 18.Billings JL, Dunitz JM, McAllister S, et al. Early colon screening of adult patients with cystic fibrosis reveals high incidence of adenomatous colon polyps. J Clin Gastroenterol 2014;48:e85–e88. [DOI] [PubMed] [Google Scholar]
- 19.Niccum DE, Billings JL, Dunitz JM, et al. Colonoscopic screening shows increased early incidence and progression of adenomas in cystic fibrosis. J Cyst Fibros 2016;15:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Than BL, Linnekamp JF, Starr TK, et al. CFTR is a tumor suppressor gene in murine and human intestinal cancer. Oncogene 2016;35(32):4179–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starr TK, Allaei R, Silverstein KA, et al. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science 2009;323(5922):1747–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustafsson JK, Ermund A, Ambort D, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 2012;209:1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borowitz D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr Pulmonol 2015;50(Suppl 40):S24–S30. [DOI] [PubMed] [Google Scholar]
- 24.Kreda SM, Davis CW, Rose MC. CFTR, mucins, and mucus obstruction in cystic fibrosis. Cold Spring Harb Perspect Med 2012;2(9):a009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norkina O, Burnett TG, De Lisle RC. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect Immun 2004;72(10):6040–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munck A. Cystic fibrosis: evidence for gut inflammation. Int J Biochem Cell Biol 2014;52:180–183. [DOI] [PubMed] [Google Scholar]
- 27.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Limited Use, Nov 2002 Sub (1973–2000). National Cancer Institute DCCPS Surveillance Research Program Cancer Statistics Branch. https://seer.cancer.gov/faststats/selections.php. Published April 2003, based on November 2002 submission. Accessed January 2017. [Google Scholar]
- 28.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2014 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation, 2015. [Google Scholar]
- 29.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016;315(23):2595–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gini A, Zauber AG, Cenin DR, et al. Cost effectiveness of screening individuals with cystic fibrosis for colorectal cancer. Gastroenterology 2018;154:556–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SurveyMonkey Inc. www.surveymonkey.com. Accessed October 2016.
- 32.Buzzetti R, Alicandro G, Minicucci L, et al. Validation of a predictive survival model in Italian patients with cystic fibrosis. J Cyst Fibros 2012;11:24–29. [DOI] [PubMed] [Google Scholar]
- 33.ASGE Standards of Practice Committee, Fisher DA, Maple JT, et al. Complications of colonoscopy. Gastrointest Endosc 2011;74:745–752. [DOI] [PubMed] [Google Scholar]
- 34.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666–689. [DOI] [PubMed] [Google Scholar]
- 35.Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110:223–262; quiz 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer KC, Francois ML, Thomas HK, et al. Colon cancer in lung transplant recipients with CF: increased risk and results of screening. J Cyst Fibros 2011;10:366–369. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–857. [DOI] [PubMed] [Google Scholar]
- 38.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-Second Official Adult Lung and Heart-Lung Transplantation Report—2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1264–1277. [DOI] [PubMed] [Google Scholar]
- 39.Atkin WS, Valori R, Kuipers EJ, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition—Colonoscopic surveillance following adenoma removal. Endoscopy 2012;44(Suppl 3):SE151–SE163. [DOI] [PubMed] [Google Scholar]
- 40.Menees SB, Kim HM, Elliott EE, et al. The impact of fair colonoscopy preparation on colonoscopy use and adenoma miss rates in patients undergoing outpatient colonoscopy. Gastrointest Endosc 2013;78:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chokshi RV, Hovis CE, Hollander T, et al. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc 2012;75:1197–1203. [DOI] [PubMed] [Google Scholar]
- 42.Atassi T, Thuluvath PJ. Risk of colorectal adenoma in liver transplant recipients compared to immunocompetent control population undergoing routine screening colonoscopy. J Clin Gastroenterol 2003;37:72–73. [DOI] [PubMed] [Google Scholar]
- 43.Organ Procurement and Transplantation Network Data. https://optn.transplant.hrsa.gov/data/. Accessed September 30, 2016.
- 44.Rex DK. Optimal bowel preparation—a practical guide for clinicians. Nat Rev Gastroenterol Hepatol 2014;11:419–425. [DOI] [PubMed] [Google Scholar]
- 45.Rex DK. Bowel preparation for colonoscopy: entering an era of increased expectations for efficacy. Clin Gastroenterol Hepatol 2014;12:458–462. [DOI] [PubMed] [Google Scholar]
- 46.Hassan C, Bretthauer M, Kaminski MF, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2013;45:142–150. [DOI] [PubMed] [Google Scholar]
- 47.Samarasena JB, Muthusamy VR, Jamal MM. Split-dosed MiraLAX/Gatorade is an effective, safe, and tolerable option for bowel preparation in low-risk patients: a randomized controlled study. Am J Gastroenterol 2012;107:1036–1042. [DOI] [PubMed] [Google Scholar]
- 48.Hjelkrem M, Stengel J, Liu M, et al. MiraLAX is not as effective as GoLytely in bowel cleansing before screening colonoscopies. Clin Gastroenterol Hepatol 2011;9:326–332 e321. [DOI] [PubMed] [Google Scholar]
- 49.Siddique S, Lopez KT, Hinds AM, et al. Miralax with gatorade for bowel preparation: a meta-analysis of randomized controlled trials. Am J Gastroenterol 2014;109:1566–1574. [DOI] [PubMed] [Google Scholar]
- 50.Nagler J, Poppers D, Turetz M. Severe hyponatremia and seizure following a polyethylene glycol-based bowel preparation for colonoscopy. J Clin Gastroenterol 2006;40:558–559. [DOI] [PubMed] [Google Scholar]
- 51.Lochhead P, Chan AT. Statins and colorectal cancer. Clin Gastroenterol Hepatol 2013;11:109–118; quiz e113–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.