Abstract
Wolfram syndrome is a rare genetic disorder largely caused by pathogenic variants in the WFS1 gene and manifested by diabetes mellitus, optic nerve atrophy, and progressive neurodegeneration. Recent genetic and clinical findings have revealed Wolfram syndrome as a spectrum disorder. Therefore, a genotype-phenotype correlation analysis is needed for diagnosis and therapeutic development. Here, we focus on the WFS1 c.1672C>T, p.R558C variant, which is highly prevalent in the Ashkenazi Jewish population. Clinical investigation indicated that patients carrying the homozygous WFS1 c.1672C>T, p.R558C variant showed mild forms of Wolfram syndrome phenotypes. Expression of WFS1 p.R558C was more stable compared with the other known recessive pathogenic variants associated with Wolfram syndrome. Human induced pluripotent stem cell–derived (iPSC-derived) islets (SC-islets) homozygous for WFS1 c.1672C>T variant recapitulated genotype-related Wolfram syndrome phenotypes. Enhancing residual WFS1 function through a combination treatment of chemical chaperones mitigated detrimental effects caused by the WFS1 c.1672C>T, p.R558C variant and increased insulin secretion in SC-islets. Thus, the WFS1 c.1672C>T, p.R558C variant causes a mild form of Wolfram syndrome phenotypes, which can be remitted with a combination treatment of chemical chaperones. We demonstrate that our patient iPSC–derived disease model provides a valuable platform for further genotype-phenotype analysis and therapeutic development for Wolfram syndrome.
Keywords: Endocrinology, Genetics
Keywords: Beta cells, Diabetes, Genetic diseases
Introduction
Wolfram syndrome is a rare, monogenic, life-threatening disease largely caused by pathogenic variants in the Wolfram syndrome (WFS1) gene, or in a small fraction of patients, pathogenic variants in the CDGSH iron sulfur domain protein 2 gene (1–3). There is currently no treatment to delay, halt, or reverse the progression of this disease. Wolfram syndrome is well characterized by juvenile-onset insulin-dependent diabetes, optic nerve atrophy, and progressive neurodegeneration (4, 5). Many patients also develop other symptoms, ranging from hearing loss and endocrine deficiencies to neurological and psychiatric conditions (4, 6). Accordingly, recent clinical and genetic findings have revealed that Wolfram syndrome is best characterized as a spectrum disorder (7). Of the approximately 200 WFS1 variants associated with Wolfram syndrome, approximately 35% are missense, 25% are nonsense, 21% are frameshift, 13% are in-frame insertions or deletions, and 3% are splice-site variants (8, 9). Most of these variants are predicted to be inactivating, loss-of-function variants, but extensive molecular characterization of individual alleles is sparse. Hence, there is a great need for genotype-phenotype correlation data to guide diagnostic interpretation of WFS1 variants.
WFS1 encodes an ER transmembrane protein. The ER is a central cell organelle responsible for protein folding, Ca2+ storage, and lipid synthesis. It has been reported that WFS1 regulates Ca2+ homeostasis in the ER, which is crucial in the synthesis and secretion of neurotransmitters and hormones such as insulin (10, 11). WFS1 deficiency in the ER causes Ca2+ homeostasis disruption, leading to chronic ER stress followed by the unfolded protein response (UPR) (12, 13). WFS1 also negatively regulates activating transcription factor 6 (ATF6), a UPR molecule, inhibiting hyperactivation of ATF6 and consequent cell apoptosis (14). Furthermore, a recent study suggested that WFS1 affects mitochondrial function by transporting Ca2+ from the ER to the mitochondria via the mitochondria-associated ER membrane (15).
Several Wfs1-knockout rodents were developed as disease models of Wolfram syndrome, which generated insight into the etiology and provided opportunities to test therapeutic agents (11, 16–19). The models display progressive glucose intolerance due to impaired glucose-stimulated insulin secretion (GSIS) and increased pancreatic β cell death (16, 18–20). However, the onset of diabetes in these rodent models is delayed relative to the human phenotype, with further variation between each model based on rodent strain (16, 19). Also, to the best of our knowledge, there are no transgenic animals with phenotypes and variants corresponding to the pathogenic WFS1 variants found in patients with Wolfram syndrome. As a result, these rodent models may not fully capture the spectrum of Wolfram phenotypes. By contrast, patient induced pluripotent stem cells (iPSCs) differentiated into disease-relevant cell types have been demonstrated as suitable models for genotype-phenotype correlation analysis (21, 22).
To date, significant efforts have been invested to develop novel Wolfram syndrome treatments (23). Compounds such as valproic acid and glucagon-like peptide-1 receptor agonists were identified as possible drug candidates based on preclinical studies in immortalized cell and rodent models (24–26). In addition, we recently conducted a phase Ib/IIa clinical trial of dantrolene sodium, an ER Ca2+ stabilizer, which demonstrated efficacy in a subset of patients with Wolfram syndrome (11, 27). However, additional therapeutic candidates for patients with Wolfram syndrome are still needed.
Here, we focus on the missense variant, WFS1 c.1672C>T, p.R558C, which is enriched in the Ashkenazi Jewish population (allele frequency 1.4%) (28). We characterize this variant multidimensionally through clinical investigation, biochemical studies, and patient iPSC–derived disease models. Further, we demonstrate the potential efficacy of a combination treatment of chemical chaperones, sodium 4-phenylbutyrate (4-PBA) and tauroursodeoxycholic acid (TUDCA), as a potentially novel therapeutic approach for Wolfram syndrome.
Results
WFS1 c.1672C>T, p.R558C is enriched in the Ashkenazi Jewish population and causes a mild form of Wolfram phenotypes.
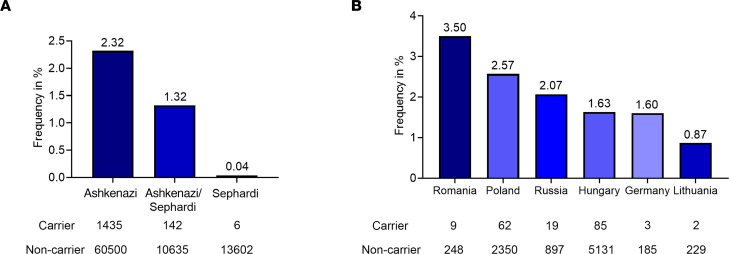
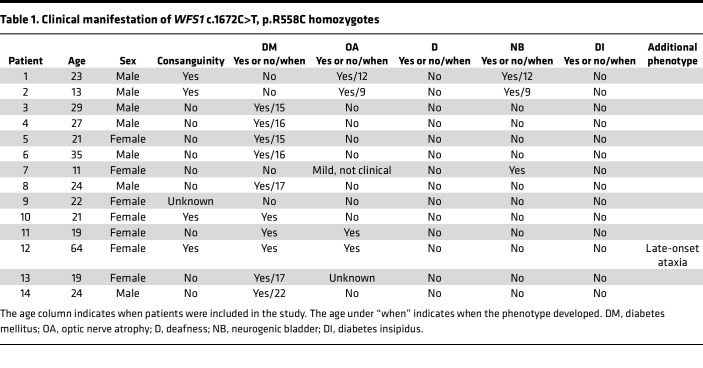
To determine the carrier frequency for WFS1 c.1672C>T, p.R558C variant in the Jewish population, we genotyped 87,093 patients from several Jewish populations. In the original data set, each patient was classified by self-identification as Ashkenazi, Sephardi, Ashkenazi/Sephardi, convert, and unknown. Samples from convert and unknown origin summed 773 and were excluded from analysis. The observed frequency of WFS1 c.1672C>T, p.R558C carriers reached 2.32% (1:43) in Ashkenazi Jewish patients, 1.32% (1:76) for Ashkenazi/Sephardi patients, and 0.04% (1:2,268) in Sephardi Jewish patients (Figure 1A and Supplemental Table 1A; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.156549DS1). To elucidate if WFS1 c.1672C>T, p.R558C was present at higher rates in the Jewish population from various countries, we classified data based on self-reported ancestry of 4 grandparents. Patients who stated 2 or more countries of mixed origin were removed from analysis. In cases where South Africa was provided as the country of origin, the samples were redefined as Lithuanian, as South African Jews are primarily of Lithuanian origin (29). Patients who had Israel or United States listed in their ancestry were also removed because the Jewish people residing in these countries often have mixed Ashkenazi origins (30). Patients who did not provide any information on grandparental origin or stated unknown were removed. Patients with Ukrainian origin were merged into the Russian group. Patients with Belarus and Czechia origin were removed from analysis because they totaled fewer than 100 patients and a small sample group can produce spurious signals. In data classified by the country of origin, the frequencies occurred as follows: Romania 3.50% (1:29), Poland 2.57% (1:39), Russia 2.07% (1:48), Hungary 1.63% (1:61), Germany 1.60% (1:63), and Lithuania 0.87% (1:116) (Figure 1B and Supplemental Table 1B). Clinical investigation revealed that most patients carrying the homozygous WFS1 c.1672C>T, p.R558C variant developed diabetes mellitus; however, the age at diagnosis was greater than that of typical Wolfram syndrome (approximately 6 years) (4) (Table 1). Only 4 patients were clinically diagnosed with optic nerve atrophy. Their optic nerve atrophy was mild, and no case was diagnosed as legally blind (Table 1). Additionally, no patient developed hearing loss or diabetes insipidus (Table 1). Together, WFS1 c.1672C>T, p.R558C variant was enriched in the Ashkenazi Jewish population, especially those originated from Romania, and the variant led to mild or less severe phenotypes of Wolfram syndrome.
Figure 1. Carrier frequencies and clinical manifestation of WFS1 c.1672C>T, p.R558C variant.
(A) Carrier frequencies for WFS1 c.1672C>T, p.R558C in patients of Ashkenazi, Ashkenazi/Sephardi, and Sephardi descent. (B) Carrier frequencies for WFS1 c.1672C>T, p.R558C by country of origin.
Table 1. Clinical manifestation of WFS1 c.1672C>T, p.R558C homozygotes.
The WFS1 p.R558C variant is degraded more than wild-type but less than WFS1 p.P885L variant.
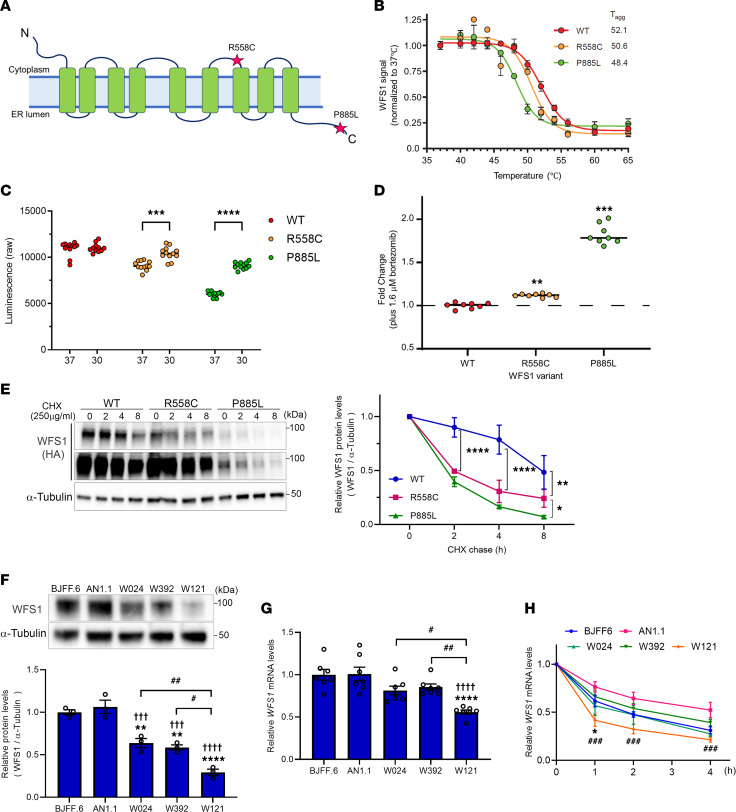
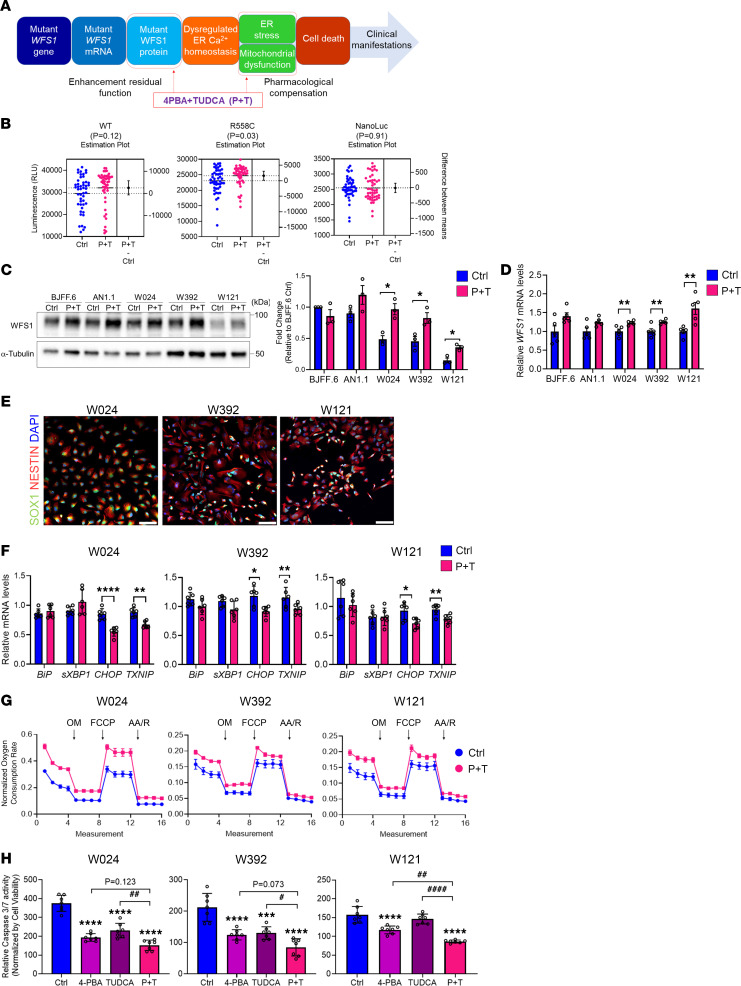
Pathogenic WFS1 variants are classified based on their effect on WFS1 expression: class A, depleted WFS1 protein or reduced, defective WFS1 protein, which leads to loss of function or incomplete function, or class B, expression of defective WFS1 protein leading to gain of function. Class A is further divided into 3 subclasses: class A1, WFS1 depletion due to WFS1 mRNA degradation (nonsense-mediated decay, NMD); class A2, WFS1 depletion due to WFS1 protein degradation; or class A3, WFS1 depletion due to mRNA and protein degradation (31, 32) (Supplemental Figure 1). To determine the class specification of the WFS1 c.1672C>T, p.R558C variant, we investigated the thermal stability of WFS1 p.R558C and p.P885L by appending a HiBiT-based tag (Promega) to detect the variant in cells (33). The p.R558C variant showed less thermal stability than wild-type WFS1, suggesting an altered folding state, but more stability compared with the known autosomal recessive variant p.P885L, which is pathogenic and is associated with a typical form of Wolfram syndrome (34, 35) (Figure 2, A and B). Both p.R558C and p.P885L expression could be rescued by incubating cells at reduced temperature, supporting a folding defect conferred by the variants (Figure 2C). Treatment with a proteasome inhibitor, bortezomib, increased WFS1 protein levels from both variants, and the fold change for p.P885L was higher than for p.R558C (Figure 2D), indicating that proteasomal degradation of p.R558C is less than p.P885L. To confirm this observation, we performed a cycloheximide (CHX) chase assay using HA-tagged WFS1 variants. After inhibiting translation of nascent protein by CHX treatment, the protein levels of p.R558C and p.P885L were rapidly decreased within 2 hours (Figure 2E). However, the rate of p.P885L decay was higher than p.R558C (Figure 2E). Also, the basal expression of p.P885L was lower before CHX treatment compared with wild-type and p.R558C, all consistent with more rapid degradation of p.P885L (Figure 2E).
Figure 2. WFS1 p.R558C is more stable in the cell compared with p.P885L variant.
(A) Diagram of WFS1 protein showing the location of 2 variants, R558C and P885L. (B) Thermal profiles of WFS1 variants (WT, R558C, and P885L) measured using SplitLuc-tagged reporters expressed in HEK293T cells (data from 3 independent experiments). (C) Luminescence intensities of WFS1 variants in cells incubated at 30°C and 37°C for 24 hours (n = 12, ***P < 0.001 and ****P < 0.0001 by unpaired t test). (D) Fold change of luminescence intensities of WFS1 variants treated with a proteasome inhibitor, bortezomib, for 24 hours (**P < 0.01 and ***P < 0.001 by unpaired t test compared with untreated). (E) (Left) Representative blotting image of WFS1 (HA) and α-Tubulin in CHX chase assay. Lower panel of WFS1 (HA) is long-exposure image. (Right) A quantification of relative WFS1 protein level normalized with α-Tubulin. (n =3, *P < 0.05, **P < 0.01, and ****P < 0.0001 by 2-way ANOVA.) (F) (Upper) Representative blotting image of WFS1 and α-Tubulin in iPSCs. (Lower) Quantification of relative WFS1 protein level normalized with α-Tubulin (n = 3, **P < 0.01 and ****P < 0.0001 by 1-way ANOVA compared with BJFF.6, †††P < 0.001 and ††††P < 0.0001 by 1-way ANOVA compared with AN1.1, #P < 0.05 and ##P < 0.01 by 1-way ANOVA). (G) Relative mRNA level of WFS1 in iPSCs. (n = 7, ****P < 0.0001 by 1-way ANOVA compared with BJFF.6, ††††P < 0.0001 by 1-way ANOVA compared with AN1.1, #P < 0.05 and ##P < 0.01 by 1-way ANOVA.) (H) Relative mRNA level of WFS1 in ActD chase assay (n = 3, *P < 0.05 by 1-way ANOVA compared with BJFF.6, ###P < 0.001 by 1-way ANOVA compared with AN1.1).
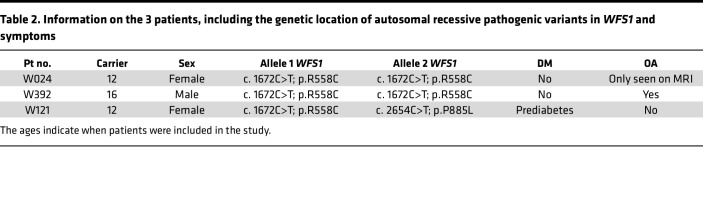
Next, we examined if the WFS1 variants endogenously expressed in cells would show similar posttranslational stabilities. We obtained peripheral blood mononuclear cells (PBMCs) from 3 patients carrying pathogenic variants in the WFS1 gene (W024: c.1672C>T, c.1672C>T; W392: c.1672C>T, c.1672C>T; W121: c.1672C>T, c.2654C>T) and generated iPSCs (Table 2 and Supplemental Figure 2). Consistent with our clinical investigation, patients W024, W392, and W121 had mild phenotypes of Wolfram syndrome (Table 2). Western blot analysis revealed a reduction in WFS1 protein levels for W024, W392, and W121 compared with 2 control iPSC lines (BJFF.6 and AN1.1) (Figure 2F). Of the 3 patient lines, WFS1 protein level in W121 was less than W024 and W392 (Figure 2F). WFS1 mRNA was not significantly decreased in W024 and W392 compared to control lines but was reduced for W121 (Figure 2G). We also performed the actinomycin D (ActD) chase assay to determine WFS1 mRNA stabilities in each iPSC line. WFS1 mRNA decay was higher than that in control line AN1.1, but similar to control line BJFF.6 (Figure 2H). On the other hand, corresponding to endogenous WFS1 expression, WFS1 mRNA in W121 was more unstable than both control lines (Figure 2H). Taken together, the WFS1 c.1672C>T, p.R558C variant led to reduced expression of defective WFS1 protein, which was driven by posttranslation protein degradation, but not mRNA alterations, designating the variant as class A2.
Table 2. Information on the 3 patients, including the genetic location of autosomal recessive pathogenic variants in WFS1 and symptoms.
A combination treatment of 4-PBA and TUDCA ameliorates cellular function in neural progenitor cells with c.1672C>T, p.R558C variant.
Wolfram syndrome is recognized as an ER disorder (6, 36, 37). Given that the ER and mitochondria interact both physiologically and functionally to maintain cellular homeostasis and determine cell fate under pathophysiological conditions, pathogenic WFS1 variants cause not only ER dysfunction but also dysregulation of mitochondrial dynamics and appropriate function (38, 39). This evidence suggests that a combination drug modulating multiple targets simultaneously in the cell could be an effective treatment candidate for Wolfram syndrome. Chemical chaperones, such as 4-PBA and TUDCA, are well known to rescue or stabilize the native conformation of proteins by interacting with exposed hydrophobic segments of the unfolded protein (40). In addition to its chaperone activity, 4-PBA exhibits histone deacetylase–inhibitory (HDAC-inhibitory) activity, which transcriptionally induces the expression of molecular chaperones (41). TUDCA has been reported to reduce reactive oxygen species formation (42), prevent mitochondrial dysfunction (43), and inhibit apoptosis through the intrinsic (44) and extrinsic pathways (45). It has been shown that 4-PBA can improve insulin synthesis in iPSC-derived β cells from patients with typical Wolfram syndrome (46). We, therefore, hypothesized that a combination treatment of 4-PBA and TUDCA would have an additive effect on the restoration of reduced WFS1 expression and organelle dysfunction. Specifically, the combination treatment would be expected to affect pathogenic WFS1 variants that target protein-level degradation, such as WFS1 p.R558C (Figure 3A).
Figure 3. A combination treatment with 4-PBA and TUDCA mitigates detrimental effect of WFS1 c.1672C>T, p.R558C variant.
(A) A schematic of Wolfram syndrome etiology and the targets to modulate by a combination treatment of 4-PBA and TUDCA (P+T). (B) Expression of HiBiT-tagged WFS1 protein after treatment with 500 μM 4-PBA and 50 μM TUDCA (P+T) for 24 hours. NanoLuc levels, expressed from an identical plasmid backbone, were examined (n = 48, P value by unpaired t test). (C) (Left) Representative blotting images of WFS1 and α-Tubulin in iPSCs treated with or without P+T for 48 hours. (Right) Quantification of WFS1 protein levels normalized with α-Tubulin. (n = 3, *P < 0.05 by unpaired t test compared with Ctrl.) (D) Relative mRNA levels of WFS1 in iPSCs treated with or without P+T for 48 hours (n = 5, **P < 0.01 by unpaired t test compared with Ctrl). (E) Representative immunofluorescence images of neural progenitor cell (NPC) markers in NPCs differentiated from patient-derived iPSCs. Scale bar: 100 μm. (F) Quantitative PCR analysis of ER stress–related genes in NPCs treated with or without P+T for 48 hours. (n = 6, *P < 0.05, **P < 0.01, and ****P < 0.0001 by unpaired t test compared with Ctrl.) (G) Mitochondrial respiration of NPCs treated with or without P+T for 48 hours represented as percentage of baseline oxygen consumption rate (OCR) measurements. Respiration was interrogated by measuring changes in relative OCR multiple times, every 8.5 minutes, after injection with oligomycin (OM), FCCP, and antimycin A (AA)/rotenone (R) (n = 3, W024: ***P < 0.001, W392: *P < 0.05, and W121: *P < 0.05 by unpaired t test compared with Ctrl AUC). (H) Caspase-3/7 activity normalized by cell viability in NPCs treated with or without either of 4-PBA, TUDCA, or P+T for 48 hours. (n = 7; ***P < 0.001 and ****P < 0.0001 by 1-way ANOVA compared with Ctrl; #P < 0.05, ##P < 0.01, and ####P < 0.0001 by 1-way ANOVA.)
We first tested if P+T stabilized WFS1 protein using the HiBiT-tagged reporters. The incubation with P+T significantly increased the steady-state levels of WFS1 p.R558C protein but not WT or a NanoLuc control expressed from an identical plasmid backbone (Figure 3B). Although the treatment slightly increased the steady-state levels of WFS1 p.P885L as well, it was not statistically significant (P = 0.0697, Supplemental Figure 3A). We also screened the NCATS Pharmaceutical Collection (~2,000 compounds), which includes approved drugs as well as 4-PBA, but not TUDCA, and found a small number of compounds that increased WFS1 p.R558C protein level, of which disulfiram was the top hit, but the magnitude of effect was similar to P+T (Supplemental Figure 3, B and C, and Supplemental Data 1). We next compared endogenous WFS1 protein levels in iPSCs treated with P+T. The P+T treatment significantly increased WFS1 protein levels in iPSCs derived from all 3 patient lines (Figure 3C). Of note, WFS1 protein levels in W024 and W392 were restored as great as control lines (Figure 3C). Additionally, mRNA level was increased by the P+T treatment (Figure 3D). We previously described organelle dysfunction preceding cell death in neural progenitor cells (NPCs) differentiated from iPSCs derived from patients with typical Wolfram syndrome (11). NPCs differentiated from the 3 patient lines and control line (AN1.1) expressed NPC markers NESTIN and SOX1 (Figure 3E and Supplemental Figure 4A). They showed a similar pattern of WFS1 expression in iPSCs of these lines (Supplemental Figure 4B). Interestingly, the expression of ER stress marker genes, BiP and spliced XBP1 (sXBP1), was not greatly changed among the lines, but CHOP, an ER stress–induced apoptosis gene, was significantly increased in each of the 3 patient lines (Supplemental Figure 4C). The other ER stress–induced apoptosis gene, TXNIP, was increased in W392 and W121 compared with AN1.1 (Supplemental Figure 4C).
Next, we examined if a combination treatment of 4-PBA and TUDCA would restore organelle functions in NPCs derived from the 3 patient iPSC lines. The expression of BiP and sXBP1 was not affected by the P+T treatment, whereas CHOP and TXNIP were significantly decreased in each of the 3 patient lines (Figure 3F). We also measured the oxygen consumption rate (OCR) of NPCs to further assess mitochondrial function. Increased OCRs were observed throughout the assay in each of the 3 patient lines with the P+T treatment (Figure 3G). To investigate whether increased OCR was caused by increased mitochondrial number or improved mitochondrial function, we measured mitochondrial DNA contents and mitochondrial membrane potentials in the NPCs treated with or without P+T. Interestingly, mitochondrial DNA was increased in W024 and W392 NPCs by the treatment (Supplemental Figure 5A), whereas the mitochondrial membrane potentials were not affected (Supplemental Figure 5B). In W121 NPCs, both mitochondrial DNA and membrane potentials were increased by the treatment (Supplemental Figure 5, A and B). Besides these improvements, the P+T treatment inhibited apoptosis, as indicated by caspase-3/7 activity and cleaved caspase-3 protein levels, in each of the 3 patient lines (Figure 3H and Supplemental Figure 5C).
To define if a combination treatment of 4-PBA and TUDCA is valuable, we compared P+T efficacies with a single treatment with either 4-PBA or TUDCA. The P+T effect on endogenous WFS1 protein levels was the greatest in each of the 3 patient iPSC lines (Supplemental Figure 6A). Of note, WFS1 protein levels were significantly increased in W392 and W121 by the P+T treatment compared with a single treatment with each compound (Supplemental Figure 6A). The expression of ER stress–induced apoptosis genes was similar regardless of single or combination treatments in W024 and W392 NPCs, whereas only the P+T treatment significantly decreased ER stress–induced apoptosis gene expression in W121 (Supplemental Figure 6B). Mitochondrial DNA was not greatly changed by any single treatment in each of the 3 patient lines (Supplemental Figure 5A). Although we confirmed the increase of mitochondrial membrane potentials in W121 NPCs by the P+T treatment, it was not observed by a single treatment with each compound (Supplemental Figure 5B). Last, a single treatment with 4-PBA inhibited apoptosis in all 3 patient lines, and TUDCA also decreased apoptosis in W392 (Figure 3H and Supplemental Figure 5C). However, the magnitude of inhibition was the largest in the P+T treatment in each of 3 patient lines (Figure 3H and Supplemental Figure 5C). In addition, we confirmed the P+T treatment reduced caspase-3/7 activity in NPCs derived from patients with typical Wolfram syndrome (Supplemental Figure 7, A and B). In summary, a combination treatment of 4-PBA and TUDCA increased WFS1 expression and inhibited apoptosis by mitigating ER stress and mitochondrial dysfunction, which was more beneficial than a single treatment of either 4-PBA or TUDCA alone, though there were some variabilities among cell lines.
A combination treatment of 4-PBA and TUDCA improves insulin secretion and survival in stem cell–derived β cells with WFS1 c.1672C>T, p.R558C variant.
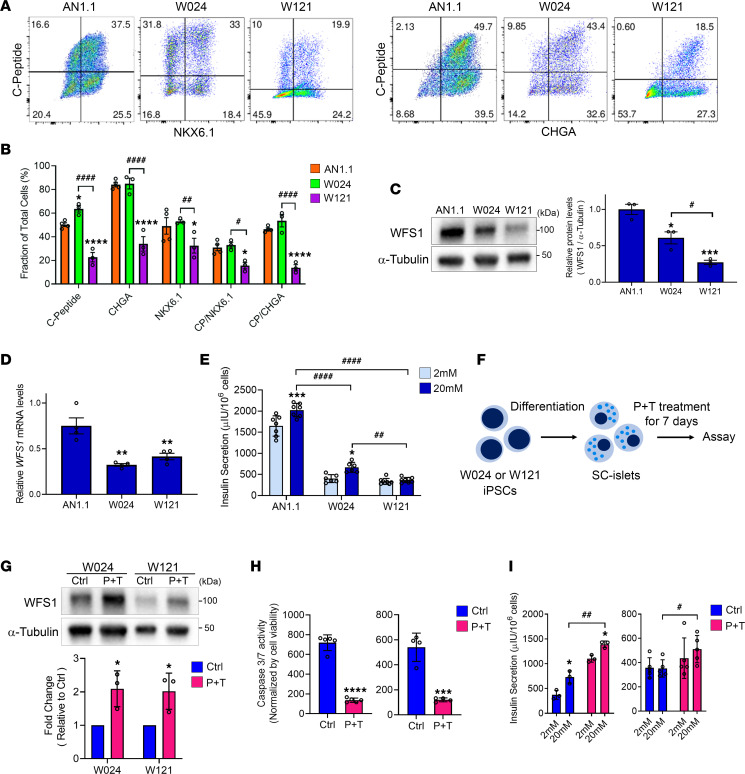
The majority of patients with Wolfram syndrome develop diabetes mellitus due to the pathogenic WFS1 variants causing detrimental effects in pancreatic β cells (13, 14, 20). To evaluate the impact of the WFS1 c.1672C>T, p.R558C variant on β cells, we generated stem cell–derived pancreatic islets (SC-islets) from W024 and W121 iPSCs and AN1.1 iPSCs as a control. We previously developed a 6-stage differentiation strategy, incorporating cytoskeleton modulation, to produce SC-islets containing hormone-secreting endocrine cell types, including insulin-positive stem cell–derived β (SC-β), glucagon-positive stem cell–derived α, and somatostatin-positive stem cell–derived δ cells (47, 48) (Supplemental Figure 8A). The W024 and W121 stage 6 SC-islets produced C-peptide+ cells coexpressing β cell differentiation marker (NKX6.1) and committed endocrine cell marker (chromogranin A, CHGA). The β cell population was similar between W024 and control SC-islets but reduced in the W121 line (Figure 4, A and B). WFS1 protein was expressed in SC-islets derived from all 3 lines, with greater expression detected in control SC-islets (Figure 4C). Of note, WFS1 protein level was significantly higher in W024 SC-islets when compared with W121 (Figure 4C). However, both patient-derived SC-islets (W024 and W121) showed a significant reduction of WFS1 mRNA levels (Figure 4D), which was not observed in W024 iPSCs and NPCs (Figure 2G and Supplemental Figure 4B). We previously demonstrated the robust increase of WFS1 expression during the SC-islet differentiation from stage 5 to stage 6 (49), suggesting that WFS1 expression in SC-islets could be much higher than iPSCs and NPCs. Previous studies showed WFS1 deficiency causes mild dilation of the ER in β cells (19, 50, 51). Electron microscopic analyses displayed well-formed ER structures in AN1.1 SC-islets (Supplemental Figure 9). On the other hand, ERs in W024 and W121 SC-islets were distorted, fragmented, and dilated (Supplemental Figure 9). We tested the functional capacity of the SC-islets in response to high glucose (20 mM) using the GSIS assay. Throughout GSIS, W024 and W121 SC-islets secreted less insulin compared with control SC-islets. W024 SC-islets were able to increase their insulin secretion in response to the glucose stimulus, whereas W121 SC-islets were not capable of a glucose-stimulated response (Figure 4E). These data suggest the WFS1 c.1672C>T, p.R558C variant has a milder effect on β cell insulin secretion than the WFS1 c.2654C>T, p.P885L variant.
Figure 4. Insulin secretion is increased by a combination treatment of 4-PBA and TUDCA in SC-islets with WFS1 c.1672C>T, p.R558C variant.
(A) Representative flow cytometry dot plots and (B) quantified fraction of cells expressing or coexpressing pancreatic β cell or committed endocrine cell markers for AN1.1 (n = 4), W024 (n = 3), and W121 (n = 3) stage 6 SC-islets (*P < 0.05 and ****P < 0.0001 by 2-way ANOVA compared with AN1.1; #P < 0.05, ##P < 0.01, and ####P < 0.0001 by 2-way ANOVA). (C) (Left) Representative blotting image of WFS1 and α-Tubulin in stage 6 SC-islets. (Right) Quantification of relative WFS1 protein level normalized with α-Tubulin (n = 3, *P < 0.05 and ***P < 0.001 by 1-way ANOVA compared with AN1.1; #P < 0.05 by 1-way ANOVA). (D) Relative mRNA levels of WFS1 in stage 6 SC-islets (n = 4, **P < 0.01 by 1-way ANOVA compared with AN1.1). (E) Static GSIS functional assessment of AN1.1 (n = 7), W024 (n = 6), and W121 (n = 8) stage 6 SC-islets (*P < 0.05 and ***P < 0.001 by 2-way ANOVA compared with 2 mM of each line; ##P < 0.01 and ####P < 0.0001 by 2-way ANOVA). (F) A schematic of P+T verification in SC-islets. (G) (Upper) Representative blotting images of WFS1 and α-Tubulin in stage 6 SC-islets treated with or without P+T for 7 days. (Lower) Quantification of WFS1 protein levels normalized with α-Tubulin. (n = 3, *P < 0.05 by unpaired t test compared with Ctrl.) (H) Caspase-3/7 activity normalized by cell viability in stage 6 SC-islets treated with or without P+T for 7 days (n = 3, ***P < 0.001 and ****P < 0.0001 by unpaired t test compared with Ctrl). (I) Static GSIS functional assessment of W024 (n = 5) and W121 (n = 4) treated with or without P+T for 7 days. (*P < 0.05 by by unpaired t test compared with 2 mM of each condition; #P < 0.05 and ##P < 0.01 by 2-way unpaired t test.) CP, C-peptide.
Next, we tested if the P+T treatment is effective in ameliorating W024 and W121 SC-islet dysfunction (Figure 4F). WFS1 protein expression was restored in the treated SC-islets, as observed in both the W024 and W121 iPSCs (Figure 4G). We observed the dilated ER was remitted in some W024 and W121 SC-islet cells treated with P+T (Supplemental Figure 9). In addition, the P+T treatment greatly inhibited cell death in the W024 and W121 SC-islets (Figure 4H). As expected with greater WFS1 protein, the insulin secretion of W024 and W121 SC-islets in low and high glucose was increased by the P+T treatment (Figure 4I). However, insulin content was increased only in W121 SC-islets by the P+T treatment (Supplemental Figure 8B). The proinsulin/insulin ratio was not changed in either W024 or W121 SC-islets by the treatment (Supplemental Figure 8C), suggesting P+T does not alter insulin processing. We also measured OCRs of W024 and W121 treated with P+T. Although W024 showed a trend of slight increase in oxygen consumption over the assay by P+T treatment, neither W024 nor W121 showed significant improvement by the treatment (Supplemental Figure 8D). In summary, P+T treatment restored WFS1 expression and increased insulin secretion capabilities of W024 and W121 SC-islets.
Cellular stress is mitigated by a combination treatment of 4-PBA and TUDCA in SC-islets with WFS1 c.1672C>T, p.R558C variant.
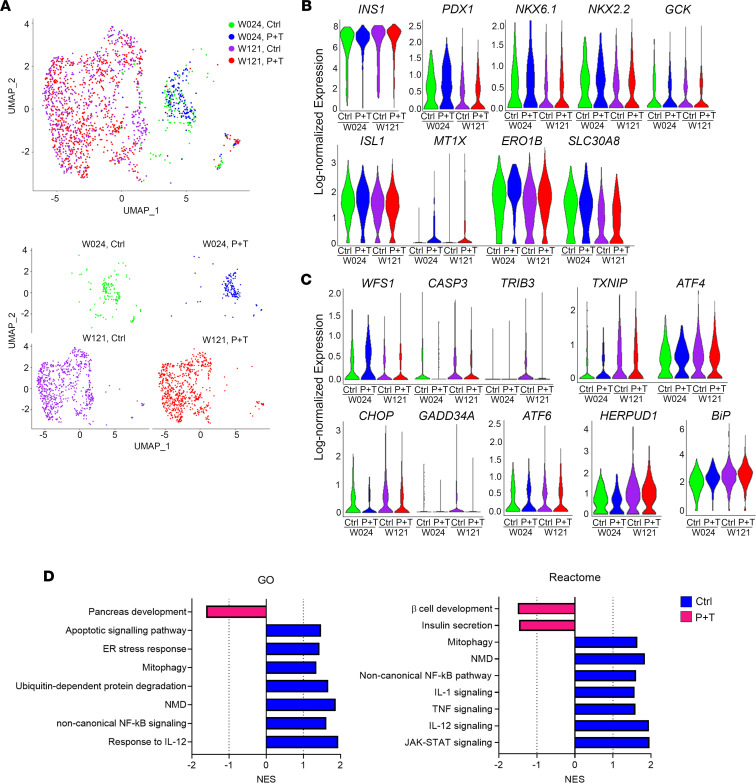
We performed multiplexed single-cell RNA sequencing (scRNA-Seq) using the 10x Genomics platform to investigate genotype-phenotype correlations and the efficacy of the P+T combination treatment on SC-β cells more precisely. We utilized cell hashing, which applies oligo-tagged antibodies to the cell surface proteins of individual samples, thus allowing detection of individual samples within a pooled cell population (52). We sequenced 4 biological replicates per cell line from independent differentiations, treated with or without P+T for 7 days. In total, we sequenced 16 samples with 8 samples in each pooled population, which were submitted separately based on the cell line. In total, we sequenced 13,951 stage 6 SC-islet cells differentiated from W024 and W121 iPSCs to study the effects of P+T treatment (W024: 2,619 cells; W024, P+T: 3,158 cells; W121: 3,625 cells; and W121, P+T: 4,549 cells; 4 biological replicates for each sample). The scRNA-Seq data were analyzed using dimensionality reduction and unsupervised clustering to classify individual cells into cell populations based on similarities in their transcriptome profiles. The cell types were identified by aligning the top upregulated genes in each cell cluster population with published pancreatic transcriptome data (53, 54). After identifying the β cell population in each sample, we combined the 2,329 SC-β cells from the 4 experimental conditions (W024: 377 cells; W024, P+T: 220 cells; W121: 749 cells; and W121, P+T: 680 cells) and performed principal component analysis and unsupervised clustering. The β cells clustered together based on genetic background, regardless of combination treatment (Figure 5A), suggesting the β cell transcriptional profile was not greatly changed in response to P+T.
Figure 5. Single-cell transcriptional evaluation of a combination treatment with 4-PBA and TUDCA on SC-β cells.
(A) Uniform manifold approximation and projection (UMAP) plot from unsupervised clustering of combined transcriptional data from scRNA-Seq of W024, Ctrl (green); W024, P+T (blue); W121, Ctrl (purple); and W121, P+T (red) SC-β cell populations. Lower plots are UMAP plots split by experimental conditions. (B) Violin plots detailing log-normalized gene expression of β cell genes in the same populations as A. Log fold change and P values for violin plots are available in Supplemental Table 2. (C) Violin plots detailing log-normalized gene expression of ER stress and apoptotic genes in the same populations as A. Log fold change and P values for violin plots are available in Supplemental Table 3. (D) Gene Ontology (GO) and Reactome GSEA, quantified by the normalized enrichment score (NES), for pathways upregulated in the combined population of W024 and W121 SC-β cells treated with (pink) or without (blue) P+T. NES values, P values, FDR q values, and gene set lists are available in Supplemental Table 4.
Next, we evaluated the key β cell genes and ER stress markers. WFS1 pathogenic variants cause ER stress, resulting in altered expression of β cell genes in SC-β cells (49). Expression of the insulin gene (INS), crucial transcription factors for β cell differentiation (ISL1, NKX6.1, NKX2.2, and PDX1), β cell maturation genes (MT1X and ERO1B), and a β cell function gene (GCK) was similar between untreated W024 and W121 SC-β cells (Figure 5B and Supplemental Table 2). Interestingly, the expression of SLC30A8, an alternate β cell maturation gene, was significantly higher in W024 SC-β cells compared with W121 (Figure 5B and Supplemental Table 2). The P+T treatment did not change the gene expression for many genes in W024 SC-β cells (Figure 5B and Supplemental Table 2). On the other hand, MT1X and ERO1B were highly expressed in W121 SC-β cells treated with P+T compared with untreated (Figure 5B and Supplemental Table 2). Unlike WFS1 protein levels in SC-islets, WFS1 transcription within the SC-β cell population was similar between both W024 and W121 SC-β cells (Figure 4C, Figure 5C, and Supplemental Table 3). Some ER stress markers (TXNIP and BiP) were highly expressed in W121 SC-β cells compared with W024 (Figure 5C and Supplemental Table 3), whereas other ER stress markers (ATF6, ATF4, CHOP, GADD34A, TRIB3, and HERPUD1) and apoptotic (CASP) genes were not statistically different between both W024 and W121 SC-β cells (Figure 5C and Supplemental Table 3). The expression of WFS1, ER stress markers, and apoptotic genes was not statistically altered by the P+T treatment in either W024 or W121 SC-β cells (Figure 5C and Supplemental Table 3).
We performed gene set enrichment analysis (GSEA) on the SC-β cells. Gene sets pertaining to NMD, ubiquitination-mediated protein degradation, and oxidative stress were enriched in the untreated SC-β cells compared with the P+T-treated SC-β cells (Figure 5D and Supplemental Table 4). Interestingly, we found the inflammation and the selective mitochondrial autophagy (mitophagy) pathways were also enriched in the untreated SC-β cell population (Figure 5D, Supplemental Figure 10, and Supplemental Table 4). Although we did not observe major changes in the expression of ER stress markers and apoptotic genes in W024 and W121 SC-β cells treated with P+T, gene sets pertaining to apoptosis and ER stress were enriched in the untreated SC-β cells (Figure 5D, Supplemental Figure 10, and Supplemental Table 4). Of note, gene sets related to insulin secretion and β cell development were enriched in the P+T-treated SC-β cell population (Figure 5D, Supplemental Figure 10, and Supplemental Table 4). Additionally, gene sets related to regulation of cytosolic K+ and Ca2+ levels were increased, and these levels play an important role in β cell differentiation and function (55–57) (Supplemental Figure 10 and Supplemental Table 4). Collectively, P+T treatment mitigated cellular stress increased by pathogenic WFS1 variants without changing β cell identity, which resulted in increased β cell and insulin secretion in W024 and W121 SC-β cells.
A combination treatment of 4-PBA and TUDCA delays the diabetic phenotype progression in Wfs1-deficient mice.
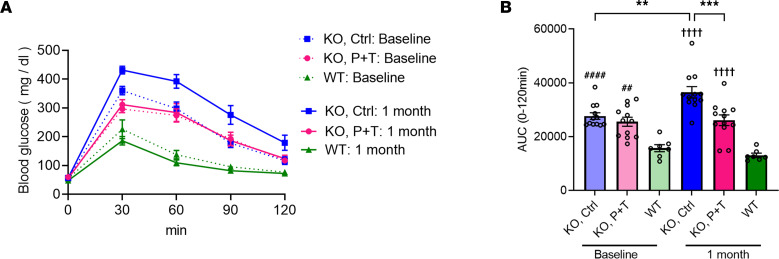
Finally, we verified the efficacy of our combination treatment with chemical chaperones with an in vivo study. The field lacks a c.1672C>T, p.R558C variant WFS1 mutation mouse model. Therefore, we employed 129S6 whole-body Wfs1-knockout (Wfs1-KO) mice. This mouse model develops progressive glucose intolerance during adolescence, hence a mouse model of Wolfram syndrome (20). We showed Wfs1-KO mice developed glucose intolerance at 5–6 weeks old (Figure 6, A and B). In addition, Wfs1-KO mice did not show glucose-stimulated increase of the serum insulin level, which was lower than that of WT (Supplemental Figure 11A). We treated the mice at 5–6 weeks old with P+T chow (4-PBA: 0.338% and TUDCA: 0.225%) for 1 month. Both groups of Wfs1-KO mice consumed similar amounts of chow (Supplemental Figure 11B). After feeding for 1 month, Wfs1-KO mice fed with control chow developed more severe glucose intolerance (Figure 6, A and B). Conversely, an IP-GTT blood glucose curve was similar to the baseline outcome in Wfs1-KO mice fed with P+T chow (Figure 6, A and B), indicating that P+T chow delayed the progression of the diabetic phenotype. Body weight and insulin sensitivity were not greatly changed by the P+T chow (Supplemental Figure 11, C–E). The basal level of serum insulin (0 minutes) was higher in Wfs1-KO mice fed with P+T chow as compared with control chow (Supplemental Figure 11F). Compared with baseline, serum insulin level at 30 minutes following glucose injection was decreased in Wfs1-KO mice fed with control chow, whereas it was similar in the mice fed with P+T chow (Supplemental Figure 11G). Collectively, we observed delays in the Wolfram diabetic phenotype in Wfs1-KO mice when using the P+T treatment. Therefore, we expect the combination treatment to be efficacious against diabetic Wolfram phenotypes caused by the WFS1 c.1672C>T, p.R558C variant in vivo.
Figure 6. In vivo verification of a combination treatment with chemical chaperones.
(A) Intraperitoneal glucose tolerance test (IP-GTT) with WT or Wfs1-KO mice at baseline and 1 month after feeding with either control chow or food containing 4-PBA: 0.338% and TUDCA: 0.225% (P+T chow). (B) AUCs of the IP-GTT (KO, Ctrl: n = 12; KO, P+T: n = 12; WT: n = 7; **P < 0.01 and ***P < 0.001 by 1-way ANOVA; ##P < 0.01 and ####P < 0.0001 by 1-way ANOVA compared with WT: Baseline; ††††P < 0.0001 by 1-way ANOVA compared with WT: 1 month).
Discussion
In this study, we characterize a likely unique pathogenic WFS1 c.1672C>T, p.R558C variant, which is associated with a mild form of Wolfram syndrome and highly prevalent in the Ashkenazi Jewish population. Molecular investigation revealed WFS1 p.R558C had a greater posttranslational stability in cells compared with another pathogenic variant, WFS1 c.2654C>T, p.P885L. Based on the molecular characterization of WFS1 p.R558C, we hypothesized a combination treatment of 2 chemical chaperones, 4-PBA and TUDCA, could provide a treatment for Wolfram syndrome. We demonstrated that a combination treatment of 4-PBA and TUDCA increased WFS1 expression within the cell, ameliorating organelle dysfunction and the associated apoptosis. We also identified that patient SC-islets demonstrated genotype-phenotype relationships that correlated with clinical observations, and a combination treatment with 4-PBA and TUDCA improved insulin secretion in this cellular model of Wolfram syndrome.
Wolfram syndrome is a very rare genetic disorder. In the United Kingdom, the prevalence is 1:770,000, and the carrier frequency of pathogenic WFS1 variants is 1:354 (4). The prevalence in the North American population is estimated to be 1:100,000 (58). We observed greater carrier frequency of the WFS1 c.1672 C>T, p.R558C variant in the Ashkenazi Jewish population with a frequency of 1:43 as compared with 1:76 in the Ashkenazi/Sephardi Jewish population and 1:2,268 in the Sephardi population. Although present in the Sephardi population, as shown in 6 carriers out of 13,608 Sephardi patients tested, we suspect that the low frequency of WFS1 c.1672 C>T, p.R558C variant might be explained by historical admixture with Ashkenazim. Forgotten or suppressed admixture of Gentile and Jewish people was previously exhibited in a study focusing on genome-wide Jewish genetic signature (59).
WFS1 is subjected to a ubiquitin-proteasomal degradation by SMAD specific E3 ubiquitin protein ligase 1 (Smurf1), a HECT-type ligase, which recognizes the degron within the C-terminal luminal region of WFS1 (aa 671–700) (60). Missense mutations in the degron or truncating mutations lacking the degron are resistant to Smurf1-mediated degradation (60). Other WFS1 variants that retain the functional degron can lead to complete depletion or degradation of the WFS1 protein by NMD or proteasomal degradation (31, 32). Our study demonstrated WFS1 p.R558C protein is also subjected to proteasomal degradation. However, the degradation rate was lower than the WFS1 p.P885L variant. Additionally, WFS1 p.R558C protein was detected while conducting in vivo and in vitro experiments, indicating that a portion of the WFS1 p.R558C protein escapes proteasomal degradation. The remaining WFS1 p.R558C protein may compensate for the loss of wild-type WFS1 function. This hypothesis is consistent with the observation that clinically, patients carrying the homozygous WFS1 c.1672 C>T, p.R558C variant have mild or less severe phenotypes of Wolfram syndrome. Our findings strongly suggest that alternate degron-retaining pathogenic WFS1 variants require further protein expression characterization to determine the genotype-phenotype correlation.
Most of the studies conducting experimental genotype-phenotype correlation analysis employ overexpression of mutant WFS1 proteins in transfected cell lines such as HEK293T, HeLa, and COS7 (60, 61). This system is incapable of evaluating detrimental effects of pathogenic WFS1 variants on physiological functions in disease-relevant cells. In pursuit of more reliable models and achieving a deeper understanding of Wolfram phenotypes, we have studied patient-derived, disease-relevant cells. SC-islets, containing SC-β cells, differentiated from patient iPSCs with homozygous WFS1 c.1672C>T, p.R558C variant (W024), displayed insufficient β cell function and similar differentiation efficiency compared to control SC-islets. W024 SC-islet phenotypes were milder than W121 SC-islets differentiated from the heterozygous WFS1 c.1672C>T, c.2654C>T variant iPSC line, indicating that our SC-islets capture Wolfram phenotypes based on pathogenic WFS1 variants and could model genotype-phenotype correlation analysis and drug discovery in vitro. The diabetes stem cell field has recently leveraged this technology to correct the WFS1 pathogenic variant using CRISPR/Cas9 gene-edited SC-islets from a patient with Wolfram syndrome to reverse preexisting diabetes in a mouse (49). Stem cell technology and genetic engineering have also been used for correcting and studying other pathogenic variants (62).
We demonstrated that a combination treatment of 4-PBA and TUDCA was efficacious against the WFS1 c.1672C>T variant. In our screening, the disulfiram molecule stabilized the WFS1 protein. The stabilization is likely due to the inhibitory function of disulfiram on NPL4, an adaptor of p97/VCP segregase, which is essential for ER-associated degradation (ERAD) (63, 64). ERAD is a crucial system aiming to mitigate ER stress. Indeed, some studies showed that disulfiram induces ER stress followed by apoptosis (65, 66). On the other hand, 4-PBA and TUDCA stabilize proteins by facilitating protein folding thereby mitigating ER stress. Numerous studies have demonstrated that 4-PBA and TUDCA are promising for the treatment of diabetes mellitus and other ER stress–related neurodegenerative diseases (67–70). However, most chaperone treatment studies used an individual chaperone and a single-dose treatment. A combination treatment with these compounds has rarely been attempted. Combining several compounds is a common cancer treatment strategy, aiming to yield additive or synergistic efficacy (71). Recently, a study reported that a combination treatment of 4-PBA and TUDCA resulted in slower functional decline of patients with amyotrophic lateral sclerosis, an ER stress–related disease (72). In our study, 4-PBA and TUDCA increased WFS1 protein as well as mRNA levels in the patient-derived iPSCs. We predict this increase of WFS1 mRNA level is induced by 4-PBA activity as an HDAC inhibitor, which selectively promotes gene transcription (41, 73). Alternatively, WFS1 and ER stress marker expression in SC-β cells was not statistically significantly altered by the combination treatment with 4-PBA and TUDCA in our scRNA-Seq analysis. This could be due to variability in the scRNA-Seq data set. Genes not in a consistent steady-state manner cause variable detection, which is commonly observed in ER stress–inducible genes (74, 75). Additionally, current scRNA-Seq technology detects only approximately 10% of the cellular mRNA molecules, resulting in difficulties in detecting genes with small expression within a single cell (76). Therefore, the scRNA-Seq data set may be limited in the number of gene transcript copies detected for ER stress markers and WFS1, thus underestimating the impact of 4-PBA and TUDCA on ER stress in the SC-β cells.
GSEA revealed a combination treatment of 4-PBA and TUDCA downregulated mitophagy, ER stress, and apoptosis. Mitophagy mediates clearance of damaged mitochondria in the cells (77). Pathogenic WFS1 variants have been reported to cause mitochondrial dysfunction (38, 39), and we showed a combination treatment of 4-PBA and TUDCA restored mitochondrial function in NPCs, implying that mitophagy could be reduced by preventing mitochondrial dysfunction. Additionally, in GSEA, several inflammatory pathways were reduced by a combination treatment of 4-PBA and TUDCA. We and others recently reported elevated expression and serum levels of inflammatory cytokines in patients with Wolfram syndrome (27, 78). ER stress and mitochondrial dysfunction have been well known to cause inflammation (79–82). It is possible that the several inflammatory pathways were downregulated as a consequence of reduced ER stress and restored organelle function by a combination treatment of 4-PBA and TUDCA. In summary, a combination treatment of 4-PBA and TUDCA mitigated various cellular stresses the WFS1 c.1672C>T, p.R558C variant caused, resulting in improved insulin secretion.
We previously reported on the restoration of Wolfram phenotypes by correcting pathogenic WFS1 variants with CRISPR/Cas9 in SC-islets derived from patients with typical Wolfram syndrome (49). When comparing the functional assessments and scRNA-Seq analyses, a combination treatment of 4-PBA and TUDCA had a milder effect compared with CRISPR/Cas9 gene correction. This is expected because CRISPR/Cas9 correction directly intervenes on the cause of Wolfram syndrome. However, the combination treatment of 4-PBA and TUDCA as a therapeutic is advantageous for ease of administration. Furthermore, prolonged 4-PBA and TUDCA administration could delay additional symptom onset, including hearing loss and neurodegeneration, which typically manifests at later stages of the disease. Therefore, verifying efficacy of the combination treatment on other relevant cell types and pathogenic WFS1 variants is warranted.
This study harnesses multiple iPSC-derived in vitro disease models and demonstrates efficacy of a combination treatment of 4-PBA and TUDCA. The benefits of this treatment may apply to other genetic ER stress–related diseases, such as Wolcott-Rallison syndrome. Moreover, our differentiation technology for in vitro disease models could be leveraged as a drug screening tool to discover new therapies or pave the way to personalized medicine strategies for patients with Wolfram syndrome.
Methods
iPSC lines.
To generate iPSCs, we obtained PBMCs from patients with Wolfram syndrome. The iPSC lines were generated by the Genome Engineering and iPSC Center (GEiC) at Washington University in St. Louis with Sendai viral reprogramming. Control iPSC lines BJFF.6 and AN1.1 were obtained from the GEiC at Washington University in St. Louis. HEK293 cells were obtained from ATCC. Validation of pluripotency was performed using Pluripotent Stem Cell 4-Marker Immunocytochemistry Kit (Thermo Fisher Scientific; A24881).
Chemical chaperones.
We obtained 4-PBA and TUDCA from Amylyx Pharmaceuticals Inc. We dissolved 4-PBA and TUDCA in PBS and used them at final concentrations of 500 μM and 50 μM, respectively. Control conditions were treated with only PBS. The treatment time is described in the figure legends. Stock reagents were kept at 4°C and made fresh every 2 weeks. For animal experiments, we ordered Envigo custom-made chow containing 4-PBA and TUDCA (4-PBA: 34 mg/kg and TUDCA: 23 mg/kg in Teklad global 18% protein rodent diet). The animals ate approximately 4 g of diet per day, with a goal of 6 g 4-PBA/kg body weight/d and 4 g TUDCA/kg body weight/d. The same base diet without chemical chaperones was used as the control chow.
NPC differentiation.
Undifferentiated stem cells were plated down on tissue culture plastic in mTeSR1 (StemCell Technologies; 05850) and cultured in a humidified 5% CO2 and 37°C tissue culture incubator. NPC differentiation was performed as described previously (83, 84). Briefly, 4 × 104 to 6 × 104 cells per well were seeded onto V-bottom, 96-well plates (Corning) in NPC differentiation medium suppled with 10 μM Y27632 (Tocris; 1254) to generate embryonic bodies (EBs) (day 0). On day 4, EBs were plated on 6 cm dishes coated with poly-l-ornithine solution (20 μg/mL, MilliporeSigma) and laminin (1 μg/mL, Thermo Fisher Scientific) in NPC differentiation medium. On day 12, neural rosettes were detached from the dish using STEMdiff Neural Rosette Selection Reagent (StemCell Technologies; 05832), and dissociated cells (NPCs) were plated on 6 cm dishes coated with Matrigel and laminin (5 μg/mL). NPC differentiation medium consists of Neurobasal-A (Life Technologies; 10888022), 1× B27 supplement without vitamin A (Life Technologies; 12587), 1% nonessential amino acids (Life Technologies; 11140050), 0.1% 2-mercaptoethanol (Life Technologies; 21985-023), 1% PenStrep (Life Technologies; 15140122), 1% Glutamax (Life Technologies 35050061), 10 μM SB-431542 (Tocris; 1614), and 100 nM LDN-193189 (Tocris; 6053). After the differentiation, medium was changed every other day in the whole differentiation process. NPCs were maintained in STEMdiff Neural Progenitor Medium (StemCell Technologies; 05833). Passage was performed with Accutase (MilliporeSigma; A6964) and medium was changed every day.
SC-islet differentiation.
SC-islet differentiation was performed similarly to as we have previously reported (47, 48). Undifferentiated stem cells were plated down on tissue culture plastic in mTeSR1 and cultured in a humidified 5% CO2, 37°C tissue culture incubator. Stem cell passaging occurred every 3–4 days, with TrypLE (Life Technologies; 12-604-039) used for single-cell dispersion and Vi-Cell XR (Beckman Coulter) for counting. Undifferentiated stem cells were seeded at 0.52 × 106 cells/cm2 in mTeSR1 + 10 μM Y27632 (Abcam; ab120129) on Matrigel-coated (Corning; 356230) plates. On stage 6 day 7, cells were single-cell dispersed with TrypLE and seeded in a 6-well plate at 5 × 106 cells per well with 4 mL of stage 6 enriched serum-free medium per well. Cells continued culturing on an orbi-shaker (Benchmark) set at 100 rpm in a humidified tissue culture incubator at 5% CO2 and 37°C. Supplemental Table 7 contains subsequent feeding schedule, media formulations, and differentiation factors. Assays were carried out between stage 6 day 9 and 20.
Data availability.
RNA-Seq data were deposited in the NCBI’s Gene Expression Omnibus database (GEO accession GSE212256).
Statistics.
Statistical analysis was performed by 1-tailed unpaired and paired t tests and 1- and 2-way ANOVA with Tukey’s or Dunnett’s tests. Statistical tests are specified in figure legends. P < 0.05 was considered statistically significant. Data are shown as means ± SEM unless otherwise noted.
Study approval.
For human study, patients and their parents or legal guardians, as appropriate, provided written informed consent before participating in this study, which was approved by the Human Research Protection Office at Washington University School of Medicine in St. Louis (IRB ID 201107067). Animal experimentation was performed according to procedures approved by the Institutional Animal Care and Use Committee at the Washington University School of Medicine in St. Louis (20-0334).
Further methods details are available in the Supplemental Methods.
Author contributions
RAK, KGM, JRM, and FU conceived the experimental design. YH, MMJ, TW, JE, and FU conducted clinical investigation. WY, MJH, and AS performed WFS1 SplitLuc assays and screening. KGM differentiated β cells and performed functional assays. KGM and PA performed sequencing analysis. RAK and CMB performed animal experiments. RAK differentiated NPCs and RAK, CMB, and KK performed the other in vitro experiments. JC, JK, and KL examined data on 4-PBA+TUDCA. JRM and FU supervised the data. RAK, KGM, and FU wrote the manuscript. All authors edited and reviewed the manuscript.
Supplementary Material
Acknowledgments
This work was partly supported by grants from the NIH/National Institute of Diabetes and Digestive and Kidney Diseases (DK112921, DK020579, DK114233, DK127497) and NIH/NCATS (TR002065, TR000448); JDRF (5-CDA-2017-391-A-N); and philanthropic support from the Silberman Fund, the Ellie White Foundation for Rare Genetic Disorders, the Snow Foundation, the Unravel Wolfram Syndrome Fund, the Stowe Fund, the Feiock Fund, the Cachia Fund, the Gildenhorn Fund, the Eye Hope Foundation, Ontario Wolfram League, Associazione Gentian — Sindrome di Wolfram Italia, Alianza de Familias Afectadas por el Sindrome Wolfram Spain, Wolfram Syndrome UK, and Association Syndrome de Wolfram France to FU. Research reported in this publication was also supported, in part, by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the NIH/NCATS. WY, AS, and MJH were supported by the intramural research program of the NIH/NCATS. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
We thank all the members of the Washington University Wolfram Syndrome Study and Research Clinic for their support (https://wolframsyndrome.wustl.edu) and all the participants in the Wolfram Syndrome International Registry and Clinical Study, Research Clinic, and Clinical Trials for their time and efforts. We would also like to thank Chinyere Onwumere at Washington University School of Medicine in St. Louis for her support concerning in vivo experiments, Kesavan Meganathan at Washington University School of Medicine in St. Louis for helping us to establish NPC differentiation, David Chao and Richard Suderman at Stowers Institute for Medical Research for providing the plasmids, and Sarah Gladstone and Beth White for scientific feedback.
Version 1. 09/22/2022
Electronic publication
Footnotes
Conflict of interest: FU is an inventor of 3 patents related to Wolfram syndrome treatment, US 9,891,231 “Soluble MANF in pancreatic beta-cell disorders,” and US 10,441,574 and US 10,695,324 “Treatment for Wolfram syndrome and other endoplasmic reticulum stress disorders.” JRM is an inventor on a patent and patent application relating to stem cell–derived pancreatic islets, US 10,030,229 “SC-β cells and compositions and methods for generating the same” and US application PCT/US2019/032643 “Methods and Compositions for Generating Cells of Endodermal Lineage and Beta Cells and Uses Thereof.” FU is a founder and president of CURE4WOLFRAM, Inc. JRM is a consultant for Sana Biotechnology. JC and JK are co-CEOs of and report personal fees from and equity holdings in Amylyx Pharmaceuticals Inc. JC and JK have 2 patents, “Compositions for improving cell viability and methods of use thereof” (US 9,872,865; US Patent 10,251,896; AU 2014242123; EP 14775675.3; and JP2016515575A) and “For improving the composition of cell viability and using the method for the composition” (CN105050593B), issued to Amylyx Pharmaceuticals Inc. JL reports personal fees from and equity holdings in Amylyx Pharmaceuticals Inc.
Copyright: © 2022, Asada Kitamura et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: JCI Insight. 2022;7(18):e156549.https://doi.org/10.1172/jci.insight.156549.
Contributor Information
Kristina G. Maxwell, Email: kristinawernes@gmail.com.
Wenjuan Ye, Email: wenjuan.ye@nih.gov.
Kelly Kries, Email: kellykries@gmail.com.
Cris M. Brown, Email: crisbrown@wustl.edu.
Punn Augsornworawat, Email: punn@wustl.edu.
Yoel Hirsch, Email: yh@doryeshorim.org.
Martin M. Johansson, Email: mmj@doryeshorim.org.
Tzvi Weiden, Email: tzviden@doryes.com.
Justin Klee, Email: justin_klee@amylyx.com.
Kent Leslie, Email: kentleslie8@gmail.com.
Anton Simeonov, Email: asimeono@mail.nih.gov.
Mark J. Henderson, Email: mark.henderson2@nih.gov.
Jeffrey R. Millman, Email: jmillman@wustl.edu.
Fumihiko Urano, Email: urano@wustl.edu.
References
- 1.Wolfram DJ, Wagener HP. Diabetes mellitus and simple optic atrophy among siblings: report of four cases. May Clin Proc. 1938;13:715–718. [Google Scholar]
- 2.Inoue H, et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome) Nat Genet. 1998;20(2):143–148. doi: 10.1038/2441. [DOI] [PubMed] [Google Scholar]
- 3.Amr S, et al. A homozygous mutation in a novel zinc-finger protein, ERIS, is responsible for Wolfram syndrome 2. Am J Hum Genet. 2007;81(4):673–683. doi: 10.1086/520961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett TG, Bundey SE. Wolfram (DIDMOAD) syndrome. J Med Genet. 1997;34(10):838–841. doi: 10.1136/jmg.34.10.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett TG, et al. Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet. 1995;346(8988):1458–1463. doi: 10.1016/S0140-6736(95)92473-6. [DOI] [PubMed] [Google Scholar]
- 6.Urano F. Wolfram syndrome: diagnosis, management, and treatment. Curr Diab Rep. 2016;16(1):6. doi: 10.1007/s11892-015-0702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Franco E, et al. Dominant ER stress-inducing WFS1 mutations underlie a genetic syndrome of neonatal/infancy-onset diabetes, congenital sensorineural deafness, and congenital cataracts. Diabetes. 2017;66(7):2044–2053. doi: 10.2337/db16-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van ven Ouweland JM, et al. Molecular characterization of WFS1 in patients with Wolfram syndrome. J Mol Diagn. 2003;5(2):88–95. doi: 10.1016/S1525-1578(10)60457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanim F, et al. WFS1/wolframin mutations, Wolfram syndrome, and associated diseases. Hum Mutat. 2001;17(5):357–367. doi: 10.1002/humu.1110. [DOI] [PubMed] [Google Scholar]
- 10.Takei D, et al. WFS1 protein modulates the free Ca(2+) concentration in the endoplasmic reticulum. FEBS Lett. 2006;580(24):5635–5640. doi: 10.1016/j.febslet.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Lu S, et al. A calcium-dependent protease as a potential therapeutic target for Wolfram syndrome. Proc Natl Acad Sci U S A. 2014;111(49):E5292–E5301. doi: 10.1073/pnas.1421055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara T, et al. Calcium efflux from the endoplasmic reticulum leads to β-cell death. Endocrinology. 2014;155(3):758–768. doi: 10.1210/en.2013-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonseca SG, et al. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem. 2005;280(47):39609–39615. doi: 10.1074/jbc.M507426200. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca SG, et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest. 2010;120(3):744–755. doi: 10.1172/JCI39678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angebault C, et al. ER-mitochondria cross-talk is regulated by the Ca 2+ sensor NCS1 and is impaired in Wolfram syndrome. Sci Signal. 2018;11(553):eaaq1380. doi: 10.1126/scisignal.aaq1380. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara H, et al. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet. 2004;13(11):1159–1170. doi: 10.1093/hmg/ddh125. [DOI] [PubMed] [Google Scholar]
- 17.Luuk H, et al. Distribution of Wfs1 protein in the central nervous system of the mouse and its relation to clinical symptoms of the Wolfram syndrome. J Comp Neurol. 2008;509(6):642–660. doi: 10.1002/cne.21777. [DOI] [PubMed] [Google Scholar]
- 18.Plaas M, et al. Wfs1- deficient rats develop primary symptoms of Wolfram syndrome: insulin-dependent diabetes, optic nerve atrophy and medullary degeneration. Sci Rep. 2017;7(1):10220. doi: 10.1038/s41598-017-09392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riggs AC, et al. Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia. 2005;48(11):2313–2321. doi: 10.1007/s00125-005-1947-4. [DOI] [PubMed] [Google Scholar]
- 20.Abreu D, et al. Wolfram syndrome 1 gene regulates pathways maintaining beta-cell health and survival. Lab Invest. 2020;100(6):849–862. doi: 10.1038/s41374-020-0408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young JE, et al. Elucidating molecular phenotypes caused by the SORL1 Alzheimer’s disease genetic risk factor using human induced pluripotent stem cells. Cell Stem Cell. 2015;16(4):373–385. doi: 10.1016/j.stem.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anastasaki C, et al. Human iPSC-derived neurons and cerebral organoids establish differential effects of germline NF1 gene mutations. Stem Cell Reports. 2020;14(4):541–550. doi: 10.1016/j.stemcr.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abreu D, Urano F. Current landscape of treatments for Wolfram syndrome. Trends Pharmacol Sci. 2019;40(10):711–714. doi: 10.1016/j.tips.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kakiuchi C, et al. Valproate, a mood stabilizer, induces WFS1 expression and modulates its interaction with ER stress protein GRP94. PLoS One. 2009;4(1):e4134. doi: 10.1371/journal.pone.0004134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toots M, et al. Preventive treatment with liraglutide protects against development of glucose intolerance in a rat model of Wolfram syndrome. Sci Rep. 2018;8(1):10183. doi: 10.1038/s41598-018-28314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo M, et al. Activation of GLP-1 receptor signalling alleviates cellular stresses and improves beta cell function in a mouse model of Wolfram syndrome. Diabetologia. 2018;61(10):2189–2201. doi: 10.1007/s00125-018-4679-y. [DOI] [PubMed] [Google Scholar]
- 27.Abreu D, et al. A phase Ib/IIa clinical trial of dantrolene sodium in patients with Wolfram syndrome. JCI Insight. 2021;6(15):145188. doi: 10.1172/jci.insight.145188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal V, et al. Identification of a missense variant in the WFS1 gene that causes a mild form of Wolfram syndrome and is associated with risk for type 2 diabetes in Ashkenazi Jewish individuals. Diabetologia. 2018;61(10):2180–2188. doi: 10.1007/s00125-018-4690-3. [DOI] [PubMed] [Google Scholar]
- 29.Meiner V, et al. A common Lithuanian mutation causing familial hypercholesterolemia in Ashkenazi Jews. Am J Hum Genet. 1991;49(2):443–449. [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen GM, et al. The Tay-Sachs disease gene in North American Jewish populations: geographic variations and origin. Am J Hum Genet. 1983;35(6):1258–1269. [PMC free article] [PubMed] [Google Scholar]
- 31.De Heredia ML, et al. Genotypic classification of patients with Wolfram syndrome: insights into the natural history of the disease and correlation with phenotype. Genet Med. 2013;15(7):497–506. doi: 10.1038/gim.2012.180. [DOI] [PubMed] [Google Scholar]
- 32.Rigoli L, et al. Genetic and clinical aspects of Wolfram syndrome 1, a severe neurodegenerative disease. Pediatr Res. 2018;83(5):921–929. doi: 10.1038/pr.2018.17. [DOI] [PubMed] [Google Scholar]
- 33.Martinez NJ, et al. A widely-applicable high-throughput cellular thermal shift assay (CETSA) using split Nano Luciferase. Sci Rep. 2018;8(1):9472. doi: 10.1038/s41598-018-27834-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardy C, et al. Clinical and molecular genetic analysis of 19 Wolfram syndrome kindreds demonstrating a wide spectrum of mutations in WFS1. Am J Hum Genet. 1999;65(5):1279–1290. doi: 10.1086/302609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian X, et al. Phenotype prediction of pathogenic nonsynonymous single nucleotide polymorphisms in WFS1. Sci Rep. 2015;5:14731. doi: 10.1038/srep14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urano F. Diabetes: Targeting endoplasmic reticulum to combat juvenile diabetes. Nat Rev Endocrinol. 2014;10(3):129–130. doi: 10.1038/nrendo.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urano F. Wolfram syndrome iPS cells: the first human cell model of endoplasmic reticulum disease. Diabetes. 2014;63(3):844–846. doi: 10.2337/db13-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koks S, et al. Silencing of the WFS1 gene in HEK cells induces pathways related to neurodegeneration and mitochondrial damage. Physiol Genomics. 2013;45(5):182–190. doi: 10.1152/physiolgenomics.00122.2012. [DOI] [PubMed] [Google Scholar]
- 39.La Morgia C, et al. Calcium mishandling in absence of primary mitochondrial dysfunction drives cellular pathology in Wolfram syndrome. Sci Rep. 2020;10(1):4785. doi: 10.1038/s41598-020-61735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cortez L, Sim V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion. 2014;8(2):197–202. doi: 10.4161/pri.28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright JM, et al. Gene expression profile analysis of 4-phenylbutyrate treatment of IB3-1 bronchial epithelial cell line demonstrates a major influence on heat-shock proteins. Physiol Genomics. 2004;16(2):204–211. doi: 10.1152/physiolgenomics.00160.2003. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigues CM, et al. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med. 1998;4(3):165–178. doi: 10.1007/BF03401914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodrigues CM, et al. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101(12):2790–2799. doi: 10.1172/JCI1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues CM, et al. Tauroursodeoxycholic acid prevents Bax-induced membrane perturbation and cytochrome C release in isolated mitochondria. Biochemistry. 2003;42(10):3070–3080. doi: 10.1021/bi026979d. [DOI] [PubMed] [Google Scholar]
- 45.Azzaroli F, et al. Ursodeoxycholic acid diminishes Fas-ligand-induced apoptosis in mouse hepatocytes. Hepatology. 2002;36(1):49–54. doi: 10.1053/jhep.2002.34511. [DOI] [PubMed] [Google Scholar]
- 46.Shang L, et al. β-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes. 2014;63(3):923–933. doi: 10.2337/db13-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hogrebe NJ, et al. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat Biotechnol. 2020;38(4):460–470. doi: 10.1038/s41587-020-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hogrebe NJ, et al. Generation of insulin-producing pancreatic β cells from multiple human stem cell lines. Nat Protoc. 2021;16(9):4109–4143. doi: 10.1038/s41596-021-00560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maxwell KG, et al. Gene-edited human stem cell-derived β cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci Transl Med. 2020;12(540):eaax9106. doi: 10.1126/scitranslmed.aax9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akiyama M, et al. Increased insulin demand promotes while pioglitazone prevents pancreatic beta cell apoptosis in Wfs1 knockout mice. Diabetologia. 2009;52(4):653–663. doi: 10.1007/s00125-009-1270-6. [DOI] [PubMed] [Google Scholar]
- 51.Hatanaka M, et al. Wolfram syndrome 1 gene (WFS1) product localizes to secretory granules and determines granule acidification in pancreatic beta-cells. Hum Mol Genet. 2011;20(7):1274–1284. doi: 10.1093/hmg/ddq568. [DOI] [PubMed] [Google Scholar]
- 52.Stoeckius M, et al. Cell Hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol. 2018;19(1):224. doi: 10.1186/s13059-018-1603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muraro MJ, et al. A single-cell transcriptome atlas of the human pancreas. Cell Syst. 2016;3(4):385–394. doi: 10.1016/j.cels.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baron M, et al. A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure. Cell Syst. 2016;3(4):346–360. doi: 10.1016/j.cels.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark R, Proks P. ATP-sensitive potassium channels in health and disease. Adv Exp Med Biol. 2010;654:165–192. doi: 10.1007/978-90-481-3271-3_8. [DOI] [PubMed] [Google Scholar]
- 56.Zeng H, et al. An isogenic human ESC platform for functional evaluation of genome-wide-association-study-identified diabetes genes and drug discovery. Cell Stem Cell. 2016;19(3):326–340. doi: 10.1016/j.stem.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klec C, et al. Calcium signaling in ß-cell physiology and pathology: a revisit. Int J Mol Sci. 2019;20(24):6110. doi: 10.3390/ijms20246110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinsley BT, et al. Morbidity and mortality in the Wolfram syndrome. Diabetes Care. 1995;18(12):1566–1570. doi: 10.2337/diacare.18.12.1566. [DOI] [PubMed] [Google Scholar]
- 59.Need AC, et al. A genome-wide genetic signature of Jewish ancestry perfectly separates individuals with and without full Jewish ancestry in a large random sample of European Americans. Genome Biol. 2009;10(1):R7. doi: 10.1186/gb-2009-10-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo X, et al. The E3 ligase Smurf1 regulates Wolfram syndrome protein stability at the endoplasmic reticulum. J Biol Chem. 2011;286(20):18037–18047. doi: 10.1074/jbc.M111.225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gong Y, et al. A novel mutation of WFS1 gene leading to increase ER stress and cell apoptosis is associated an autosomal dominant form of Wolfram syndrome type 1. BMC Endocr Disord. 2021;21(1):76. doi: 10.1186/s12902-021-00748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maxwell KG, Millman JR. Applications of iPSC-derived beta cells from patients with diabetes. Cell Rep Med. 2021;2(4):100238. doi: 10.1016/j.xcrm.2021.100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skrott Z, et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature. 2017;552(7684):194–199. doi: 10.1038/nature25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding N, Zhu Q. Disulfiram combats cancer via crippling valosin-containing protein/p97 segregase adaptor NPL4. Transl Cancer Res. 2018;7(suppl 4):S495–S499. doi: 10.21037/tcr.2018.03.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah O’Brien P, et al. Disulfiram (Antabuse) activates ROS-dependent ER stress and apoptosis in oral cavity squamous cell carcinoma. J Clin Med. 2019;8(5):611. doi: 10.3390/jcm8050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X, et al. Induction of autophagy-dependent apoptosis in cancer cells through activation of ER stress: an uncovered anti-cancer mechanism by anti-alcoholism drug disulfiram. Am J Cancer Res. 2019;9(6):1266–1281. [PMC free article] [PubMed] [Google Scholar]
- 67.Mimori S, et al. 4-Phenylbutyric acid protects against neuronal cell death by primarily acting as a chemical chaperone rather than histone deacetylase inhibitor. Bioorg Med Chem Lett. 2013;23(21):6015–6018. doi: 10.1016/j.bmcl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Chaudhuri TK, Paul S. Protein-misfolding diseases and chaperone-based therapeutic approaches. FEBS J. 2006;273(7):1331–1349. doi: 10.1111/j.1742-4658.2006.05181.x. [DOI] [PubMed] [Google Scholar]
- 69.Ricobaraza A, et al. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacology. 2009;34(7):1721–1732. doi: 10.1038/npp.2008.229. [DOI] [PubMed] [Google Scholar]
- 70.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yap TA, et al. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592–1605. doi: 10.1200/JCO.2011.37.6418. [DOI] [PubMed] [Google Scholar]
- 72.Paganoni S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919–930. doi: 10.1056/NEJMoa1916945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cuadrado-Tejedor M, et al. Defining the mechanism of action of 4-phenylbutyrate to develop a small-molecule-based therapy for Alzheimer’s disease. Curr Med Chem. 2011;18(36):5545–5553. doi: 10.2174/092986711798347315. [DOI] [PubMed] [Google Scholar]
- 74.Zheng Y, et al. SCC: an accurate imputation method for scRNA-seq dropouts based on a mixture model. BMC Bioinformatics. 2021;22(1):5. doi: 10.1186/s12859-020-03878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reich S, et al. A multi-omics analysis reveals the unfolded protein response regulon and stress-induced resistance to folate-based antimetabolites. Nat Commun. 2020;11(1):2936. doi: 10.1038/s41467-020-16747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Papalexi E, Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol. 2018;18(1):35–45. doi: 10.1038/nri.2017.76. [DOI] [PubMed] [Google Scholar]
- 77.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1):31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Panfili E, et al. Novel mutations in the WFS1 gene are associated with Wolfram syndrome and systemic inflammation. Hum Mol Genet. 2021;30(3–4):265–276. doi: 10.1093/hmg/ddab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.West AP. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology. 2017;391:54–63. doi: 10.1016/j.tox.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 81.Oslowski CM, et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16(2):265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lerner AG, et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16(2):250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meganathan K, et al. Regulatory networks specifying cortical interneurons from human embryonic stem cells reveal roles for CHD2 in interneuron development. Proc Natl Acad Sci U S A. 2017;114(52):E11180–E11189. doi: 10.1073/pnas.1712365115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewis EMA, et al. Cellular and molecular characterization of multiplex autism in human induced pluripotent stem cell-derived neurons. Mol Autism. 2019;10:51. doi: 10.1186/s13229-019-0306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-Seq data were deposited in the NCBI’s Gene Expression Omnibus database (GEO accession GSE212256).