Abstract
Purpose:
Myopia is the most common type of refractive error and the leading cause of functional visual loss. Increased risk of myopic maculopathy, retinal detachment, glaucoma and cataract has been seen with a myopia of as low as −1D. This study was done to determine the effect of atropine 0.01% eye drops on the progression of myopia in children >5 years.
Methods:
This was a single-blind, prospective, randomized case–control study which included children of 5–15 years with myopia of >2D and were divided into treatment group (group 1) and placebo group (group 2). Children under treatment group were treated with application of 0.01% atropine at night. Children with history of any ocular surgery, chronic ophthalmic illness, squint and amblyopia were excluded from the study. The follow-up for myopia progression was done for two years.
Results:
This study showed a significant difference in increase of spherical equivalent and axial length among treatment and placebo groups after a duration of two years. Total duration of follow up was twenty-four months. Mean increase in axial length of group 1 and 2 was 0.115 mm and 0.32 mm, respectively. Mean increase in refraction of groups 1 and 2 was −0.30 D and −0.88 D, respectively, showing significant change in axial length and refraction (P < 0.0001).
Conclusion:
This study supports the use of atropine 0.01% eye drops in reducing the progression of myopia.
Keywords: Atropine, axial length, myopia, spherical equivalent
Myopia is the leading cause of functional visual loss among all known refractive errors.[1] It is predicted that there will be an increase in myopia from 2.6 billion in 2020 to 4.7 billion in 2050.[2] Myopia is characterized by excessive enlargement of the axial length because of an increase in the depth of the vitreous chamber, which causes light from distant objects to focus in front of the retina, ultimately leading to blurred image formation. Myopia is regulated by multiple factors like environmental and genetic factors[3] and occurs due to failure to maintain the normal process of emmetropization.[4] Environmental factors like time spent outdoors,[5] near work,[6] prolonged and intense education,[7] and urbanization[8] play a significant role in the development of myopia.
Childhood myopia occurs between the ages of 6 and 12 years.[9] According to the American Academy of Ophthalmology (AAO), the mean rate of myopia progression is 0.5 D per year.[10] Risk of myopic maculopathy, retinal detachment, glaucoma and cataract increases with increase in myopia of as low as 1 diopter (− 1D).[11] Prevalence of myopia was maximum among students of 16–18 years of age in Asia.[12]
Atropine has affinity for all subtypes of acetylcholine muscarinic receptors (MR1–MR5), and thus, it has been assumed that it exerts its effect mainly through the MRs. Various side effects of atropine like photophobia, pupillary dilatation, dryness of mouth, restlessness, irritability or delirium, tachycardia and flushed skin of the face and neck are not seen with atropine 0.01%.
Atropine is thought to block the signal for axial elongation. It is assumed that it involves both M4 and M1 muscarinic receptor signalling pathways.[13] MRs are found in cornea, iris, ciliary body and ciliary muscles,[14] epithelium of lens,[15] retina, retinal pigment epithelium,[16] choroid and sclera (in scleral fibroblasts).[17,18] It’s been seen that it exerts the structural integrity of the sclera.[19] Atropine exerts its effects via non accommodative pathway in the retina or sclera.[20,21]
Methods
In this single-blind, prospective, randomized, case–control study, we included children aged 5–15 years[22] with myopia of >2 D presenting at the tertiary care hospital between September 2018 and September 2020. A total of 150 children were included in study, out of which 5 patients did not complete the study due to loss to follow-up. Children with history of any ocular surgery, chronic ophthalmic illness, squint and amblyopia were excluded from the study. Children who met the eligibility criteria were enrolled and randomized to receive atropine 0.01% once nightly (72 children) or receive placebo drugs (73 children) in an allocation ratio of 1:1. The progression of myopia after two-year follow-up was analyzed among the case and control groups. Approval for the study was granted by the ethical committee of S. N. Medical College, Agra, and informed consent was obtained from the parents of the children.
A detailed medical and ocular examination was recorded of the children. Visual acuity was done using the Snellen chart. Patients with subnormal visual acuity were investigated further. Wet retinoscopy was done in such cases. Each patient was examined over slit lamp, pupil was dilated and fundus examination was done. Anterior segment examination was conducted with the help of slit lamp. The lids, eyelashes, conjunctiva, cornea, iris and pupil were examined. In case of any abnormality, the diagnosis was specified. Posterior segment was examined using direct and indirect ophthalmoscope with +20 D lens after dilatation of pupil, and any abnormality was recorded. Wet retinoscopy was done and axial length was measured using A-scan by contact technique. Wet retinoscopy was performed in a semi-dark room at a distance of 75 cm with the help of streak retinoscope after dilatation of pupil with topical eye drops like homatropine 2% in children aged 5–8 years and cyclopentolate 1% in children aged 8–15 years. The pupil in each eye was dilated with two drops of homatropine or cyclopentolate, depending on the patient’s age, at an interval of five minutes. After twenty minutes if pupillary reflex was still present, a third drop was administered. Light reflex and pupil dilatation were evaluated after an additional fifteen minutes. Cycloplegia was considered complete if the pupil was dilated to 6 mm or more and light reflex was absent. The power of the lenses at neutralization point was noted at both the meridian. Post mydriatic refraction was done later depending on the drug used. Children who were divided into treatment and placebo groups were followed up on the fourteenth day after initiation of treatment; then they were reviewed every one month for the first three months, then quarterly for twenty-four months. At each visit, cycloplegic refraction and axial length was measured. Slit-lamp and fundus examinations were done. It was done in all children with decreased visual acuity. BCVA and spherical equivalent (spherical +1/2 cylindrical power) were recorded.
Data was collected and after data collection, the whole data was compiled on Microsoft Office Excel spreadsheet, and Chi-squared test was used to analyze mean increase in spherical equivalent and axial length in groups 1 and 2. P value < 0.05 was considered significant.
Results
The study comprised of 150 myopic patients; 73 patients were categorized into group 1 and 72 into group 2. Only 145 patients completed the schedule and turned up for follow-up during the study period.
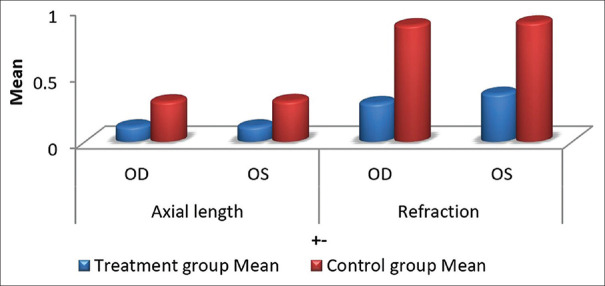
After 24 months, the mean axial length of group 1 and group 2 is 24.62 mm and 24.85 mm, respectively, and the mean refraction of group 1 and group 2 is 4.26D and 4.98D, respectively [Table 1]. In comparison to the baseline [Table 2], group 1 exhibits a smaller increase in axial length than group 2, which is 0.115mm and 0.32mm. Similarly, the increase in refraction for group 1 is lower than that for group 2, which is 0.30D and 0.88D, respectively, after 24 months. (P<0.0001). [Table 3 and Fig. 1].
Table 1.
Table showing axial length and refraction of atropine 0.01% treated eyes and placebo treated eyes at 2 years
| 2 years | Treatment group | Control group | t | P | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean | SD | Mean | SD | |||
| Axial length | ||||||
| OD | 24.65 | 0.62 | 24.85 | 0.74 | 1.772 | 0.0785 |
| OS | 24.56 | 0.71 | 24.86 | 0.84 | 2.3283 | 0.0213 |
| Refraction | ||||||
| OD | −4.21 | 1 | −4.93 | 1.21 | 3.8718 | 0.0002 |
| OS | −4.31 | 0.93 | −5.03 | 1.2 | 4.0325 | 0.0001 |
Mean axial length of group 1 is 24.62 mm and that of group 2 is 24.85 mm. Mean refraction of group 1 is−4.26 D and that of group 2 is−4.98 D. Mean increase in axial length of group 1 is by 0.11 D and that of group 2 is by 0.32 D as compared to baseline, and mean increase in refraction of group 1 is by 0.39 D and that of group 2 is by 0.88 D as compared to baseline which is highly significant
Table 2.
Table showing axial length and refraction of atropine 0.01% treated eyes and placebo treated eyes at 0 weeks
| 0 weeks | Treatment group | Control group | t | P | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean | SD | Mean | SD | |||
| Axial length | ||||||
| OD | 24.54 | 0.64 | 24.58 | 0.79 | 0.321 | 0.7487 |
| OS | 24.5 | 0.66 | 24.49 | 0.65 | 0.463 | 0.6481 |
| Refraction | ||||||
| OD | −3.92 | 1.009 | −4.05 | 1.25 | −0.678 | 0.4986 |
| OS | −3.76 | 1.54 | −4.13 | 1.237 | −1.579 | 0.1165 |
Mean axial length of group 1 is 24.52 mm and that of group 2 is 24.53 mm. Mean refraction of group 1 is−3.84 D and that of placebo treated eyes is−4.09 D
Table 3.
Table showing mean change in axial length and refraction of atropine 0.01% treated eyes and placebo treated eyes
| Final | Treatment group | Control group | t | P | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean | SD | Mean | SD | |||
| Axial length | ||||||
| OD | 0.115 | 0.11 | 0.303 | 0.12 | 9.8822 | < 0.0001 |
| OS | 0.115 | 0.127 | 0.306 | 0.13 | 8.9181 | < 0.0001 |
| Refraction | ||||||
| OD | 0.29 | 0.31 | 0.876 | 0.217 | 13.1466 | < 0.0001 |
| OS | 0.36 | 0.308 | 0.897 | 0.238 | 11.7239 | < 0.0001 |
Mean increase in axial length of group 1 is 0.115 mm and that of group 2 is 0.32 mm. Mean increase in refraction of group 1 is−0.30 D and that of group 2 is−0.88 D showing significant change in axial length and refraction
Figure 1.
Mean increase in axial length and refraction of treatment and control group after 24 months of treatment with atropine 0.01% and placebo drug
Discussion
Since myopia is increasing significantly in public health, it makes it a matter of concern. The goal of the study was to reduce the progression of myopia using topical atropine 0.01%. In this Randomized controlled trials (RCT), we compared the results of atropine 0.01% drug in the treatment and placebo groups. It is suggested that a nightly dose of 0.01% atropine seems to be a safe and effective regimen for slowing myopia progression in children, with minimal impact on visual function and without any photophobia. The current study was based on a total of 145 patients, out of which 72 belonged to the treatment group and 73 belonged to the placebo group. The mean difference in Spherical equivalent (SE) between treatment and placebo-treated eyes were 0.40 D and 0.72 D after one year and two years, respectively (P < 0.0001), which is quite significant and similar with the Atropine for the Treatment of Myopia (ATOM) study in Singapore and the study done by Joaschimsen L et al.[23] where there was a significant reduction in mean progression in SE of treatment and placebo groups.
Mean increase in refractive error of group 1 was −0.32 D and that of group 2 was −0.88 D, and mean increase in axial length of group 1 was 0.11 mm and that of group 2 was 0.30 mm showing significant change in refraction and axial length which was consistent with a study done by Kennedy et al.[24] Any increase greater than 0.04 mm or approximately 0.1 D was clinically meaningful.
In our study, most patients under group 1 experienced slowing of myopic progression with only 22% progressing more than 0.50 D after two years. In contrast, 84% of patients under group 2 experienced progression more than 0.50 D after two years. These results were both statistically significant and clinically meaningful, given that the progression beyond 0.75 D would indicate the need for a new spectacle prescription. The effect of atropine 0.01% on myopia progression that we observed concurs with the aforementioned randomized controlled trials from Asia, that is, ATOM2[25] and Low-Concentration Atropine for Myopia Progression (LAMP).[26]
Mean progression in axial length of group 1 was 0.115 ± 0.12 and that of group 2 was 0.303 ± 0.12 (P < 0.0001), which is similar in the LAMP[26] and ATOM 2[25] studies that showed significant decrease in mean progression of axial length of treatment group and control group.
No safety concerns with atropine 0.01% were evident in our study. Despite a relatively small sample size, the impact of low-concentration treatment was statistically significant (P < 0.0001). Still, the exact mechanism of action for atropine to reduce myopic progression is currently not known. Our study confirmed the efficacy and safety of atropine 0.01% eye drops in retarding the progression of axial length and spherical equivalent in atropine 0.01% treated eyes when compared to eyes treated with a placebo drug. Nevertheless, several limitations of the study must be admitted. Firstly, it was a single-center study with a somewhat small sample size, thereby reducing the power of statistical test. The use of atropine 0.01% is a new modality in the treatment of myopia progression. Various studies have been conducted to establish the use of atropine in myopia progression. This study also showed that atropine 0.01% eye drops can achieve the best balance between efficacy and safety in the prevention and treatment of myopia and is an effective treatment modality to control the progression of myopia.
Conclusion
Data from this study supports the use of atropine 0.01% eye drops in the progression of myopia. Group 1 showed that atropine 0.01% eye drops provided significant reduction of increase in axial length and increase in refractive error as compared with group 2, with no side effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bourne RR, Steven GA, White RA, Smith JL, Flaxman SR, Price H, et al. Causes of vision loss worldwide, 1990-2010:A systematic analysis. Lancet Glob Health. 2013;1:e339–49. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 2.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Saw SM, Shankar A, Tan SB, Taylor H, Tan DT, Stone RA, et al. A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci. 2006;47:1839–44. doi: 10.1167/iovs.05-1081. [DOI] [PubMed] [Google Scholar]
- 4.Troilo D, Gottlieb MD, Wallman J. Visual deprivation causesmyopia in chicks with optic nerve section. Curr Eye Res. 1987;6:993–9. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- 5.Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120:1080–5. doi: 10.1016/j.ophtha.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Saw SM, Zhang MZ, Hong RZ, Fu Z-F, Pang M-H, Tan DTH, et al. Near work activity, night –lights, and myopia in the Singapore –China study. Arch Ophthalmol. 2002;120:620–7. doi: 10.1001/archopht.120.5.620. [DOI] [PubMed] [Google Scholar]
- 7.Dirani M, Shekhar SN, Baird PN. The role of educational attainment in refraction;The genes in myopia (GEM) twin study. Invest Ophthalmol Vis Sci. 2008;49:534–8. doi: 10.1167/iovs.07-1123. [DOI] [PubMed] [Google Scholar]
- 8.Morgan I, Rose K. How genetic is school myopia?Prog Retin Eye Res. 2005;24:1–38. doi: 10.1016/j.preteyeres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Grosvenor T, Perrigin DM, Perrigin J, Maslovitz B. Houston Myopia Control Study:A randomized clinical trial Part II. Final report by the patient care team. Am J Optom Physiol Opt. 1987;64:482–98. [PubMed] [Google Scholar]
- 10.Jensen H. Myopia progression in young school children and intraocular pressure. Doc Ophthalmol. 1992;82:249–55. doi: 10.1007/BF00160772. [DOI] [PubMed] [Google Scholar]
- 11.Filtcroft DI. The complex interactions of retinal, optical and environmental factors in myopiaaetiology. Prog Retin Eyes. 2012;31:622–60. doi: 10.1016/j.preteyeres.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese school children:1983 to 2000. Ann Acad Med Singapore. 2004;33:27–33. [PubMed] [Google Scholar]
- 13.Arumugam B, McBrien NA. Muscarinic antagonist control of myopia:Evidence for M4 and M1 receptor –based pathways in the inhibition of experimentally-induced axial myopia in the tree shrew. Invest Ophthalmol Vis Sci. 2012;53:5827–37. doi: 10.1167/iovs.12-9943. [DOI] [PubMed] [Google Scholar]
- 14.Gil DW, Krauss HA, Bogardus AM, WoldeMussie E. Muscarinic receptor subtypes in human iris-ciliary body measured by immunoprecipitation. Invest Ophthalmol Vis Sci. 1997;38:1434–42. [PubMed] [Google Scholar]
- 15.Collison DJ, Coleman RA, James RS, Carey J, Duncan G. Characterization of muscarinic receptors in human lens cells by pharmacologic and molecular techniques. Invest Ophthalmol Vis Sci. 2000;41:2633–41. [PubMed] [Google Scholar]
- 16.Friedman Z, Hackett SF, Campochiaro PA. Human retinal pigment epithelial cells possess muscarinic receptors coupled to calcium mobilization. Brain Res. 1988;446:11–6. doi: 10.1016/0006-8993(88)91291-7. [DOI] [PubMed] [Google Scholar]
- 17.Barathi VA, Weon SR, Beuerman RW. Expression of muscarinic receptors in human and mouse sclera and their role in the regulation of sclera fibroblasts proliferation. Mol Vis. 2009;15:1277–1293. [PMC free article] [PubMed] [Google Scholar]
- 18.Qu J, Zhou X, Xie R, et al. The presence of M1 to M5 receptors in human sclera:Evidence of the sclera as a potential site of action for muscarinic receptor antagonists. Curr Eye Res. 2006;31:587–597. doi: 10.1080/02713680600770609. [DOI] [PubMed] [Google Scholar]
- 19.Grytz R, Siegwart JT., Jr Changing material properties of the tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2015;56:2065–78. doi: 10.1167/iovs.14-15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mc Brien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eyeenlargement via a non–accommodative mechanism. Invest Ophthalmol Vis Sci. 1993;34:205–15. [PubMed] [Google Scholar]
- 21.Stone RA, Lin T, Laties AM. Muscarinic antagonis effects on experimental chick myopia. Exp Eyes Res. 1991;52:755–8. doi: 10.1016/0014-4835(91)90027-c. [DOI] [PubMed] [Google Scholar]
- 22.Sorsby A, Leary GA. A longitudinal study of refraction and it's components during growth. Spec Rep Ser Med Res Counc (GB) 1969;309:1–41. [PubMed] [Google Scholar]
- 23.Joachimsen L, Bohringer D, Gross NJ, Reich M, Stifter J, Reinhard T, et al. A pilot study on the efficacy and safety of 0.01% atropine in German school children with progressive myopia. Ophthalmol Ther. 2019;8:427–33. doi: 10.1007/s40123-019-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy RH, Dyer JA, Kennedy MA, Parulkar S, Kurland LT, Herman DC, et al. Reducing the progression of myopia with atropine:A long term cohort study of Olmsted Country students. Binocul Vis Strabismus Q. 2000;15:281–304. [PubMed] [Google Scholar]
- 25.Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, et al. Atropine for the treatment of childhood myopia:Safety and efficacy of 0.5%, 0.1% and 0.01% doses (Atropine for the Treatment of Myopia 2) Ophthalmology. 2012;119:347–54. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Yam JC, Jiang Y, Tang SM, Law AK, Chan JJ, Wong E, et al. Low –concentration Atropine for Myopia Progression (LAMP) Study:A randomized double blinded, placebo-controlled trial of 0.05%, 0.025%, 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126:113–24. doi: 10.1016/j.ophtha.2018.05.029. [DOI] [PubMed] [Google Scholar]