Abstract
Erysipelothrix rhusiopathiae is a causal agent of swine erysipelas, which is of economic importance in the swine industry by virtue of causing acute septicemia, chronic arthritis, and endocarditis. However, little is known about the genetic properties of its protective antigens. Recently, a surface protective antigen (SpaA) gene was identified from serotype 2 in a mouse model. We cloned spaA from virulent strain Fujisawa (serotype 1a) and determined that the N-terminal 342 amino acids without C-terminal repeats of 20 amino acids have the ability to elicit protection in mice. Fusions of 342 amino acids of Fujisawa SpaA and histidine hexamer (HisSpa1.0) protected pigs against challenge with both serotype 1 and serotype 2, the most important serotypes in the swine industry. Pigs immunized with HisSpa1.0 reacted well with both HisSpa1.0 and intact SpaA by enzyme-linked immunosorbent assay and immunoblotting. Serum collected at the time of challenge from a pig immunized with HisSpa1.0 markedly enhanced the in vitro phagocytic and killing activity of pig neutrophils against the bacteria. DNA sequences of protective regions of spaA genes from five strains of serotypes 1 and 2 were almost identical. The full DNA sequences also seemed to be conserved among strains of all 12 serotype reference strains harboring the spaA gene by restriction fragment length polymorphism analysis of PCR products. These results indicates that SpaA is a common protective antigen of serotypes 1 and 2 of E. rhusiopathiae in swine and will be a useful tool for development of new types of vaccines and diagnostic tools for effective control of the disease.
Erysipelothrix rhusiopathiae (formerly E. insidiosa) is a small gram-positive rod that causes erysipelas mainly in swine (34) and turkeys (2) and less frequently in other animals and humans. E. rhusiopathiae was once thought to be the only member of genus Erysipelothrix and was classified into 23 serotypes and type N based on peptidoglycan antigens of the cell wall (9, 34). The genus now contains two species, E. rhusiopathiae and E. tonsillarum, and other (two) genetically distinct unclassified groups (24, 25). Among 15 serotypes of E. rhusiopathiae (25), serotypes 1 (subdivided into 1a and 1b) and 2 (subdivided into 2a and 2b) are the most important in the pig industry (3, 4, 17, 26, 32, 34). Species other than E. rhusiopathiae are of low virulence in swine (25). Because of the importance of swine erysipelas, killed and attenuated live vaccines having been used extensively. However, despite widely practiced vaccination, the importance of this disease has not decreased (34). In Japan, annually about 2,000 pigs are affected with acute and subacute septicemia and about 2,000 pigs are condemned by meat inspection authorities because of arthritis.
There are many reports on the characterization of protective antigens of E. rhusiopathiae. A mouse protective antigen was identified in culture supernatant (30, 31) and in 10 mM NaOH extracts of bacterial cells (18, 19). Neuraminidase was also considered a protective antigen, because mice were protected by passive immunization with rabbit antiserum against E. rhusiopathiae neuraminidase (16). In 1 mM EDTA extract of T28 (serotype 2), one major polysaccharide capsular antigen of 14.4 to 22 kDa and two main protein antigens of 64 and 48 kDa were revealed by immunoblotting with rabbit antiserum (10). However, the protective activity of these antigens was not examined in the work reported. Groschup et al. (7) showed that protective antisera from pigs recognized prominent bands of 64 to 66 and 39 to 40 kDa in 1 mM EDTA and 10 mM NaOH extracts of T28. Both antigens were trypsin sensitive and contained no detectable polysaccharide. Mice immunized with preparations of the 64- to 66-kDa band were protected against challenge with Frankfurt XI (serotype N). They also described the enhanced production of these protective antigens in serum-free modified Feist broth (6). However, some questions remain as to whether the band of 64 to 66 kDa is the only protective antigen of the bacteria, this band contains only one kind of protective antigen, and this antigen can elicit protection in swine.
Galan and Timoney first identified a mouse protective antigen gene in a 5.4-kb EcoRI fragment of chromosomal DNA of virulent strain E1-6P (serotype 1a) (5). Guinea pig antiserum against the recombinant clone of this gene reacted with E. rhusiopathiae protein antigens of 66, 64, and 43 kDa. These proteins are of the same size as the protective proteins mentioned above. However, the DNA sequences of the gene were not described, and it is also not known whether the clone contained only one gene. Recently, a novel surface protective antigen (SpaA) of E. rhusiopathiae was identified from serotype 2 in a mouse model using a monoclonal antibody recognizing 64-kDa proteins of most serotypes of E. rhusiopathiae (13). Mice immunized with live recombinant Escherichia coli intraperitoneally survived after challenge with the same strain of E. rhusiopathiae. In this study, the presence of 20-amino-acids repeat units at the C terminus was shown to be essential for protection.
In contrast to protein antigens, 14.4- to 22-kDa capsular antigen appears to be not necessary for protection. A live acapsular mutant created by insertion and excision of Tn916, which is avirulent in mice, could confer complete protective immunity to mice (21).
The existence of a common protective antigen among serotypes of E. rhusiopathiae was identified experimentally and practically. Although killed vaccines are prepared from serotype 1a and live vaccines are from serotype 2, both vaccines can cross-protect pigs against challenge with strains of serotypes 1 and 2 (1, 23, 33). In this study, we cloned spaA from a Sau3AI library of virulent strain Fujisawa (serotype 1a), determined that the N-terminal 342 amino acids are necessary to elicit protection in mice, and evaluated the ability of SpaA of strain Fujisawa (SpaA/Fujisawa) to elicit cross-protection in pigs against challenge with serotypes 1 and 2 by the use of fusion of truncated SpaA/Fujisawa with a histidine hexamer (HisSpa1.0).
MATERIALS AND METHODS
Bacterial strains, vectors, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. E. rhusiopathiae Fujisawa was used for cloning of spaA, preparation of intact SpaA for enzyme-linked immunosorbent assay (ELISA), and challenge of mice and pigs. For challenge of pigs, E. rhusiopathiae 82-875 was also used. Vector plasmid pBluescript II SK+ (Stratagene) was used for cloning of spaA, and expression vector pQE32 (Qiagen) was used to construct HisSpa1.0, in which the histidine hexamer tag was placed at the N terminus of the protein. E. coli XL1-Blue was used as the host strain for these plasmids.
TABLE 1.
Strains and plasmids used in this study
| Strains and plasmids | Relevant characteristic | Reference or source |
|---|---|---|
| E. rhusiopathiae (serotype) | ||
| Fujisawa (1a) | Japanese official challenge strain | 20 |
| Koganei (1a) | Japanese official live vaccine strain | 20 |
| SE-9 (2a) | U.S. official bacterin strain | 6 |
| ATCC 19414T (2b) | Type strain | American Type Culture Collection |
| Marienfelde (1a) | Growth agglutination strain | 29 |
| ME-7 (1a), 422/1E1 (1b), R32E11 (2a), NF4E1 (2b), Pécs 67 (5), Goda (8), Kaparek (9), Pécs 9 (12), Pécs 3597 (15), Tanzania (16), 545 (17), MEW 22 (N) | Reference strains for serotyping | 25 |
| E. coli XL1-Blue | Host strain for plasmids | Stratagene |
| Plasmids | ||
| pBluescript II | Cloning vector | Stratagene |
| pQE32 | His6 vector | Qiagen |
| Recombinant plasmids | ||
| pA, pB | Recombinant pBluescript II of spaA/Fujisawa | This study |
| pA1.0 | Recombinant pQE32 of spaA/Fujisawa | This study |
| Recombinant E. coli XL1-Blue (pA1.0) | This study |
E. rhusiopathiae was grown on brain heart infusion broth or agar (pH 7.8) supplemented with 0.3% Tris and 0.1% Tween 80 at 37°C. For the isolation of E. rhusiopathiae from experimentally infected animals, selective media were also used. Selective enrichment medium was further supplemented with kanamycin (500 μg/ml) and gentamicin (25 μg/ml), and selective agar medium was supplemented with 0.002% crystal violet and 0.02% sodium azide. For the preparation of intact SpaA, E. rhusiopathiae Fujisawa was grown on modified Feist broth at 37°C. Recombinant E. coli was grown on L agar or L broth supplemented with ampicillin (100 μg/ml) at 37°C.
Preparation of antisera.
A pig was first immunized with live vaccine and then injected with 107 live cells of Fujisawa intradermally once and with 108 to 109 live cells intravenously three times at weekly intervals. Serum was collected 3 weeks after the final injection. A rabbit was immunized by weekly injection of 1 mg of purified HisSpa1.0 subcutaneously three times with Freund’s complete adjuvant and after 2 weeks intravenously twice without adjuvant. Serum was collected 2 weeks after the final injection.
DNA extractions.
Chromosomal DNA of E. rhusiopathiae for use in cloning and PCR was extracted and purified as described previously (5, 12). Plasmid DNA was prepared by a modified alkaline lysis-polyethylene glycol precipitation procedure in a dye terminator cycle sequencing protocol (Perkin-Elmer).
Determination of protective region of spaA.
Two spaA recombinant plasmids, pA and pB, obtained from a Sau3AI library of Fujisawa were used. Lysates of recombinant E. coli carrying these plasmids could elicit protection in mice. The protective region of the gene was determined by analyzing the Exo-Mung deletion mutants of these plasmids created as instructed by the manufacturer (Stratagene) by immunoblotting and confirmed by a mouse protection test.
Expression and purification of fusion protein.
A KpnI fragment of recombinant plasmid pA containing bp 266 to 1,294 of spaA was ligated into the compatible site of expression vector pQE32 to construct pA1.0 as shown in Fig. 1. One KpnI site was located in spaA, and another was located in the multicloning site of pBluescript II. Recombinant fusion protein HisSpa1.0 was expressed in E. coli XL1-Blue(pA1.0) and purified as specified by the manufacturer (Qiagen) under denaturing conditions. The culture was inoculated with a 1:50 dilution of overnight culture of recombinant E. coli, grown at 37°C to mid-exponential phase (A600 = 0.8), and then induced with 2 mM isopropyl-β-d-thiogalactopyranoside for 5 h with vigorous shaking. Cells were harvested and resuspended in 6 M guanidine buffer (pH 8.0) at 0.2 g (wet weight)/ml and stirred for 1 h at room temperature to solubilize the fusion protein. The slurry was centrifuged at 10,000 × g for 15 min; then the fusion protein in the supernatant was filter sterilized and adsorbed on Ni-nitrilotriacetic acid (NTA)-Sepharose column. The Ni-NTA column was washed with guanidine buffer (pH 8.0), 8 M urea buffer (pH 8.0), and 8 M urea buffer (pH 6.3); then the fusion protein was eluted with urea buffer (pH 4.5) and dialyzed against 10 mM phosphate-buffered saline, pH 7.2 (PBS).
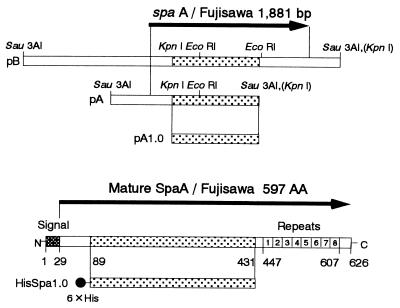
FIG. 1.
Map of spaA/Fujisawa in recombinant plasmids pA, pB, and pA1.0 and map of HisSpa1.0 in intact SpaA. Protective regions are indicated as dotted boxes.
Mouse immunization and challenge.
Four-week-old ddY female mice were immunized subcutaneously with 500 μg of sonicated extract of recombinant E. coli in Freund’s incomplete adjuvant or with 50 μg of purified HisSpa1.0 in complete adjuvant twice and challenged with 100 50% lethal doses of E. rhusiopathiae Fujisawa subcutaneously 3 weeks after immunization. The infections were monitored for 12 days, and the cause of death was confirmed by isolation of the organism.
Pig immunization and challenge.
Four-week-old specific-pathogen-free (SPF) pigs obtained from a farm free from swine erysipelas where no pigs were vaccinated against the disease were used. Six pigs were divided into three groups and immunized intramuscularly with 0, 100, and 500 μg of purified HisSpa1.0 in Freund’s complete adjuvant twice at 3-week intervals and 2 weeks later challenged intradermally with 4 × 107 Fujisawa (serotype 1a) organisms. Another four pigs were divided into two groups and immunized with 0 and 100 μg of purified HisSpa1.0 twice at 4-week intervals and 2 weeks later challenged with 8 × 107 82-875 (serotype 2b) bacteria. Dead pigs were autopsied on the day of death, and pigs that survived were euthanized and autopsied 1 week after challenge. Organs (heart, lung, liver, spleen, kidney, lymph nodes, and tonsils) and skin erythema lesions of all pigs were examined by bacterial isolation. Sera were collected from all pigs every week through the experiment, and antibody was titrated by double-antibody sandwich ELISA with intact SpaA, indirect ELISA with HisSpa1.0, and growth agglutination test.
Preparation of intact SpaA in alkaline extract of E. rhusiopathiae.
E. rhusiopathiae Fujisawa was cultured in modified Feist broth at 37°C overnight (6). Cells were harvested and washed with distilled water, then resuspended in 10 mM NaOH at 0.1 g (wet weight)/ml, and incubated at 4°C overnight with gentle shaking (7). After neutralization, the suspension was centrifuged at 10,000 rpm for 30 min; then the supernatant was sterilized by filtration and kept at −20°C. The extract was used in double-antibody sandwich ELISA.
SDS-PAGE and immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (11) on 10% gels. After semidry electrophoretic transfer of the antigens to a nitrocellulose membrane, the membrane was blocked with 3% skim milk in PBS supplemented with 0.05% Tween 20 (PBS-Tw) for 30 min, incubated with pig antiserum diluted 1:100 with 1% skim milk in PBS-Tw for 1 h, washed with PBS-Tw three times for 15 min, and then incubated with anti-pig immunoglobulin G (IgG)-peroxidase conjugate (Rockland) diluted 1:1,000 with 1% skim milk in PBS-Tw for 1 h. Membrane was washed as described above and then developed with 0.05% 4-chloro-1-naphthol and 0.01% hydrogen peroxide in PBS-Tw for 15 to 30 min. The reaction was stopped by washing the membrane with PBS-Tw.
ELISA to detect antibody response in pigs against SpaA.
In sandwich ELISA, rabbit antiserum against HisSpa1.0 was used to capture intact SpaA in the alkaline extract of E. rhusiopathiae Fujisawa to the ELISA plate well. All steps were done with a 100-μl reaction volume and 1-h incubation at room temperature. Between each step, the plate was washed three times with PBS-Tw.
Rabbit antiserum was diluted 1:1,000 with 0.05 M carbonate-bicarbonate buffer (pH 9.6) and dispensed to high-adsorption ELISA plate wells (Immulon 600; Greiner). The plate was successively incubated with alkaline extract diluted 1:100, pig sera diluted 1:100, and then anti-pig IgG-peroxidase conjugate diluted 1:12,000 (all dilutions were with 1% skim milk in PBS-Tw). Finally substrate solution (0.02% tetramethyl benzidine and 0.01% hydrogen peroxide in 0.1 M disodium phosphate–0.05 M citric acid buffer [pH 4.5]) was added, and the wells were incubated for 30 min. After the reaction was stopped by addition of 100 μl of 2 N sulfuric acid, the A450 was read.
In indirect ELISA, purified HisSpa1.0, diluted 10 μg/ml with 0.05 M carbonate-bicarbonate buffer (pH 9.6) and dispensed to a medium adsorption plate (Immunon 200), was used as the antigen. After incubation and washing, the plate was blocked by incubation with 150 μl of 3% skim milk in PBS-Tw for 30 min. Subsequent procedures were the same as for the sandwich ELISA method.
Growth agglutination test.
A modification of the method of Wellmann (29) was used. To prepare the serum dilution and bacterial suspension, tryptic soy broth (pH 7.8) (Difco) supplemented with 0.1% Tween 80, 0.3% Tris, kanamycin (500 μg/ml), and gentamicin (25 μg/ml) (S-TSB) was used. Each 50 μl of pig serum was serially diluted with 50 μl of S-TSB in a sterile round-bottomed microplate and then mixed with an equal volume of overnight culture of E. rhusiopathiae Marienfelde diluted 1:100 with S-TSB. The plate was sealed and incubated at 37°C overnight. The agglutination titer was expressed as the reciprocal of the highest final serum dilution giving agglutination.
In vitro phagocytosis assay.
Peripheral blood was collected from an SPF pig not immunized against swine erysipelas. Mononuclear cells and neutrophils were isolated by standard density gradient centrifugation on Ficoll-Paque (Pharmacia). The mononuclear cell fraction was washed and resuspended at 5 × 106/ml (monocytes = 106/ml) in 5 ml of RPMI 1640 medium (Sigma) supplemented with 10% fetal calf serum and 25 mM HEPES (pH 7.2) (RPMI-FCS). Monocytes were regarded as 20% of the mononuclear cells and were not separated from lymphocytes. Neutrophils were purified by ammonium chloride lysis of erythrocytes and after washing resuspended in 5 ml of RPMI-FCS at 106/ml. An overnight culture of Fujisawa was centrifuged and resuspended at 108/ml in 0.5 ml of a 1:1 mixture of inactivated pig serum, collected from an immunized pig or nonimmunized control pig at the time of challenge, and fresh germfree pig serum. The suspension was incubated at 37°C for 1 h with gentle agitation to opsonize the bacteria, then added to the each phagocyte suspension at a final concentration of 107 CFU/ml, and incubated at 37°C with gentle shaking. After 30 min, the phagocytes were washed with medium twice, resuspended in 10 ml of RPMI-FCS containing penicillin (10 U/ml) to inhibit the growth of E. rhusiopathiae in the medium, and reincubated at 37°C. Each 2 ml of the suspension was collected after 0, 2, 4, and 18 h; 1 ml was used for Giemsa staining, and another 1 ml was used for counting the bacteria surviving in phagocytes.
PCR cloning of protective region of spaA.
Each DNA fragment flanking the 1,026-bp protective region of spaA was amplified by PCR from chromosomal DNA of Koganei (serotype 1a, Japanese official live vaccine strain), SE-9 (serotype 2a, U.S. official vaccine strain), and ATCC 19414T (serotype 2b, type strain of E. rhusiopathiae), and cloned into pBluescript II. DNA sequences of these fragments were determined by cycle sequencing on a model 373S automated DNA sequencer (Applied Biosystems).
PCR-RFLP of spaAs of diverse serotypes.
spaAs of almost full size (bp 16 to 1871) were amplified by PCR with a primer set designed from sequences of spaA/Fujisawa from chromosomal DNA of Fujisawa (serotype 1a), Koganei (serotype 1a), SE-9 (serotype 2a), ATCC 19414T (serotype 2b) and E. rhusiopathiae reference strains of all 12 serotypes harboring this gene. Each 1 μg of the purified PCR products was digested with restriction enzymes EcoRI, HpaII, KpnI, PstI, and SacI, respectively, and restriction fragment length polymorphism (RFLP) was analyzed by 1% agarose gel electrophoresis.
Nucleotide sequence accession number.
The nucleotide sequence of spaA/Fujisawa will appear in the DDBJ/EMBL/GenBank nucleotide sequence databases with accession no. AB019124. Nucleotide sequences of protective regions of spaA/Koganei, spaA/ATCC 19414T, and spaA/SE-9 will appear in the DDBJ/EMBL/GenBank nucleotide sequence databases with accession no. AB024082, AB024083, and AB024084, respectively.
RESULTS
Determination of protective region of spaA.
The spaA/Fujisawa recombinant plasmids pA and pB had bp 1 to 1,294 of spaA in a 1.7-kb insert and bp 1 to 1,881 of full-length spaA in a 3.8-kb insert, as shown in Fig. 1. Analysis of Exo-Mung deletion mutants of them showed that an approximately 1.0 kb C-terminal region of the insert of pA was necessary to elicit protection in mice. This was confirmed by immunizing mice with 50 μg of purified HisSpa1.0 in complete adjuvant twice and successive challenge. After challenge, four of five mice survived.
Purification of truncated SpaA by affinity chromatography.
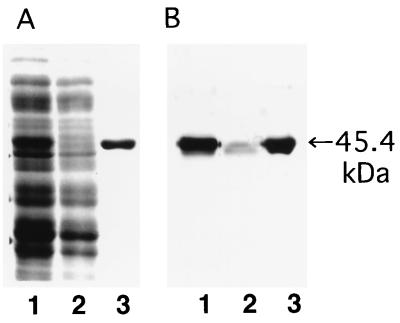
The elution profile of HisSpaA1.0 at each stage of the purification procedure analyzed by SDS-PAGE and immunoblotting (Fig. 2). Purified HisSpa1.0 showed the predicted molecular size of 45.5 kDa and was reactive with pig antiserum immunized with E. rhusiopathiae. Both mouse and pig antisera against HisSpa1.0 reacted well with the 69.0-kDa intact SpaA in the alkaline extract and with a 43-kDa SpaA fragment in the culture supernatant of Fujisawa (data not shown).
FIG. 2.
SDS-PAGE and immunoblot analysis of affinity purification of HisSpa1.0 on an Ni-NTA-agarose column. Lanes: 1, crude extract; 2, flowthrough fraction; 3, purified protein eluted at pH 4.5. (A) Coomassie blue staining of polyacrylamide gel; (B) immunoblot detection with pig serum immunized with live E. rhusiopathiae.
Pig protection assay.
All pigs immunized with purified HisSpa1.0 in Freund’s complete adjuvant were protected completely against intradermal challenge with virulent strain Fujisawa (serotype 1a) and strain 82-875 (serotype 2b) (Table 2). They showed no clinical symptoms and no urticarial lesions at the injection site. Bacterial isolation 1 week after challenge was also negative by both direct culture and enrichment culture.
TABLE 2.
Protection of pigs immunized with HisSpa1.0 against challenge with Fujisawa (serotype 1a) and 82-875 (serotype 2b)
| Challenge strain | Immuniza-tion dose (μg, given twice) | Result for pig no. 1/pig no. 2
|
|
|---|---|---|---|
| Symptom after challenge | Isolation of E. rhusio-pathiae at necropsya | ||
| Fujisawa | 0 | Death/death | +++/+++ |
| 100 | −/− | −/− | |
| 500 | −/− | −/− | |
| 82-875 | 0 | Septicemia/generalized erythema | +++/+++ (tonsils) |
| +/(+) (spleen) | |||
| NT/(+) (erythema) | |||
| (+)/− (heart, lung, lymph nodes) | |||
| 100 | −/− | −/− | |
Results for all body organs examined, otherwise indicated. −, no isolates by direct and enrichment culture; + and +++, few and numerous of colonies isolated; (+), isolation positive only by enrichment culture; NT, not tested (no erythema lesion).
All two control pigs challenged with Fujisawa died from septicemia 3 to 4 days after challenge, and numerous E. rhusiopathiae bacteria were isolated from all body organs examined. In contrast, both of the control pigs challenged with 82-875 survived, although they showed systemic symptoms such as severe septicemia or generalized skin erythema. Many E. rhusiopathiae bacteria were isolated from the tonsils of these pigs by direct culture 1 week after challenge. By enrichment culture, E. rhusiopathiae was isolated from the spleen, heart, lungs, and lymph nodes of one pig which showed septicemia and from spleen and skin erythema lesions of another pig which showed generalized skin erythema.
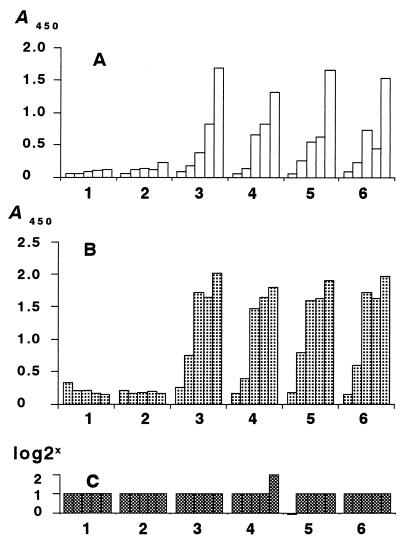
The antibody response of all pigs immunized with HisSpa1.0 was sensitively detected by double-antibody sandwich ELISA using intact SpaA and by indirect ELISA using HisSpa1.0 from 2 weeks after the first immunization. The specificity of the antibody response was confirmed by immunoblotting. On the other hand, a conventional growth agglutination test could not detect the antibody response in pigs immunized with HisSpa1.0. The results are shown in Fig. 3.
FIG. 3.
Antibody response of pigs immunized with two injections of various doses of HisSpa1.0 at weeks 0 and 3 of the experiment. Sera were collected at 0, 1, 2, 3, and 5 weeks, and pigs were challenged at 5 weeks of the experiment. (A) Sandwich ELISA with intact SpaA; (B) indirect ELISA with HisSpa1.0; (C) growth agglutination test. Pigs 1 and 2, 0 μg; pigs 3 and 4, 100 μg; pigs 5 and 6, 500 μg.
Effect of pig serum immunized with HisSpa1.0 on in vitro phagocytosis.
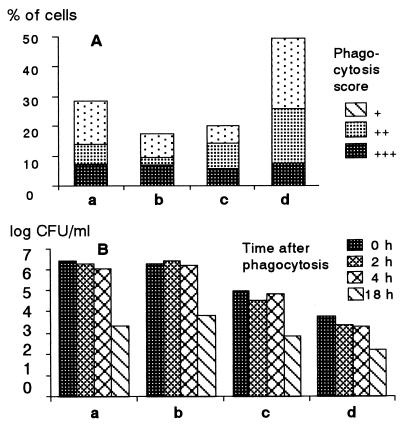
In vitro phagocytosis activity of swine neutrophils observed by Giemsa staining was significantly enhanced by opsonizing Fujisawa cells with pig antiserum immunized with HisSpa1.0. Nearly 50% of neutrophils showed phagocytosis when the cells were opsonized, but only 20% showed phagocytosis when the cells were not opsonized. On the other hand, phagocytosis of monocytes was not affected by opsonization of the bacteria; 18 and 29% of monocytes showed phagocytosis.
In contrast, the number of live bacteria in neutrophils was much less than that in monocytes. Furthermore, upon opsonization of the cells, the number of live bacteria in neutrophils decreased significantly, from 105 to less than 104 CFU/ml. On the other hand, the viable bacterial count in monocytes was not affected by opsonization, being about 106 CFU/ml regardless of opsonization. From these results (Fig. 4), we attributed the major protection mechanism of pigs immunized with HisSpa1.0 to the enhancement of bacterial killing activity of neutrophils by opsonizing the bacteria with antibody.
FIG. 4.
Effect of pig serum immunized with HisSpa1.0 on in vitro phagocytosis of monocytes (columns a and b) and neutrophils (columns c and d) of a nonimmunized SPF pig. (A) Percentage of cells showing the indicated phagocytosis score; (B) number of E. rhusiopathiae bacteria surviving in phagocytes. Bacteria were treated with control serum (columns a and c) or immunized serum (columns b and d) collected at the time of challenge.
DNA sequence analysis of diverse spaAs.
The DNA sequences of the protective regions of spaAs of four strains of serotypes 1 and 2, Fujisawa, Koganei, SE-9, ATCC 19414T, and Tama, were almost identical, as shown by alignment of amino acid sequences (Fig. 5).
FIG. 5.
Alignment of the 342-amino-acid sequences of protective regions (amino acids 76 to 438) of SpaA proteins of E. rhusiopathiae Fujisawa (serotype 1a), Koganei (1a), SE-9 (2a), ATCC 19414T (2a), and Tama-96 (2). Identical and different amino acids are marked with asterisks and dots, respectively.
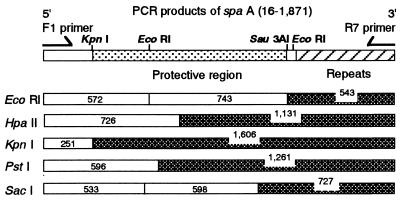
By PCR, all spaA genes of 16 strains of E. rhusiopathiae representing 12 serotypes were amplified well, and the sizes of all PCR products and restriction fragments were the same as for spaA/Fujisawa except in two strains, R32E11 and Kaparek. From these results, the nucleic acid sequences of diverse spaAs seemed to be highly conserved among different serotypes (Fig. 6).
FIG. 6.
RFLP analysis of PCR products of spaA (bp 16 to 1,871) of Fujisawa (serotype 1a), Koganei (1a), SE-9 (2a), ATCC 19414T (2b), and reference strains of E. rhusiopathiae, representing all 12 serotypes harboring the gene. Products of all strains but two showed the same restriction fragment size as Fujisawa with all enzymes used; products of R32E11 (2a) and Kaparek (9) gave about 300-bp longer and about 100-bp shorter C-terminal fragments, respectively, with all enzymes used. Restriction fragments showing size variation are indicated as checked boxes.
In R32E11 the PCR products and all C-terminal restriction fragments were about 300 bp longer, and in Kaparek they were about 100 bp shorter, compared to spaA/Fujisawa. Because these size variations were observed in the EcoRI C-terminal fragments of PCR products, encoding all C-terminal amino acid repeating units and four additional nucleic acids, we concluded that they reflect the number of amino acid repeating units as observed in Tama. These size variations of spaAs agreed with the immunoblotting results for intact SpaAs (data not shown).
DISCUSSION
In this study, we showed that purified fusions of truncated SpaA/Fujisawa, constructed with the N-terminal 342 amino acids (90 to 431) and histidine hexamer, could elicit complete protection in swine against challenge with virulent strains of E. rhusiopathiae of serotypes 1 and 2, and the C-terminal amino acid repeats of SpaA were not necessary for protection. In contrast, Makino et al. (13) emphasized the importance of the C-terminal amino acid repeats for protection, because in their study only recombinant E. coli expressing complete SpaA could elicit protection in mice. Although they created many Exo-Mung deletion derivatives, including two clones harboring complete spaA, they could not show protection with any of them (13). The contradiction in results can be attributed to the experimental method used by Makino et al. They immunized mice by intraperitoneal injection of a large dose of viable recombinant E. coli, and most mice died from endotoxin shock. In the paper they mentioned that their protection assay was very difficult to perform. It appears difficult to obtain reproducible results by their method.
The C-terminal 20-amino-acid repeat region of E. rhusiopathiae SpaA was shown by Makino et al. (13) to be necessary for SpaA to bind tightly to the bacterial surface like other gram-positive bacteria. This region has high sequence homology with the C-terminal amino acid repeats of pneumococcal surface protein A (PspA) (44.9% over 225 amino acids) and Streptococcus pneumoniae secretory IgA binding protein (SpsA) (40.1% over 227 amino acids) (8, 35). On the other hand, the protective region of SpaA is located at the N-terminal region, like that of PspA. In PspA, epitopes eliciting protection in mice were present in the 43-kDa α-helical N-terminal half of the native 84-kDa molecule (27) and in amino acids 192 to 260 and 192 to 588 (14, 28). Despite the high diversity of the α-helical protective region of PspA (15, 22), a recombinant PspA of one strain can elicit cross-protection against pneumococci of different capsular types and PspA serological types (15, 28). In contrast to PspA, the DNA sequences of protective region of SpaA of E. rhusiopathiae of five strains of serotypes 1 and 2, the most important serotypes in pigs, were almost identical and highly conserved. Also, nucleic acid sequences of spaA of all serotypes of E. rhusiopathiae harboring this gene seemed to be well conserved when examined by PCR-RFLP. Although Makino et al. (13) showed strain differences of EcoRI fragment size in the spaA gene, such as 0.7 or 2 kb, by Southern hybridization, in this study all of these stains gave the same 0.7-kb fragment upon EcoRI digestion by PCR-RFLP. These results indicate that spaA genes are highly conserved among different serotypes of E. rhusiopathiae and this characteristic provides a major mechanism of the cross-protection activity of SpaA against challenge with different serotypes. Although the number of C-terminal amino acid repeats appears to vary among strains, this repeat has no role in protection.
Pigs immunized with recombinant truncated SpaA were completely protected against challenge with virulent strains of E. rhusiopathiae. By in vitro phagocytosis assay, we determined that the major protection mechanism in these pigs seemed to be the enhancement of the activity of neutrophils to phagocytose and kill the bacteria by effective opsonization with antibody produced against SpaA. On the other hand, the bacteria phagocytosed by monocytes of nonimmunized pigs tended to resist killing. This result suggested that activation of monocytes and macrophages may be also necessary for ready clearance of the bacteria. In immunized pigs, macrophages seemed to be also activated readily after challenge.
The antibody response of all pigs immunized with HisSpa1.0 was sensitively detected by ELISA with intact SpaA/Fujisawa and HisSpa1.0 from 2 weeks after the first immunization. In contrast, the conventional growth agglutination test widely used in Japan did not detected the antibody response to HisSpa1.0, although the test is considered useful to assay the protective antibody in pigs (20). These results indicates that the growth agglutination test cannot directly detect the antibody response against SpaA, the protective antigen of E. rhusiopathiae, and can detect antibody responses against other antigens.
By immunoblotting analysis with pig antiserum against HisSpa1.0, it was shown that SpaA, like the 64- to 66-kDa protective protein described by Groschup and Timoney (6), was produced in larger amounts in modified Feist broth than in brain heart infusion, and SpaA was produced consistently in larger amounts by SE-9 than by Fujisawa.
In this study, we found that purified recombinant SpaA/Fujisawa can elicit protective immunity in pigs, the N-terminal 342 amino acids are necessary for protection in pigs, the nucleic acid sequences of this region are highly conserved among strains of serotypes 1 and 2, which are the most important serotypes in pigs, truncated SpaA of serotype 1 can elicit cross-protective immunity against challenge with serotype 2, and the sequences of full-size spaA also seemed to be highly conserved among all serotypes of E. rhusiopathiae harboring this gene. From these results, we conclude that this truncated SpaA may be useful for development of new types of vaccines such as component, vector, and DNA vaccines and of new diagnostic techniques such as ELISA to assay protective antibody of vaccinated pigs and maternal protective antibody of piglets.
ACKNOWLEDGMENT
We thank T. Takahashi for providing E. rhusiopathiae 82-875.
REFERENCES
- 1.Bricker J M, Saif Y M. Use of a live oral vaccine to immunize turkeys against erysipelas. Avian Dis. 1988;32:668–673. [PubMed] [Google Scholar]
- 2.Bricker J M, Saif Y M. Erysipelas. In: Calnek B W, et al., editors. Diseases of poultry. Ames, Iowa: Iowa State University Press; 1991. pp. 247–257. [Google Scholar]
- 3.Cross G M J, Claxton P D. Serological classification of Australian strains of Erysipelothrix rhusiopathiae isolated from pigs, sheep, turkeys and man. Aust Vet J. 1979;55:77–81. doi: 10.1111/j.1751-0813.1979.tb15170.x. [DOI] [PubMed] [Google Scholar]
- 4.Eamens G J, Turner M J, Catt R E. Serotypes of Erysipelothrix rhusiopathiae in Australian pigs, small ruminants, poultry, and captive wild birds and animals. Aust Vet J. 1988;65:249–252. doi: 10.1111/j.1751-0813.1988.tb14311.x. [DOI] [PubMed] [Google Scholar]
- 5.Galan J E, Timoney J F. Cloning and expression in Escherichia coli of a protective antigen of Erysipelothrix rhusiopathiae. Infect Immun. 1990;58:3116–3121. doi: 10.1128/iai.58.9.3116-3121.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groschup M H, Timoney J F. Modified Feist broth as a serum-free alternative for enhanced production of protective antigen of Erysipelothrix rhusiopathiae. J Clin Microbiol. 1990;28:2573–2575. doi: 10.1128/jcm.28.11.2573-2575.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groschup M H, Cussler K, Weiss R, Timoney J F. Characterization of a protective protein antigen of Erysipelothrix rhusiopathiae. Epidemiol Infect. 1991;107:637–649. doi: 10.1017/s0950268800049335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammerschmidt S, Talay S R, Brandtzaeg P, Chhatwal G S. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–1124. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- 9.Kucsera G. Proposal for standardization of the designations used for serotypes of Erysipelothrix rhusiopathiae (Migula) Buchanan. Int J Syst Bacteriol. 1973;23:184–188. [Google Scholar]
- 10.Lachmann P G, Deicher H. Solubilization and characterization of surface antigenic components of Erysipelothrix rhusiopathiae T28. Infect Immun. 1986;52:818–822. doi: 10.1128/iai.52.3.818-822.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Makino S, Okada Y, Maruyama T, Ishikawa K, Takahashi T, Nakamura M, Ezaki T, Morita H. Direct and rapid detection of Erysipelothrix rhusiopathiae DNA in animals by PCR. J Clin Microbiol. 1994;32:1526–1531. doi: 10.1128/jcm.32.6.1526-1531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makino S, Yamamoto K, Murakami S, Shirahata T, Uemura K, Sawada T, Wakamoto H, Morita Y. Properties of repeat domain found in a novel protective antigen, SpaA, of Erysipelothrix rhusiopathiae. Microb Pathog. 1998;25:101–109. doi: 10.1006/mpat.1998.0216. [DOI] [PubMed] [Google Scholar]
- 14.McDaniel L S, Ralph B A, McDaniel D O, Briles D E. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb Pathog. 1994;17:323–337. doi: 10.1006/mpat.1994.1078. [DOI] [PubMed] [Google Scholar]
- 15.McDaniel L S, McDaniel D O, Hollingshead S K, Briles D E. Comparison of the PspA sequence from Streptococcus pneumoniae EF5668 to the previously identified PspA sequence from strain Rx1 and ability of PspA from EF5668 to elicit protection against pneumococci of different capsular types. Infect Immun. 1998;66:4748–4754. doi: 10.1128/iai.66.10.4748-4754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller H E. The protection of mice by a specific anti-Erysipelothrix insidiosa-neuraminidase-antiserum against Erysipelothrix insidiosa infection. Med Microbiol Immunol. 1974;159:301–308. doi: 10.1007/BF02123740. [DOI] [PubMed] [Google Scholar]
- 17.Nørrung V, Munch B, Larsen H E. Occurrence, isolation and serotyping of Erysipelothrix rhusiopathiae in cattle and pig slurry. Acta Vet Scand. 1987;28:9–14. doi: 10.1186/BF03548251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothe F. Das protektive Antigen des Rotlaufbakteriums (Erysipelothrix rhusiopathiae). 1. Mitteilung: Spezifischer Nachweis des protektiven Antigens. Arch Exp Veterinaermed. 1982;36:243–253. [PubMed] [Google Scholar]
- 19.Rothe F. Das protektive Antigen des Rotlaufbakteriums (Erysipelothrix rhusiopathiae). 2. Mitteilung: Die weitere Charakterisierung des protektiven Antigens. Arch Exp Veterinaermed. 1982;36:255–267. [PubMed] [Google Scholar]
- 20.Sawada T, Muramatsu M, Seto K. Response of growth agglutinating antibody and protection of pigs inoculated with swine erysipelas live vaccine. Jpn J Vet Sci. 1979;41:593–600. doi: 10.1292/jvms1939.41.593. [DOI] [PubMed] [Google Scholar]
- 21.Shimoji Y, Mori Y, Sekizaki T, Shibahara T, Yokomizo Y. Construction and vaccine potential of acapsular mutants of Erysipelothrix rhusiopathiae: use of excision of Tn916 to inactivate a target gene. Infect Immun. 1998;66:3250–3254. doi: 10.1128/iai.66.7.3250-3254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swiatlo E, Brooks-Walter A, Briles D E, McDaniel L S. Oligonucleotides identify conserved and variable regions of pspA and pspA-like sequences of Streptococcus pneumoniae. Gene. 1997;188:279–284. doi: 10.1016/s0378-1119(96)00823-2. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, Takagi M, Sawada T, Seto K. Cross protection in mice and swine immunized with live erysipelas vaccine to challenge exposure with strains of Erysipelothrix rhusiopathiae of various serotypes. Am J Vet Res. 1984;45:2115–2118. [PubMed] [Google Scholar]
- 24.Takahashi T, Fujisawa T, Benno Y, Tamura Y, Sawada T, Suzuki S, Muramatsu M, Mitsuoka T. Erysipelothrix tonsillarum sp. nov. isolated from tonsils of apparently healthy pigs. Int J Syst Bacteriol. 1987;37:166–168. [Google Scholar]
- 25.Takahashi T, Fujisawa T, Tamura Y, Suzuki S, Muramatsu M, Sawada T, Benno Y, Mitsuoka T. DNA relatedness among Erysipelothrix rhusiopathiae strains representing all twenty-three serovars and Erysipelothrix tonsillarum. Int J Syst Bacteriol. 1992;42:469–473. doi: 10.1099/00207713-42-3-469. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T, Nagamine N, Kijima M, Suzuki S, Takagi M, Tamura Y, Nakamura M, Muramatsu M, Sawada T. Serovars of Erysipelothrix strains isolated from pigs affected with erysipelas in Japan. J Vet Med Sci. 1996;58:587–589. doi: 10.1292/jvms.58.587. [DOI] [PubMed] [Google Scholar]
- 27.Talkington D F, Crimmins D L, Voellinger D C, Yother J, Briles D E. A 43-kilodalton pneumococcal surface protein, PspA: isolation, protective abilities, and structural analysis of the amino-terminal sequence. Infect Immun. 1991;59:1285–1289. doi: 10.1128/iai.59.4.1285-1289.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tart R C, McDaniel L S, Ralph B A, Briles D E. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J Infect Dis. 1996;173:380–386. doi: 10.1093/infdis/173.2.380. [DOI] [PubMed] [Google Scholar]
- 29.Wellman G. Die subklinische Rotlaufinfektion und ihre Bedeutung für die Epidemiologie des Schweinerotlaufs. Zentralbl Bakteriol Orig A. 1955;162:265–274. [PubMed] [Google Scholar]
- 30.White R R, Verwey W F. Isolation and characterization of a protective antigen-containing particle from culture supernatant fluids of Erysipelothrix rhusiopathiae. Infect Immun. 1970;1:380–386. doi: 10.1128/iai.1.4.380-386.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White R R, Verwey W F. Solubilization and characterization of a protective antigen of Erysipelothrix rhusiopathiae. Infect Immun. 1970;1:387–393. doi: 10.1128/iai.1.4.387-393.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood R L, Harrington R., Jr Serotypes of Erysipelothrix rhusiopathiae isolated from swine and from soil and manure of swine pens in the United States. Am J Vet Res. 1978;39:1833–1840. [PubMed] [Google Scholar]
- 33.Wood R L, Booth G D, Cutlip R C. Susceptibility of vaccinated swine and mice to generalized infection with specific serotypes of Erysipelothrix rhusiopathiae. Am J Vet Res. 1981;42:608–614. [PubMed] [Google Scholar]
- 34.Wood R L. Erysipelas. In: Leman A D, et al., editors. Diseases of swine. Ames, Iowa: Iowa State University Press; 1992. pp. 475–486. [Google Scholar]
- 35.Yother J, Briles D E. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J Bacteriol. 1992;174:601–609. doi: 10.1128/jb.174.2.601-609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]