Abstract
Of the various manifestations of ocular chemical burns (OCBs), ischemia of the limbus and the peri-limbal sclera indicates poor prognosis and in severe cases threaten the integrity of the globe. Tenonplasty is a surgical procedure which involves advancing the Tenon’s capsule over the ischemic areas to provide a vascular supply and to enable migration of the conjunctival epithelium. This review aims to provide an overview of the diagnosis of limbal ischemia and its management with Tenonplasty. A literature review was conducted using the keywords “Tenonplasty,” “Tenon’s capsule,” “ocular chemical injury,” “ocular thermal injury,” “Tenon advancement,” “scleral ischemia,” and “limbal ischemia,” and outcomes were studied from seven selected articles. In addition to clinical evaluation, in vivo imaging techniques such as anterior segment optical coherence tomography angiography can provide an objective method of measuring and monitoring the ischemia and re-perfusion of the peri-limbal vasculature. Tenonplasty can be performed in eyes with acute OCBs with scleral or limbal ischemia by dissecting the Tenon’s layer from the orbit and securing it to the limbus. The indications, mechanism of action, peri-operative considerations, surgical technique, and post-operative care of Tenonplasty are discussed in detail. The average time for post-operative re-epithelization ranges from 1 to 6 months with the formation of a symblepharon being the most common complication. In conclusion, Tenonplasty is a globe-salvaging procedure in cases with severe limbal and scleral ischemia because of OCBs and has good anatomical outcomes priming the globe for subsequent re-constructive and vision-restoring surgeries.
Keywords: Limbal ischemia, ocular chemical burns, scleral ischemia, Tenon’s capsule, Tenonplasty
Ocular chemical burns (OCBs) constitute 0.1–15% of all the ocular emergency conditions and require immediate management to prevent long-term sequelae which may result in irreversible corneal blindness.[1] The spectrum of damage caused by ocular chemical or thermal injury can range from conjunctival hyperemia to ocular surface epithelial defects which may progress to form a corneal perforation and ultimately result in phthisis bulbi.[2,3] This is largely determined by the nature of the inciting chemical agent, the duration between injury and presentation, the degree of penetration of the chemical agent in the eye, associated limbal involvement, scleral ischemia, and adnexal damage.
Deeper penetration or a strong chemical agent can cause extensive damage to the conjunctival and episcleral vessels, causing limbal ischemia and underlying scleral necrosis.[2,3] The presence of limbal ischemia is detrimental to the healing of the conjunctival and corneal epithelium, resulting in non-healing epithelial defects, and corneal and scleral melt in severe cases. Thus, limbal ischemia is an important prognostic marker in chemical injury, and eyes with larger and deeper ischemic damage tend to have a poorer prognosis.[4] Several attempts have been made to address limbal and scleral ischemia with conjunctival autografts (CAG), amniotic membrane transplantation (AMT), and tarsorrhaphy. However, in the absence of an underlying vascularized bed, these interventions have a limited role in promoting epithelialization.[3,5] In 1989, Teiping and Reim described a surgical technique called Tenonplasty in an attempt to restore blood supply over the ischemic sclera, thus promoting conjunctival and limbal perfusion, thereby allowing re-epithelization over the conjunctiva and the cornea.[6] Since then, this technique has been extensively used to address limbal and scleral ischemia following chemical injury and other surgical procedures. In this review, we attempt to provide an overview of the indications, clinical presentation, surgical techniques, and outcomes of Tenonplasty.
Methods
A review of literature was conducted on PubMed in September 2021 using the following keywords and their variations: “Tenonplasty,” “Tenon’s capsule,” “ocular chemical injury,” “ocular thermal injury,” “Tenon advancement,” “scleral ischemia,” and “limbal ischemia”. A total of 2731 articles were retrieved. After excluding articles which were not relevant to our review and articles from which outcomes of tenonplasty could not be gauged, seven articles were included for the purpose of the current review.
Anatomy and Pathophysiology
The sclera forms the protective tough outer coat of the eyeball. The scleral matrix is composed of compact arrangement of collagen fibrils, surrounded by the extracellular matrix, predominantly composed of proteoglycans, glycoproteins, and elastin fibers. The sclera is avascular and receives its blood supply from the underlying choroidal plexus and the overlying vascular plexus of the Tenon’s capsule and the episcleral vessels. These vessels pass through the emissary channels within the scleral stroma, supplying the sclera.[7]
Tenon’s capsule is a dense fibrovascular connective tissue that surrounds the eyeball and is densely adherent to the episcleral tissue throughout its extent. It extends from about 1.5 mm behind the corneo-scleral junction anteriorly, moves posteriorly fusing with the fascial sheaths of the extra-ocular muscle, and finally merging with the meningeal sheaths around the optic nerve and surrounding sclera. Tenon’s capsule is divided into anterior and posterior parts by the insertion of recti muscles. The anterior part of the Tenon’s capsule consists of collagen, elastin, and smooth muscle fibers, whereas the posterior part of the Tenon’s capsule predominantly consists of condensed collagen fibrils.[8]
In acute OCBs, damage to the anterior segment vasculature results in limbal and scleral ischemia. This may cause damage directly to the limbal epithelial stem cells (LESCs) or indirectly to the surrounding niche, both of which can result in limbal stem cell deficiency (LSCD). This prevents epithelization of the corneal epithelium, resulting in persistent corneal epithelial defects and corneal melt, which are often associated with poor visual outcomes.[9] Damage to the adjacent conjunctival epithelium and the underlying sclera can lead to further scleral melt. This scleral ischemia is further worsened by release of destructive enzymes and inflammatory mediators from the surrounding necrotic conjunctival and episcleral tissues, thus causing cornea-scleral melt and compromising globe integrity.[9]
Role of Limbal Ischemia in Classification Systems
Classification for OCBs was first given by Hughes in 1946 and later modified by Ballen in 1964.[10,11] Roper–Hall in 1965 proposed a classification, where prognosis was primarily based on the extent of limbal ischemia and clinical appearance of the cornea at the time of presentation.[12] As per this classification, all cases with >50% limbal ischemia were identified as severe grade of chemical injury and conjunctival injury was not taken into consideration. The classification was again modified by Dua et al.[13] in 2001, where conjunctival involvement and limbal involvement were the primary indicators determining the severity and prognosis of ocular surface injury. However, the diagnosis of limbal ischemia purely based on clinical judgement in the early stages of chemical injury can be erroneous.[14]
Diagnosis of Limbal and Scleral Ischemia
Based on clinical examination
In the acute phase of OCBs, the presence of conjunctival chemosis and blanching of conjunctival vessels may often make it difficult to identify the underlying areas of limbal and scleral ischemia and the vitality of the residual tissue.[9] Once the chemosis and edema subside, it is easier to visualize the ischemic areas on clinical evaluation. Hence, limbal ischemia and surrounding scleral ischemia may not be evident within the first 3–4 days after an acute burn. Scleral ischemia is seen as a blanched-out sclera, devoid of any blood vessels [Fig. 1]. Depending on the extent of chemical/thermal injury, scleral ischemia may be sectoral involving 1–3 quadrants of the sclera or may be total with involvement of 360 degrees of sclera adjacent to the limbus. Severe cases often present with complete loss of scleral vasculature. Some cases may present with a column of stagnant blood in the vessels. It is difficult to differentiate between irreversible damage to the vascular endothelial cells and a transient vascular endothelial damage resulting in vascular stasis in the deeper vessels by clinical examination alone.[9,15]
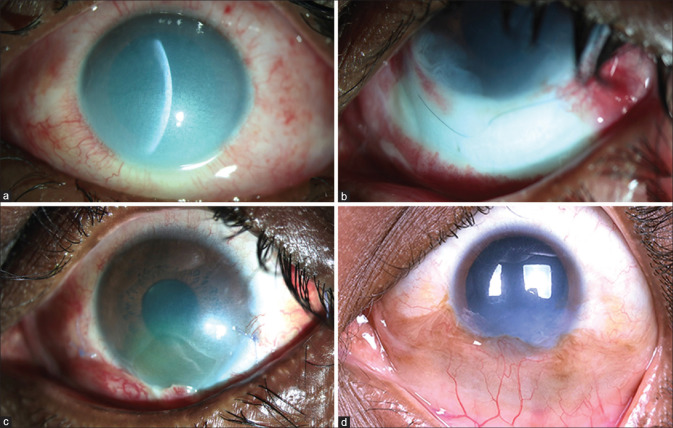
Figure 1.
Inferior sectoral tenonplasty following alkali chemical burns: Slit-lamp photo-micrographs (a–d) of a 27-year-old gentleman with ocular alkali burns. The inferior limbal ischemia that appears subtle at presentation (a) is more evident at follow-up on day 10 that persisted despite amniotic membrane transplantation after 3 days of injury (b). Images (c and d) show the post-op recovery at 4 weeks and 4 months following tenonplasty, respectively
The extent of limbal ischemia is determined by the area of fluorescein staining and appearance of the limbal vasculature on slit-lamp biomicroscopy. Considering that limbal ischemia is an important prognostic marker for the likelihood of development of LSCD, corneal scarring, and permanent visual loss, clinical examination alone may not be a suitable technique because it is subjective and gives variable results.[14,15] It can neither determine the viability of the involved LESCs nor can it inform us if the loss of limbal vasculature is transient or permanent.
Use of ancillary tests
The signs on clinical examination are often subjective and non-specific and may be masked by the extensive inflammation that often accompanies cases of OCBs. To overcome these limitations, ocular surface angiography has played a pivotal role in identifying areas of ischemia. Ocular surface video fluorescein angiography (FA) was first described by Kuckelkorn et al.[16] in 1997 to identify limbal and scleral ischemia. This study illustrated the various patterns of perfusion during the early and late phases of the angiograms in the eyes with severe chemical injury and compared these angiographic patterns with those seen in the normal eyes. Necrotic tissues showed a complete absence of perfusion and appeared as dark areas throughout the angiogram. The vessels that were damaged but retained some function showed hyper-flourescence during the late phase of the angiogram because of delayed leakage. Clear de-lineation between the perfused and non-perfused areas was established in all the eyes. FA has also been used to determine the extent of tenonplasty to be performed and to monitor the post-operative recovery. Although this method has shown promising results, it is an invasive technique. The risks of adverse reactions to sodium fluorescein dye such as anaphylaxis, discoloration of body fluids, and pruritus at the site of drug administration are rare, but they still impose a challenge, especially in resource limited settings. Also, repeated booster doses of the dye are required because of the rapid transit time of the dye, and the images can be obscured by leakage of the dye from the inflamed vessels. Complementary results have been seen with anterior segment angiography using indocyanine green and low-dose fluorescein dye in early detection of vaso-occlusive areas seen in scleral inflammation.[17]
A non-invasive technique that shows promise is anterior segment optical coherence tomography angiography (AS-OCTA). AS-OCTA has been used to determine the anterior segment blood supply based on the flow of erythrocytes in the blood vessels. It has been used to study the anatomical pattern of vessels in the cornea, conjunctiva, sclera, and iris.[15,18] In the eyes with acute ocular chemical burns, it can quantify objectively the extent of limbal ischemia and the pattern of vascular re-perfusion in the recovery phase [Fig. 2]. It is not dependent on loss of the overlying conjunctival-limbal epithelium, as determined by fluorescein staining. In a study by Fung et al.,[15] the extent of clinically determined limbal ischemia determined by slit-lamp evaluation was significantly less than that determined by AS-OCTA, thus underestimating the extent of injury. They also concluded that the area of ischemia was significantly less than the area of the conjunctival epithelial defect determined by clinical evaluation after fluorescein staining. Using AS-OCTA, the extent of ischemia in the deeper scleral vessels, underlying an area of superficial conjunctival ischemia, could also be ascertained. The results of AS-OCTA were found to have better correlation with the final visual acuity, as compared to extent of damage ascertained by clinical evaluation, during the acute phase. Ang et al.[18] also found that the results obtained on AS-OCTA were more reliable, when compared to assessment of these parameters on clinical examination. Disruption of the limbal vasculature in the acute phase was also associated with an increased risk of corneal vascularization in the chronic phase.
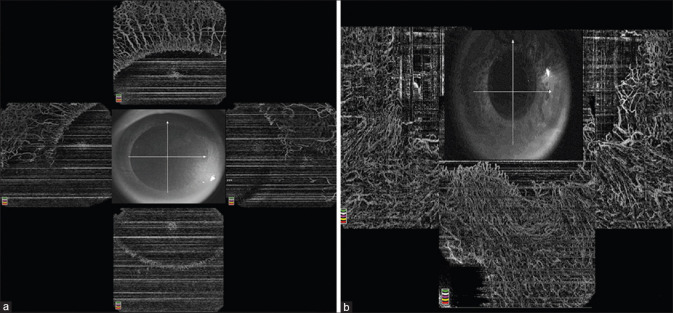
Figure 2.
AS-OCTA images of the patient in figure 1. The AS-OCTA compilation of 360 degrees limbus (a) prior to tenonplasty shows compromised vascularity in the inferior quadrant that improved in terms of vascular density 5 months post tenonplasty (b)
In an animal model, a graded chemical injury was induced, and the pattern of re-perfusion was studied using AS-OCTA.[19] The vessel density, calculated using image software, correlated with the severity of the injury and the duration of onset. The extrapolation of such studies to human trials can lead to parameters measured by AS-OCTA being incorporated in chemical injury classifications. This in turn can help better prognosticate these eyes.
Thus, AS-OCTA has been found to be a rapid technique which provides high-quality detailed evaluation of the different layers of vascular perfusion during the acute phase of chemical injury and helps to objectively quantify the area of limbal ischemia. It is independent of the need for a dye and its associated systemic risks as discussed above.[15,18] A limitation is the inability to detect the permeability of the vessels, as seen with dye-based angiography techniques and the motion artifacts affecting the image quality. Also, considering that AS-OCTA detects movement of RBCs in blood vessels, temporary vasospasm of conjunctival vessels in the acute phase may give a false impression of ischemia.
Indications
The primary indication of tenonplasty is limbal ischemia with scleral ischemia.[3] Scleral ischemia and thinning with associated corneal melt are most commonly seen in severe cases of acute ocular chemical and thermal burns. Most of the severe chemical injuries that required tenonplasty have been reported to be caused by alkali, acids, and liquid metal injury.[20]
Apart from being used in acute OCBs, tenonplasty has also been extensively used in cases of chronic scleral ischemia, scleral necrosis, and melt seen after pterygium excision, especially in cases where CAG was not used to cover the bare sclera, and in cases where adjuvants such as beta-irradiation and Mitomycin C were used.[21,22] Isolated case reports have also mentioned tenonplasty as an effective globe-preserving surgery in treating scleral necrosis and melt in cases of bleb leak, following glaucoma filtration surgery, retinal detachment surgery, and sterile corneal and scleral melts seen in severe cases of connective tissue disorders.[23,24,25]
Mechanism of Action
Tenonplasty or Tenon’s advancement is a surgical procedure where the connective tissue is advanced from the orbit and used to cover the area of scleral ischemia, thus allowing a scaffold over which the conjunctival epithelium can grow to cover the defect. The Tenon’s tissue also acts as a bridge to allow the conjunctival epithelium to grow over the cornea, accelerating the process of conjunctivalization of the cornea [Fig. 3]. In the presence of LSCD, this process is a protective mechanism, thus preventing the catastrophic sequelae of corneal stromal melt and perforation, which is devastating in the setting of limbal ischemia.
Figure 3.
Successful outcome post 360-degree tenonplasty: A 34-year-old patient with 360-degree limbal ischemia (a) following acid chemical injury presented 1 month after the acute injury and underwent a 360-degree tenonplasty with an improved surface at 1 month following the procedure (b). He later underwent simple limbal epithelial transplantation (c) at the 4-month follow-up and a subsequent penetrating keratoplasty with cataract surgery 1 year following injury. (d) shows the clinical picture at 10 months post corneal transplant with a clear graft and a stable ocular surface
However, the Tenon’s tissue is not just an inert platform for surface epithelialization. The tissue itself acts as a source of vasculature for the underlying ischemic sclera and prevents its necrosis. They also help sustain the overlying conjunctival epithelial sheet. Additionally, the fibroblasts within the Tenon’s tissue help modulate the inflammatory response and contribute to a faster healing response.[26]
Case Selection
Although tenonplasty is indicated in cases of limbal and scleral ischemia, not all the eyes with these two features warrant surgery. In mild cases, the patients can be closely followed up and the procedure is performed only if established ischemia is observed. In the eyes with suspicious areas of ischemia, soon after the diagnosis of ocular chemical burn, a trial of intensive topical steroids is warranted to reduce the ocular surface inflammation. In the acute phase, within 1 week of acute ocular chemical burns, if medical management in the form of topical steroids has been started within 24–48 hours of the injury, it is always prudent to observe for re-perfusion in areas suspected to have ischemia. The use of AS-OCTA in such cases is particularly useful as it provides an objective measurement of the degree of ischemia which may not correlate with the clinical signs. At the other end of the spectrum lie cases with very severe burns involving all quadrants of the limbus and sclera [Fig. 4]. In such eyes, the Tenon’s tissue is likely to be non-perfused, and hence, performing tenonplasty is associated with poor outcomes. We provide an algorithm for appropriate case selection for the eyes that may require tenonplasty [Fig. 5]. All the eyes scheduled to undergo tenonplasty require pre-operative and post-operative topical intensive topical steroids.
Figure 4.
Outcome of tenonplasty based on initial severity of chemical injury: A 27-year-old patient with a 1-month-old chemical injury with molten aluminum alloy with extensive limbal ischemia of 7 clock hours with a persisting total corneal epithelial defect (a). The surface shows successful complete epithelization with the conjunctival epithelium following tenonplasty and amniotic membrane transplantation 4 months post surgery, leading to successful globe salvage (b). An eye with a severe acid burn with extensive 360-degree limbal ischemia till the fornices with ischemia of the tarsal area (c and d)
Figure 5.
Algorithmic approach for evaluation and decision making regarding tenonplasty in the eyes with acute ocular chemical burns with emphasis on the time of presentation post injury
Pre-Surgical Considerations
Anesthesia
In children and apprehensive patients, this procedure should be performed under general anesthesia. In cases of severe chemical injury, especially in the eyes with 360 degrees of involvement, it is preferable to perform the procedure under general anesthesia. For sectoral tenonplasty, local anesthesia may suffice.
Patient counseling
The purpose of the surgery must be explained to the patient, and the fact that the primary purpose of tenonplasty is to preserve the tectonic support of the globe should be emphasized. The patient should also be counseled regarding the need for subsequent surgeries to restore the ocular surface integrity and for visual rehabilitation.
Pre-operative vasoconstrictors
It is advisable to avoid using any vasoconstrictors as the end point of dissection of the necrotic tissue is the bleeding encountered from the healthy conjunctival tissue at the edge of the necrotic tissue. Adrenaline should not be added in any of the fluids that are utilized during surgery.
Surgical Technique
Exposure of the surgical field
Although a traditional lid speculum can be used, it may not provide the surgical exposure needed to dissect the Tenon’s capsule. The lids can be retracted using 4-0 silk sutures passed 2–3 mm behind the lid margins, which are then secured to the surgical drape with artery forceps. Traction sutures with 6-0 polyglactin sutures at the limbus also provide adequate exposure in the quadrant where tenonplasty is to be attempted. If sectoral tenonplasty is to be attempted inferiorly, a stay suture can be passed at the inferior limbus at 6 o clock and the globe can be rotated superiorly to provide access to the inferior sclera and the inferior fornix.
Steps of the procedure
Excision of the necrotic tissue
Under general or local anesthesia, the extent of limbal/scleral damage is first assessed. Staining of the ocular surface with 2% fluorescein dye helps determine the extent of de-epithelized ocular surface. All the devitalized conjunctival and episcleral tissues are excised. If the necrotic tissue is adjacent to the recti muscles, the muscle sheath and fibers should be hooked with a muscle hook, and blunt dissection should be performed carefully all around, avoiding damage to the muscle fibers. Active bleeding from the conjunctival vessels marks the posterior extent of dissection and is suggestive of tissue viability. The amount of necrotic tissue that needs to be excised should be determined clinically to avoid the inadvertent excision of the healthy tissue.
Tenonplasty
Careful blunt dissection is performed in two planes, first between the conjunctiva and the Tenon’s capsule and then between the Tenon’s capsule and the underlying sclera. This dissection is performed till the depth of the fornix in order to free as much Tenon’s capsule so that it can be sutured to the limbus without traction. The Tenon’s capsule is then advanced from the equatorial region till the limbus and anchored with 7-0 polyglactin sutures at the limbus. The surface of the Tenon’s capsule should be smooth to allow the conjunctival tissue to grow over it. Extensive dissection of the conjunctival and episcleral tissues is avoided to prevent forniceal shortening and symblepharon formation in the post-operative period.
Anchoring amniotic membrane (AM)
To cover the adjacent corneal epithelial defect, which may be present in the eyes which require tenonplasty, a human amniotic membrane (hAM) is used and secured with fibrin glue. The hAM can be sutured at the limbus 360 degrees in cases with a large total corneal epithelial defect. A soft therapeutic bandage contact lens (BCL) is placed on the cornea at the end of the surgery.
Tarsorrhaphy
A simultaneous tarsorrhaphy is recommended to prevent re-traction of the Tenon’s flap.[27] Tarsorrhaphy may also help to prevent exposure, thus allowing faster epithelization of the corneal and conjunctival surfaces. In cases that require sectoral tenonplasty, the tarsorrhaphy can be medial or lateral paramedian depending on the area of limbal ischemia. In the eyes that require 360 degrees of tenonplasty, a central broad tarsorrhaphy is required to minimize exposure and to allow for faster epithelization of a larger area of the ocular surface. The tarsorrhaphy can be temporary or permanent. However, a permanent tarsorrhaphy is useful in the eyes with larger areas of ischemia as the ocular surface in these cases takes longer to epithelize and a permanent tarsorrhaphy will stay intact for the duration required. The tarsorrhaphy can be released later once the epithelization is complete and a surgery for visual rehabilitation is planned. A video of the surgical technique can be found at https://www.youtube.com/watch?v=5OoBbWBUrJE&t=1s
Modifications of the technique
Instead of sutures, fibrin glue can also be used to secure the Tenon’s pedicle graft at the limbus. This reduces the surgical time and can be used in cases where extensive dissection is not required. However, it should be ensured that the Tenon’s capsule is completely free from both overlying and underlying attachments. This prevents the Tenon’s capsule from retracting from the limbus in the post-operative period, especially in cases when only fibrin glue has been used.
Selective tenonplasty is a procedure where limbal peritomy is performed in selected areas of limbal and scleral non-perfusion which are localized by FA.[28] Studies on this modification of tenonplasty have shown re-established perfusion, which was also confirmed on FA.[16] This procedure is less invasive when compared to a complete 360 degree limbal peritomy and may prevent the inadvertent removal of healthy limbal and conjunctival tissues.[16,28] However, the procedure is dependent on FA or AS-OCTA confirmation of the ischemic area, and unavailability of these devices will limit the feasibility of the procedure.
The combined use of an oral buccal mucous membrane with tenonplasty has been described in severe cases of scleral ischemia. The oral buccal mucosa acts as an additional source of epithelial cells and helps accelerate the time taken for surface epithelialization. In a series by Wang et al.,[29] preservation of globe integrity was reported following this combined approach.
The simultaneous use of a lamellar corneal graft or a multi-layered AM has also been described to address areas of scleral defects before covering the entire surface with the Tenon’s flap.[30]
Post-operative medications
Post-operatively, prednisolone acetate 1% eye drops 6–8 times/day, moxifloxacin 0.5% eye drops 4 times/day, and lubricating eye drops are prescribed. At every visit, re-epithelization should be re-assessed under the BCL and the tarsorrhaphy. It is also important to look for the integrity of hAM at every post-operative visit.
Normal Post-Operative Course
As mentioned previously, the conjunctival epithelial cells migrate over the Tenon’s layer to cover the bare sclera. As LSCD is a concurrent feature in most of these eyes, the spread of the conjunctival epithelium continues over the corneal surface as there is no barrier preventing their migration. Complete epithelization of the ocular surface marks the end of the process and is associated with significant vascularization and scarring of the cornea. The newly formed ocular surface appears vascular and fleshy, not unlike a recurrent pterygium, and gives the eye a congested appearance. Also, depending upon the extent of Tenon’s capsule and the fibrous tissue dissected intra-operatively, there may be symblepharon formation.
Surgical Outcomes
Table 1 gives an overview of all the studies where the outcomes of tenonplasty have been discussed in eyes with acute OCBs. All were non-comparative case series,[3,5,9,20,27,28,31] with the primary indication being conjunctival and scleral ischemia in severe cases of acute chemical injury (McCulley and Dua’s Grade IV and worse), except in the study by Peng W et al.,[5] where tenonplasty was performed in less severe grades of chemical injury (Dua’s grade 3 or better). These studies cumulatively included 222 eyes where a total tenonplasty was performed in 197 eyes (88.7%) and selective tenonplasty was performed in 25 eyes (11.3%). Of the eyes (26.5%; 59/222) where the causative chemical agent was known, 26 eyes (44%) had alkali injuries, 24 eyes (40.6%) had acid injuries, and eight eyes (13.5%) had thermal injuries. The mean follow-up period ranged from 6 to 82 months, with a successful outcome being defined as complete re-epithelization over the conjunctival and corneal surfaces. This re-epithelization rate was 100% in all the studies, except in a study by Gupta et al.,[31] where complete conjunctival and corneal epithelial regeneration was seen in 69% eyes (9/13). No intra-operative complications were reported during the procedure. A repeat tenonplasty was required in seven eyes (3%) because of retraction of the Tenon’s tissue. The time for complete epithelial regeneration showed significant variation among the different studies, from as early as 5 weeks after the procedure to a maximum period of 6 months. One or more secondary surgical interventions for surface reconstruction and visual rehabilitation were carried out in 24.7% eyes (55/222).
Table 1.
A review of studies which describe the outcomes of tenonplasty in acute ocular chemical burns (studies with outcomes of ≥5 eyes with ocular chemical burns were included)
| Author, year | Eyes/Grade of chemical injury | Surgery performed | Post -operative follow-up duration (months) | Outcomes (conjunctival and corneal epithelial re-generation) after tenonplasty | Post-operative complications | Additional surgeries required |
|---|---|---|---|---|---|---|
| Reim M et al.,[9] 1992 | 24 eyes, total conjunctival necrosis, scleral ischemia | Tenonplasty | 6-42 months | 100% eyes (24) in 7 weeks | NA | NA |
| Kuckelkorn R et al.,[3] 1995 | 64 eyes with grade IV chemical/thermal burns (McCulley classification), total conjunctival and scleral necrosis | Tenonplasty | 6-84 months | Epithelization over the Tenon’s flap – 100% eyes (64) at 5 weeks Corneal re-epithelization was seen in 25% eyes (16) |
Symblepharon (22%, 13 eyes) | Keratoplasty |
| Kuckelkorn R et al.,[20]1997 | 75 eyes, grade IV McCulley classification, Alkali burns (most common etiology) | 43 eyes – tenonplasty in all four quadrants; 7 eyes – tenonplasty in three quadrants; 25 eyes – tenonplasty in two quadrants | 9-82 months | Epithelization over the Tenon’s flap – 100% eyes (75) at 8 weeks Corneal re-epithelization in 30.7% eyes (23) | Moderate symblepharon (28%, 21 eyes) Complete obliteration of fornix with broad symblepharon (26.7%, 20 eyes) |
Penetrating keratoplasty in 42.7% eyes (32) for corneal ulceration |
| Iyer G et al.,[27] 2012 | 21 eyes, grade V and VI Dua’s classification Acid injury – 17 Thermal injury – 3 Alkali injury – 1 |
Four-quadrant tenonplasty with AMT | 27.37±14.5 months | 100% eyes – complete conjunctival epithelization in 5.4±4.03 months (repeat tenonplasty was required in 6 eyes) | Phthisis bulbi (4.8%, 1 eye) Evisceration following microbial keratitis (4.8%, 1 eye) Uncontrolled glaucoma (4.8%, 1 eye) |
Surface re-construction in 47.6% eyes (10) for visual rehabilitation - Ex vivo LSCT with keratoplasty (14%, 3 eyes) -MOOKP (23.8%, 5 eyes) - Boston type 1 keratoprosthesis (9.5%, 2 eyes) - Keratoplasty with KLAL (4.7%, 1 eye) |
| Tabatabaei SA et al.,[28] 2017 | 6 eyes, Grade V and VI Dua’s classification Acid injury – 2 Alkali injury – 3 | Selective tenonplasty* | 24 months | 100% eyes – complete epithelization (repeat tenonplasty was required in 1 eye) | Symblepharon (50%, 3 eyes) | Surface re-construction for visual rehabilitation in all the six eyes |
| Gupta N et al.,[31] 2018 | 13 eyes, Grade IV, V and VI Dua’s classification Alkali injury – 6 Acid injury – 3 Thermal injury – 4 |
Tenonplasty+AMT | 12 months | 69% eyes (9) – complete epithelization at 6 months | LSCD (69%, 9 eyes) Phthisis bulbi (15%, 2 eyes) Hypotony with LSCD (15%, 2 eyes) | SLET in 53.8% eyes (7) |
| Peng W et al.,[5] 2020 | 19 eyes, Grade III and less, Dua’s classification Alkali injury – 16 Acid injury – 2 Thermal injury – 1 | Selective tenonplasty + AMT ^ | NA | 100% eyes – complete conjunctival with corneal epithelization in 26–156 days | Focal pterygium (26.3%, 5 eyes) Secondary glaucoma (36.8%, 7 eyes) Secondary cataract (42%, 8 eyes) Symblepharon (15.7%, 3 eyes) Eyelid entropion (26.3%, 5 eyes) |
None |
*Selective limbal peritomy and Tenon’s advancement were performed over limited areas of limbal and scleral non-perfusion identified using fluorescein angiography. ^Selective tenonplasty was performed in patients with persistent corneal epithelial, conjunctival, and Tenon’s tissue defects as well as ischemic sclera based on clinical evaluation. The time interval from the chemical burn to the surgery varied between 3 to 91 days (mean: 37.4±24.5 days). NA=not available; AMT=amniotic membrane transplantation; LSCT=limbal stem cell transplantation; MOOKP=modified osteo-odonto keratoprosthesis; KLAL=kerato-limbal allograft; LSCD=limbal stem cell deficiency; SLET=simple limbal epithelial transplantation; AS-OCTA=anterior segment optical coherence tomography angiography
Post-Operative Complications
None of the studies have reported serious complications post tenonplasty. The most common complication was obliteration of the conjunctival fornix and symblepharon formation seen in 31% eyes (69/222).[6,20,28] Other complications reported were retraction of the Tenon’s pedicle flap,[28] diplopia, and restriction of extra-ocular movements because of extensive dissection of the necrotic tissue, especially if the Tenon’s flap is taken from the nasal quadrant.[27,30] Most complications related to tenonplasty are known to develop within 3 months after the procedure.[20]
Conclusion
Limbal ischemia and scleral ischemia herald the onset of serious acute and chronic complications in eyes with chemical injuries. Relying only on clinical examination for the identification of these signs has several fallacies which can be subverted using diagnostic tools such as FA and AS-OCTA. However, these also have their own inherent limitations, and an objective method of diagnosing ischemia is yet to be standardized. Incorporation of in-built tools within these devices will help confirm the presence of ischemia, quantify the degree of severity, plan surgical interventions, and monitor the response to the same. Furthermore, integrating the results of these imaging modalities within the existing classifications can make prognosticating these cases more robust.
Tenonplasty is an extremely useful technique to salvage eyes with severe acute ocular chemical burns with limbal ischemia and scleral melt. If performed at an appropriate time, it can prevent grave complications in the setting of ischemic necrosis, which are otherwise exceedingly difficult to manage. However, quantifying the efficacy of this surgical technique in the absence of controlled studies is challenging. Complications following this procedure are rare with the formation of a symblepharon being the most common one. Once the globe has been salvaged with adequate epithelization of the ocular surface, further surgical interventions for visual rehabilitation such as penetrating keratoplasty with limbal stem cell transplantation and keratoprosthesis can be undertaken.
Financial support and sponsorship
This work was funded by the Hyderabad Eye Research Foundation Hyderabad, India. The sponsoring organization had no role in the design or conduct of this research.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ahmmed AA, Ting DSJ, Figueiredo FC. Epidemiology, economic and humanistic burdens of ocular surface chemical injury:A narrative review. Ocul Surf. 2021;20:199–211. doi: 10.1016/j.jtos.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 2.White WL, Hollsten DA. Burns of the ocular adnexa. Curr Opin Ophthalmol. 1994;5:74–7. [PubMed] [Google Scholar]
- 3.Kuckelkorn R, Schrage N, Reim M. Treatment of severe eye burns by tenonplasty. Lancet. 1995;345:657–8. doi: 10.1016/s0140-6736(95)90564-2. [DOI] [PubMed] [Google Scholar]
- 4.Duke-Elder S MP. Non mechanical injuries. In: Duke-Elder S, editor. System of Ophthalmology Vol. XIV. St. Louis, MO: Mosby; 1972. [Google Scholar]
- 5.Peng WY, He LW, Zeng P, Chen DC, Zhou SY. Tenonplasty combined with amniotic membrane transplantation for patients with severe ocular burns induced anterior segment necrosis. J Burn Care Res. 2020;41:668–73. doi: 10.1093/jbcr/iraa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teping C, Reim M. [Tenonplasty as a new surgical principle in the early treatment of the most severe chemical eye burns. Klin Monbl Augenheilkd. 1989;194:1–5. doi: 10.1055/s-2008-1046325. [DOI] [PubMed] [Google Scholar]
- 7.Watson PG, Young RD. Scleral structure, organisation and disease. A review. Exp Eye Res. 2004;78:609–23. doi: 10.1016/s0014-4835(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 8.Kakizaki H, Takahashi Y, Nakano T, Asamoto K, Ikeda H, Ichinose A, et al. Anatomy of Tenons capsule. Clin Exp Ophthalmol. 2012;40:611–6. doi: 10.1111/j.1442-9071.2011.02745.x. [DOI] [PubMed] [Google Scholar]
- 9.Reim M. The results of ischaemia in chemical injuries. Eye (Lond) 1992;6:376–80. doi: 10.1038/eye.1992.77. [DOI] [PubMed] [Google Scholar]
- 10.Hughes WF., Jr Alkali burns of the eye;Clinical and pathologic course. Arch Ophthalmol. 1946;36:189–214. doi: 10.1001/archopht.1946.00890210194005. [DOI] [PubMed] [Google Scholar]
- 11.Ballen PH. Treatment of chemical burns of the eye. Eye Ear Nose Throat Mon. 1964;43:57–61. [PubMed] [Google Scholar]
- 12.Roper-Hall MJ. Thermal and chemical burns. Trans Ophthalmol Soc UK. 1965;85:631–53. [PubMed] [Google Scholar]
- 13.Dua HS, King AJ, Joseph A. A new classification of ocular surface burns. Br J Ophthalmol. 2001;85:1379–83. doi: 10.1136/bjo.85.11.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kam KW, Patel CN, Nikpoor N, Yu M, Basu S. Limbal ischemia:Reliability of clinical assessment and implications in the management of ocular burns. Indian J Ophthalmol. 2019;67:32–6. doi: 10.4103/ijo.IJO_945_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung SSM, Stewart RMK, Dhallu SK, Sim DA, Keane PA, Wilkins MR, et al. Anterior segment optical coherence tomographic angiography assessment of acute chemical injury. Am J Ophthalmol. 2019;205:165–74. doi: 10.1016/j.ajo.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Kuckelkorn R, Remky A, Wolf S, Reim M, Redbrake C. Video fluorescein angiography of the anterior eye segment in severe eye burns. Acta Ophthalmol Scand. 1997;75:675–80. doi: 10.1111/j.1600-0420.1997.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwenhuizen J, Watson PG, Emmanouilidis-van der Spek K, Keunen JE, Jager MJ. The value of combining anterior segment fluorescein angiography with indocyanine green angiography in scleral inflammation. Ophthalmology. 2003;110:1653–66. doi: 10.1016/S0161-6420(03)00487-1. [DOI] [PubMed] [Google Scholar]
- 18.Ang M, Foo V, Ke M, Tan B, Tong L, Schmetterer L, et al. Role of anterior segment optical coherence tomography angiography in assessing limbal vasculature in acute chemical injury of the eye. Br J Ophthalmol. 2021 doi: 10.1136/bjophthalmol-2021-318847. doi:10.1136/bjophthalmol-2021-318847. [DOI] [PubMed] [Google Scholar]
- 19.Tey KY, Gan J, Foo V, Tan B, Ke MY, Schmetterer L, et al. Role of anterior segment optical coherence tomography angiography in the assessment of acute chemical ocular injury:A pilot animal model study. Sci Rep. 2021;11:16625. doi: 10.1038/s41598-021-96086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuckelkorn R, Redbrake C, Reim M. Tenonplasty:A new surgical approach for the treatment of severe eye burns. Ophthalmic Surg Lasers. 1997;28:105–10. [PubMed] [Google Scholar]
- 21.Dougherty PJ, Hardten DR, Lindstrom RL. Corneoscleral melt after pterygium surgery using a single intraoperative application of mitomycin-C. Cornea. 1996;15:537–40. [PubMed] [Google Scholar]
- 22.Tsai YY, Lin JM, Shy JD. Acute scleral thinning after pterygium excision with intraoperative mitomycin C:A case report of scleral dellen after bare sclera technique and review of the literature. Cornea. 2002;21:227–9. doi: 10.1097/00003226-200203000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Sangwan VS, Jain V, Gupta P. Structural and functional outcome of scleral patch graft. Eye (Lond) 2007;21:930–5. doi: 10.1038/sj.eye.6702344. [DOI] [PubMed] [Google Scholar]
- 24.Ozcan AA, Bilgic E, Yagmur M, Ersöz TR. Surgical management of scleral defects. Cornea. 2005;24:308–11. doi: 10.1097/01.ico.0000141228.10849.17. [DOI] [PubMed] [Google Scholar]
- 25.Oh JH, Kim JC. Repair of scleromalacia using preserved scleral graft with amniotic membrane transplantation. Cornea. 2003;22:288–93. doi: 10.1097/00003226-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Xi X, McMillan DH, Lehmann GM, Sime PJ, Libby RT, Huxlin KR, et al. Ocular fibroblast diversity:Implications for inflammation and ocular wound healing. Invest Ophthalmol Vis Sci. 2011;52:4859–65. doi: 10.1167/iovs.10-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iyer G, Srinivasan B, Agarwal S, Barbhaya R. Visual rehabilitation with keratoprosthesis after tenonplasty as the primary globe-saving procedure for severe ocular chemical injuries. Graefes Arch Clin Exp Ophthalmol. 2012;250:1787–93. doi: 10.1007/s00417-012-2030-8. [DOI] [PubMed] [Google Scholar]
- 28.Tabatabaei SA, Soleimani M, Mirshahi R, Zandian M, Ghasemi H, Hashemian MN, et al. Selective localized tenonplasty for corneal burns based on the findings of ocular surface fluorescein angiography. Cornea. 2017;36:1014–7. doi: 10.1097/ICO.0000000000001256. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Tian Y, Zhu H, Cheng Y, Zheng X, Wu J. Tenonplasty combined with free oral buccal mucosa autografts for repair of sclerocorneal melt caused by chemical burns. Cornea. 2015;34:1240–4. doi: 10.1097/ICO.0000000000000576. [DOI] [PubMed] [Google Scholar]
- 30.Casas VE, Kheirkhah A, Blanco G, Tseng SC. Surgical approach for scleral ischemia and melt. Cornea. 2008;27:196–201. doi: 10.1097/ICO.0b013e31815ba1ae. [DOI] [PubMed] [Google Scholar]
- 31.Gupta N, Singh A, Mathur U. Scleral ischemia in acute ocular chemical injury:Long-term impact on rehabilitation with limbal stem cell therapy. Cornea. 2019;38:198–202. doi: 10.1097/ICO.0000000000001807. [DOI] [PubMed] [Google Scholar]