Abstract
Remimazolam (CNS7056) is a novel benzodiazepine for intravenous sedation; it has an ultra-short duration of action and was recently approved for use in procedural sedation and general anaesthesia. It acts on γ-aminobutyric acid type A receptors and is rapidly converted into an inactive metabolite by tissue esterase enzymes. Remimazolam has been successfully used in endoscopic inspection or surgery and general anaesthesia induction and maintenance with fast and predictable onset and recovery times, high procedure success rates, and minor respiratory and hemodynamic fluctuations and without serious drug-related adverse reactions. If needed, the effects of remimazolam can be reversed by flumazenil, which allows prompt termination of sedation. Although remimazolam has great potential for sedation in patients admitted to intensive care units, future studies are needed to evaluate its efficacy and safety in patients requiring sedation for a long period, and numerous studies are warranted to explore the optimal dose in different application scenarios. The review aimed to provide an introduction to the process of remimazolam synthesis and its current clinical uses and future clinical developments.
Keywords: sedation, endoscopy, general anaesthesia, ICU
Introduction
Sedation is an essential procedure used to relieve anxiety, pain, and discomfort among patients undergoing invasive procedures for diagnosis and treatment.1 Sedatives such as benzodiazepines (BDZs) (eg, midazolam, a well-known short-acting BDZ), propofol, etomidate, and dexmedetomidine have been commonly used in recent years;2,3 however, all of these sedatives have some drawbacks. For example, long-term infusion of midazolam results in respiratory depression and increases the time to recovery;4 propofol causes significant cardiorespiratory depression and injection pain;5 etomidate causes myoclonus, nausea and vomiting, and injection pain;6 and dexmedetomidine causes adverse reactions such as hypotension and bradycardia.7 Ideal, fast-onset anaesthetics and sedatives with sedative–hypnotic properties, a high therapeutic ratio of toxic dose to a minimum effective dose, and the least possible risk of side-effects when meeting sedation requirements is warranted in the anaesthesia and critical care domains.1,8,9
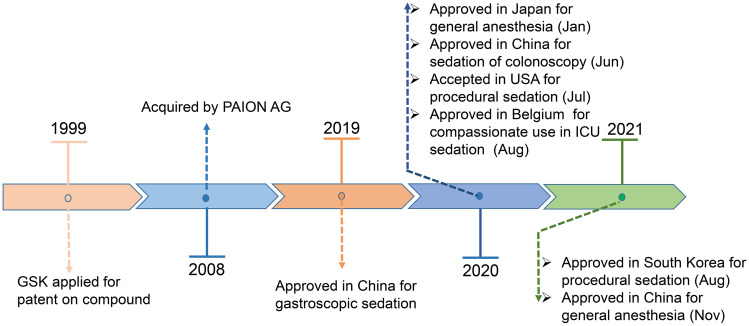
Remimazolam (CNS7056) is a new ultra-short-acting intravenous (IV) BDZ developed by the pharmaceutical company PAION AG and acts on γ-aminobutyric acid type A (GABAA) receptors.10 Owing to its “soft drug” (self-metabolising and organ-independent) design, it is rapidly hydrolyzed in the body as an inactive carboxylic acid (CNS7054) by tissue esterase enzymes present in the blood.11 Remimazolam was approved in December 2019 for sedation during gastroscopy and for colonoscopy in June 2020 in China; it was also approved for procedural sedation in the United States in July 2020 and the European Union and South Korea in August 2021.12,13 Remimazolam was first approved for use in general anaesthesia in Japan on 23 January, 2020, and then in China in November 2021.14 It was also approved for compassionate use in intensive care unit (ICU) sedation in Belgium in August 2020.15 (Figure 1).
Figure 1.
The main milestones in the development of remimazolam.
Remimazolam has been successfully used for the induction and maintenance of procedural sedation and general anaesthesia owing to its fast onset, short and predictable duration of sedative action, short recovery time, rare accumulation after long-term infusion, and less serious side effects as compared with those of other currently used BDZs.16,17 These characteristics render remimazolam a very promising sedative for use among a wide range of patients, including critically ill ones.16
This review is aimed at providing updated information on the synthesis, pharmacokinetics and pharmacodynamics, and current and future clinical applications of remimazolam as a novel sedative and anesthetic to provide a theoretical basis for further clinical applications.
The Pharmacology of Remimazolam
Chemical Structure
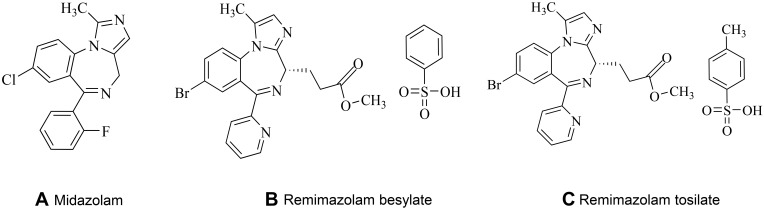
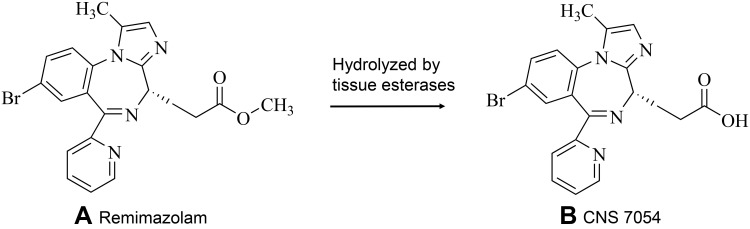
Remimazolam is the newest benzodiazepine and possesses the properties of a combination of two existing drugs used in anaesthesia: midazolam and remifentanil.18 Its structure is similar to that of midazolam; it has a high-affinity, selective ligand for the BZD site on the GABAA receptor but no affinity for other sites.19 From the soft drug standpoint, medicinal chemists have been inspired by the structures of etomidate and remifentanil. A new type of ultra-short-acting sedative/ anesthetic has been synthesized by introducing a carboxylic ester side group into the chemical structure of midazolam (Figure 2).18,19 In vivo, this carboxylic ester is rapidly broken down by tissue esterases into the inactive metabolite CNS7054 (Figure 3); compared with CNS7056, CNS7054 shows at least 300 times lower affinity with GABAA,17,20 and this mechanism is responsible for the ultra-short-action of the drug.21
Figure 2.
Chemical structures: (A) midazolam, (B) remimazolam besylate, (C) remimazolam tosilate.
Figure 3.
Chemical structure of (A) remimazolam and the carboxylic acid metabolite (B) CNS7054.
Pharmacokinetics and Pharmacodynamics
Pharmacokinetics
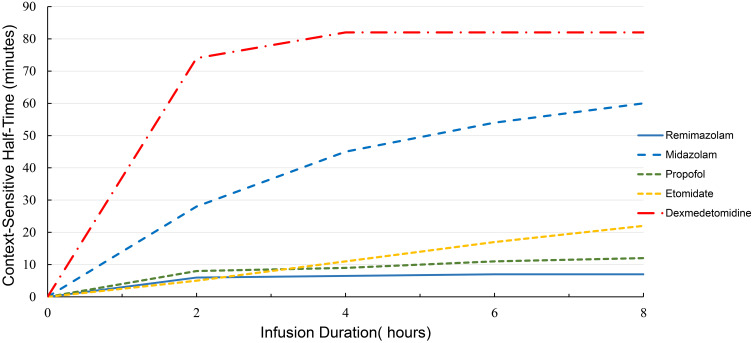
Remimazolam is rapidly distributed upon intravenous administration. According to a phase I ascending-dose study conducted among healthy adults administered with 1-min intravenous dose of 0.01–0.3 mg/kg remimazolam, the maximum effect of sedation was achieved within 3 min of injection.17,22 Part I of this study discovered that remimazolam had an approximately three times greater systemic clearance (CL) than midazolam {0.075 mg/kg [(70.3±13.9) L/h vs (23.0±4.5) L/h]}, whereas its steady-state volume (Vss) of distribution was 50% of that of midazolam [(34.8±9.4) L vs (81.8±27.1) L], and its terminal half-life (T1/2) was also shorter than that of midazolam (0.75±0.15 vs 2.89±0.65 h). Thus, the mean retention time of remimazolam was one-seventh of that of midazolam (0.51 vs 3.6 h) (Table 1).17 Part II of this study demonstrated that the context-sensitive half-times (the time required for the plasma level of the drug to decrease by 50% after the infusion is stopped) of remimazolam (50 mg/h) were much shorter than those of midazolam (0.075 mg/kg/h), the pharmacokinetics (PKs) of remimazolam were linear within the dose range, and the clearance of remimazolam from the body was not associated with a body weight of 65–90 kg.22 The context-sensitive half-times of remimazolam facilitate its rapid removal; remimazolam can reach a steady-state value of 7–8 min after an approximate infusion of 2 h and appears to not be affected by the infusion duration10,22,23 (Figure 4).
Table 1.
Pharmacokinetic Parameters of Remimazolam and Midazolam
| Drug | Onset (Min) | Recovery (Min) | Duration (Min) | CL (L/h) | Vss (L) | T1/2 (Hours) | MRT (Hours) |
|---|---|---|---|---|---|---|---|
| Remimazolam17 | 1–3 | 5.5–20 | 8.0 | 70.3±13.9 | 34.8±9.4 | 0.75±0.15 | 0.51 |
| Midazolam17,28 | 3–5 | 40.0 | 12.0 | 23.0±4.5 | 81.8±27.1 | 2.89±0.65 | 3.6 |
Abbreviations: CL, systemic clearance; Vss, volume of distribution at steady state; T1/2, terminal half-life; MRT, mean retention time.
Figure 4.
The context-sensitive half-times (the time required for the plasma level of the drug to decrease 50% after the infusion is stopped) for the sedatives remimazolam, midazolam, propofol, etomidate, and dexmedetomidine.
Notes: Data from Whizar et al,10 Wiltshire et al,22 Schüttler et al,23 and Iirola et al.33
Remimazolam showed no significant effects on the PR interval and QRS duration, and the largest change in the QTcI interval from baseline never exceeded 10 ms. Thus, no clinically significant effect of remimazolam on cardiac repolarization was observed during procedural sedation and anaesthesia.23 Moreover, remimazolam has no clinical effects on heart rate (HR), electrocardiogram (ECG), blood pressure (BP), and respiratory rate.17 Further, according to a preclinical study conducted in sheep, no burst suppression patterns and isoelectric electroencephalogram (EEG) results were observed for remimazolam.24 It can reduce the incidence of delirium and postoperative cognitive dysfunction and ensure the rapid return of neurological function to baseline.4,25 Remimazolam undergoes organ-independent metabolism and is rapidly hydrolyzed by tissue esterases (mainly catalyzed by carboxylesterases in the liver) to an inactive carboxylic acid metabolite (CNS7054), bypassing the action of cytochrome-dependent hepatic pathways (particularly P450-mediated metabolism).19,22,23 Owing to the absence of the P450-mediated metabolism of remimazolam, clinically significant metabolic drug interactions are unlikely.26 Approximately 99.7% of remimazolam is transformed into CNS7054 in the plasma and excreted in urine.27 Because remimazolam shows no accumulation and CNS7054 is pharmacologically inactive, it does not affect liver and kidney function and can be safely used among patients with impaired liver or renal function.28,29
No significant differences were observed in the pharmacokinetics of remimazolam between healthy individuals and patients with end-stage renal disease. Remimazolam can be used in patients with varying degrees of renal impairment without any requirement for dose adjustment. However, AUC(0-∞), Vss, T1/2, and recovery times (171 ng·h/mL; 1.01 L/kg; 109 min; 16.7 min) were increased in patients with severe hepatic impairment (Child-Pugh scores ≥10) as compared with that in patients with normal liver function (132 ng·h/mL; 0.329 L/kg; 43.1 min; 8.0 min); thus, remimazolam should be cautiously used during anaesthesia or sedation maintenance in such patients.13,29 Lower doses of infusion should be considered for the fragile elderly or American Society of Anesthesiologists (ASA) class 3+ patients, although the PK of remimazolam is unaffected by age, ASA class, sex, and race.23,30,31
Pharmacodynamics
Previous phase I pharmacokinetics and pharmacodynamics trials demonstrated that, in healthy volunteers, rapid-onset sedation for remimazolam was initially observed at a dose of 0.05 mg/kg and peak sedation effect was observed within 1–2 min after injection at doses of ≥ 0.075 mg/kg.32 At higher doses of 0.075–0.2 mg/kg, remimazolam showed a greater sedation effect while still maintaining a shorter recovery time, as compared with midazolam (0.075 mg/kg); that is, the duration of sedation and the median time for patients to be fully alert were shorter than those for midazolam (8 vs 12 min; 5.5–20 vs 40 min) (Table 1).17,32 Recovery took up to 50 min with higher doses of remimazolam (0.25, 0.3, and 0.4 mg/kg); such doses are not suitable for short-term procedures or diagnostic sedation but may be used for anaesthesia induction. In other words, remimazolam is a safe, well-tolerated, rapid-onset option with dose-dependent pharmacodynamics properties.17,32
A study on the pharmacokinetics and pharmacodynamics of remimazolam after continuous infusion was published in 2020 [sedation was assessed based on the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale]. Remimazolam was continuously injected into 20 healthy males at 5 mg/min for 5 min, at 3 mg/min for the next 15 min, and at 1 mg/min for a further 15 min; remimazolam showed a low Vss (35.4±4.2 L) and a high clearance (1.15±0.12 L/min), consistent with that reported in a previously published study.23 The context-sensitive decrement times of remimazolam were short and reached 6.8±2.4 min after infusion for 4h. All the subjects lost consciousness within 5±1 min (MOAA/S score decreased from 5 to <2), and full alertness (MOAA/S score, 5) was recovered 19±7 min after the infusion was stopped.23
Owing to its organ-independent metabolism and its first-order pharmacokinetics, the degree and duration of remimazolam sedation are dose-dependent, and long-term infusions or higher doses will not result in cumulative and extended sedative effects.4 Moreover, remimazolam sedation can be reversed by flumazenil (1 min flumazenil vs 10.5 min placebo) without re-sedation.34 These characteristics make its use as a sedative agent suitable as an intravenous anesthetic and in the ICU.4
Clinical Uses
Sedation in Endoscopy
Upper Gastrointestinal Endoscopy
Gastrointestinal endoscopy is a commonly performed medical procedure that is better accomplished with sedation to relieve discomfort, pain, or anxiety.35,36 Several published clinical trials have used remimazolam as a sedative for upper gastrointestinal endoscopy (Table 2). A phase IIa clinical study of remimazolam for sedation in gastroscopy was published in 2015 (NCT00869440). Patients were randomly allocated to four groups (25 patients per group) and intravenously treated with a single dose of remimazolam (0.10, 0.15, and 0.20 mg/kg) or midazolam (0.075 mg/kg). The time to onset (MOAA/S score, ≤3) was faster than in the remimazolam groups than in the midazolam group (1.5–2.5 vs 5 min), and the mean time to becoming fully alert (MOAA/S score, 5) was also shorter in the remimazolam groups (6.8–9.9 vs 11.5 min); moreover, the procedural success rates in the remimazolam groups (0.10, 0.15, and 0.20 mg/kg groups) were 32%, 56%, and 64% as compared with that in the midazolam group (44%). There were no significant differences in the safety of using remimazolam and midazolam. The study results showed that a single dose of remimazolam (0.10–0.20 mg/kg) can be used safely and effectively for procedure sedation; however, initial loading doses of at least 0.15 mg/kg of remimazolam needed further studies for detailed verification of the findings.37 Another phase III trial published in 2021 (NCT03425474) enrolled 384 Chinese patients aged 18–60 years to compare the efficacy and safety of remimazolam tosilate (RT) with propofol for use in gastroscopy. Patients were randomly assigned to receive 5.0/2.5 mg of remimazolam (initial dose of 5.0 mg, supplemental dose of 2.5mg), 1.5/0.5 mg/kg of propofol, or 0.5 μg/kg of fentanyl before sedative medication. The sedation success rate in the remimazolam group was non-inferior to that of propofol (97.34% vs 100.00%; difference in rate: −2.66%; 95% CI: −4.96 to 0.36; meeting the criteria for non-inferiority). Compared with those in the propofol group, patients in the remimazolam group exhibited a longer time to adequate sedation (1.3 vs 2.0 min) but a shorter time to become fully alert (5.75 vs 6.71 min). The percentage of adverse events (AEs) that occurred in the remimazolam group was less than that in the propofol group (39.15% vs 60.32%). Compared with propofol, remimazolam showed a rapid recovery time but a lower potential to cause cardiorespiratory depression among patients undergoing gastroscopy.38 Recently, a phase III trial enrolled elderly patients aged >60 years who required upper gastrointestinal endoscopy and evaluated the effects of RT on early cognitive function in those patients (ChiCTR2100042084). All patients received 10 g of lidocaine viscous orally and butorphanol (0.01 mg/kg) intravenously before receiving the test sedatives. The researchers administered the sedative drugs intravenously according to the group allocations [a dose of 0.10 mg/kg (R1) or 0.20 mg/kg (R2) RT; or 1.0–1.5 mg/kg propofol].39 Differences in the postoperative cognitive functions, sedation times, and recovery times between the patients in the R1 and propofol groups were not statistically significant. The R2 group demonstrated worsened postoperative results than baseline upon immediate recall (−2.79±4.04 vs 1.64±3.97, P<0.001) and short-delayed recall (−0.97±1.83 vs 0.61±1.47, P=0.003). Besides, the R2 group exhibited a longer sedation time (12.09±3.60 vs 8.21±2.85, P<0.01) and recovery time (6.85±4.29 vs 4.33±2.97 min, P<0.01) compared with the propofol group. The incidence of hypotension was lower in the R1 group than in the R2 and propofol groups (3.0% vs 21.2% vs 48.5%, P<0. 05). The results indicated that 0.1 mg/kg RT suitable for sedation in elderly patients undergoing upper gastrointestinal endoscopy exhibited not only significantly stable hemodynamics but also acceptable neuropsychiatric functions.39
Table 2.
Main Clinical Trials of Remimazolam for Sedation Under Gastroscopy
| Author (Year) | Phase | N | Dose of Remimazolam | Age (Years) | Primary Endpoint | Comparator | Co-Treatment | Location (s) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Borkett et al 201937 | IIa | 100 | Three dose of group: 0.10, 0.15, or 0.20 mg/kg | 18–65 | Safety and efficacy of different single doses for sedation in upper gastrointestinal endoscopy | Midazolam 0.075 mg/kg |
N/A | USA | A single dose of remimazolam (0.1–0.2mg/kg) was able to induce rapider sedation and faster recovery than midazolam in patients undergoing upper gastrointestinal endoscopy |
| Chen et al 202138 | III | 384 | 5.0 mg plus 2.5 top-ups | 18–60 | Compare efficacy and safety of remimazolam tosilate with propofol for sedation in upper gastrointestinal endoscopy | Propofol 1.5 mg plus 0.5 mg/kg top-ups | Fentanyl (0.5 μg/kg); lidocaine viscous oral liquid 10g |
China | The sedation successful rate in remimazolam was non-inferior to propofol but shorter time to recovery from sedation, safety profile appears to be superior to propofol |
| Tan et al 202239 | III | 99 | Initial dose: two dose of group (0.1 mg/kg or 0.2 mg/kg), supplemental dose: 0.05 mg/kg |
>60 | Efficacy and safety of remimazolam tosilate in patients undergoing bronchoscopy | Propofol 1.0–1.5 mg plus 0.5mg/kg top-ups | N/A | China | A dose of 0.1 mg/kg remimazolam tosilate suitable for sedation in elderly patients undergoing upper gastrointestinal endoscopy |
| Cao et al 202240 | III | 148 | 0.107 mg/kg | 18–65 | Efficacy and safety of remimazolam tosilate sedation in patients with liver cirrhosis undergoing gastroscopy | Propofol 2 mg/kg |
Sufentanil 0.15 μg/kg | China | Remimazolam tosilate combined with sufentanil adjuvant is better than propofol with sufentanil in patients with liver cirrhosis undergoing gastroscopy |
Abbreviations: N, number of subjects; N/A, not available/applicable.
A 2022 clinical study recruited 148 Chinese patients with liver cirrhosis (Child-Pugh class A) undergoing gastroscopy to evaluate the efficacy and safety of remimazolam sedation as compared with propofol sedation (ChiCTR2100042179). Patients were divided into two groups to receive either 0.107 mg/kg of RT or 2 mg/kg of propofol within 30s. All patients required 0.15 μg/kg of intravenous sufentanil before sedative medication. If the sedation was insufficient, an additional dose of 20 mg propofol was administered. The mean time to sedation (MOAA/S score, ≤1) was longer among patients in the RT group than among those in the propofol group (88.3 vs 62.7s, P<0.001); however, the RT group had a shorter time to recovery (44.7 vs 64.6s, P<0.001). The sedation satisfaction rate during the procedures was significantly higher in the RT group than in the propofol group (90.5% vs 77.0%, P=0.026); moreover, a lower incidence of respiratory depression (2.7% vs 17.6%, P=0.003), body movement (8.1% vs 23.0%, P=0.013), and hypotension (4.1% vs 14.9%, P=0.025) were noted in the RT group than in the propofol group. This trial suggested that RT combined with sufentanil adjuvant is better than propofol combined with sufentanil for patients with liver cirrhosis undergoing gastroscopy.40
Colonoscopy
Several clinical trials have used remimazolam as a sedative during colonoscopy (Table 3). A 2018 phase III study evaluated the safety and efficacy of remimazolam in patients undergoing colonoscopy; the study enrolled 461 patients with ASA class I, II, or III (NCT02290873). All patients were treated with fentanyl at doses of 50–75 μg before the study. The patients were randomly allocated to receive 5.0/2.5 mg of remimazolam or an equal volume of placebo, whereas patients in the midazolam group received an initial dose of 1.75 mg. In addition, midazolam was the rescue medication in case of treatment failure. The success rate of the completion of the procedure was higher in the remimazolam group (91.3% vs 1.7% vs 25.5%) than in the placebo and midazolam groups. The procedure times (time from the start of medication administration until reaching an MOAA/S of 3) were shorter (5.1 vs 20.3 vs 16.9 min) for remimazolam. The mean time from the end of the procedure to becoming fully alert was also shorter in the remimazolam group than in the other two groups (7.35 vs 21.95 vs 15.84 min). The total dose of fentanyl was lower with remimazolam (88.6 vs 121.3 vs 106.9 μg). This clinical trial demonstrated that remimazolam is a safe and effective sedative for use in patients undergoing colonoscopy.41
Table 3.
Main Clinical Trials of Remimazolam for Sedation Undergoing Colonoscopy
| Author (Year) | Phase | N | Dose of Remimazolam | Age (Years) | Primary Endpoint | Comparator | Co-Treatment | Location (s) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Rex et al 201841 | III | 461 | 5.0 mg plus 2.5 top-ups | 18–74 | Efficacy and safety of remimazolam in patients undergoing colonoscopy | Midazolam [1.75 mg plus 0.5 mg (≥60 years) or 1.0 mg (<60 years) top-ups] | Fentanyl (75 μg) | USA | Patients in remimazolam group had Procedural success rate, faster mean procedure time and recovery time, less needed of fentanyl, less incidence of hypotension |
| Liu et al 202142 | III | 260 | 0.15mg/kg plus 0.075 mg/kg top-ups | 65–75 | Efficacy and safety of remimazolam in elderly outpatients undergoing colonoscopy | Etomidate-Propofol (0.1 mL/kg plus 0.05 mL/kg top-ups) | Fentanyl (0.15 mg/kg plus 0.5 mg/kg top-ups, the maximum dose of 150 μg) | China | The procedure success rate in remimazolam group was non-inferior to etomidate-propofol group (96.52% vs 100.00%; difference in rate −3.48%, 95% CI −6.81% to −0.15%), and has a higher safety for elderly patients |
| Rex et al 202143 | III | 79 | 2.5–5.0 mg plus 1.25–2.5 mg top-ups | 42–84 | Safety and efficacy of remimazolam in ASA class III/IV patients undergoing colonoscopy | Midazolam (1.0 mg plus 0.5 mg top-ups) | Fentanyl (50 μg, top up doses of 25 μg, the maximum dose of 200 μg) | USA | Procedure success for patients treated with remimazolam, placebo, midazolam was 87.1%, 0%, 13.3%, the shorter time to sedation and recovery in remimazolam group, and no serious AEs were reported. it can be efficacy and safety for sedation in ASA III/IV patients |
| Yao et al 202244 | III | 132 | 0.2 mg/kg plus 6 mg top-ups | 18–65 | Discharge readiness after remimazolam versus propofol for colonoscopy | Propofol (1 mg/kg plus 30 mg top-ups) | Sufentanil (5 mg) | China | Remimazolam provided a similar sedative efficacy and safer profile in patients undergoing colonoscopy, compared with propofol |
Abbreviations: N, number of subjects; N/A, not available/applicable.
A 2021 clinical trial compared the efficacy and safety of RT with that of etomidate–propofol in 260 elderly outpatients undergoing colonoscopy (ChiCTR2000041524). Patients received 0.15 mg/kg of RT (n=115) or 0.1 mL/kg of etomidate–propofol (10 mL of 20 mg etomidate + 10 mL of 100 mg of propofol; n=117). The procedure success rate in the remimazolam group was non-inferior to that in the etomidate–propofol group (96.52% vs 100.00%; difference in rate −3.48%; 95% CI −6.81% to −0.15%). The onset time (41 vs 32s, P=0.001) was significantly higher, whereas the time to becoming fully alert (3.0 vs 4.0 min, P=0.001), time to becoming ready for discharge (8.7 vs 9.38 min, P=0.001), and time to hospital discharge (13.92 vs 14.57 min, P=0.002) were all significantly lower in the remimazolam group than in the etomidate–propofol group. Muscular tremors and pain during injection were less frequently recorded in the remimazolam group. The authors found that a combination of fentanyl and remimazolam exhibited a non-inferior efficacy and a higher safety profile than etomidate–propofol among elderly patients undergoing colonoscopy.42 A 2021 phase III study evaluated the security and efficacy of remimazolam in patients with ASA class III and IV undergoing colonoscopy (NCT02532647). A total of 79 patients were randomly assigned to receive remimazolam (2.5–5.0 mg, n=32), placebo (2.5–5.0 mg, n=16), or midazolam (1.0 mg + 0.5 mg top-ups, n=31) if sedation (MOAA/S score, ≤ 3) was not achieved; up to four repeated top-up doses of 1.25–2.5 mg remimazolam or placebo within a 15-min period and up to two repeated top-up doses of 0.5 mg midazolam within a 12-min period were allowed. The study showed a significantly superior procedure success rate with remimazolam (87.1%) versus placebo (0%) and midazolam (13.3%); the rate of no-rescue midazolam administration in the remimazolam group was lower than that in the placebo and midazolam groups (90.3%, 0%, 20.0%), and the mean time to onset of sedation was faster in the remimazolam group (8.0 vs 20 vs 18.6 min, P<0.00001); moreover, the mean time to become fully alert was shorter with remimazolam (3.0 vs 5.3 vs 7.0 min) than with placebo and midazolam. No treatment-emergent AEs were reported in the patients treated with remimazolam. This indicated that remimazolam was safe and efficient for use in sedation among high-risk patients with ASA III/IV undergoing colonoscopy.43 A randomised, double-blinded trial evaluated the discharge time of patients administered remimazolam versus propofol for colonoscopy; the study included 132 participants with ASA class I–II and was published in 2022 (ChiCTR2100048678). The participants were randomly allocated 1:1 to either the remimazolam or propofol group, and an initial dose of 0.2 mg/kg of remimazolam or 1 mg/kg of propofol was then administered over 30s to achieve adequate sedation (MOAA/S score, ≤3); otherwise, 6 mg of remimazolam or 30 mg of propofol was administered no less than 2 min apart. The results showed that remimazolam leads to a non-inferior discharge time (24 vs 21 min; median difference, 2 min; 95% CI, 0 to 4 min, P=0.038), lower occurrence of hypotension (20% vs 47%, P<0.001) and bradycardia (6% vs 20%, P=0.019), and higher patient satisfaction scores in ambulatory colonoscopy as compared with propofol.44 All the clinical trials confirmed the potential of remimazolam as a valuable medication for gastrointestinal endoscopy with a sedation successful rate non-inferior to that of propofol but higher than that of midazolam and with better safety.
Bronchoscopy
In a randomised, double-blinded, parallel-group trial on remimazolam for sedation during flexible bronchoscopy (NCT02296892), patients received an initial single intravenous dose of 5.0 mg of remimazolam (n=310) or an equal volume of placebo (n=63) or midazolam (1–1.75 mg; n=63). The success rate (procedure completed using the sedation protocol described above, no need for rescue sedatives) was higher in the remimazolam group than in the placebo (80.6% vs 4.8%, P<0.0001) and midazolam (32.9%) groups; the time to starting bronchoscopy was sooner in patients treated with remimazolam than in those treated with placebo (6.4±5.82 vs 17.2±4.15 min, P<0.0001) and midazolam (16.3±8.6 min); and the mean time to becoming fully alert was significantly shorter in with remimazolam than in those treated with placebo and midazolam (6.0 vs 13.6 vs 12.0 min, P=0.0001 for placebo). Furthermore, as compared with placebo and midazolam, remimazolam showed superior neuropsychiatric function 5 min after becoming fully alert.45 A clinical trial used effective dosages of RT for moderate sedation in patients (n=50, ASA class I or II) undergoing bronchoscopy. The first patient was administered a dose of 0.18 mg/kg of RT; the dosages were increased or decreased 1:1, and bronchoscopy was started when the MOAA/S score was <1 and eyelash reflex disappeared after the intravenous administration of a trial dose of RT + 0.1 μg/kg sufentanil; a sedation rate of 1 mg/kg/h of RT was maintained. The 50% effective dose (ED50) and the 95% effective dose (ED95) concentrations of RT were 0.174 mg/kg and 0.219 mg/kg, respectively, and the time to sedation (MOAA/S score, <1) was 50±11 s; the time to becoming fully alert after the use of flumazenil (0.2 mg) was 56±16 s.46 A prospective, randomised, double-blinded trial indicated that RT leads to effective and safe sedation among patients with ASA class I–II who are undergoing bronchoscopy (ChiCTR2000041524). Patients received RT 12 mg/kg/h (n=73) or dexmedetomidine 0.5 μg/kg (n=73), and sedation (MOAA/S score, <3) was maintained using 1–2 mg/kg/h of RT or 0.2–0.7 μg/kg/h of dexmedetomidine; all the patients were treated with 0.2 mg of flumazenil at the end of the procedure.47 The success rate of completing the procedure with RT was non-inferior to that with dexmedetomidine (94.52% vs 91.78%; difference in rate, 2.74%; 95% CI: 1.70%–3.90%), and the onset time started sooner with remimazolam than with dexmedetomidine (13.22±1.70 vs 15.12±2.07 min, P<0.05); moreover, the time to becoming fully alert (2.52±1.11 vs 3.62±1.28 min, P<0.05) and time to discharge (18.58±2.98 vs 21.21±3.60 min, P<0.05) were significantly shorter in the RT group. RT provided better time metrics and hemodynamic stability than dexmedetomidine (P<0.05).47 A summary of the published clinical trials is presented in Table 4.
Table 4.
Main Clinical Trials of Remimazolam for Sedation Under Bronchoscopy
| Author (Year) | Phase | N | Dose of Remimazolam | Age (Years) | Primary Endpoint | Comparator | Co-Treatment | Location (s) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Pastis et al 201945 | III | 446 | 5.0 mg plus 2.5 top-ups | ≥18 | Safety and efficacy for moderate sedation during bronchoscopy | Midazolam (1–1.75 mg plus 0.5–1.0 mg top-ups) |
Fentanyl (25–75 mg, top-up doses of 25 μg, the maximum dose of 200 μg) |
USA | Remimazolam achieved moderate procedural sedation efficacy and safety during flexible bronchoscopy, with a rapid onset and rapid return of cognitive function |
| Jia et al 202146 | III | 50 | The first patient was given a dose of 0.18 mg/kg, Maintenance dose: 1mg/kg/h | 18–65 | Observation of effective dosage of remimazolam tosilate used for moderate sedation in bronchoscopy | N/A | Sufentanil (0.1 μg/kg); 2% lidocaine (5 mL, airway nebulization); flumazenil (0.2 mg) |
China | The ED50 and the ED95 of remimazolam tosilate for moderate sedation in bronchoscopy were 0.174 mg/kg and 0.219 mg/kg, combined with Sufentanil 0.1 μg/kg |
| Chen et al 202247 | III | 146 | Initial dose: 12 mg/kg Maintenance dose: 1–2mg/kg/h | 45–65 | Efficacy and safety of remimazolam tosilate in patients undergoing bronchoscopy | Dexmedeto-midine (0.5 μg/kg, maintained by 0.2–0.7 μg/kg) | Remifentanil (0.05–0.2 μg/kg/min) |
China | Remimazolam tosilate has non-inferior efficacy, better time metrics and hemodynamic stability than dexmedetomidine combine with remifentanil in outpatients undergoing bronchoscopy |
Abbreviations: N, number of subjects; N/A, not available/applicable.
Induction and Maintenance of General Anaesthesia
In January 2020, remimazolam besylate was approved for use in the induction and maintenance of general anaesthesia in Japan.48 Several clinical trials were conducted to assess the use of remimazolam for general anaesthesia (Table 5). Two clinical trials conducted at Hamamatsu University Hospital in Japan discussed the application of remimazolam in the induction and maintenance of general anaesthesia in patients undergoing surgery (JapicCTI number: 121973). The first study enrolled 391 patients with ASA class I or II and randomly allocated them into three groups. Anaesthesia was induced with 6 mg/kg/h (n=158) or 12 mg/kg/h (n=156) of remimazolam, maintained at a rate of 1–2 mg/kg/h. The patients in the propofol group (n=77) were induced with 2–2.5 mg/kg of propofol, and maintenance was achieved with 4–10 mg/kg/h. The efficacy rate in each group was 100%, and the time to LoC was significantly shorter in the propofol group than in the 6 mg/kg/h and 12 mg/kg/h remimazolam groups (78.7±38.4 vs 102.0±26.6 vs 88.7±22.7 s); the extubation time was also shorter in the propofol group than in the two remimazolam groups (13.1 vs 19.2 min, P<0.05). However, hypotension was higher in the propofol group than in the two other groups (49.3% vs 20% vs 24%), and the patients in that group needed more vasopressors. Those in the remimazolam groups had no injection site pain as compared with those in the propofol group (18.7%). The authors confirmed that remimazolam was well-tolerated and non-inferior to propofol in terms of efficacy as a sedative for general anaesthesia.49 A similarly designed second trial (JapicCTI number: 121977) included high-risk patients (ASA class III) undergoing elective general surgery; this study demonstrated a comparable efficacy and safety profile of remimazolam, as seen in the ASA class I–II patients.50
Table 5.
Main Clinical Trials of Remimazolam for Sedation in General Anesthesia
| Author (Year) | Phase | N | Dose of remimazolam | Age (years) | Primary endpoint | Comparator | Co-treatment | Location (s) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Doi et al 202049 | IIb/III | 391 | Initial dose: 6 mg/kg/h or 12 mg/kg/h Maintenance dose: 1–2 mg/kg/h |
≥ 20 | Efficacy and safety | Propofol was started at 2.0–2.5 mg/kg, followed by 4–10 mg/kg/h | Remifentanil (0.25 and 0.5 μg/kg/min, maintained by 0.25–2.0 µg/kg/min) |
Japan | Remimazolam showed well tolerated and non-inferior to propofol in term of efficacy as a sedative for general anesthesia, but the time to LoC, recovery, and extubation were both longer than propofol |
| Doi et al 202050 | III | 67 | Initial dose: 6 mg/kg/h or 12 mg/kg/h Maintenance dose: 1–2 mg/kg/h |
≥ 20 | Efficacy and safety | N/A | N/A | Japan | Remimazolam could be used safely and effectively in high-risk surgical patients (ASA Class III), time to LoC was significantly shorter with dose of 12 mg/kg/h |
| Chae et al 202251 | III | 120 | Six dose groups of 0.02, 0.07, 0.12, 0.17, 0.22, and 0.27 mg/kg | >18 | Determine the ED50 and ED95 Of IV remimazolam for LoC |
N/A | N/A | Korea | A bolus injection of remimazolam can be administered safely without causing significant hemodynamic instability. The optimal doses of 0.25–0.33, 0.19–0.25, and 0.14–0.19 mg. kg-1 in 95% patients aged <40, 60–80, and >80 years |
| Shimamoto et al 202252 | N/A | 76 | Initial dose: 0.2 mg/kg Maintenance dose: 1–2mg/kg/h | 20–80 | Efficacy and safety | Propofol was started at 2 mg/kg, followed by 4–10 mg/kg/h | Fentanyl citrate (3 μg/kg, maintained by remifentanil 0.25 µg/kg/min) | China | Remimazolam tosilate can provide satisfactory anesthetic effects for EVL and combination with flumazenil can induce quick recovery from anesthesia |
Abbreviations: N, number of subjects; N/A, not available/applicable.
Dosing guidelines for the induction of general anaesthesia should consider not only the loss of consciousness (LoC) but also safety issues.51 The above two clinical trials conducted in Japan demonstrated that the most effective and safe dose for continuously infusing remimazolam for general anaesthesia is 6 or 12 mg/kg/h until LoC is noted and then maintenance at doses of 1–2 mg/kg/h.49,50 A dose-finding and safety study evaluated the intravenous administration of bolus remimazolam during anaesthesia induction (NCT04901871) and reported that LoC and respiratory depression were dose-dependent and that elderly patients were more vulnerable to LoC and respiratory depression as compared with younger patients. Dose estimation was based on ED95; the authors recommended optimal doses of 0.25–0.33, 0.19–0.25, and 0.14–0.19 mg/kg in patients aged <40, 60–80, and >80 years. The study results suggested that bolus injection of remimazolam can be administered safely without causing significant hemodynamic instability.51 Moreover, remimazolam was proven effective for use in general anaesthesia among patients undergoing cardiac surgery by reducing hemodynamic fluctuations; moreover, the amount of norepinephrine was significantly lower, as compared with that in the propofol group.52,53 It was concluded that remimazolam can safely avoid the risk of cardiac suppression when used in general anaesthesia among patients with severe aortic stenosis.54
RT was first compared with propofol for use in general anaesthesia among patients with liver cirrhosis (ASA class II or III) undergoing endoscopic variceal ligation (EVL) (ChiCTR2100045710). Patients were randomly administered a bolus of 0.2 mg/kg of remimazolam for induction and then 1–2 mg/kg/h for maintenance (n=38); otherwise, they were induced with a bolus of 2.0 mg/kg of propofol and then given 4–10 mg/kg/h for maintenance (n=38). A bolus of 0.5 mg flumazenil was administered to the remimazolam group at the end of surgery.55 The time to LoC (MOAA/S score, ≤1) was longer in the remimazolam group than in the propofol group (65.9±4.7 vs 45.7±5.3 s, P>0.05). However, the time to return of consciousness (MOAA/S score, ≥4) (67.1±9.6 vs 503.3±59.6 s, P<0.05) and time to extubation (115.7±12.5 vs 524.7±57.8 s, P<0.05) were significantly shorter in the remimazolam group than in the propofol group. Bispectral index (BIS) scores were higher in the remimazolam group (P<0.05), whereas the fluctuation of mean arterial BP and HR and the incidence of postoperative low SpO2 were lower in the remimazolam group than in the propofol group (P<0.05). The study showed that the administration of RT + flumazenil was a better alternative than propofol for general anaesthesia in cirrhotic patients during EVL.55
Recently, a study assessed the factors affecting prolonged time to extubation in patients administered remimazolam.56 Patients were induced with a bolus dose of 1 mg/kg of remimazolam and maintained with 0.2–1.0 mg/kg/h of remimazolam. The patients were divided into two different groups [long period group (LP), ≥ 15 min, n=31; and short period group (SP), <15 min, n=34] based on the time to extubation. The LP group patients had older age, a higher BMI, and lower plasma albumin concentrations as compared with those in the SP group (P<0.05). There were no significant differences in sex, body weight, height, ASA class, total duration of general anaesthesia, duration of surgery, or the total dose of remimazolam administered (P > 0.05). Logistic regression analysis demonstrated that patients with a BMI of >22.0 kg/m2, age >79.0 years, and plasma albumin concentration of <3.60 g/dl exhibit increased time to extubation and that lower doses of remimazolam should be administered to these patients.56 A phase III trial on general anaesthesia to explore the possibility of weight-independent dosing is underway in Europe (NCT 03661489).57 All of the clinical studies confirmed the safety and efficacy of remimazolam in the induction and maintenance of general anaesthesia without any serious AEs.49–55
Sedation in Pediatric or Other Medical Procedures
Only a few studies have reported on the use of remimazolam in pediatric procedures since it was introduced in the market (Table 6).58 Despite its potential advantages, remimazolam is not approved for use in children. Horikosi et al reported on the use of remimazolam in general anaesthesia in a 4-year-old patient with Duchenne muscular dystrophy.59 Petkus et al also reported its use as an adjunct to propofol in general anaesthesia in a 6-year-old girl with a suspected family history of malignant hyperthermia; they reported that remimazolam reduced the dose of propofol with a rapid recovery.58 Moreover, remimazolam was successfully used in a 12-year-old girl who required supratentorial glioma resection in general anaesthesia under intraoperative direct cortical motor-evoked potential monitoring. Remimazolam was found to be beneficial in maintaining the stability of cardiovascular system.60 Remimazolam could be used in a pediatric patient with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome in general anaesthesia without causing seizures and delayed emergence.61 Furthermore, we searched four clinical trials assessing the use of remimazolam for sedation in pediatric patients at ClinicalTrials.gov (NCT04621305, NCT04851717, NCT04720963, and NCT05527314).62–65
Table 6.
Main Articles About Other Clinical Application of Remimazolam
| Author (Year) | Procedure | Dose | Age (years) | Primary endpoint | Comparator | Co-treatment | Results |
|---|---|---|---|---|---|---|---|
| Horikoshi et al 202159 | Single-incision laparoscopic percutaneous extraperitoneal closure in a pediatric with DMD | Induction: 3 mg Maintenance: 15 mg/h |
4 | Safety | N/A | Fentanyl (100 μg), maintained by remifentanil 1.0 µg/kg/min | Remimazolam can be safely used for general anesthesia in a pediatric patient with DMD |
| Petkus H et al 202258 | Dental rehabilitation procedure in a pediatric suspected with family history of MH | 5–7 µg/kg/min | 6 | Safety | N/A | Propofol (50 µg/kg/min) | Remimazolam was effective as an adjunct to propofol during general anesthesia in a pediatric suspected with family history of with MH |
| Kamata, K et al 202260 | Supratentorial glioma resection under dc-MEP monitoring | 0.9 mg/kg/h | 12 | Safety | N/A | Remifentanil (0.35 μg/kg/min) | Direct cortical MEP was successfully recorded in the pediatric patient under remimazolam anesthesia with cardiovascular stability |
| Yamadori, Y et al 202261 | Open gastrostomy in a pediatric with MELAS | Induction: 0.2 mg/kg Maintenance: 2.0mg/kg/h |
10 | Safety | N/A | Remifentanil (0.3 μg/kg/min) | Remimazolam could be used in a pediatric with MELAS under general anesthesia without causing seizures and delayed emergence |
Abbreviations: ERCP, endoscopic retrograde cholangiopancreatography; MELAS, mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes; DMD, Duchenne muscular dystrophy; N, number of subjects; N/A, not available/applicable.
The use of remimazolam in a patient with MELAS who underwent MitraClip implantation is of particular interest, and the study results may provide new insights into sedation for patients with MELAS syndrome having heart failure.66 The successful use of remimazolam in tracheostomy has been reported in a patient with amyotrophic lateral sclerosis, a patient with mitochondrial encephalomyopathy who underwent cochlear implantation, and patients who underwent hysteroscopy.67–69 Further clinical trials or case reports that shed light on the use of remimazolam in clinical practice are expected in the coming years.
ICU Sedation
Remimazolam is eliminated by first-order pharmacokinetics and rapidly hydrolysed by tissue esterase enzymes to an inactive metabolite (CNS 7054) in the body, which may reduce the burden on the liver and kidney, making it suitable for use as an intravenous anesthetic and a sedative for use in the ICU.4,19,70 A phase II study on the preclinical profile of remimazolam and the pharmacokinetics of 49 Japanese patients admitted to the ICU showed that all patients were successfully sedated and no significant AEs were reported; seven of those patients who received continuous infusions of >24 h had higher than expected plasma remimazolam concentrations.71 The change in remimazolam CL was not related to the cumulative dose but rather to time; the remimazolam CL decreased by 25% after infusion for 22 h. The time-related changes in the high plasma concentrations in CL indicated some level of tolerance development if the remimazolam treatment was administered for 1 day or more; however, high concentrations in patients admitted to the ICU will not cause excessive sedation if they are treated for ≤24 h.72 A study evaluated patient tolerance to long-term sedation with remimazolam in a miniature pig model and reported that remimazolam dose increases over 28 days (0–3-fold increase) were lower than those for midazolam (2–4-fold increase), albeit in the same range; moreover, the tolerance to remimazolam after 28 days of sedation was weaker than that to midazolam, indicating that the dose increase of remimazolam in the patients may have been lower than that of midazolam.73
Owing to shortages in hypnotic sedative medications resulting from the COVID-19 crisis, remimazolam was approved for compassionate use in ICU sedation in August 2020 in Belgium.15 The safety and suitability of remimazolam for prolonged sedation in critically ill patients admitted to the ICU remains to be confirmed. In 2021 in China, RT for injection was approved for sedation in phase II and III clinical trials in ICU (Acceptance number: CXHL2101106). Currently, a few clinical trials (NCT04947345, NCT04815265, ChiCTR2100053106) that are underway are recruiting participants for evaluating the efficacy and safety of remimazolam for sedation in patients admitted to the ICU with mechanical ventilation; however, the trials have not reported any findings yet.70,74–78
Safety Data
The most frequently reported AEs in procedural sedation include fluctuations in BP and HR, respiratory depression, body movement, nausea or vomiting, and dizziness or headache; however, these AEs are generally mild and require no reversal treatment.15,34,39,40,79 Remimazolam is associated with a low risk of hypotension and respiratory depression and injection site pain in procedural sedation as compared with propofol,38,80 and pre-treatment with remimazolam is known to reduce the incidence and intensity of propofol-induced injection pain.81 Furthermore, there were no significant differences in the treatment-related AEs of remimazolam and midazolam.41,45
The most common treatment-emergent AEs reported in the induction and maintenance of general anaesthesia include hypotension, bradycardia, tachycardia, hypoxia, and vomiting and nausea.1 However, the incidence of requiring vasopressor treatment for hypotension was lower with remimazolam than with propofol; moreover, bradycardia required treatment, and the injection site pain was lower with remimazolam than with propofol. Remimazolam decreased the incidence of hypotension and low SpO2.49,52 The safety data of ASA class III patients were in line with the corresponding data from patients with ASA class I and II.50 Remimazolam could reduce the incidence of postoperative nausea and vomiting in the patients after laparoscopic gynaecological surgery under general anaesthesia as compared with desflurane (27% vs 60%, P=0.02) during the early postoperative period.82
A randomized crossover trial to evaluate the abuse liability of intravenous remimazolam concluded that remimazolam cannot cause abuse via injection; single intravenous doses of 5 mg and 10 mg were comparable to or lower than midazolam (a drug known to have a low potential for intravenous abuse) doses of 2.5 mg and 5 mg in terms of the abuse potential owing to the shorter time‐averaged positive effects and the patients’ lower willingness to take the drug again.83
In short, remimazolam exerts little influence on the circulatory and respiratory systems with a small fluctuation range of BP and HR and causes no fatal, serious, or severe AEs during the infusion phase; moreover, remimazolam was safe and well-tolerated in all the assessed studies.22,23,32,38,49,50,79,80,83 The use of remimazolam is recommended in combination with saline, and its use in combination with Ringer’s acetate solution should be avoided because it does not completely dissolve in this solution and forms a precipitate.84,85
Conclusion
In conclusion, remimazolam is a novel rapidly metabolized BDZ that combines the structural features of midazolam and remifentanil; its characteristics include a rapid onset of action, an ultra-short duration, fast recovery time without serious drug-related AEs, and good tolerance. It has broad application prospects as an effective and safe sedative for procedural sedation, induction and maintenance of general anaesthesia, and sedation in patients admitted to the ICU and also has potential for use in the sedation of pediatric patients. However, further studies are warranted to assess the efficacy and safety of long-term sedation with remimazolam and the sedation of specific conditions in patients admitted to the ICU. Moreover, future studies should aim to explore the optimal dose of remimazolam in different clinical scenarios.
Funding Statement
This review did not receive any grant from funding agencies.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, search literature, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting and writing, revised the article, reviewed and agreed on all versions of the article before the article has been submitted, final version accepted for publication, and any significant changes introduced at the proofing stage, gave final approval of the version to be published, and agree to be accountable for all aspects of the review.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Chen W, Chen S, Huang Y. Induction and maintenance of procedural sedation in adults: focus on remimazolam injection. Expert Rev Clin Pharmacol. 2021;14(4):411–426. doi: 10.1080/17512433.2021.1901575 [DOI] [PubMed] [Google Scholar]
- 2.Dominguini D, Steckert AV, Michels M, et al. The effects of anaesthetics and sedatives on brain inflammation. Neurosci Biobehav Rev. 2021;127:504–513. doi: 10.1016/j.neubiorev.2021.05.009 [DOI] [PubMed] [Google Scholar]
- 3.Barr J, Donner A. Optimal intravenous dosing strategies for sedatives and analgesics in the intensive care unit. Crit Care Clin. 1995;11(4):827–847. doi: 10.1016/S0749-0704(18)30041-1 [DOI] [PubMed] [Google Scholar]
- 4.Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016;36(9):1021–1027. doi: 10.1002/phar.1806 [DOI] [PubMed] [Google Scholar]
- 5.Mertens MJ, Olofsen E, Burm AG, Bovill JG, Vuyk J. Mixed-effects modeling of the influence of alfentanil on propofol pharmacokinetics. Anesthesiology. 2004;100(4):795–805. doi: 10.1097/00000542-200404000-00008 [DOI] [PubMed] [Google Scholar]
- 6.Choi GJ, Kang H, Baek CW, Jung YH, Ko JS. Etomidate versus propofol sedation for electrical external cardioversion: a meta-analysis. Curr Med Res Opin. 2018;34(11):2023–2029. doi: 10.1080/03007995.2018.1519501 [DOI] [PubMed] [Google Scholar]
- 7.Kim KN, Lee HJ, Kim SY, Kim JY. Combined use of dexmedetomidine and propofol in monitored anesthesia care: a randomized controlled study. BMC Anesthesiol. 2017;17(1):34. doi: 10.1186/s12871-017-0311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanious MK, Beutler SS, Kaye AD, Urman RD. New hypnotic drug development and pharmacologic considerations for clinical anesthesia. Anesthesiol Clin. 2017;35(2):e95–e113. doi: 10.1016/j.anclin.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 9.Goudra B, Gouda G, Mohinder P. Recent developments in drugs for gi endoscopy sedation. Dig Dis Sci. 2020;65(10):2781–2788. doi: 10.1007/s10620-020-06044-5 [DOI] [PubMed] [Google Scholar]
- 10.Whizar V, Garnica C, Gastelum R. Remimazolam: a new ultra short acting benzodiazepine. J Anesth Critic Care. 2016;4:1. [Google Scholar]
- 11.Rogers WK, McDowell TS. Remimazolam, a short-acting GABA(A) receptor agonist for intravenous sedation and/or anesthesia in day-case surgical and non-surgical procedures. IDrugs. 2010;13(12):929–937. [PubMed] [Google Scholar]
- 12.PAION AG. PAION announces submission of the marketing authorization application for remimazolam in procedural sedation to the European Medicines Agency. PAION AG. Available from: https://www.paion.com/. Accessed Nov 20, 2019. [Google Scholar]
- 13.Keam SJ. Remimazolam: first approval. Drugs. 2020;80(6):625–633. doi: 10.1007/s40265-020-01299-8 [DOI] [PubMed] [Google Scholar]
- 14.PAION AG. Mundipharma receives market approval for Anerem® (remimazolam) in general anesthesia in Japan. PAION AG. Available from: https://www.paion.com/. Accessed Jan 23, 2020. [Google Scholar]
- 15.Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021;12:690875. doi: 10.3389/fphar.2021.690875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oka S, Satomi H, Sekino R, et al. Sedation outcomes for remimazolam, a new benzodiazepine. J Oral Sci. 2021;63(3):209–211. doi: 10.2334/josnusd.21-0051 [DOI] [PubMed] [Google Scholar]
- 17.Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115(2):274–283. doi: 10.1213/ANE.0b013e31823f0c28 [DOI] [PubMed] [Google Scholar]
- 18.Goudra BG, Singh PM. Remimazolam: the future of its sedative potential. Saudi J Anaesth. 2014;8(3):388–391. doi: 10.4103/1658-354X.136627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology. 2007;107(1):60–66. doi: 10.1097/01.anes.0000267503.85085.c0 [DOI] [PubMed] [Google Scholar]
- 20.Sneyd JR. Remimazolam: new beginnings or just a me-too? Anesth Analg. 2012;115(2):217–219. doi: 10.1213/ANE.0b013e31823acb95 [DOI] [PubMed] [Google Scholar]
- 21.Freyer N, Knöspel F, Damm G, et al. Metabolism of remimazolam in primary human hepatocytes during continuous long-term infusion in a 3-D bioreactor system. Drug Des Devel Ther. 2019;13:1033–1047. doi: 10.2147/DDDT.S186759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012;115(2):284–296. doi: 10.1213/ANE.0b013e318241f68a [DOI] [PubMed] [Google Scholar]
- 23.Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–651. doi: 10.1097/ALN.0000000000003103 [DOI] [PubMed] [Google Scholar]
- 24.Upton RN, Somogyi AA, Martinez AM, Colvill J, Grant C. Pharmacokinetics and pharmacodynamics of the short-acting sedative CNS 7056 in sheep. Br J Anaesth. 2010;105(6):798–809. doi: 10.1093/bja/aeq260 [DOI] [PubMed] [Google Scholar]
- 25.Deng Y, Qin Z, Wu Q, et al. Efficacy and safety of remimazolam besylate versus dexmedetomidine for sedation in non-intubated older patients with agitated delirium after orthopedic surgery: a randomized controlled trial. Drug Des Devel Ther. 2022;16:2439–2451. doi: 10.2147/DDDT.S373772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saari TI, Uusi-Oukari M, Ahonen J, Olkkola KT. Enhancement of GABAergic activity: neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology. Pharmacol Rev. 2011;63(1):243–267. doi: 10.1124/pr.110.002717 [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Hu P, Jiang J. Metabolite characterization of a novel sedative drug, remimazolam in human plasma and urine using ultra high-performance liquid chromatography coupled with synapt high-definition mass spectrometry. J Pharm Biomed Anal. 2017;137:78–83. doi: 10.1016/j.jpba.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 28.Liu T, Lai T, Chen J, et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double-blind, controlled trial. Pharmacol Res Perspect. 2021;9(5):e00851. doi: 10.1002/prp2.851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127(3):415–423. doi: 10.1016/j.bja.2021.05.027 [DOI] [PubMed] [Google Scholar]
- 30.Lee A, Shirley M. Remimazolam: a review in procedural sedation. Drugs. 2021;81(10):1193–1201. doi: 10.1007/s40265-021-01544-8 [DOI] [PubMed] [Google Scholar]
- 31.Lohmer LL, Schippers F, Petersen KU, Stoehr T, Schmith VD. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020;60(4):505–514. doi: 10.1002/jcph.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng XY, Liang Y, Yang XY, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76(3):383–391. doi: 10.1007/s00228-019-02800-3 [DOI] [PubMed] [Google Scholar]
- 33.Iirola T, Ihmsen H, Laitio R, et al. Population pharmacokinetics of dexmedetomidine during long-term sedation in intensive care patients. Br J Anaesth. 2012;108(3):460–468. doi: 10.1093/bja/aer441 [DOI] [PubMed] [Google Scholar]
- 34.Worthington MT, Antonik LJ, Goldwater DR, et al. A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117(5):1093–1100. doi: 10.1213/ANE.0b013e3182a705ae [DOI] [PubMed] [Google Scholar]
- 35.Vicari JJ. Sedation in the ambulatory endoscopy center: optimizing safety, expectations and throughput. Gastrointest Endosc Clin N Am. 2016;26(3):539–552. doi: 10.1016/j.giec.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 36.Moon SH, Kim HK, Myung DS, Yoon SM, Moon W. 진정내시경시환자의 감시및관련장비 [Patient monitoring and associated devices during endoscopic sedation]. Korean J Gastroenterol. 2017;69(1):64–67. Korean. doi: 10.4166/kjg.2017.69.1.64 [DOI] [PubMed] [Google Scholar]
- 37.Borkett KM, Riff DS, Schwartz HI, et al. A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120(4):771–780. doi: 10.1213/ANE.0000000000000548 [DOI] [PubMed] [Google Scholar]
- 38.Chen SH, Yuan TM, Zhang J, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, phase III trial. J Gastroenterol Hepatol. 2021;36(2):474–481. doi: 10.1111/jgh.15188 [DOI] [PubMed] [Google Scholar]
- 39.Tan Y, Ouyang W, Tang Y, Fang N, Fang C, Quan C. Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. J Gastroenterol Hepatol. 2022;37(3):576–583. doi: 10.1111/jgh.15761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao Y, Chi P, Zhou C, Lv W, Quan Z, Xue FS. Remimazolam tosilate sedation with adjuvant sufentanil in Chinese patients with liver cirrhosis undergoing gastroscopy: a randomized controlled study. Med Sci Monit. 2022;28:e936580. doi: 10.12659/MSM.936580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–437.e426. doi: 10.1016/j.gie.2018.04.2351 [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Ding B, Shi F, et al. The efficacy and safety of remimazolam tosilate versus etomidate-propofol in elderly outpatients undergoing colonoscopy: a prospective, randomized, single-blind, non-inferiority trial. Drug Des Devel Ther. 2021;15:4675–4685. doi: 10.2147/DDDT.S339535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rex DK, Bhandari R, Lorch DG, Meyers M, Schippers F, Bernstein D. Safety and efficacy of remimazolam in high risk colonoscopy: a randomized trial. Dig Liver Dis. 2021;53(1):94–101. doi: 10.1016/j.dld.2020.10.039 [DOI] [PubMed] [Google Scholar]
- 44.Yao Y, Guan J, Liu L, Fu B, Chen L, Zheng X. Discharge readiness after remimazolam versus propofol for colonoscopy: a randomised, double-blind trial. Eur J Anaesthesiol. 2022;39(12):911–917. doi: 10.1097/EJA.0000000000001715 [DOI] [PubMed] [Google Scholar]
- 45.Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155(1):137–146. doi: 10.1016/j.chest.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 46.Jia Z, Ren LX, Fan YT, Tan ZM. 甲苯磺酸瑞马唑仑用于纤维支气管镜检查中深度镇静的有效剂量观察 [Observation of effective dosage of remimazolam tosilate used for moderate-to-deep sedation in fiberoptic bronchoscopy]. Zhonghua Yi Xue Za Zhi. 2021;101(11):813–816. Chinese. doi: 10.3760/cma.j.cn112137-20200901-02524 [DOI] [PubMed] [Google Scholar]
- 47.Chen X, Xin D, Xu G, Zhao J, Lv Q. The efficacy and safety of remimazolam tosilate versus dexmedetomidine in outpatients undergoing flexible bronchoscopy: a prospective, randomized, blind, non-inferiority trial. Front Pharmacol. 2022;13:902065. doi: 10.3389/fphar.2022.902065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masui K. Remimazolam besilate, a benzodiazepine, has been approved for general anesthesia!! J Anesth. 2020;34(4):479–482. doi: 10.1007/s00540-020-02755-1 [DOI] [PubMed] [Google Scholar]
- 49.Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543–553. doi: 10.1007/s00540-020-02788-6 [DOI] [PubMed] [Google Scholar]
- 50.Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34(4):491–501. doi: 10.1007/s00540-020-02776-w [DOI] [PubMed] [Google Scholar]
- 51.Chae D, Kim HC, Song Y, Choi YS, Han DW. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: a randomised, prospective, double-blind study. Br J Anaesth. 2022;129(1):49–57. doi: 10.1016/j.bja.2022.02.040 [DOI] [PubMed] [Google Scholar]
- 52.Tang F, Yi JM, Gong HY, et al. Remimazolam benzenesulfonate anesthesia effectiveness in cardiac surgery patients under general anesthesia. World J Clin Cases. 2021;9(34):10595–10603. doi: 10.12998/wjcc.v9.i34.10595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wahler S Difference in vasopressor use and usage patterns in patients undergoing cardiac surgery with remimazolam vs propofol/sevoflurane for general anesthesia. Paper presented at: Anesthesiology. 2015.
- 54.Furuta M, Ito H, Yamazaki M. Anaesthetic management using remimazolam in a patient with severe aortic stenosis: a case report. BMC Anesthesiol. 2021;21(1):202. doi: 10.1186/s12871-021-01422-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi F, Chen Y, Li H, Zhang Y, Zhao T. Efficacy and safety of remimazolam tosilate versus propofol for general anesthesia in cirrhotic patients undergoing endoscopic variceal ligation. Int J Gen Med. 2022;15:583–591. doi: 10.2147/IJGM.S345390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimamoto Y, Sanuki M, Kurita S, Ueki M, Kuwahara Y, Matsumoto A. Factors affecting prolonged time to extubation in patients given remimazolam. PLoS One. 2022;17(5):e0268568. doi: 10.1371/journal.pone.0268568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paion UKL, Services OC, Simbec R; Creative Clinical Research Gmb H. Efficacy and safety of remimazolam (CNS7056) compared to propofol for intravenous anesthesia during elective surgery. Available from: https://clinicaltrials.gov/show/NCT03661489. Accessed July 28, 2022.
- 58.Petkus H, Willer BL, Tobias JD. Remimazolam in a pediatric patient with a suspected family history of malignant hyperthermia. J Med Cases. 2022;13(8):386–390. doi: 10.14740/jmc3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horikoshi Y, Kuratani N, Tateno K, et al. Anesthetic management with remimazolam for a pediatric patient with Duchenne muscular dystrophy. Medicine. 2021;100(49):e28209. doi: 10.1097/MD.0000000000028209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamata K, Asagi S, Shimoda Y, et al. Successful recording of direct cortical motor-evoked potential from a pediatric patient under remimazolam anesthesia: a case report. JA Clin Rep. 2022;8(1):66. doi: 10.1186/s40981-022-00555-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamadori Y, Yamagami Y, Matsumoto Y, et al. General anesthesia with remimazolam for a pediatric patient with MELAS and recurrent epilepsy: a case report. JA Clin Rep. 2022;8(1):75. doi: 10.1186/s40981-022-00564-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu H. Remimazolam reduces emergence delirium in preschool children undergoing laparoscopic surgery by sevoflurane anesthesia. Avaliable from: https://clinicaltrials.gov/ct2/show/NCT04621305?term=NCT04621305&draw=2&rank=1. Accessed September 28, 2022.
- 63.Fox G, Walker JL. Investigation of remimazolam in children undergoing sedation for medical procedures; 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT04851717?term=remimazolam&cond=Pediatric&draw=2&rank=1. Accessed September 28, 2022.
- 64.Liu H, Cai Y. Intranasal remimazolam for premedication in pediatric patient. Available from: https://clinicaltrials.gov/ct2/show/NCT04720963?term=remimazolam&cond=Pediatric&draw=2&rank=2. Accessed September 28, 2022.
- 65.Hua F, Fang Y. Effect of remimazolam vs sevoflurane anesthesia on incidence of emergence agitation and complications in children undergoing ophthalmic surgery. Available from: https://clinicaltrials.gov/ct2/show/NCT05527314?term=remimazolam&cond=Pediatric&draw=2&rank=3. Accessed September 28, 2022.
- 66.Kitaura A, Kosumi R, Iwamoto T, Nakao S. Remimazolam anesthesia for transcatheter mitral valve repair in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome: a case report. JA Clin Rep. 2022;8(1):38. doi: 10.1186/s40981-022-00528-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishihara N, Tachibana S, Ikeshima M, Ino A, Yamakage M. Remimazolam enabled safe anesthetic management during tracheostomy in a patient with amyotrophic lateral sclerosis: a case report. JA Clin Rep. 2022;8(1):25. doi: 10.1186/s40981-022-00514-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki Y, Doi M, Nakajima Y. General anesthesia with remimazolam in a patient with mitochondrial encephalomyopathy: a case report. JA Clin Rep. 2021;7(1):51. doi: 10.1186/s40981-021-00454-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang S, Wang J, Ran R, Peng Y, Xiao Y. Efficacy and safety of remimazolam tosylate in hysteroscopy: a randomized, single-blind, parallel controlled trial. J Clin Pharm Ther. 2022;47(1):55–60. doi: 10.1111/jcpt.13525 [DOI] [PubMed] [Google Scholar]
- 70.Liu S, Su L, Zhang B, et al. The availability and safety study of remimazolam besylate for injection on sedation of ERAS Patients under mechanical ventilation in ICU: protocol for a randomized, open-label, controlled trial. Front Med. 2021;8:735473. doi: 10.3389/fmed.2021.735473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petersen KU Pre-clinical profile of Remimazolam and pharmacokinetics. 3rd annual meeting of the Japan society for clinical anesthesia.Tokyo, Japan; 2017.
- 72.Zhou J, Leonowens C, Ivaturi VD, et al. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020;66:109899. doi: 10.1016/j.jclinane.2020.109899 [DOI] [PubMed] [Google Scholar]
- 73.Io T, Saunders R, Pesic M, Petersen KU, Stoehr T. A miniature pig model of pharmacological tolerance to long-term sedation with the intravenous benzodiazepines; midazolam and remimazolam. Eur J Pharmacol. 2021;896:173886. doi: 10.1016/j.ejphar.2021.173886 [DOI] [PubMed] [Google Scholar]
- 74.Choi HR, Song IA. Review of remimazolam and sedatives in the intensive care unit. Acute Crit Care. 2022;37(2):151–158. doi: 10.4266/acc.2022.00619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song X, Wang F, Dong R, Zhu K, Wang C. Efficacy and safety of remimazolam tosilate combined with esketamine for analgesic sedation in mechanically ventilated ICU patients: a single-arm clinical study protocol. Front Med. 2022;9:832105. doi: 10.3389/fmed.2022.832105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peking Union Medical College H, Yichang Humanwell Pharmaceutical Co L. The availability and safety study of remimazolam besylate for injection on sedation of ERAS patients. Available from: https://clinicaltrials.gov/show/NCT04947345. Accessed July 1, 2022.
- 77.Tianjin Nankai H. Remimazolam for sedation in ICU patients undergoing mechanical ventilation. Available from: https://clinicaltrials.gov/show/NCT04815265. Accessed June 30, 2022.
- 78.Xuan S. Remazolam toluenesulfonate combined with esketamine for the efficacy and safety of analgesic sedation in ICU patients on mechanical ventilation: a single arm trial clinical study protocol. Available from: http://www.chictr.org.cn/showproj.aspx?proj=136350. Accessed July 28, 2022.
- 79.Guo J, Qian Y, Zhang X, Han S, Shi Q, Xu J. Remimazolam tosilate compared with propofol for gastrointestinal endoscopy in elderly patients: a prospective, randomized and controlled study. BMC Anesthesiol. 2022;22(1):180. doi: 10.1186/s12871-022-01713-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen S, Wang J, Xu X, et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res. 2020;12(8):4594–4603. [PMC free article] [PubMed] [Google Scholar]
- 81.Guan X, Jiao Z, Gong X, et al. Efficacy of pre-treatment with remimazolam on prevention of propofol-induced injection pain in patients undergoing abortion or curettage: a prospective, double-blinded, randomized and placebo-controlled clinical trial. Drug Des Devel Ther. 2021;15:4551–4558. doi: 10.2147/DDDT.S334100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hari Y, Satomi S, Murakami C, et al. Remimazolam decreased the incidence of early postoperative nausea and vomiting compared to desflurane after laparoscopic gynecological surgery. J Anesth. 2022;36(2):265–269. doi: 10.1007/s00540-022-03041-y [DOI] [PubMed] [Google Scholar]
- 83.Schippers F, Pesic M, Saunders R, et al. Randomized crossover trial to compare abuse liability of intravenous remimazolam versus intravenous midazolam and placebo in recreational central nervous system depressant users. J Clin Pharmacol. 2020;60(9):1189–1197. doi: 10.1002/jcph.1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sasaki H, Hoshijima H, Mizuta K. Ringer’s acetate solution-induced precipitation of remimazolam. Br J Anaesth. 2021;126(3):e87–e89. doi: 10.1016/j.bja.2020.11.021 [DOI] [PubMed] [Google Scholar]
- 85.Matsuo M, Okada K, Onuki Y, Yamazaki M. Incompatibility of remimazolam besylate with Ringer’s acetate infusion resulting in total occlusion of an intravenous catheter. BMJ Case Rep. 2021;14(4):e241622. doi: 10.1136/bcr-2021-241622 [DOI] [PMC free article] [PubMed] [Google Scholar]