Abstract
Adenylate cyclase (AC) toxin from Bordetella pertussis intoxicates eukaryotic cells by increasing intracellular cyclic AMP (cAMP) levels. In addition, insertion of AC toxin into the plasma membrane causes efflux of intracellular K+ and, in a related process, hemolysis of sheep erythrocytes. Although intoxication, K+ efflux, and hemolysis have been thoroughly investigated, there is little information on the nature of the interaction of this toxin with intact target cells. Using flow cytometry, we observe that binding of AC toxin to sheep erythrocytes and Jurkat T lymphocytes is dependent on posttranslational acylation of the toxin. Extracellular calcium is also necessary, with a steep calcium concentration dependence similar to that required for intoxication and hemolysis. Binding of AC toxin is concentration dependent but unsaturable up to 50 μg/ml, suggesting that if there is a specific receptor molecule with which the toxin interacts, it is not limiting. Visualization of cells by fluorescence microscopy supports the data obtained by flow cytometry and reveals a peripheral pattern of toxin distribution. AC toxin binds to erythrocytes at both 0 and 37°C; however, the total binding at 0°C is less than that at 37°C. In human erythrocytes, AC toxin does not cause an increase in K+ efflux or hemolysis. While AC toxin exhibits reduced potency to increase cAMP in these cells than in sheep erythrocytes, there is only a modest reduction in the binding of the toxin as measured by flow cytometry. Further use of this technique will provide new approaches for dynamic and functional analysis of the early steps involved in intoxication, K+ efflux, and hemolysis produced by AC toxin.
Adenylate cyclase (AC) toxin is an essential virulence factor produced by Bordetella pertussis, the causative agent of whooping cough (12, 17, 33, 44). This toxin is secreted as a single polypeptide of 1,706 amino acids, which is converted to an active toxin by posttranslational modification of an internal lysine (18), in a process requiring the product of an accessory gene, cyaC (1, 24). The active toxin is able to interact with target cells and insert its catalytic domain, resulting in synthesis of cyclic AMP (cAMP) unregulated by the host cell (8). In this report this process is termed intoxication; however, other investigators refer to this activity as cytotoxicity, cell penetration, or cAMP accumulation (35–37). Insertion of the toxin into the cytoplasmic membrane of the host cell results in the creation of a defect that allows the efflux of intracellular potassium; in a related process requiring a higher concentration of toxin and more time, AC toxin causes lysis of erythrocytes (RBC) (16). The N-terminal catalytic domain (amino acids 1 to 400) is activated by endogenous calmodulin, resulting in an increase in its enzymatic activity (4). The remainder of the molecule (amino acids 401 to 1706) of AC toxin is required for the delivery of the catalytic domain and can act independently as a hemolysin (10, 38). This region is homologous to Escherichia coli hemolysin and other members of the RTX (repeat in toxin) family of bacterial toxins (9, 20).
AC toxin is a calcium-binding protein that undergoes a conformational change in response to the binding of calcium (23, 37). The repeat region (amino acids 1007 to 1612) contains 35 to 45 glycine- and aspartate-rich nonameric repeats implicated in the binding of calcium and the resultant conformational change (14, 37). Intoxication is absolutely calcium dependent, requiring a extracellular calcium concentration greater than 200 μM (3, 19, 22, 23, 36, 37). However, efflux of K+ from sheep RBC and hemolysis have been shown to occur in the presence of EDTA or EGTA, but only when the toxin has been renatured in the presence of calcium before its addition to cells in calcium chelators (16, 34, 37). Rose et al. suggest that under these conditions (renatured in the presence of calcium but assayed in the presence of EGTA), three to four calcium ions are tightly bound to the high-affinity sites of the protein and cannot be removed without denaturation; they proposed that these calcium ions are sufficient for membrane binding and lysis of RBC which occur under these conditions (37).
Although the requirements for functional and enzymatic activities of AC toxin have been well characterized, study of the first step in the interaction of toxin with target cells, namely, binding to the cell surface, has been limited for several reasons. First, no specific receptor has been identified for AC toxin binding. Second, due to loss of activity when AC toxin is tagged with iodine or other labels, standard approaches to binding, such as those used for hormones and growth factors, have not been possible. The majority of studies of AC toxin binding have either (i) measured the presence of AC enzymatic activity associated with target cell membranes, or (ii) used reactivity of toxin with antitoxin antibody on Western blots following sodium dodecyl sulfate-polyacrylamide gel electrophoresis for detection of toxin bound to target cell membranes. Several investigators have used both of these approaches to characterize the requirements for toxin binding to lymphocytes and RBC (19, 24, 34, 35, 37). In addition, Iwaki et al., using data obtained from quantitation of enzyme activity associated with cell membranes, proposed that AC toxin does not have a dedicated membrane binding domain and that both structural integrity of the whole toxin molecule and posttranslational acylation are necessary for target cell association (25). While both of these approaches have proved useful, they do not allow the study of the dynamic role of AC toxin binding to intact target cells and the consequent structural changes.
To begin to study the changes in toxin structure that occur during the complex sequence of events required for full expression of the activities of AC toxin, we have adapted an assay using flow cytometric analysis to measure the binding of toxin to intact target cells. This method has contributed to the understanding of toxin-cell interactions for other toxins (26, 32) and has the important advantage of allowing detection and/or measurement of only cell-bound toxin. With this approach, we have further characterized the requirements for and characteristics of toxin binding to intact cells.
MATERIALS AND METHODS
Production and purification of AC toxin.
Recombinant AC toxin was produced as previously described (39), with minor modifications. Escherichia coli XL-1 Blue cells (Stratagene, La Jolla, Calif.) containing plasmid pT7CACT1 for expression of wild-type AC toxin, or plasmid pACT7 for production of nonacylated (due to omission of cyaC) AC toxin, were grown to an optical density at 600 nm of 0.2 at 37°C in 2× YT medium (1.6% Bacto Tryptone, 1% Bacto Yeast Extract, 85 mM NaCl) containing ampicillin (150 μg/ml). Isopropyl-β-d-thiogalactopyranoside (1 mM) was added to the cultures, which were then incubated for 4 h. Cultures were centrifuged, and the resulting pellet was stored at −70°C overnight. Pellets were resuspended in 50 mM Tris (pH 7.5), sonicated, and extracted with 8 M urea. Urea-extracted AC toxin was purified on a calmodulin affinity column as previously described (23). AC toxin was stored at −70°C in 8 M urea–10 mM Tricine–0.5 mM EDTA–0.5 mM EGTA (pH 8.0).
Flow cytometric analysis of binding of AC toxin to Jurkat cells.
Jurkat cells, a human T-helper cell line, were maintained in RPMI 1640 supplemented with 5% fetal bovine serum (FBS) and 5% Serxtend (Irvine) at 37°C in 5% CO2. For binding experiments, Jurkat cells were washed twice in 10 mM HEPES–140 mM NaCl–5 mM KCl–1% glucose–3 mM MgCl2–2 mM EDTA (pH 7.4) (buffer A). Jurkat cells were resuspended at 2.5 × 106/ml in buffer A supplemented with 5% FBS. When indicated, 3.2 mM CaCl2 was added to cells, resulting in 1.2 mM free calcium. AC toxin was added to cells and incubated at 37°C for 30 min. Cells were washed three times. For samples containing calcium, all washes and incubation were done with buffer A with 5% FBS and 3.2 mM CaCl2. Samples without calcium were washed, and incubation was carried out in buffer A containing 5% FBS. Pellets were resuspended in 100 μl of polyclonal antibody against AC toxin at a 1:500 dilution for 1 h at 4°C. Cells were washed three times; the pellet was resuspended in 100 μl of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) (Sigma) diluted 1:25 and incubated for 45 min at 4°C. Jurkat cells were washed three times, and 10,000 cells were assayed in a FACScan flow cytometer (Becton Dickson Immunocytometry Systems, San Jose, Calif.). Background samples assayed in each experiment consisted of cells incubated with polyclonal antibody against AC toxin and FITC-conjugated goat anti-mouse IgG, but no AC toxin. Where indicated, background values were subtracted from experimental values.
Flow cytometric analysis of binding of AC toxin to RBC.
Blood was drawn, immediately placed in Alsever’s solution (Sigma), and centrifuged to remove serum and buffy coat. RBC were washed three times and resuspended at 107/ml in buffer A. Addition of AC toxin and incubations were identical to those used for Jurkat cells, with the following exceptions. Washes and incubations were performed with buffer A with or without the addition of 3.2 mM CaCl2, and polyclonal antibody against AC toxin was added at a 1:100 dilution. For experiments addressing time course, temperature, and concentration dependence, cells were washed and incubations were carried out in Hanks’ balanced salt solution (HBSS). In experiments dealing with temperature dependence, cells in HBSS containing 75 mM sucrose to prevent hemolysis were maintained at the indicated temperature before and during the incubation with AC toxin. All washes and incubations after this were performed at 0°C in HBSS–75 mM sucrose.
Immunofluorescence microscopy.
RBC were prepared as described above for flow cytometry. After final washing, cells were mounted on slides with VectaShield (Vector Laboratories, Burlingam, Calif.). Images were obtained with a Nikon epifluorescence microscope.
Production and characterization of the polyclonal antibody against AC toxin.
Sp2/0-Ag14 mouse myeloma cells were injected into syngeneic BALB/c mice immunized with AC toxin to produce polyclonal ascites by a method similar to those described previously (27, 29, 41). The titer of this polyclonal antibody against wild-type AC toxin and nonacylated AC toxin in both the absence and presence of 1 mM CaCl2 was determined by enzyme-linked immunosorbent assay. The titer, defined as the dilution of polyclonal antibody eliciting an optical density of greater than 1.0, was 1:200,000 for the four conditions mentioned above. To confirm that this polyclonal antibody bound to all the major domains of the toxin, Western blot analysis was performed with a panel of deletion mutants (25, 28). We found the polyclonal antibody used in this study bound to the catalytic, hydrophobic, and repeat regions of the toxin molecule.
Intoxication of RBC.
Blood was obtained as described above and used immediately. RBC were washed three times in HBSS, resuspended at 107 RBC/ml, and incubated with AC toxin for 30 min at 37°C. Cells were washed 3 times in HBSS, and 10% trichloroacetic acid was added to lyse the cells and precipitate the hemoglobin. The supernatant containing the cellular cAMP was removed and extracted three times with H2O-saturated ether to remove residual trichloroacetic acid. HCl was added to yield a final concentration of 0.1 N, and cAMP measured by an automated radioimmunoassay (7).
Renaturation of AC toxin in the presence of calcium.
AC toxin stored in 8 M urea–0.5 mM EDTA–0.5 mM EGTA was renatured by a >15-fold dilution of toxin solution into HBSS. The urea concentration present after this dilution was <500 mM, and the free calcium concentration was 1.1 mM (16).
RESULTS AND DISCUSSION
Previous observations of binding of AC toxin to target cells, including those from our laboratory, have been limited by the available methodologies. These include identification of bound toxin by Western blotting or quantitation of cell-associated enzymatic activity (24, 34, 37). These approaches involve cell disruption and fractionation and, while useful, cannot fully evaluate the complex sequence of functional steps in the interaction of AC toxin with intact cells. To overcome these problems and begin to address additional features of the interaction of the toxin with intact cells, we have used flow cytometry. The advantages of this technique are that (i) only cell-bound toxin is detected, (ii) the presence of the catalytic domain is not necessary, and (iii) intact target cells, not cell membranes, are used.
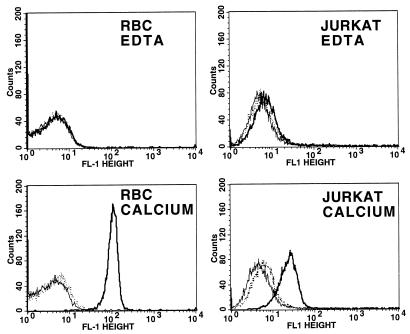
Calcium- and CyaC-mediated activation is necessary for the functional activities of all RTX toxins, but their role in binding of these toxins with target cells is less clear. Boehm et al., using an immunoblot procedure, found that binding of E. coli hemolysin to sheep RBC is both calcium and HlyC dependent (5, 6). Subsequently, using a modification of the same procedure, Bauer and Welch found that hemolysin association with sheep RBC is independent of both calcium and HlyC activation (2). Consistent with this report, Soloaga et al. showed that binding of E. coli hemolysin to liposomal membranes is independent of HlyC-mediated acylation (42), and Ostoloza and Goni reported that E. coli hemolysin binds to lipid vesicles in a Ca2+-independent manner (31). In addition, Moayeri and Welch, using flow cytometric analysis similar to that used in this study, found that binding of E. coli hemolysin to sheep RBC is independent of HlyC-mediated acylation (30). Previously, using both Western blot analysis and quantitation of membrane-associated enzymatic activity, we observed that AC toxin was bound to Jurkat cells in the absence of both calcium and acylation (24). When these experiments were repeated under more stringent washing conditions, binding of AC toxin to target cells appeared not to occur in the absence of calcium and acylation. This finding is consistent with results obtained from other groups indicating that AC toxin binding to cells requires both acylation and calcium (25, 37). Figure 1 shows the flow cytometric data on binding of AC toxin to sheep RBC and Jurkat cells, a human T-cell leukemia line, with or without acylation and with or without calcium. These results clearly indicate that both the acylation and the availability of calcium are required for toxin binding to cells under these conditions. In addition, AC toxin bound to cells in the presence of calcium could not be washed off with 2 mM EDTA (data not shown). This observation, along with the fact that toxin-treated target cells are washed nine times prior to analysis by flow cytometry, suggests a stable interaction between AC toxin and the target cell, which could include partial insertion into the membrane.
FIG. 1.
Binding of AC toxin to Jurkat cells and sheep RBC is both calcium and acylation dependent. Cells in 2 mM EDTA or 1.2 mM free calcium were treated with AC toxin (10 μg/ml) or unacylated (by virtue of omission of cyaC) AC toxin (10 μg/ml) for 30 min at 37°C as indicated in Materials and Methods. Solid line, flow cytometric histogram of control cells; bold solid line, AC toxin; dotted line, unacylated AC toxin. Data presented are representative of seven separate flow cytometry experiments for sheep RBC and two separate experiments for Jurkat cells.
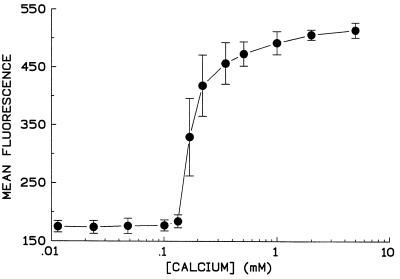
Functional evaluation of AC toxin has revealed a striking calcium concentration dependence for both intoxication and hemolysis, with little or no activity below 100 μM calcium and a steep rise to maximal activity at approximately 1 mM calcium (11, 23). Therefore, it was important to determine whether the absence of toxin function at calcium concentrations below 100 μM reflected a calcium requirement for binding as well. The calcium concentration dependence of binding illustrated in Fig. 2 shows a steep calcium concentration dependence similar to that for intoxication and hemolysis and establishes that the absence of toxin activities at low calcium levels is essentially due to lack of binding under these conditions.
FIG. 2.
Binding of AC toxin to sheep RBC shows a steep calcium concentration dependence. Sheep RBC were washed three times in buffer A and resuspended at 107/ml in the same buffer. Calcium was added to achieve the indicated free calcium concentrations. AC toxin (10 μg/ml) was added and incubated for 30 min at 37°C. Cells were washed and incubated with polyclonal antibody as described in Materials and Methods. Data are expressed as mean fluorescence obtained from flow cytometry. Each point represents mean ± standard deviation from three similar experiments.
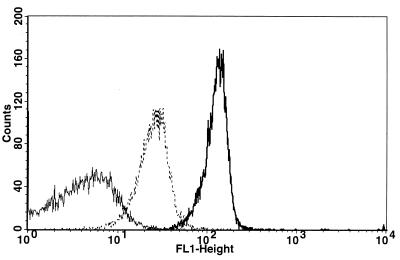
Intoxication of target cells by AC toxin is dependent on an extracellular calcium concentration greater than 200 μM (23). However, K+ efflux and hemolysis can occur, although at a reduced level, in the presence of EDTA or EGTA, but only when the toxin is renatured in the presence of calcium before its addition to cells in media containing these calcium chelators (16, 34, 37). Figure 3 illustrates that AC toxin, renatured in the presence of calcium before addition to cells in the presence of EDTA, binds to sheep RBC. Although binding of toxin to intact target cells under this condition is less than the binding of toxin renatured in calcium when added to RBC in the presence of calcium, it is greater than the binding seen when AC toxin is both renatured and incubated with cells in the presence of EDTA. Intoxication, however, did not occur when AC toxin was renatured in calcium and added to sheep RBC in an excess of EDTA (Table 1). The absence of intoxication in the presence of K+ efflux and hemolysis cannot be explained by reduction in binding, since the concentration of toxin required for intoxication is 10- to 100-fold less than that required for K+ efflux and hemolysis (16). These results suggest that the binding seen when toxin is renatured in calcium but assayed in EDTA represents a conformation of the toxin molecule able to bind and insert into the target cell membrane and elicit both K+ efflux and hemolysis but unable to delivery the catalytic domain. Use of monoclonal antibodies directed against specific regions of the toxin, along with flow cytometry, will be extremely helpful to determine the structure of the toxin molecule under this condition as well as a variety of others.
FIG. 3.
AC toxin renatured in the presence of calcium before addition to sheep RBC in EDTA binds to cells but at a reduced level. Sheep RBC were washed and resuspended in buffer A containing 2 mM EDTA or buffer A containing 3.2 mM calcium. AC toxin (10 μg/ml) was added to RBC as indicated below for 30 min at 37°C. Solid line, toxin renatured in the presence of EDTA upon its addition to cells in buffer A containing 2 mM EDTA; dotted line, AC toxin renatured in the presence of calcium as described in Materials and Methods but incubated with sheep RBC in buffer A containing EDTA; bold solid line, AC toxin renatured in the presence of calcium upon its addition to RBC in buffer A containing 3.2 mM calcium. Data are representative of five separate experiments.
TABLE 1.
AC toxin (10 μg/ml) renatured in calcium but assayed in the presence of excess EDTA does not elicit an increase in intracellular cAMP levels in sheep RBCa
| Renatured in: | Assayed in: | Mean intracellular cAMP (pmol/107 RBC) ± SD |
|---|---|---|
| EDTA | EDTA | 1.96 ± 1.24 |
| Calcium | EDTA | 2.01 ± 0.18 |
| EDTA | Calcium | 193.0 ± 2.00 |
| Calcium | Calcium | 189.0 ± 3.60 |
Background, 1.90 ± 0.15.
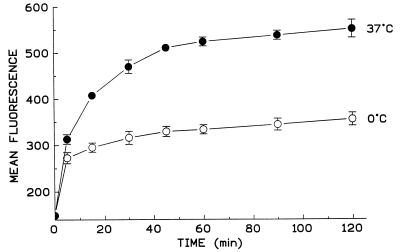
The interaction of AC toxin with sheep RBC has been shown to occur over a wide range of temperatures (4 to 36°C), while intoxication requires temperatures above 20°C (34). In addition, K+ efflux is only modestly affected at 0 to 2°C, but hemolysis, which occurs at this reduced temperature, is slowed considerably (16). Figure 4 shows that binding of AC toxin to sheep RBC does occur at 0°C; however, the total binding is less than that at 37°C. Interestingly, at 0°C the binding of AC toxin is maximal at 15 min, while binding at 37°C is not maximal until 45 min. The reason for this is unclear. The reduction seen at 0°C could be due to a difference in the conformation of the toxin molecule on the cell surface, with a decrease in the number of available epitopes exposed per cell. Alternatively, this reduction in binding could simply be due to a decrease in the amount of AC toxin bound to each cell. To address this question, we incubated AC toxin with a polyclonal antiserum before adding it to cells. We found that this procedure blocked the toxin’s ability to bind to sheep RBC and inhibited intoxication in sheep RBC, human RBC, and Jurkat cells by greater than 98%. While direct fluorophore labeling of AC toxin would be preferred to address this concern and other aspects of toxin action presented here, in our laboratory and others, direct labeling with a variety of fluorophores has yielded AC toxin with impaired functional activities and thus is inappropriate for studies such as these.
FIG. 4.
AC toxin binds to sheep RBC at 0°C, but at a reduced level. Sheep RBC at 0 or 37°C were incubated with AC toxin (15 μg/ml) for the indicated time. Data represent mean fluorescence ± standard deviation from three similar experiments. Sheep RBC treated with polyclonal antibody against AC toxin and FITC-conjugated goat anti-mouse IgG served as background fluorescence. The mean fluorescence of the background is represented as the zero time point.
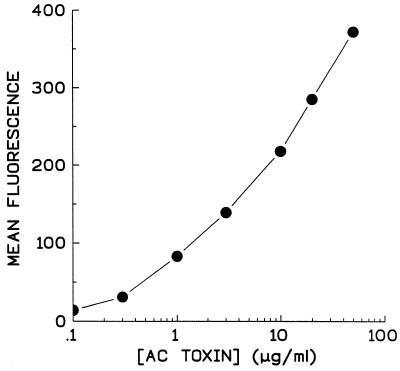
Bacterial toxins with a specific protein receptor are often limited to interacting only with cell types possessing that receptor. AC toxin, however, interacts with a wide variety of cell types (13, 21, 35, 40), which suggests that it may not need a specific cellular receptor or the molecules with which it interacts are ubiquitous. Gordon et al. (15) showed that treatment of Chinese hamster ovary cells with trypsin or cycloheximide reduced anthrax AC toxin-induced cAMP accumulation but had no effect on intoxication by pertussis AC toxin. These data led to the conclusion that anthrax toxin, which enters cells by receptor-mediated endocytosis, requires the presence of certain target cells surface proteins, while AC toxin was not affected by modification of target cell proteins. However, incubation of AC toxin with ganglioside before its addition to target cells markedly inhibited cAMP accumulation by pertussis AC toxin but had no effect on anthrax AC toxin (15). In addition, we have shown that negatively charged lipids are necessary for pore formation by AC toxin in an artificial lipid bilayer system (43). Consistent with this, we found that binding of AC toxin to sheep RBC is concentration dependent but unsaturable up to 50 μg/ml (Fig. 5). This concentration of toxin is more than fivefold higher than that necessary for maximal intoxication, suggesting that AC toxin interacts with some abundant species, such as one or more phospholipids, which is not limiting within the range of toxin concentration tested.
FIG. 5.
Binding of AC toxin to sheep RBC is concentration dependent but unsaturable up to 50 μg/ml. AC toxin at indicated concentrations was incubated with sheep RBC for 30 min at 37°C as described in Materials and Methods. Data are representative of five separate experiments and are expressed as mean fluorescence minus background.
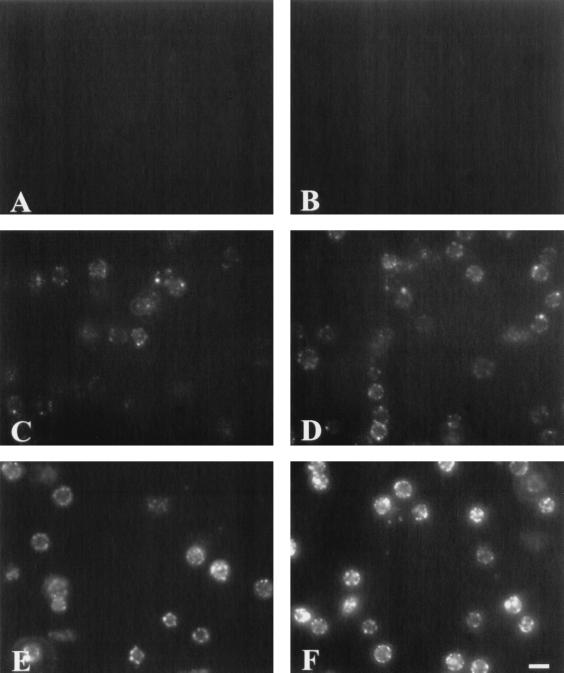
To understand better the lack of saturation of binding and the distribution of toxin on the cells, sheep RBC prepared for flow cytometry were visualized by fluorescence microscopy (Fig. 6). To determine background fluorescence for this technique, cells were incubated with polyclonal antibody against AC toxin and FITC-conjugated goat anti-mouse IgG (Fig. 6A). In addition, Fig. 6B illustrates that cells incubated with AC toxin in the presence of 2 mM EDTA show no apparent binding of AC toxin to the RBC. Consistent with the flow cytometric data, increases in fluorescence intensity were observed when sheep RBC were incubated with increasing concentrations of AC toxin (5 to 50 μg/ml) in the presence of extracellular calcium (Fig. 6C to F). Interestingly, sheep RBC treated with AC toxin exhibited a peripheral pattern of fluorescence, consistent with toxin being localized at the cell surface.
FIG. 6.
Immunofluorescence micrographs show that AC toxin binding is dependent on both the presence of calcium and concentration of toxin and exhibits a peripheral pattern of distribution on the cell membrane. Sheep RBC were treated with AC toxin for 30 min at 37°C as described in Materials and Methods. (A) Cells without toxin treatment; (B) cells treated with AC toxin (30 μg/ml) in the presence of 2 mM EDTA; (C to F) cells treated with AC toxin (5, 10, 30, and 50 μg/ml, respectively) in the presence of 1.2 mM free calcium. Bar, 10 μm.
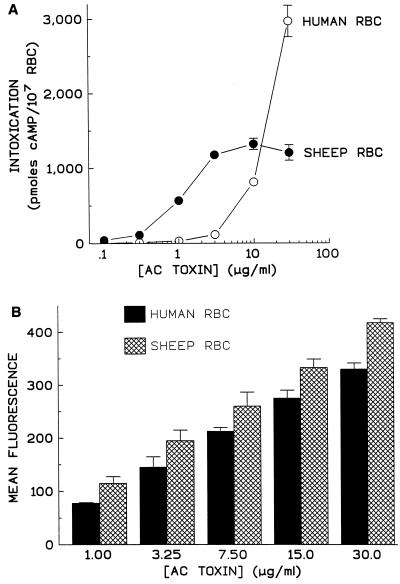
AC toxin elicits increases in intracellular cAMP in human RBC, yet these cells are resistant to increases in K+ efflux and hemolysis (16, 35). Rogel et al. reported that cAMP accumulation in human RBC in response to AC toxin was 2- to 3-fold lower than in RBC from other sources but found the binding of AC toxin to human RBC membranes, using membrane-bound enzymatic activity, was 28- to 32-fold lower than in other RBC membranes (35). Figure 7 demonstrates AC toxin’s ability to bind to and intoxicate human and sheep RBC. Results in Fig. 7A indicate that intoxication of human RBC is 10-fold less than that of sheep RBC. Intoxication of sheep RBC incubated with AC toxin was maximal at a toxin concentration of 3 μg/ml, while at 30 μg of toxin per ml, cAMP accumulation in human RBC was still increasing. Human RBC are larger than sheep RBC (mean corpuscular volumes, measured by an Abbott [Abbott Park, Ill.] CellDyn 4000 were 91.5 fl for human RBC and 36.1 fl for sheep RBC). Since the experiments illustrated in Fig. 7A were performed with equal numbers of sheep and human RBC, the difference in volume and presumably quantity of ATP could explain the difference in magnitude of cAMP response but not the difference in potency. Binding of AC toxin to sheep and human RBC was measured by flow cytometry and is shown in Fig. 7B. Binding of AC toxin to sheep RBC was significantly different from that to human RBC at each toxin concentration tested (unpaired two-tailed t test; P = 0.0004 to 0.0404). Since fluorescence is not linear, percent reduction a given toxin concentration could not be determined. However, the mean fluorescence of human RBC treated with 30 μg of AC toxin per ml was not significantly different from the mean fluorescence seen in sheep RBC treated with 15 μg of toxin per ml (P = 0.8162). Similarly, mean fluorescence of human RBC treated with 15 μg of AC toxin per ml was not significantly different from that of sheep RBC treated with 7.5 μg of toxin per ml (P = 0.4464). The same correlation held true for human RBC treated with 7.5 μg of toxin per ml and sheep RBC treated with 3.25 μg of toxin per ml (P = 0.2226). This finding suggests that binding of AC toxin to human RBC is about twofold lower than that to sheep RBC. Nevertheless, this reduction in binding in human RBC does not in itself explain the decreased potency in intoxication seen in these cells. Visualization of human RBC treated with AC toxin showed a peripheral pattern of fluorescence indistinguishable from that of sheep RBC (data not shown). Why these results differ from those reported earlier by Rogel et al. (35) is unclear, but binding in the latter study was quantitated by the presence of the catalytic domain. It is possible that the catalytic domain is more susceptible to proteolysis than the rest of the toxin molecule in human RBC. This consideration demonstrates the need for an alternative approach, not dependent on the catalytic domain, to measure binding.
FIG. 7.
While AC toxin exhibits reduced potency in its ability to increase cAMP in human RBC, there is only a modest reduction in binding of the toxin to these cells. Human and sheep blood was drawn the day of the experiment, and RBC were isolated and washed as described in Materials and Methods and then incubated with AC toxin at indicated concentrations for 30 min at 37°C. (A) Intracellular cAMP was measured as described in Materials and Methods. Data represent the mean and standard deviation of triplicate samples from a single experiment representative of three separate experiments. (B) Binding of AC toxin to sheep and human RBC was measured as described in Materials and Methods. Data are expressed as mean fluorescence minus background and represent the mean ± standard deviation from three similar experiments.
Flow cytometry of AC toxin-treated intact target cells has provided a new perspective on the calcium, acylation, and temperature requirements for binding seen previously in target cell membranes and adds a new approach, unencumbered by the requirement for certain domains of the toxin molecule for its detection. In the future, use of this technique, in combination with a panel of monoclonal antibodies directed against different domains of the toxin molecule (28), will allow the study of changes in toxin structure which occur upon binding to target cells for expression of one or all of its activities.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI1800 to E.L.H. and National Institutes of Health grant P30CA44579 to the University of Virginia Cancer Center.
We gratefully acknowledge the contribution of D. Haverstick to this study.
REFERENCES
- 1.Barry E M, Weiss A A, Ehrmann I E, Gray M C, Hewlett E L, Goodwin M S. Bordetella pertussis adenylate cyclase toxin and hemolytic activities require a second gene, cyaC, for activation. J Bacteriol. 1991;173:720–726. doi: 10.1128/jb.173.2.720-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer M E, Welch R A. Association of RTX toxins with erythrocytes. Infect Immun. 1996;64:4665–4672. doi: 10.1128/iai.64.11.4665-4672.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellalou J, Sakamoto H, Ladant D, Geoffroy C, Ullmann A. Deletions affecting hemolytic and toxin activities of Bordetella pertussis adenylate cyclase. Infect Immun. 1990;58:3242–3247. doi: 10.1128/iai.58.10.3242-3247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkowitz S A, Goldhammer A R, Hewlett E L, Wolff J. Activation of prokaryotic adenylate cyclase by calmodulin. Ann N Y Acad Sci. 1980;1:356–360. doi: 10.1111/j.1749-6632.1980.tb29626.x. [DOI] [PubMed] [Google Scholar]
- 5.Boehm D F, Welch R A, Snyder I S. Calcium is required for binding of Escherichia coli hemolysin (HlyA) to erythrocyte membranes. Infect Immun. 1990;58:1951–1958. doi: 10.1128/iai.58.6.1951-1958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehm D F, Welch R A, Snyder I S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990;58:1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooker G, Harper J F, Terasaki W L, Moylan R D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- 8.Confer D L, Slungaard A S, Graf E, Panter S S, Eaton J W. Bordetella adenylate cyclase toxin: entry of bacterial adenylate cyclase into mammalian cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:183–187. [PubMed] [Google Scholar]
- 9.Coote J G. Structural and functional relationships among the RTX toxin determinants of gram-negative bacteria. FEMS Microbiol Rev. 1992;88:137–162. doi: 10.1111/j.1574-6968.1992.tb04961.x. [DOI] [PubMed] [Google Scholar]
- 10.Ehrmann I, Weiss A, Goodwin M S, Gray M C, Barry E, Hewlett E L. Enzymatic activity of adenylate cyclase toxin from Bordetella pertussis is not required for hemolysis. FEBS Lett. 1992;304:51–56. doi: 10.1016/0014-5793(92)80587-7. [DOI] [PubMed] [Google Scholar]
- 11.Ehrmann I E, Gray M C, Gordon V M, Gray L S, Hewlett E L. Hemolytic activity of adenylate cyclase toxin from Bordetella pertussis. FEBS Lett. 1991;278:79–83. doi: 10.1016/0014-5793(91)80088-k. [DOI] [PubMed] [Google Scholar]
- 12.Endoh M, Takezawa T, Nakase Y. Adenylate cyclase activity of Bordetella organisms. I. Its production in liquid medium. Microbiol Immunol. 1980;24:95–104. doi: 10.1111/j.1348-0421.1980.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 13.Gentile F, Raptis A, Knipling L G, Wolff J. Bordetella pertussis adenylate cyclase. Penetration into host cells. Eur J Biochem. 1988;175:447–453. doi: 10.1111/j.1432-1033.1988.tb14215.x. [DOI] [PubMed] [Google Scholar]
- 14.Glaser P, Ladant D, Sezer O, Pichot F, Ullmann A, Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988;2:19–30. [PubMed] [Google Scholar]
- 15.Gordon V M, Young W W, Jr, Lechler S M, Gray M C, Leppla S H, Hewlett E L. Adenylate cyclase toxins from Bacillus anthracis and Bordetella pertussis. Different processes for interaction with and entry into target cells. J Biol Chem. 1989;264:14792–14796. [PubMed] [Google Scholar]
- 16.Gray M, Szabo G, Otero A S, Gray L, Hewlett E. Distinct mechanisms for K+ efflux, intoxication, and hemolysis by Bordetella pertussis AC toxin. J Biol Chem. 1998;273:18260–18267. doi: 10.1074/jbc.273.29.18260. [DOI] [PubMed] [Google Scholar]
- 17.Guiso N, Rocancourt M, Szatanih S, Alonso J. Bordetella adenylate cyclase is a virulence associated factor and an immunoprotective antigen. Microb Pathog. 1989;7:373–380. doi: 10.1016/0882-4010(89)90040-5. [DOI] [PubMed] [Google Scholar]
- 18.Hackett M, Guo L, Shabanowitz J, Hunt D F, Hewlett E L. Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science. 1994;266:433–435. doi: 10.1126/science.7939682. [DOI] [PubMed] [Google Scholar]
- 19.Hanski E, Farfel Z. Bordetella pertussis invasive adenylate cyclase. Partial resolution and properties of its cellular penetration. J Biol Chem. 1985;290:5526–5532. [PubMed] [Google Scholar]
- 20.Hewlett, E. L., and M. C. Gray. Bacterial protein toxins. In K. Aktories and I. Just (ed.), Handbook of experimental pharmacology, in press. Springer-Verlag, Heidelberg, Germany.
- 21.Hewlett E L, Gordon V M. Adenylate cyclase toxin of Bordetella pertussis. In: Wardlaw A C, Parton R, editors. Pathogenesis and immunity in pertussis. New York, N.Y: John Wiley & Sons; 1988. pp. 193–209. [Google Scholar]
- 22.Hewlett E L, Gordon V M, McCaffery J D, Sutherland W M, Gray M C. Adenylate cyclase toxin from Bordetella pertussis. Identification and purification of the holotoxin molecule. J Biol Chem. 1989;264:19379–19384. [PubMed] [Google Scholar]
- 23.Hewlett E L, Gray L, Allietta M, Ehrmann I, Gordon V M, Gray M C. Adenylate cyclase toxin from Bordetella pertussis. Conformational change associated with toxin activity. J Biol Chem. 1991;266:17503–17508. [PubMed] [Google Scholar]
- 24.Hewlett E L, Gray M C, Ehrmann I E, Maloney N J, Otero A S, Gray L, Allietta M, Szabo G, Weiss A A, Barry E M. Characterization of adenylate cyclase toxin from a mutant of Bordetella pertussis defective in the activator gene cyaC. J Biol Chem. 1993;268:7842–7848. [PubMed] [Google Scholar]
- 25.Iwaki M, Ullmann A, Sebo P. Identification by in vitro complementation of regions required for cell-invasive activity of Bordetella pertussis adenylate cyclase toxin. Mol Microbiol. 1995;17:1015–1024. doi: 10.1111/j.1365-2958.1995.mmi_17061015.x. [DOI] [PubMed] [Google Scholar]
- 26.Iwamori M, Shimomura J, Nagai Y. Specific binding of cholera toxin to rat erythrocytes revealed by analysis with a fluorescence-activated cell sorter. J Biochem. 1985;97:729–735. doi: 10.1093/oxfordjournals.jbchem.a135112. [DOI] [PubMed] [Google Scholar]
- 27.Kurpisz M, Pupta S K, Fulgham D L, Alexander N J. Production of large amounts of mouse polyclonal antisera. J Immunol Methods. 1988;115:195–198. doi: 10.1016/0022-1759(88)90288-8. [DOI] [PubMed] [Google Scholar]
- 28.Lee S J, Gray M C, Sebo P, Hewlett E L. Epitope mapping of monoclonal antibodies against Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1999;67:2090–2095. doi: 10.1128/iai.67.5.2090-2095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manahan W, Paraf A. Mice ascites as a source of polyclonal and monoclonal antibodies. J Immunol Methods. 1993;161:187–192. doi: 10.1016/0022-1759(93)90294-h. [DOI] [PubMed] [Google Scholar]
- 30.Moayeri M, Welch R A. Prelytic and lytic conformations of erythrocyte-associated Escherichia coli hemolysin. Infect Immun. 1997;65:2233–2239. doi: 10.1128/iai.65.6.2233-2239.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostolaza H, Goni F M. Interaction of the bacterial protein toxin alpha-haemolysin with model membranes: protein binding does not always lead to lytic activity. FEBS Lett. 1995;371:303–306. doi: 10.1016/0014-5793(95)00927-2. [DOI] [PubMed] [Google Scholar]
- 32.Ostolaza H, Soloaga A, Goni F M. The binding of divalent cations to Escherichia coli alpha-haemolysin. Eur J Biochem. 1995;228:39–44. [PubMed] [Google Scholar]
- 33.Pearson R D, Symes P, Conboy M, Weiss A A, Hewlett E L. Inhibition of monocyte oxidative responses by Bordetella pertussis adenylate cyclase toxin. J Immunol. 1987;139:2749–2754. [PubMed] [Google Scholar]
- 34.Rogel A, Hanski E. Distinct steps in the penetration of adenylate cyclase toxin of Bordetella pertussis into sheep erythrocytes. Translocation of the toxin across the membrane. J Biol Chem. 1992;267:22599–22605. [PubMed] [Google Scholar]
- 35.Rogel A, Meller R, Hanski E. Adenylate cyclase toxin from Bordetella pertussis. The relationship between induction of cAMP and hemolysis. J Biol Chem. 1991;266:3154–3161. [PubMed] [Google Scholar]
- 36.Rogel A, Schultz J E, Brownlie R M, Coote J G, Parton R, Hanski E. Bordetella pertussis adenylate cyclase: purification and characterization of the toxic form of the enzyme. EMBO J. 1989;8:2755–2760. doi: 10.1002/j.1460-2075.1989.tb08417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose T, Sebo P, Bellalou J, Ladant D. Interaction of calcium with Bordetella pertussis adenylate cyclase toxin. Characterization of multiple calcium-binding sites and calcium-induced conformational changes. J Biol Chem. 1995;270:26370–26376. doi: 10.1074/jbc.270.44.26370. [DOI] [PubMed] [Google Scholar]
- 38.Sakamoto H, Bellalou J, Sebo P, Ladant D. Bordetella pertussis adenylate cyclase toxin. Structural and functional independence of the catalytic and hemolytic activities. J Biol Chem. 1992;267:13598–13602. [PubMed] [Google Scholar]
- 39.Sebo P, Glaser P, Sakamoto H, Ullmann A. High-level synthesis of active adenylate cyclase toxin of Bordetella pertussis in a reconstructed Escherichia coli system. Gene. 1991;104:19–24. doi: 10.1016/0378-1119(91)90459-o. [DOI] [PubMed] [Google Scholar]
- 40.Selfe S, Hunter D D, Shattuck R L, Nathanson N M, Storm D R. Alteration of intracellular cAMP levels and beating rates of cultured chick cardiac cells by Bordetella pertussis adenylate cyclase. Mol Pharmacol. 1987;31:529–534. [PubMed] [Google Scholar]
- 41.Shulman M, Wilde C D, Kohler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978;276:269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- 42.Soloaga A, Ostolaza H, Goni F M, de la Cruz F. Purification of Escherichia coli pro-haemolysin, and a comparison with the properties of mature α-haemolysin. Eur J Biochem. 1996;238:418–422. doi: 10.1111/j.1432-1033.1996.0418z.x. [DOI] [PubMed] [Google Scholar]
- 43.Szabo G, Gray M C, Hewlett E L. Adenylate cyclase toxin from Bordetella pertussis produces ion conductance across artificial lipid bilayers in a calcium- and polarity-dependent manner. J Biol Chem. 1994;269:22496–22499. [PubMed] [Google Scholar]
- 44.Weiss A A, Hewlett E L, Myers G A, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42:33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]