Abstract
The serum level of the soluble Receptor for Advanced Glycation End-products (sRAGE) is a promising blood biomarker for the development, severity, and progression of chronic obstructive pulmonary disease (COPD). However, cigarette smoking causes a nearly instant drop in circulating sRAGE levels, strongly impacting on the variability in sRAGE levels. In the current study, we investigated the possible mechanism behind the sudden drop in sRAGE upon smoking. We showed that the number of activated neutrophils in blood significantly increases within two hours upon smoking three cigarettes within one hour. Furthermore, an increased expression of the leukocyte activation marker CD11b, which is a known ligand for RAGE, was observed upon smoking. Additionally, the in vitro activation of neutrophils increased their capacity to bind sRAGE. Together, these data indicate that smoking activates neutrophils in the circulation with concomitant upregulation of the RAGE ligand CD11b, leading to reduced levels of sRAGE in serum.
Keywords: COPD, Biomarkers, sRAGE, Cigarette smoke, CD11b
Introduction
Chronic obstructive pulmonary disease (COPD), is a severe lung disease characterized by chronic, mostly neutrophilic, inflammation of the lower airways (chronic bronchitis), as well as progressive destruction of the alveoli (emphysema). To date, one of the best blood biomarkers for the development, severity, and progression of COPD is the soluble receptor for advanced glycation end-products (sRAGE) [1, 2]. sRAGE is released into the bloodstream upon cleavage of the full-length pro-inflammatory pattern recognition receptor RAGE by proteases [3]. Additionally, a specific splice variant lacking the transmembrane and cytoplasmic domains, endogenous secretory (es)RAGE is directly secreted upon translation, adding to the circulating sRAGE pool [4]. Over the past years, it has been consistently shown that the levels of sRAGE are lower in COPD patients compared to healthy controls [2, 5, 6]. This was confirmed by a meta-analysis combining serum sRAGE data from multiple large cohorts [7]. Moreover, serum sRAGE levels are more associated with emphysema, especially centrilobular emphysema, compared to chronic bronchitis [6, 8]. Additionally, a genetic variant was identified (rs2070600) that significantly affects circulating levels of sRAGE [6, 9, 10]. In contrast, circulating sRAGE levels are not significantly affected by basic patient characteristics, such as age, sex, or body mass index [6, 7, 10]. Recently, we showed a strong decrease in the serum levels of sRAGE within 2 h upon smoking [11]. The mechanism of the cigarette smoke-induced decrease in blood sRAGE is currently unknown. As the decrease in serum sRAGE levels upon smoking occurs nearly instantly, sRAGE is likely to be captured and bound upon smoking. Previously, it has been described that RAGE can bind to the i-domain of CD11b [12], an important activation marker of several leukocytes, including neutrophils [13]. Binding of RAGE to CD11b within the Macrophage-1 antigen (Mac-1) complement receptor induces a pro-inflammatory response and the recruitment of leucocytes [14]. In the current study, we hypothesized that cigarette smoking increases the number of CD11b+ activated neutrophils in blood, which can bind sRAGE, lowering the measurable levels in serum.
Methods
In order to assess the effect of smoking on neutrophil counts and their activation status, blood was collected from 77 young intermittent smokers without airway obstruction [15]. The subjects were between 21 and 62 years of age, 66% was male and all subjects had normal lung function as defined by a forced expiratory volume in 1 s divided by the forced vital capacity (FEV1/FVC) of over 75% [15]. Blood samples were collected after at least two days without smoking (before smoking) and two hours after smoking three cigarettes within one hour (after smoking). The study was approved by the medical ethics committee of the university medical center Groningen (UMCG), and all participants provided written informed consent (Clinicaltrials.gov Identifier: NCT00807469). Furthermore, all clinical procedures were performed according to the standards set by the latest Declaration of Helsinki. The number of neutrophils and CD11b+ leukocytes in patient blood and the amount of RAGE bound to neutrophils in in vitro assays were determined with flow cytometry as described before [15]. In short, before and after smoking, peripheral blood was collected in tubes containing sodium heparin. Red blood cells were lysed and washed with 5 mL cold PBS + 10 g/L albumin + 0.32% sodium citrate. Cells were primed for 5 min at 37 °C before being stained for 1 h at 4 °C with antibodies against Mac-1 (CD11b, clone 2LPM19c, DAKO, Copenhagen, Denmark), L-selectin (CD62L, clone DREG56, BD Pharmingen, San Diego, CA, USA), and RAGE (MAB11451, clone 176,902, R&D Systems, Abingdon, U.K.). Neutrophils were classified as CD62Lhigh expressing granulocytes, the latter being selected based on forward and sideward scatter characteristics. sRAGE levels in serum and in vitro assays were measured using ELISA (R&D Systems, DY1145, Minneapolis, MN, USA).
Results
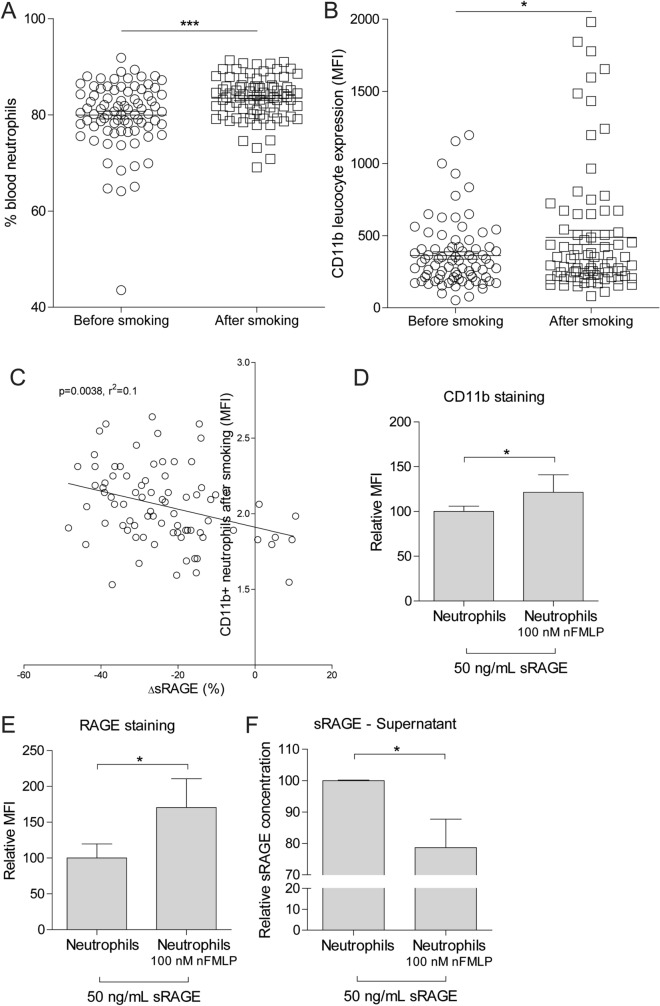
Here, it was found that smoking three cigarettes acutely increased the number of neutrophils and the number of CD11b+ leukocytes in blood (Fig. 1A–B). Additionally, a more pronounced decrease in sRAGE levels upon smoking was associated with a higher expression of CD11b on neutrophils in blood (Fig. 1C). Next, neutrophils were isolated from healthy controls and in vitro incubated in RPMI cell culture medium containing 50 ng/mL recombinant sRAGE (Merck, SRP6051, Kenilworth, New Jersey). Activation of the neutrophils by stimulation with 100 nM N-Formylmethionyl-leucyl-phenylalanine (fMLP) for one hour increased the surface expression of CD11b as well as the amount of sRAGE which was bound to the neutrophils (Fig. 1D–E). Of note, activation of neutrophils in the absence of recombinant sRAGE did not result in any surface expression of RAGE, indicating that RAGE is not noteworthy expressed on the surface of neutrophils (data not shown). To further confirm that activated neutrophils bind sRAGE, the levels of sRAGE in supernatant of non-activated and fMLP-activated neutrophils were measured. Here, a significant decrease in residual sRAGE levels was observed in the supernatant of activated neutrophils (Fig. 1F).
Fig. 1.
Activated neutrophils display increased surface binding of sRAGE. A The number of neutrophils was measured in blood of 77 intermittent smokers without airway obstruction. Blood was collected after at least two days without smoking (before smoking) and two hours after smoking three cigarettes within one hour (after smoking). The percentage of neutrophils was determined by flow cytometry. The granulocyte population was 7 isolated using forward and sideward scatter characteristics and the neutrophil population was characterized as CD62Lhigh expressing granulocytes. B CD11b expression was assessed on blood leucocytes before and after smoking using flow cytometry. C The association between the percentage decrease in serum sRAGE levels before and after smoking with the expression of CD11b on neutrophils after smoking. D–E Neutrophils were isolated from healthy controls (n = 5) and incubated for one hour in RPMI with 10% fetal calf serum and 50 ng/mL recombinant sRAGE. The effect of activation by 100 nM N-Formylmethionyl-leucyl-phenylalanine (fMLP) for hour on the surface expression of CD11b and RAGE was assessed by flow cytometry. F The residual levels of sRAGE after activation with 100 nM fMLP for one hour were assessed in cell-free supernatant using ELISA. Data are shown as individual data points or mean ± SEM. Statistical significance was assessed using a Mann–Whitney U test to compare conditions or with a linear regression analysis to assess associations, * p < 0.05, *** P < 0.001
Discussion
This study contributes to the knowledge that cigarette smoking activates neutrophils and that these activated neutrophils can bind sRAGE. Previously, it was shown that smoking acutely decreases the serum levels of the COPD biomarker sRAGE up to 50% [11]. This finding has strong implications for the effectiveness and usability of sRAGE as COPD biomarker, as this may induce large variations in sRAGE values based upon the smoking habits of the subjects [16–18]. Therefore, it is important to understand the mechanism behind the cigarette smoke-induced drop in serum sRAGE levels. The current study shows that the number of activated neutrophils increases in blood within two hours after smoking three cigarettes. Additionally, using in vitro experiments, we showed that activated neutrophils are capable of binding sRAGE on their surface and reducing the levels of sRAGE from the surrounding fluid. These data indicate that activated neutrophils can be responsible for the cigarette-smoke-induced drop in serum sRAGE levels. Furthermore, the binding of sRAGE to activated neutrophils may also contribute to the widely observed decrease in serum sRAGE levels in COPD patients, a predominantly neutrophilic inflammatory disease. Future studies should investigate whether also other surface-markers of activated neutrophils, besides the known RAGE ligand, CD11b [13], are capable of binding sRAGE. Together, this study indicates that refraining from smoking for at least a day prior to blood sampling may decrease the variability and increase the strength of sRAGE as a COPD biomarker.
Authors Contributions
Sample collection: NHTtH, DJS, MvdB Performing measurements: VRW, SJMH, SDP Data analysis: VRW, SDP, Manuscript draft: SDP, Manuscript editing: SDP, VRW.
Funding
Not applicable.
Declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethical Approval
The study was approved by the medical ethics committee of the university medical center Groningen (UMCG), and all participants provided written informed consent (Clinicaltrials.gov Identifier: NCT00807469). Furthermore, all clinical procedures were performed according to the standards set by the latest Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zemans RL, Jacobson S, Keene J, Kechris K, Miller BE, Tal-Singer R, et al. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir Res. 2017;18:117. doi: 10.1186/s12931-017-0597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pratte KA, Curtis JL, Kechris K, Couper D, Cho MH, Silverman EK, et al. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COPD. Respir Res. 2021;22:127. doi: 10.1186/s12931-021-01686-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz VV, Kojro E, Rat D, Postina R. Induction of RAGE shedding by activation of G protein-coupled receptors. PLoS ONE. 2012;7:e41823. doi: 10.1371/journal.pone.0041823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koyama H, Shoji T, Yokoyama H, Motoyama K, Mori K, Fukumoto S, et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:2587–2593. doi: 10.1161/01.ATV.0000190660.32863.cd. [DOI] [PubMed] [Google Scholar]
- 5.Smith DJ, Yerkovich ST, Towers MA, Carroll ML, Thomas R, Upham JW. Reduced soluble receptor for advanced glycation end-products in COPD. Eur Respir J. 2011;37:516–522. doi: 10.1183/09031936.00029310. [DOI] [PubMed] [Google Scholar]
- 6.Klont F, Horvatovich P, Bowler RP, van Rikxoort E, Charbonnier J-P, Kwiatkowski M, et al. Plasma sRAGE levels strongly associate with centrilobular emphysema assessed by HRCT scans. Respir Res. 2022;23:15. doi: 10.1186/s12931-022-01934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratte KA, Curtis JL, Kechris K, Couper D, Cho MH, Silverman EK, et al. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COPD. Respir Res [Internet]. 2021;22:127. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33906653 [DOI] [PMC free article] [PubMed]
- 8.Yonchuk JG, Silverman EK, Bowler RP, Agustí A, Lomas DA, Miller BE, et al. Circulating soluble receptor for advanced glycation end products (sRAGE) as a biomarker of emphysema and the RAGE axis in the lung. Am J Respir Crit Care Med. 2015;192:785–792. doi: 10.1164/rccm.201501-0137PP. [DOI] [PubMed] [Google Scholar]
- 9.Kinjo T, Kitaguchi Y, Droma Y, Yasuo M, Wada Y, Ueno F, et al. The Gly82Ser mutation in AGER contributes to pathogenesis of pulmonary fibrosis in combined pulmonary fibrosis and emphysema (CPFE) in Japanese patients. Sci Rep. 2020;10:12811. doi: 10.1038/s41598-020-69184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faiz A, Rathnayake SNH, ten Hacken NHT, Guryev V, van den Berge M, Pouwels SD. Single-nucleotide polymorphism rs2070600 regulates AGER splicing and the sputum levels of the COPD biomarker soluble receptor for advanced glycation end-products. ERJ Open Res. 2021 doi: 10.1183/23120541.00947-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouwels SD, Klont F, Kwiatkowski M, Wiersma VR, Faiz A, van den Berge M, et al. Cigarette smoking acutely decreases serum levels of the chronic obstructive pulmonary disease biomarker sRAGE. Am J Respir Crit Care Med. 2018;198:1456–1458. doi: 10.1164/rccm.201807-1249LE. [DOI] [PubMed] [Google Scholar]
- 12.Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Yamamoto H. Controlling the receptor for advanced glycation end-products to conquer diabetic vascular complications. J Diabetes Investig. 2012;3:107–114. doi: 10.1111/j.2040-1124.2011.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf D, Anto-Michel N, Blankenbach H, Wiedemann A, Buscher K, Hohmann JD, et al. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nat Commun. 2018;9:525. doi: 10.1038/s41467-018-02896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoonhorst SJ, Timens W, Koenderman L, lo Tam Loi AT, Lammers J-WJ, Boezen HM, et al. Increased activation of blood neutrophils after cigarette smoking in young individuals susceptible to COPD. Respir Res. 2014;15:121. doi: 10.1186/s12931-014-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pouwels SD, Klont F, Kwiatkowski M, Wiersma VR, Faiz A, van den Berge M, et al. Reply to biswas: acute and chronic effects of cigarette smoking on sRAGE. Am J Respir Crit Care Med. 2019;199:806–807. doi: 10.1164/rccm.201812-2257LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biswas SK. Acute and chronic effects of cigarette smoking on sRAGE. Am J Respir Crit Care Med. 2019;199:805. doi: 10.1164/rccm.201811-2169LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pouwels SD, Klont F, Bischoff R, ten Hacken NHT. Confounding factors affecting sRAGE as a biomarker for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2019;200:114. doi: 10.1164/rccm.201902-0356LE. [DOI] [PMC free article] [PubMed] [Google Scholar]