Abstract
Detoxified mutants of the Escherichia coli heat-labile toxin (LT) act as mucosal adjuvants to intranasally presented coadministered antigens. Here, we compare the adjuvant activity of a panel of detoxified derivatives of LT, using both intranasal (i.n.) and oral (p.o.) routes of administration. The mutants used as adjuvants varied in sensitivity to proteases and toxicity. With keyhole limpet hemocyanin (KLH) as the bystander antigen, the immune responses to i.n. immunizations were consistently higher than the equivalent p.o.-delivered proteins. LT-G192, a mutant which demonstrates a 10-fold reduction in toxicity in vitro, demonstrated wild-type adjuvant activity both i.n. and p.o., inducing similar titers of KLH specific antibody in the sera and immunoglobulin A in local mucosal secretions as wild-type LT. In line with previous data, the nontoxic holotoxoid LT-K63 induced intermediate immune responses in both the serum and mucosal secretions which were lower than those achieved with wild-type LT but at least 10-fold higher than those measured when the antigen was administered with LT-B. Although significant levels of local and systemic anti-KLH antibodies were induced following p.o. immunization with LT-K63, cellular proliferative responses to KLH was poor or undetectable. In contrast, LT and LT-G192 induced significant T-cell responses to KLH following p.o. immunization. These proliferating cells secreted both gamma interferon and interleukin-5, suggesting that the type of immune response induced following p.o. coimmunization with LT and purified protein is a mixed Th1/Th2 response.
Escherichia coli heat-labile toxin (LT) and cholera toxin (CT) are potent mucosal immunogens, inducing systemic and mucosal responses following administration to mucosal surfaces. These immune responses are so potent that they can activate an enhanced immune response to coadministered foreign bystander antigens which are normally poor mucosal immunogens (1, 12, 14). Although LT and CT have the potential to act as mucosal adjuvants, their use in the development of new mucosal vaccines has been restricted mainly to studies in rodents (17, 19). This is because humans are exquisitely sensitive to these toxins, which cause the debilitating watery secretions typical of cholera and traveler’s diarrhea, respectively (13).
The generation of fully defined and safe mucosal adjuvants for humans could have enormous impact on vaccine development and in the treatment of diseases, which result from the induction of an inappropriate immunological response leading to immune system-mediated pathology rather than a protective response (21). However, since many antigens are poor immunogens when delivered mucosally, development of practical mucosal vaccines has been slow. In response to these limitations, considerable effort has been focused on the mucosal adjuvant activities of LT and CT. It would be of value to reduce the toxicity of these molecules while maintaining useful aspects of their immunomodulatory activity. Recombinant, enzymatically inactive forms of both LT and CT toxins have been generated and some of the mutant derivatives retain some adjuvant or immunomodulatory activity while having either greatly reduced or undetectable toxicity (2, 3, 8, 11, 22). LT and CT derivatives with reduced toxicity are potentially suitable for clinical evaluation as mucosal adjuvants in volunteers. In general, most work describing the immunogenicity and adjuvanticity of these toxin derivatives has used the intranasal (i.n.) route of immunization, as rodents are much more sensitive to i.n. than to oral (p.o.) immunization (6). Indeed, so much material is needed for p.o. immunization experiments that such studies with defined adjuvants and bystander antigens have proved logistically difficult for many research teams. Factors such as stomach acid and proteolytic breakdown of both the holotoxin and the bystander are likely to affect significantly the success of p.o. compared to i.n. immunization. Despite these problems, clearly it would be desirable to obtain comparative information on the mucosal adjuvant activity of some of the nontoxic LT and CT derivatives following p.o. compared to i.n. immunization.
One property which appears to significantly influence the ability of mutant toxins to act as mucosal adjuvants is the inherent stability of the mutant holotoxin derivatives to proteases or pH changes. The position and type of amino acid substitution can significantly influence the stability of the toxin structure (16). Some amino acid substitutions in LT, such as K63 (Ser 63 to Lys), appear to have little or no impact on holotoxin integrity, while others, including K7 (Arg 7 to Lys) and K112 (Glu 112 to Lys), result in a reduction in holotoxin stability. Clearly, protein stability could influence the ability of candidate molecules to reproducibly act as mucosal adjuvants. These factors will obviously have greater impact on antigens presented p.o. than those given i.n. Interestingly, some LT mutations, such as G192 (Arg 192 to Gly), have the potential to increase the stability of the holotoxin structure (3). Peptide cleavage at position 192 of the A subunit is essential for LT toxicity, and amino acid substitution here can alter the proteolytic susceptibility of the holotoxin to proteases. These mutants may potentially be more suitable for immunization via the p.o. route. However, these mutants do appear to retain some toxic activity both in Y1 adrenal cell assays and in vivo in rabbit ileal loop tests (10).
In this study, we investigated the ability of a panel of mutant derivatives of LT to act as mucosal adjuvants for coadministered keyhole limpet hemocyanin (KLH). KLH was chosen in this comparative study as we have shown previously that several other antigens (including ovalbumin, tetanus toxoid, and purified surface antigen from influenza virus) induce very poor and variable immune responses when coadministered to the gastrointestinal tract with LT (7). We describe specifically the adjuvant activity of two mutant proteins with single mutations in LT at positions 63 (LT-K63) and 192 (LT-G192) and a double toxin mutant, LT-K63/G192. Using both the i.n. and p.o. routes of immunization, we compared the abilities of these mutant toxins to induce both local and systemic immune responses to KLH. In addition, we have studied aspects of the cellular immune response to KLH following p.o. immunization with the modified forms of toxin.
MATERIALS AND METHODS
Antigens and toxin derivatives.
Wild-type LT, the site-directed mutants LT-K63, LT-G192, and LT-K63/G192, and recombinant LT-B (rLT-B) were constructed and purified as described previously (10, 18). KLH was purchased from Calbiochem (Nottingham, United Kingdom).
Toxicity tests.
Toxicity of the mutants was determined in vitro by using Y1 adrenal cells and in vivo by using the rabbit ileal loop model (10).
Proteolytic breakdown of mutant toxins.
Proteolytic breakdown of LT or mutants derivatives was determined by using trypsin or intestinal wash as a source of protease. Briefly, trypsin sensitivity was determined by first incubating 60 μg of LT or mutant with 0.6 μg of trypsin in a final volume of 300 μl of TEAN buffer (50 mM Tris, 200 mM NaCl, 1 mM EDTA, 3 mM NaN3 [pH 7.5]) at 37°C for 90 min. The enzymatic reaction was stopped by heating the samples at 95°C, and treated subunits were separated on sodium dodecyl sulfate–15% polyacrylamide gels before transfer to nitrocellulose filters. Samples were analyzed by Western blotting using polyclonal anti-LT sera as described previously (6). The protease cleavage experiment was repeated under denaturing conditions by addition of 3.5 M urea. These samples were incubated for 5 or 15 min before the reaction was stopped as described above. Subunits were visualized by Western blotting as described elsewhere (6). Resistance to intestinal protease was determined by incubation of 7 μg of LT or mutant in 20 μl of intestinal wash (derived from two naive mice) at 37°C for 30 min. Breakdown of subunits was determined by Western blotting as described above.
Immunization of mice.
Female BALB/c mice 6 to 8 weeks of age were obtained from Harlan Olac (Bicester, United Kingdom). The immunogenicity and adjuvanticity of mutants toxins were determined by using either the i.n. or p.o. route of immunization. For i.n. immunization, mice were immunized as described previously (8), using 1 μg of toxin and 10 μg of KLH. For p.o. immunization, mice were starved for 2 h and then treated with 400 μl of 0.1 M sodium bicarbonate (Sigma). Thirty minutes after treatment with bicarbonate, mice were immunized with a 0.2-ml volume containing 50 μg of toxin and 5 mg of KLH. All proteins were resuspended in phosphate-buffered saline for immunizations. Animals were immunized on days 1, 21, and 35. Immune responses were monitored by using blood samples taken on day 0, 20, 34, and terminally on day 49. Mucosal lavage samples were taken from the nasal and pulmonary secretions on day 49 as described previously (8). Intestinal washes were performed on day 49 as follows. The small intestine was removed from the stomach to the cecum and cut into sections of approximately 5 cm. Large debris was then removed from the intestine by gentle manipulation, and the intestine was washed by insertion of a fine-tipped pastette into the lumen of the intestine. Each section of the gut was washed through twice with 0.1% bovine serum albumin–1 mM phenylmethylsulfonyl fluoride; the entire gut section from each mouse was washed in the same 1-ml volume of lavage.
ELISA.
Estimation of KLH-specific total antibody in the serum and local immunoglobulin A (IgA) in the mucosal secretions was determined as described previously (6) except that KLH was used as the coating antigen at 5 μg/ml. IgG subclass of the response to KLH was determined by enzyme-linked immunosorbent assay (ELISA) as described previously (6).
Cellular proliferative responses.
The responses were measured by using splenocytes taken from mice on day 49. Single-cell suspensions were generated by crushing individual spleens between frosted-ended slides. Erythrocytes were lysed in ammonium citrate buffer; remaining cells were then washed twice and finally resuspended in Dulbecco modified Eagle medium (DMEM) containing 5% fetal calf serum 2 mM l-glutamine, penicillin-streptomycin, and 2-mercaptoethanol). Splenocytes were counted in a hemocytometer, and the cells were added to wells in a 96 U-bottomed plates at 5 × 105 cells/well. Cells were stimulated with KLH in DMEM at concentrations of 10, 1, and 0.1 μg/ml. Wells in which either DMEM alone was used to determine nonspecific proliferation or concanavalin A was used as a nonspecific mitogen served as the negative or positive control, respectively. Cells were incubated with antigen for 72 h before addition of 20 μl of [3H]thymidine (1 mCi/ml) per well. Six hours after addition of thymidine, cells were harvested under vacuum onto filter mats. The filter mats were allowed to dry before addition of scintillant. Thymidine incorporation was measured with a beta plate harvester and counter (ES&G Berthold Wallac, Milton Keynes, United Kingdom). Proliferation is expressed as stimulation indices, which were calculated by dividing the incorporation of radioactivity in stimulated wells by the incorporation of radioactivity in wells in which similar cells were stimulated with medium alone.
Measurement of cytokine release.
Cytokines released from cells stimulated in vitro were measured in media taken from duplicates of the samples used to measure proliferation; 100 μl of supernatant was removed from each well and stored at −70°C. Levels of cytokines were determined by ELISA using anti-murine cytokine-specific antibodies (Pharmingen, San Diego, Calif.). In brief, anti-mouse gamma interferon (IFN-γ) and interleukin-5 (IL-5) antibodies were coated onto 96-well plates in 0.1 M bicarbonate buffer overnight at 4°C. Plates were washed and then blocked with 1% bovine serum albumin for 1 h 37°C; 50 μl of supernatant was added to each well and allowed to incubate for 24 h at 37°C. A standard curve was also created by using recombinant IFN-γ and IL-5 (R&D Systems). Detection of bound cytokine was determined by using biotinylated anti-mouse IFN-γ or IL-5 (Pharmingen). Horseradish peroxidase-streptavidin (Dako) was then added to each well (1:1,000) and incubated for 1 h at 37°C. Bound antibody was visualized with o-phenylenediamine substrate. The enzymatic reaction was stopped with 3 M sulfuric acid, and absorbancies were read at 490 nm. Calculation of the cytokine release was determined by the generation of a standard curve from known amounts of recombinant cytokine.
Statistical analysis.
Specific antibody responses were compared by the student t test, where differences in the P values of <0.05 were considered to be significantly different.
RESULTS
Proteolytic breakdown of mutant forms of LT.
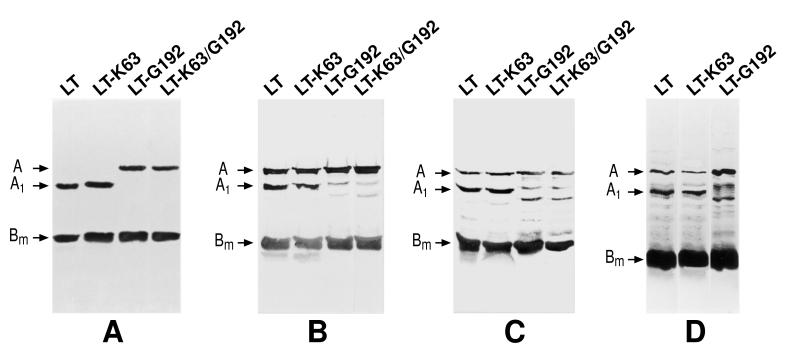
LT-G192 and LT-K63/G192 produced significantly altered proteolytic breakdown products compared to wild-type LT when digested with trypsin under both nondenaturing and denaturing conditions (Fig. 1). Under nondenaturing conditions both LT-G192 and LT-K63/G192 failed to show any obvious cleavage of the A subunit after 90-min treatment with trypsin. In contrast, both LT and LT-K63 show a complete reduction and loss of the A subunit to the A1 form. Under denaturing conditions with trypsin, the A subunit of LT and LT-K63 was proteolytically cleaved and showed the typical A and A1 forms on the gel. In contrast, the A subunits of LT-G192 and LT-K63/G192 appeared to be broken down into two atypical bands. This demonstrates the effect of the mutation on the normal proteolytic site at position 192. Treatment of LT derivatives with intestinal washes from mice confirmed the atypical proteolytic breakdown pattern of LT-G192, which was significantly different from that of LT and LT-K63.
FIG. 1.
Sensitivity of LT, LT-K63, LT-G192, and LT-K63/G192 protease activity. The structural subunits of LT are highlighted. The smallest protein is the monomeric form of the B subunit (Bm). The remaining two proteins are the uncleaved enzymatically active A subunit and the proteolytically cleaved A1 subunit. (A) Proteolytic breakdown products after treatment of the proteins with trypsin for 90 min under nondenaturing conditions; (B) breakdown pattern observed after incubation with trypsin for 5 min under denaturing conditions; (C) breakdown pattern observed after incubation with trypsin for 15 min under denaturing conditions; (D) proteolytic breakdown pattern of the proteins after incubation with protease-rich intestinal lavage fluid.
Mucosal adjuvanticity of LT derivatives determined by i.n. immunization.
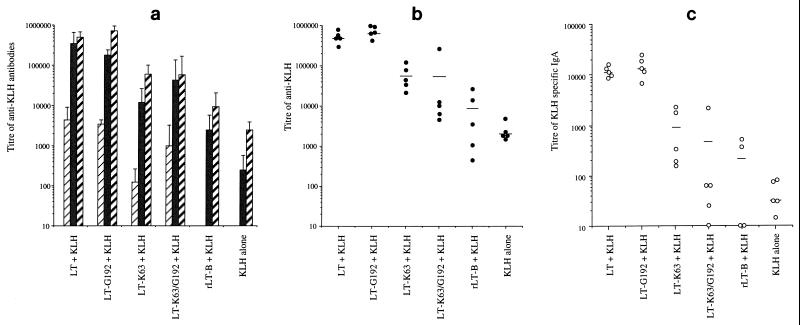
Adjuvant activities of the different LT derivatives were determined by using the i.n. route of immunization with KLH as the bystander antigen (Fig. 2). In general, mice immunized with KLH together with LT had 100-fold-higher titers of KLH-specific antibody in their sera compared to mice immunized with KLH alone (P = 0.00016 [significantly different]) (Fig. 2a). LT-G192 was a potent mucosal adjuvant, inducing an anti-KLH serum antibody response which was not significantly different from that observed when LT was used as the adjuvant (P = 0.117). LT-K63 induced a serum antibody level to KLH which was approximately 10-fold less than that generated by LT (LT/LT-K63 P = 0.0004 [significantly different]) but approximately 10-fold greater than that induced by KLH alone or KLH in the presence of rLT-B (LT-K63/rLT-B P = 0.025 [significantly different]). The individual serum anti-KLH responses within the groups of mice immunized with LT, LT-G192, and LT-K63 were very consistent (Fig. 2b). This is in contrast to the response observed in the group immunised with the double mutant LT-K63/G192, in which the mean response is skewed significantly by one mouse which gave a high anti-KLH response. In addition, we detected high levels of anti-KLH IgA in the nasal and pulmonary lavage samples from animals immunized with LT or LT-G192, intermediate levels in mice immunized with LT-K63, and low levels in mice immunized with either the double mutant (LT-K63/G192), rLT-B, or KLH alone (Fig. 2c). These data show that LT and LT-G192 had a greater i.n. adjuvant activity than LT-K63, which had greater activity than LT-K63/G192 and rLT-B.
FIG. 2.
Serum and local antibody responses to KLH in i.n.-immunized mice. (a) Total anti-KLH response in sera of mice on days 0 (□), 20 ( ), 34 (■), and 49 (
), 34 (■), and 49 ( ). The results are mean titers calculated from individual animals. Error bars represent the standard deviation of the mean response. (b) Variation in individual titers of KLH specific antibody in the sera of mice at a single time point on day 49. (c) Mean IgA response detected in the lung lavage samples from these mice on day 49. Bar represent mean titers for each group.
). The results are mean titers calculated from individual animals. Error bars represent the standard deviation of the mean response. (b) Variation in individual titers of KLH specific antibody in the sera of mice at a single time point on day 49. (c) Mean IgA response detected in the lung lavage samples from these mice on day 49. Bar represent mean titers for each group.
Mucosal adjuvanticity of LT derivatives determined by p.o. immunization.
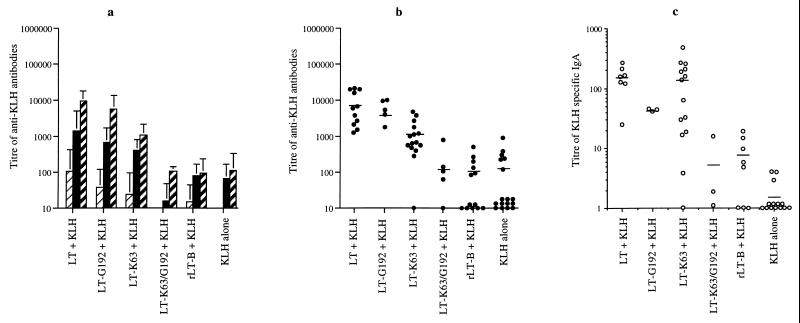
The anti-KLH serum antibody responses generated following intragastric immunization using KLH with or without LT or LT mutant derivatives were all consistently at least 100-fold lower than those detected following i.n. immunization, despite the fact that 50 times the amount of toxin and 500 times the amount of KLH were administered by p.o. compared to i.n. immunizations. Since so much more material is required to immunize p.o. compared to i.n., great care was taken to ensure that mice receiving p.o. doses were not accidentally additionally immunized i.n. due to regurgitation or inhalation of antigen. As a routine, anti-KLH nasal and lung IgA titers were screened in all mice following p.o. immunization, and mice that exhibited high anti-KLH IgA (titer greater than 10) in these secretions were eliminated from the experiments, as we previously determined that the p.o. route is a comparatively poor route for inducing significant nasal and lung IgA responses (6). These results represent data generated from at least three independent experiments. Interestingly, the immune response to KLH after p.o. immunization reflected the response observed following i.n. immunization (Fig. 3a). Mice immunized with LT or LT-G192 showed high and equivalent levels of anti-KLH response in the sera (LT/LT-G192 P = 0.448 [not significantly different]). Mice immunized with LT-K63 and KLH, showed an intermediate response which was approximately 10-fold lower than that after LT immunization (LT/LT-K63 P = 0.032 [significantly different]), while no or poor adjuvant activity was observed when either LT-K63/G192 or rLT-B (LT-K63/rLT-B P = 0.004 [significantly different]) was used as the adjuvant. Again, study of the individual immune responses (Fig. 3b) showed that immunization with LT-K63/G192 or rLT-B resulted in very variable responses. KLH-specific IgA titers detected in intestinal washes from mice immunized via the p.o. route are shown in Fig. 3c. In general, mice immunized with LT plus KLH showed a significant KLH-specific IgA response in the intestine. In contrast, mice immunized with KLH alone or with rLT-B as an adjuvant failed to induce detectable anti-KLH IgA in intestinal washes. Mice immunized with LT-K63 showed a very variable anti-KLH response; in some animals the response was low and almost undetectable, while in other animals the response was as high as that observed when wild-type LT was used for immunization.
FIG. 3.
Serum and local antibody responses to KLH in p.o.-immunized mice. (a) Mean antibody response of p.o.-immunized mice on days 0 (□), 20 ( ), 34 (■), and 49 (
), 34 (■), and 49 ( ). The results are mean titers calculated from individual animals. Error bars represent standard deviations of the mean responses. (b) Variation in individual titers of KLH-specific antibody in sera of mice on day 49. (c) Titers of KLH-specific IgA from intestinal washes from individual animals on day 49. Limited intestinal samples were available for testing from groups immunized with LT-G192 or LT-K63/G192.
). The results are mean titers calculated from individual animals. Error bars represent standard deviations of the mean responses. (b) Variation in individual titers of KLH-specific antibody in sera of mice on day 49. (c) Titers of KLH-specific IgA from intestinal washes from individual animals on day 49. Limited intestinal samples were available for testing from groups immunized with LT-G192 or LT-K63/G192.
Cellular proliferative responses to KLH.
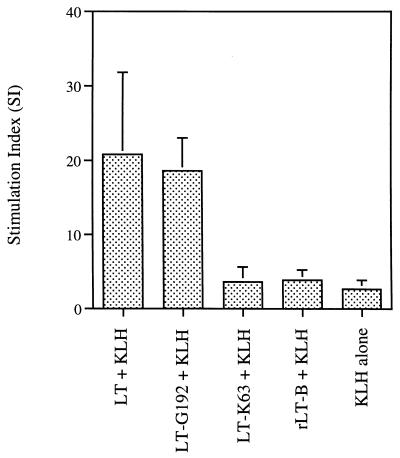
Specific cellular proliferative responses were measured by using splenocytes isolated from p.o.-immunized mice Responses in individual mice were determined and were found to be dependent on the concentration of stimulating antigen. The results shown in Fig. 4 represent the mean stimulation of cells at the optimal dose (10 μg/ml). As expected, mice immunized with either LT or LT-G192 showed strong proliferative responses when stimulated with KLH in vitro. In contrast, LT-K63 failed to generate a good proliferative response to KLH despite being able to generate a significant serum and local KLH-specific antibody response. This response was no greater than that observed when either rLT-B was used as the adjuvant or mice were immunized with KLH alone.
FIG. 4.
T-cell proliferative responses to KLH in vitro. Splenocytes from individual mice were isolated and stimulated with various concentrations of KLH. Each bar represents the mean stimulation index for a group of mice at the optimal antigen stimulating concentration of 10 μg/ml; Error bars represent standard deviations of the means.
Secretion of cytokines by splenocytes following stimulation with KLH.
Cytokines were detected only in the supernatants of cells from mice immunized with either LT or LT-G192 (Table 1). The level of cytokines measured was toward the limit of the detection assay. However, detection of both IL-5 and IFN-γ suggests that the immune response generated in mice immunized with LT is a mixed Th1/Th2 response. Interestingly, although the splenocytes from mice immunized with LT-K63 did not detectably proliferate, they generated very low but reproducibly detectable levels of IL-5. In contrast, cells from mice immunized with LT-G192 secreted both IL-5 and IFN-γ, although the reduction in the amount of IFN-γ did appear to be considerably lower than that observed in the cells from mice immunized with LT.
TABLE 1.
Cytokine release from splenic cells
| Group | Amt released (pg/ml)a
|
|
|---|---|---|
| IL-5 | IFN-γ | |
| LT + KLH | 178 | 251 |
| LT-G192 + KLH | 127 | 30 |
| LT-K63 + KLH | 35 | ND |
| rLT-B + KLH | ND | ND |
| KLH alone | ND | ND |
Monitored in culture supernatants removed 72 h after addition of KLH to splenocytes isolated from immunized animals and calculated from standard curves created by use of purified recombinant material. Results represent means for at least two individual mice in which responses were measured in triplicate. ND, not detectable.
Subclass of antibody.
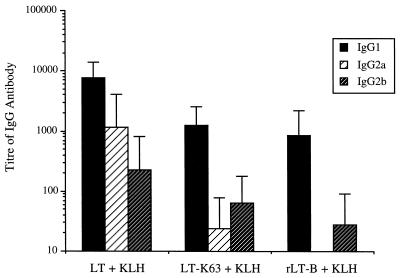
Despite the production of both IL-5 and IFN-γ by T cells stimulated with antigen, the IgG isotype of KLH-specific antibody which predominates is IgG1 (Fig. 5), which is indicative of a Th2 response. Interestingly, as the toxins used as adjuvants become less toxic, the isotype of antibody which is appears to be most affected is IgG2a.
FIG. 5.
IgG subclass analysis of the anti-KLH response in sera taken on day 49 of the experiment from p.o.-immunized mice. Mean antibody titers of KLH-specific IgG1 (■), IgG2a ( ), and IgG2b (▨) were calculated from at least eight mice per group. Error bars represent standard deviations of the mean responses.
), and IgG2b (▨) were calculated from at least eight mice per group. Error bars represent standard deviations of the mean responses.
DISCUSSION
Data presented here provide evidence that LT, LT-G192, and LT-K63 can act as mucosal adjuvants to p.o. as well as in i.n.-delivered, coadministered KLH. These toxins induce both systemic and local immune responses to the bystander antigen. Mice that were immunized with these detoxified derivative showed significant levels of anti-KLH antibodies in their blood and detectable anti-KLH IgA in local lavage fluids. Both LT and LT-G192 were able to induce higher levels of anti-KLH responses compared to LT-K63, in agreement with previously published data comparing LT and LT-K63 (5, 8). The data are in agreement with the work of Dickinson and Clements (3), who demonstrated that p.o. priming of the immune system to ovalbumin was possible with LT-G192. However, in these experiments, priming was demonstrated by boosting the animals intraperitoneally with small amounts of ovalbumin. With KLH (which appears less susceptible to proteolytic breakdown), immune responses could be determined without the need for a parenteral boost. In contrast, the double mutant LT-K63/G192 was a very poor adjuvant both i.n. and p.o. The serum anti-KLH responses generated by using LT-K63/G192 was very variable within the group and were no better than those generated by rLT-B alone. This finding provides the first detailed description of a comparison between the adjuvant activity of different LT mutant derivatives following administration via the p.o. route. In a previous report on the p.o. adjuvanticity of a mutant LT, Lycke et al. described an association between the retention of ADP-ribosyltransferase activity and mucosal adjuvant activity of LT (15). In this study, a different LT derivative, E112, was coadministered p.o. with ovalbumin. The reasons for lack of response to ovalbumin observed in these experiments are not known. Although we do not know whether E112 and ovalbumin are able to resist degradation in the intestine, we have found that ovalbumin in particular is a poor p.o. bystander antigen (7). More recently, E112 has been described as having adjuvant activity when immunized i.n. with hemagglutinin from influenza virus (20), suggesting that the lack of response resulted from the route of immunization rather than the mutation in the toxin.
In addition, the exquisite sensitivity of the i.n. route of immunization for these toxins should always be considered when the p.o. adjuvanticity of these proteins is being described. During p.o. dosing experiments, it is important to assess whether accidental immunization of the respiratory surface has also occurred. In our hands, such cross-contamination with minute amounts of toxin appear to significantly alter the immune responses generated (data not shown). Interestingly, in our experiments mice which had detectable levels of anti-KLH IgA in the nasal and pulmonary lavage fluids (suggesting that they had been immunized both p.o. and i.n.) failed to induce high levels of KLH-specific IgA in intestinal washes (data not shown). Variability in responses generated by the two routes of immunization suggests that great care is required for interpretation of data from such experiments. In particular, the use of pooled serum or lavage samples for analysis of results may lead to misinterpretation. In our hands, one strongly responding individual mouse can significantly skew the mean titer scores for a pooled group. This is of high relevance for mucosal compared to parenteral immunization, as the efficiency of antigen delivery to immune response-inductive sites is of critical importance in mucosal immunization studies. This factor does not have great relevance with respect to LT derivatives (such as LT-G192 and LT-K63) which work well as effective mucosal adjuvants; data from these groups are generally consistent. However, it does bear on mutants such as LT-K63/G192 and LT-B, which show only moderate or minimal adjuvant activity. In fact, this type of interpretation may contribute to the confusion that exists regarding the adjuvanticity of the LT-B, as in our hands, over many experiments, this protein has generated the most variable results.
It is possible that the deficiency in the response generated by LT-K63 can be overcome by altering the dose of the antigen given, as suggested by recent i.n. experiments in which the dose of the mutant toxin was increased (11). This finding also confirms that the i.n. route of immunization is far more sensitive for delivery of such antigens than the p.o. route. Others have recently found this to be true also for antigens expressed by attenuated bacterial vaccine strains (9). The i.n. route is obviously potentially attractive for practical immunization since it avoids the hostile environment of the stomach and would also require administration of relatively small amounts of antigen per dose. However, although this route is attractive, the concern remains that immunization of these surfaces may result in induction of potentially lethal inflammation or allergic responses at the respiratory surface. In addition, it is unclear whether mucosal responses generated following i.n. immunization can induce protective immune responses at another mucosal location such as the gut. The idea of a common mucosal system is an attractive one; in reality, however, there appear to be limitations on the extent of the links between the mucosal surfaces. We have previously reported that following i.n. immunization, mucosal IgA responses can be detected in the vaginas of mice (4). However, following intravaginal immunization, responses in the nasal and lung mucosa were fairly poor. In addition, we have shown that although relatively high levels of anti-toxin IgA can be detected in the nasal and lung mucosa following i.n. immunization, only poor responses were detected in intestinal washes from the same mice (6). This work suggests a need for the development of mucosal adjuvants that can act independently at each mucosal surface to induce protective responses. Using these toxins, Marchetti et al. generated protective responses in mice at the mucosal surface to subsequent challenge with Helicobacter pylori (17). Such studies suggest that the usefulness of such toxins for vaccination will be important in the future. However, only clinical trials now being initiated in humans will indicate whether such an approach can radically alter the field of vaccinology.
Interestingly, proliferation of splenocytes from mice immunized with LT-K63 and KLH was poor on restimulation, with only a proportion of individuals showing stimulation indices greater than 5. In contrast, all mice immunized with either LT-G192 or wild-type LT showed stimulation indices greater than 10. The lack of response of systemic T cells could reflect a reduction in the activity of the nontoxic LT mutant action on T cells or indicate that these LT derivatives stimulate T cells at a low levels that is not within the sensitivity range of this assay. Despite the low levels of proliferation, IL-5 could still be detected in the supernatants from these cells. In contrast, the increase in adjuvanticity of the enzymatically active wild-type LT appeared to be associated with increased levels of IL-5 and the presence of IFN-γ, which suggests that the increase in response generated T-cell help to B cells. This finding is in agreement with reports of others that LT can generate a mixed Th1/Th2 response in mice and is also supported by the profiles of IgG isotypes observed in these mice. IgG1 was the predominant antibody in all groups of mice; however, as the toxicity of the mutant decreased, there was a loss of detectable IgG2a in the sera. This also coincided with a loss of detection of IFN-γ in culture supernatants. This loss of IgG2a has been previously observed by this group following i.n. immunization with LT derivatives and ovalbumin (data not shown). In these experiments, IgG2a was found to be present in higher titers than IgG1 when mice were immunized with the wild-type toxin (5). However, in mice immunized with mutants which had reduced toxicity, there was a coincidental reduction in the titers of IgG2a generated. Further work is required to confirm this observation and may begin to provide additional clues to the action of these toxins at the mucosal surface. This effort will assist in the development of novel chimeric proteins in which the features identified as important with regard to mucosal adjuvanticity can be incorporated. These proteins will hopefully form the basis of a new generation of mucosal adjuvants.
ACKNOWLEDGMENTS
This work was in part supported by EC grant TS3*-CT96-0144 and in part by grant 043139/B95/Z from the Wellcome Trust (London, United Kingdom).
REFERENCES
- 1.Clements J D, Hartzog N M, Lyon F L. Adjuvant activity of Escherichia coli heat labile enterotoxin and effect of induction of oral tolerance in mice to unrelated antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 2.de Haan L, Feil I K, Verweij W R, Holtrop M, Hol W G, Agsteribbe E, Wilschut J. Mutational analysis of the role of ADP-ribosylation activity and GM1-binding activity in the adjuvant properties of the Escherichia coli heat-labile enterotoxin towards intranasally administered keyhole limpet hemocyanin. Eur J Immunol. 1998;28:1243–1250. doi: 10.1002/(SICI)1521-4141(199804)28:04<1243::AID-IMMU1243>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris M-T. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douce G, Fontana M, Pizza M, Rappuoli R, Dougan G. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect Immun. 1997;65:2821–2818. doi: 10.1128/iai.65.7.2821-2828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douce G, Giuliani M M, Giannelli V, Pizza M G, Rappuoli R, Dougan G. Mucosal immunogenicity of genetically detoxified derivatives of heat labile toxin from Escherichia coli. Vaccine. 1998;16:1065–1073. doi: 10.1016/s0264-410x(98)80100-x. [DOI] [PubMed] [Google Scholar]
- 7.Douce G, Pizza M, Roberts M, Rappuoli R, Dougan G. Mutant pertussis and Escherichia coli heat labile toxins as adjuvants for enhancing local and systemic immune responses to coadministered, nonliving antigens. In: Levine M M, Woodrow G C, Kaper J, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker; 1997. pp. 253–263. [Google Scholar]
- 8.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galen J E, Gomez-Duarte O G, Losonsky G A, Halpern J L, Lauderbaugh C S, Kaintuck S, Reymann M K, Levine M M. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine. 1997;15:700–708. doi: 10.1016/s0264-410x(96)00227-7. [DOI] [PubMed] [Google Scholar]
- 10.Giannelli V, Fontana M R, Giuliani M M, Guangcai D, Rappuoli R, Pizza M. Protease susceptibility and toxicity of heat-labile enterotoxins with a mutation in the active site or in the protease-sensitive loop. Infect Immun. 1997;65:331–334. doi: 10.1128/iai.65.1.331-334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliani M M, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson R J, Fujihashi K, Xu-Amano J, Kiyono H, Elson C O, McGhee J R. Optimizing oral vaccines: induction of systemic and mucosal B-cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infect Immun. 1993;61:4272–4279. doi: 10.1128/iai.61.10.4272-4279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine M M, Kaper J B, Black R E, Clements M L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47:510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 15.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 16.Magagnoli C, Manetti R, Fontana M R, Giannelli V, Giuliani M M, Rappuoli R, Pizza M. Mutations in the A subunit affect yield, stability, and protease sensitivity of nontoxic derivatives of heat-labile enterotoxin. Infect Immun. 1996;64:5435–5438. doi: 10.1128/iai.64.12.5434-5438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetti M, Rossi M, Giannelli V, Giuliani M M, Pizza M, Censini S, Covacci A, Massari P, Pagliaccia C, Manetti R, Telford J L, Douce G, Dougan G, Rappuoli R, Ghiara P. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–37. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 18.Pizza M, Fontana M R, Giuliani M M, Domenighini M, Magagnoli C, Giannelli V, Nucci D, Hol W, Manetti R, Rappuoli R. A genetically detoxified derivative of heat-labile Escherichia coli enterotoxin induces neutralizing antibodies against the A subunit. J Exp Med. 1994;180:2147–2153. doi: 10.1084/jem.180.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanCott J L, Kobayashi T, Yamamoto M, Pillai S, McGhee J R, Kiyono H. Induction of pneumococcal polysaccharide-specific mucosal immune response by oral immunisation. Vaccine. 1996;14:392–398. doi: 10.1016/0264-410x(95)00198-a. [DOI] [PubMed] [Google Scholar]
- 20.Verweij W R, de Haan L, Holtrop M, Agsteribbe E, Brands R, Van Scharrenburg G J, Wilschut J. Mucosal immunoadjuvant activity of recombinant Escherichia coli heat-labile enterotoxin and its B subunit: induction of systemic IgG and secretory IgA response in mice by intranasal immunisation with influenza virus surface antigen. Vaccine. 1998;16:2069–2076. doi: 10.1016/s0264-410x(98)00076-0. [DOI] [PubMed] [Google Scholar]
- 21.Wiedermann U, Jahn-Schmid B, Fritsch R, Bauer L, Renz H, Kraft D, Ebner C. Effects of adjuvants on the immune response to allergens in a murine model of allergen inhalation: cholera toxin induces a Th1-like response to Bet v 1, the major birch pollen antigen. Clin Exp Immunol. 1998;111:144–151. doi: 10.1046/j.1365-2249.1998.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto S, Kiyono H, Yamamoto Y, Imaoka K, Fujihashi K, Van Ginkel F W, Noda M, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]