Abstract
Myelin, the membrane surrounding neuronal axons, is critical for central nervous system (CNS) function. Injury to myelin-forming oligodendrocytes (OL) in chronic neurological diseases (e.g. multiple sclerosis) ranges from sublethal to lethal, leading to OL dysfunction and myelin pathology, and consequent deleterious impacts on axonal health that drive clinical impairments. This is regulated by intrinsic factors such as heterogeneity and age, and extrinsic cellular and molecular interactions. Here, we discuss the responses of OLs to injury, and perspectives for therapeutic targeting. We put forward that targeting mature OL health in neurological disease is a promising therapeutic strategy to support CNS function.
Subject terms: Neuroimmunology, Neurological disorders
In this Perspective, Molina-Gonzalez and co-authors discuss the contribution of oligodendrocyte injury to neurological disease and the potential for their therapeutic targeting.
Introduction
In the central nervous system (CNS), oligodendrocytes (OL) support neuronal health and function by extending myelinating processes and providing trophic and metabolic support to the underlying axon1,2. OLs are derived from the differentiation of oligodendrocyte progenitor cells (OPCs) throughout the lifespan3–7. However, most are generated in early development, within the first decade in humans and before 2 months of age in mice. Tracking of OL dynamics under homeostasis has indicated 100-fold more annual OL turnover in mice (>36%) vs humans (0.3%)7. What is consistent between species, however, is the highly dynamic turnover of myelin4,5,7. Injury to, and loss of, mature OLs and their processes are a prominent feature of myelin damage (demyelination) underpinning an array of clinical disorders across the lifespan.
The most common myelin disorder of the CNS is multiple sclerosis (MS), characterized by motor, sensory and cognitive impairments resulting from demyelinating lesions, considered to be initiated by transgression of immune constituents across blood-brain and cerebrospinal fluid (CSF) barriers. In active/demyelinating MS lesions, the numbers of mature OLs are comparable to the numbers in the NAWM, suggesting a relative preservation of OLs, in contrast to the significant loss in chronic lesions linked with disease progression8,9. Surviving OLs in acute lesions show “dying back” of processes with intact somas, suggesting sub-lethal injury that may precede their subsequent progressive loss10. This may be induced by systemic sources, such as fibrinogen11, that access the CNS via the blood-brain or CSF-brain barriers. In addition, there may be a contribution from local sources, including soluble products released by reactive microglia and astrocytes12–15 and the breakdown products of injured myelin16–18. Conversely, there could be lack of elements needed to maintain OL and myelin integrity (e.g., sources of energy, lipids)19. Discussed below in more detail are the potential mediators and mechanisms that underlie the above observations.
OL dysfunction/loss is now implicated in contributing to a wide array of neurological/psychiatric disorders. Pathogenesis of multiple system atrophy (MSA), characterized by a variable combination of parkinsonism, cerebellar impairment, and autonomic dysfunction, is ascribed to the accumulation of aggregated α-synuclein in OLs, which leads to severe neuronal loss, yet only a modest reduction in OLs20,21. Studies of the leukodystrophy Vanishing White Matter disease, in which mutations occur in subunits comprising the eukaryotic initiation factor 2B (eIF2B), demonstrate OL-directed cytotoxic function of astrocytes for reasons that are not completely understood22. Acquired pathology of OLs is also described in Parkinson’s Disease23, Alzheimer’s Disease24, and social isolation syndromes25,26, and dysfunctional OL-neuron interactions during development are described in Schizophrenia and Autism Spectrum Disorders27–29. There is evidence for varying susceptibility to injury based on the maturity of OL lineage cells. For instance, mature OLs are relatively preserved following perinatal white matter injury (e.g., periventricular leukomalacia, hypoxic-ischemic encephalopathy) in response to inflammatory and/or hypoxic-ischemic damage, as opposed to late-stage OL progenitors being highly susceptible to oxidative stress-induced apoptosis30. In addition, our analysis of MS tissues indicated a relatively greater loss of progenitors than mature OLs within active MS lesions31.
The OL/myelin deficits observed in the above neurological disorders highlight the critical need for therapeutics to preserve mature OLs, promote new oligodendrogenesis, and support the regeneration of myelin (‘remyelination’). In this Perspective, we cover the range of injury to OLs and intrinsic versus extrinsic influences on OLs, and reflect on how this knowledge may be harnessed for therapeutic development.
Types of OL injury in MS
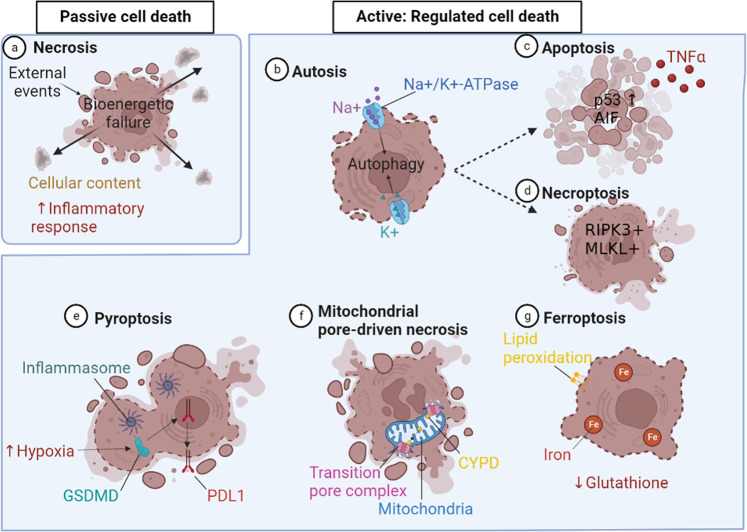
Mechanisms underlying cell death have been categorized under two broad processes, namely passive or active (the latter also referred to as ‘programmed’ or ‘regulated cell death’) (1). As summarized by Galluzzi et al.32, the former commonly results from exposure to external events such as excessive temperatures, shear forces and/or pressures, and reflects mechanical disassembly of cellular constituents not involving molecular machinery. This cellular response is referred to as necrosis, and as stated by Edinger and Thompson33, is the end result of a bioenergetic catastrophe to a level incompatible with cell survival. Disruption of the plasma membrane induces cellular contents to escape to the extracellular space, evoking an inflammatory response. Apoptosis was the initial example of regulated cell death, involving a genetically programmed cell death that does not evoke a secondary inflammatory response. Now recognized are an array of regulated cell death processes, i.e., involving molecular mechanisms, many of which have now been demonstrated to contribute to OL death in experimental or in vitro models, and are being implicated in the MS disease process (Fig. 1). This category now also includes injury mechanisms that result in the features of cell necrosis described above.
Fig. 1. Types of cell death.
Passive cell death; a Necrosis is a form of death where external events induce bioenergetic failure and plasma membrane breakdown, promoting the cellular contents to be released into the extracellular space, inducing an inflammatory response. Active: Regulated cell death; b Autosis is a form of excess autophagy mediated via Na+/K+-ATPase, which results in swelling and plasma membrane breakdown. c Apoptosis is regulated by the transcription factor p53 and is triggered by inflammatory mediators such as TNFα that activate the apoptosis-inducing factor mitochondria associated 1 (AIF). d Necroptosis is a form of controlled necrosis activated by death receptors like Toll-like and TNF receptor-1 (TNFR1) that act on RIPK1 and activate necroptosome forming elements, RIPK3 and MLKL. e Pyropotosis is a form of controlled cell death mediated by inflammasome activation, and triggered by hypoxia, activating gasderminD (GSDMD) leading to nuclear translocation of the programmed death ligand 1 (PDL1). f Mitochondrial permeability transition-driven necrosis is activated by metabolic stress which influences the opening of the permeability transition pore complex at the inner and outer mitochondrial membranes driven by the binding of cyclophilin D (CYPD). g Ferroptosis if a form of cell death that takes place under glutathione deficiency and induces an increase in cellular iron, altering the intracellular environment and causing lipid peroxidation. Created using BioRender.com.
OL death in acute MS lesions
Barnett and Prineas observed dying OLs in a small subset of brain stem lesions which showed morphological features typically associated with apoptotic cell death; however, only 2% of the apoptotic OLs were stated to be immuno-reactive for activated caspase-334. The authors did put forward however, the challenges of detecting caspase-3 expression in OL in post-mortem tissue due to the potential rapid disappearance of dying cells, or tissue preservation artefacts. This OL pathology was stated to resemble the type III lesions described by Lucchinetti et al.9,35. A concept suggested by Casaccia and Bodekke is that OL death in MS lesions could reflect a continuum of injury mechanisms dependent upon the specific insults and its severity36. We have shown that multiple types of cytotoxic immune cells found in acute lesions, including γδ T cells, activated NK cells37, CD56 + NKG2C + CD4 + T cells38, and CD4 + Th17 cells39 can mediate non-MHC restricted cell-cell contact lethal injury of human adult brain-derived mature OLs in vitro (Fig. 2). In addition, Jamann et al. showed that for CD4 + Th17 cells, OL cytotoxicity was perforin/granzyme-dependent40.
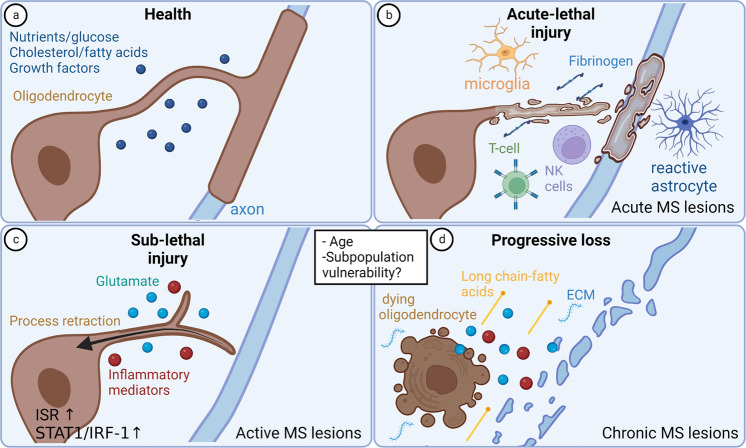
Fig. 2. OL injury in Multiple Sclerosis.
a Oligodendrocytes in healthy conditions rely on nutrients (e.g., glucose), lipids (e.g., cholesterol, fatty acids), and growth factors to support their survival and myelination. b Acute-lethal injury is observed in acute MS lesions driven by cytotoxic T and NK cells (contact dependent), reactive astrocytes, microglia and increased fibrinogen. c Sub-lethal injury to oligodendrocytes in active MS lesions involves process retraction with retention of cell bodies, which can be reversed by returning them to optimal conditions. Important contributors to sub-lethal injury include metabolic stress, glutamate, and inflammatory mediators. Susceptibility to such injury may be influenced by age, and oligodendrocyte subpopulations may be specifically vulnerable. d Combination of insults and age can convert sublethal responses to progressive loss. Ongoing loss of oligodendrocytes is regulated via cell death mechanisms during disease progression, changes to the extracellular milieu such as modifications to the extracellular matrix (ECM) and presence of cytotoxic long chain fatty acids. Created using BioRender.com.
Sub-lethal OL injury
Rodriguez et al.10 identified dying back of OL processes with preserved cell bodies in acute MS lesion biopsies, as had first been described in early phases of demyelination in the cuprizone mouse model by Ludwin et al.41. Such retraction would impair participation in any recovery response. We reproduced this potentially reversible response by human adult mature OLs in both dissociated cell culture and microfibre ensheathment assays, using an array of insults implicated in MS pathogenesis including the pro-inflammatory molecules interferon (IFN)-γ and tumor necrosis factor (TNF)-α, the excitatory neutrotransmitter glutamate, and metabolic stress conditions involving nutrient deprivation and low glucose19,42. RNA sequencing revealed that process retraction can occur via a number of distinct mechanisms, reflecting the mediators of injury (Fig. 2). First, IFNγ or TNF-α induced the STAT1/IRF-1 signaling pathway; this pathway has been shown to impair process outgrowth in neurons and regulate glycolysis - the major source of of ATP in mature OLs which contributes to protein and lipid production needed for myelin biosynthesis. Some studies have also proposed that TNFα modulates the Rho GTPase pathways regulating the cytoskeleton through the activation of the autophagy pathway, providing a link to a stress response42–44. Second, glutamate-induced few changes in gene expression, implicating a direct effect on OL processes. Third, metabolic insult-induced OL process retraction was associated with induction of the integrated stress response (ISR)42, which can either be protective or contribute to cell injury, depending on its level and duration of activation. Addition of the ISR agonist Sephin1 to cultured human OL—maintained under control conditions or exposed to ongoing metabolic stress—reduced process outgrowth42, suggesting that the ISR regulates the protein synthesis machinery required for OL process outgrowth and maintenance. Consistent with this postulate, inhibiting the ISR after stress induction enhanced OL process outgrowth42. Expression of ElF2α, a major regulator of the ISR pathway, is increased in oligodendrocytes in active MS lesions compared to levels in normal appearing white matter or control tissue42.
Further to be considered are the effects of combined insults as a link between sublethal injury and subsequent OL cell death. As an example, we have previously shown that adenovirus-mediated induction in human adult OLs of low levels of p53, a transcription factor that regulates apoptosis and can be detected in OLs in actively demyelinating MS lesions, renders these cells susceptible to subsequent FasL- and TRAIL-medicated killing45. P53 can be induced by multiple stress stimuli, including inflammatory cytokines/inflammation and ischemia/hypoxia. As observed in OPCs in MS lesions46, it is tempting to speculate that cellular senescence may play a role in regulating OL responses, as senescent cells are resistant to cell death; this postulate remains to be investigated.
OL loss in progressive MS
In contrast to acute lesions, there is consensus of significant oligodendroglial cell loss in the center of chronic active lesions as indicated by neuropathological observations of post-mortem tissue8 and single nuclei sequencing47. However, although a number of studies have tried to identify the pathways involved, the precise mechanisms of OL death remain to be established and are being investigated in ongoing studies using experimental in vivo and in vitro models. An increasing number of regulated cell death mechanisms are being defined by their distinct molecular signatures, and linked to specific inducing signals or conditions (Fig. 2). These pathways can be triggered by specific receptor engagement, such as by immune/infectious constituents and/or by metabolic insults, all of which are implicated in the MS disease process. For instance, apoptosis can be triggered by inflammatory signals or metabolic stress. However, Prineas and Barnett observed in two secondary-progressive MS cases that surviving OLs present amongst the microglia and macrophages at the lesion edge rarely showed apoptotic nuclei48.
Necroptosis is a form of necrosis that depends on a membrane pore-forming complex termed the necroptosome. Necroptosis is usually triggered via Toll-like receptors or death receptors that include TNF receptor-1 (TNFR1)49 acting via RIPK1 to sequentially activate RIPK3 and MLKL. Ofengeim et al. (2015) implicated activation of the necroptosis pathway as contributing to progressive MS, finding that ~50% genes upregulated in chronic active lesions are regulators of this process50. Necroptosome markers RIPK1 and MLKL were expressed in OLs and microglia, in MS lesions, the latter consistent with our findings of RIPK3+ and MLKL + microglia in remyelinating MS lesions51. Ripk3−/− mice had increased resistance to cuprizone-induced demyelination, and OLs derived from these mice were less susceptible to TNF-α-mediated necroptosis50. However, Zhang et al. found that cuprizone-induced injury reflected an MLKL-dependent, but RIPK3-independent, mechanism of injury acting via myeloid cells52. Zelic et al. then showed that RIPK1 activation in microglia and astrocytes induces a detrimental neuroinflammatory program that contributes to the neurodegenerative environment in progressive MS53. Of note, we could not inhibit cell death in human mature OLs exposed to metabolic stress with necroptosis inhibitors19. Jurewicz et al. found that TNF treatment of human OLs in culture induces apoptosis-inducing factor mitochondria associated 1 (AIF)-mediated cell death, also known as parthanatosis, involving hyperactivation of DNA damage response machinery54. TNFα was also shown to induce human OL progenitor cells to undergo death by pyroptosis, a pro-inflammatory programmed cell death mediated by inflammasome complex activation55. OLs in MS lesions were shown to express a pyroptosis mediator, gasderminD (GSDMD), and inflammasome inhibition reduces injury to the OL lineage in EAE55. Hypoxia, a feature of a subtype of MS lesions35, can induce GSDMD expression through phosphorylation of STAT3, which promotes nuclear translocation of programmed death ligand 1 (PDL1)56.
Both histological and imaging analyses implicate metabolic stress as a contributor to lesion evolution in MS57–64, with reduced focal cerebral blood flow associated with chronic hypoxia, lesion formation and axonal degeneration, and evidence of axonal metabolic dysfunction. Autophagy is a process of degradation of cellular components by the lysosomal pathway as an adaptive response to stress, to mediate cytoprotective responses and enhance energy production. Blocking autophagy of metabolically stressed OLs in vitro by using chloroquine results in increased cell death65. This could potentially reflect reduced energy production and/or failure of the auto-phagosomal/lysosomal pathway to clear tissue injury-mediating products. Excess autophagy activation itself can contribute to cell death mediated via Na+/K+-ATPase, resulting in cell swelling and plasma membrane rupture (a process known as autosis)66 and by activating other selected cell death pathways, such as apoptosis67 and necroptosis68. Metabolic stress can also trigger mitochondrial permeability transition-driven necrosis which involves the opening of a supramolecular complex (‘permeability transition pore complex’) at the junctions between inner and outer mitochondrial membranes; OLs in MS lesions express cyclophilin D (CYPD), a driver of mitochondrial pore-driven necrosis; deletion protects against neurodegeneration in demyelinating experimental models of MS69.
Other types of cell death may be critical in regulating OL death but have not yet been demonstrably shown to do so in human demyelinating disease. Experimental OL death and demyelination induced by the copper chelator cuprizone involves ferroptosis70—an iron-dependent form of cell death induced by perturbations of the intracellular environment, leading to lipid peroxidation under conditions of glutathione deficiency. This likely reflects an impact on OL metabolism, as copper-containing iron-oxidizing enzymes (ferroxidases) are involved in mitigating oxidative stress. Indeed, OL loss in the cuprizone model can be rescued by treatment with a lipid radical scavenging molecule, ferrostatin 170. Overall, the mechanisms by which human OLs undergo cell death are still being resolved, with the potential for new regulated cell death mechanisms to be uncovered and the development of novel therapeutic interventions. Whether these distinct forms of cell death related to distinct pathologies, CNS regions, age, or subpopulations of OLs remains to be defined.
Intrinsic regulation of oligodendrocyte responses
Multiple variables may influence the intrinsic properties of oligodendrocytes which in turn contributes to their responses to injury, including heterogeneity and age, and OL differentiation state (Fig. 3).
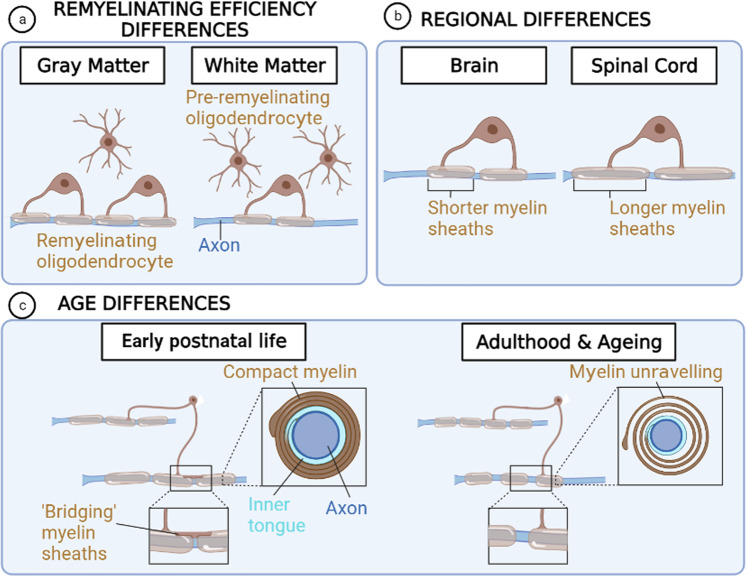
Fig. 3. Oligodendrocyte Heterogeneity.
a Remyelinating efficiency differences are observed between the gray matter and the white matter, oligodendrocytes in the former remyelinate more efficient than the latter, where oligodendrocytes have a higher capacity to differentiate. b Oligodendrocytes show regional differences, in the brain forming shorter myelin sheaths than in the spinal cord. c Oligodendrocyte differences exist during the lifespan, when during early postnatal life oligodendrocytes form compact myelin and longer myelin sheaths, and can create bridges forming sequential myelin sheaths known as ‘bridging myelin sheaths’. In adulthood and ageing, myelin sheaths are shorter and uncompact, leading to myelin unravelling and loss of the bridging myelin sheaths. Created using BioRender.com.
Heterogeneity
OLs show intrinsic heterogeneity as has recently been demonstrated at the transcriptional level using single cell/nuclei RNA sequencing, with 6–9 subpopulations of OLs identified in mouse and human brain under healthy conditions and in the context of demyelination (EAE and MS)71,72. A significant amount of overlap between human and mouse clusters has been observed. Of interest, mature OL clusters expressing genes suggestive of immune function have been identified, for example supporting an MHC-class II-mediated response71. In this regard, a recent study revealed a human-specific OL subpopulation enriched in the spinal cord with predicted immune function73. Examples of regional differences in OL function include observations that spinal cord OLs form longer myelin sheaths on microfibres than their cortical counterparts, recapitulating in vivo myelin properties74. OLs show faster differentiation yet slower remyelination in white matter vs gray matter in mouse models and MS lesions, and reduced remyelination in spinal cord vs deep white matter75–80. However, white matter OL lineage cells are more intrinsically regulated vs those in the gray matter, as transplantations into the adult mouse cerebral cortex revealed that white matter-derived cells differentiated into mature OLs in both niches with equal efficiency, whereas gray matter-derived cells did not75. Moreover, a distinct OL population (‘peri-neuronal OL’) has been identified in gray matter regions81.
In the context of human neurodegenerative diseases such as MS and Alzheimer’s disease (AD), OLs undergo a shift in gene expression and subpopulations compared to healthy controls71,82,83. Single RNA sequencing of the most extensive MS cohort to date revealed differences in OL heterogeneity in white matter vs gray matter, and vulnerability of a specific OL subpopulation84. OL subpopulation profiles allowed for the stratification of MS samples into 3 types: (1) having OL subpopulations largely similar to that observed in normal appearing white matter, (2) showing an increase in an OL subpopulation with a stressed profile, and (3) indicating a block in OL differentiation84. Of note, pathway analysis of OLs in MS gray and white matter lesions indicated engagement of pathways which include those related to injury responses (e.g., p53 and metabolism)84,85.
Age
Myelination is tightly regulated over the lifespan, with onset in humans in the third trimester and peaking in the first decade, and continuing on in adulthood to support learning and memory processes86. 14C -dating studies in humans indicate that OLs are long-lived cells, yet there is dynamic turnover of their myelin membranes7.
Myelin undergoes structural changes with age. In mice, OLs born later in life elaborate increased numbers of myelin internodes, yet these are shorter than those generated in early postnatal life3. We have shown that myelination capacity of human OL lineage cells decreases postnatally through to adulthood87. With adulthood and ageing, there is a reduced amount of new myelin formed in the context of a learning task or following demyelination88,89, in association with impaired oligodendrogenesis. In addition, myelin structure becomes dysregulated particularly in the white matter, with myelin thickening, outfoldings, unravelling, enlargement of inner tongues90–92. Degeneration of myelin sheaths occurs with aging5, with particular vulnerability of newly discovered ‘bridging’ myelin sheaths, by which OLs generate sheaths via an extension of the outer tongue of an existing sheath93. Age is associated with poor efficiency of remyelination in animal models, and is a predominant risk factor for MS progression94.
The molecular underpinnings of OL and myelin changes with ageing are still being elucidated. These findings may reflect in part the epigenetic changes in the OL lineage with ageing, which contribute to poor remyelination95. Transcriptional profiling of rodent OLs with ageing suggests increased engagement of pathways related to antigen presentation, phagocytosis, and interferon signaling96,97. Our own transcriptomic data indicated that pediatric human OLs (<5 years of age), while expressing mature lineage markers, still express developmental markers suggesting they are less mature than their adult counterparts98. The pediatric OLs showed increased expression of immune regulatory genes, as observed in comparative studies with mature OLs99. Our study also showed increased expression of metabolism-related genes in human adult vs pediatric OLs98, perhaps reflecting the extended state of myelin production in the former.
With regards to metabolism, under optimal conditions, adult rat OLs preferentially use glycolysis whereas in development, OPCs and newly differentiated OLs mainly utilize oxidative phosphorylation to produce Adenosine 5′-triphosphate (ATP). We observed that human mature OLs also preferentially use glycolysis but overall have a significantly lower rate of metabolic activity compared to their rat counterparts100. Neumann et al compared metabolic properties of OPCs in culture derived from young versus aged adult rats, and found lower ATP levels and cellular respiration in the latter, likely reflecting mitochondrial dysfunction101. Our in vitro studies indicated that mature human OLs derived from young-middle aged adult brain undergo non-apoptotic death in response to metabolic stress culture conditions in vitro19; conversely, OLs and progenitors from pediatric and fetal sources show a measurable apoptotic response, with differences ascribed to relative expression of effector versus protective members of the BCL-2 family65.
Extrinsic regulation of oligodendrocyte responses
OL responses are regulated by extrinsic influences throughout the lifespan, including in response to CNS injury. This includes being dependent on factors in the microenvironment and cell-cell interactions for their health and function (Figs. 2, 3).
Lipid regulation of OL responses
As OLs extend up to 60 myelinated processes and can generate up to 50 × 103 µm3 of membrane per day, myelination and remyelination are highly demanding processes. Up to 80% of myelin membrane is formed of lipids as main structural components, and the integration of cholesterol and fatty acids into myelin membranes is a critical aspect of myelin growth during development and remyelination102–104. For instance, cholesterol in oligodendrocytes is an essential building block for efficient myelin growth102. However, OLs do not solely rely on their own production of lipids for myelination. Conditional deletion of genes in OLs critical for lipid synthesis leads to reduced OL number and impaired myelination, yet these eventually normalize, indicating compensation through uptake from neighboring cells102,105. Indeed, astrocyte conditional knockouts which impair their lipid synthesis in development result in hypomyelination, and myelin defects only persist when both OL and astrocyte lipid synthesis are inhibited105. However, dietary lipid supplementation improves neurological deficits caused by the lack of lipid generation by astrocytes106.
Furthermore, both cholesterol and fatty acids can act as signaling units and energy sources to regulate the survival of oligodendrocyte lineage cells in development and during remyelination (Fig. 2)104,107. They form membrane lipid rafts which house pro-survival receptors such as integrin receptors108. Cholesterol intermediates such as 8,9-unsaturated sterols and desmosterol have recently been shown to regulate OPC differentiation during myelination and early remyelination109–113. Microglia provide such intermediates to OPCs during remyelination110, and this can occur through transfer of lipid-containing extracellular vesicles (EVs)114; lipids can be recycled by microglia for export following phagocytosis of myelin debris.
Dysregulated lipogenesis leads to accumulation of toxic substrates that cause neuron degeneration, loss of myelin, and/or OL death. A balanced lipid ratio is crucial for the function and health of OLs. For instance, n-3 polyunsaturated fatty acids (n-3-FA) are pro-resolving lipid mediators. N-3-FA are produced from n-6-FA via Fat-1. Efficient conversion in Fat-1 controls low levels of n-6/n-3, which support OL generation during remyelination, although in part through regulation of microglial inflammation115. Moreover, extracellular lipids can also have toxic effects on OLs. Long-chain saturated free fatty acids (FFAs) produced by reactive astrocytes promote OL death in culture116. Oxidised forms of cholesterol (oxysterols) which accumulate in aging and AD promote OL death117. In addition, oxidized phosphatidyl cholines—markers of oxidative stress—are detected in MS lesions, mediate OL death in culture, and induce demyelination when injected in vivo118.
The importance of lipids in regulating OL responses is highlighted by the dysregulation of their synthesis or transport in neurological disorders characterized by OL injury and dysfunction. For instance, the cholesterol biosynthesis pathway is downregulated in MS and in astrocytes in the poorly remyelinating model EAE119, and single nuclei sequencing of chronic active MS lesions indicate altered lipid storage/response genes in microglia and astrocytes47. Decreased cholesterol levels observed in Smith-Lemli-Optiz Syndrome (SLOS) due to 7-dehydrocholesterol reductase (DHCR7) dysfunction are associated with abnormal developmental myelination120,121. Conversely, cholesterol accumulation can lead to hypomyelination and decompacted myelin as observed in some leukodystrophies. For instance, Pelizaeus-Merbacher disease is caused by a mutation in the myelin gene PLP1, which results in the accumulation of cholesterol and membrane raft components in lysosomal compartments due to mistrafficking of these myelin elements122–125. In addition, in Niemann-Pick disease type C, caused by mutations in regulators of cholesterol transport NPC1 or NPC2, results in cholesterol accumulated in the lysosomes126–128. Cholesterol accumulation can also lead to demyelination, as seen in Cerebrotendinous Xanthomatosis, caused by mutations in the sterol 27-hydroxylase gene (CYP27A1)129,130. In addition, deficiencies in production of other lipids, such as sphingolipids, important for oligodendrocyte maturation, and fatty acids, lead to hypomyelination131–135.
Growth factors and extracellular matrix regulation of OLs
Many extracellular factors have been implicated in regulating the responses of immature cells of the OL lineage, including IGF-1, LIF, CNTF, CSPGs, and laminins136–139. OLs are also influenced by neighbouring cells that shape the microenvironment by secreting growth factors and components of the extracellular matrix (ECM). For instance, platelet-derived growth factor (PDGF) can prevent apoptosis of newly formed OLs to support myelination and remyelination, while also supporting OPC proliferation and differentiation140–145. However, expression of its receptor PDGFRα needs to be tightly controlled, as it is normally downregulated with OL maturation and preventing this via overexpression drives myelin defects146. Fibroblast growth factor (FGF) signaling also regulates OLs, with FGFR2 ligation stimulating process outgrowth and regulating myelin thickness via ERK1/2 MAPK signaling147,148. Knockout of FGFR1 in mature OLs increases their numbers and promotes remyelination in EAE, while promoting myelination in vitro149,150. Of interest, FGF2 expression by astrocytes is increased in active MS lesions, and is known to downregulate myelin proteins, cause loss of mature OLs, and inhibit myelin formation150–152. ECM components are also important regulators of OL responses, and their dysregulation after injury contributes to remyelination failure153,154. Cell surface integrin receptors on OLs such as β1 integrin interact with ECM ligands on axons to amplify growth factor signaling, regulating the survival of pre-myelinating and myelinating OLs, increasing myelin membrane formation, influencing axonal selection for myelination in an diameter-dependent manner, and regulating myelin gene translation155–160. Astrocytes contribute to the ECM, as the main source of fibronectin after demyelination, which can inhibit OL differentiation and remyelination161; fibronectin is increased in the plasma of pre-symptomic and symptomatic people with MS, while low levels are observed in remyelinating MS lesions161. Astrocytes also produce hyaluronan during chronic demyelination, impairing remyelination by arresting OL differentiation via Toll-like receptor 2 ligation162–164; of note, hyaluronan is increased in several demyelinating diseases162,163,165. Moreover, ECM may influence OLs through regulation of tissue stiffness, which regulates OL differentiation166, and is increased in chronic demyelination yet decreased during remyelination167.
Immune and glial cell interactions regulating OLs
OL responses are directly and indirectly influenced by cell-cell interactions with immune cells and other glia. For instance, differentiation of OL lineage cells generated from MS patient iPSCs occurs efficiently in vitro and in vivo168, yet is inhibited by exposure to peripheral blood mononuclear cell supernatants, with CD4+ T cell-derived interferon (IFN)-γ being a prime contributor169. IFNγ can also induce OPC death indirectly, via enhanced CD8+ T cell infiltration and subsequent antigen presentation by OPCs99. Primary human adult OLs undergo cell death in vitro following exposure to several T cell populations and natural killer cells, occurring in part through NKG2C/D receptor-ligand interactions37,38; OLs express the ligands in MS lesions thereby making them susceptible to immune mediated cytotoxicity37,38. Furthermore, direct contact of OLs with T helper (Th) 17 cells promotes glutamate release that alters OL cholesterol biosynthesis, thereby causing stress, reducing process formation, and increasing death39. In EAE, ultrastructural analysis demonstrated infiltrating monocytes appearing to pull myelin off the axon170; together with the known requirement of monocytes to initiate disease in EAE171, this suggests an important interaction between monocytes and myelin processes in inducing demyelination. Accordingly, macrophage depletion via small molecule inhibition of the pro-survival receptor CSF1R is sufficient to reduce OL loss and demyelination in the cuprizone model, and administration of the ligand CSF1 is sufficient to cause demyelination172. Microglia are in close communication with astrocytes to drive demyelination, as seen in EAE13, through secretion of pro-inflammatory cytokines, complement signaling47, and semaphorin and ephrin signaling173. Although direct mechanisms by which microglia interact with mature OLs to regulate their responses are not clear, a study in zebrafish showed that microglia engulf myelin sheaths to regulate sheath number in developmental myelination174. In addition, microglia in the presence of astrocytes induce OL death through TNFα after exposure to the pro-inflammatory stimuli lipopolysaccharide (LPS)175. Astrocytes may also contribute to demyelination indirectly via induction of BBB breakdown and expression of chemokines that recruit peripheral immune cells176–180. Human OLs are however resistant to toxic effects of conditioned media from astrocytes preconditioned by immune cell supernatants, whereas immature OL lineage cells are susceptible to impaired differentiation and/or death181. Conversely, immune cells can have protective effects on OLs. Astrocytes indirectly promote OL survival by inducing apoptosis in CD4+ T cells182, and peripheral immune cells in MS produce leukaemia inhibitor factor (LIF) which protects OLs from TNFα-induced death in vitro183. In addition, microglia and astrocytes support OL lineage responses during myelination and remyelination, in part via secretion of growth factors that can act on OL receptors51,138,184,185. Altogether these studies suggest that cell-cell interactions with OLs can have a major influence on OL and myelin pathology in neuroinflammatory diseases such as MS.
Outlook
Therapies currently approved for myelin disorders such as MS are focused on dampening initial myelin damage via immune-modulation. Whereas these drugs can reduce the frequency of clinical relapses, they do not prevent MS progression and clinical decline, likely due to the continued impairment in remyelination and consequent axonal dysfunction and loss. This highlights the importance of complementing immune modulation with neuroprotective and pro-remyelination therapies186,187. Clinical trials are underway assessing drugs targeting neuronal survival and OPC differentiation into remyelinating OLs. We put forward that therapeutic targeting of mature OLs to reduce their injury and dysfunction is a complementary strategy that may support neural health. However, several factors need to be taken into consideration to achieve successful therapeutic targeting of OLs in neurological disease.
First, targeting of mature OLs without off-targets on the immature stages of the OL lineage may be important, given their differential susceptibilities to injury and responses to extrinsic factors. Although mature OLs were long considered not to contribute to remyelination, recent work has surprisingly demonstrated the contrary. Studies have demonstrably shown that OLs surviving demyelination can extend processes to remyelinate, as shown in cats, mouse, and zebrafish188–190. Consistent with these findings, 14C-labeling suggests that remyelinating MS lesions contain ‘old’ OLs191, implying that existing OLs mediated remyelination (although non-proliferating OPC-derived OLs may have also contributed). However, in an elegant study performed in zebrafish larvae enabling imaging of OL behavior in real time, Neely et al. revealed that OLs surviving demyelination remyelinate poorly compared to newly generated OLs, extending fewer processes and mistargeting myelin to neuronal cell bodies190; importantly, this mistargeted myelin profile was also found in MS lesions190. These studies suggest that therapeutic targeting of OL survival would need to also take into account the potential for ensuing poor quality remyelination. Revealing the mechanisms underpinning surviving OL-mediated remyelination, and how this can be improved, is key in harnessing the regenerative potential of these cells. This may be particularly important if drugs promoting OPC differentiation lead to eventual depletion of the progenitor pool, and considering that aged OPCs have decreased capacity to mature and remyelinate, and are less responsive to pro-remyelinating drugs101.
Second, one may need to consider targeting OL subpopulations. While specific functions of OL subpopulations remain to be elucidated, the shift in OL populations in disease may contribute to pathology and therefore may need to be considered for targeted therapy. For instance, an OL subpopulation with alteration in pathways related to inflammation and lipid metabolism has been found to emerge in several models of demyelination (toxin induced, autoimmune-mediated, and an amyloidosis model of Alzheimer’s disease), identified by high expression of C4b and Serpina3n72,192–194; accordingly, OLs expressing complement have been observed in numerous neurodegenerative diseases. Should this population be associated with increased vulnerability to injury or contribution to myelin pathology, targeting this subpopulation would be of interest. Moreover, the differential responses of the OL lineage in white vs gray matter, particularly in the context of remyelination, suggests the importance of considering distinct regional responses of OLs to therapeutics.
Third, sex differences in the OL lineage and myelination have been well described in rodents, although less so in humans195,196. Of note, MS is of lower prevalence but worse severity in males versus females. Although the OL subpopulation changes in MS could not be explained by sex differences84, the potential for differential responses to injury needs to be further investigated.
In summary, we put forward that targeting mature OLs is an untapped therapeutic avenue to support CNS health in the context of neurological disorders. This holds promise not only in the treatment of neurological disorders typically considered to be those of the white matter like MS, but also other neurodegenerative conditions where OL and myelin pathology have been described, such as Alzheimer’s disease, ALS, Huntington’s Disease, and Schizophrenia27–29,197,198. Furthering our understanding of the mechanisms driving sublethal and lethal injury of OLs is therefore a critical priority in expanding effective therapeutic options for a broad spectrum of neurological disorders across the lifespan.
Acknowledgements
I.M.-G. and V.E.M. are supported by a Medical Research Council Senior Non-Clinical Fellowship (to V.E.M.) and the United Kingdom Dementia Research Institute at The University of Edinburgh in partnership with Astex Pharmaceuticals. V.E.M. is supported by the John David Eaton Chair in Multiple Sclerosis Research at the Barlo Multiple Sclerosis Centre. J.A. received support from the Progressive MS Alliance MSIF. We thank Tanja Kuhlmann for critical feedback. Diagrams were created using BioRender.com.
Author contributions
I.M.-G., V.E.M., and J.P.A. co-wrote the manuscript. I.M.-G. made the figures.
Peer review
Peer review information
Communications Biology thanks Jason Plemel, Marta Fumagalli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Eliana Scemes, Karli Montague-Cardoso and Christina Karlsson Rosenthal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Funfschilling U, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saab AS, Tzvetanova ID, Nave KA. The role of myelin and oligodendrocytes in axonal energy metabolism. Curr. Opin. Neurobiol. 2013;23:1065–1072. doi: 10.1016/j.conb.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Young KM, et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 2018;21:696–706. doi: 10.1038/s41593-018-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill RA, Li AM, Grutzendler J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 2018;21:683–695. doi: 10.1038/s41593-018-0120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeung MS, et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Hess K, et al. Lesion stage-dependent causes for impaired remyelination in MS. Acta Neuropathol. 2020;140:359–375. doi: 10.1007/s00401-020-02189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prineas JW, Parratt JD. Oligodendrocytes and the early multiple sclerosis lesion. Ann. Neurol. 2012;72:18–31. doi: 10.1002/ana.23634. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez M, Scheithauer BW, Forbes G, Kelly PJ. Oligodendrocyte injury is an early event in lesions of multiple sclerosis. Mayo Clin. Proc. 1993;68:627–636. doi: 10.1016/S0025-6196(12)60597-7. [DOI] [PubMed] [Google Scholar]
- 11.Ryu JK, et al. Fibrin-targeting immunotherapy protects against neuroinflammation and neurodegeneration. Nat. Immunol. 2018;19:1212–1223. doi: 10.1038/s41590-018-0232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothhammer V, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557:724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheeler MA, et al. MAFG-driven astrocytes promote CNS inflammation. Nature. 2020;578:593–599. doi: 10.1038/s41586-020-1999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler MA, Rothhammer V, Quintana FJ. Control of immune-mediated pathology via the aryl hydrocarbon receptor. J. Biol. Chem. 2017;292:12383–12389. doi: 10.1074/jbc.R116.767723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lampron A, et al. Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J. Exp. Med. 2015;212:481–495. doi: 10.1084/jem.20141656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 2006;26:328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Healy LM, et al. MerTK is a functional regulator of myelin phagocytosis by human myeloid cells. J. Immunol. 2016;196:3375–3384. doi: 10.4049/jimmunol.1502562. [DOI] [PubMed] [Google Scholar]
- 19.Cui QL, et al. Sublethal oligodendrocyte injury: a reversible condition in multiple sclerosis? Ann. Neurol. 2017;81:811–824. doi: 10.1002/ana.24944. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann A, et al. Oligodendroglial alpha-synucleinopathy-driven neuroinflammation in multiple system atrophy. Brain Pathol. 2019;29:380–396. doi: 10.1111/bpa.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meissner WG, et al. Multiple system atrophy: recent developments and future perspectives. Mov. Disord. 2019;34:1629–1642. doi: 10.1002/mds.27894. [DOI] [PubMed] [Google Scholar]
- 22.Bugiani M, Vuong C, Breur M, van der Knaap MS. Vanishing white matter: a leukodystrophy due to astrocytic dysfunction. Brain Pathol. 2018;28:408–421. doi: 10.1111/bpa.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azevedo C, et al. Parkinson’s disease and multiple system atrophy patient iPSC-derived oligodendrocytes exhibit alpha-synuclein-induced changes in maturation and immune reactive properties. Proc. Natl Acad. Sci. USA. 2022;119:e2111405119. doi: 10.1073/pnas.2111405119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasrabady SE, Rizvi B, Goldman JE, Brickman AM. White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 2018;6:22. doi: 10.1186/s40478-018-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eluvathingal TJ, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- 26.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7:272–281. doi: 10.1016/S2215-0366(19)30302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raabe, F. J. et al. Oligodendrocytes as a new therapeutic target in schizophrenia: from histopathological findings to neuron-oligodendrocyte interaction. Cells8, 10.3390/cells8121496 (2019). [DOI] [PMC free article] [PubMed]
- 29.Galvez-Contreras, A. Y., Zarate-Lopez, D., Torres-Chavez, A. L. & Gonzalez-Perez, O. Role of oligodendrocytes and myelin in the pathophysiology of autism spectrum disorder. Brain Sci.10, 10.3390/brainsci10120951 (2020). [DOI] [PMC free article] [PubMed]
- 30.van Tilborg E, et al. Impaired oligodendrocyte maturation in preterm infants: Potential therapeutic targets. Prog. Neurobiol. 2016;136:28–49. doi: 10.1016/j.pneurobio.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Cui QL, et al. Oligodendrocyte progenitor cell susceptibility to injury in multiple sclerosis. Am. J. Pathol. 2013;183:516–525. doi: 10.1016/j.ajpath.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 35.Lucchinetti CF, Bruck W, Lassmann H. Evidence for pathogenic heterogeneity in multiple sclerosis. Ann. Neurol. 2004;56:308. doi: 10.1002/ana.20182. [DOI] [PubMed] [Google Scholar]
- 36.Casaccia P, Boddeke E. Foreword. Glia. 2020;68:1551–1553. doi: 10.1002/glia.23841. [DOI] [PubMed] [Google Scholar]
- 37.Saikali P, et al. NKG2D-mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis. J. Neurosci. 2007;27:1220–1228. doi: 10.1523/JNEUROSCI.4402-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaguia F, et al. Cytotoxic NKG2C+ CD4 T cells target oligodendrocytes in multiple sclerosis. J. Immunol. 2013;190:2510–2518. doi: 10.4049/jimmunol.1202725. [DOI] [PubMed] [Google Scholar]
- 39.Larochelle, C. et al. Pro-inflammatory T helper 17 directly harms oligodendrocytes in neuroinflammation. Proc. Natl Acad. Sci. USA118, 10.1073/pnas.2025813118 (2021). This study demonstrated that direct contact between Th17 T lymphocytes and oligodendrocytes induces oligodendrocyte stress, reduces myelinaton, and increases cell death. [DOI] [PMC free article] [PubMed]
- 40.Jamann, H. et al. Contact-dependent granzyme B-mediated cytotoxicity of Th17-polarized cells toward human oligodendrocytes. Front. Immunol.13, 10.3389/fimmu.2022.850616 (2022). [DOI] [PMC free article] [PubMed]
- 41.Ludwin SK, Johnson ES. Evidence for a “dying-back” gliopathy in demyelinating disease. Ann. Neurol. 1981;9:301–305. doi: 10.1002/ana.410090316. [DOI] [PubMed] [Google Scholar]
- 42.Pernin F, et al. Diverse injury responses of human oligodendrocyte to mediators implicated in multiple sclerosis. Brain. 2022 doi: 10.1093/brain/awac075. [DOI] [PubMed] [Google Scholar]
- 43.Kast DJ, Dominguez R. The Cytoskeleton-Autophagy Connection. Curr. Biol. 2017;27:R318–R326. doi: 10.1016/j.cub.2017.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, et al. Raf-1/CK2 and RhoA/ROCK signaling promote TNF-alpha-mediated endothelial apoptosis via regulating vimentin cytoskeleton. Toxicology. 2017;389:74–84. doi: 10.1016/j.tox.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Wosik K, et al. Oligodendrocyte injury in multiple sclerosis: a role for p53. J. Neurochem. 2003;85:635–644. doi: 10.1046/j.1471-4159.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- 46.Nicaise AM, et al. Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc. Natl Acad. Sci. USA. 2019;116:9030–9039. doi: 10.1073/pnas.1818348116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Absinta M, et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature. 2021;597:709–714. doi: 10.1038/s41586-021-03892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prineas JW, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann. Neurol. 2001;50:646–657. doi: 10.1002/ana.1255. [DOI] [PubMed] [Google Scholar]
- 49.Dhuriya YK, Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J. Neuroinflammation. 2018;15:199. doi: 10.1186/s12974-018-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ofengeim D, et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015;10:1836–1849. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lloyd AF, et al. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci. 2019;22:1046–1052. doi: 10.1038/s41593-019-0418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang S, et al. RIP1 kinase inhibitor halts the progression of an immune-induced demyelination disease at the stage of monocyte elevation. Proc. Natl Acad. Sci. USA. 2019;116:5675–5680. doi: 10.1073/pnas.1819917116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zelic M, et al. RIPK1 activation mediates neuroinflammation and disease progression in multiple sclerosis. Cell Rep. 2021;35:109112. doi: 10.1016/j.celrep.2021.109112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jurewicz A, et al. Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain. 2005;128:2675–2688. doi: 10.1093/brain/awh627. [DOI] [PubMed] [Google Scholar]
- 55.McKenzie BA, et al. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc. Natl Acad. Sci. USA. 2018;115:E6065–E6074. doi: 10.1073/pnas.1722041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou J, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020;22:1264–1275. doi: 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 58.Lassmann H. Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J. Neurol. Sci. 2003;206:187–191. doi: 10.1016/S0022-510X(02)00421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lassmann H, van Horssen J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim Biophys. Acta. 2016;1862:506–510. doi: 10.1016/j.bbadis.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 60.Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–291. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- 61.D’Haeseleer M, et al. Cerebral hypoperfusion: a new pathophysiologic concept in multiple sclerosis. J. Cereb. Blood Flow. Metab. 2015;35:1406–1410. doi: 10.1038/jcbfm.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Witte ME, Mahad DJ, Lassmann H, van Horssen J. Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol. Med. 2014;20:179–187. doi: 10.1016/j.molmed.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Davies AL, et al. Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann. Neurol. 2013;74:815–825. doi: 10.1002/ana.24006. [DOI] [PubMed] [Google Scholar]
- 64.Desai RA, et al. Cause and prevention of demyelination in a model multiple sclerosis lesion. Ann. Neurol. 2016;79:591–604. doi: 10.1002/ana.24607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernandes MGF, et al. Age-related injury responses of human oligodendrocytes to metabolic insults: link to BCL-2 and autophagy pathways. Commun. Biol. 2021;4:20. doi: 10.1038/s42003-020-01557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y, et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl Acad. Sci. USA. 2013;110:20364–20371. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 68.Bonapace L, et al. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J. Clin. Invest. 2010;120:1310–1323. doi: 10.1172/JCI39987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forte M, et al. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl Acad. Sci. USA. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jhelum P, et al. Ferroptosis mediates cuprizone-induced loss of oligodendrocytes and demyelination. J. Neurosci. 2020;40:9327–9341. doi: 10.1523/JNEUROSCI.1749-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jakel S, et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature. 2019;566:543–547. doi: 10.1038/s41586-019-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Falcao AM, et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 2018;24:1837–1844. doi: 10.1038/s41591-018-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seeker, L. A. et al. Marked regional glial heterogeneity in the human white matter of the central nervous system. Preprint at bioRxiv10.1101/2022.03.22.485367 (2022).
- 74.Bechler ME, Byrne L, Ffrench-Constant C. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol. 2015;25:2411–2416. doi: 10.1016/j.cub.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vigano F, Mobius W, Gotz M, Dimou L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat. Neurosci. 2013;16:1370–1372. doi: 10.1038/nn.3503. [DOI] [PubMed] [Google Scholar]
- 76.Bai CB, et al. A mouse model for testing remyelinating therapies. Exp. Neurol. 2016;283:330–340. doi: 10.1016/j.expneurol.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gudi V, et al. Regional differences between grey and white matter in cuprizone induced demyelination. Brain Res. 2009;1283:127–138. doi: 10.1016/j.brainres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 78.Strijbis EMM, Kooi EJ, van der Valk P, Geurts JJG. Cortical remyelination is heterogeneous in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2017;76:390–401. doi: 10.1093/jnen/nlx023. [DOI] [PubMed] [Google Scholar]
- 79.Chang A, et al. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann. Neurol. 2012;72:918–926. doi: 10.1002/ana.23693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albert M, Antel J, Bruck W, Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17:129–138. doi: 10.1111/j.1750-3639.2006.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szuchet S, et al. The genetic signature of perineuronal oligodendrocytes reveals their unique phenotype. Eur. J. Neurosci. 2011;34:1906–1922. doi: 10.1111/j.1460-9568.2011.07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mathys H, et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570:332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sadick JS, et al. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer’s disease. Neuron. 2022 doi: 10.1016/j.neuron.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Macnair, W. et al. Single nuclei RNAseq stratifies multiple sclerosis patients into three distinct white matter glia responses. bioRxiv10.1101/2022.04.06.487263 (2022).
- 85.Ladiwala U, Li H, Antel JP, Nalbantoglu J. p53 induction by tumor necrosis factor-alpha and involvement of p53 in cell death of human oligodendrocytes. J. Neurochem. 1999;73:605–611. doi: 10.1046/j.1471-4159.1999.0730605.x. [DOI] [PubMed] [Google Scholar]
- 86.Williamson JM, Lyons DA. Myelin dynamics throughout life: an ever-changing landscape. Front. Cell Neurosci. 2018;12:424. doi: 10.3389/fncel.2018.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luo JXX, et al. Human oligodendrocyte myelination potential; relation to age and differentiation. Ann. Neurol. 2022;91:178–191. doi: 10.1002/ana.26288. [DOI] [PubMed] [Google Scholar]
- 88.Wang F, et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 2020;23:481–486. doi: 10.1038/s41593-020-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen JF, et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron. 2021;109:2292–2307.e2295. doi: 10.1016/j.neuron.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie F, Liang P, Fu H, Zhang JC, Chen J. Effects of normal aging on myelin sheath ultrastructures in the somatic sensorimotor system of rats. Mol. Med. Rep. 2014;10:459–466. doi: 10.3892/mmr.2014.2228. [DOI] [PubMed] [Google Scholar]
- 91.Peters A, Sethares C, Killiany RJ. Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J. Comp. Neurol. 2001;435:241–248. doi: 10.1002/cne.1205. [DOI] [PubMed] [Google Scholar]
- 92.Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J. Comp. Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- 93.Call, C. L. et al. Oligodendrocytes form paranodal bridges that generate chains of myelin sheaths that are vulnerable to degeneration with age. Preprint at bioRxiv10.1101/2022.02.16.480718 (2022). This study revealed anovel mechanism of myelination of distal axons by oligodendrocytes, by creatingbridges between myelin internodes; these are particularly vulnerable todegeneration with ageing relative to internodes stemming from theoligodendrocyte cell body.
- 94.Neumann B, Segel M, Chalut KJ, Franklin RJ. Remyelination and ageing: reversing the ravages of time. Mult. Scler. 2019;25:1835–1841. doi: 10.1177/1352458519884006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen S, et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ximerakis M, et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat. Neurosci. 2019;22:1696–1708. doi: 10.1038/s41593-019-0491-3. [DOI] [PubMed] [Google Scholar]
- 97.Dulken BW, et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature. 2019;571:205–210. doi: 10.1038/s41586-019-1362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yaqubi M, et al. Regional and age-related diversity of human mature oligodendrocytes. Glia. 2022;70:1938–1949. doi: 10.1002/glia.24230. [DOI] [PubMed] [Google Scholar]
- 99.Kirby L, et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat. Commun. 2019;10:3887. doi: 10.1038/s41467-019-11638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rone MB, et al. Oligodendrogliopathy in multiple sclerosis: low glycolytic metabolic rate promotes oligodendrocyte survival. J. Neurosci. 2016;36:4698–4707. doi: 10.1523/JNEUROSCI.4077-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neumann B, et al. Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell. 2019;25:473–485 e478. doi: 10.1016/j.stem.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saher G, et al. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005;8:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- 103.Voskuhl RR, et al. Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proc. Natl Acad. Sci. USA. 2019;116:10130–10139. doi: 10.1073/pnas.1821306116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dimas, P. et al. CNS myelination and remyelination depend on fatty acid synthesis by oligodendrocytes. Elife8, 10.7554/eLife.44702 (2019). [DOI] [PMC free article] [PubMed]
- 105.Camargo N, et al. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017;15:e1002605. doi: 10.1371/journal.pbio.1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Camargo N, et al. High-fat diet ameliorates neurological deficits caused by defective astrocyte lipid metabolism. FASEB J. 2012;26:4302–4315. doi: 10.1096/fj.12-205807. [DOI] [PubMed] [Google Scholar]
- 107.Montani L. Lipids in regulating oligodendrocyte structure and function. Semin Cell Dev. Biol. 2021;112:114–122. doi: 10.1016/j.semcdb.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 108.Decker L, Baron W, Ffrench-Constant C. Lipid rafts: microenvironments for integrin-growth factor interactions in neural development. Biochem Soc. Trans. 2004;32:426–430. doi: 10.1042/bst0320426. [DOI] [PubMed] [Google Scholar]
- 109.Hubler Z, et al. Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination. Nature. 2018;560:372–376. doi: 10.1038/s41586-018-0360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Berghoff SA, et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat. Neurosci. 2021;24:47–60. doi: 10.1038/s41593-020-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martin, E. et al. Teriflunomide promotes oligodendroglial 8,9-unsaturated sterol accumulation and CNS remyelination. Neurol. Neuroimmunol. Neuroinflamm.8, 10.1212/NXI.0000000000001091 (2021). [DOI] [PMC free article] [PubMed]
- 112.Hubler Z, et al. Modulation of lanosterol synthase drives 24,25-epoxysterol synthesis and oligodendrocyte formation. Cell Chem. Biol. 2021;28:866–875.e865. doi: 10.1016/j.chembiol.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sax JL, Hubler Z, Allimuthu D, Adams DJ. Screening reveals sterol derivatives with pro-differentiation, pro-survival, or potent cytotoxic effects on oligodendrocyte progenitor cells. ACS Chem. Biol. 2021;16:1288–1297. doi: 10.1021/acschembio.1c00461. [DOI] [PubMed] [Google Scholar]
- 114.Lombardi M, et al. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol. 2019;138:987–1012. doi: 10.1007/s00401-019-02049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Penkert H, et al. Proteomic and lipidomic profiling of demyelinating lesions identifies fatty acids as modulators in lesion recovery. Cell Rep. 2021;37:109898. doi: 10.1016/j.celrep.2021.109898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guttenplan KA, et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature. 2021;599:102–107. doi: 10.1038/s41586-021-03960-y. [DOI] [PubMed] [Google Scholar]
- 117.Badreddine, A. et al. Argan oil-mediated attenuation of organelle dysfunction, oxidative stress and cell death induced by 7-ketocholesterol in murine oligodendrocytes 158N. Int. J. Mol. Sci.18, 10.3390/ijms18102220 (2017). [DOI] [PMC free article] [PubMed]
- 118.Dong Y, et al. Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat. Neurosci. 2021;24:489–503. doi: 10.1038/s41593-021-00801-z. [DOI] [PubMed] [Google Scholar]
- 119.Itoh N, et al. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: focus on astrocytes. Proc. Natl Acad. Sci. USA. 2018;115:E302–E309. doi: 10.1073/pnas.1716032115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marcos J, Shackleton CH, Buddhikot MM, Porter FD, Watson GL. Cholesterol biosynthesis from birth to adulthood in a mouse model for 7-dehydrosterol reductase deficiency (Smith-Lemli-Opitz syndrome) Steroids. 2007;72:802–808. doi: 10.1016/j.steroids.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dang Do AN, Baker EH, Warren KE, Bianconi SE, Porter FD. Spontaneously regressing brain lesions in Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. A. 2018;176:386–390. doi: 10.1002/ajmg.a.38563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stumpf SK, et al. Ketogenic diet ameliorates axonal defects and promotes myelination in Pelizaeus-Merzbacher disease. Acta Neuropathol. 2019;138:147–161. doi: 10.1007/s00401-019-01985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Osorio MJ, Goldman SA. Neurogenetics of Pelizaeus-Merzbacher disease. Handb. Clin. Neurol. 2018;148:701–722. doi: 10.1016/B978-0-444-64076-5.00045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simons M, et al. Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: implications for Pelizaeus-Merzbacher disease. J. Cell Biol. 2002;157:327–336. doi: 10.1083/jcb.200110138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Laukka JJ, Kamholz J, Bessert D, Skoff RP. Novel pathologic findings in patients with Pelizaeus-Merzbacher disease. Neurosci. Lett. 2016;627:222–232. doi: 10.1016/j.neulet.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Davies-Thompson J, Vavasour I, Scheel M, Rauscher A, Barton JJ. Reduced myelin water in the white matter tracts of patients with niemann-pick disease type C. Am. J. Neuroradiol. 2016;37:1487–1489. doi: 10.3174/ajnr.A4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang F, et al. Proteomics of the corpus callosum to identify novel factors involved in hypomyelinated Niemann-Pick Type C disease mice. Mol. Brain. 2019;12:17. doi: 10.1186/s13041-019-0440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vanier MT. Niemann-Pick disease type C. Orphanet J. Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang CC, et al. Multi-parametric neuroimaging evaluation of cerebrotendinous xanthomatosis and its correlation with neuropsychological presentations. BMC Neurol. 2010;10:59. doi: 10.1186/1471-2377-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tao QQ, et al. Clinical and genetic characteristics of Chinese patients with cerebrotendinous xanthomatosis. Orphanet J. Rare Dis. 2019;14:282. doi: 10.1186/s13023-019-1252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davis DL, et al. Dynamics of sphingolipids and the serine palmitoyltransferase complex in rat oligodendrocytes during myelination. J. Lipid Res. 2020;61:505–522. doi: 10.1194/jlr.RA120000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J, et al. Myelin lipid abnormalities in the aspartoacylase-deficient tremor rat. Neurochem Res. 2009;34:138–148. doi: 10.1007/s11064-008-9726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pant DC, et al. Loss of the sphingolipid desaturase DEGS1 causes hypomyelinating leukodystrophy. J. Clin. Invest. 2019;129:1240–1256. doi: 10.1172/JCI123959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bieberich E. There is more to a lipid than just being a fat: sphingolipid-guided differentiation of oligodendroglial lineage from embryonic stem cells. Neurochem Res. 2011;36:1601–1611. doi: 10.1007/s11064-010-0338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marin-Valencia I, Roe CR, Pascual JM. Pyruvate carboxylase deficiency: mechanisms, mimics and anaplerosis. Mol. Genet. Metab. 2010;101:9–17. doi: 10.1016/j.ymgme.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 136.Plemel JR, Liu WQ, Yong VW. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat. Rev. Drug Discov. 2017;16:617–634. doi: 10.1038/nrd.2017.115. [DOI] [PubMed] [Google Scholar]
- 137.Ghorbani S, Yong VW. The extracellular matrix as modifier of neuroinflammation and remyelination in multiple sclerosis. Brain. 2021;144:1958–1973. doi: 10.1093/brain/awab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lloyd AF, Miron VE. The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol. 2019;15:447–458. doi: 10.1038/s41582-019-0184-2. [DOI] [PubMed] [Google Scholar]
- 139.Miron VE. Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination. J. Leukoc. Biol. 2017;101:1103–1108. doi: 10.1189/jlb.3RI1116-494R. [DOI] [PubMed] [Google Scholar]
- 140.Zhou, L. et al. Gab1 mediates PDGF signaling and is essential to oligodendrocyte differentiation and CNS myelination. Elife9, 10.7554/eLife.52056 (2020). [DOI] [PMC free article] [PubMed]
- 141.Watzlawik JO, Warrington AE, Rodriguez M. PDGF is required for remyelination-promoting IgM stimulation of oligodendrocyte progenitor cell proliferation. PLoS ONE. 2013;8:e55149. doi: 10.1371/journal.pone.0055149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Medved J, et al. Novel guanidine compounds inhibit platelet-derived growth factor receptor alpha transcription and oligodendrocyte precursor cell proliferation. Glia. 2021;69:792–811. doi: 10.1002/glia.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yao ZF, et al. Transplantation of PDGF-AA-overexpressing oligodendrocyte precursor cells promotes recovery in rat following spinal cord injury. Front. Cell Neurosci. 2017;11:79. doi: 10.3389/fncel.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sherafat A, Pfeiffer F, Reiss AM, Wood WM, Nishiyama A. Microglial neuropilin-1 promotes oligodendrocyte expansion during development and remyelination by trans-activating platelet-derived growth factor receptor. Nat. Commun. 2021;12:2265. doi: 10.1038/s41467-021-22532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Younsi A, et al. Three growth factors induce proliferation and differentiation of neural precursor cells in vitro and support cell-transplantation after spinal cord injury in vivo. Stem Cells Int. 2020;2020:5674921. doi: 10.1155/2020/5674921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cardona HJ, Somasundaram A, Crabtree DM, Gadd SL, Becher OJ. Prenatal overexpression of platelet-derived growth factor receptor A results in central nervous system hypomyelination. Brain Behav. 2021;11:e2332. doi: 10.1002/brb3.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Furusho M, Dupree JL, Nave KA, Bansal R. Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J. Neurosci. 2012;32:6631–6641. doi: 10.1523/JNEUROSCI.6005-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Furusho M, Ishii A, Bansal R. Signaling by FGF receptor 2, not FGF receptor 1, regulates myelin thickness through activation of ERK1/2-MAPK, which promotes mTORC1 activity in an Akt-independent manner. J. Neurosci. 2017;37:2931–2946. doi: 10.1523/JNEUROSCI.3316-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rajendran R, Giraldo-Velasquez M, Stadelmann C, Berghoff M. Oligodendroglial fibroblast growth factor receptor 1 gene targeting protects mice from experimental autoimmune encephalomyelitis through ERK/AKT phosphorylation. Brain Pathol. 2018;28:212–224. doi: 10.1111/bpa.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thummler K, et al. Polarizing receptor activation dissociates fibroblast growth factor 2 mediated inhibition of myelination from its neuroprotective potential. Acta Neuropathol. Commun. 2019;7:212. doi: 10.1186/s40478-019-0864-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fortin D, Rom E, Sun H, Yayon A, Bansal R. Distinct fibroblast growth factor (FGF)/FGF receptor signaling pairs initiate diverse cellular responses in the oligodendrocyte lineage. J. Neurosci. 2005;25:7470–7479. doi: 10.1523/JNEUROSCI.2120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Butt AM, Dinsdale J. Fibroblast growth factor 2 induces loss of adult oligodendrocytes and myelin in vivo. Exp. Neurol. 2005;192:125–133. doi: 10.1016/j.expneurol.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 153.de Jong, J. M., Wang, P., Oomkens, M. & Baron, W. Remodeling of the interstitial extracellular matrix in white matter multiple sclerosis lesions: Implications for remyelination failure. J. Neurosci. Res. 98, 1370–1397 (2020). [DOI] [PubMed]
- 154.Malekzadeh A, et al. Plasma proteome in multiple sclerosis disease progression. Ann. Clin. Transl. Neurol. 2019;6:1582–1594. doi: 10.1002/acn3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol. Cell Neurosci. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- 156.Colognato H, et al. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat. Cell Biol. 2002;4:833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- 157.Laursen LS, Chan CW, Ffrench-Constant C. Translation of myelin basic protein mRNA in oligodendrocytes is regulated by integrin activation and hnRNP-K. J. Cell Biol. 2011;192:797–811. doi: 10.1083/jcb.201007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Camara J, et al. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J. Cell Biol. 2009;185:699–712. doi: 10.1083/jcb.200807010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Decker L, ffrench-Constant C. Lipid rafts and integrin activation regulate oligodendrocyte survival. J. Neurosci. 2004;24:3816–3825. doi: 10.1523/JNEUROSCI.5725-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Relvas JB, et al. Expression of dominant-negative and chimeric subunits reveals an essential role for beta1 integrin during myelination. Curr. Biol. 2001;11:1039–1043. doi: 10.1016/S0960-9822(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 161.Stoffels JM, et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain. 2013;136:116–131. doi: 10.1093/brain/aws313. [DOI] [PubMed] [Google Scholar]
- 162.Back SA, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 163.Bugiani M, et al. Hyaluronan accumulation and arrested oligodendrocyte progenitor maturation in vanishing white matter disease. Brain. 2013;136:209–222. doi: 10.1093/brain/aws320. [DOI] [PubMed] [Google Scholar]
- 164.Sloane JA, et al. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc. Natl Acad. Sci. USA. 2010;107:11555–11560. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Q, et al. High level of serum and cerebrospinal fluid of heparan sulfate and hyaluronic acid might be a biomarker of severity of neuromyelitis optica. Front. Immunol. 2021;12:705536. doi: 10.3389/fimmu.2021.705536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jagielska A, et al. Mechanical strain promotes oligodendrocyte differentiation by global changes of gene expression. Front. Cell Neurosci. 2017;11:93. doi: 10.3389/fncel.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Urbanski MM, Brendel MB, Melendez-Vasquez CV. Acute and chronic demyelinated CNS lesions exhibit opposite elastic properties. Sci. Rep. 2019;9:999. doi: 10.1038/s41598-018-37745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mozafari, S. et al. Multiple sclerosis iPS-derived oligodendroglia conserve their properties to functionally interact with axons and glia in vivo. Sci. Adv.6, 10.1126/sciadv.abc6983 (2020). [DOI] [PMC free article] [PubMed]
- 169.Starost L, et al. Extrinsic immune cell-derived, but not intrinsic oligodendroglial factors contribute to oligodendroglial differentiation block in multiple sclerosis. Acta Neuropathol. 2020;140:715–736. doi: 10.1007/s00401-020-02217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yamasaki R, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 172.Marzan DE, et al. Activated microglia drive demyelination via CSF1R signaling. Glia. 2021;69:1583–1604. doi: 10.1002/glia.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Clark, I. C. et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science372, 10.1126/science.abf1230 (2021). [DOI] [PMC free article] [PubMed]
- 174.Hughes AN, Appel B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 2020;23:1055–1066. doi: 10.1038/s41593-020-0654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li J, et al. Tumor necrosis factor alpha mediates lipopolysaccharide-induced microglial toxicity to developing oligodendrocytes when astrocytes are present. J. Neurosci. 2008;28:5321–5330. doi: 10.1523/JNEUROSCI.3995-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Argaw AT, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim RY, et al. Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2014;274:53–61. doi: 10.1016/j.jneuroim.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Meares GP, Ma X, Qin H, Benveniste EN. Regulation of CCL20 expression in astrocytes by IL-6 and IL-17. Glia. 2012;60:771–781. doi: 10.1002/glia.22307. [DOI] [PubMed] [Google Scholar]
- 179.Nitsch L, et al. Astrocyte-specific expression of interleukin 23 leads to an aggravated phenotype and enhanced inflammatory response with B cell accumulation in the EAE model. J. Neuroinflammation. 2021;18:101. doi: 10.1186/s12974-021-02140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hridi, S. U. et al. Increased levels of IL-16 in the central nervous system during neuroinflammation are associated with infiltrating immune cells and resident glial cells. Biology10, 10.3390/biology10060472 (2021). [DOI] [PMC free article] [PubMed]
- 181.Moore CS, et al. Direct and indirect effects of immune and central nervous system-resident cells on human oligodendrocyte progenitor cell differentiation. J. Immunol. 2015;194:761–772. doi: 10.4049/jimmunol.1401156. [DOI] [PubMed] [Google Scholar]
- 182.Sanmarco LM, et al. Gut-licensed IFNgamma(+) NK cells drive LAMP1(+)TRAIL(+) anti-inflammatory astrocytes. Nature. 2021;590:473–479. doi: 10.1038/s41586-020-03116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vanderlocht J, et al. Leukemia inhibitory factor is produced by myelin-reactive T cells from multiple sclerosis patients and protects against tumor necrosis factor-alpha-induced oligodendrocyte apoptosis. J. Neurosci. Res. 2006;83:763–774. doi: 10.1002/jnr.20781. [DOI] [PubMed] [Google Scholar]
- 184.Molina-Gonzalez I, Miron VE. Astrocytes in myelination and remyelination. Neurosci. Lett. 2019;713:134532. doi: 10.1016/j.neulet.2019.134532. [DOI] [PubMed] [Google Scholar]
- 185.Dillenburg A, et al. Activin receptors regulate the oligodendrocyte lineage in health and disease. Acta Neuropathol. 2018;135:887–906. doi: 10.1007/s00401-018-1813-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lubetzki C, Zalc B, Williams A, Stadelmann C, Stankoff B. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 2020;19:678–688. doi: 10.1016/S1474-4422(20)30140-X. [DOI] [PubMed] [Google Scholar]
- 187.Stangel M, Kuhlmann T, Matthews PM, Kilpatrick TJ. Achievements and obstacles of remyelinating therapies in multiple sclerosis. Nat. Rev. Neurol. 2017;13:742–754. doi: 10.1038/nrneurol.2017.139. [DOI] [PubMed] [Google Scholar]
- 188.Duncan ID, et al. The adult oligodendrocyte can participate in remyelination. Proc. Natl Acad. Sci. USA. 2018;115:E11807–E11816. doi: 10.1073/pnas.1808064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bacmeister CM, et al. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat. Neurosci. 2020;23:819–831. doi: 10.1038/s41593-020-0637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Neely SA, et al. New oligodendrocytes exhibit more abundant and accurate myelin regeneration than those that survive demyelination. Nat. Neurosci. 2022;25:415–420. doi: 10.1038/s41593-021-01009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yeung MSY, et al. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature. 2019;566:538–542. doi: 10.1038/s41586-018-0842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shen K, et al. Multiple sclerosis risk gene Mertk is required for microglial activation and subsequent remyelination. Cell Rep. 2021;34:108835. doi: 10.1016/j.celrep.2021.108835. [DOI] [PubMed] [Google Scholar]
- 193.Zhou Y, et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 2020;26:131–142. doi: 10.1038/s41591-019-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kirby L, Castelo-Branco G. Crossing boundaries: Interplay between the immune system and oligodendrocyte lineage cells. Semin Cell Dev. Biol. 2021;116:45–52. doi: 10.1016/j.semcdb.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 195.Liu F, Vidarsson L, Winter JD, Tran H, Kassner A. Sex differences in the human corpus callosum microstructure: a combined T2 myelin-water and diffusion tensor magnetic resonance imaging study. Brain Res. 2010;1343:37–45. doi: 10.1016/j.brainres.2010.04.064. [DOI] [PubMed] [Google Scholar]
- 196.Darling JS, Daniel JM. Pubertal hormones mediate sex differences in levels of myelin basic protein in the orbitofrontal cortex of adult rats. Neuroscience. 2019;406:487–495. doi: 10.1016/j.neuroscience.2019.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Raffaele, S., Boccazzi, M. & Fumagalli, M. Oligodendrocyte dysfunction in amyotrophic lateral sclerosis: mechanisms and therapeutic perspectives. Cells10, 10.3390/cells10030565 (2021). [DOI] [PMC free article] [PubMed]
- 198.Ferrari Bardile C, et al. Intrinsic mutant HTT-mediated defects in oligodendroglia cause myelination deficits and behavioral abnormalities in Huntington disease. Proc. Natl Acad. Sci. USA. 2019;116:9622–9627. doi: 10.1073/pnas.1818042116. [DOI] [PMC free article] [PubMed] [Google Scholar]