Abstract
Background.
Population-based short-term air pollution health studies often have limited spatiotemporally representative exposure data, leading to concerns of exposure measurement error.
Objective.
To compare the use of monitoring and modeled exposure metrics in time-series analyses of air pollution and cardiorespiratory emergency department (ED) visits.
Methods.
We obtained daily counts of ED visits for Atlanta, GA during 2009–2013. We leveraged daily ZIP code level concentration estimates for eight pollutants from nine exposure metrics. Metrics included central monitor (CM), monitor-based (inverse distance weighting, kriging), model-based [community multiscale air quality (CMAQ), land use regression (LUR)], and satellite-based measures. We used Poisson models to estimate air pollution health associations using the different exposure metrics. The approach involved: (1) assessing CM-based associations, (2) determining if non-CM metrics can reproduce CM-based associations, and (3) identifying potential value added of incorporating full spatiotemporal information provided by non-CM metrics.
Results.
Using CM exposures, we observed associations between cardiovascular ED visits and carbon monoxide, nitrogen dioxide, fine particulate matter, elemental and organic carbon, and between respiratory ED visits and ozone. Non-CM metrics were largely able to reproduce CM-based associations, although some unexpected results using CMAQ- and LUR-based metrics reduced confidence in these data for some spatiotemporally-variable pollutants. Associations with nitrogen dioxide and sulfur dioxide were only detected, or were stronger, when using metrics that incorporate all available monitoring data (i.e., inverse distance weighting and kriging).
Significance.
The use of routinely-collected ambient monitoring data for exposure assignment in time-series studies of large metropolitan areas is a sound approach, particularly when data from multiple monitors are available. More sophisticated approaches derived from CMAQ, LUR, or satellites may add value when monitoring data are inadequate and if paired with thorough data characterization. These results are useful for interpretation of existing literature and for improving exposure assessment in future studies.
Keywords: Air pollution, criteria pollutants, epidemiology, exposure modeling, particulate matter, population-based studies
INTRODUCTION
Substantial evidence supports short-term associations of ambient air pollution and health, including cardiovascular and respiratory illnesses (1, 2). Contributing to this body of evidence are population-based studies that relate day-to-day changes in ambient concentrations to indicators of population health for a community, such as daily counts of deaths or hospitalizations. These studies have had considerable weight in setting of National Ambient Air Quality Standards (NAAQS) in the US (3). Ideally, communities represented in these studies are reflective of all populations of concern; this includes populations of different geographic extents ranging from those living in specific neighborhoods within a city, to entire metropolitan statistical areas, and rural regions.
A common challenge in short-term health effect studies is the limited availability of spatially representative air pollution exposure data for the study population. The most commonly available data are compliance monitoring data routinely-collected by state and federal agencies. Monitoring data provide highly accurate time series of pollutant concentrations at the monitoring site, and thus make these data highly relevant for temporal studies. However, these measurements are inherently limited in that they may not represent concentrations across space. Their ability to reflect population-level exposure for a given study area depends on the density (numbers and placement) of the monitors and pollutant variability over space. For pollutants that vary little over space [e.g., ozone (O3), a secondary pollutant, or fine particulate matter (PM2.5), which has predominant contributions from secondary particle components, such as sulfate and nitrate] (4), limited monitors may represent concentrations across a large study area reasonably well. For pollutants that vary substantially over space such as primary pollutants emitted directly from mobile or stationary sources [e.g., nitrogen dioxide (NO2) or sulfur dioxide (SO2)] (4), limited measurement locations are unlikely to accurately represent concentrations across a large study area over which health outcomes are aggregated.
For studies relying on air monitoring alone, the exposure and health outcome data are often misaligned in space, leading to concerns of exposure measurement error (5). Spatial misalignment can contribute to exposure measurement error that biases observed health effect associations to different degrees for different pollutants (5–7). Simulation studies in the Atlanta metropolitan area have estimated that error due to uncaptured pollutant spatial variability leads to 43–68% reductions in observed health effect estimates for primary pollutants (e.g., NO2, SO2) compared to only 16% for secondary pollutants (e.g., O3) when assigning exposures based on central monitors (6). Exposure measurement error due to spatial misalignment is likely to be non-constant across days; for example, the higher spatial contrast in traffic pollutant concentrations when traffic pollution is high will result in greater measurement error compared to when traffic pollution is low.
Approaches to handle such measurement error include: 1) restriction of study areas to specific distances from monitors (8–10); 2) application of statistical methods to estimate and adjust health effects estimates for spatial misalignment error (5, 11); and 3) application of modeled daily air pollution estimates that provide greater study area coverage (12–19). The latter presents opportunities for directly limiting exposure measurement error in population-based time-series studies with geographically expansive study areas. However, the impact on epidemiologic findings of different types of exposure metrics across different patient populations with varying spatiotemporal profiles is understudied. Specifically, few studies to our knowledge have compared and interpreted the use of different types of exposure metrics in time-series analyses of multiple pollutants and outcomes (15, 20, 21).
To address this gap, we leveraged a long-standing study of air pollution and emergency department (ED) visits in Atlanta, Georgia (22–27). Our study area includes the 20-county Atlanta metropolitan statistical area (1999 definition), comprising 15,790 square kilometers and a population of 5,110,183 persons in 2010 (28). While we do not include rural counties outside of the MSA, the Atlanta MSA includes territories and populations that may be rural. County population density ranges from relatively high (e.g., 2,586 persons/sq. mile in DeKalb County) to relatively low (126 persons/sq. mile in Pickens County). While the majority of the population (66%) resides in the five most densely populated central counties of the city (Clayton, Cobb, DeKalb, Fulton, Gwinnett), a sizeable percent of the population (44%) resides in outer counties.
Building on previous exposure modeling efforts (29, 30), Yu and colleagues developed a set of nine daily exposure metrics, using a range of monitoring- and model-based approaches, for eight pollutants for the 2009–2013 period for our study area (31). Here, we applied these outputs to epidemiologic analyses of cardiorespiratory ED visits, and compared the resulting health effect estimates across exposure metrics. Our assessment involved a 3-step approach: (1) estimating associations using concentrations from a central monitor (CM), a common method for characterizing daily air pollution in time-series studies (32); (2) determining if non-CM exposure metrics can reproduce CM-based associations; and (3) identifying potential value added of incorporating the full spatiotemporal information provided by non-CM exposure metrics.
MATERIALS AND METHODS
Emergency Department Visit Data
We obtained daily counts of cardiorespiratory ED visits for patients living in 192 residential ZIP codes located wholly or partially within the 20-county Atlanta area for the period January 1, 2009 to December 15, 2013 (n=1800 days). Data were aggregated from individual-level hospital billing records obtained through the Georgia Hospital Association (26, 33). During the 2009–2013 time period, 38 of 39 hospitals were represented, capturing over 95% of all ED visits in the study area. We identified outcomes based on patient billing records with primary International Classification of Diseases, 9th Revision (ICD-9) diagnosis codes for: cardiovascular disease (CVD, ICD-9 codes: 410–414, 427, 428, 433–437, 440, 443–445, 447) and respiratory disease (RESP, ICD-9 codes 460–519).
Air Pollution Exposure Metrics
Air pollution exposure metric estimation approaches are provided in detail by Dharmalingam and colleagues (31). Brief descriptions of the nine metrics and how we applied them for this analysis, are provided below. Daily pollutant measures from each metric included 1-hour maximum concentrations of carbon monoxide (CO), NO2, and SO2; 8-hour average maximum O3; and 24-hour average PM2.5, PM2.5 sulfate (SO4), PM2.5 elemental carbon (EC), and PM2.5 organic carbon (OC).
1. Central Monitor (CM).
The Jefferson St. (JST) site, part of the SouthEastern Aerosol Research and Characterization (SEARCH) network, was selected as the central monitoring site. The JST site is located in a central urbanized area of Atlanta approximately 2.3 km away from the nearest major roadway and provided daily concentrations for all pollutants of interest.
2. Site Average (SA).
The SA metric averaged daily concentration data from all monitors located inside the 20-county Atlanta region, obtained from the U.S. Environmental Protection Agency (USEPA) and the Southeastern Aerosol Research and Characterization (SEARCH) study (34, 35). Data were available from 19 monitors, with the number of monitors per pollutant varying: CO=4, NO2=5, SO2=5, O3=10, PM2.5=14, SO4=3, EC=3, and OC=3 [Supplemental Table S1; see also Dharmalingam et al. (2022) Figure S2 (31)].
3. Inverse Distance Weighting (IDW).
IDW is a spatial interpolation method in which pollutant concentrations are inferred at unknown locations. In this study, daily pollutant concentration data were interpolated to the centroids of each of the 192 Atlanta study area ZIP codes using observations collected from all USEPA and SEARCH monitors located within the 200 km radius of the study area. Data were available from 141 monitors, with the number of monitors per pollutant varying: CO=12, NO2=12, SO2=23, O3=76, PM2.5=88, SO4=27, EC=27, and OC=24 [see Dharmalingam et al. (2022) Figure S2 (31)].
4. Kriging (KRIG).
Kriging is also a spatial interpolation method, which unlike IDW, uses optimal weights derived from observed spatial dependence. For each pollutant, Kriging prediction was based on a parametric variogram, estimated using binned empirical semivariance. The fitted variogram model for each pollutant was then used to estimate daily pollutant concentrations at ZIP code centroids.
5. Community Multiscale Air Quality Model (CMAQ).
CMAQ is a chemical transport air quality model driven by emissions and meteorology. Here, we used a 12 km gridded product generated by the USEPA and the Centers for Disease Control and Prevention as part of the Public Health Air Surveillance Evaluation study (36, 37). The 12 km resolution CMAQ data were mapped to ZIP codes via area-weighting to obtain daily concentrations for each pollutant at each ZIP code. Since the CMAQ model itself generally does not incorporate observations, daily temporal variability in pollutant concentrations is purely modeled and is generally not reproduced well (31). As a result, we do not consider raw CMAQ as a directly suitable metric for daily time-series applications, however we include it as a comparison with the next two CMAQ-based metrics that do incorporate monitoring information.
6. CMAQ-Kriging (CMAQKR).
The CMAQKR approach uses observations and Kriging to adjust CMAQ model outputs. Specifically, daily ratios of CMAQ/observations were calculated at all monitor locations within the 200 km radius study domain. These ratios were then interpolated to all 12 km CMAQ grid cell centroids using Kriging. Daily CMAQ concentrations at each grid cell were adjusted proportionately using the interpolated ratios, and then mapped to ZIP codes via area-weighting to obtain daily concentrations for each pollutant at each ZIP code.
7. CMAQ Data Fusion (CMAQDF).
We collected a ready-to-use CMAQDF data product (developed by the Georgia Institute of Technology) for this study for 2009–2013 (29, 38). The CMAQDF approach is designed to correct for modeling biases/errors within CMAQ data. It also accounts for the potential for specific locations being consistently impacted by very local sources leading to observations higher than surrounding areas. In CMAQDF, two concentration fields were developed. The first field assimilated temporal variations of observation data and spatial structure from CMAQ results, and the second field incorporated spatial and temporal variations of CMAQ data which were also scaled using observation data to correct for modeling biases. The two developed fields were then fused together using weighting factors that vary over space based on their ability to predict temporal variations.
8. Land Use Regression (LUR) – NO2 and PM2.5 only.
LUR is a potentially computationally-intensive empirical supervised multiple regression approach to simulate traffic-related pollutant concentration fields when sufficient measurement locations are available. Here, sufficient locations and computing resources were available to estimate NO2 and PM2.5. In LUR, concentration measurement data are related to spatially or temporally varying candidate predictor variables. Candidate predictor variables included land use (elevation, population, land cover types and roadway types), meteorological parameters (visibility, boundary layer height, precipitation, radiations, pressure, humidity, temperature, wind), pollutant emission data and output data from the CMAQ model. Using LUR, we estimated daily concentrations of NO2 and PM2.5 at 1 km grid cells covering the study area, and then mapped concentrations to ZIP codes via area-weighting.
9. Aerosol Optical Depth (AOD) – PM2.5 only.
Use of satellite observational data has gained popularity in exposure modeling over the past decade due to their broad spatial coverage (39–41). Essentially, the AOD approach is based on a regression that associates satellite AOD data with available ground-based observational data. In this study, we applied a Bayesian-based calibration method (42) with 1-km resolution AOD data obtained from the Multiangle Implementation of Atmospheric Correction (MAIAC) (43) downscaled from MODIS data. Using this approach, we estimated daily concentrations of PM2.5 at 1 km grid cells covering the study area, and then mapped concentrations to ZIP codes via area-weighting.
Epidemiological Study Approach
The goal of this study was to estimate and compare associations of air pollution and cardiorespiratory ED visits across exposure metrics. The epidemiologic model used to estimate the associations is described below. The study approach consisted of three steps that facilitated the comparison of effect estimates across exposure metrics.
Step 1. CM Analysis.
First, we estimated the pollutant-outcome relationships for the central monitor. The daily CM value of each pollutant, from the JST monitoring station, was assigned to all 192 study area ZIP codes (i.e., same daily values for each ZIP code). With the CM metric, any potential spatiotemporal concentration variability in the study area is neglected.
Step 2. Central ZIP Analysis.
Second, we determined if the non-CM exposure metrics were able to reproduce the CM-based associations. For this analysis, we assigned the daily value of each pollutant from the non-CM exposure metrics at the ZIP code that houses the JST central monitor (ZIP code 30318) to all 192 ZIP codes (i.e., same daily values for each ZIP code). Therefore, this approach again neglects any potential spatiotemporal concentration variabilities. This step is analogous to Step 1, but with exposures assigned using each of the non-CM metrics estimated at the same location. If the non-CM metrics estimate daily concentrations near the CM well, observed health effect estimates should be similar.
Step 3. Full Spatial Analysis.
Finally, to assess potential value added of utilizing exposure metrics with spatial information, for each non-CM exposure metric we assigned the daily ZIP code-specific pollution value to each study area ZIP code (i.e., allowing different daily values for each ZIP code). Unlike the previous two steps, this approach incorporates spatiotemporal information in health effects estimation and therefore we anticipated either similar or stronger health effect estimates compared to the previous two steps. [Note that the SA metric results from this approach were identical to those from the Central ZIP approach (Step 2) given that the SA provided only one value per day for the entire study area].
To facilitate comparisons, exposure metrics were matched for missing values by pollutant. For example, if data for a certain pollutant (e.g., NO2) were missing for one exposure metric (e.g., CM) for a specific date, the data for the other exposure metrics for that pollutant were also set to missing for that date; the resulting sample size (ZIP code-days) was the same for all metrics for a given pollutant.
Epidemiologic Model
Main Model.
We used Poisson generalized linear models to estimate associations between daily measures of air pollution and daily counts of ED visits for cardiovascular and respiratory outcomes, following the 3-step epidemiological study approach. For each study approach, the form of the model was the same:
| Eq. 1 |
where Ykt was the count of ED visits in ZIP code k on day t for CVD or RESP. Pollution referred to the moving average (of lag days 0–7) concentrations for a given pollutant-metric-assignment of interest in ZIP code k on day t. The geographical area (zip) from which ED counts were spatially aggregated was represented by indicator variables, to control for spatially-varying factors and enable the analysis to rely solely on temporal contrasts; this also stringently controlled for spatial autocorrelation in the baseline ED visits across ZIP codes. The models included indicator variables for hospital to account for any entry and exit of hospitals during the study period, day of week and federal holidays (dow_h), season (Winter=Dec-Feb, Spring=Mar-May, Summer=June-Aug, Autumn=Sep-Nov), an interaction between dow_h and season, and additional holiday indicators (holiday_oth) for New Year’s Day, Independence Day, Veterans Day, Thanksgiving, and Christmas Day when different from the date of the federal holiday. Meteorology was controlled using indicator variables for same-day (lag 0) maximum temperature (maxT_l0, for each degree Celsius), and cubic terms for the moving averages of minimum temperature (lags 1–7, minT_ma17) and mean dew point temperature (lags 0–7, meanDT_ma07). Finally, long-term trends in case presentation rates (time) were controlled with parametric cubic splines with monthly knots for CVD ED visits and bi-monthly (2 knots per month) for RESP ED visits. Variance estimates were scaled to account for Poisson overdispersion. These modeling decisions (e.g., choice of a priori lags, meteorological control, and time trend control) were derived largely from our previous analyses (15, 22, 23, 45–47). We ran this model for each outcome, pollutant, exposure metric, and study approach combination. We scaled rate ratios (RR) and 95% confidence intervals (CI) to increases in pollutant concentrations as follows: 250 ppb for CO; 10 ppb for NO2; 4 ppb for SO2; 15 ppb for O3; 5 μg/m3 for PM2.5; 1 μg/m3 for SO4; 0.5 μg/m3 for EC; and 1 μg/m3 for OC.
Sensitivity Analyses.
To assess robustness of our results to different model specifications, we considered two sets of sensitivity analyses based on the ‘Full Spatial Analysis’ approach. We tested different lag structures for air pollution: lag 0, moving average of lag days 0–2, and moving average of lag days 0–5, and compared the results to the moving average of lag days 0–7 in our base model (Eq. 1). Meteorological controls were adjusted to match the lag structure of air pollution exposure in these analyses. In addition, we tested different specifications of temporal controls compared to our base model (Eq. 1): controlling for time using parametric cubic splines with two knots per month (for CVD ED visits) and one knot per month (for RESP ED visits); controlling for temperature with cubic terms for the moving average of mean temperature (lag days 0–7); and adding product terms for season*temperature. All epidemiological analyses were conducted in SAS V9.4 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
ED Visit Summary
During 2009–2013, there were 230,718 ED visits (average of 128 visits/day) for CVD and 1,068,026 ED visits (average of 593 visits/day) for RESP. Figure 1 presents time-series plots of daily visit counts for each outcome. The visits were made by patients residing across the 192 ZIP codes, with ZIP code-level daily averages ranging from <1 to 2 for CVD and <1 to 11 for RESP. ED visit counts among the outcomes were similarly distributed over the study area (Supplemental Figure S1), with a high spatial correlation of total ZIP code-level visit counts between the two outcomes (r = 0.896). While similarly distributed over space, the outcomes were weakly correlated over time; the average ZIP code-level Pearson correlation of daily CVD and RESP ED visit counts was 0.016 (Supplemental Figure S1).
Figure 1.
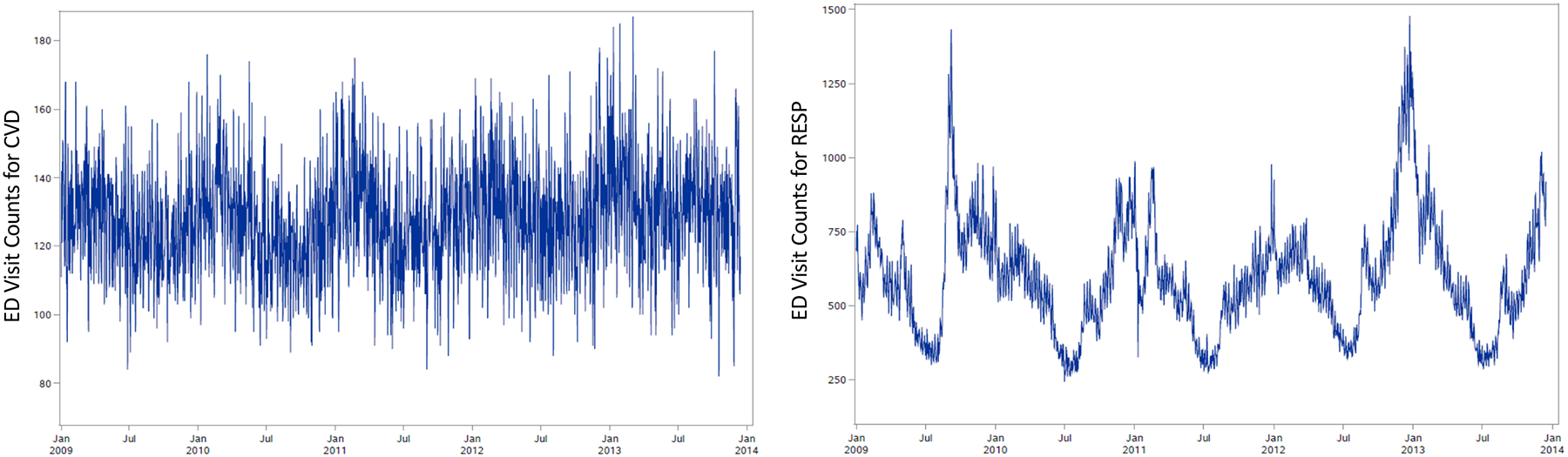
Time-series plots of daily ED visit counts for cardiovascular diseases (left) and respiratory diseases (right) in 20-county Atlanta during January 1, 2009 to December 15, 2013.
Air Pollution Levels
Table 1 presents summary concentrations for each pollutant-metric combination. Median levels and IQRs of primary pollutants (e.g., CO, NO2, SO2) differed across metrics more than for pollutants with secondary contributions (e.g., O3, PM2.5). Maps of ZIP code average pollutant concentrations by exposure metric are presented by Dharmalingam et al. (31). Note that data availability for OC (N of 86,976 ZIP code-days) was less than for other pollutants (N>340,000 ZIP code-days), due to lower monitoring coverage.
Table 1.
Summary of pollutant concentrations across all ZIP code-days for each exposure metric.1
| 1-hr max CO (ppb) | 1-hr max NO2 (ppb) | 1-hr max SO2 (ppb) | 8-hr max O3 (ppb) | 24-hr avg PM2.5 (μg/m3) | 24-hr avg SO4 (μg/m3) | 24-hr avg EC (μg/m3) | 24-hr avg OC (μg/m3) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=345,600 | N=343,680 | N=345,216 | N=344,640 | N=345,216 | N=342,528 | N=340,608 | N=86,976 | |||||||||||||||||
| Metric | Median | IQR | Max | Median | IQR | Max | Median | IQR | Max | Median | IQR | Max | Median | IQR | Max | Median | IQR | Max | Median | IQR | Max | Median | IQR | Max |
| CM | 378.5 | 326.2 | 2309.6 | 29.7 | 17.9 | 82.5 | 3.0 | 7.4 | 86.3 | 39.6 | 22.6 | 103.6 | 9.7 | 5.9 | 39.8 | 2.1 | 1.5 | 11.0 | 0.67 | 0.48 | 4.32 | 2.6 | 1.6 | 12.2 |
| SA | 458.2 | 332.6 | 1879.3 | 16.8 | 14.3 | 70.8 | 2.6 | 5.1 | 64.9 | 39.9 | 18.6 | 89.0 | 10.2 | 6.4 | 31.6 | 2.0 | 1.5 | 7.4 | 0.59 | 0.56 | 3.57 | 2.4 | 1.5 | 8.5 |
| IDW | 490.9 | 315.0 | 3287.1 | 19.5 | 14.2 | 82.2 | 2.8 | 4.8 | 128.3 | 39.4 | 19.3 | 120.1 | 10.2 | 6.3 | 62.1 | 2.0 | 1.5 | 11.0 | 0.61 | 0.45 | 4.31 | 2.4 | 1.5 | 12.2 |
| KRIG | 472.6 | 335.1 | 4287.8 | 17.3 | 13.5 | 79.5 | 2.6 | 4.6 | 122.5 | 39.3 | 19.4 | 118.4 | 10.1 | 6.5 | 63.3 | 2.0 | 1.5 | 11.0 | 0.61 | 0.44 | 4.28 | 2.3 | 1.5 | 12.1 |
| CMAQ | 359.2 | 340.1 | 2516.7 | 20.9 | 22.8 | 119.9 | 1.6 | 1.9 | 46.3 | 41.6 | 20.8 | 108.6 | 9.1 | 6.8 | 121.0 | 1.7 | 1.3 | 10.1 | 0.83 | 0.89 | 11.53 | 1.8 | 1.9 | 38.1 |
| CMAQKR | 373.0 | 325.0 | 4051.8 | 12.8 | 16.0 | 88.7 | 2.5 | 4.6 | 575.9 | 41.0 | 19.0 | 272.5 | 9.5 | 6.5 | 57.2 | 1.9 | 1.5 | 12.4 | 0.47 | 0.42 | 4.52 | 2.2 | 1.7 | 13.1 |
| CMAQDF | 490.7 | 318.8 | 3559.8 | 16.7 | 15.4 | 117.3 | 2.1 | 2.6 | 177.9 | 40.2 | 18.7 | 118.6 | 9.1 | 4.5 | 63.5 | 1.9 | 1.3 | 10.1 | 0.51 | 0.37 | 2.80 | 2.0 | 1.0 | 8.9 |
| LUR | 13.1 | 14.7 | 75.9 | 10.2 | 4.0 | 57.3 | ||||||||||||||||||
| AOD | 10.4 | 6.0 | 47.6 | |||||||||||||||||||||
Exposure metrics included: Central Monitor (CM), Site Average (SA), Inverse Distance Weighting (IDW), Kriging (KRIG), Community Multiscale Air Quality model (CMAQ), CMAQ-kriging adjustment with observations (CMAQKR), CMAQ combined with monitoring data in a data fusion (CMAQDF), Land Use Regression (LUR), and satellite Aerosol Optical Depth (AOD).
At the Central ZIP code (ZIP 30318), pollutant concentrations from the different exposure metrics were generally well correlated with the CM (Supplemental Table S2, blue shading). IDW, KRIG, and CMAQKR values were the most correlated with CM values for all pollutants (r>0.97). SA values were also correlated with CM values for all pollutants (r>0.80), but at slightly lower levels due to the influence of monitors located away from the CM. CMAQ values were least correlated with CM values (r = 0.47–0.79).
When considering the full spatial information of the exposure metrics data, pollutant concentrations from the different exposure metrics were also well correlated (Supplemental Table S2, red shading), although not as strongly as at the Central ZIP code. ZIP code-level correlations previously reported by Dharmalingam et al. (31) showed that for many pollutants, correlations were strongest in the city center and weakest towards the outskirts. In general, for spatiotemporally heterogeneous pollutants (such as CO, NO2, SO2, EC), correlations among exposure metrics were more variable than for spatiotemporally homogeneous pollutants (such as O3, PM2.5, SO42−, and OC).
Associations of Air Pollution and Cardiovascular ED Visits
Estimated associations of air pollution and ED visits for cardiovascular diseases are presented in Figure 2 and Supplemental Table S3. Rate ratios from models using the CM analysis approach (Figure 2, black RRs) were positive for several pollutants (i.e., CO, NO2, PM2.5, EC and OC).
Figure 2. Associations of air pollution and ED visits for cardiovascular diseases, comparing among pollutants, exposure metrics, and study approaches.
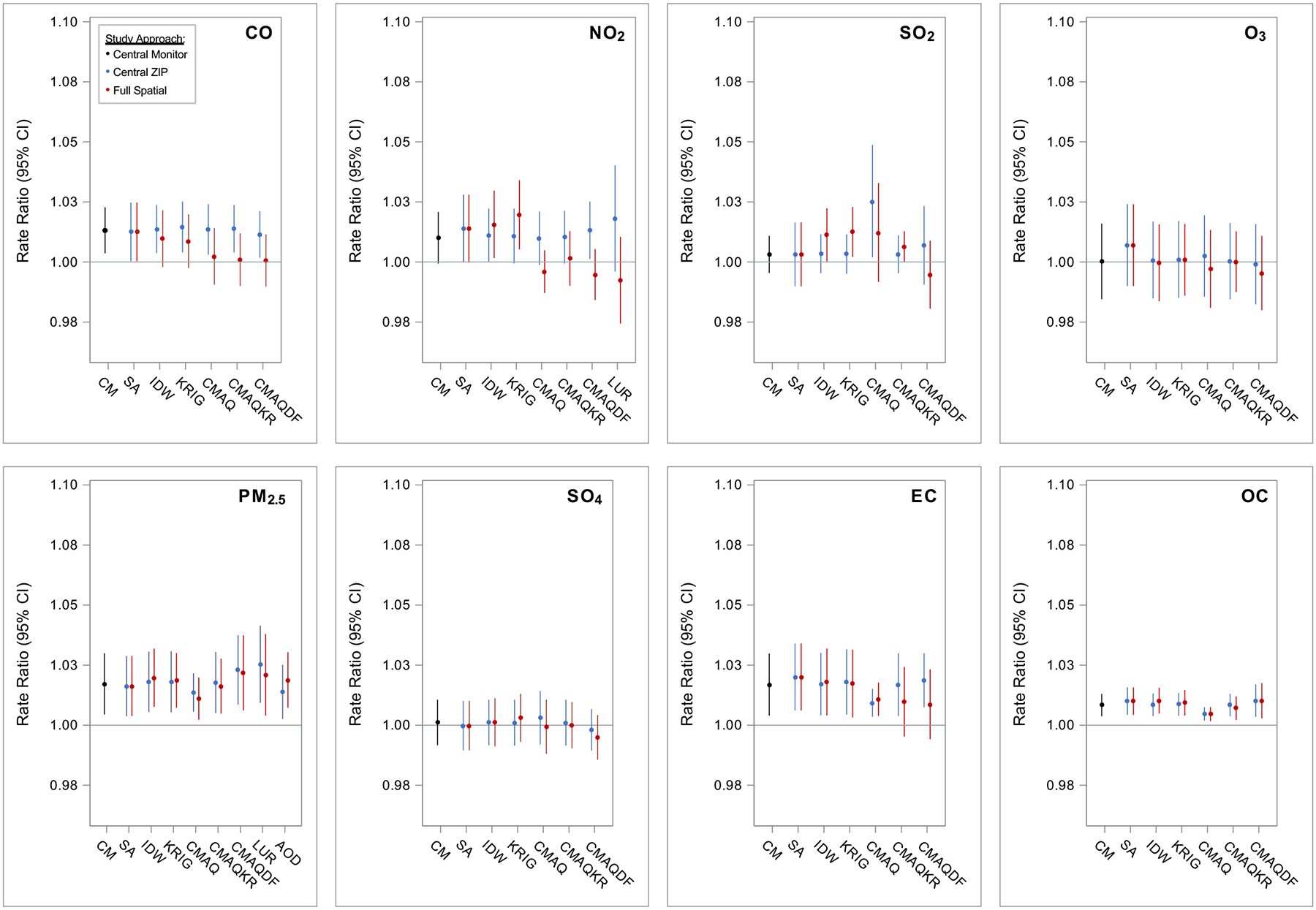
Exposure metrics included: Central Monitor (CM), Site Average (SA), Inverse Distance Weighting (IDW), Kriging (KRIG), Community Multiscale Air Quality model (CMAQ), CMAQ-kriging adjustment with observations (CMAQKR), CMAQ combined with monitoring data in a data fusion (CMAQDF), Land Use Regression (LUR), and satellite Aerosol Optical Depth (AOD). Study approaches included assigning: Central Monitor values to all 192 ZIP codes by day (black), Central ZIP Code values for each exposure metric to all 192 ZIP codes by day (blue), and the Full Spatial information of the exposure metrics to the 192 ZIP codes by day (red). All models controlled for time and meteorology as specified by the base model. Rate ratios (RR) and confidence intervals (CI) scaled to standard units: per 250 ppb CO, 10 ppb NO2, 4 ppb SO2, 15 ppb O3, 5 μg/m3 PM2.5, 1 μg/m3 SO42−, 0.5 μg/m3 EC, and 1 μg/m3 OC.
When applying Central ZIP values from the non-CM exposure metrics, models largely reproduced the CM-based associations (Figure 2, blue RRs). For NO2, for example, all RRs were positive and ranged from 1.010 (95% CI: 0.999–1.021) to 1.018 (95% CI: 0.996–1.040) per 10 ppb increase across metrics. The reproducibility of CM-based associations lend support to the non-CM exposure metrics in their ability to capture health-relevant temporal contrasts similar to the CM. The only exception was for CMAQ SO2, for which Central ZIP exposures showed a positive association with CVD ED visits; given the lack of observed association using CM SO2 data, this result was unexpected and reduces our confidence in the use of CMAQ SO2 in this health study application.
When utilizing the full spatial information of exposure metrics (Figure 2, red RRs), estimated associations were largely similar to those estimated with Central ZIP data. However, for several pollutants (CO, NO2, and EC), estimated associations – especially the three CMAQ-derived estimates and LUR – were considerably weaker when utilizing the full spatial data.
Associations of Air Pollution and Respiratory ED Visits
Estimated associations of air pollution and ED visits for respiratory diseases are presented in Figure 3 and Supplemental Table S3. Rate ratios from models using the CM analysis approach were positive only for O3 (Figure 3, black RRs).
Figure 3. Associations of air pollution and ED visits for respiratory diseases, comparing among pollutants, exposure metrics, and study approaches.
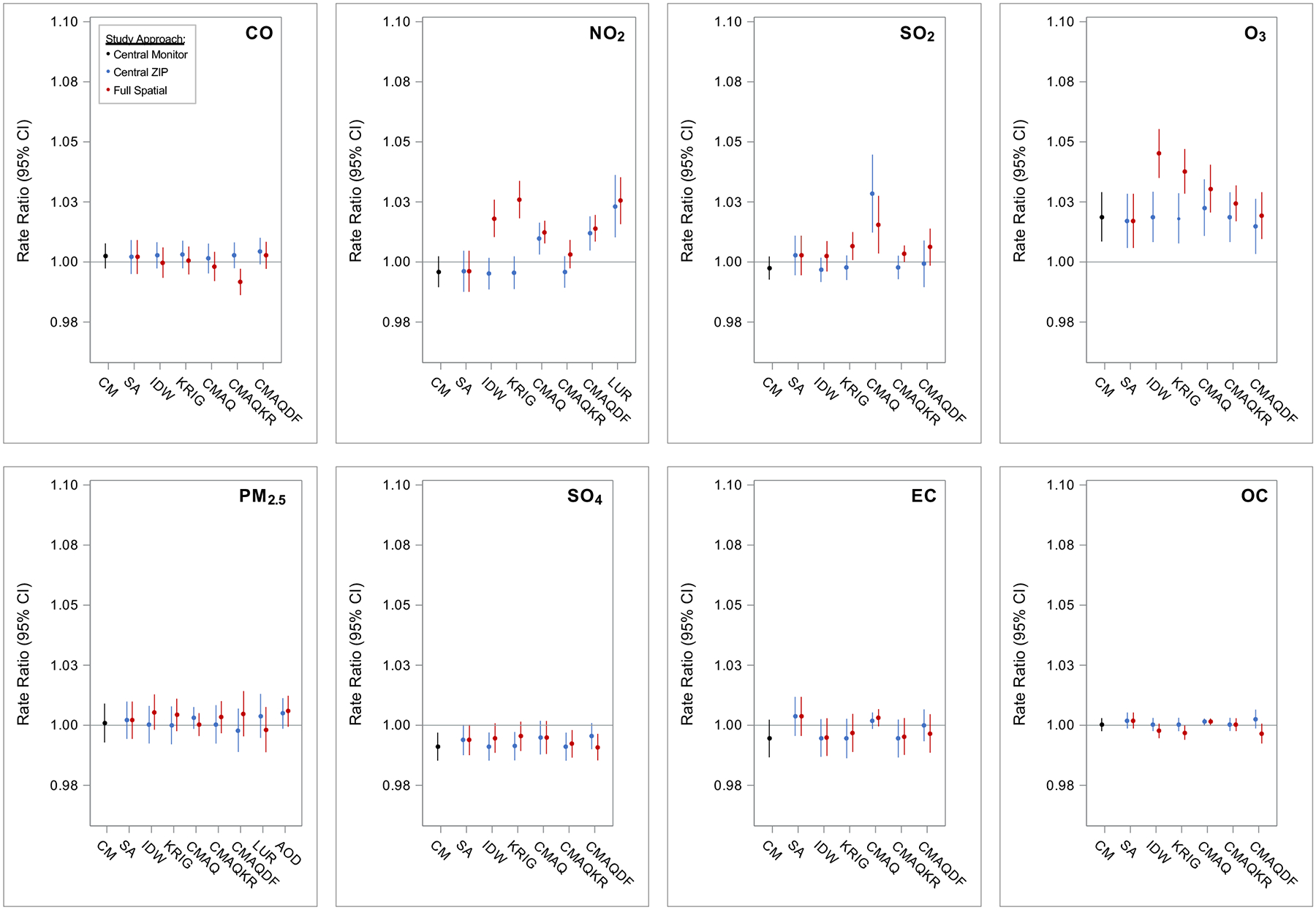
Exposure metrics included: Central Monitor (CM), Site Average (SA), Inverse Distance Weighting (IDW), Kriging (KRIG), Community Multiscale Air Quality model (CMAQ), CMAQ-kriging adjustment with observations (CMAQKR), CMAQ combined with monitoring data in a data fusion (CMAQDF), Land Use Regression (LUR), and satellite Aerosol Optical Depth (AOD). Study approaches included assigning: Central Monitor values to all 192 ZIP codes by day (black), Central ZIP Code values for each exposure metric to all 192 ZIP codes by day (blue), and the Full Spatial information of the exposure metrics to the 192 ZIP codes by day (red). All models controlled for time and meteorology as specified by the base model. Rate ratios (RR) and confidence intervals (CI) scaled to standard units: per 250 ppb CO, 10 ppb NO2, 4 ppb SO2, 15 ppb O3, 5 μg/m3 PM2.5, 1 μg/m3 SO42−, 0.5 μg/m3 EC, and 1 μg/m3 OC.
When applying Central ZIP values from the non-CM exposure metrics, models again largely reproduced the CM-based associations (Figure 3, blue RRs). For some pollutant-metrics (i.e., CMAQ NO2, CMAQDF NO2, LUR NO2, CMAQ SO2, and CMAQ EC), estimated associations were unexpectedly different from the CM-based associations. For example, while the RR for CM NO2 [0.996 (95% CI: 0.989–1.002) per 10 ppb increase] was consistent with the null, RRs for NO2 when using CMAQ [1.010 (95% CI: 1.003–1.016)], CMAQDF [1.012 (95% CI: 1.005–1.019)], and LUR [1.023 (95% CI: 1.010–1.036)] were considerably stronger. Again, these results reduce our confidence in the CMAQ- and LUR-based metrics for these pollutants in this health study application.
When utilizing the full spatial resolution of exposure metrics (Figure 3, red RRs), estimated associations showed largely similar patterns across exposure metrics as those using Central ZIP code assignment. Some important differences were noted. In particular, associations with NO2 and SO2 were only detected when assigning IDW and KRIG exposures utilizing their full spatial information; associations with O3 were also stronger when utilizing these metrics. For example, the RR for full spatial KRIG NO2 [1.026 (95% CI: 1.018–1.034) per 10 ppb increase] was considerably stronger than that for Central ZIP KRIG NO2 [0.996 (95% CI: 0.989–1.002)].
Sensitivity Analyses
The observed pattern of associations across exposure metrics for both outcomes was largely unchanged when considering different exposure windows (Supplemental Figures S2 and S3), with the moving average of lag days 0–7 exposure window generally providing the strongest estimated association among different outcome-pollutant-exposure metric combinations. Similarly, the observed pattern of associations across exposure metrics was largely unchanged when considering different temporal control specifications in the model (Supplemental Figures S4 and S5). In general, less stringent time control (monthly knots for splines) led to stronger observed outcome-pollutant associations for RESP, and less stringent temperature control (moving average of mean temperature for lag days 0–7) also led to stronger observed outcome-pollutant associations.
DISCUSSION
In this study, we compared associations of cardiorespiratory ED visits and exposure to 8 ambient air pollutants estimated using multiple approaches in 20-county Atlanta, GA during 2009–2013. We utilized exposure metrics that varied considerably in complexity, both in terms of inputs required, computational resources needed, ease of implementation, and availability and ease of use to public health researchers (31). Using central monitor data, the simplest and common metric for characterizing daily air pollution in time-series studies (32), we observed a number of anticipated associations, including between CVD and combustion-related gases and particles (CO, NO2, PM2.5, EC and OC) as well as between RESP and O3. To understand whether the effort and resources required for more complex exposure metrics development is warranted for the purpose of short-term air pollution health effects studies, we compared the use of the CM metric to non-CM metrics in a step-wise approach.
In the Central ZIP analysis, we conducted analyses with exposures for all 192 study area ZIP codes assigned using values from the ZIP code that houses the JST central monitor. We anticipated similar health effect estimates for a given pollutant comparing results using non-CM exposures metrics from this approach to those using CM exposure data. That is, if the non-CM metrics estimate daily concentrations near the CM well, observed health effect estimates should be similar. More specifically, this approach enabled an assessment of whether temporal over-smoothing, a recognized concern for predictions from statistical models (e.g., KRIG, CMAQ) because of regression to the mean (44), affected health effect estimates compared to the CM that reflects accurate temporal variability in its specific location. Indeed, estimated associations were largely similar across metrics at the Central ZIP. The reproducibility of CM-based associations lend support to the non-CM exposure metrics in their ability to capture health-relevant temporal contrasts similar to the CM for most pollutants.
Exceptions to this were several unexpected results for both CVD and RESP associations with NO2, SO2, and EC exposures from CMAQ, CMAQDF, and LUR metrics. CMAQ and LUR data in this study were developed at 12 km and 1 km grid cell resolutions, respectively, thus providing good spatial characterization of pollutant concentrations (31). However, the ability of CMAQ and LUR to capture daily variation in pollutant concentrations at ZIP codes relies mostly on input emissions and meteorology data (and associated chemical transport and transformation processes for CMAQ), all of which are expected to contain considerable uncertainties; hence their estimated temporal concentration variability may be poor. In previous work, for example, we found that raw CMAQ data do not perform as well in reproducing temporal pollutant concentration variations in the study region due to modeling biases and errors (31). Given that CMAQ and LUR in this study would not be anticipated to provide good estimates of temporal variability (30, 31), we would have expected similar or weaker health effect associations compared with those of other metrics. However, we in fact observed stronger associations of CVD-SO2, RESP-NO2, RESP-SO2, and RESP-EC with these metrics. While epidemiologic models of this study controlled strictly for time, the results may be highlighting complex temporal confounding, when pairing ED visit outcomes with modeled exposure metrics that have poor estimated temporal concentration variability. As such, health effect estimates derived from raw CMAQ and LUR data should be interpreted with caution. We note that CMAQKR and CMAQDF may partially overcome this shortfall when paired with rigorous time control in epidemiologic models, given their fusion to monitoring data, and were among the metrics with better performance in the evaluation by Dharmalingam et al. (31).
The Full Spatial analysis enabled a qualitative assessment of the potential value added in health effect estimation when incorporating the full spatial information provided by non-CM exposure metrics. We anticipated that use of full spatial data from the more complex multi-monitor and model-based exposure metrics would result in stronger health effect estimates compared to CM or Central ZIP exposure assignment, as these were specifically developed to provide a better estimate of population exposure for our large study area. We observed some anticipated results. In particular, associations for RESP and NO2, SO2, and O3 were only detected or stronger when using IDW and KRIG full spatial data than when utilizing Central ZIP estimates for these pollutants. These results suggest that use of data from all monitors in and extended beyond the study area to generate ZIP code-specific concentration estimates, compared to relying on just on the central monitor may be important in observing health effects in some cases. We also observed some unanticipated results, most strikingly the weaker estimated associations for CVD when using full spatial CO, NO2, and EC CMAQ-based and LUR metrics compared to those from the Central ZIP approach. It is possible that relatively low pollution levels with less variation in the outskirts of the city (31) contributed to the weaker observed health effect associations for CVD when using full spatial resolution data, and the results may be indicative of non-linear pollutant-CVD health associations.
There are several limitations to consider in this study. Some differences in associations observed among pollutants and metrics may be impacted by uncertainties in scaling to some degree; we selected a common increment by which to standardize rate ratios for each pollutant. However, because of different exposure distributions by different metrics, the scaling may not reflect expected changes in exposure equally well. It is important to note that scaling does not impact direction of effects (i.e., positive vs. negative) or significance. It is also important to note that this particular analysis focused on time-series design, where minimizing temporal errors in the metrics is the most important, and other study designs may be more affected by spatial errors.
Conclusions
We conducted a time-series study of cardiorespiratory ED visits and ambient air pollutants estimated using a variety of approaches in 20-county Atlanta, GA during 2009–2013. Ultimately, associations observed with using the simplest CM exposure assignment were largely similar to those using more complex metrics. We found some evidence of better representation, in particular for NO2 and SO2, by monitoring-based metrics that incorporate all available monitoring data and interpolate to locations between monitors (i.e., IDW, KRIG). These findings are likely driven by the ability of air monitors to accurately characterize temporal contrasts at their location, which is the key exposure contrast for time-series health studies. Even though air monitors for most pollutants are often sparsely distributed over metropolitan regions in the US, our results suggest monitoring-based metrics are a sound exposure assignment approach. In locations where monitoring data are inadequate, our results provide some support for the use of CMAQ-, LUR-, and satellite-based metrics in time-series health studies. However, unexpected results using CMAQ- and LUR-based metrics for some pollutants (in particular, NO2, SO2, and EC) in their inability to reproduce associations observed using CM data temper our confidence. Use of these exposure metrics should be paired with thorough data characterization and validation within health analyses. We anticipate that the results of this research will be useful for interpretation of existing health effects literature and for improving exposure assessment in future air pollution epidemiology studies. Additional studies in other locations are warranted, especially for locations with different population spatial structures and monitoring availability and placement.
Supplementary Material
IMPACT STATEMENT.
This study compared and interpreted the use of monitoring and modeled exposure metrics in a daily time-series analysis of air pollution and cardiorespiratory emergency department visits. The results suggest that the use of routinely-collected ambient monitoring data in population-based short-term air pollution and health studies is a sound approach for exposure assignment in large metropolitan regions. CMAQ-, LUR-, and satellite-based metrics may allow for health effects estimation when monitoring data are sparse, if paired with thorough data characterization. These results are useful for interpretation of existing health effects literature and for improving exposure assessment in future air pollution epidemiology studies.
ACKNOWLEDGEMENTS
This publication is based in part upon information obtained from the Georgia Hospital Association (GHA). We are grateful for the support of the GHA and their member hospitals. We would also like to acknowledge Drs. Annette Rohr and Chloe Kim of the Electric Power Research Institute for their helpful feedback.
FUNDING
This research was supported by funding from the Electric Power Research Institute (10009553). This publication was also made possible by grants to Emory University from the National Institute of Environmental Health Sciences of the National Institutes of Health under award numbers R01ES027892 and P30ES019776. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Electric Power Research Institute or the National Institutes of Health.
Footnotes
ETHICAL APPROVAL
Use of the ED data was in accordance with agreements with the Georgia Hospital Association and this study was approved prior to its conduct by the Emory University Institutional Review Board.
COMPETING INTERESTS
We have no competing financial interests in relation to the work to declare.
DATA AVAILABILITY
This study was based on air pollution data from a variety of sources and on hospital billing records. We will make the air pollution exposure metrics data applied in this study, and relevant documentation and metadata available without cost to researchers. Individuals and parties must agree to the conditions of use governing access to the public release data, including reporting responsibilities, restrictions on redistribution of the data to third parties, proper acknowledgement of the data resource, and restrictions on use for commercial purposes. We will not be able to make the hospital records data (neither patient-level nor aggregate forms) available to external investigators given restrictions in our data use agreements between Emory University and the Georgia Hospital Association. The exposure-response functions generated in this study are available in the Supplemental Material for incorporation into meta-analyses, health impact assessments, or other analyses by external investigators.
REFERENCES
- 1.Thurston G, Lippmann M. Ambient particulate matter air pollution and cardiopulmonary diseases. Seminars in Respiratory and Critical Care Medicine. 2015;36(3):422–32. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N Engl J Med. 2019;381(8):705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.USEPA. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2019). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-19/188, 2019. 2019. [Google Scholar]
- 4.Wade KS, Mulholland JA, Marmur A, Russell AG, Hartsell B, Edgerton E, et al. Effects of instrument precision and spatial variability on the assessment of the temporal variation of ambient air pollution in Atlanta, Georgia. Journal of the Air & Waste Management Association. 2006;56(6):876–88. [DOI] [PubMed] [Google Scholar]
- 5.Peng RD, Bell ML. Spatial misalignment in time series studies of air pollution and health data. Biostatistics. 2010;11(4):720–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman GT, Mulholland JA, Russell AG, Srivastava A, Strickland MJ, Klein M, et al. Ambient air pollutant measurement error: characterization and impacts in a time-series epidemiologic study in Atlanta. Environmental Science & Technology. 2010;44(19):7692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman GT, Mulholland JA, Russell AG, Gass K, Strickland MJ, Tolbert PE. Characterization of ambient air pollution measurement error in a time-series health study using a geostatistical simulation approach. Atmospheric Environment. 2012;57:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA : the journal of the American Medical Association. 2006;295(10):1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai L, Zanobetti A, Koutrakis P, Schwartz JD. Associations of fine particulate matter species with mortality in the United States: a multicity time-series analysis. Environmental health perspectives. 2014;122(8):837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanobetti A, Dominici F, Wang Y, Schwartz JD. A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environ Health. 2014;13(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang HH, Peng RD, Dominici F. Estimating the acute health effects of coarse particulate matter accounting for exposure measurement error. Biostatistics. 2011;12(4):637–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloog I, Zanobetti A, Nordio F, Coull BA, Baccarelli AA, Schwartz J. Effects of airborne fine particles (PM2.5) on deep vein thrombosis admissions in the northeastern United States. Journal of Thrombosis and Haemostasis. 2015;13(5):768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu MM, Guo YM, Zhang YJ, Westerdahl D, Mo YZ, Liang FC, et al. Spatiotemporal analysis of particulate air pollution and ischemic heart disease mortality in Beijing, China. Environmental Health. 2014;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloog I, Nordio F, Zanobetti A, Coull BA, Koutrakis P, Schwartz JD. Short term effects of particle exposure on hospital admissions in the Mid-Atlantic states: a population estimate. PLoS One. 2014;9(2):e88578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarnat SE, Sarnat JA, Mulholland J, Isakov V, Ozkaynak H, Chang HH, et al. Application of alternative spatiotemporal metrics of ambient air pollution exposure in a time-series epidemiological study in Atlanta. Journal of Exposure Science & Environmental Epidemiology. 2013;23(6):593–605. [DOI] [PubMed] [Google Scholar]
- 16.Mannshardt E, Sucic K, Jiao W, Dominici F, Frey HC, Reich B, et al. Comparing exposure metrics for the effects of fine particulate matter on emergency hospital admissions. J Expo Sci Environ Epidemiol. 2013;23(6):627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent O, Pedrono G, Filleul L, Segala C, Lefranc A, Schillinger C, et al. Influence of Socioeconomic Deprivation on the Relation Between Air Pollution and beta-Agonist Sales for Asthma. Chest. 2009;135(3):717–23. [DOI] [PubMed] [Google Scholar]
- 18.Choi J, Fuentes M, Reich BJ. Spatial-temporal association between fine particulate matter and daily mortality. Computational Statistics & Data Analysis. 2009;53(8):2989–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurent O, Pedrono G, Segala C, Filleul L, Havard S, Deguen S, et al. Air pollution, asthma attacks, and socioeconomic deprivation: A small-area case-crossover study. American Journal of Epidemiology. 2008;168(1):58–65. [DOI] [PubMed] [Google Scholar]
- 20.Strickland MJ, Darrow LA, Mulholland JA, Klein M, Flanders WD, Winquist A, et al. Implications of different approaches for characterizing ambient air pollutant concentrations within the urban airshed for time-series studies and health benefits analyses. Environ Health. 2011;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Wilhelm M, Chung J, Ritz B. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environmental Research. 2011;111(5):685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. American Journal of Respiratory & Critical Care Medicine. 2010;182(3):307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winquist A, Kirrane E, Klein M, Strickland M, Darrow LA, Sarnat SE, et al. Joint effects of ambient air pollutants on pediatric asthma emergency department visits in Atlanta, 1998–2004. Epidemiology. 2014;25(5):666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Lenick CR, Winquist A, Mulholland JA, Friberg MD, Chang HH, Kramer MR, et al. Assessment of neighbourhood-level socioeconomic status as a modifier of air pollution-asthma associations among children in Atlanta. J Epidemiol Community Health. 2017;71(2):129–36. [DOI] [PubMed] [Google Scholar]
- 25.Ye D, Klein M, Chang HH, Sarnat JA, Mulholland JA, Edgerton ES, et al. Estimating acute cardiorespiratory effects of ambient volatile organic compounds. Epidemiology. 2017;28(2):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye D, Klein M, Mulholland JA, Russell AG, Weber R, Edgerton ES, et al. Estimating Acute Cardiovascular Effects of Ambient PM2.5 Metals. Environmental health perspectives. 2018;126(2):027007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumberg AH, Ebelt ST, Liang D, Morris CR, Sarnat JA. Ambient air pollution and sickle cell disease-related emergency department visits in Atlanta, GA. Environ Res. 2020;184:109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Census. U.S. Census Bureau, Population Division; Release Date: September 2011; Table 1. Intercensal Estimates of the Resident Population for Counties of Georgia, Alabama, Texas, Illinois, and Missouri: April 1, 2000 to July 1, 2010 (CO-EST00INT-01–13, CO-EST00INT-01–01, CO-EST00INT-01–48, CO-EST00INT-01–17, and CO-EST00INT-01–29). 2011.
- 29.Friberg MD, Zhai X, Holmes HA, Chang HH, Strickland MJ, Sarnat SE, et al. Method for fusing observational data and chemical transport model simulations To estimate spatiotemporally resolved ambient air pollution. Environ Sci Technol. 2016;50(7):3695–705. [DOI] [PubMed] [Google Scholar]
- 30.Yu H, Russell A, Mulholland J, Odman T, Hu Y, Chang HH, et al. Cross-comparison and evaluation of air pollution field estimation methods. Atmos Environ. 2018;179:49–60. [Google Scholar]
- 31.Dharmalingam S, Senthilkumar N, D’Souza RR, Hu Y, Chang HH, Ebelt S, et al. Developing air pollution concentration fields for health studies using multiple methods: Cross-comparison and evaluation. Environ Res. 2022;207:112207. [DOI] [PubMed] [Google Scholar]
- 32.Ozkaynak H, Baxter LK, Dionisio KL, Burke J. Air pollution exposure prediction approaches used in air pollution epidemiology studies. J Expo Sci Environ Epidemiol. 2013;23(6):566–72. [DOI] [PubMed] [Google Scholar]
- 33.Winquist A, Grundstein A, Chang HH, Hess J, Sarnat SE. Warm season temperatures and emergency department visits in Atlanta, Georgia. Environ Res. 2016;147:314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgerton ES, Hartsell BE, Saylor RD, Jansen JJ, Hansen DA, Hidy GM. The Southeastern Aerosol Research and Characterization Study: Part II. Filter-based measurements of fine and coarse particulate matter mass and composition. J Air Waste Manag Assoc. 2005;55(10):1527–42. [DOI] [PubMed] [Google Scholar]
- 35.Edgerton ES, Hartsell BE, Saylor RD, Jansen JJ, Hansen DA, Hidy GM. The Southeastern Aerosol Research and Characterization Study, part 3: Continuous measurements of fine particulate matter mass and composition. Journal of the Air & Waste Management Association. 2006;56(9):1325–41. [DOI] [PubMed] [Google Scholar]
- 36.Appel W, Roselle SJ, Pouliot G, Eder BK, Pierce TE, Mathur R, et al. Performance Summary of the 2006 Community Multiscale Air Quality (CMAQ) Simulation for the AQMEII Project: North American Application. Chapter 84, Steyjn Douw G. & Castelli Silvia Trini (ed.), NATO/SPS/ International Technical Meeting on Air Pollution Modeling and its Application. Springer Netherlands,, Netherlands, Series C:505–511. 2011. [Google Scholar]
- 37.Appel KW, Pouliot GA, Simon H, Sarwar G, Pye HOT, Napelenok SL, et al. Evaluation of dust and trace metal estimates from the Community Multiscale Air Quality (CMAQ) model version 5.0, Geosci. Model Dev., 6, 883–899, 10.5194/gmd-6-883-2013. 2013. [DOI] [Google Scholar]
- 38.Hao H, Chang HH, Holmes HA, Mulholland JA, Klein M, Darrow LA, et al. Air Pollution and Preterm Birth in the U.S. State of Georgia (2002–2006): Associations with Concentrations of 11 Ambient Air Pollutants Estimated by Combining Community Multiscale Air Quality Model (CMAQ) Simulations with Stationary Monitor Measurements. Environmental health perspectives. 2016;124(6):875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu X, Waller LA, Lyapustin A, Wang Y, Liu Y. 10-year spatial and temporal trends of PM2.5 concentrations in the southeastern US estimated using high-resolution satellite data. Atmos Chem Phys. 2014;14(12):6301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu X, Waller LA, Lyapustin A, Wang Y, Liu Y. Improving satellite-driven PM2.5 models with Moderate Resolution Imaging Spectroradiometer fire counts in the southeastern U.S. J Geophys Res Atmos. 2014;119(19):11375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lv B, Hu Y, Chang HH, Russell AG, Bai Y. Improving the Accuracy of Daily PM2.5 Distributions Derived from the Fusion of Ground-Level Measurements with Aerosol Optical Depth Observations, a Case Study in North China. Environ Sci Technol. 2016;50(9):4752–9. [DOI] [PubMed] [Google Scholar]
- 42.Chang HH, Hu X, Liu Y. Calibrating MODIS aerosol optical depth for predicting daily PM2.5 concentrations via statistical downscaling. J Expo Sci Environ Epidemiol. 2014;24(4):398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyapustin A, Wang Y, Laszlo I, Kahn R, Korkin S, Remer L, et al. Multiangle implementation of atmospheric correction (MAIAC): 2. Aerosol algorithm. J Geophys Res. 2011;116:D03211. [Google Scholar]
- 44.Szpiro AA, Sheppard L, Lumley T. Efficient measurement error correction with spatially misaligned data. Biostatistics. 2011;12(4):610–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarnat SE, Klein M, Sarnat JA, Mulholland J, Russell AG, Flanders WD, et al. An examination of exposure measurement error from air pollutant spatial variability in time-series studies. Journal of Exposure Science and Environmental Epidemiology. 2010;20:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gass K, Balachandran S, Chang HH, Russell AG, Strickland MJ. Ensemble-based source apportionment of fine particulate matter and emergency department visits for pediatric asthma. American Journal of Epidemiology. 2015;181(7):504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bi J, D’Souza RR, Rich DQ, Hopke PK, Russell AG, Liu Y, et al. Temporal changes in short-term associations between cardiorespiratory emergency department visits and PM2.5 in Los Angeles, 2005 to 2016. Environ Res. 2020;190:109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study was based on air pollution data from a variety of sources and on hospital billing records. We will make the air pollution exposure metrics data applied in this study, and relevant documentation and metadata available without cost to researchers. Individuals and parties must agree to the conditions of use governing access to the public release data, including reporting responsibilities, restrictions on redistribution of the data to third parties, proper acknowledgement of the data resource, and restrictions on use for commercial purposes. We will not be able to make the hospital records data (neither patient-level nor aggregate forms) available to external investigators given restrictions in our data use agreements between Emory University and the Georgia Hospital Association. The exposure-response functions generated in this study are available in the Supplemental Material for incorporation into meta-analyses, health impact assessments, or other analyses by external investigators.


