Abstract
Extracorporeal membrane oxygenation (ECMO) is an increasingly utilized intervention for cardiopulmonary failure. Analgosedation during ECMO support is essential to ensure adequate pain and agitation control and ventilator synchrony, optimize ECMO support, facilitate patient assessment, and minimize adverse events. Although the principles of analgosedation are likely similar for all critically ill patients, ECMO circuitry alters medication pharmacodynamics and pharmacokinetics. Lack of clinical guidelines for analgosedation during ECMO, especially at times of medication shortage, can affect patient management. Here, we review pharmacological considerations, protocols, and special considerations for analgosedation in critically ill adults receiving ECMO support.
Introduction
Extracorporeal membrane oxygenation (ECMO) is an increasingly used form of prolonged mechanical cardiopulmonary support. The two main configurations of ECMO, veno-venous (VV) and veno-arterial (VA), provide extracorporeal gas exchange and circulatory support in patients with refractory respiratory and cardiac failure, respectively. ECMO support has been shown to improve clinical outcomes1–5.
Optimal analgosedation is critical in patients on ECMO support. The main goals for analgosedation during ECMO support are pain control, agitation prevention and treatment, ventilator synchrony, optimization of ECMO flows, lowering metabolic demand, as well as facilitation of patient communication and neurological examination, early liberation, and optimization of long-term functional outcomes. Both inadequate and excessive analgosedation may lead to potential harm and worsen short- and long-term functional and cognitive outcomes6.
Several clinical guidelines have been published for analgosedation in acute respiratory distress syndrome and severe cardiogenic shock patients7,8. Although the principles of analgosedation are likely similar for all critically ill patients, no specific guidelines exist for patients receiving ECMO support, and deviations from current international guidelines are not unexpected in this complex population. ECMO circuitry can alter the pharmacokinetics of analgosedation medications9,10. In addition, ECMO initiation and maintenance require adequate pain, agitation, and movement control to optimize ventilatory support and gas exchange in VV-ECMO, lower metabolic demand of VA-ECMO patients, and prevent potential harm11–14. Therefore, ECMO patients commonly receive higher doses of analgesic and sedative medications than patients with similar disease severity who are not receiving ECMO support15. Lack of evidence-based practice guidelines is more conspicuous during increased demand and medication shortage, as experienced in the ongoing COVID-19 pandemic. This article reviews analgosedation in critically ill adults receiving ECMO support.
Pharmacological Considerations
The analgosedation choice varies based on clinical goals and patient-specific factors such as hemodynamics and renal and hepatic function. In addition, the ECMO circuit alters medication pharmacokinetics and pharmacodynamics16 depending on the physicochemical properties of the drug, such as lipophilicity, protein binding, molecular size, and ionization degree17. Pharmacokinetic properties of commonly used analgesic and sedative agents with considerations in ECMO are summarized in Table 1. Of note, practitioners must be cautious when interpreting this data as much of the available literature is extrapolated from ex vivo and neonatal studies with limited evidence regarding clinical outcomes as well as the differences in body composition and immature renal and hepatic function in the neonatal population17.
Table 1:
Pharmacokinetic properties of analgesic and sedative agents and considerations for use in ECMO.
| Class | Medication | Protein Binding | Lipophilicity (logP)* | Sequestration in ECMO Circuit | Considerations for Use in ECMO |
|---|---|---|---|---|---|
| Opioid | Hydromorphone | 8–19% | 1.1 | Limited (<25%) sequestration | Standard dosing or slightly increased dosing is likely sufficient |
| Fentanyl | 80–85% | 4.1 | Highly sequestered (up to 97%) | May require high doses | |
| Oxycodone | 45% | 0.7 | Unlikely to be sequestered but no PK data | Requires adequate gastrointestinal absorption May reduce IV opioid requirements but limited utility in patients with high opioid requirements |
|
| Morphine | 35% | 0.9 | Not sequestered | Not preferred due to adverse effects and accumulation in renal dysfunction | |
| Methadone | 85–90% | 3.9 | Likely sequestered, but no PK data | Requires QTc monitoring Unpredictable sequestration and long half-life may lead to adverse effects | |
| Non-benzodiazepine sedative | Dexmedetomidine | 94% | 2.8 | Likely sequestered | Increased dosing may be necessary to achieve sedation goals but maximum 1.5 mcg/kg/hr still recommended due to the risk of bradycardia and hypotension |
| Propofol | 95–99% | 3.8 | Highly sequestered | Increased dosing may be necessary to achieve sedation goals but avoid prolonged high doses Risk of propofol-related infusion syndrome, especially with ECMO decannulation |
|
| Ketamine | 54% | 2.2 | Likely sequestered | Avoid if presence or risk of myocardial ischemia, decompensated heart failure, catecholamine depletion, tachycardia, or arrhythmias | |
| Benzodiazepine | Clonazepam | 82–86% | 2.4 | Likely moderately sequestered, but no PK data | Requires adequate gastrointestinal absorption Slightly higher doses may be required to achieve sedation goals |
| Lorazepam | 85% | 2.4 | Moderately sequestered (59%) | Monitor for signs and symptoms of propylene glycol toxicity, including osmolal gap, especially in patients receiving prolonged or high doses | |
| Midazolam | 97% | 4.3 | Highly sequestered (87%) | May require high doses to achieve sedation goals | |
| Diazepam | 98–99% | 2.8 | Highly sequestered (88%) | Not recommended due to long half-life and high sequestration | |
| Adjunctive agent | Gabapentin | <3% | 1.3 | Unlikely to be sequestered but no PK data | Standard dosing is likely sufficient |
| Quetiapine | 83% | 2.8 | Likely moderately sequestered but no PK data | No data guiding use; dose to clinical effects with QTc monitoring | |
| Haloperidol | ∼90% | 4.3 | Likely moderately sequestered but no PK data | No data guiding use; dose to clinical effects with QTc monitoring |
PK= pharmacokinetic
LogP: log of the octanol/water partition coefficient, which measures lipophilicity. High positive values indicate lipophilic compounds.
Alterations in Volume of Distribution
ECMO can increase the volume of distribution (Vd) and decrease serum levels of certain medications17 that can significantly increase the analgosedation requirement. Components of the ECMO circuit, such as the membrane oxygenator and polyvinyl chloride (PVC) tubing, increase the surface area for drug sequestration and adsorption, although the oxygenator contribution to sequestration is minimal17,18. The degree of sequestration typically depends on the medication lipophilicity and protein binding19, but the effects of molecular size and ionization degree are not well-characterized18. Lipophilic medications with a higher positive n-octanol/water partition coefficient (typically with a log P of ≥2) have a higher risk of sequestration than hydrophilic medications due to solubility in organic materials, such as PVC tubing16,17,20. Sequestration is also higher with highly protein-bound medications, likely due to the binding of blood or the priming solution proteins to the circuit16. Critical illness may also alter plasma concentrations of highly protein-bound medications due to decreased albumin and increased α1-acid glycoprotein concentrations20. However, less medication may be necessary over time after the binding sites on circuit surfaces become saturated17. Conversely, increased drug dosing may be required when circuit components are exchanged18. The circuit acts as a reservoir and slowly releases sequestered drugs after medication discontinuation resulting in a prolonged duration of action17,18. The Vd for hydrophilic medications also increases in ECMO from hemodilution due to priming solutions or volume resuscitation or by inducing a systemic inflammatory response with leaky capillaries17,20. Hemodilution can reduce concentrations of plasma proteins leading to higher free concentrations of highly protein-bound medications and toxicity20. Liberation from ECMO will decrease the Vd necessitating a significant empiric reduction in medication dosing to avoid toxicity20.
Alterations in Medication Clearance
Medication clearance is typically reduced in patients receiving ECMO due to renal and hepatic hypoperfusion or insufficiency, resulting in the accumulation of medications and metabolites17. However, clearance may initially increase due to augmented cardiac output from volume resuscitation, inotropic support, and cardiac support with VA-ECMO17,20.
Analgosedation Medications
Opioids
Clinical practice guidelines for preventing and managing pain, agitation/sedation, delirium, immobility, and sleep disruption in critically ill adults acknowledge that opioids remain the cornerstone for pain management in most settings20. These guidelines support an analgesia-first approach to minimize the use of sedatives. Using the lowest effective dose of opioid with careful titration as part of a multimodal analgesia regimen is recommended21. Daily sedative interruption and nursing-protocolized targeted sedation can be utilized to maintain light levels of sedation and minimize medication-related adverse effects21. The choice of opioid and the frequency of dosing (e.g., intermittent bolus, continuous infusion) depend on goals of care, frequency and severity of pain or agitation, and pharmacokinetic factors.
Fentanyl is commonly utilized in intensive care settings based on its rapid onset and ease of titration; however, its high sequestration level in ECMO circuits makes it a less desirable agent in this population.10 The anilidopiperidines (such as fentanyl, alfentanil, remifentanil, and sufentanil) are highly lipophilic and highly protein-bound; therefore, extensive binding to components of the ECMO circuit occurs (Table 1)10. An ex vivo study evaluated fentanyl concentrations in crystalloid- and albumin-primed circuits and demonstrated a significant 97% loss of fentanyl at 24 hours compared to baseline12. Another ex vivo study compared crystalloid- and blood-primed circuits and showed fentanyl losses of 87% and 100%, respectively, at 24 hours22. Based on these findings, high doses of fentanyl would be required to be effective for ECMO patients, and alternative/additional agents may be considered18. A strategy recently described in the literature is to initiate fentanyl as the first-line opioid and convert it to hydromorphone if fentanyl doses reach 400 mcg/hr without achieving adequate analgesia23. In a retrospective cohort study of 81 (38 obese and 43 non-obese) patients receiving VV-ECMO, 31 (38%) required a switch to hydromorphone within the first seven days23.
Hydromorphone may be considered an initial opioid agent in patients requiring ECMO or a second-line agent for patients who have failed alternative agents. The onset of hydromorphone is slightly longer than fentanyl; however, it is hydrophilic and not highly protein-bound, which may be preferable in patients receiving ECMO. An ex vivo analysis of a pediatric ECMO circuit demonstrated hydromorphone losses at 12 hours were 23.5% compared to fentanyl losses of 55.4%24. A single-center propensity-matched study, comparing ECMO patients who received hydromorphone versus fentanyl, demonstrated significantly lower median (interquartile range; IQR) daily fentanyl equivalents [555 mcg (287–905) vs. 2291 mcg (1053–4023), p<0.005], and an increased number of delirium-free and coma-free days (53.2% vs. 42.1%, p=0.006) in patients receiving hydromorphone25.
Morphine is hydrophilic and not highly protein-bound; and, therefore, is not sequestered in the ECMO circuit. An ex vivo study of morphine concentrations in ECMO circuits primed with crystalloid, albumin, and fresh whole blood demonstrated no significant loss of morphine at 24 hours compared to baseline (103% vs. 97%)12. However, the risk of adverse effects with morphine administration may outweigh the benefits in critically ill patients. Morphine and its active metabolites may accumulate in patients with renal dysfunction, and adverse effects include prolonged sedation, neurotoxicity, and histamine release resulting in hypotension and bronchospasm16,26.
Intravenous opioids are recommended first-line in patients with non-neuropathic pain and offer rapid onset and titration advantages without concern for erratic absorption16,26. However, enteral opioids and non-opioid analgosedation can also be considered in patients with appropriate gastrointestinal functions26. In the setting of intravenous analgesia and sedative shortages, optimization of enteral therapy can reduce the dose requirements of intravenous agents but may also require large and frequent enteral dosing to achieve an equianalgesic effect (Table 1). While the onset of enteral opioid therapy is slower than intravenous medications, hence not appropriate for acute pain, their prolonged duration may benefit certain patients. Enteral treatments such as oxycodone and methadone may be considered, but data is minimal. No data are available regarding the serum concentrations or clinical outcomes in ECMO patients receiving oxycodone; however, due to its hydrophilic nature and lack of high protein-binding, the sequestration in the ECMO circuit is likely limited. Methadone is lipophilic and highly protein-bound; therefore, it is not ideal for patients receiving ECMO. It is also associated with QT prolongation, and the risks may outweigh the benefit in high doses. Two case reports of methadone use in patients receiving ECMO showed decreased intravenous opioid and sedative infusion doses after initiating methadone at doses of 30 mg four times per day and 10 mg three times per day27. The authors suggested reserving methadone for patients with high sedative requirements, demonstration of opioid tolerance, and need for long-term support.
Benzodiazepines
Benzodiazepines are lipophilic medications; however, they vary slightly in lipophilicity, onset and duration of action, and metabolism. While benzodiazepines may help manage agitation or facilitate mechanical ventilation, non-benzodiazepine sedatives are preferred to improve short- and long-term outcomes in critically ill patients. However, based on the limitations of pharmacological parameters of analgosedation agents in patients receiving ECMO, the use of benzodiazepine agents may sometimes be necessary when adequate analgosedation cannot be achieved with non-benzodiazepine sedatives or is limited by untoward effects.
Midazolam is lipophilic, highly protein-bound, and binds extensively to the ECMO circuit. An ex vivo study demonstrated that midazolam losses at 24 hours were 87%, while another ex vivo study showed similar losses of 46% at 30 minutes and 89% at 24 hours (p=0.01)9,12. Accumulation of midazolam and its active metabolites occurs in patients with renal or hepatic insufficiency or prolonged administration, resulting in protracted sedation. Lorazepam is less lipophilic and protein-bound than midazolam and may be preferable in patients receiving ECMO16. An ex vivo evaluation determined that lorazepam losses in the ECMO circuit at 48 hours were less than midazolam, 59% vs. 83%28. However, propylene glycol may be present in parenteral and enteral solution forms of lorazepam resulting in osmolar gap metabolic acidosis, seizures, respiratory depression, and renal insufficiency29–32. Monitoring the osmolal gap and substituting tablet formulations can lower propylene glycol toxicity in patients receiving prolonged therapy or high doses. No data exist regarding the use of clonazepam in ECMO patients; however, it may be a reasonable enteral alternative to lorazepam due to similar lipophilicity and protein-binding characteristics. Diazepam is highly lipophilic and protein-bound with 88% sequestration in the circuit and, therefore, is not a preferred agent in patients with ECMO.
Non-benzodiazepine Sedatives
Alpha-2 Agonists:
Dexmedetomidine is commonly utilized as a part of the sedation regimen in the intensive care unit. Dexmedetomidine exerts its action as a selective alpha-2 receptor agonist, allowing the patient to be easily aroused with minimal respiratory depressant effects26. There is limited clinical data on the use of dexmedetomidine in patients receiving ECMO. In vitro studies have shown significant sequestration of dexmedetomidine in the ECMO circuit. Blood sampling at various time intervals showed continuous drug loss, with more than 80% of the medication lost at 24 hours, without a significant difference between new and old circuits or between the pre-and post-oxygenator samples, suggesting the contribution of PVC tubing to drug loss33. However, a more recent in vitro study showed that predominant drug extracted by the oxygenator with 41% and 96% recovery at 24 hours with and without the oxygenator, respectively34. The in vitro studies used bolus rather than continuous dexmedetomidine administration. While dexmedetomidine may be sequestered by the PVC tubing and/or the oxygenator in the ECMO circuit, it is still a practical option when light sedation is desired. In a small study of 26 ECMO patients, dexmedetomidine was used in 92% of patients at a median dose of 0.7 mcg/kg/hr without increasing doses needed for prolonged use35.
Propofol:
Propofol is a gamma-aminobutyric acid -agonist with sedative, hypnotic, and anxiolytic properties. Propofol is highly lipophilic with a short duration of action, making it another commonly used sedative in the intensive care unit26. Concerns for oxygenator failure with the use of propofol have been raised since it is a lipid emulsion. In a study of 43 patients on ECMO, nearly 40% received propofol for a median duration of four days with no difference in the rate of oxygenator exchange in those receiving propofol versus those who did not.36 In addition, benzodiazepine and opioid use were significantly lower in the propofol group, suggesting propofol’s efficacy as a sedative37. Another study evaluated 122 patients with a propofol-based sedation strategy, compared to midazolam, and found no difference in oxygenator duration between the strategies. Propofol sequestration in the ECMO circuit has also been reported. In an ex vivo circuit, propofol concentrations decreased by 70% only 30 minutes after administration, where the control sample of propofol in polypropylene tubes had a negligible loss at the same time. This study also demonstrated that higher oxygen concentrations led to further propofol degradation9. Since much of propofol is sequestered, patients will likely need higher doses to achieve their sedation targets. Similarly, with the discontinuation of ECMO, a dose reduction is probably warranted. One case report described the development of propofol infusion syndrome upon cessation of ECMO without empiric propofol weaning38.
Ketamine:
Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist which may reduce hyperalgesia and opioid tolerance. Ketamine is lipophilic but not highly protein-bound, so it is unclear whether high doses are necessary to achieve adequate sedation and reduce opioid requirements in patients receiving ECMO. A retrospective, single-center cohort study of 26 ECMO patients reported the median starting dose of ketamine at 50 mg/hr (range 6–150), leading to a reduction in sedative and opioid infusions within two hours of ketamine initiation in more than a third of the patients without a significant difference in sedation 24 hours after initiation39. A randomized trial of 20 VV-ECMO patients receiving standard sedation versus low-dose ketamine plus standard sedation did not find a difference in opioid or sedative requirement. However, the findings may be attributed to the deep level of sedation, lack of standardized sedation protocol, and possible inadequate ketamine dosing. Data supporting alterations in ketamine pharmacokinetic parameters during ECMO is limited to case reports. One case showed reduced sedative use in a VV-ECMO patient receiving ketamine infusion, initiated at 0.06 mg/kg/hr and titrated to 0.6 mg/kg/hr40. In a VV-ECMO patient who had been started on ketamine infusion at 0.22 mg/kg/hr and titrated to 0.625 mg/kg/hr for mild sedation, the mean ketamine level at the maximum dose was 402 ng/mL, lower than a reference range of 500–6500 ng/mL for non-ECMO patients41. Another case report evaluated ketamine pharmacokinetics at high doses (initiated at 0.5 mg/kg/hr and escalated to 2 mg/kg/hr) in a VV-ECMO patient and found the mean steady-state concentration at 1018.7 ng/mL with an estimated Vd of 14.2 L/kg. This level is almost seven times greater than healthy adults but similar to non-ECMO critically ill patients42. Patients with lighter sedation levels need to be monitored for side effects such as hallucination.
Adjunctive agents
Adjunctive agents such as gabapentin may be helpful in the management of neuropathic pain. Gabapentin is recommended as an adjunct pain medication after cardiac surgery21. Despite the lack of clinical data, it may also be a reasonable adjunct drug during ECMO given its favorable pharmacokinetics with negligible protein-binding and low lipophilicity43. Although not part of the standard sedation practices in the intensive care unit, both typical (such as haloperidol) and atypical (such as quetiapine) anti-psychotics, may also be helpful to control severe agitation and delirium, when necessary, in ECMO patients21.
Analgosedation Protocols
Analgosedation monitoring is required to ensure the adequacy of treatment and minimize medication use. It is critical to define, measure, document, and daily reassess the analgosedation goal, and make adjustments based on the patient’s individual needs at various stages of critical illness. Analgosedation monitoring in critically ill patients has been reviewed extensively. Clinical monitoring includes subjective bedside assessment and objective evaluations using standard and validated scoring systems such as Richmond Agitation/Sedation Scale (RASS). Electrophysiological techniques using electroencephalography, electromyography, and evoked potential signals are commonly employed where clinical monitoring is unreliable or insufficient in non-communicating, deeply sedated, and paralyzed patients44,45. Advanced processing of electroencephalography signals, such as frequency, power, or burst-suppression analysis, provides objective and continuous monitoring of brain function and sedation. However, additional studies are required to prove their validity and reliability in ECMO patients. The proposed analgosedation protocol, summarized in Figure 1, applies the abovementioned principles and pharmacological considerations.
Figure 1:
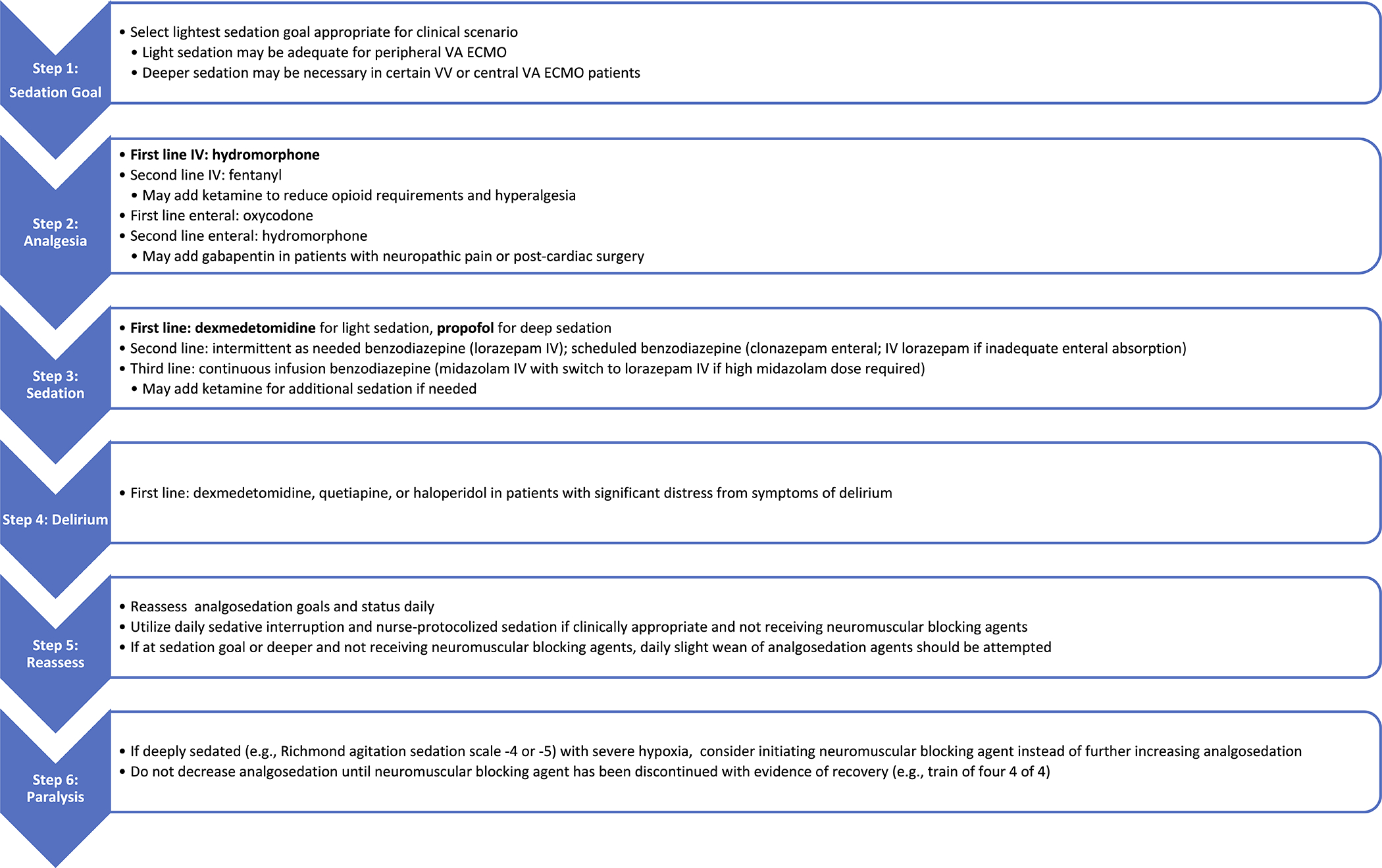
Suggested Analgosedation Protocol during ECMO Support. Refer to Table 1 for important considerations regarding each medication listed in the Figure.
A large number of patients will already be deeply sedated and often paralyzed at the time of ECMO consideration due to the severity of their cardiopulmonary disease. While fentanyl, midazolam, and propofol are the most common analgosedation agents used in the critical care setting, they are not optimal during ECMO support due to their high sequestration.
Intermittent as-needed analgesic and sedative medications are preferred over intermittent scheduled or continuous infusion medications to minimize sedative use. The protocol suggests analgesia-first with hydromorphone infusion as the first line; if a continuous infusion is required, we recommend a starting dose of 1 mg/hr. The alternative opioids are shown in Figure 1. We also recommend prioritizing non-benzodiazepine sedatives, such as ketamine infusion, before initiation of benzodiazepines administered either as a continuous infusion or intermittently as needed. Ketamine infusion can be initiated at 0.5 mg/kg/hr. For patients requiring light sedation, dexmedetomidine is preferred, whereas propofol can achieve deep sedation. In patients with intolerance to non-benzodiazepine sedatives or inadequate sedation, a benzodiazepine may be required; we recommend clonazepam as the first-line agent, to start at 3 mg enteral twice daily (roughly equivalent to lorazepam 1mg/hr). Although lorazepam sequestration is lower than midazolam (30 versus 87%), it is not recommended as a first-line agent due to the associated propylene glycol.
Deeper levels of sedation, frequently with neuromuscular blockade, are often required during the first 24–48 hours of ECMO cannulation to optimize ECMO support. Sedation can be lightened once a steady-state has been achieved and recovery from neuromuscular blockade has been demonstrated. The overall goal should be the maintenance of analgosedation as light as tolerated to enable accurate neurologic assessments and decrease potential side effects. The patient should be awakened when possible, particularly when early mobilization and rehabilitation are desired, such as a bridge to transplant. In such cases, ketamine or low-dose hydromorphone infusion is preferred. The addition of enteral opiates (such as oxycodone) and benzodiazepines (such as clonazepam) can be considered to reduce the total infusion requirements in patients with adequate gastrointestinal absorption. Enteral and second-line intravenous analgosedative agents can also be considered in case of medication shortage. Of note, enteral analgosedation agents may have limited utility in patients with high intravenous analgosedation requirements depending on the pill burden associated with an equivalent enteral dose.
Not uncommonly, weaning analgosedation is delayed due to pain and agitation. Administration of the first-line agents is often titrated to prevent self-injury and interference with the ECMO flows. Adjunct agents, such as dexmedetomidine and quetiapine, are often added to improve delirium and agitation control. At times, agents with higher sequestration, such as propofol, may be required. After a period of analgosedation maintenance, a slower step-wise weaning approach may be more feasible than traditional waking trials when clinical improvement is detected. Weaning the opioid and ketamine doses on alternating days with benzodiazepine doses may effectively reduce sedation without increasing agitation. Complete liberation from all analgosedation medications is often not feasible or necessary. A low dose of dexmedetomidine or other agents in the regimen can be continued through clinical improvement and the ECMO weaning process. Once the patient is decannulated, analgosedation medications shall be carefully adjusted. A more traditional weaning protocol can be adopted without the circuit effects with the administration of agents like fentanyl and midazolam. Adherence to established principles of analgosedation induction, maintenance, and weaning/liberation in critically-ill patients is essential.
Special Considerations and Future Directions
Renal Replacement Therapy
Acute kidney injury is prevalent in ECMO patients due to multiple factors, including predisposing comorbidities, hemodynamic lability, coagulation alterations, systemic inflammation, multi-organ failure, and administration of nephrotoxic agents46–49. Acute kidney injury is also associated with worsened clinical outcomes of ECMO patients48–55. Accordingly, renal replacement therapy is commonly indicated to treat ECMO patients with acute kidney injury, metabolic derangements, and volume overload51–53,56–59. Renal replacement therapy during ECMO is safe, feasible, and life-saving and facilitates weaning ECMO support; however, it is unclear whether it improves survival56. However, the addition of renal replacement therapy can further complicate medication pharmacokinetics, especially considering variations in the renal replacement techniques10. Various renal replacement modalities can be used, including integration into the ECMO circuit or a separate circuit56,60. Continuous renal replacement therapy is more commonly applied due to hemodynamic instability of ECMO patients61–63. In the absence of clinical and population pharmacokinetics studies, close monitoring of analgesic and sedative medications’ clinical effects, adverse events, and therapeutic levels, when possible, is recommended.
Therapeutic hypothermia
Induced therapeutic hypothermia and targeted temperature management are commonly employed after cardiac arrest to improve cardiac and neurological recovery. The increasing use of extracorporeal cardiopulmonary resuscitation (E-CPR) has led to increased concomitant hypothermia in ECMO patients, although its impact on neurological outcome is understudied and unclear at this time64. Hypothermia can further affect several aspects of pharmacokinetics by decreasing medication metabolism and excretion and absorption and distribution65. Therefore, parenteral, short-acting, and rapid-onset analgosedation administration is reasonable during hypothermia65.
Obesity
ECMO support is feasible and effective in obese patients66,67. However, achieving optimal analgosedation during ECMO support can be more challenging in obese patients. As described above, the chemical properties, particularly lipophilic and protein-binding characteristics, can significantly affect medical sequestration. Obesity can further affect medication pharmacokinetics by several mechanisms. Fat tissue in obese patients increases the volume of distribution for lipophilic agents68,69. Increased kidney and liver mass and blood flow, as well as decreased expression of hepatic and intestinal cytochrome P450 in critically-ill obese patients, may also enhance drug clearance70,71. Together these mechanisms can result in higher analgosedation requirements9,19,33,72,73. However, the findings of previous reports have been inconsistent. While some studies suggested an increased need for lipophilic agents such as fentanyl and midazolam during ECMO support, the relationship is not linear with the body mass and was only observed earlier during medication administration23,68. Other studies did not find a significant change in midazolam requirement in obese ECMO patients23.
Medication Shortage
Medication shortage at times of crisis due to decreased production, impaired delivery, or increased consumption can further complicate optimal analgosedation in ECMO patients. For instance, during the COVID-19 pandemic, a surge in mechanical ventilation and ECMO usage was accompanied by a worldwide medication shortage, including analgesics, sedatives, and paralytics74–77. A better understanding of medication pharmacodynamics, pharmacokinetics, and safety profile, as reviewed above, provides the opportunity to use alternative medications despite the lack of previous studies and even despite existing clinical guidelines. During the COVID-19 pandemic, some institutions opted to use intravenous benzodiazepines more frequently for deep sedation or administer more available but otherwise less commonly used agents such as oral opiates, benzodiazepines, and alpha-2 agonist (clonidine) as well as volatile agents (such as isoflurane, sevoflurane, and desflurane) and oral and/or intravenous barbiturates (such as phenobarbital and pentobarbital), non-steroidal anti-inflammatory agents (such as ibuprofen and ketorolac), anti-psychotics, and anti-seizure medications (such as gabapentin, pregabalin, and carbamazepine) with sedative properties74,75. Similar strategies may be adopted in future crises.
Awake ECMO and Spontaneous Breathing
Recently, there has been a growing interest in ECMO in spontaneously breathing and awake patients. Awake ECMO enables the initiation and maintenance of ECMO support in non-intubated patients and is even an alternative to mechanical ventilation78,79. Awake ECMO requires lighter analgosedation, and at least in theory, can reduce delirium, duration of mechanical ventilation, hospital/ICU length of stay, and short- and long-term adverse effects of prolonged analgosedation and mechanical ventilation80,81. It can also enhance neurological assessment, patient communication, early rehabilitation, and even improve mortality82,83. However, close monitoring with adequate analgosedation is essential to avoid pain, discomfort, and self-harm. Accumulating evidence supports safety, feasibility, and favorable outcomes of awake ECMO in VV-ECMO for ARDS and patients with cardiogenic shock on VA-ECMO support. A study of 12 patients with severe ARDS extubated after a median of 10.2 days of ECMO showed no increased mortality associated with awake ECMO while awaiting native lung recovery84. Several studies have shown that awake ECMO as a bridge to lung transplantation is safe and can improve the duration of mechanical ventilation, respiratory distress, rehabilitation, and survival85–88. Similarly, in a series of 231 VA-ECMO patients, 39% of the patients underwent awake ECMO (i.e., invasive mechanical ventilation used in ≤50% of the VA-ECMO duration) that was associated with significantly lower rates of analgosedation use, tracheostomy, pneumonia, and antibiotic use, renal replacement therapy, and mortality89,90. Awake ECMO can also facilitate a bridge to left ventricular assist device implantation with improved mortality91.
Conclusions
Critically ill patients often have significant variability in pharmacokinetics and pharmacodynamics with altered hemodynamics and physiology. The addition of the ECMO circuit poses special challenges in understanding drug metabolism, clearance, and distribution. We provided a comprehensive review of available literature on the pharmacokinetics of commonly used analgosedative medications in ECMO patients. We also provided recommendations on the preferred analgosedative regimen, although the optimal analgosedation medication choice, dosing, duration, and weaning strategy are unknown due to limited data. Also, there are no studies comparing outcomes among different analgosedation strategies. Further research is warranted to understand better the effectiveness of analgosedative medications in ECMO patients and their impact on overall outcomes.
Footnotes
Disclosures/Conflicts of Interest: The authors report no conflicts of interests to declare.
References
- 1.Thiagarajan RR, Barbaro RP, Rycus PT, et al. : Extracorporeal Life Support Organization Registry International Report 2016 ASAIO J 63: 60–67, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Peek GJ, Mugford M, Tiruvoipati R, et al. : Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial Lancet 374: 1351–1363, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Abrams D, Brodie D: Extracorporeal Membrane Oxygenation for Adult Respiratory Failure: 2017 Update Chest 152: 639–649, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Karagiannidis C, Brodie D, Strassmann S, et al. : Extracorporeal membrane oxygenation: evolving epidemiology and mortality Intensive Care Medicine 42: 889–896, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Combes A, Hajage D, Capellier G, et al. : Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome N Engl J Med 378: 1965–1975, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Bhaskar S, Sharma D, Walker AH, et al. : Acute Neurological Care in the COVID-19 Era: The Pandemic Health System REsilience PROGRAM (REPROGRAM) Consortium Pathway Front Neurol 11: 579, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bateman RM, Sharpe MD, Jagger JE, et al. : 36th International Symposium on Intensive Care and Emergency Medicine Crit Care 20: 94, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho Y-J, Moon JY, Shin E-S, et al. : Clinical Practice Guideline of Acute Respiratory Distress Syndrome Tuberc Respir Dis 79: 214–233, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemaitre F, Hasni N, Leprince P, et al. : Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood Critical Care 19, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shekar K, Fraser JF, Smith MT, Roberts JA: Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation J Crit Care 27: 741.e9–18, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Zwischenberger JB, Extracorporeal Life Support Organization: ECMO: Extracorporeal Cardiopulmonary Support in Critical Care Extracorporeal Life Support Organization, 2000. [Google Scholar]
- 12.Shekar K, Roberts JA, Mcdonald CI, et al. : Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation Critical Care 16: R194, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skelton PA, Lillyblad MP, Eckman PM, et al. : Clinical outcomes associated with sedation and analgesia in patients supported with venoarterial extracorporeal membrane oxygenation Int J Artif Organs 43: 277–282, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Sanfilippo F, Ippolito M, Santonocito C, et al. : Long-term functional and psychological recovery in a population of acute respiratory distress syndrome patients treated with VV-ECMO and in their caregivers Minerva Anestesiologica 85, 2019. [DOI] [PubMed] [Google Scholar]
- 15.Alhazzani W, Møller MH, Arabi YM, et al. : Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satyapriya SV, Lyaker ML, Rozycki AJ, Papadimos: Sedation, Analgesia Delirium in the ECMO Patient, in: Firstenberg MS (ed) Extracorporeal Membrane Oxygenation. Rijeka: IntechOpen, 2016. [Google Scholar]
- 17.Cheng V, Abdul-Aziz M-H, Roberts JA, Shekar K: Optimising drug dosing in patients receiving extracorporeal membrane oxygenation J Thorac Dis 10: S629–S641, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha MA, Sieg AC: Evaluation of Altered Drug Pharmacokinetics in Critically Ill Adults Receiving Extracorporeal Membrane Oxygenation Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 37: 221–235, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Shekar K, Roberts JA, Mcdonald CI, et al. : Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study Critical Care 19, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dzierba AL, Abrams D, Brodie D: Medicating patients during extracorporeal membrane oxygenation: the evidence is building Crit Care 21: 66, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devlin JW, Skrobik Y, Gélinas C: Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the … Crit Care, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Mehta NM, Halwick DR, Dodson BL, Thompson JE, Arnold JH: Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment Intensive Care Medicine 33: 1018–1024, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Verkerk BS, Dzierba AL, Muir J, et al. : Opioid and Benzodiazepine Requirements in Obese Adult Patients Receiving Extracorporeal Membrane Oxygenation Annals of Pharmacotherapy 54: 144–150, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Heith CS, Hansen LA, Bakken RM, et al. : Effects of an Ex Vivo Pediatric Extracorporeal Membrane Oxygenation Circuit on the Sequestration of Mycophenolate Mofetil, Tacrolimus, Hydromorphone, and Fentanyl The Journal of Pediatric Pharmacology and Therapeutics 24: 290–295, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landolf KM, Rivosecchi RM, Goméz H, et al. : Comparison of Hydromorphone versus Fentanyl-based Sedation in Extracorporeal Membrane Oxygenation: A Propensity-Matched Analysis Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 40: 389–397, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Barr J, Fraser GL, Puntillo K, et al. : Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit Crit Care Med 41: 263–306, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Dong E, Fellin R, Ramzy D, et al. : Role of Methadone in Extracorporeal Membrane Oxygenation: Two Case Reports J Extra Corpor Technol 50: 252–255, 2018 [PMC free article] [PubMed] [Google Scholar]
- 28.Harthan AA, Buckley KW, Heger ML, Fortuna RS, Mays K: Medication Adsorption into Contemporary Extracorporeal Membrane Oxygenator Circuits The Journal of Pediatric Pharmacology and Therapeutics 19: 288–295, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller MA, Forni A, Yogaratnam D: Propylene glycol-induced lactic acidosis in a patient receiving continuous infusion pentobarbital Ann Pharmacother 42: 1502–1506, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Hayman M, Seidl EC, Anderson JA, Harchelroad F: MONITORING LORAZEPAM INDUCED PROPYLENE GLYCOL TOXICITY IN A CRITICAL CARE POPULATION: A PROSPECTIVE CASE SERIES Critical Care Medicine 30: A157, 2002 [Google Scholar]
- 31.Zosel A, Egelhoff E, Heard K: Severe Lactic Acidosis After an Iatrogenic Propylene Glycol Overdose Pharmacotherapy 30: 219–219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson KC, Reardon C, Theodore AC, Farber HW: Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines: a case series and prospective, observational pilot study Chest 128: 1674–1681, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Wagner D, Pasko D, Phillips K, Waldvogel J, Annich G: In vitro clearance of dexmedetomidine in extracorporeal membrane oxygenation Perfusion 28: 40–46, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Dallefeld SH, Sherwin J, Zimmerman KO, Watt KM: Dexmedetomidine extraction by the extracorporeal membrane oxygenation circuit: results from an in vitro study Perfusion 35: 209–216, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel M, Altshuler D, Lewis TC, et al. : Sedation Requirements in Patients on Venovenous or Venoarterial Extracorporeal Membrane Oxygenation Annals of Pharmacotherapy 54: 122–130, 2020 [DOI] [PubMed] [Google Scholar]
- 36.Hohlfelder B, Szumita PM, Lagambina S, Weinhouse G, Degrado JR: Safety of Propofol for Oxygenator Exchange in Extracorporeal Membrane Oxygenation ASAIO J 63: 179–184, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Hohlfelder B, Szumita PM, Lagambina S, Weinhouse G, Degrado JR: Safety of Propofol for Oxygenator Exchange in Extracorporeal Membrane Oxygenation ASAIO Journal 63: 179–184, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Lal A, Nabzdyk C, Ramakrishna H, Radosevich M: Consider Heightened Awareness of Propofol Infusion Syndrome after Extracorporeal Membrane Oxygenation (ECMO) Decannulation Journal of Cardiothoracic and Vascular Anesthesia 34: 2174–2177, 2020 [DOI] [PubMed] [Google Scholar]
- 39.Tellor B, Shin N, Graetz TJ, Avidan MS: Ketamine infusion for patients receiving extracorporeal membrane oxygenation support: a case series F1000Res 4: 16, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Floroff CK, Hassig TB, Cochran JB, Mazur JE: High-Dose Sedation and Analgesia During Extracorporeal Membrane Oxygenation: A Focus on the Adjunctive Use of Ketamine J Pain Palliat Care Pharmacother 30: 36–40, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Farrokh S, Cho S-M, Kim BS, Geocadin R: 932: KETAMINE INFUSION FOR SEDATION IN A PATIENT ON EXTRACORPOREAL MEMBRANE OXYGENATION Crit Care Med 47: 445, 2019 [Google Scholar]
- 42.Lam E, Rochani A, Kaushal G, et al. : Pharmacokinetics of Ketamine at Dissociative Doses in an Adult Patient With Refractory Status Asthmaticus Receiving Extracorporeal Membrane Oxygenation Therapy Clinical Therapeutics 41: 994–999, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P: A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin Clin Pharmacokinet 49: 661–669, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Hajat Z, Ahmad N, Andrzejowski J: The role and limitations of EEG-based depth of anaesthesia monitoring in theatres and intensive care Anaesthesia 72 Suppl 1: 38–47, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Romagnoli S, Franchi F, Ricci Z: Processed EEG monitoring for anesthesia and intensive care practice Minerva Anestesiol 85: 1219–1230, 2019 [DOI] [PubMed] [Google Scholar]
- 46.Kilburn DJ, Shekar K, Fraser JF: The Complex Relationship of Extracorporeal Membrane Oxygenation and Acute Kidney Injury: Causation or Association? BioMed Research International 2016: 1–14, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SW, Yu M-Y, Lee H, et al. : Risk Factors for Acute Kidney Injury and In-Hospital Mortality in Patients Receiving Extracorporeal Membrane Oxygenation PLoS One 10: e0140674, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kielstein JT, Heiden AM, Beutel G, et al. : Renal function and survival in 200 patients undergoing ECMO therapy Nephrology Dialysis Transplantation 28: 86–90, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Yan X, Jia S, Meng X, et al. : Acute kidney injury in adult postcardiotomy patients with extracorporeal membrane oxygenation: evaluation of the RIFLE classification and the Acute Kidney Injury Network criteria Eur J Cardiothorac Surg 37: 334–338, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Lin C-Y, Chen Y-C, Tsai F-C, et al. : RIFLE classification is predictive of short-term prognosis in critically ill patients with acute renal failure supported by extracorporeal membrane oxygenation Nephrology Dialysis Transplantation 21: 2867–2873, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Han S-S, Kim HJ, Lee SJ, et al. : Effects of Renal Replacement Therapy in Patients Receiving Extracorporeal Membrane Oxygenation: A Meta-Analysis The Annals of Thoracic Surgery 100: 1485–1495, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Antonucci E, Lamanna I, Fagnoul D, Vincent J-L, De Backer D, Silvio Taccone F: The Impact of Renal Failure and Renal Replacement Therapy on Outcome During Extracorporeal Membrane Oxygenation Therapy Artif Organs 40: 746–754, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Wu V-C, Tsai H-B, Yeh Y-C, et al. : Patients Supported by Extracorporeal Membrane Oxygenation and Acute Dialysis: Acute Physiology and Chronic Health Evaluation Score in Predicting Hospital Mortality Artificial Organs 34: 828–835, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Chen Y-C, Tsai F-C, Chang C-H, et al. : Prognosis of patients on extracorporeal membrane oxygenation: the impact of acute kidney injury on mortality Ann Thorac Surg 91: 137–142, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Aubron C, Cheng AC, Pilcher D, et al. : Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study Crit Care 17: R73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen H, Yu R-G, Yin N-N, Zhou J-X: Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review Crit Care 18: 675, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt M, Bailey M, Kelly J, et al. : Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation Intensive Care Medicine 40: 1256–1266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Askenazi DJ, Selewski DT, Paden ML, et al. : Renal Replacement Therapy in Critically Ill Patients Receiving Extracorporeal Membrane Oxygenation Clinical Journal of the American Society of Nephrology 7: 1328–1336, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yap H-J, Chen Y-C, Fang J-T, Huang C-C: Combination of Continuous Renal Replacement Therapies (CRRT) and Extracorporeal Membrane Oxygenation (ECMO) for Advanced Cardiac Patients Renal Failure 25: 183–193, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Fleming GM, Askenazi DJ, Bridges BC, et al. : A multicenter international survey of renal supportive therapy during ECMO: the Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) group ASAIO J 58: 407–414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ostermann M, Joannidis M, Pani A, et al. : Patient Selection and Timing of Continuous Renal Replacement Therapy Blood Purification 42: 224–237, 2016 [DOI] [PubMed] [Google Scholar]
- 62.Ostermann M, Wald R, Bagshaw SM: Timing of Renal Replacement Therapy in Acute Kidney Injury Contributions to Nephrology: 106–120, 2016. [DOI] [PubMed] [Google Scholar]
- 63.Ostermann M, Connor M, Kashani K: Continuous renal replacement therapy during extracorporeal membrane oxygenation Current Opinion in Critical Care 24: 493–503, 2018 [DOI] [PubMed] [Google Scholar]
- 64.Jacquot A, Lepage X, Merckle L, Girerd N, Levy B: Protocol for a multicentre randomised controlled trial evaluating the effects of moderate hypothermia versus normothermia on mortality in patients with refractory cardiogenic shock rescued by venoarterial extracorporeal membrane oxygenation (VA-ECMO) (HYPO-ECMO study) BMJ Open 9: e031697, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeilmaker GA, Pokorna P, Mian P, et al. : Pharmacokinetic considerations for pediatric patients receiving analgesia in the intensive care unit; targeting postoperative, ECMO and hypothermia patients Expert Opinion on Drug Metabolism & Toxicology 14: 417–428, 2018 [DOI] [PubMed] [Google Scholar]
- 66.Swol J, Buchwald D, Dudda M, Strauch J, Schildhauer TA: Veno-venous extracorporeal membrane oxygenation in obese surgical patients with hypercapnic lung failure Acta Anaesthesiologica Scandinavica 58: 534–538, 2014 [DOI] [PubMed] [Google Scholar]
- 67.Belliato M, Cremascoli L, Aliberti A, Pagani M, Pellegrini C, Iotti GA: A case of veno-venous extracorporeal membrane oxygenation for severe respiratory failure in a superobese patient Clin Case Rep 4: 1147–1150, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shibutani K, Inchiosa MA, Sawada K, Bairamian M: Pharmacokinetic mass of fentanyl for postoperative analgesia in lean and obese patients † †Presented in abstract form at the Annual Meeting of the American Society of Anesthesiologists, Las Vegas, October 26, 2004 British Journal of Anaesthesia 95: 377–383, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Lemmens HJM, Ingrande J: Pharmacology and Obesity International Anesthesiology Clinics 51: 52–66, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Ulvestad M, Skottheim IB, Jakobsen GS, et al. : Impact of OATP1B1, MDR1, and CYP3A4 Expression in Liver and Intestine on Interpatient Pharmacokinetic Variability of Atorvastatin in Obese Subjects Clinical Pharmacology & Therapeutics 93: 275–282, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Kolwankar D, Vuppalanchi R, Ethell B, et al. : Association Between Nonalcoholic Hepatic Steatosis and Hepatic Cytochrome P-450 3A Activity Clinical Gastroenterology and Hepatology 5: 388–393, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Shekar K, Roberts JA, Mullany DV, et al. : Increased Sedation Requirements in Patients Receiving Extracorporeal Membrane Oxygenation for Respiratory and Cardiorespiratory Failure Anaesthesia and Intensive Care 40: 648–655, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Ahsman MJ, Hanekamp M, Wildschut ED, Tibboel D, Mathot RAA: Population pharmacokinetics of midazolam and its metabolites during venoarterial extracorporeal membrane oxygenation in neonates Clin Pharmacokinet 49: 407–419, 2010 [DOI] [PubMed] [Google Scholar]
- 74.Ammar MA, Sacha GL, Welch SC, et al. : Sedation, Analgesia, and Paralysis in COVID-19 Patients in the Setting of Drug Shortages J Intensive Care Med 36: 157–174, 2021 [DOI] [PubMed] [Google Scholar]
- 75.Adams CD, Altshuler J, Barlow BL, et al. : Analgesia and Sedation Strategies in Mechanically Ventilated Adults with COVID-19 Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 40: 1180–1191, 2020 [DOI] [PubMed] [Google Scholar]
- 76.Pettus K, Cleary JF, de Lima L, Ahmed E, Radbruch L: Availability of Internationally Controlled Essential Medicines in the COVID-19 Pandemic J Pain Symptom Manage 60: e48–e51, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karamchandani K, Dalal R, Patel J, Modgil P, Quintili A: Challenges in Sedation Management in Critically Ill Patients with COVID-19: a Brief Review Current Anesthesiology Reports 11: 107–115, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langer T, Santini A, Bottino N, et al. : “Awake” extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering Critical Care 20, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swol J, Shekar K, Protti A, et al. : Extubate before VV ECMO decannulation or decannulate while remaining on the ventilator? The EuroELSO 2019 weaning survey ASAIO J, 2020 [DOI] [PubMed] [Google Scholar]
- 80.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y: Incidence, risk factors and consequences of ICU delirium Intensive Care Medicine 33: 66–73, 2007 [DOI] [PubMed] [Google Scholar]
- 81.Hager DN, Dinglas VD, Subhas S, et al. : Reducing Deep Sedation and Delirium in Acute Lung Injury Patients Critical Care Medicine 41: 1435–1442, 2013 [DOI] [PubMed] [Google Scholar]
- 82.Lehr CJ, Zaas DW, Cheifetz IM, Turner DA: Ambulatory Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation Chest 147: 1213–1218, 2015 [DOI] [PubMed] [Google Scholar]
- 83.Schweickert WD, Pohlman MC, Pohlman AS, et al. : Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial The Lancet 373: 1874–1882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xia J, Gu S, Li M, et al. : Spontaneous breathing in patients with severe acute respiratory distress syndrome receiving prolonged extracorporeal membrane oxygenation BMC Pulmonary Medicine 19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Biscotti M, Gannon WD, Agerstrand C, et al. : Awake Extracorporeal Membrane Oxygenation as Bridge to Lung Transplantation: A 9-Year Experience Ann Thorac Surg 104: 412–419, 2017 [DOI] [PubMed] [Google Scholar]
- 86.Crotti S, Bottino N, Ruggeri GM, et al. : Spontaneous Breathing during Extracorporeal Membrane Oxygenation in Acute Respiratory Failure Anesthesiology 126: 678–687, 2017 [DOI] [PubMed] [Google Scholar]
- 87.Nosotti M, Rosso L, Tosi D, et al. : Extracorporeal membrane oxygenation with spontaneous breathing as a bridge to lung transplantation Interact Cardiovasc Thorac Surg 16: 55–59, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schechter MA, Ganapathi AM, Englum BR, et al. : Spontaneously Breathing Extracorporeal Membrane Oxygenation Support Provides the Optimal Bridge to Lung Transplantation Transplantation 100: 2699–2704, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Montero S, Huang F, Rivas-Lasarte M, et al. : Awake venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock European Heart Journal Acute Cardiovascular Care 10: 585–594, 2021 [DOI] [PubMed] [Google Scholar]
- 90.Alozie A, Kische S, Birken T, et al. : Awake Extracorporeal Membrane Oxygenation (ECMO) as Bridge to Recovery After Left Main Coronary Artery Occlusion: A Promising Concept of Haemodynamic Support in Cardiogenic Shock Heart, Lung and Circulation 23: e217–e221, 2014 [DOI] [PubMed] [Google Scholar]
- 91.Mori M, McCloskey G, Geirsson A, et al. : Improving Outcomes in INTERMACS Category 1 Patients with Pre-LVAD, Awake Venous-Arterial Extracorporeal Membrane Oxygenation Support ASAIO Journal 65: 819–826, 2019 [DOI] [PubMed] [Google Scholar]


