Abstract
Ovarian combined serous borderline tumor/low-grade serous carcinomas (SBT/LGSC) and mesonephric-like adenocarcinomas (MLA) have been previously reported and the presence of identical oncogenic somatic mutations in both components supports the concept that at least some of MLAs arise from a Müllerian origin. We report two cases of ovarian combined SBT/LGSC and mesonephric-like lesion. Case 1 was a 70 year-old woman presented with a liver lesion and omental carcinomatosis. Histological examination revealed biphasic tumors in bilateral ovaries consisting of conventional SBT and invasive MLA with extraovarian spread. The right ovary also had a component of cribriform variant of SBT/non-invasive LGSC. The SBT/LGSC component was diffusely positive for Pax8, WT-1 and ER, focally positive for PR, and negative for Gata3, while the MLA component was diffusely positive for Gata3 but negative for WT-1, ER, and PR. Molecular analysis revealed a KRAS G12V mutation in both the SBT/LGSC and MLA components, indicating their clonal origin. Case 2 was a 58-year-old woman who presented with conventional type SBT in both ovaries. In addition, the left ovarian tumor demonstrated a few areas (each less than 5 mm) of mesonephric-like differentiation/hyperplasia in close proximity to the serous-type epithelium, with an immunophenotype of focal Gata3 expression, luminal pattern of CD10 staining and negative WT-1, ER and PR staining. This phenomenon has been reported in endometrioid borderline tumor but not in any serous type lesions. The findings in case 1 provide further evidence to demonstrate the clonal relationship between these morphologically and immunophenotypically distinct components. It also supports the theory that, unlike cervical MCs originating from mesonephric remnants, MLAs are derived from a Müllerian-type lesion with differentiation into mesonephric lineage. The presence of a hyperplastic mesonephric-like lesion/differentiation in case 2 indicates that a precursor lesion in the same lineage with the potential to develop into MLA exists in the ovary.
Keywords: RAS mutation, low-grade serous carcinoma, mesonephric-like adenocarcinoma, mesonephric-like differentiation, serous borderline tumor
Introduction
Mesonephric-like adenocarcinomas (MLAs), commonly occurring in the uterine corpus and ovary, are rare malignant neoplasms displaying mesonephric differentiation [1]. As newly described entities, MLAs have been added to the recent 2020 World Health Organization (WHO) Classification of Female Genital Tumors [2]. Despite the absence of obvious mesonephric/Wolffian-type precursor lesions, MLAs exhibit similar histological and immunohistochemical features as well as molecular alterations to those of mesonephric carcinomas (MCs) of the uterine cervix which is thought to arise from mesonephric remnants [3–5]. Both MLAs and MCs feature a variety of morphologies including tubular, glandular/pseudoendometrioid, ductal, retiform, papillary or solid patterns. Small glands and tubules with eosinophilic intraluminal secretions are frequently seen. These tumors are characterized by nuclear expression of Pax8, Gata3 and TTF-1, luminal staining of CD10, lack of ER/PR expression, and a wild-type p53 staining pattern [6]. At a molecular level, KRAS somatic mutations has been reported in a high proportion of both MLAs and MCs, with up to 89% in the former and 100% in the latter [7–11].
Although the cell of origin of primary MLAs is unknown, the literature indicates that at least some of these tumors are derived from transdifferentiation of Müllerian-type lesions into those with Wolffian/mesonephric lineage. Indeed, some of these tumors are associated with Müllerian-type lesions in the ovary including endometriosis [12–14], serous cystadenoma [15], endometrioid borderline tumor [13], and serous borderline tumor/low-grade serous carcinoma (SBT/LGSC) [7, 15–17]. Thus far, 7 cases of ovarian combined SBT/LGSC and MLA [7, 15–17] or mesonephric-like carcinosarcoma [18] have been reported and the presence of identical KRAS, NRAS, or PIK3CA mutations in both components provides convincing evidence that these distinct components are clonally related. On the other hand, the presence of private mutations in each component indicates lineage-specific differentiation with distinct morphology and immunophenotype. Here, we report two cases of ovarian combined serous tumor and mesonephric-like lesion: one is a mixed SBT/LGSC and MLA, and the other is an SBT with focal mesonephric-like differentiation/hyperplasia. The observations in the latter case have not been reported previously.
Case 1. SBT/LGSC with coexisting MLA
Clinicopathological findings.
The first case was a 70 year-old woman with a liver lesion and omental carcinomatosis. The liver biopsy was consistent with metastatic carcinoma of gynecologic origin. Subsequently she underwent total hysterectomy and bilateral salpingo-oophorectomy (TH-BSO) and omentectomy. On gross examination, the right ovary measured 6.2 × 4.6 × 3.6 cm and the left ovary measured 2.9 × 2.1 × 1.2 cm. Both ovaries showed adherent papillary excrescences on their external surface. Examination of the omentum revealed abundant tumor nodules (involving 40% of specimen) ranging in size from 1.1 cm to 4.2 cm in greatest dimension. Microscopic examination of the right ovary revealed a conventional type serous borderline tumor/atypical proliferative serous tumor (SBT/APST, 75%, Figure 1A) with a focal cribriform variant SBT/non-invasive low-grade serous carcinoma (SBT/niLGSC, 20%, Figures 1B and 1C). An invasive carcinoma (5%, Figure 1D) exhibiting an admixture of tubulocystic, glandular, papillary and focal growth patterns was identified near the surface of the ovary and within proximity to the SBT/niLGSC. Intraluminal eosinophilic material was present in some glands. The tumor cells contained scant eosinophilic cytoplasm, vesicular nuclei with nuclear overlap, irregular nuclear membranes and inconspicuous to variably prominent nucleoli (Figure 1E). The architecture and cytological features were in keeping with an MLA. The left ovary also featured a conventional type SBT/APST (95%) with foci of invasive MLA (5%). The vast majority of tumor in the omentum was metastatic MLA (95%) with the remaining 5% composed of adjacent invasive implants/invasive LGSC (Figure 1F).
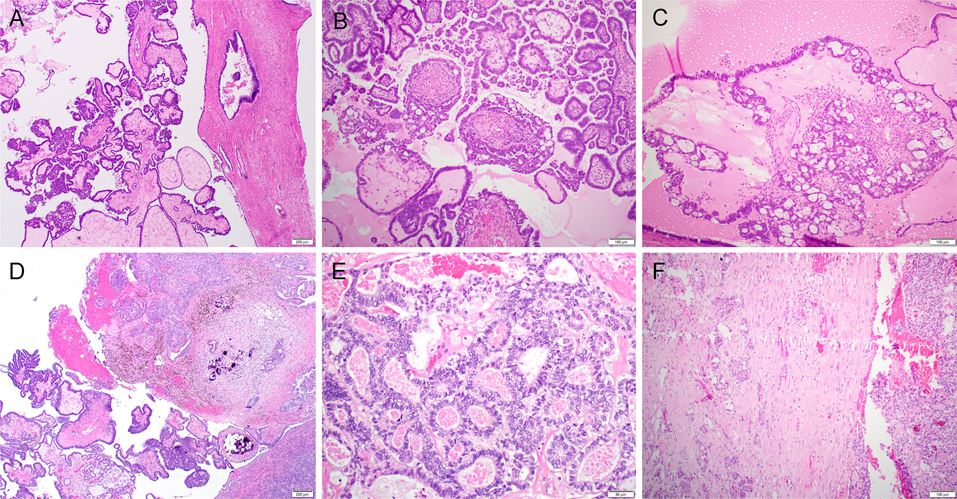
Figure 1.
Serous borderline tumor/non-invasive low-grade serous carcinoma (SBT/niLGSC) with co-existing mesonephric-like adenocarcinoma (MLA) (case 1). Microscopic examination of the right ovary revealed a conventional type-SBT/atypical proliferative serous tumor (SBT/APST, 75%, A) with focal cribriform variant-SBT/niLGSC (20%, B and C). Towards the ovarian surface there was invasive MLA (5%, D). The tumor cells contained scant eosinophilic cytoplasm, vesicular nuclei with nuclear overlap, irregular nuclear membrane and inconspicuous to variably prominent nucleoli (E). Metastatic MLA (right) with adjacent invasive implants/invasive LGSC component (left) was present in the omentum (F).
Immunohistochemical study.
Immunohistochemically, the SBT/niLGSC component (Figure 2A) was diffusely positive for Pax8, WT-1 (Figure 2B) and ER (Figure 2D), focally positive for PR, and negative for Gata3 (Figure 2C) while the MLA component (Figure 2E) was diffusely positive for Gata3 (Figure 2G) but negative for WT-1 (Figure 2F), ER (Figure 2H), and PR. Both components displayed focal/patchy positivity of p16, a wild-type pattern of p53, and negative staining for Napsin A, TTF-1 and CD10. Some areas contained a serous-type tumor intimately admixed with scattered foci of MLA (Figure 2I) with distinct immunoprofile for each component (Figures 2J: WT-1; 2K: Gata3; and 2L: ER). Immunostaining for mismatch repair proteins (MLH1, PMS2, MSH2, and MSH6) revealed intact expression. The tumor cells were also negative for PD-L1 staining.
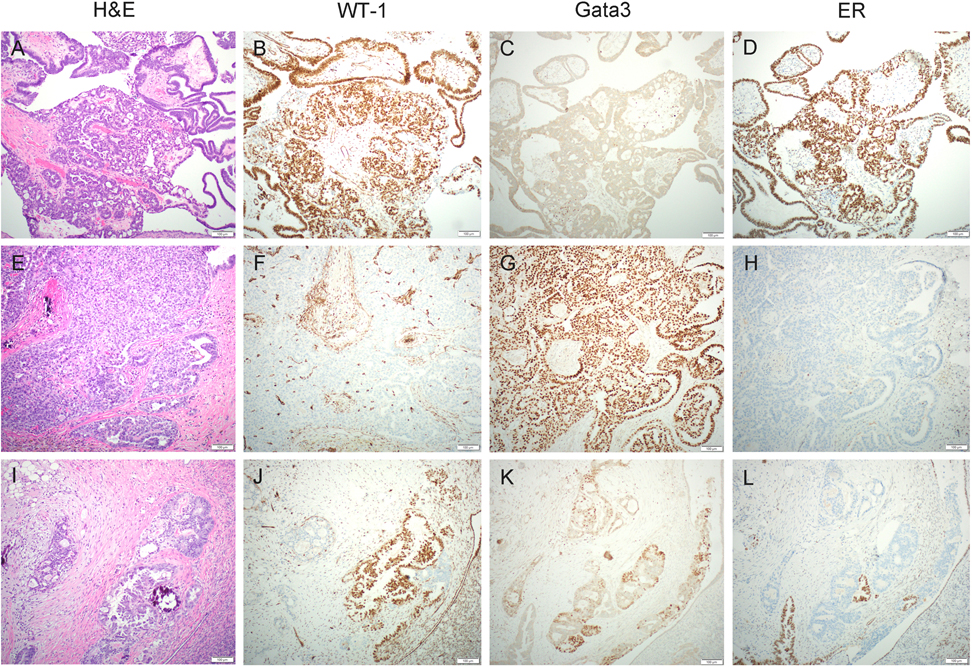
Figure 2.
Immunohistochemical results from case 1. The serous borderline tumor/non-invasive low-grade serous carcinoma (SBT/niLGSC) component (A) was diffusely positive for WT-1 (B) and ER (D) but negative for Gata3 (C), while the mesonephric-like adenocarcinoma (MLA) component (E) was diffusely positive for Gata3 (G) but negative for WT-1 (F) and ER (H). Some areas contained a serous-type tumor intimately admixed with scattered foci of MLA (I) with distinct immunoprofile for each component (J: WT-1; K: Gata3; L: ER).
Molecular analysis.
To identify genetic alterations, the omental tumor (mixed MLA and invasive LGSC) was sequenced using the Tempus xT platform (Tempus, Chicago, IL) which included a 648-gene panel that is enriched for clinically relevant genes and genes of emerging clinical relevance as well as whole transcriptome RNA sequencing with validated fusion detection. A KRAS c.35G>T (p.G12V) mutation (pathogenic) and a NOTCH1 c.383G>C (p.R128P) mutation (variant of unknown significance) were detected. To further characterize lineage-specific alterations, the KRAS G12V mutation was further analyzed by Sanger sequencing. The following primers were used for amplification: 2F1: 5’- GTGTATTAACCTTATGTGTGACA-3’, 2R1: 5’- TGGTCAGAGAAACCTTTATCTG-3’, and 2R2: 5’- TGGTCCTGCACCAGTAATATGC-3’. The PCR products amplified by 2F1/2R1 primers were diluted 10 times and were used as templates for the second PCR amplification (nested PCR) utilizing another pair of primers 2F1/2R2. DNA sequencing of the purified DNA products showed that the KRAS G12V mutation was present in both the SBT/niLGSC and the MLA, indicating their clonal origin (Figure 3).
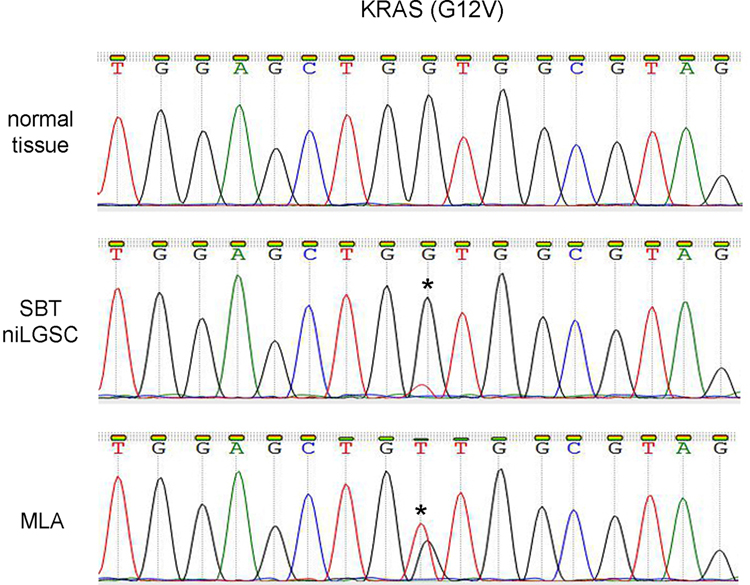
Figure 3.
Molecular study of case 1. Sanger sequencing showed that the KRAS G12V mutation was present in both the serous borderline tumor/non-invasive low-grade serous carcinoma (SBT/niLGSC) and the mesonephric-like adenocarcinoma (MLA), indicating their clonal origin. Star (*) indicates mutational site.
Case 2. SBT/APST with mesonephric-like differentiation
Clinicopathological findings.
The second case was a 58-year-old woman who presented with bilateral ovarian masses. She underwent TH-BSO, omentectomy and staging biopsies. Gross examination revealed a 30 × 23 × 14 cm unilocular cystic lesion in the left ovary and a 17 × 15 × 11 cm multilocular cystic mass in the right ovary. Similar to case 1, both ovaries exhibited papillary excrescences on their external surface. Histological examination revealed conventional type SBT/APST in both ovaries. In addition, the left ovarian tumor demonstrated a few areas of glandular proliferations composed of small tubules lined by cuboidal cells with a moderate degree of cytologic atypia and eosinophilic colloid-like secretions in the lumen (Figures 4A and 4B). Each area measured less than 5 mm in greatest dimension. This component, classified as mesonephric-like tubules, displayed some hyperplastic features that were considered insufficient to establish a diagnosis of MLA. In particular, neither glandular confluence nor desmoplastic reaction were present. In some areas, individual mesonephric-like glands and a few glandular clusters were seen in close proximity to the serous-type epithelium (Figure 4G). The epithelial cells in the mesonephric-like glands displayed mild to moderate atypia and lacked cilia. Non-invasive implants were found in the right pelvic sidewall, but evidence of invasive carcinoma or extra-ovarian benign/hyperplastic mesonephric-like proliferation was not identified.
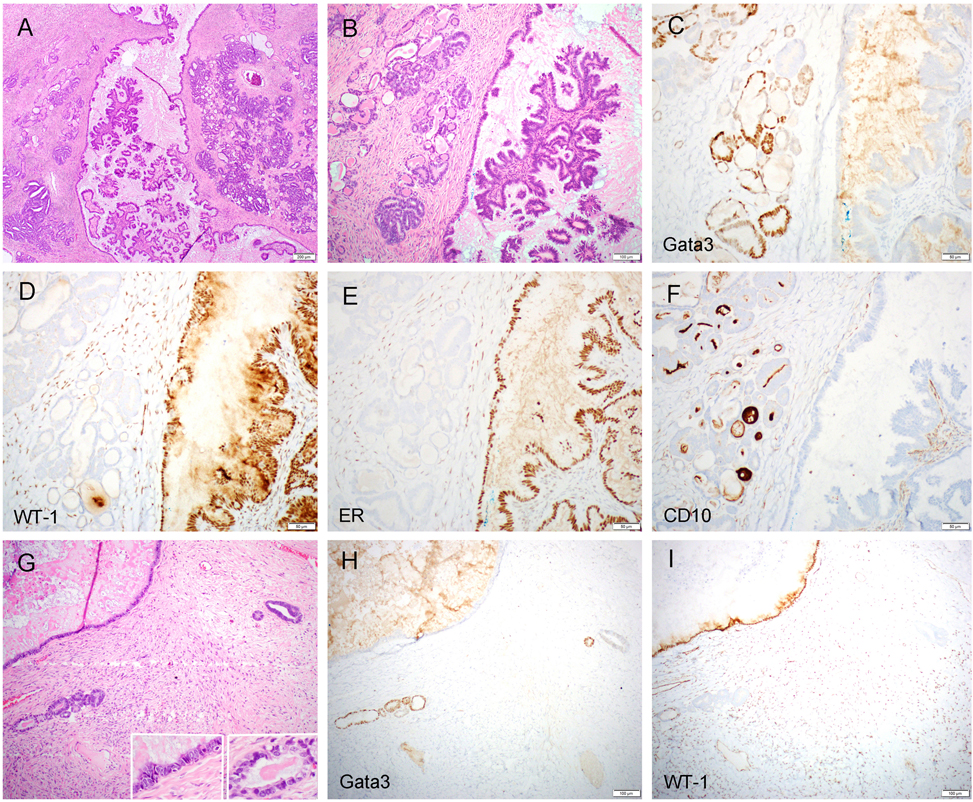
Figure 4.
Serous borderline tumor/atypical proliferative serous tumor (SBT/APST) with mesonephric-like differentiation/hyperplasia (case 2). The left ovarian tumor demonstrated conventional type SBT/APST and a few areas (each area measured less than 5 mm) of mesonephric-like differentiation/hyperplasia (A and B). The former component was negative for Gata3 (C) but diffusely positive for WT-1 (D) and ER (E), while the latter component was focally positive for Gata3 (C) but negative for WT-1 (D) and ER (E). A luminal pattern of CD10 staining was present in the mesonephric-like tubules but not in the SBT/APST (F). In some areas, individual mesonephric-type glands or few glandular clusters were seen in close proximity to the serous-type epithelium (G; left inset: serous epithelium; right inset: mesonephric-like epithelium), displaying distinct immunoprofile for each component (H: Gata3; I: WT-1).
Immunohistochemical study.
Similar to the first case, both the SBT/APST and the mesonephric-like tubules displayed focal/patchy positivity of p16 and a wild-type pattern of p53 staining. The former component was negative for Gata3 (Figure 4C), diffusely positive for WT-1 (Figure 4D) and ER (Figure 4E), and focally positive for PR, and while the mesonephric-like component was focally positive for Gata3 (Figure 4C) and negative for WT-1 (Figure 4D), ER (Figure 4E), and PR. A luminal pattern of CD10 staining was present in the mesonephric-like tubules but not in the SBT/APST (Figure 4F). Individual mesonephric-type glands and adjacent serous-type epithelium (Figure 4G) displayed a distinct immunoprofile for each component (Figures 4H: Gata3; 4I: WT-1).
Review of published cases of ovarian combined SBT/LGSC and mesonephric-like lesions
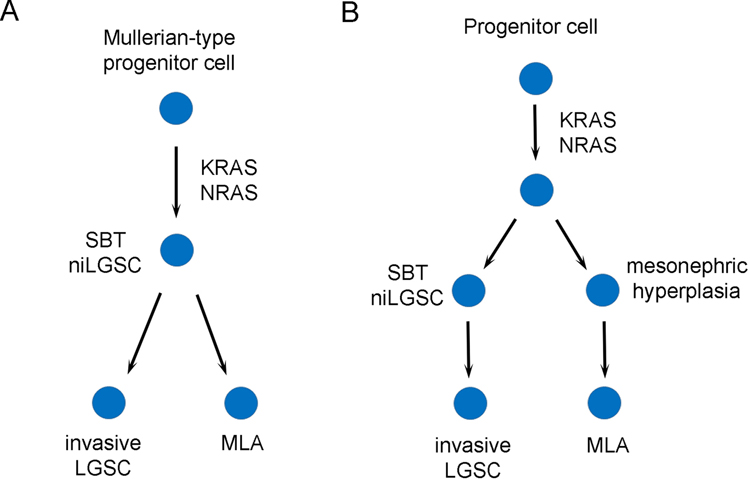
Six cases of ovarian combined SBT/LGSC with MLA and 1 case of SBT/LGSC with co-existent mesonephric-like carcinosarcoma have been reported in the literature [7, 15–18]. Clinicopathologic features are summarized in Table 1 and immunohistochemical and molecular findings are summarized in Table 2. The patients with mesonephric-like tumor, including 7 published cases and 1 case from this report, ranged in age from 61 to 80 years (mean, 67; median, 64). All 8 cases with a malignant mesonephric-like component, with or without co-existing LGSC, developed metastatic disease involving the abdomen/pelvis. Other sites involved by metastatic tumor included liver, lung, and lymph nodes (pelvic and intrathoracic). Based on the morphologic findings and molecular changes, two models were proposed to explain the histopathogenesis of the mixed tumors in our report as well as in other studies (Figures 5A and 5B).
Table 1.
Clinicopathological features in the current and published cases of ovarian combined SBT/LGSC and mesonephric-like lesion
| Case | Age (years) | Clinical presentation | Surgical procedure | Tumor site and size | Serous component | Mesonephric component | Extraovarian disease |
|---|---|---|---|---|---|---|---|
| 1 This study | 70 | Liver lesion and carcinomatosis | TH-BSO, omentectomy | Right ovary: 6.2 cm; left ovary: 2.9 cm | Right ovary: SBT/LGSC; left ovary: SBT | MLA | Liver metastasis; omental metastasis with mixed MLA (95%) and invasive LGSC (5%) |
| 2 This study | 58 | Bilateral ovarian masses | TH-BSO, omentectomy, staging biopsy | Left ovary: 30cm; right ovary: 17 cm | Bilateral SBT | Mesonephric-like differentiation/hyperplasia | Non-invasive implants |
| 3 Chapel et al. [16] | 80 | 2 months of abdominal pain | TH-BSO, omentectomy and tumor debulking after neoadjuvant chemotherapy | Right ovary: 10.6 cm | SBT and invasive LGSC | MLA | Metastatic MLA: sigmoid mesentery, rectosigmoid serosa and splenic omentum; Mixed MLA (major) and LGSC (minor): uterine serosa and right pelvic sidewall, and in additional metastatic foci on the omentum and rectosigmoid serosa and epiploica |
| 4 McCluggage et al. [15] | 61 | Pelvic pain and vaginal discharge | TH-BSO, omentectomy, resection of peritoneum from the Pouch of Douglas, and bilateral pelvic lymphadenectomy | Left ovary: 6 cm; Right ovary: 3 cm | Bilateral SBT | MLA | Invasive LGSC (invasive implants) in the Pouch of Douglas and 1 pelvic lymph node |
| 5 Dundr et al. [17] | 61 | Advanced tumor of the left ovary with abdominal spread and liver metastases | TH-BSO, omentectomy, portion of liver, diaphragm, appendectomy, a resection of an umbilical metastasis | Left ovary: 3.5 cm | SBT | MLA | Omentum and liver with metastatic MLA |
| 6 da Silva et al. [7] | 66 | - | - | Ovary: 5.4 cm | SBT | MLA | Left rectal mesentery (both components) |
| 7 da Silva et al. [7] | 62 | - | - | Bowel (metastasis): 7.5 cm | Invasive LGSC | MLA | Bowel (both components) |
| 8 da Silva et al. [7] | 61 | - | - | Ovary: 4.1 cm | SBT/LGSC | MLA | Peritoneal and omental carcinomatosis (both components), lung, bowel, liver, abdominal and intrathoracic lymph nodes |
| 9 d'Amati et al. [18] | 74 | Abdominal discomfort; RSO for an ovarian serous cystadenoma at age of 27 | TH-LSO, omentectomy, large-bowel segmental resection, and resection of two peritoneal nodules | Left ovary: 4.5 cm | SBT and invasive LGSC | Mesonephric-like carcinosarcoma | Metastatic MLA: omental and peritoneal nodules; carcinosarcoma: large bowel serosa nodule |
BSO: bilateral salpingo-oophorectomy; LGSC: low-grade serous carcinoma; LSO: left salpingo-oophorectomy; MLA: mesonephric-like adenocarcinoma; RSO: right salpingo-oophorectomy; SBT: serous borderline tumor; TH: total hysterectomy
IHC: immunohistochemistry; LGSC: low-grade serous carcinoma; SBT: serous borderline tumor
Table 2.
Immunohistochemical and molecular findings in the current and published cases of ovarian combined SBT/LGSC and mesonephric-like lesion
| Case | Shared positive IHCs | Private positive IHCs (serous) | Private positive IHCs (mesonephric-like) | Shared genetic alteration | Private genetic alteration (serous) | Private genetic alteration (mesonephric-like) |
|---|---|---|---|---|---|---|
| 1 This study | Pax8, p16 (focal), p53 (wild-type) | WT-1, ER, PR (focal) | Gata3 | KRAS p.G12V | - | - |
| 2 This study | p16 (focal), p53 (wild-type) | WT-1, ER, PR (focal) | Gata3 (focal), CD10 (luminal) | - | - | - |
| 3 Chapel et al. [16] | CK7, EMA, Pax8, p16 (focal), p53 (wild-type) | WT-1, ER (focal), PR | Gata3, TTF-1, CD10 (luminal), Inhibin (focal), p63 (focal) | NRAS p.Q61R; gains in 1q and 18p and losses in chromosomes 1p, 18q, and 22 | KDM5A p.Y1017_A1018delins; STAG2 p.S941P (subclones) | BCOR p.Q1248fs; BCOR p.N1057fs; AMER1 p.E371fs |
| 4 McCluggage et al. [15] | Pax8, p53 (wild-type) | WT-1, ER | Gata3 (focal), TTF-1 (rare positive cells), CD10 (luminal) | KRAS p.G12D | - | - |
| 5 Dundr et al. [17] | Pax8, p53 (wild-type) | ER, PR (focal) | Gata3 (focal), TTF-1, CD10 (luminal) | KRAS p.G12C, PIK3CA p.E545K, CHEK2 exon 9–10 deletion (germline) | - | MYCN p.P44L |
| 6* da Silva et al. [7] | p53 (wild-type) | WT-1 | Gata3, TTF-1 | NRAS Q61R, ATR p.A2014V (in metastasis), TNFAIP3 p.F783I (in metastasis) | - | - |
| 7* da Silva et al. [7] | Pax8 | WT-1, ER | Gata3 | KRAS p.G12V, DIS3 p.P635S, PLCG2 p.E877D, FANCA p.T126R, NOTCH3 p.A1020P, GNAS p.P376L, ATM p.X974_splice | - | - |
| 8 da Silva et al. [7] | CK7, Pax8, HNF-1b | WT-1, ER, PR | TTF-1 | NRAS p.Q61R, 1q gain, losses of chromosomes 4 and 18 | - | HIST1H3I p.G103R, gains of chromosomes 6p and 17 |
| 9** d'Amati et al. [18] | CK7, Pax8, p53 (wild-type) | ER, PR | Gata3, TTF-1, Calretinin, CD10 (luminal) | - | - | - |
mesonephric-like/serous borderline tumors were dissected and sequenced together.
mesonephric-like adenocarcinoma and sarcoma displayed the same immunophenotype.
Figure 5.
Based on the morphologic findings and molecular changes, two models are proposed to explain the histopathogenesis of the mixed tumors in our report as well as in other studies. In theory, a Müllerian-type progenitor cell can acquire KRAS or NRAS mutations and develop into a serous borderline tumor/non-invasive low-grade serous carcinoma (SBT/niLGSC) that continues to give rise to both invasive LGSC and mesonephric-like adenocarcinoma (MLA) (A). Our case 1 supports this model. Alternatively, the tumor with mixed histology may originate from a pluripotent stem cell in the embryonic ridge which acquires KRAS or NRAS mutations and differentiates in parallel along both Müllerian (SBT/niLGSC to invasive LGSC) and mesonephric lineage (mesonephric hyperplasia to MLA) (B). The presence of a non-malignant mesonephric-like component in case 2 indicates the latter possibility does theoretically exist, although a carcinomatous component, either serous or mesonephric-like, is not present in this case.
Discussion
This report documents two cases of mixed serous tumor and mesonephric-like lesions in the ovary. To the best of our knowledge, our case 1 represents the eighth reported case of ovarian malignant mesonephric-like tumor coexisting with SBT/LGSC. Strikingly, all these tumors display similar histomorphology, immunophenotype, molecular alterations, and clinical behavior. The serous component of case 1 was composed of both cribriform SBT/niLGSC and conventional SBT/APST, rather than a pure conventional borderline tumor. For micropapillary/cribriform type SBT, we favor designation as niLGSC since women with niLGSC are more likely to develop invasive serous carcinoma than women with conventional SBT/APST [19, 20]. Consistently, a component of LGSC, either non-invasive or invasive, was present in 4 of 7 reported cases (Table 1). Immunohistochemically, this component was characterized by WT-1 and ER/PR expression and lack of expression of mesonephric markers Gata3, TTF-1 and CD10 (Table 2). Interestingly, the median age of the patents with these mixed tumors was 64 years, which is similar to those reported in MCs and pure MLAs, but decade older than those of pure SBT/APST or LGSC [20].
The other component in the biphasic lesion of case 1 exhibited typical features of MLA, illustrated by a mixed growth pattern, intraluminal eosinophilic secretions and a Gata3-positive, WT-1/ER/PR-negative immunophenotype. Similar to other reported cases, both components in our case showed patchy p16 expression and wild-type p53. Although only 5% of tumor was MLA in the ovary, this component accounted for more than 95% of the metastatic tumor in the omentum. A similar observation has been reported in other cases [16, 18]. Our case—as well as reported cases with a malignant mesonephric-like component, with or without co-existing LGSC—all developed metastatic disease involving the abdomen/pelvis and some cases had spread outside of the abdominal cavity, indicating their aggressive behavior.
At a molecular level, NGS and Sanger sequencing analysis revealed a common KRAS G12V driver mutation in both the SBT/niLGSC and MLA components in case 1. In fact, since its first description [16], there are several published reports with molecular analysis confirming a clonal origin for these mixed tumors (Table 2). The identical activating KRAS mutations, including G12D [15], G12C [17], and G12V (case 1), and NRAS Q61R mutation [7, 16] have been reported in both SBT/LGSC and MLA components. Some reported cases had mixed histology but it was impossible to dissect the distinct components entirely due to the intimate association [7]. These cases, in which both SBT/LGSC and MLA components were dissected and sequenced together, demonstrated a KRAS G12V mutation in one case and a NRAS Q61R in the other. In addition to common KRAS and NRAS mutations, other shared genetic alterations have been reported in both components, including a PIK3CA p.E545K mutation, gains in chromosome 1q and 18p and losses in chromosomes 1p, 4, 18q, and 22.
It is conceivable that, despite having a common clonal origin with shared genetic alterations, there must be other distinct genetic changes to explain their distinct phenotypes in a mixed tumor. Consistent with this assumption, additional private aberrations were detected either only in SBT/LGSC (KDM5A and STAG2) or only in MLAs (BCOR, AMER1, MYCN, HIST1H3I, gains of chromosomes 6p and 17) [7, 16, 17].
The literature indicates that KRAS somatic mutations are the most common genetic alteration of both ovarian (up to 87%) and endometrial (up to 92%) MLAs [7]. These mutations were also detected in our case 1 and 3 of 6 reported cases which had molecular analysis. Ras/Raf/MEK/MAPK pathway alterations are postulated to be drivers of both MCs and MLAs [7]. Consistently, it has been demonstrated that MCs or MLAs that lack KRAS mutations may harbor hotspot mutations in other RAS/RAF family genes such as NRAS or BRAF. However, compared with KRAS mutation, NRAS or BRAF mutations in these tumors are very rare – only one pure cervical MC with NRAS mutation [9] and one pure endometrial MLA with BRAF mutation [7] have been reported. Several studies demonstrated that NRAS is a critical oncogenic driver in the progression of SBT/APSTs to LGSCs. In one study, 5 of 58 (9%) invasive tumors with adjacent SBT harbored activating NRAS mutations [21]. Another study demonstrated that NRAS mutations were detected in 26.3% of LGSCs, but none were detected in the SBT/APST cohort [22]. Similarly, our previous study showed that NRAS Q61R mutations were detected in 2 of 56 (3.6%) invasive LGSCs but not in any of the SBT/APSTs or niLGSCs [23]. Interestingly, while NRAS p.Q61R driver mutations are not common in either LGSCs or MC/MLAs, 3 of 7 mixed MLAs and SBT/LGSCs with molecular testing had this mutation.
It is conceivable that acquisition of an NRAS mutation—similar to, or perhaps more effectively than, a KRAS mutation—defines the invasive nature of SBT/APST, causing its progression into a malignant tumor with distinct serous and mesonephric lineages [16].
In case 2, we report a conventional type SBT/APST with mesonephric-like differentiation/hyperplasia, a finding which has not been previously described. The predominant component was SBT/APST in which the tumor cells were positive for WT-1, ER, and PR, but negative for Gata3, TTF-1, and CD10. In this 30 cm tumor, few microscopic foci of small tubular clusters, each measuring less than 5 mm, also were present. These small tubules displayed typical mesonephric morphology and immunophenotype with positive Gata3 expression and a luminal pattern of CD10 staining. The degree of glandular crowding was consistent with hyperplasia but considered insufficient to establish a diagnosis of MLA. Although a similar phenomenon has not been reported in any serous type lesions, a recent case series described a case of ovarian endometrioid borderline tumor where the glands focally exhibited “mesonephric-like” differentiation [13].
It has been well accepted that at least some MLAs, if not all, arise from a Müllerian origin. In a tumor with mixed serous and mesonephric morphology, the presence of SBT/APST with an associated biphasic invasive carcinoma suggests a Müllerian origin for the entire malignant process. In theory, a Müllerian-type progenitor cell can acquire KRAS or NRAS mutations and develop into an SBT/APST that continues to give rise to both invasive LGSC and MLA (Figure 5A). Alternatively, it is also plausible that the tumor with mixed histology may originate from a pluripotent stem cell in the embryonic ridge which acquires KRAS or NRAS mutations and then differentiates in parallel into both Müllerian and mesonephric lineage (Figure 5B) [16]. The presence of a non-malignant mesonephric-like component in case 2 indicates the latter possibility does theoretically exist. It has been demonstrated that, in serous type lesions, endosalpingiosis is frequently associated with SBT/APST and even harbors the same KRAS/BRAF mutation as the ovarian tumor despite its benign morphology [24]. Likewise, as the precursor lesion, the individual mesonephric-like glands in case 2 may represent an analogous phenomenon to endosalpingiosis and may already contain KRAS or NRAS mutation, although this is purely speculative.
In summary, we report two cases of ovarian combined SBT/LGSC and MLA or mesonephric-like differentiation/hyperplasia. In concordance with previously reported cases, case 1 contained identical KRAS mutations in both tumor components. These findings provide further evidence to demonstrate the clonal relationship between these morphologically and immunophenotypically distinct components. It also supports the theory that, unlike cervical MCs originating from mesonephric remnants, MLAs are derived from Müllerian-type lesions which differentiates into the mesonephric lineage. The presence of a hyperplastic mesonephric-like lesion/differentiation in case 2 indicates that a precursor lesion in the same lineage with the potential to develop into MLA exists in the ovary.
Acknowledgement
We thank Ms. Roberta Knox and the supporting staff in the JHH Pathology Department for their assistance.
Grant support: This study is supported by a Clinician Scientist Award at The Johns Hopkins University School of Medicine (D.X.) and partially supported by the Pilot Project Award by the Cervical Cancer SPORE program at Johns Hopkins (D.X.).
Footnotes
Conflicts of Interest: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Contributor Information
Neshat Nilforoushan, Department of Pathology, The Johns Hopkins Medical Institutions, Baltimore, MD
Lian Liu, Department of Pathology, Sky Ridge Medical Center, Lone Tree, CO /Forward Pathology Solutions, Denver Division, CO.
Brian S. Finkelman, Department of Pathology, The Johns Hopkins Medical Institutions, Baltimore, MD
John Andersen, Department of Pathology, The Johns Hopkins Medical Institutions, Baltimore, MD
Ying Liu, Department of Pathology, The Johns Hopkins Medical Institutions, Baltimore, MD
Jeffrey James, Colorado Gynecologic Oncology Specialists at Sky Ridge Medical Center, Lone Tree, CO
Chien-Fu Hung, Department of Pathology, The Johns Hopkins Medical Institutions, Baltimore, MD Department of Gynecology and Obstetrics, The Johns Hopkins Medical Institutions, Baltimore, MD; Department of Oncology, The Johns Hopkins Medical Institutions, Baltimore, MD.
T.-C. Wu, Department of Pathology, The Johns Hopkins Medical Institutions, Baltimore, MD Department of Gynecology and Obstetrics, The Johns Hopkins Medical Institutions, Baltimore, MD; Department of Oncology, The Johns Hopkins Medical Institutions, Baltimore, MD.
Russell Vang, Department of Pathology, The Johns Hopkins Medical Institutions, Baltimore, MD Department of Gynecology and Obstetrics, The Johns Hopkins Medical Institutions, Baltimore, MD.
Deyin Xing, Department of Pathology, The Johns Hopkins Medical Institutions, Baltimore, MD Department of Gynecology and Obstetrics, The Johns Hopkins Medical Institutions, Baltimore, MD; Department of Oncology, The Johns Hopkins Medical Institutions, Baltimore, MD.
References
- 1.McFarland M, Quick CM, and McCluggage WG, Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: report of a series of mesonephric-like adenocarcinomas. Histopathology, 2016. 68(7): p. 1013–20. [DOI] [PubMed] [Google Scholar]
- 2.WHO Classification of Tumours Editorial Board.WHO Classification of Tumours: Female Genital Tumours 5th ed. Lyon: International Agency for Research on Cancer;. 2020. [Google Scholar]
- 3.Kenny SL, et al. , Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-beta. Am J Surg Pathol, 2012. 36(6): p. 799–807. [DOI] [PubMed] [Google Scholar]
- 4.Bague S, Rodriguez IM, and Prat J, Malignant mesonephric tumors of the female genital tract: a clinicopathologic study of 9 cases. Am J Surg Pathol, 2004. 28(5): p. 601–7. [DOI] [PubMed] [Google Scholar]
- 5.Silver SA, et al. , Mesonephric adenocarcinomas of the uterine cervix: a study of 11 cases with immunohistochemical findings. Am J Surg Pathol, 2001. 25(3): p. 379–87. [DOI] [PubMed] [Google Scholar]
- 6.Howitt BE, et al. , GATA3 Is a Sensitive and Specific Marker of Benign and Malignant Mesonephric Lesions in the Lower Female Genital Tract. Am J Surg Pathol, 2015. 39(10): p. 1411–9. [DOI] [PubMed] [Google Scholar]
- 7.da Silva EM, et al. , Mesonephric and mesonephric-like carcinomas of the female genital tract: molecular characterization including cases with mixed histology and matched metastases. Mod Pathol, 2021. 34(8): p. 1570–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Na K and Kim HS, Clinicopathologic and Molecular Characteristics of Mesonephric Adenocarcinoma Arising From the Uterine Body. Am J Surg Pathol, 2019. 43(1): p. 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirkovic J, et al. , Targeted genomic profiling reveals recurrent KRAS mutations and gain of chromosome 1q in mesonephric carcinomas of the female genital tract. Mod Pathol, 2015. 28(11): p. 1504–14. [DOI] [PubMed] [Google Scholar]
- 10.Mirkovic J, et al. , Targeted Genomic Profiling Reveals Recurrent KRAS Mutations in Mesonephric-like Adenocarcinomas of the Female Genital Tract. Am J Surg Pathol, 2018. 42(2): p. 227–233. [DOI] [PubMed] [Google Scholar]
- 11.Howitt BE and Nucci MR, Mesonephric proliferations of the female genital tract. Pathology, 2018. 50(2): p. 141–150. [DOI] [PubMed] [Google Scholar]
- 12.Seay K, et al. , Mesonephric-like adenocarcinoma of the ovary with co-existent endometriosis: A case report and review of the literature. Gynecol Oncol Rep, 2020. 34: p. 100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deolet E, et al. , Extrauterine Mesonephric-like Neoplasms: Expanding the Morphologic Spectrum. Am J Surg Pathol, 2021. [DOI] [PubMed] [Google Scholar]
- 14.McCluggage WG, Endometriosis-related pathology: a discussion of selected uncommon benign, premalignant and malignant lesions. Histopathology, 2020. 76(1): p. 76–92. [DOI] [PubMed] [Google Scholar]
- 15.McCluggage WG, Vosmikova H, and Laco J, Ovarian Combined Low-grade Serous and Mesonephric-like Adenocarcinoma: Further Evidence for A Mullerian Origin of Mesonephric-like Adenocarcinoma. Int J Gynecol Pathol, 2020. 39(1): p. 84–92. [DOI] [PubMed] [Google Scholar]
- 16.Chapel DB, et al. , An Ovarian Adenocarcinoma With Combined Low-grade Serous and Mesonephric Morphologies Suggests a Mullerian Origin for Some Mesonephric Carcinomas. Int J Gynecol Pathol, 2018. 37(5): p. 448–459. [DOI] [PubMed] [Google Scholar]
- 17.Dundr P, et al. , Ovarian mesonephric-like adenocarcinoma arising in serous borderline tumor: a case report with complex morphological and molecular analysis. Diagn Pathol, 2020. 15(1): p. 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.d’Amati A, et al. , Mesonephric-Like Carcinosarcoma of the Ovary Associated with Low-Grade Serous Carcinoma: A Case Report. Diagnostics (Basel), 2021. 11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannibal CG, et al. , A nationwide study of ovarian serous borderline tumors in Denmark 1978–2002. Risk of recurrence, and development of ovarian serous carcinoma. Gynecol Oncol, 2017. 144(1): p. 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vang R, et al. , Long-term Behavior of Serous Borderline Tumors Subdivided Into Atypical Proliferative Tumors and Noninvasive Low-grade Carcinomas: A Population-based Clinicopathologic Study of 942 Cases. Am J Surg Pathol, 2017. 41(6): p. 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emmanuel C, et al. , Genomic classification of serous ovarian cancer with adjacent borderline differentiates RAS pathway and TP53-mutant tumors and identifies NRAS as an oncogenic driver. Clin Cancer Res, 2014. 20(24): p. 6618–30. [DOI] [PubMed] [Google Scholar]
- 22.Hunter SM, et al. , Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget, 2015. 6(35): p. 37663–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing D, et al. , Mutation of NRAS is a rare genetic event in ovarian low-grade serous carcinoma. Hum Pathol, 2017. 68: p. 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chui MH and Shih IM, Oncogenic BRAF and KRAS mutations in endosalpingiosis. J Pathol, 2020. 250(2): p. 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]