Abstract
Background
The neutrophil-to-lymphocyte ratio (NLR) may predict poor outcomes in adult anti-NMDAR encephalitis (NMDARE). The association of NLR with outcomes in pediatric NMDARE was examined.
Methods
Pediatric NMDARE patients (N = 36) were retrospectively studied.
Results
High NLR (>6) had a higher proportion of tumors (43% versus 7%) and higher intubation rates (100% versus 38%, p = 0.008). Multivariate analyses showed that high NLR did not correlate with one-year outcomes, inpatient length of stay (LOS), or with tumor, but was associated with intubation and rehabilitation LOS.
Conclusion
NLR is associated with intubation and rehabilitation LOS. Further investigation is needed for prognostic biomarkers in NMDARE.
Keywords: Anti-NMDA receptor encephalitis, Autoimmune encephalitis, Pediatric autoimmune encephalitis, Anti-NMDA receptor antibodies, Neutrophil to lymphocyte ratio
1. Introduction
Anti-NMDA receptor encephalitis (NMDARE) is an autoimmune encephalitis characterized by a range of psychiatric symptoms, cognitive impairment, seizures, and dyskinesias (Dalmau et al., 2019). NMDARE is rare, but can occur in about 3% of patients with ovarian teratomas (Li et al., 2021a). Movement disorders, atypical focal deficits such as cerebellar ataxia or hemiparesis, and irritability are more likely to occur in children as compared to adults (Dalmau et al., 2019; Titulaer et al., 2013). Cerebrospinal fluid (CSF) demonstration of IgG antibodies against NMDA receptors, specifically against the GluN1 subunit, is currently the only specific diagnostic test for NMDARE (Dalmau et al., 2019). NMDARE typically responds to immunotherapy, although approximately 20% of patients will experience severe disease and patients are at an increased risk for long-term sequelae, including neurocognitive dysfunction (Titulaer et al., 2013; Hacohen et al., 2014; Linnoila et al., 2019). However, prognostic biomarkers are limited in NMDARE, especially in children. While the anti-NMDA receptor IgG antibodies are pathogenic (Moscato et al., 2014), antibody titers in serum or in CSF do not correlate with outcomes and trending titers also are not helpful in predicting outcomes (Gresa-Arribas et al., 2014).
We have previously published that age (Howarth et al., 2019), electroencephalography (EEG) characteristics (Lin et al., 2021), and the anti-NMDA receptor encephalitis one-year outcome score (NEOS) (Loerinc et al., 2021) may predict outcomes as a group, but is not applicable to an individual patient. Neutrophil-to-lymphocyte ratio (NLR) has been used in patients with critical illness to reflect alterations in the balance between the innate (neutrophils) and adaptive (lymphocytes) immunological response to infection, inflammation, or stress (Zahorec, 2001). NLR identified as a potential predictive biomarker for poor outcomes and disease severity in adult NMDARE (Huang et al., 2021; Yu et al., 2021) and autoimmune encephalitis (Qiu et al., 2019; Zeng et al., 2019). While high NLR has been shown to correlate with worse outcomes and disease severity in adult patient populations with autoimmune encephalitis at 2 months, to our knowledge, no prior studies have explored the NLR as a prognostic biomarker in pediatric anti-NMDA receptor encephalitis patients. This retrospective study aims to determine whether neutrophil-to-lymphocyte ratio (NLR) can be used as a biomarker to predict outcomes in pediatric anti-NMDA receptor encephalitis.
2. Methods
Institutional Review Board approval was obtained for this study. Retrospective chart review of pediatric NMDARE patients presenting to Children’s Healthcare of Atlanta (CHOA) between January 1, 2010 and June 30, 2021 were included if initial white blood cell (WBC) count and differential were available to calculate the NLR at time of presentation and prior to immunotherapy. CHOA is a free-standing quaternary referral pediatric hospital. The diagnosis of NMDARE was defined as a positive anti-NMDA receptor antibody in the cerebrospinal fluid (CSF) and having at least one of six symptoms: 1) behavioral changes, 2) speech dysfunction, 3) seizures, 4) movement disorder, 5) decreased level of consciousness, or 6) autonomic dysfunction/ central hypoventilation (Graus et al., 2016).
Out of 55 patients in the NMDARE database at CHOA, 36 had initial WBC and differential available. Only the initial WBC that was pretreated was included in the study as treatments including steroids can affect NLR. Average of prior WBCs did not occur as usually only one pretreated CBC was available for each patient. Clinical information, other laboratory tests including imaging and electroencephalography (EEG), treatments, intensive care unit (ICU) admission, and hospital length of stay were included. Firstline treatments including intravenous (IV) steroids, intravenous immunoglobulin (IVIG), and plasmapheresis (PLEX), with second-line treatments including rituximab and cyclophosphamide (Nosadini et al., 2021). NLR was treated as a continuous variable, but also as a dichotomous variable of high and low, with the cutoff value for NLR was 6 as designated by prior studies (Zahorec, 2001). One-year outcomes as defined by the pediatric modified Rankin Score (mRS) from 0 to 6 (de Haan et al., 1995) were available in a subset of patients and the additional diagnoses unrelated to NMDARE were not identified.
2.1. Statistical analysis
Descriptive statistics included mean and standard deviation for normally distributed populations and median and interquartile ranges for skewed populations. Statistical analysis including logistic regression, linear regression, Chi-square and Fischer’s exact tests were performed using SAS (Cary, NC). Some patients have been previously published (Howarth et al., 2019; Lin et al., 2021; Loerinc et al., 2021).
3. Results
Thirty-six patients were included in this study with one-year follow up mRS outcome scores in 28 patients. The age range was from 2.0 to 18.4 years. Clinical characteristics of the entire cohort are included in Table 1, and comparisons of clinical characteristics in the high versus low NLR are in Table 2. We then stratified NLR as high versus low. High NLR (defined as 6 or greater) was observed 7/36 (19%). The high NLR patients were older (mean 15.2 years, SD 3.5 years, range 8.5 to 18.0) than the low NLR patients (mean 10.3 years, SD 5.1, range 2.0 to 18.4, Student’s t-test p = 0.021). White blood cell (WBC) count was also higher in the high NLR patients (mean 12.6, SD 4.1) versus low NLR (mean 8.3, SD 2.9, Student’s t-test p = 0.003). High NLR patients had a higher proportion of tumors (3/7, 43%) as compared to low NLR (2/29, 7%, p = 0.040, Fisher’s exact test). Linear regression models did not demonstrate an association of NLR correlating with hospital length of stay (LOS) or mRS at one year. A good outcome by mRS was defined as 0–2 and a poor mRS outcome was defined as 3 or greater. The proportion of NMDARE patients by mRS at one year is displayed in Table 3. Of 28 patients, 10 (39%) had poor outcomes by mRS at one year from onset. High NLR did not correlate with worse outcomes by mRS at one year.
Table 1.
Demographic and clinical information on pediatric NMDARE patients (N = 36).
| All patients | |
|---|---|
| Age (mean, SD) | 11.2 (5.2) |
| Female, N (%) | 24 (67) |
| Race, N (%) | |
| American Indian/Alaska Native | 1 (3) |
| Black | 21 (58) |
| Mixed | 1 (3) |
| Unknown | 5 (14) |
| White | 8 (22) |
| Ethnicity, N (%) | |
| Hispanic | 8 (22) |
| Non-Hispanic | 27 (75) |
| Unknown | 1 (3) |
| ICU admission, N (%) | 25 (69) |
| Intubated, N (%) | 18 (50) |
| IV steroids, N (%)a | 35 (100) |
| IVIG, N (%) | 35 (97) |
| PLEX, N (%) | 23 (64) |
| Second Line (Rituximab or cyclophosphamide), N (%) | 26 (72) |
| Tumor present, N (%) | 5 (14) |
| Prior ED visit or hospitalization before diagnosis, N (%) | 28 (78) |
| Length of hospitalizationa Days, median (IQR) | 22 (12–40) |
| MRI abnormal,a N (%) | 17 (49) |
| EEG abnormal, N (%) | 31 (86) |
| WBC Mean (SD) | 9.1 (3.6) |
| NLR Median (IQR) | 2.3 (1.3, 3.9) |
| LMR Mean (SD) | 4.8 (2.3) |
| CSF WBC Median (IQR) | 11.0 (3.0, 38.0) |
| Modified Rankin Scale at one year | 2.2 (1.2) |
SD: standard deviation, ICU: intensive care unit, IV: intravenous, IVIG: intravenous immunoglobulin, PLEX: plasmapheresis, ED: emergency department, LMR: lymphocyte to monocyte ratio; MRI: magnetic resonance imaging, EEG: electroencephalography, WBC: white blood cell, NLR: neutrophil to lymphocyte ratio, CSF: cerebrospinal fluid, IQR: interquartile range.
1 missing.
Table 2.
Comparison of patient characteristics in high versus low NLR pediatric NMDARE patients.
| High NLR N = 7 |
Low NLR N = 29 |
p-value | |
|---|---|---|---|
| Age (mean, SD) | 15.2 (3.5) | 10.3(5.1) | 0.021 |
| Female, N (%) | 4 (57) | 20 (69) | 0.664 |
| ICU admit, N (%) | 7 (100) | 18 (62) | 0.076 |
| Intubated, N (%) | 7 (100) | 11 (38) | 0.008 |
| PLEX, N (%) | 6 (86) | 17 (59) | 0.382 |
| Second Line, N (%) | 4 (57) | 22 (76) | 0.370 |
| Tumor present, N (%) | 3 (43) | 2 (7) | 0.040 |
| Length of hospitalizationa Days, mean (SD) | 40.4 (18.6) | 35.4 (51.4) | 0.804 |
| MRI abnormal,a N (%) | 4 (57) | 13 (46) | 0.691 |
| EEG abnormal, N (%) | 7 (100) | 24 (83) | 0.559 |
| WBC, Mean (SD) | 12.6 (4.1) | 8.3 (2.9) | 0.003 |
| CSF WBC, Median (IQR) | 40 (4.0, 63.0) | 6.0 (2.0, 26.0) | 0.052b |
| LMR, Mean (SD) | 2.1 (0.4) | 5.5 (2.1) | 0.0002 |
| Modified Rankin Scale at one-year, Mean (SD) | 2.4 (0.8) | 2.1 (1.4) | 0.495 |
SD: standard deviation, NLR: neutrophil to lymphocyte ratio, ICU: intensive care unit, PLEX: plasmapheresis, LMR: lymphocyte to monocyte ratio; MRI: magnetic resonance imaging, EEG: electroencephalography, WBC: white blood cell, CSF: cerebrospinal fluid.
1 missing.
Nonparametric Wilcoxon rank sum test.
Table 3.
Frequency of pediatric NMDARE patients by the pediatric modified Rankin scale (mRS) at one year (N = 28).
| mRS at one year | N | Percent |
|---|---|---|
| 0 | 4 | 14 |
| 1 | 2 | 7 |
| 2 | 11 | 39 |
| 3 | 8 | 29 |
| 4 | 2 | 7 |
| 5 | 1 | 4 |
| 6 | 0 | 0 |
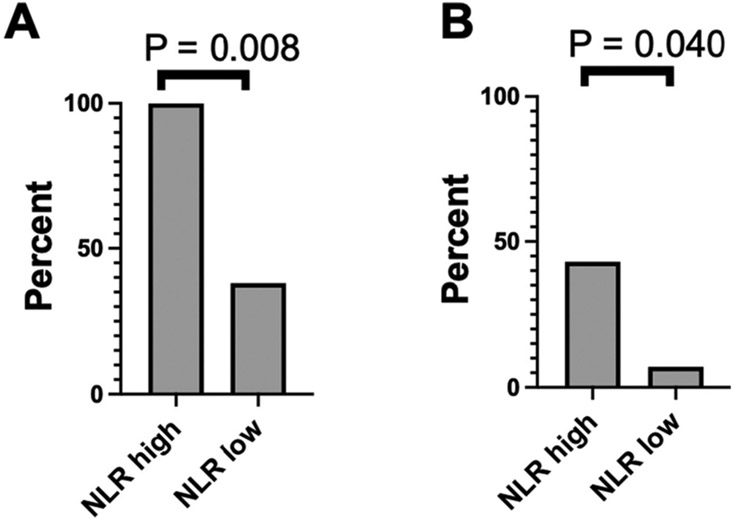
However, high NLR was associated with a higher proportion of intubations. In the high NLR group, 100% (7/7) were intubated as compared to 38% (11/29) in the low NLR group (p = 0.008, Fig. 1). We used a backward stepwise regression model to identify which variables were associated with poor outcomes at one year by mRS. This model included time to first line treatment, time to second line treatment, tumor, age, hospital length of stay, ICU admit, intubation status, NLR, and high versus low NLR. These variables did not associate with poor outcomes at one year by mRS. Including NLR as a continuous variable also did not correlate with poor mRS at one year. In a multivariate analysis for tumor, NLR was not significantly associated with tumor when adjusting for age. However, multivariate analysis for risk for intubation demonstrated an association with NLR while adjusting for age, with an odds ratio of 1.72 (95% confidence interval 1.05, 2.84, and p = 0.0321).
Fig. 1.
Proportions of NMDARE patients with high NLR versus low NLR who are A) intubated and B) have a tumor.
We then assessed whether NLR was associated with hospital length of stay (LOS) and inpatient rehabilitation LOS. As for hospital length of stay (LOS), NLR was not correlated with hospital LOS (p = 0.7099). We then performed a multiple linear regression using backward elimination with NLR, time to first line treatment, time to second line treatment, ICU admission, age, and gender, ICU admission (p = 0.0203) was the only variable associated with hospital length of stay. However, NLR did correlate with inpatient rehabilitation LOS (p = 0.0429, with R-square value of 0.1385), and on multivariate linear regression with backwards elimination (using variables NLR, time to first line treatment, ICU admission, age, and gender) resulted in only NLR as a significant covariate.
4. Discussion
In this study, we retrospectively analyzed pediatric patients with an initial diagnosis of anti-NMDA receptor encephalitis. We specifically analyzed the initial blood and CSF studies in addition to clinical features to determine potential biomarkers correlated with worse clinical outcomes. This study revealed that high NLR was associated with a higher proportion of patients who were intubated or admitted to the ICU but did not correlate with one-year outcomes.
NLR has been shown to predict outcomes in other critically ill populations, including sepsis (Huang et al., 2020), stroke (Li et al., 2021b), and coronavirus-19 syndrome (COVID-19) (Ulloque-Badaracco et al., 2021). NLR has been used as a measure of stress, since endogenous steroids are released in times of stress, including in cancer (Gibney et al., 2016). Interestingly, higher NLR was associated with presence of tumor, which were all ovarian teratomas in our cohort, and with higher WBC. While steroids – both endogenous and exogenous – can increase WBC and NLR, the WBC and NLR in our study was obtained prior to treatment. The limitations of our study include a single center retrospective study, and clinical data was not available for all patients, which could result in some bias due to missing data. Retrospective analyses are prone to bias due to limitations in data collection (Norvell, 2010). Additionally, the mRS is a crude measure of outcome and not sensitive to some possible deficits (Kapoor et al., 2017).
Prior to our study, NLR had been examined in the context of the adults with anti-NMDA receptor encephalitis as well as in other autoimmune encephalitic conditions and demyelinating diseases such as multiple sclerosis. NLR was observed to be a prognostic biomarker in their respective retrospective adult cohort studies with regard to poor outcomes (Qiu et al., 2019) and disease severity (Zeng et al., 2019). One difference is that NLR predicted a worse outcome at 2 months in the adult NMDARE, and we examined our patients at one year. Additionally, the role of NLR as a marker of neuro-inflammation was assessed in a cohort of newly diagnosed multiple sclerosis and clinically isolated syndrome patients, and no association between NLR and disease activity was found (Gelibter et al., 2021). In another study evaluating the validity of NLR as an inflammatory marker for multiple sclerosis disability and activity, NLR levels were found to be significantly higher in patient with relapse compared to those in remission and NLR was found to be significantly associated with disease disability (Fahmi et al., 2021). Similarly, NLR was proposed as a marker of severity of multiple sclerosis related neurological disability with elevated NLR strongly predicting increased disease related disability in a separate large adult cohort study (Hemond et al., 2019). Additionally, in a retrospective study of adults with neuromyelitis optica spectrum disorder (NMOSD), elevated NLR was found to be significantly associated with high relapse rates and poor recovery (Xie et al., 2021).
5. Conclusions
In conclusion, NLR was associated with older age in our cohort, and NLR does increase with age, especially in adolescents (Moosmann et al., 2022). However, increasing NLR was still associated with increased risk for intubation, even when adjusting for age. NLR, while associated with rates of intubation, did not correlate with one-year outcomes. Further investigation is needed to assess for prognostic biomarkers in pediatric NMDARE.
Funding
This work was supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Numbers UL1TR002378 and KL2TR002381, United States, and the 2019 Pediatric Research Alliance Center for Clinical and Translational Research Pilot Grant, Atlanta, GA, United States.
Abbreviations:
- NMDARE
anti-NMDA receptor encephalitis
- NLR
neutrophil-to-lymphocyte ratio
- CHOA
Children’s Healthcare of Atlanta
- mRS
modified Rankin score
Footnotes
Declaration of Competing Interest
N.K., M.M., L.L., M.B., L.B, and R.H. have nothing to declare. G.G. receives salary support from the Centers for Disease Control and Prevention for surveillance for acute flaccid myelitis.
References
- Dalmau J, Armangue T, Planaguma J, Radosevic M, Mannara F, Leypoldt F, et al. , 2019. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 18, 1045–1057. [DOI] [PubMed] [Google Scholar]
- de Haan R, Limburg M, Bossuyt P, van der Meulen J, Aaronson N, 1995. The clinical meaning of Rankin ‘handicap’ grades after stroke. Stroke. 26, 2027–2030. [DOI] [PubMed] [Google Scholar]
- Fahmi RM, Ramadan BM, Salah H, Elsaid AF, Shehta N, 2021. Neutrophil-lymphocyte ratio as a marker for disability and activity in multiple sclerosis. Mult. Scler. Relat. Disord 51, 102921. [DOI] [PubMed] [Google Scholar]
- Gelibter S, Pisa M, Croese T, Dalla Costa G, Orrico M, Preziosa P, et al. , 2021. Neutrophil-to-lymphocyte ratio: a marker of neuro-inflammation in multiple sclerosis? J. Neurol 268, 717–723. [DOI] [PubMed] [Google Scholar]
- Gibney GT, Weiner LM, Atkins MB, 2016. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 17 (e542–e51). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. , 2016. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 15, 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, et al. , 2014. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 13, 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen Y, Deiva K, Pettingill P, Waters P, Siddiqui A, Chretien P, et al. , 2014. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov. Disord 29, 90–96. [DOI] [PubMed] [Google Scholar]
- Hemond CC, Glanz BI, Bakshi R, Chitnis T, Healy BC, 2019. The neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratios are independently associated with neurological disability and brain atrophy in multiple sclerosis. BMC Neurol. 19, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth RA, Vova J, Blackwell LS, 2019. Early functional outcomes for pediatric patients diagnosed with anti-NMDA receptor encephalitis during inpatient rehabilitation. Am. J. Phys. Med. Rehabil 98 (7), 529–535. [DOI] [PubMed] [Google Scholar]
- Huang Z, Fu Z, Huang W, Huang K, 2020. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am. J. Emerg. Med 38, 641–647. [DOI] [PubMed] [Google Scholar]
- Huang XX, Zhang S, Yan LL, Tang Y, Wu J, 2021. Influential factors and predictors of anti-N-methyl-D-aspartate receptor encephalitis associated with severity at admission. Neurol. Sci 42 (9), 3835–3841. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Lanctot KL, Bayley M, Kiss A, Herrmann N, Murray BJ, et al. , 2017. “good outcome” Isn’t good enough: cognitive impairment, depressive symptoms, and social restrictions in physically recovered stroke patients. Stroke. 48, 1688–1690. [DOI] [PubMed] [Google Scholar]
- Li JH, Milla SS, Gombolay GY, 2022. Apr. Rate of Anti-NMDA Receptor Encephalitis in Ovarian Teratomas. Neuropediatrics 53 (2), 133–135. 10.1055/s-0041-1740352. Epub 2021 Dec 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Hou M, Ding Z, Liu X, Shao Y, Li X, 2021b. Prognostic value of neutrophil-to-lymphocyte ratio in stroke: a systematic review and meta-analysis. Front. Neurol 12, 686983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Elkins K, Bhalla S, Gedela S, Kheder A, Zhang G, et al. , 2021. Electroencephalography characteristics to predict one-year outcomes in pediatric anti-NMDA receptor encephalitis. Epilepsy Res. 178, 106787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnoila J, Pulli B, Armangue T, Planaguma J, Narsimhan R, Schob S, et al. , 2019. Mouse model of anti-NMDA receptor post-herpes simplex encephalitis. Neurol. Neuroimmunol. Neuroinflamm 6, e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerinc LB, Blackwell L, Howarth R, Gombolay G, 2021. Evaluation of the anti-N-methyl-D-aspartate receptor encephalitis one-year functional status score in predicting functional outcomes in pediatric patients with anti-N-methyl-D-aspartate receptor encephalitis. Pediatr. Neurol 124, 21–23. [DOI] [PubMed] [Google Scholar]
- Moosmann J, Krusemark A, Dittrich S, Ammer T, Rauh M, Woelfle J, et al. , 2022. Age- and sex-specific pediatric reference intervals for neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and platelet-to-lymphocyte ratio. Int. J. Lab. Hematol 44 (2), 296–301. [DOI] [PubMed] [Google Scholar]
- Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ, 2014. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol 76, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvell DC, 2010. Study types and bias-Don’t judge a study by the abstract’s conclusion alone. Evid. Based Spine Care J 1, 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosadini M, Thomas T, Eyre M, Anlar B, Armangue T, Benseler SM, et al. , 2021. International consensus recommendations for the treatment of pediatric NMDAR antibody encephalitis. Neurol. Neuroimmunol. Neuroinflamm 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Zhang H, Li D, Wang J, Jiang Z, Zhou Y, et al. , 2019. Analysis of clinical characteristics and poor prognostic predictors in patients with an initial diagnosis of autoimmune encephalitis. Front. Immunol 10, 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, et al. , 2013. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 12, 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloque-Badaracco JR, Ivan Salas-Tello W, Al-Kassab-Cordova A, Alarcon-Braga EA, Benites-Zapata VA, Maguina JL, et al. , 2021. Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 patients: a systematic review and meta-analysis. Int. J. Clin. Pract 75, e14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Zhao Y, Pan C, Zhang J, Zhou Y, Li Y, et al. , 2021. Association of neutrophil-to-lymphocyte ratio (NLR) with the prognosis of first attack neuromyelitis optica spectrum disorder (NMOSD): a retrospective cohort study. BMC Neurol. 21, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Wu Y, Cao X, Li J, Liao X, Wei J, et al. , 2021. The clinical features and prognosis of anti-NMDAR encephalitis depends on blood brain barrier integrity. Mult. Scler. Relat. Disord 47, 102604. [DOI] [PubMed] [Google Scholar]
- Zahorec R, 2001. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 102, 5–14. [PubMed] [Google Scholar]
- Zeng Z, Wang C, Wang B, Wang N, Yang Y, Guo S, et al. , 2019. Prediction of neutrophil-to-lymphocyte ratio in the diagnosis and progression of autoimmune encephalitis. Neurosci. Lett 694, 129–135. [DOI] [PubMed] [Google Scholar]