Abstract
Clinical algorithms stipulate that transthyretin amyloid cardiomyopathy (ATTR-CM) can be diagnosed noninvasively by technetium-99m pyrophosphate (PYP) imaging when light chain (AL) amyloidosis has been excluded. We sought to define the distribution of light chain abnormalities and final diagnosis of ATTR-CM among patients referred for PYP imaging. We conducted a retrospective cohort study of 378 sequential patients with suspected ATTR-CM, referred for PYP imaging from October 2014 to January 2019. PYP scans were adjudicated as per guidelines. We found that 97 patients (26%) had abnormal plasma cell dyscrasia (PCD) markers, including serum free light chain (FLC) and/or urine/serum immunofixation electrophoresis (IFE). After exclusions for incomplete data or known AL amyloidosis, the final study population with abnormal PCD testing was n = 82. Final adjudication of amyloidosis was determined by multidisciplinary clinical assessment and/or tissue biopsy. The median age of cohort was 75 (68 to 81) years, 88% were men, and 33% were Black. Of the 82 patients, 62 had positive PYP scans (76%) and 20 had negative PYP scans (24%). A total of 64 patients had adjudicated ATTR-CM, confirmed by tissue biopsy in 41 (64%). Of those with confirmed ATTR-CM, 44 (69%) had abnormal FLC ratio between 1.65 and 3.1 and normal IFE. In conclusion, among patients referred for technetium-99m-PYP imaging for suspected ATTR-CM, 26% exhibited abnormalities of PCD markers. An FLC ratio 1.65 to 3.1, with normal IFE was noted in 69% of those with ATTR-CM, suggesting that ATTR-CM can be diagnosed noninvasively without cardiac biopsy in patients with positive PYP scan and similar plasma cell testing results.
Introduction
Cardiac amyloidosis is a progressive infiltrative disease, characterized by extracellular deposition of misfolded protein fibrils.1 Classification of cardiac amyloidosis is based on the precursor protein that misfolds, with immunoglobulin light chain (AL) amyloidosis and transthyretin (ATTR) amyloidosis involved in the vast majority of cardiac amyloidosis cases.2
Diagnosis of ATTR or AL cardiomyopathy (CM) formerly required tissue biopsy with histologic identification of both amyloid deposits by Congo red staining and accurate typing of the amyloidogenic protein.3 Currently accepted algorithms now permit nonbiopsy diagnosis of ATTR-CM in the context of both abnormal technetium (Tc)-99m pyro-phosphate (PYP) imaging and normal blood plasma cell dyscrasia (PCD) testing that excludes the AL amyloidosis.4,5 Unfortunately, guidelines also suggest that Tc-99m-PYP imaging cannot be interpreted as diagnostic of ATTR-CM in the context of abnormal PCD testing because a proportion of AL cardiac amyloidosis cases demonstrate uptake suggestive of ATTR. In an attempt to guide clinicians, this study was designed to describe the spectrum of abnormalities in PCD testing encountered among patients referred for Tc-99m-PYP imaging and compare those results with the final adjudication of ATTR-CM. We hypothesized that a range of free light chain (FLC) testing could be identified that, although above the upper limit of normal, would be associated with confirmed ATTR-CM, thereby obviating the requirement for invasive biopsy among similar patients.
Methods
We conducted a retrospective cross-sectional study of 378 consecutive patients, referred for PYP imaging for suspected ATTR-CM at a single academic hospital from October 2014 to January 2019. PCD testing was acquired contemporaneously with Tc-99m-PYP imaging. PCD testing identified 97 eligible participants (26%) who were ≥18 years of age and had evidence of abnormal PCD testing defined as (1) FLC ratio outside of the normal range, (2) abnormal serum immunofixation electrophoresis (IFE), or (3) abnormal urine IFE. The remaining 281 patients had normal PCD testing. After exclusion (n = 15) because of incomplete data, loss of follow-up, and previously known AL amyloidosis or multiple myeloma diagnosis, the final study population was 82 participants (Figure 1). All excluded patients with previously known AL amyloidosis had FLC ratio <0.26 or >3.1 and/or abnormal IFE and all had Tc-99m-PYP scans without diagnostic uptake. The study was approved by the institutional review board and written informed consent was obtained from all participants to access clinical data for research purposes. Data were collected retrospectively after thorough review of electronic medical records and included demographics, biochemical data, imaging, and pathology. Data were complete for all measures except N-terminal prohormone of brain natriuretic peptide (available in 64 patients) and prealbumin (available in 69 patients). Chronic kidney disease (CKD) was defined by an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2, using the 4-variable MDRD formula.
Figure 1.
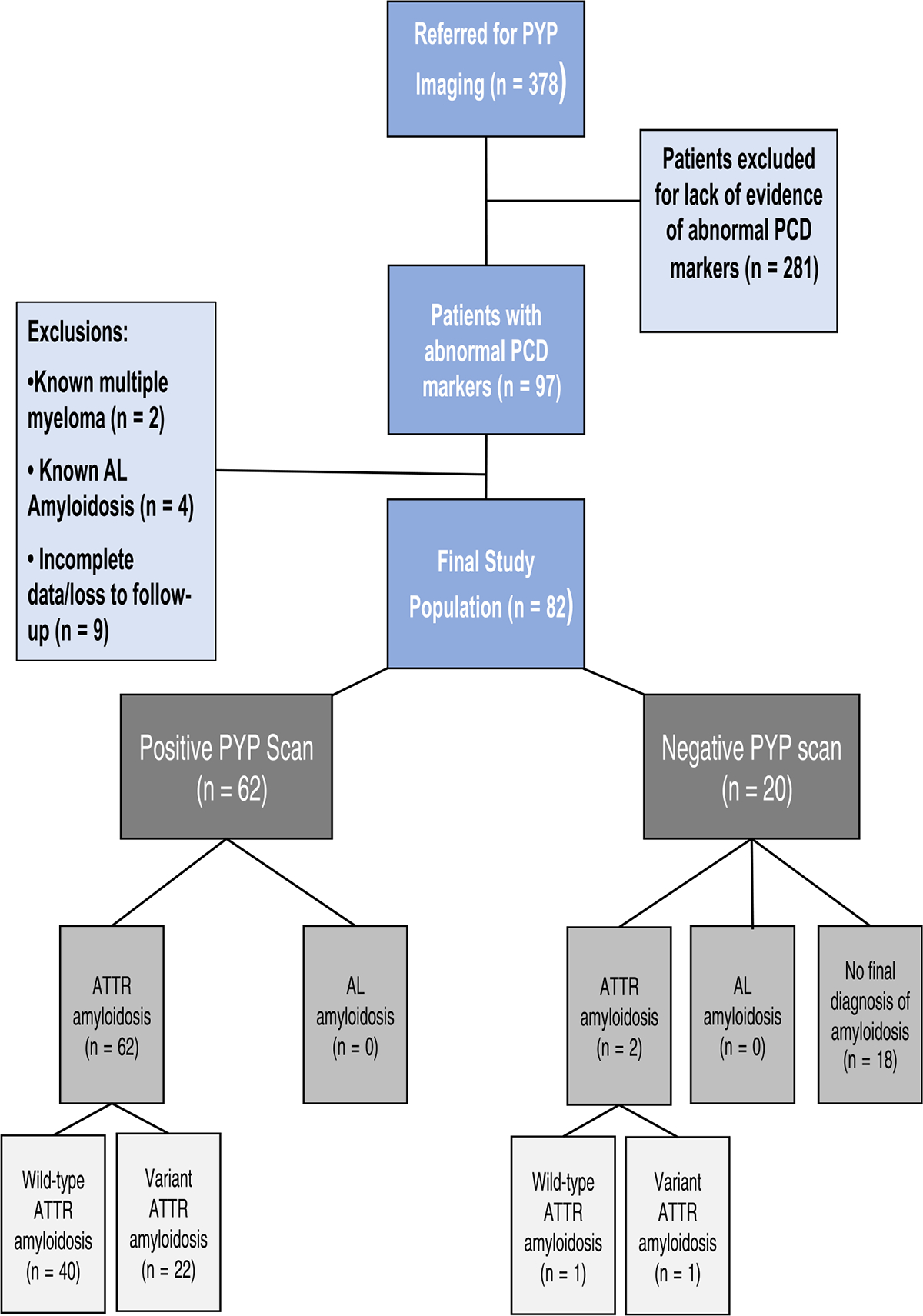
Study flow chart and breakdown by amyloid diagnosis. ATTR = amyloid transthyretin; PCD = plasma cell dyscrasia; PYP = Tc99m-pyrophosphate.
Myocardial radiotracer activity was determined after a 1-hour incubation by planar imaging and evaluated using 2 methods: (1) semiquantitative method, with a visual score comparing myocardial uptake to bone uptake (grade 0: absent cardiac uptake, grade 1: cardiac uptake is less intense than the rib signal, grade 2: cardiac uptake is similar to rib signal, and grade 3: cardiac uptake is greater than rib signal) and (2) the quantitative heart-to-contralateral lung ratio, which was calculated as the fraction of mean counts in a region of interest over the heart to mean counts in an identical size region of interest over the contralateral chest. Further assessment with single-photon emission computed tomography was performed in suspected cases to distinguish myocardial uptake from ventricular blood pool signal.
We defined a positive PYP scan as grade 2 or 3 cardiac uptake and/or a heart : contralateral ratio ≥1.5 at 1 hour after contrast injection on planar imaging, with confirmation by single-photon emission computed tomography, based on the 2019 expert consensus recommendations for multimodality imaging in cardiac amyloidosis.6
Serum FLCs, including kappa and lambda light chains, and the kappa: lambda ratio were measured (Freelite assay, The Binding Site, Birmingham, United Kingdom). Serum FLC data were available in all patients (n = 82). The reference intervals for kappa FLC are 3.3 to 19.4 mg/l and for lambda, FLC 5.7 to 26.3 mg/l respectively, and the normal range for the kappa to lambda ratio is 0.26 to 1.65. Lambda-predominant PCD was defined as FLC ratio <0.26, whereas kappa-predominant PCD was defined as FLC ratio >1.65. Serum IFE was performed in all patients and urine IFE was performed in 66 patients. PCD testing results were interpreted as abnormal if a monoclonal protein was detected in serum or urine or if the FLC ratio was outside of the normal range.
ATTR-CM was defined as follows:
positive cardiac biopsy for amyloid deposits by Congo red stain and definitive identification of transthyretin protein in the amyloid deposits by either immunohistochemistry (n = 1), immunofluorescence (n = 1), tandem mass spectrometry analysis (n = 20), or immunogold electron microscopy (n = 3), (total n = 25), or
positive extracardiac biopsy for transthyretin amyloid deposits and evidence of heart failure with an echocardiogram or cardiovascular magnetic resonance imaging, consistent with or suggestive of amyloidosis (n = 16), or
patients evaluated by the multidisciplinary team, including cardiology and hematology expertise, at the Boston University Amyloidosis Center and adjudicated as ATTR CM (n = 23).
All patients with ATTR-CM underwent bidirectional deoxyribonucleic acid sequencing by polymerase chain reaction of the transthyretin gene. Patients with normal gene testing were classified as ATTRwt amyloidosis (n = 41) and those with abnormal gene sequencing were classified as ATTRv amyloidosis (n = 23).
For statistical analysis, continuous data were expressed as mean ± SD, or median (25th and 75th percentiles [quartile 1 to quartile 3]), depending on the variable distribution, whereas categoric data were expressed as percentages of the number of cases within each group. Distribution of the data was evaluated using Shapiro-Wilk test. Continuous data were compared using the t test for normally distributed data or Wilcoxon rank-sum test for non-normally distributed variables. The association between categoric variables was examined using chi-square test or Fischer’s exact test when the observations were <5. The correlation between serum kappa/lambda FLC and eGFR in patients with ATTR-CM was tested using the Spearman correlation coefficient (r). All statistical analyses were performed using the STATA software, version 16.1 (Stata Corp, College Station, Texas). The results were considered statistically significant if p <0.05.
Results
Of 378 patients referred for PYP imaging, PCD testing was abnormal in 97 (26%). After exclusion for known AL amyloidosis, known MGUS, and loss to follow-up, the final population was n = 82. For those with known AL amyloidosis (n = 4), 3 had lambda PCD, with FLC ratio <0.26 and/or positive serum/urine IFE and 1 had kappa PCD, with FLC of 2.94 and positive urine IFE. The 2 patients with known multiple myeloma had kappa PCD and both had high FLC ratio >30.
The baseline demographics of the final study population (n = 82) are demonstrated in Table 1. Median age of cohort was 75 (range 81 to 86) years, 88% were men, 51% were White, and 33% were Black. The mean eGFR was 62.5 ± 24.3 ml/min/1.73 m2.
Table 1.
Baseline characteristics of patients referred for Tc-99m-pyrophosphate imaging with abnormal plasma cell testing
| Total patients, N | 82 |
|---|---|
| Age (years) | 75 (68–81) |
| BMI (kg/m2) | 28 (25–30) |
| Male | 72 (88%) |
| Female | 10 (12%) |
| Black | 27 (33%) |
| Hispanic | 8 (10%) |
| White | 42 (51%) |
| Other/unknown | 5 (6%) |
| eGFR (mL/min/1.73m2) | 62.5 ± 24.3 |
| Abnormal FLC ratio/normal IFE | 55 (67%) |
|
0 |
|
54 (98%) |
|
1 (2%) |
| Abnormal FLC ratio/abnormal IFE | 16 (20%) |
|
1(6%) |
|
11(69%) |
|
4(25%) |
| Normal FLC ratio/abnormal IFE | 11 (13%) |
Values are median (interquartile range), n (%), or mean ± standard deviation.
eGFR = estimated glomerular filtration rate; BMI = body mass index; FLC = free light chains; IFE = immunofixation electrophoresis.
Of the 82 patients with abnormal PCD testing, 62 (76%) had a positive scan and 20 (24%) had a negative scan. All patients with positive PYP imaging had a final diagnosis of ATTR-CM, whereas 2 patients with negative PYP imaging were diagnosed with ATTR-CM based on positive cardiac biopsy for transthyretin deposits (Figure 1).
In comparison to patients with negative PYP imaging, patients with positive PYP scan were older (median age 76 vs 67 years, p = 0.002) and had a lower body mass index (27.2 vs 30.2, p = 0.008). There were no significant differences observed between those with and without a positive PYP scan based on clinical laboratory testing, with the exception of troponin I, which was higher among those with a positive scan (0.12 vs 0.07 ng/ml, p = 0.003) (Table 2).
Table 2.
Demographics and clinical characteristics of patients with positive Tc-99m pyrophosphate (PYP) imaging versus patients with negative imaging
| Variable | PYP group | P-value | |
|---|---|---|---|
| Positive (N = 62) | Negative (N = 20) | ||
| Age at PYP scan (years) | 76 ± 7.3 | 68 ± 12.4 | <0.001 |
| BMI (kg/m2) | 27.2 ± 3.8 | 30.2 ± 5.4 | 0.008 |
| Male | 55 (89%) | 17 (85%) | 0.700 |
| Black | 17 (28%) | 10 (50%) | 0.056 |
| Hispanic | 7 (11%) | 1 (5%) | |
| White | 33 (53%) | 9 (45%) | |
| Hemoglobin (g/dL) | 13.2 (12.1–14.5) | 12.7 (11.1–14.6) | 0.44 |
| Creatinine (mg/dL) | 1.21 (1.08–1.48) | 1.18 (1.08–1.82) | 0.77 |
| eGFR (mL/min/1.73m2) | 63.5 ± 21.14 | 59.5 ± 32.92 | 0.52 |
| Troponin (ng/mL) | 0.12 (0.06–0.18) | 0.06 (0.01–0.08) | 0.003 |
| NT-ProBNP (pg/mL) | 2030 (1146–3722) | 754 (206–3041) | 0.072 |
| BNP (pg/mL) | 381 (240–757) | 336 (190–1028) | 0.85 |
| Prealbumin (mg/dL) | 20.8 ± 6.84 | 25 ± 7.39 | 0.100 |
| AST (U/L) | 28 (21–35) | 27 (19–30) | 0.72 |
| ALT (U/L) | 24 (19–32) | 3 (18–32) | 0.92 |
| Total bilirubin (mg/dL) | 0.8 (0.6–1.2) | 0.7 (0.5–0.9) | 0.081 |
| Alkaline phosphatase (U/L) | 90 (72–122) | 82 (72–95) | 0.23 |
| Echocardiography | |||
| Left atrial size (mm) | 42.2 ±6.5 | 42.1 ± 9.7 | 0.96 |
| Septal wall thickness (mm) | 15.1 ± 2.9 | 13.8 ± 4.5 | 0.13 |
| Inferolateral wall thickness (mm) | 15 ± 3.1 | 12 ± 2.3 | <0.001 |
| Left ventricular mass (g) | 277.5 ± 74.7 | 229 ± 68.6 | 0.015 |
| Left ventricular ejection fraction (%) | 49 ± 12 | 56 ± 12 | 0.019 |
| Electrocardiogram | |||
| Total voltage (mm) | 111 (92–141) | 133 (101–194) | 0.048 |
| Low voltage* | 5 (8%) | 1 (5%) | 1.00 |
Values are median (interquartile range), n (%), or mean ± standard deviation.
Low voltage defined as QRS amplitude of <5 millimeters in the limb leads and/or <10 millimeters in the precordial leads.
A total of 64 patients had adjudicated ATTR CM as a final diagnosis, including 41 (64%) with ATTRwt and 23 (36%) with ATTRv (17 with V122I, 2 with T60A, 1 with V30M, 2 with L58H, and 1 with E89Q TTR variants). Mean age of patients with ATTR-CM was 75.5 ± 7.5 years, and patients were predominantly men (89%) and White (55%).
Of 64 patients with ATTR CM and abnormal PCD testing, a total of 64% had biopsy-proved ATTR amyloidosis, including 25 (39%) with positive endomyocardial biopsy and 16 (25%) by extracardiac tissue biopsy (mainly fat aspirate). The remaining 23 patients (36%) had adjudicated ATTR-CM by clinical diagnosis based on expert opinion, without confirmatory biopsy. Of note, 82% of the patients without confirmatory biopsy (n = 23) had normal serum and urine IFE, and none had evidence of a lambda PCD, thus had FLC above the upper limit of normal. Importantly, each of these patients who had ATTR-CM adjudicated clinically also had an extracardiac biopsy using fat pad aspirate, and all were negative for amyloid deposits by Congo red staining. The baseline and clinical characteristics were similar among patients with ATTR-CM with and without confirmatory biopsy, except that patients who did not undergo biopsy were older (78.4 ± 6.2 vs 74.1 ± 7.8, p = 0.024) (Table 3).
Table 3.
Baseline and clinical characteristics of transthyretin amyloid cardiomyopathy (ATTR-CM) patients with and without confirmatory biopsy
| Variable | ATTR-CM with confirmatory biopsy | P-value | |
|---|---|---|---|
| Yes (N = 41) | No (N = 23) | ||
| Age at PYP scan (years) | 74.1 ± 7.8 | 78.4 ± 6.2 | 0.024 |
| Male | 36 (88%) | 21 (91%) | 1.000 |
| Black | 12 (29%) | 5 (22%) | 0.377 |
| White | 24 (59%) | 11 (48%) | 0.377 |
| Left ventricular ejection fraction (%) | 48.8 ± 12.7 | 49.3 ± 10.5 | 0.87 |
| Creatinine (mg/dL) | 1.21 (0.93–1.54) | 1.22 (1.08–1.82) | 0.91 |
| eGFR (mL/min/1.73m2) | 61.6 (46.4–87.5) | 62.4 (54.0–74.6) | 0.92 |
| Troponin (ng/mL) | 0.l2 (0.05–0.19) | 0.11 (0.06–0.15) | 0.67 |
| NT-ProBNP (pg/mL) | 2080 (1297–7102) | 2003 (1068–3187) | 0.34 |
| BNP (pg/mL) | 356 (206–842) | 425 (260–703) | 0.80 |
| Abnormal FLC ratio/normal IFE | 25 (61%) | 19 (82%) | 0.095 |
|
0 | 0 | |
|
25 (61%) | 19 (83%) | |
|
0 | 0 | |
| Abnormal FLC ratio/abnormal IFE | 10 (24%) | 2 (9%) | 0.095 |
|
1 (2%) | 0 | |
|
8 (20%) | 2 (9%) | |
|
1 (2%) | 0 | |
| Normal FLC ratio/abnormal IFE | 6 (15%) | 2 (9%) | 0.095 |
Values are median (interquartile range), n (%), or mean ± standard deviation.
Among those with adjudicated ATTR-CM, 44 (69%) had abnormal FLC ratio between 1.65 and 3.1 and normal IFE, 12 (19%) had abnormal FLC ratio/abnormal IFE, and 8 (12%) had normal FLC ratio/abnormal IFE (Figure 2). Of note, all patients with abnormal urine IFE results had abnormal serum IFE as well. Further information regarding PCD testing characteristics in patients with adjudicated ATTR-CM is shown in Supplementary Table 1 in the Supplementary Appendix.
Figure 2.
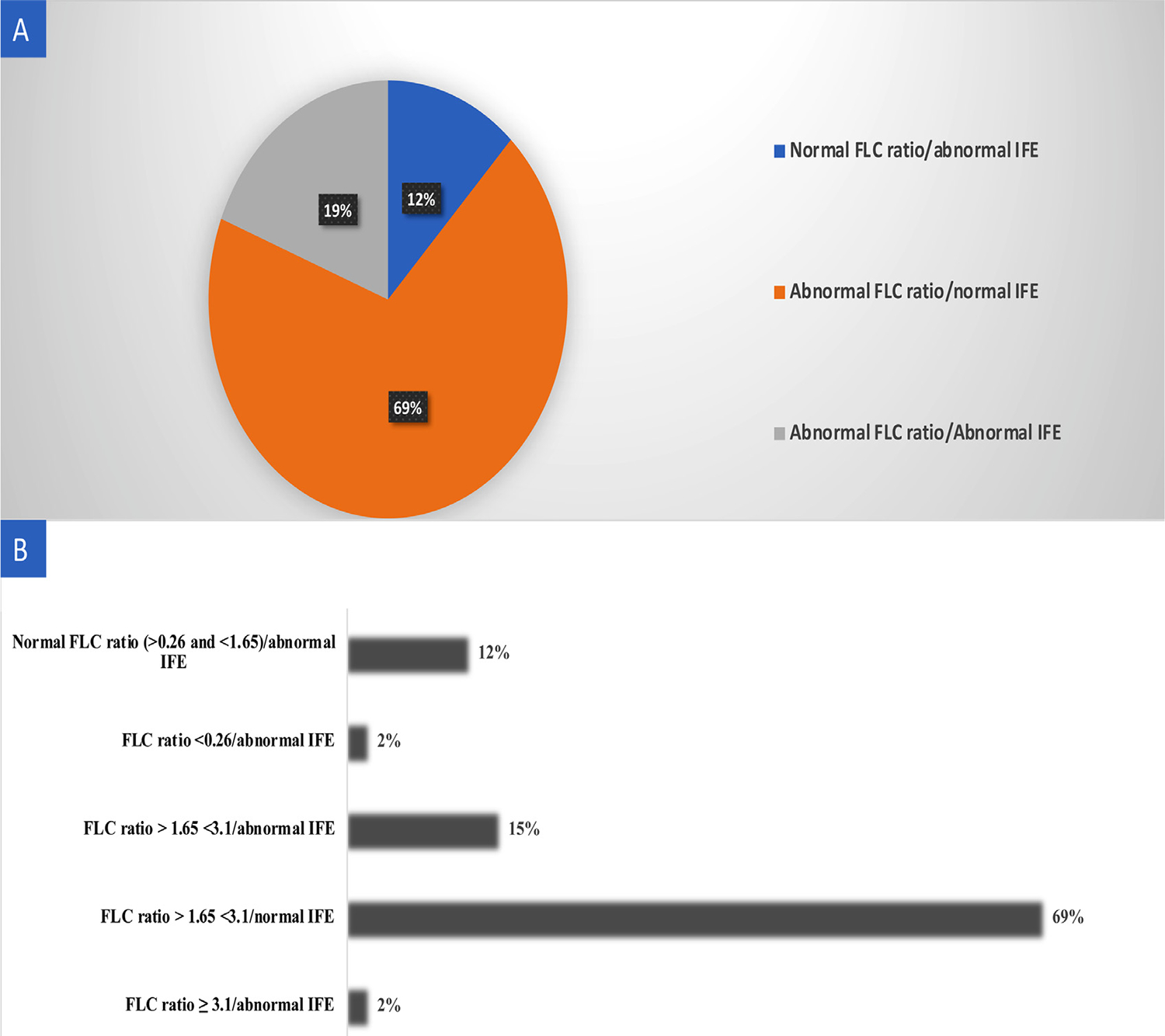
Plasma cell testing in patients with final adjudication of ATTR cardiomyopathy. (A) Pie chart showing the distribution of plasma cell dyscrasia abnormalities. (B) Histogram demonstrating that the majority (69%) of ATTR cardiomyopathy patients had an abnormal FLC ratio between 1.65 and 3.1 and normal IFE.
We demonstrated that both serum kappa FLC (ρ = −0.451, p <0.001) and serum lambda FLC (ρ = −0.466, p <0.001) were inversely correlated with eGFR, confirming that patients with reduced eGFR might have higher serum kappa and lambda FLC level at baseline. Approximately 28 patients (44%) with ATTR-CM had evidence of CKD at baseline, of whom 25 (89% of CKD) had abnormal FLC ratio between 1.65 and 3.1.
Discussion
Our study found that approximately 26% of patients referred for PYP imaging for suspected ATTR-CM had evidence of abnormal PCD testing, that, by current guidelines, would obviate the possibility of adjudicating ATTR-CM without confirmatory biopsy. We then observed that 78% (n = 64) of this population had adjudicated ATTR-CM based on positive tissue biopsy or clinical diagnosis by a multidisciplinary team assessment at an experienced amyloidosis referral center. The majority of abnormal PCD testing findings (69%) observed in those with ATTR-CM comprised abnormal FLC ratio above the upper limit of normal between 1.65 and 3.1, with normal serum and urine IFE, reflecting increased kappa light chain concentration. Our patient population was older, with a sizable proportion of CKD (44% of patients with ATTR-CM), typical of patients in whom ATTR amyloidosis is considered. We conclude that, in the context of such testing results, histologic confirmation of ATTR diagnosis may not be necessary especially in patients with known CKD at baseline. That stated, we strongly advocate for confirmatory endomyocardial biopsy in those with abnormal IFE testing or FLC ratio <0.26 (indicative of a lambda PCD) or ≥3.1, with normal or abnormal IFE, or those in whom clinical suspicion of AL amyloidosis remains high (Figure 3).
Figure 3.
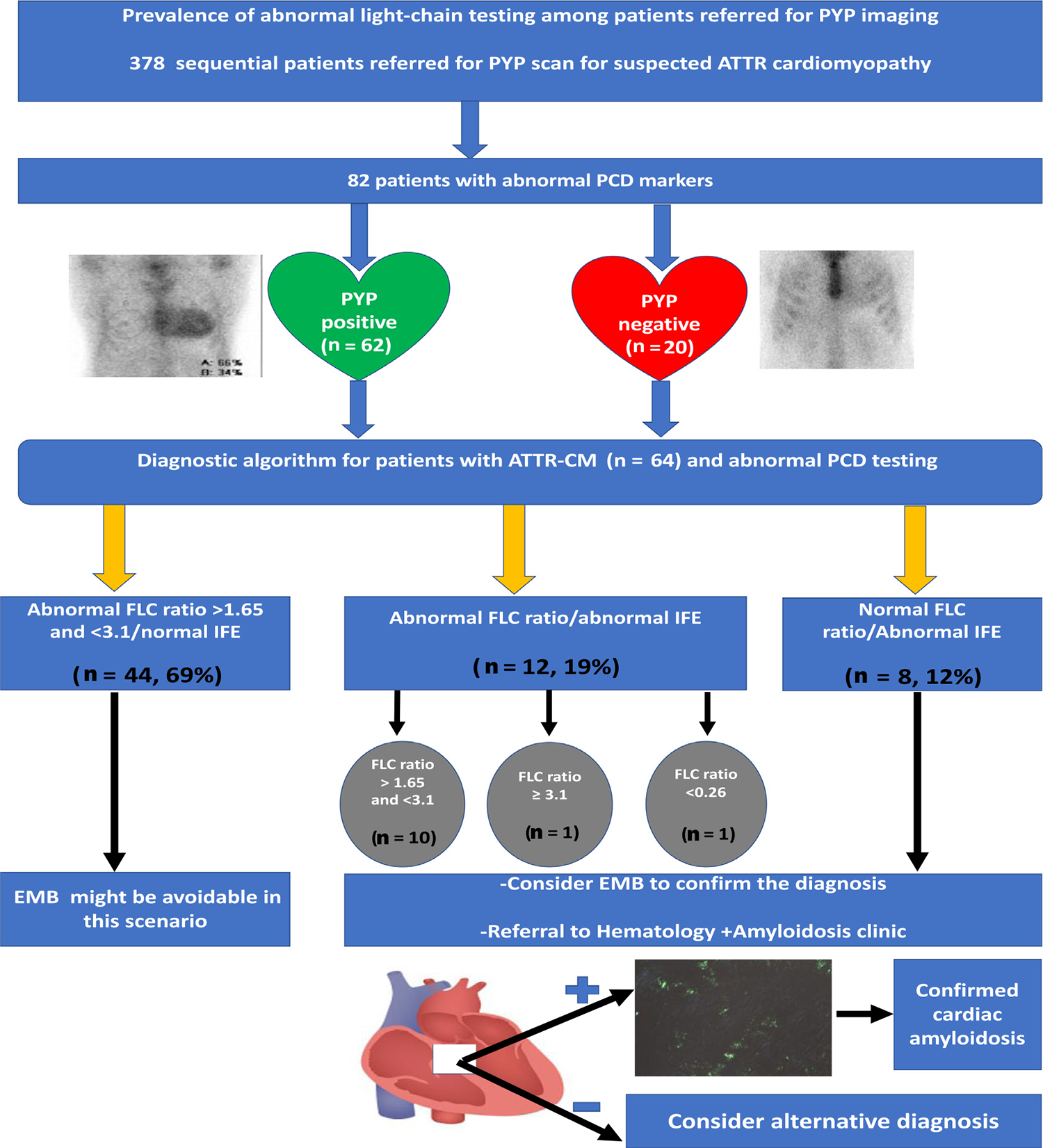
Central illustration. Light chain abnormalities and ATTR cardiomyopathy diagnosis among patients referred for PYP imaging. EMB = endomyocardial biopsy. EMB = endomyocardial biopsy; IFE = immunofixation electrophoresis; PCD = plasma cell dyscrasia; PYP = Tc99m-pyro-phosphate.
The prevalence of MGUS increases with age, affecting approximately 5.3% of patients aged >70 years.7 Although the exact prevalence of abnormal PCD testing in patients with ATTR amyloidosis remains unknown, it can range from 10% to 40% based on the previously reported retrospective analyses.8–10 We previously reported in a large retrospective study of 226 patients with tissue biopsy-proved ATTR amyloidosis, including 155 with ATTRwt and 71 with V122I ATTRv, that 39% of patients with ATTRwt and 49% of patients with ATTRv V122I had evidence of abnormal PCD testing.8 The present study adds to these observations by describing PCD test characteristics among patients with suspected but not confirmed ATTR-CM, more consistent with standard everyday practice.
Serum kappa and lambda FLC levels are mainly dependent on the balance between production and clearance. In normal circumstances, kappa FLC is produced at a rate approximately twice that of lambda FLC, but the half-life of kappa (2 hours) is shorter than lambda half-life (2 to 4 hours) because the renal clearance of kappa chains is faster than lambda chains. In cases of renal impairment, clearance of FLC is altered and will become more dependent upon the reticuloendothelial system, which shows no size preference and clears both light chains at the same rate.8 As a consequence, serum concentration of kappa light chains is disproportionately increased relative to that of lambda light chains in patients with CKD, and therefore, FLC ratio can be elevated even without evidence of monoclonal gammopathy. In fact, a previously published report suggested a new reference range of 0.37 to 3.1 for FLC ratio in patients with severe renal failure, with increased specificity from 93% to 99% and no loss of sensitivity for detecting monoclonal FLC production in these patients.11 Similarly, our study demonstrated that the majority (89%) of patients with ATTR-CM with concomitant CKD at baseline had an abnormal FLC ratio between 1.65 and 3.1, indicative of increased kappa FLC concentration. Our data support the contention that an adjusted reference range for FLC ratio in patients with CKD is warranted when approaching PCD testing interpretation in AL amyloidosis to increase the diagnostic accuracy of monoclonal gammopathy and to avoid unnecessary invasive biopsies in these patients.
To the best of our knowledge, this is the first study to examine the prevalence of abnormal PCD testing among patients with suspected ATTR-CM referred for PYP imaging. However, there are several important limitations to our study that must be acknowledged. First, we conducted a single-center, retrospective study, subjected to potential bias by selection and confounding. We attempted to correct for this limitation by reviewing data from sequential patients. Similarly, the sample size of patients with PCD abnormalities was small, which may explain why no case of AL amyloidosis, a much rarer disease, was identified. Alternatively, as our center is an experienced amyloidosis referral center, it is possible that patients with low-level paraprotein abnormalities, suspicious for AL or cases of known AL amyloidosis that were already recognized, were not referred for PYP imaging. Next, not all patients with adjudicated ATTR-CM and abnormal PCD testing had cardiac or extracardiac biopsy to confirm the diagnosis; however, all patients with ATTR-CM, without confirmatory biopsy (n = 23) had abdominal fat pad aspiration that was negative for amyloid deposits. In our experience and those of others, fat pad aspiration has an overall sensitivity of 75% to 80% for AL amyloidosis12; thus, a negative aspirate argues against AL. This information was considered in the final clinical adjudication through our multidisciplinary team approach, including an experienced hematologist, to exclude significant monoclonal gammopathy and determine the necessity of cardiac biopsy to exclude AL amyloidosis.
In conclusion, our study found that approximately 26% of patients referred for PYP imaging had evidence of abnormal PCD testing. The majority of patients (69%) with final adjudication of ATTR-CM had an abnormal FLC ratio between 1.65 and 3.1, with negative IFE. These findings suggest that endomyocardial biopsy might be avoidable in these circumstances, particularly among patients with concomitant CKD. Further validation is necessary with larger cohorts, preferably from nonamyloidosis specialty centers, to minimize risk of selection bias, before widespread clinical adaptation of our findings.
Disclosures
Dr. Ruberg acknowledges research support from Pfizer, Alnylam Pharmaceuticals, and Akcea Therapeutics, and consulting income from Alexion Therapeutics and Attralus. John Berk participates as a study investigator for Alnylam Pharmaceuticals, Ionis Pharmaceutical, Eidos Therapeutics, Corino Therapeutics and ad hoc scientific advisor for Ionis, Intellia Therapeutics, and Corino. Vaishali Sanchorawala acknowledges research support from Janssen, Celgene, Takeda, Prothena, Caelum, Sorrento; is part of the advisory board for Regeneron, Abbvie, Caelum, and Janssen; and a consultant for Pfizer. The remaining authors have no conflicts of interest to declare.
Supplementary Material
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2022.06.064.
Acknowledgment
The authors would like to thank Haili Cui, MD and Eric Burks, MD of Boston University Amyloidosis Center for processing and designing the cardiac biopsy image, demonstrating the amyloid deposits.
Dr. Ruberg acknowledges research support from National Institutes of Health/National Heart, Lung, and Blood Institute, R01 HL139671, Bethesda, Maryland.
Abbreviations:
- ATTR-CM
Transthyretin amyloidosis cardiomyopathy
- PCD
Plasma cell dyscrasia
- PYP
technetium-99m pyrophosphate
- FLC
Free light chain
Footnotes
See page 111 for disclosure information.
References
- 1.Griffin JM, Maurer MS. Cardiac amyloidosis a rare disease in older adults hospitalized for heart failure? Circ Heart Fail 2019;12: e006169. [DOI] [PubMed] [Google Scholar]
- 2.Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merlini G, Dispenzieri A, Sanchorawala V, Schönland SO, Palladini G, Hawkins PN, Gertz MA. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers 2018;4:38. [DOI] [PubMed] [Google Scholar]
- 4.Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404–2412. [DOI] [PubMed] [Google Scholar]
- 5.Nappi C, Zampella E, Volpe F, De Risi M, Piscopo L, Ponsiglione A, Imbriaco M, Acampa W, Petretta M, Cuocolo A. Identification and typing of cardiac amyloidosis by noninvasive imaging: two cases for two patterns. J Nucl Cardiol 2020;27:915–920. [DOI] [PubMed] [Google Scholar]
- 6.Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, Fontana M, Gheysens O, Gillmore JD, Glaudemans AWJM, Hanna MA, Hazenberg BPC, Kristen AV, Kwong RY, Maurer MS, Merlini G, Miller EJ, Moon JC, Murthy VL, Quarta CC, Rapezzi C, Ruberg FL, Shah SJ, Slart RHJA, Verberne HJ, expert Bourque JMASNC/ AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI. consensus recommendations for multimodality imaging in cardiac amyloidosis: part 2 of 2-diagnostic criteria and appropriate utilization. J Card Fail 2019;25:854–865. [DOI] [PubMed] [Google Scholar]
- 7.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, Dispenzieri A, Katzmann JA, Melton LJ 3rd. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006;354:1362–1369. [DOI] [PubMed] [Google Scholar]
- 8.Phull P, Sanchorawala V, Connors LH, Doros G, Ruberg FL, Berk JL, Sarosiek S. Monoclonal gammopathy of undetermined significance in systemic transthyretin amyloidosis (ATTR). Amyloid 2018;25:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, Berk JL, Seldin DC. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation 2016;133: 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geller HI, Singh A, Mirto TM, Padera R, Mitchell R, Laubach JP, Falk RH. Prevalence of monoclonal gammopathy in wild-type transthyretin amyloidosis. Mayo Clin Proc 2017;92:1800–1805. [DOI] [PubMed] [Google Scholar]
- 11.Hutchison CA, Plant T, Drayson M, Cockwell P, Kountouri M, Basnayake K, Harding S, Bradwell AR, Mead G. Serum free light chain measurement aids the diagnosis of myeloma in patients with severe renal failure. BMC Nephrol 2008;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staron A, Connors LH, Ruberg FL, Mendelson LM, Sanchorawala V. A new era of amyloidosis: the trends at a major US referral centre. Amyloid 2019;26:192–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


