Abstract
OBJECTIVE
To investigate cerebral reorganization, both structurally and functionally, occurring in patients with degenerative cervical myelopathy (DCM) following surgical decompression.
METHODS
In the current observational study with 19 subjects, high-resolution T1-weighted structural magnetic resonance imaging (MRI) and resting-state function MRI scans were collected from DCM patients pre and post-surgery and healthy controls (HCs). The resting-state fMRI data was utilized to perform ROI-to-ROI and ROI-to-voxel functional connectivity analysis and was similarly compared between and within cohorts. Macroscopic structural plasticity was evaluated by assessing for changes in cortical thickness within the DCM cohort following decompression surgery.
RESULTS
Prior to surgery, functional connectivity patterns were significantly different between DCM patients and HCs in cerebral areas responsible for postural control, motor regulation, and perception and integration of sensory information. Significantly stronger functional connectivity (FC) between the cerebellum and frontal lobes was identified in postoperative DCM patients compared to preoperative DCM patients. Additionally, increased FC between the cerebellum and primary sensorimotor areas was found to be positively associated with neurological improvement of DCM patients. No macroscopic structural changes were observed in the DCM patients following surgery.
CONCLUSIONS
These results support our hypothesis that functional changes within the brain are associated with effective post-operative recovery, particularly in regions associated with motor regulation, and with perception and integration of sensory information. In particular, increased FC between the cerebellum and the primary sensorimotor following surgery appears to associated with neurological improvement. Macroscopic morphological changes may be too subtle to be detected within three months following surgery.
Keywords: degenerative cervical myelopathy, surgical decompression, MRI, functional connectivity, morphology
Introduction
Degenerative cervical myelopathy (DCM) is a debilitating form of degenerative disc disease, affecting millions of adults over the age of 50. It is caused by progressive compressive abnormalities of the vertebral column that result in spinal cord damage due to both mechanical and biological injury. Chronic spinal cord compression causes necrosis and demyelination of gray and white matter, ultimately resulting in progressive neurological impairment and physical disability.1-3 Supraspinal alterations within the sensory and motor cortex have been demonstrated in DCM patients, commonly attributed to injury of projecting neurons.4, 5 Neural plasticity of the cortical network allows for minimization of functional impairment, and occurs via synaptic modification of pre-existing connections and/or the development of new circuitry.6, 7 Both fMRI and diffusion imaging studies have demonstrated increased functional and anatomical connectivity within primary and supplementary motor areas in DCM patients, and such reorganization is associated with worsened neurological status.8-10
While neurological function of DCM patients may be preserved by neural plasticity, the cerebral changes and reorganization following surgical decompression remain largely unexplored. Early intervention before manifestation of severe symptoms has frequently been advocated, predominantly based on the degree of neurological impairment and imaging features. Cessation of functional deterioration or even functional improvement following decompressive cervical surgery has been reported by several groups, where activation in primary sensorimotor cortices, including the precentral and postcentral gyri, was correlated with improvement.11-14 However, the specifics of the cerebral functional and structural changes associated with decompression remain understudied.
In the present study, we seek to further investigate the cerebral functional reorganization and any macrostructural changes that occur following decompressive surgery in DCM patients. Both high-resolution T1-weighted structural scans and resting-state fMRI scans were collected for all subject groups. Supraspinal differences between DCM patients that underwent surgery and neurologically intact healthy volunteers (HCs) were identified. In addition, intra-group differences between DCM patients pre- and post- surgery were elucidated. We hypothesized that DCM patients undergo rapid functional changes within the brain in clinically relevant regions proportional to the degree of post-operative recovery of neurological function, but macroscopic morphological changes may be slower and therefore too subtle to be detected within three months of recovery.
Methods
Patient Population
Nineteen DCM patients were prospectively enrolled in a cross-sectional observational study involving MR imaging paired with clinical assessment. All patients were recruited from an outpatient neurosurgery clinic. Exclusion criteria included 1) previous cervical spine surgery, 2) age < 18 or > 85, 3) clinical or radiological evidence of stroke or other neurologic disease, 4) cardiac pacemaker or other non-MRI compatible implant, 5) musculoskeletal, degenerative joint disease, or other medical cause of weakness or pain that affected use of the hands and gait, and 6) severe claustrophobia. All subjects signed Institutional Review Board-approved consent forms andmall analyses were performed in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
The study cohort included fourteen men and five women, with a mean age of 55.2 years (ranging from 41 to 74). The modified Japanese Orthopedic Association (mJOA) score was used to evaluate neurological function 15. A cohort of sixteen neurologically intact healthy volunteers (HCs) underwent the same MRI protocol, with an average age of 29 years, ranging from 20 to 64 years. Patient and healthy control demographic data is summarized in Table 1. The operative patients underwent cervical spine MRI, brain MRI, and neurological assessment at baseline and three months postoperatively.
TABLE 1.
Cohort Demographics for Diffusion Tensor Imaging Analysis.
| Subject Population |
N | Age (mean years ± SD) [min, max] |
mJOA Score (mean ± SD) [min, max] |
|
|---|---|---|---|---|
| HC Volunteers | 16 | 29.4 ± 11.3 [20, 64] |
18 | |
|
DCM Patients
|
Before Surgery | 19 | 55.2 ± 9.4 [41, 74] |
14.1 ± 2.1 [10, 17] |
| After Surgery | 16.8 ± 1.2 [14, 18] |
|||
Presenting Symptoms
The mean duration of preoperative symptoms was 8.2 months, with a range of 84 months. The most common presenting symptom in the surgical cohort was paresthesia or pain in the upper extremities, and was found in eighteen of the nineteen patients. Sixteen of the patients presented with deterioration of hand function. Sixteen of the patients endorsed a history of neck pain. Thirteen patients complained of gait abnormalities. One patient presented with a recent change in bladder function.
Physical Examination
Seven patients were found to have weakness in the upper extremities on examination, and two had weakness in the lower extremities. Eight patients had decreased sensation in the upper extremities, and two had sensory changes in the lower extremities. Hyperreflexia was the most common upper motor neuron sign, and was observed in twelve patients. Hoffman’s sign was the second most common long tract sign, and was elicited in ten patients. Four patients had clonus in the lower extremities, and one had a positive Babinski reflex.
Cervical Radiographical Imaging
Fifteen of the nineteen patients were noted to have T2-weighted signal abnormalities within the spinal cord parenchyma. Five of the patients had ossification of the posterior longitudinal ligament. Eleven of the patients had a lordotic cervical spine, six had straight, and two had a kyphotic spinal alignment.
Operative Treatment
The senior author (LTH) performed all of the surgical procedures. The surgical procedure choice was predominantly influenced by the following factors: patient age, exact location and type of pathology (e.g. OPLL), spinal alignment, and number of spinal levels involved. Eight patients underwent cervical laminectomy and fusion, seven patients had a laminoplasty performed, three underwent an anterior cervical discectomy and fusion, and had an anterior cervical corpectomy.
Anatomical Data Acquisition and Rs-fMRI
To evaluate cortical thickness and volume changes, high resolution 1 mm 3-dimensional (3D) T1-weighted structural MRIs were acquired on a 3T MR scanner (Siemens Prisma or Trio; Siemens Healthcare, Erlangen, Germany) using a 3D magnetization-prepared rapid gradient-echo sequence in either the coronal, sagittal, or axial orientation, with a repetition time (TR) of 2300 to 2500 ms, a minimum echo time (TE), an inversion time (TI) of 900 to 945 ms, a flip angle of 9° to 15°, field of view (FOV) = 240 x 320 mm and matrix size of 240 x 320, slice thickness = 1 mm. To evaluate functional connectivity, functional MR images were acquired using a Siemens Prisma 3T MRI scanner (Siemens Healthcare, Erlangen, Germany) with TR = 2000 ms; TE = 28 ms; slice thickness of 4 mm; FOV = 220 mm with an acquisition matrix of 64 x 64 for an in-plane resolution of 3.4 mm, and flip angle of 77°.
Image Preprocessing
The CONN Toolbox (https://www.nitrc.org/projects/conn),16 which implements functions from the Statistic Parametric Mapping (SPM, http://www.fil.ion.ucl.ac.uk/spm/) toolbox, was used to conduct the functional connectivity analysis of the brain. All functional MR images were preprocessed using the standard built-in preprocessing pipeline within CONN. Spatial smoothing of the functional data was performed using an 8mm full width at half maximum (FWHM) Gaussian kernel. To carry out denoising, the signal from the WM, CSF, and motion parameters was regressed from the functional data. Additional signal filtering was performed using a band-pass filter of 0.008 – infinity Hz, to reduce noise due to physiological effects, such as respiration and pulsation, and noise due to scanner drift.
Cortical segmentation and computation of cortical thickness and volume were performed using FreeSurfer (https://surfer.nmr.mgh.harvard.edu/fswiki)17-19 on the T1 images. Processed brain surfaces were smoothed with a FWHM of 10 mm, then registered to a standard space. Age, which was predominantly linearly related to cortical thickness,19-21 was included as a covariate in analyses.
Image Statistical Analysis
Following image preprocessing, a general linear model (GLM) was implemented to identify reorganizations in cortical thickness and functional connectivity (FC) following surgical decompression. We performed two-sample t-tests to evaluate inter-group differences between DCM patients who underwent surgery and healthy volunteers. We also performed paired t-tests to evaluate intra-group changes in DCM patients pre- and post- surgery. The level of significance was set at p < 0.05 with age included as a covariate.
Results
Changes in Clinical Measurements following Surgery
The mean preoperative mJOA score of the DCM cohort was 14.1 (range from 10 to 17), which significantly improved to 16.8 (range from 14 to 18) after surgery (p < 0.0001, Fig. 1). Three-month postoperative cervical spine MRI demonstrated satisfactory decompression in each case, as demonstrated by reestablishment of visible CSF around the spinal cord and no evidence of residual osseous/soft tissue in contact with the spinal cord (Fig. 2)
FIG. 1.

Graph demonstrating the range and mean mJOA scores of the study cohort pre and postoperatively. The mean mJOA score of the cohort significantly improved following surgery (p < 0.0001).
FIG. 2.

(Left) Preoperative T2 sagittal MRI demonstrating spinal cord compression in a 47 year old gentleman with DCM with a mJOA score of 13. He presented with gait and hand dysfunction. (Right) Postoperative T2 sagittal MRI demonstrating satisfactory spinal cord decompression following C3-7 laminoplasty. His mJOA score improved to 17 following the surgery.
Inter-Group Differences in Cortical Thickness and Functional Connectivity
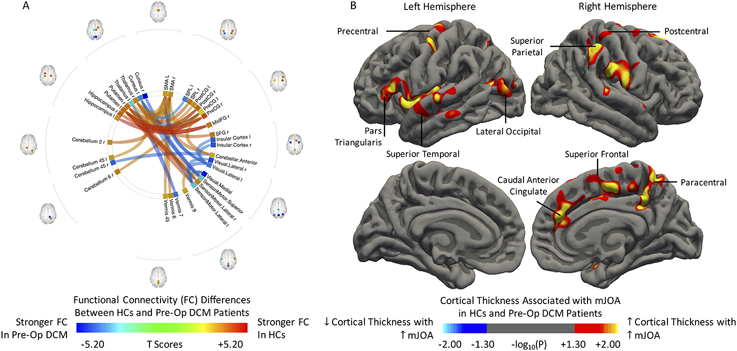
To study post-surgical structural and functional changes, we first compared the inter-group differences in cortical thickness and functional connectivity (FC) between healthy volunteers and the DCM patients that underwent surgery. Generally, HCs demonstrated stronger FC between the cerebral cortex and the thalamus, hippocampus, and putamen (Fig. 3A). Specifically, the SMA, the primary motor (precentral gyrus) and sensory (postcentral gyrus) areas displayed stronger FC with the putamen. Compared to the HCs, the visual network of preoperative DCM patients showed stronger FC to the left SMA, the sensorimotor system and the bilateral insular cortices. Additionally, the cuneus showed stronger FC to the sensorimotor system and the left superior parietal lobule in preoperative DCM patients. Significant cortical atrophy was associated with increasing neurological deficit (Fig. 3B). In the left hemisphere, clusters were observed within the pars triangularis, the precentral gyrus, the superior temporal gyrus, and the lateral occipital gyrus. In the right hemisphere, the presence of clusters including the caudal anterior cingulate, the superior parietal lobule, the superior frontal, postcentral and paracentral gyri were positively correlated with mJOA scores.
FIG. 3.
Differences in A) functional connectivity and B) morphology between preoperative DCM patients (Pre-Op DCM) and healthy controls (HCs). Red-Yellow denotes stronger FC in HCs (increasing cortical thickness with increasing mJOA score), while Blue-Light Blue denotes stronger FC in Pre-Op DCM (decreasing cortical thickness with increasing mJOA score). Position of ROIs was displayed on mid-axial slices. PreCG = Precentral Gyrus; PostCG = Postcentral Gyrus; SPL = Superior Parietal Lobule; SFG = Superior Frontal Gyrus; MidFG = Middle Frontal Gyrus; r = Right Hemisphere; l = Left Hemisphere.
Intra-Group Changes in DCM Patients following Surgery
The functional connectivity analysis revealed post-operative cerebral reorganization in DCM patients (Fig. 4), where stronger FC was observed between the right superior frontal gyrus and the left cerebellum section VI, as well as between the left superior frontal gyrus and the left cerebellum section X. In contrast, FC between the left thalamus and the left cerebellum section X, as well as between the right superior frontal gyrus and the left postcentral gyrus was only statistically significant in the preoperative DCM patients. Macroscopic morphological analysis of cortical thickness did not identify any regions showing statistically significant differences between preoperative DCM patients and postoperative DCM patients.
FIG. 4.
Differences in functional connectivity of DCM patients following surgical treatment. Red-Yellow denotes stronger FC in postoperative DCM patients (Post-Op DCM), while Blue-Light Blue denotes stronger FC in preoperative DCM patients (Pre-Op DCM). Position of ROIs was displayed on mid-axial slices. SFG = Superior Frontal Gyrus; PostCG = Postcentral Gyrus; r = Right Hemisphere; l = Left Hemisphere.
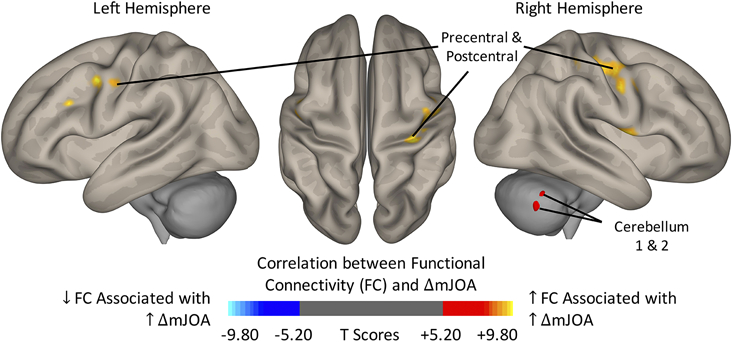
Finally, differences in FC of DCM patients following surgery was associated with improvement of neurological function. The ROI-to-ROI (Fig. 5) and ROI-to-Voxel (Fig. 6) functional connectivity analysis provided insights into the cerebral alterations. For increasing mJOA score, the left cerebellum showed increased connectivity with the sensorimotor network, including the bilateral precentral gyri and the right postcentral gyrus. In contrast, FC within the cerebellum was negatively correlated with the neurological improvement, especially within the posterior cerebellum. There was no association between cortical thickness changes and neurological improvement following surgery. Symptom duration did not demonstrate a statistically significant correlation with FC in the preoperative or postoperative fMRI within the cohort.
FIG. 5.
Correlation between region of interest (ROI)-to-ROI functional connectivity (FC) and improving neurological status (ΔmJOA) for DCM patients following surgical treatment. Yellow-Red denotes increasing FC associated with increasing ΔmJOA, while Light Blue-Blue denotes decreasing FC associated with increasing ΔmJOA. osition of ROIs was displayed on mid-axial slices. PreCG = Precentral Gyrus; PostCG = Postcentral Gyrus; r = Right Hemisphere; l = Left Hemisphere.
FIG. 6.
Brain surface display of correlation between region of interest (ROI)-to-voxel functional connectivity (FC) and improving neurological status (ΔmJOA) for DCM patients following surgical treatment. Seeding ROI was bilateral cerebellum section IV-VI. Yellow-Red denotes increasing FC associated with increasing ΔmJOA, while Light Blue-Blue denotes decreasing FC associated with increasing ΔmJOA.
Discussion
Cortical reorganization has been shown to play an important role in preserving neurological function in DCM patients. Through neuronal plasticity, the structural and functional connectivity network adapts by either modifying preexisting connections or by creating new circuitry.6, 7 Following surgical decompression, preservation and even recovery of motor function has been widely reported. It has been suggested that decompression of the compressed spinal cord induces cortical reorganization, and direct association between pre- and postoperative status in cortical sensorimotor activation in relation to behavioral improvement have been observed with fMRI.11, 12 Bhagavatula et al have reported post-surgical recruitment of cortical areas such as the postcentral gyrus, premotor cortex, and supplementary cortex, which correlated with improved dexterity.11 Furthermore, using fMRI during a three-finger pinch task, Dong et al found that the pinch-related activation in sensorimotor cortex contralateral to the movement paradigm, which was reduced in DCM patients, showed functional gains in the upper extremity following decompression surgery that approached the activation levels of the healthy controls.12
In addition to task-based fMRI, several groups also identified significant cortical reorganization using resting-state fMRI.22-24 In comparison to preoperative DCM patients, the postoperative group showed increased functional connectivity between the bilateral thalamus and posterior cingulate lobe, angular gyrus, and medial prefrontal, but significantly decreased functional connectivity between the bilateral thalamus, paracentral lobe, and precentral gyrus. Compared to the HCs, DCM patients showed significant differences in the visual and left sensorimotor cortices, the right superior frontal gyrus, and the right temporo-parietal junction following surgical decompression. These investigations highlight the improvement and adaptations of the supraspinal functional activity and connectivity that occur following the cervical decompression surgery and their potential impact on clinical recovery.
Consistent with previous findings, this study identified changes in cerebral structure and functional connectivity, supporting the hypothesis that cervical decompression can reorganize brain activity. Following surgical decompression, postoperative DCM patients demonstrated significant neurological improvement, which was closely associated with increasing FC between the cerebellum and the pre- and postcentral gyri, but no postoperative macroscopic morphological changes. These findings may have significant implications toward understanding the pathogenesis of DCM and recovery following surgical intervention.
Cerebral Alterations in DCM Patients Prior to Surgery
In the present study, we first conducted multiple analyses to identify cerebral alterations by comparing differences in morphology and functional connectivity between preoperative DCM patients and HCs. In addition to seeding common ROIs, we also included visual networks and cerebellum as seeds in the inter-group difference analysis. Consistent with previously proposed underlying biomarkers for disease severity and progression in patients with cervical spondylotic myelopathy,4, 9 we demonstrated that preoperative DCM patients, compared to HCs, have weaker FC from the putamen, thalamus, and hippocampus to the primary sensorimotor areas, and cortical atrophy associated with the worsened neurological impairment was seen in the caudal anterior cingulate, the superior frontal gyrus, as well as para-, pre- and postcentral gyri.
The importance of visual cortical activity in DCM patients has been previously demonstrated,24, 25 where FC alterations in DCM patients revealed differences between the visual cortex and the posterior cingulate gyrus. Consistent with those earlier studies, our results found greater FC between the visual network and the sensorimotor network, bilateral insular cortices and SMA prior to surgery, suggesting patients’ greater reliance upon visual inputs is a feature of their compensatory mechanism. This finding is akin to the premise of the Romberg test, which is based upon the concept that standing balance requires appropriate functioning of two of the three following senses – proprioception, vestibular function, and vision. Impairment of spinal cord proprioceptive function can be unmasked by removing visual input during the test, revealing that the patient was utilizing vision to compensate for dorsal column dysfunction.26
Cerebral Changes in DCM Patients following Surgery
We found a stronger FC between the left thalamus and the left cerebellum section X, as well as between the right superior frontal gyrus and the left postcentral gyrus in preoperative DCM patients, and increased FC between the cerebellum and the superior frontal gyrus following the surgical decompression. The superior frontal gyrus is involved in self-awareness, which is coordination with the action of the sensory system,27 while the thalamus plays an important role in relaying sensory and motor signals. In previous fMRI studies, we have demonstrated that associated with worsened neurological impairment evaluated by the mJOA score, patients with DCM exhibited increased FC between the superior frontal gyrus and the primary sensorimotor regions, but decreased FC between the cerebellum and thalamus to the anterior and posterior cingulate and frontal lobe regions. 9 In addition, Hrabalek et al. showed that DCM patients have functional activation increases not limited to the primary sensorimotor cortex but also found in the SMA, anterior cingulate, thalamus, basal ganglia, and cerebellum.28 Consistent with these findings, FC differences observed between preoperative and postoperative DCM patients indicated that compensatory inputs are actively adapted by DCM patients to preserve neurological function prior to the surgery, and ongoing functional reorganization is associated with effective post-operative recovery.
The mean mJOA score showed significant improvement of 2.7 after surgical decompression, and such improvement was closely correlated with increased FC between cerebellum and the pre- and postcentral gyri. In particular, the ROI-to-Voxel analyses identified that the activated regions within the pre- and postcentral gyri represent arm, elbow, and hand, suggesting the improvement in postural control and alleviated symptoms such as the weakness in the muscles of arms and hands following successful surgical decompression.12 The corticospinal tract plays an important role in voluntary motor control and modulating sensory information.29 Previous studies have shown that DCM patients experience white matter tract damage to the bilateral corticospinal tracts, with a significantly reduced number of streamlines compared to HCs.30
Compared to the corticospinal tract, the cerebellum has been relatively understudied in DCM patients, yet is critical for coordination of voluntary movements such as ambulation and hand dexterity, both of which are commonly impaired in DCM patients. Prior to surgery, our results showed that HCs demonstrated stronger FC between the cerebellum and the pre- and postcentral gyri when compared to preoperative DCM patients, and following surgical decompression, increased FC between the sub-regions of the cerebellum and the primary sensorimotor areas was observed in postoperative DCM patients. This recovery in FC between the cerebellum and pre – and post central gyri, which correlates with improvement in mJOA score, further highlights the beneficial effect of decompression surgery on sensorimotor function.
Limitations
Analyses based on morphology and connectivity have been used in our previous studies to demonstrate cortical reorganization of the sensorimotor system to compensate for potential functional loss due to progressive spinal cord injury. The current study investigated the cerebral neural plasticity of DCM patients undergoing surgical decompression. To account for the mean age of our HC cohort being significantly lower than that of the DCM cohort, all of our analyses, including the t-test, paired t-test and correlation analysis, have controlled for the ages of both cohorts as a covariate. Additionally, there was some value of including this particular HC cohort, as it provided a standard for optimal functional connectivity through which the surgical cohort could be contrasted. Although our results provide evidence on how surgical decompression can alter functional connectivity, longitudinal follow-up study is necessary to track continued morphological and structural changes. It is likely that further plasticity may evolve over time secondary to activity-dependent mechanisms related to motor practice and learning.10 Validation from diffusion weighted imaging could help to confirm the reorganization of such functional connectivity, as well as the formation of new connections in DCM patients who have exhausted their compensatory mechanism of recruitment and reorganization.
Conclusions
Reorganization in functional connectivity between the cerebellum and primary sensorimotor regions was associated with significant neurological improvement after successful surgical decompression. However, no macroscopic morphological changes were observed in postoperative DCM patients compared to preoperative DCM patients.
Acknowledgments
We would like to thank Mr. Craig Ehrlich for his generous research support.
Disclosures
Funding was received through the following NIH/NINDS grants: 1R01NS078494-01A1 (to LTH, NS, and BME), and 2R01NS078494-06 (to LTH, NS, and BME)
Abbreviations used in this paper:
- DCM
Degenerative cervical myelopathy
- HCs
Healthy volunteers
- mJOA
modified Japanese Orthopaedic Association
- fMRI
Functional MRI
- ROI
Region of Interest
- TR
Repetition time
- TE
Echo time
- TI
Inversion time
- FOV
Field of view
- PreCG
Precentral Gyrus
- PostCG
Postcentral Gyrus
- SPL
Superior Parietal Lobule
- SFG
Superior Frontal Gyrus
- MidFG
Middle Frontal Gyrus
- r
Right Hemisphere
- l
Left Hemisphere
References
- 1.Benzel EC, Lancon J, Kesterson L, Hadden T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. Sep 1991;4(3):286–95. [DOI] [PubMed] [Google Scholar]
- 2.Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. Aug 2009;11(2):112–8. doi: 10.3171/2009.1.SPINE08718 [DOI] [PubMed] [Google Scholar]
- 3.Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. May 2010;16(3):176–87. doi: 10.1097/NRL.0b013e3181da3a29 [DOI] [PubMed] [Google Scholar]
- 4.Woodworth DC, Holly LT, Mayer EA, et al. Alterations in Cortical Thickness and Subcortical Volume are Associated With Neurological Symptoms and Neck Pain in Patients With Cervical Spondylosis. Neurosurgery. Mar 1 2019;84(3):588–598. doi: 10.1093/neuros/nyy066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon EJ, Kim YK, Shin HI, et al. Cortical and white matter alterations in patients with neuropathic pain after spinal cord injury. Brain Res. Dec 2 2013;1540:64–73. doi: 10.1016/j.brainres.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 6.Freund P, Weiskopf N, Ward NS, et al. Disability, atrophy and cortical reorganization following spinal cord injury. Brain. Jun 2011;134(Pt 6):1610–22. doi: 10.1093/brain/awr093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holly LT, Dong Y, Albistegui-DuBois R, et al. Cortical reorganization in patients with cervical spondylotic myelopathy. J Neurosurg Spine. Jun 2007;6(6):544–51. doi: 10.3171/spi.2007.6.6.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holly LT, Wang C, Woodworth DC, et al. Neck disability in patients with cervical spondylosis is associated with altered brain functional connectivity. J Clin Neurosci. Nov 2019;69:149–154. doi: 10.1016/j.jocn.2019.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodworth DC, Holly LT, Salamon N, Ellingson BM. Resting-State Functional Magnetic Resonance Imaging Connectivity of the Brain Is Associated with Altered Sensorimotor Function in Patients with Cervical Spondylosis. World Neurosurg. Nov 2018;119:e740–e749. doi: 10.1016/j.wneu.2018.07.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun P, Murphy RK, Gamble P, et al. Diffusion Assessment of Cortical Changes, Induced by Traumatic Spinal Cord Injury. Brain Sci. Feb 17 2017;7(2)doi: 10.3390/brainsci7020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhagavatula ID, Shukla D, Sadashiva N, et al. Functional cortical reorganization in cases of cervical spondylotic myelopathy and changes associated with surgery. Neurosurg Focus. Jun 2016;40(6):E2. doi: 10.3171/2016.3.FOCUS1635 [DOI] [PubMed] [Google Scholar]
- 12.Dong Y, Holly LT, Albistegui-Dubois R, et al. Compensatory cerebral adaptations before and evolving changes after surgical decompression in cervical spondylotic myelopathy. J Neurosurg Spine. Dec 2008;9(6):538–51. doi: 10.3171/SPI.2008.10.0831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hrabalek L, Hok P, Hlustik P, et al. Longitudinal brain activation changes related to electrophysiological findings in patients with cervical spondylotic myelopathy before and after spinal cord decompression: an fMRI study. Acta Neurochir (Wien). May 2018;160(5):923–932. doi: 10.1007/s00701-018-3520-1 [DOI] [PubMed] [Google Scholar]
- 14.Tam S, Barry RL, Bartha R, Duggal N. Changes in functional magnetic resonance imaging cortical activation after decompression of cervical spondylosis: case report. Neurosurgery. Sep 2010;67(3):E863–4; discussion E864. doi: 10.1227/01.NEU.0000374848.86299.17 [DOI] [PubMed] [Google Scholar]
- 15.Yonenobu K, Abumi K, Nagata K, et al. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976). Sep 1 2001;26(17):1890–4; discussion 1895. doi: 10.1097/00007632-200109010-00014 [DOI] [PubMed] [Google Scholar]
- 16.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–41. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- 17.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. Feb 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 18.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. Sep 26 2000;97(20):11050–5. doi: 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. Jul 2004;14(7):721–30. doi: 10.1093/cercor/bhh032 [DOI] [PubMed] [Google Scholar]
- 20.Lemaitre H, Goldman AL, Sambataro F, et al. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. Mar 2012;33(3):617 e1–9. doi: 10.1016/j.neurobiolaging.2010.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. Jul 2007;17(7):1550–60. doi: 10.1093/cercor/bhl066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng X, Tan Y, He L, Ou Y. Alterations of functional connectivity between thalamus and cortex before and after decompression in cervical spondylotic myelopathy patients: a resting-state functional MRI study. Neuroreport. Oct 11 2019;doi: 10.1097/WNR.0000000000001346 [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Wang Z, Tu Y, et al. Regional Homogeneity and Multivariate Pattern Analysis of Cervical Spondylosis Neck Pain and the Modulation Effect of Treatment. Front Neurosci. 2018;12:900. doi: 10.3389/fnins.2018.00900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takenaka S, Kan S, Seymour B, et al. Towards prognostic functional brain biomarkers for cervical myelopathy: A resting-state fMRI study. Sci Rep. Jul 18 2019;9(1):10456. doi: 10.1038/s41598-019-46859-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Wang Q, Liang M, et al. Visual cortex neural activity alteration in cervical spondylotic myelopathy patients: a resting-state fMRI study. Neuroradiology. Sep 2018;60(9):921–932. doi: 10.1007/s00234-018-2061-x [DOI] [PubMed] [Google Scholar]
- 26.Khasnis A, Gokula RM. Romberg's test. J Postgrad Med. Apr-Jun 2003;49(2):169–72. [PubMed] [Google Scholar]
- 27.Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. Apr 20 2006;50(2):329–39. doi: 10.1016/j.neuron.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 28.Hrabalek L, Hlustik P, Hok P, et al. [Effects of spinal cord decompression in patients with cervical spondylotic myelopathy oncortical brain activations]. Rozhl Chir. Nov 2014;93(11):530–5. Efekt dekomprese krcni michy pri spondylogenni myelopatii na korove funkce mozku. [PubMed] [Google Scholar]
- 29.Jang SH. The corticospinal tract from the viewpoint of brain rehabilitation. J Rehabil Med. Mar 2014;46(3):193–9. doi: 10.2340/16501977-1782 [DOI] [PubMed] [Google Scholar]
- 30.Bernabeu-Sanz A, Molla-Torro JV, Lopez-Celada S, et al. MRI evidence of brain atrophy, white matter damage, and functional adaptive changes in patients with cervical spondylosis and prolonged spinal cord compression. Eur Radiol. Jul 26 2019;doi: 10.1007/s00330-019-06352-z [DOI] [PubMed] [Google Scholar]