Abstract
This article reviews evidence showing that neurochemical modulators can regulate the relative participation of the hippocampus and striatum in learning and memory tasks. For example, relative release of acetylcholine increases in the hippocampus and striatum reflects the relative engagement of these brain systems during learning of place and response tasks. Acetylcholine release is regulated in part by available brain glucose levels, which themselves are dynamically modified during learning. Recent findings suggest that glucose acts through astrocytes to deliver lactate to neurons. Brain glycogen is contained in astrocytes and provides a capacity to deliver energy substrates to neurons when needed, a need that can be generated by training on tasks that target hippocampal and striatal processing mechanisms. These results integrate an increase in blood glucose after epinephrine release from the adrenal medulla with provision of brain energy substrates, including lactate released from astrocytes. Together, the availability of peripheral and central energy substrates regulate the processing of learning and memory within and across multiple neural systems. Dysfunctions of the physiological steps that modulate memory—from hormones to neurotransmitters to metabolic substrates—may contribute importantly to some of the cognitive impairments seen during normal aging and during neurodegenerative diseases.
Keywords: acetylcholine, glycogen, astrocytes, glia, plasticity, metabolism, striatum, hippocampus, learning, memory
INTRODUCTION
As reflected in this Special Issue, there is extensive evidence for multiple memory systems, with each system responsible for processing different attributes of learning and memory. The view that these memory systems are distinct has diverse bases, including findings obtained after brain damage as well as a breadth of correlational measures and pharmacological manipulations. In rats, damage to different brain regions impairs particular cognitive functions, sometimes with extraordinary specificity (cf., White, 2008; Kesner, 2009). McDonald and White (1993) found that damage to the hippocampal formation, striatum, and amygdala impaired learning on three variants of food-motivated maze learning—win-shift, win-stay, and conditioned cue preference, respectively. Importantly, learning of two of the three variants was not impaired by each lesion. Similarly, Kesner et al. (1993) demonstrated a triple-dissociation between lesions of hippocampus, caudate, and extrastriate cortex for memory of spatial locations, motor responses, and objects, respectively, again with each lesion not impairing learning of the other two tasks.
Of course, most tasks and most experiences are not as specifically linked to independent memory systems, as found with these carefully devised tasks, but must instead involve interplay between these systems. If separate memory systems process different classes of information, it is easy to see that interactions across systems will be very important to the quality of learned information. There are many examples of competition between memory systems, often using the hippocampus (win-shift or place learning) and striatum (win-stay or response learning) as the brain systems of interest. In these experiments, down-regulation or damage to one system enhances learning and memory processed by another system (cf., Packard and Cahill, 2001; Gold, 2004; White, 2008). Other results highlight additional relationships, such as cooperation and independence across neural systems; many examples can be found within this Special Issue. Moreover, the relationships themselves are dynamic, changing for example with age (Barnes et al., 1980), stress (Sadowski et al., 2011), anxiety (Packard, 2009), ethanol administration (Matthews et al., 1999), and estrogen status (Korol and Kolo, 2002; Korol, 2004), and as described below, as rats develop different learning strategies from early to late learning trials.
The research described here is based mainly on pharmacological and neurochemical approaches to understanding the biological bases of the use of different neural systems responsible for learning and memory. Two brain areas, the hippocampus and striatum, are used as exemplars to study the mechanisms responsible for the controlling the balance between neural systems and the relative contributions of these brain regions in different learning and memory situations. Throughout, the studies use in vivo neurochemical measures obtained during learning and memory testing to identify profiles related to the participation of the hippocampus and striatum toward cognition. One neurochemical approach examines release of acetylcholine in the hippocampus and striatum during learning and memory tests in mazes that tap the function of one or both neural systems. A second neurochemical approach, directed by findings obtained in the acetylcholine studies, examines the mechanisms by which glucose acts directly on these brain regions to regulate learning and memory functions.
ACETYLCHOLINE
Spontaneous alternation mazes take advantage of the rats’ propensity to move nonrandomly through the maze, using a pattern in which there is a balance between entering recent and nonrecent arms. For example, in a Y-shaped maze (Fig. 1A), a rat that has visited arm A and then B is more likely to select arm C than A or B; an alternation is scored if all three arms are selected on three consecutive entries. Typically, the alternation score is between chance, 50% in this example, and full alternation, here 100%. In rats, alternation scores are typically near 75% in this task. In a four-arm version of this task (Fig. 1B), we generally calculate an alternation as four different arms in five consecutive choices, with typical scores above chance (>44%). In both versions of the task, the animal uses spatial cues for navigation and alternation reflects spatial working memory: performance declines if delays are interposed between arm selections or if the task is conducted in a room with poor extramaze cues.
FIGURE 1.

Left: Three-arm spontaneous alternation maze, with example of alternation scoring method. Right: Four-arm spontaneous alternation maze, with example of alternation scoring method.
Often, the task does not include the use of extrinsic behavioral reinforcement. When acetylcholine release in the hippocampus is assessed during nonrewarded alternation performance, release increases and is sustained throughout the test period (Ragozzino et al., 1996, 1998; Stefani et al., 2001), and is correlated across conditions with alternation scores (Ragozzino and Gold, 1995). We have also examined alternation behavior and acetylcholine release in both hippocampus and striatum (Pych et al., 2005a) in a rewarded version of the Y-maze. A piece of Frosted Cheerio® was placed at the end of each arm and was replaced as the rat left for another arm. An interesting behavior pattern emerged across arm choices during a 20-min test session. During the first 5 min, rats showed alternation scores of ~73%, similar to the scores seen in nonrewarded testing. However, later in the rewarded test session, the rats began to move more and more quickly through the maze using a repetitive turning-based strategy in which they almost always selected the arm to the right (or left) of the one they entered, resulting in alternation scores that approached 100% (Fig. 2, right axis, triangles). Unlike the behavior in similar non-rewarded tasks or the behavior seen here on early trials, performance on later trials appeared very automatic and repetitive.
FIGURE 2.
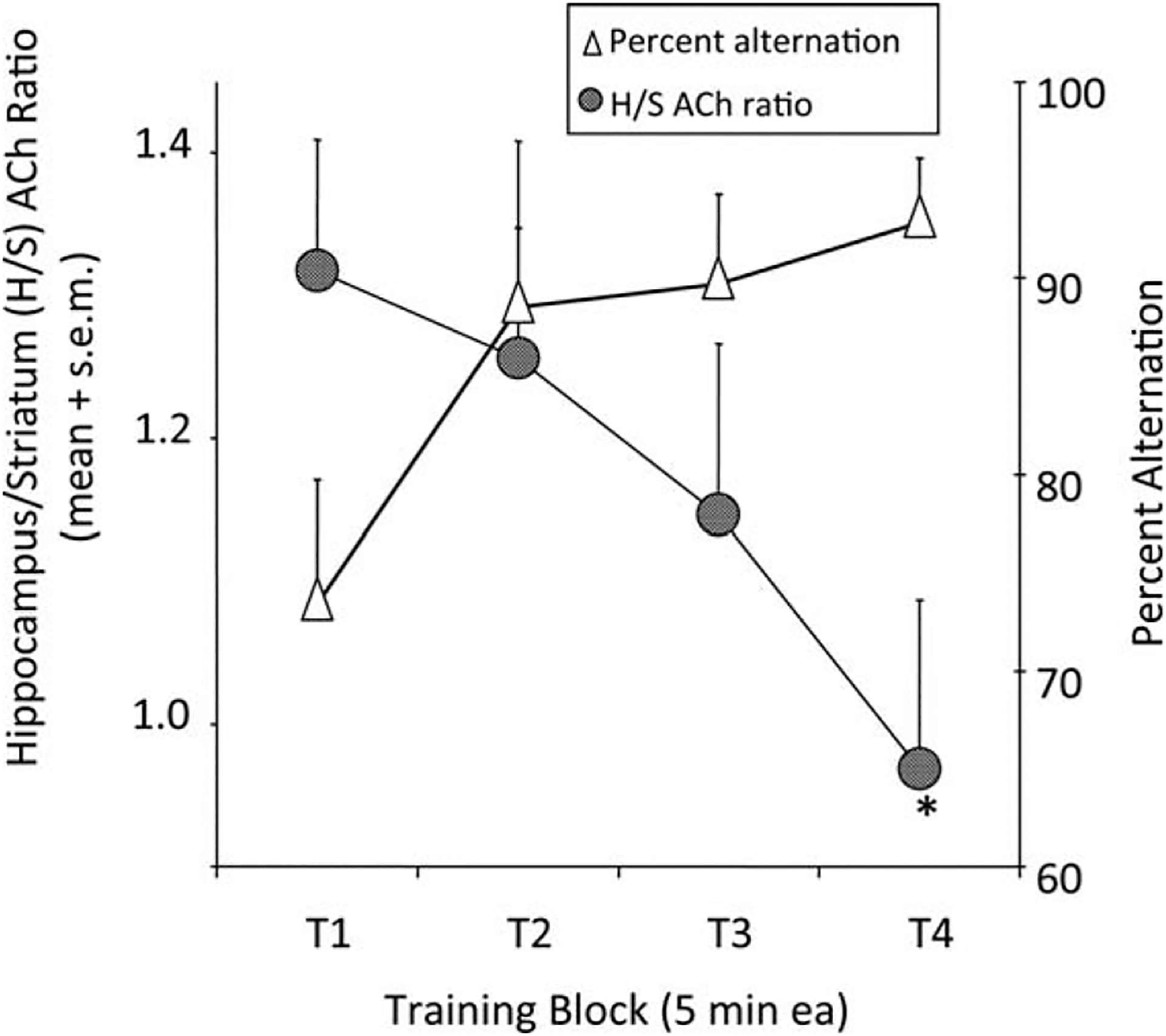
Changes in alternation scores and the ratio of acetylcholine (ACh) release in the hippocampus and striatum. Rats were tested on a food-rewarded spontaneous alternation task. Alternation scores rose during the testing session, beginning with what appears to be a spatial alternation strategy early and ending with a turning response strategy later in testing. In concert with this change in behavior, the ratio of ACh release in the hippocampus:striatum, measured simultaneously in the two brain areas, decreased during testing. From Pych et al., 2005a.
During the maze testing, acetylcholine release was measured simultaneously in the hippocampus and striatum, with samples collected every 5 min (i.e., four samples during the 20-min test). The neurochemical results showed that acetylcholine release increased in both the hippocampus and striatum when rats were tested for alternation performance. However, release in the hippocampus decreased during testing while release in the striatum increased during testing. The changes in acetylcholine release in these brain areas resulted in a decrease in the ratio of acetylcholine release in hippocampus:striatum across time on the maze at the same time that alternation performance increased from what we interpret to be hippocampus-based allocentric navigation to striatum-based egocentric or automatic navigation (Fig. 2, left axis, circles).
The shift in relative release of acetylcholine in the hippocampus and striatum during alternation testing is similar to that seen during training on a dual-solution T-maze. In this task (Tolman et al., 1946; Restle, 1957), rats are trained to find food in, for example, the arm to the right of the start arm. Rats learn rather quickly to solve this simple maze that can be solved effectively using either a place (reward in same place in the room) or a response (reward obtained after turn to right [or left]) strategy. There is no obvious behavioral difference during training that identifies whether rats are using a place or response strategy to find the food reward. To identify the strategy used, a probe trial is conducted after training in which the start arm is rotated 180°. If a rat is using a place strategy, it will choose the arm located in the same position in the room. If a rat is using a response strategy, it will choose the arm that requires the same turning movement as on original training. The relative expression of place vs. response solutions is shifted by lidocaine inactivation of the hippocampus and striatum, respectively (Packard and McGaugh, 1996); lidocaine is a sodium-channel blocker that acts as a local anesthetic. Of interest, rats used place solutions when tested after 8 days of training but response solutions when tested after 16 days of training.
Gonadal hormones also influence the selection of place vs. response solutions in the T-maze in female rats (Korol and Gold, 2007). When tested at different times through the estrous cycle, rats exhibited place solutions when ovarian hormones are high, i.e., at proestrus, and response solutions when ovarian hormones are low, i.e., at estrus (Korol et al., 2004). In addition, intrahippocampal infusions of the GABA agonist, muscimol, interacted with changes in preferred learning strategy through the stages of the estrous cycle (McElroy and Korol, 2005). Administration of muscimol directly into the hippocampus resulted in the rats preferring response solutions in the T-maze not only at estrus but also at proestrus, importantly without an effect on rate of learning per se.
We measured acetylcholine release simultaneously in the hippocampus and striatum while male rats learned this task (Chang and Gold, 2003). To accommodate the in vivo microdialysis methods, we compressed training into a single session of 100 trials, with probe trials given after every 20 trials and acetylcholine release measured in 1-min intervals that coincided with timed trials on the maze. As seen by Packard and McGaugh (1996), rats gradually shifted their strategy selection on probe trials from early use of place solutions to later use of response solutions (Fig. 3A, top). The main new question was how would the profile of acetylcholine release change during the transition from place to response solutions (Fig. 3B, bottom). The results showed that acetylcholine release increased in the hippocampus on the first trial and essentially remained high throughout the rest of training, even on late trials when the response strategy dominated probe behavior. Related to this result, it is interesting to note that if lidocaine is injected into the striatum late in training, rats revert to a place solution and not to chance behavior (Packard and McGaugh, 1996), suggesting that the information in the hippocampus remains available though the expression of that information is eclipsed by striatal functions. The maintained high release of acetylcholine in the hippocampus, even after response solutions are used behaviorally, is consistent with this view. The temporal pattern of acetylcholine release was different in the striatum than in the hippocampus. Acetylcholine release in the striatum was slower to rise across trials than was seen in the hippocampus, with striatal increases generally corresponding to the change in strategy selection on probe trials. Therefore, it appears that the response solution develops as the striatum comes “on-line” sufficiently to control the strategy used during the probe tests. These findings are consistent with those described above during rewarded spontaneous alternation performance and suggest that acetylcholine may dynamically reflect and perhaps control the relative participation of the hippocampus and striatum during the performance of learned behaviors.
FIGURE 3.
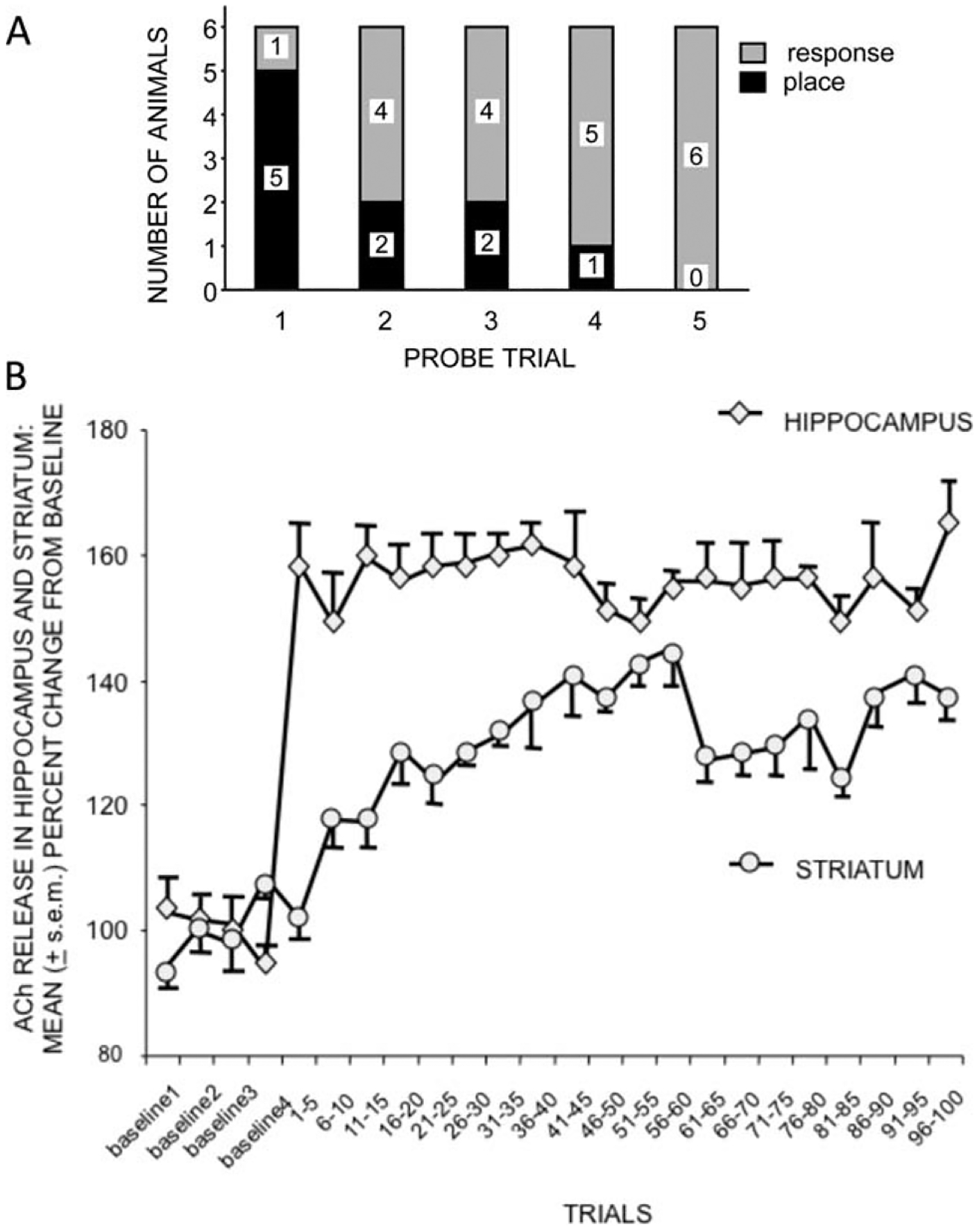
A. Change from place to response strategies during training on a dual-solution T-maze. 100 trials were conducted within a single session. The strategies were assessed on probe trials administered after sets of 20 trials. B. Changes in release of acetylcholine (ACh) in the hippocampus and striatum. Note that release of ACh in the hippocampus increased with the start of training to a level maintained throughout training. Acetylcholine (ACh) release in the striatum increased more slowly, reaching its asymptote about the time rats changed the strategy used to solve the maze. From Chang and Gold, 2003.
In a subsequent experiment (Pych et al., 2005b), we examined release of acetylcholine in the hippocampus and striatum of rats while they were trained on one of two versions of a four-arm plus-shaped maze. One version can be solved well using a place strategy and the other using a response strategy (Korol and Kolo, 2002). In both versions of the maze, rats are started from either the north or south arm. In the place version of the maze, the food reward is found at the end of the arm in one location (e.g., east) in the room regardless of start location. In the response version of the maze, the food reward is found at the end of the arm reached by a specific turn (e.g., right) when leaving the start arm. The place and response versions of these tasks are based on hippocampus and striatum functions, respectively, as defined by both damage and pharmacological manipulations (e.g., Packard and White, 1991; Kesner et al., 1993; Devan and White, 1999; Zurkovsky et al., 2007). The question here was whether acetylcholine release in the hippocampus and striatum would differ by task. As shown in Figure 4A (top), acetylcholine release in the hippocampus increased and was maintained about equally during training on both tasks. Thus, acetylcholine release in the hippocampus did not differ by task, increasing to the same extent when rats were trained on the canonical task, the place maze, as on the non-canonical task, the response maze. In this set of tasks, it was the striatum that showed differential profiles of release of acetylcholine by task, with acetylcholine release in the striatum greater during response training than during place training (Fig. 4B, bottom). Across tasks—rewarded spontaneous alternation, dual-solution, and place and response mazes—it appears that the hippocampus is engaged nondifferentially by task but acetylcholine release in the striatum corresponds to the use of response vs. place strategies.
FIGURE 4.
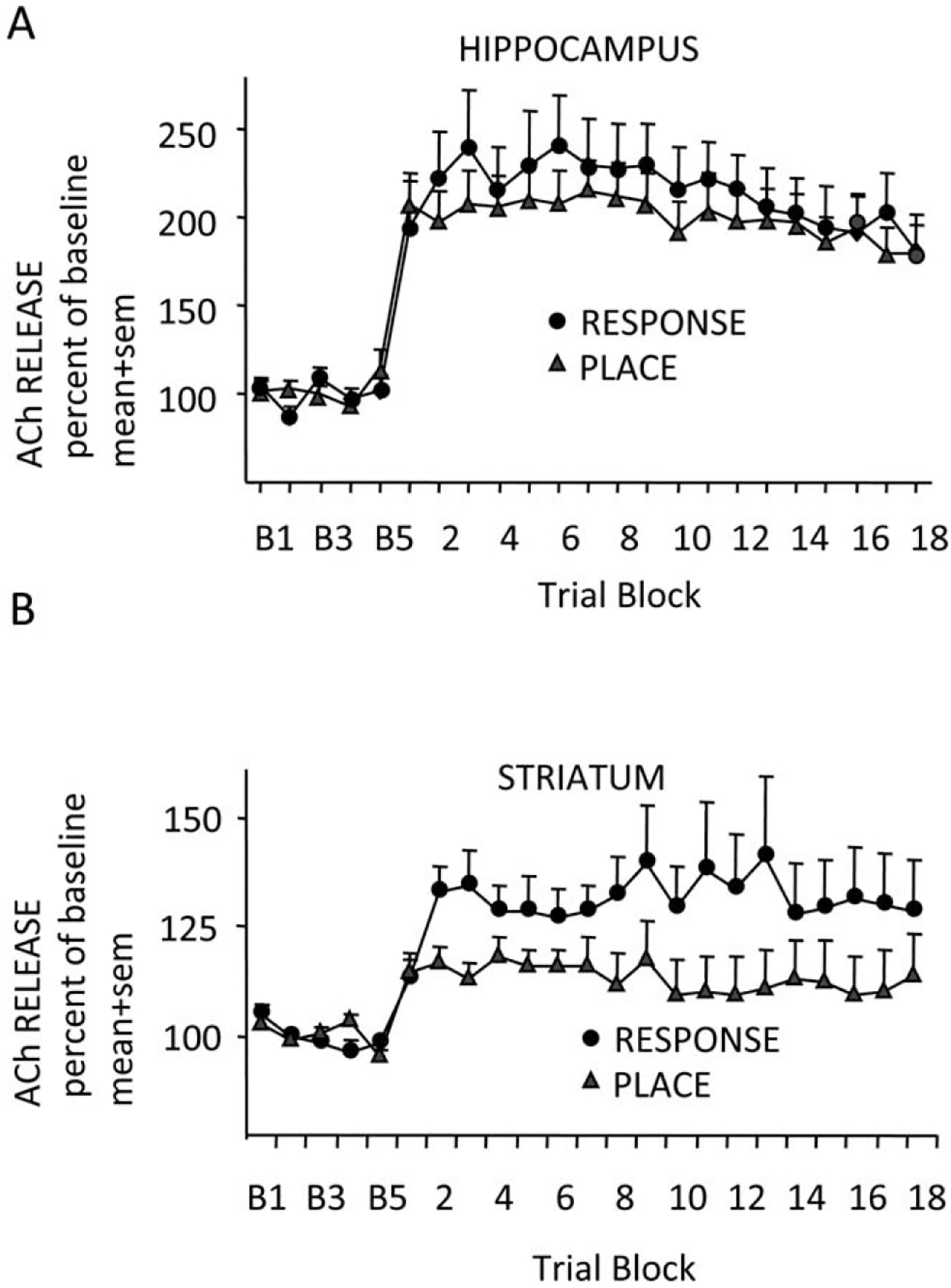
Acetylcholine (ACh) release in the hippocampus (A) and striatum (B) of rats measured simultaneously while rats were trained in either a place or response task. Response training caused significantly more striatum ACh release in the striatum was significantly higher during response training than during place training. From Pych et al., 2005b.
FROM ACETYLCHOLINE TO GLUCOSE
The studies above provide evidence that acetylcholine modulates learning and memory processing in the hippocampus and striatum and may participate in regulating the relative participation of these brain regions during learning and memory. Of related interest is the evidence linking peripheral modulators of memory, like epinephrine, to central release of acetylcholine. Epinephrine, released into blood from the adrenal medulla, is a particularly potent modulator of memory processing (Gold and van Buskirk, 1975; Gold, 1991, 2008; McGaugh and Roozendaal, 2002; Gold and Korol, 2012). Because under most conditions epinephrine does not itself enter the brain from blood in large extent (Axelrod et al., 1959), the hormone likely enhances memory through a peripheral intermediate mechanism. Considerable evidence shows that epinephrine-induced increases in blood glucose levels represent a key step between epinephrine and its effects on brain processes including learning and memory (Gold, 1991, 2008; Korol and Gold, 2007; Gold and Korol, 2012). A classic physiological action of epinephrine is to bind to adrenergic receptors on hepatocytes and then to initiate breakdown of glycogen stores (Sutherland and Rall, 1960), leading to the subsequent release of glucose into blood. With regard to effects on memory, glucose injections themselves enhance memory at doses that result in blood glucose levels comparable to those achieved with memory-enhancing doses of epinephrine. In addition, epinephrine loses its potency in enhancing memory in rats that have low liver glycogen levels and therefore little glycogen available for breakdown. These findings are seen in rats that have been food-deprived (Talley et al., 2000) and in rats that are senescent (Morris et al., 2010). Related findings come from studies showing that peripherally injected adrenergic receptor antagonists block, in parallel, the effects of epinephrine on memory and on epinephrine-induced increases in blood glucose levels (Hall and Gold, 1986; Gold et al., 1986). Under each of these conditions—food deprivation, aging, pharmacological block of liver glycogen breakdown—glucose itself retains its efficacy in enhancing memory.
Acetylcholine release during learning and memory is regulated in part by glucose availability. In an early study of this type, Ragozzino et al. (1996) found that enhancement of spontaneous alternation scores with systemic injection of glucose was accompanied by augmentation of acetylcholine release in the hippocampus. In addition, infusions of glucose directly into the hippocampus also enhanced memory and simultaneously augmented acetylcholine release in this brain area (Ragozzino et al., 1998). Glucose is also effective at enhancing memory and release of acetylcholine in aged rats as well as in young rats, about equally effective as epinephrine in young rats and somewhat better than epinephrine in aged rats (Fig. 5; Morris et al., 2010). A further link between glucose and acetylcholine actions on memory is that glucose appears to act through nicotinic α7 receptors. The findings supporting this are based on the interpretation of pharmacological results of interactions between glucose and several acetylcholine receptor antagonists. The logic is that when glucose can reverse the effects of blockade of a receptor class, that receptor is not downstream of glucose actions. Conversely, if glucose cannot reverse the effects of an acetylcholine receptor antagonist, that receptor is downstream of glucose actions and may mediate those actions. We have found that glucose administration reverses impairments of memory produced by muscarinic antagonists such as scopolamine and atropine (Stone et al., 1988, 1995). The ability of glucose to reverse the effects of these drugs suggests that glucose effects on memory are not mediated by muscarinic receptors. Also, glucose reverses impairments of memory produced by the nicotinic α4β2 receptor antagonist, dihydro-beta-erythroidinem, again suggesting that glucose effects on memory are not mediated by this receptor class. However, glucose does not reverse the impairments produced by the α7 nicotinic receptor antagonist, methyllycaconitine (Morris et al., 2012), suggesting that the α7 nicotinic receptor may be downstream of glucose actions and, when viewed together with the ability of glucose to augment acetylcholine release, suggest further that the α7 nicotinic receptor may mediate the effects of glucose on memory. A comparison of the interactions of glucose with the nicotinic receptor antagonists on spontaneous alternation scores is shown in Figure 6; similar results were obtained with post-training injections of glucose administered after inhibitory avoidance training (Morris et al., 2012).
FIGURE 5.

Acetylcholine (ACh) release in 10-min microdialysis samples collected from the hippocampus before (B 1–4) and after (P 1–6) one-trial inhibitory avoidance training in young adult and 2-yr-old rats. Systemic injections of epinephrine or glucose were administered immediately after training. Both enhance memory in young rats; glucose enhances memory in aged rats and is more effective than epinephrine in doing so in aged rats (not shown). In young rats, either epinephrine or glucose augments the duration of ACh release. In old rats, glucose augments the duration of ACh release after training and is more effective than is epinephrine. From Morris et al., 2010.
FIGURE 6.
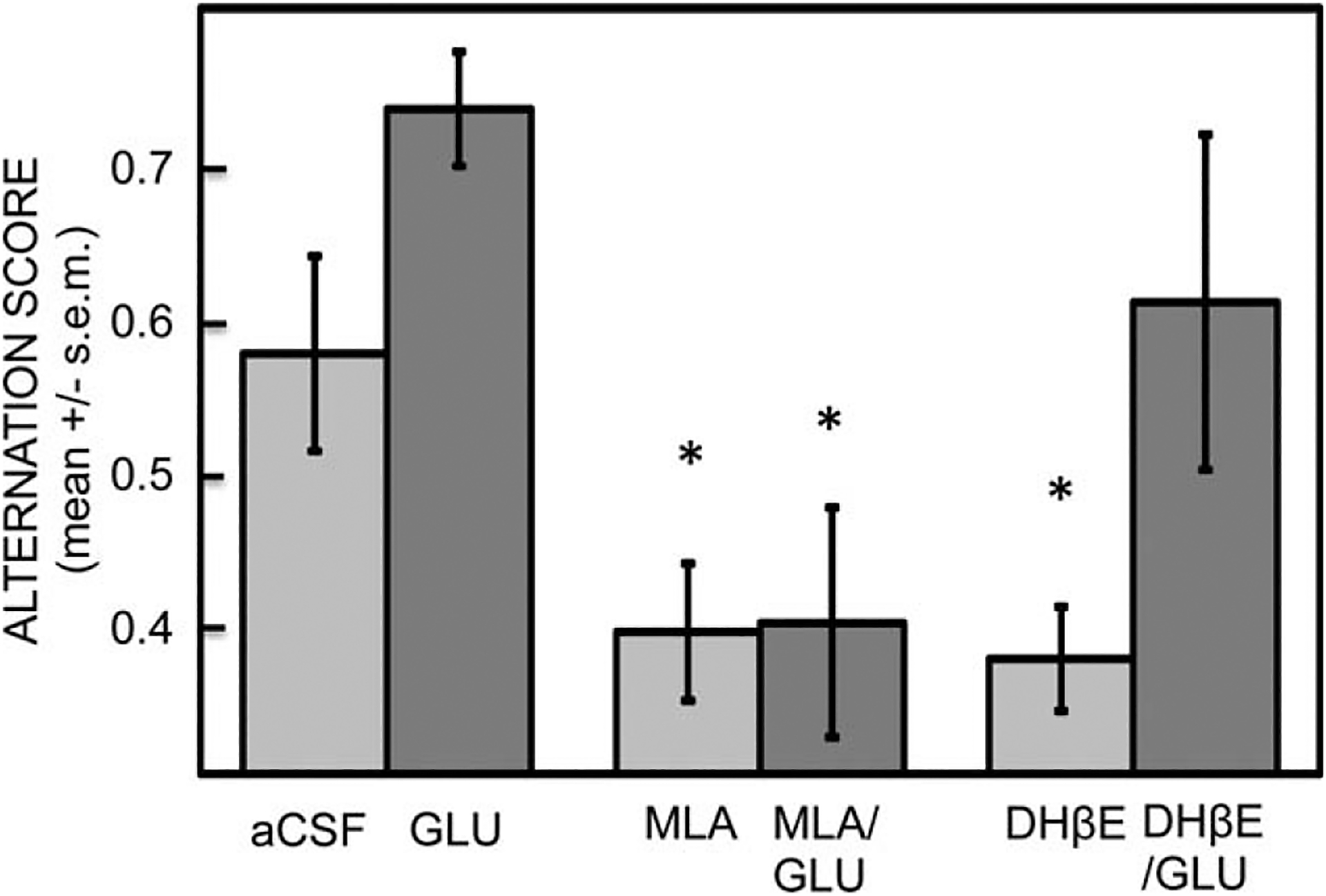
Effects of glucose and nicotinic antagonists on memory during spontaneous alternation testing. Glucose enhanced memory and significantly attenuated the memory impairment produced by dihydro-beta-erythroidinem (DHβE), an α4β2 receptor antagonist. However, glucose did not reverse the impairments produced by methyllycaconitine (MLA), an α7 nicotinic receptor antagonist. The later finding suggests that α7 nicotinic receptors may act on memory downstream from glucose effects on memory. [*P < 0.05 vs. aCSF.] From Morris et al., 2012.
The relationships between epinephrine, glucose and acetylcholine have led to our attempts to integrate the fields of modulation of memory with multiple memory systems. There are many demonstrations showing that direct injections of glucose into any of several brain areas can enhance learning and memory. These include pretesting injections of glucose into the hippocampus or medial septal region to enhance spontaneous alternation scores (Ragozzino and Gold, 1995; Parent and Gold, 1997; Parent et al., 1997; Ragozzino et al., 1995, 1998; Stefani et al., 1999; Stefani and Gold, 1998, 2001; Newman et al., 2011) and post-training injections of glucose into the hippocampus to enhance inhibitory avoidance memory assessed 7 days after training (Morris and Gold, 2013). Much more work is needed to examine effects of direct brain injections of glucose into brain areas that are associated with specific tasks, i.e., in a multiple memory system framework.
ROLE OF ASTROCYTES IN MEDIATING GLUCOSE EFFECTS ON MEMORY
One potential role for glucose in regulating learning and memory is to support the energy demands of the neurons engaged in processing the storage of new information. Importantly, extracellular glucose levels in the hippocampus are depleted when rats are tested on a four-arm spontaneous alternation maze (McNay et al., 2000, 2001; McNay and Gold, 2001; McNay and Sherwin, 2004; Newman et al., 2011, see below). While enhancing memory, glucose injections ameliorate the behavior-induced depletion in extracellular glucose levels. The findings that glucose is depleted during cognitive activity suggest that administration of glucose can supplement available energy. Glucose contributions to brain energy needs could be accomplished by two mechanisms, either by increasing glucose available for direct use by neurons or by increasing glucose available for astrocytes for conversion to lactate delivered to neurons as a energy substrate (Dringen et al., 1993; Magistretti et al., 1999; Pellerin, 2003; Ransom et al., 2003; Brown and Ransom, 2007).
Astrocytes, but not neurons, store glycogen in the brain (Maxwell and Kruger, 1965; Petersen, 1969; Pfeiffer-Guglielmi et al., 2003; Brunet et al., 2009; Newman et al., 2011). The glycogen stores can therefore be a supplement for glucose, with evidence that upon activation of a brain area the glycogen is broken down to lactate. Lactate in turn is shuttled from astrocytes to neurons where it can be used for oxidative metabolism. There is a good deal of evidence indicating that lactate may serve a function of “energy on demand” (Magistretti et al., 1999). One example of this comes from evidence obtained upon stimulation of somatosensory barrel cortex during whisker stimulation (Chuquet et al., 2101). Without stimulation, uptake of a glucose analog was about equal in both neurons and astrocytes. However, upon whisker stimulation, uptake of the glucose analog increased in astrocytes, while glucose uptake in neurons was unchanged by the stimulation. Thus, the increase in glucose utilization upon activation was largely confined to astrocytes rather than neurons.
Figure 7 illustrates a model describing cell mechanisms through which glucose can regulate neural function by acting through astrocytes. This model is based heavily on views proposed by several others (Magistretti, 2006; Brown and Ransom, 2007; Pellerin et al., 2007). Upon uptake by astrocytes, glucose can be converted directly to lactate by glycolysis or glucose can first be converted to glycogen for storage, available via glycogenolysis for conversion to lactate. Whether derived from glycolysis or glycogenolysis followed by glycolysis, lactate is then shuttled from astrocytes to neurons via monocarboxylate transporters on each cell type (Pierre and Pellerin, 2005; Bergersen, 2007).
FIGURE 7.
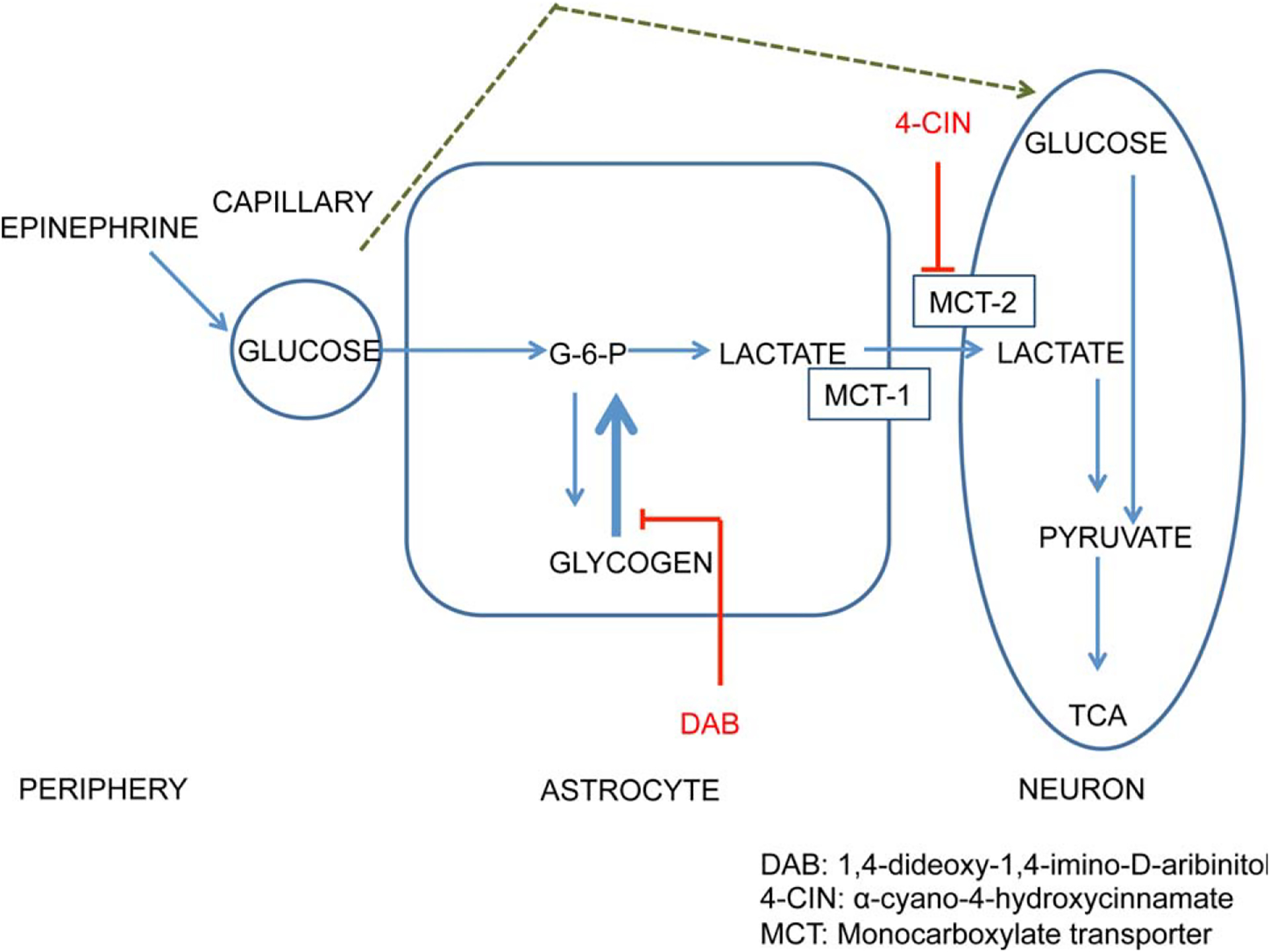
Schematic model linking epinephrine through glucose to regulation of memory via astrocyte production of lactate. In this model, glucose acts directly on neurons as an energy substrate during times of low energy demand and also is stored as glycogen in astrocytes. During times of activation by cognitive functioning, glycogen stores and newly uptaken glucose are metabolized to lactate, which is shuttled to neurons as an energy substrate. At this time, it is unclear whether to position acetylcholine and/or norepinephrine receptors in this model on astrocytes or on neurons. Based on Newman et al., 2011.
We recently began to test this model as a basis by which glucose might regulate learning and memory processing. Memory in spontaneous alternation tasks is sensitive to hippocampal lesions and drug manipulations (Johnson et al., 1977; Parent et al., 1997; Ragozzino et al., 1998; Stefani and Gold, 2001; cf. Lalonde, 2002). With prior evidence that spontaneous alternation testing of working memory drained extracellular glucose levels in the hippocampus (McNay et al., 2000, 2001; McNay and Gold, 2001; McNay and Sherwin, 2004; Newman et al., 2011), we examined the role of lactate derived from glycogen stores in astrocytes in regulating memory in this task (Newman et al., 2011). McNay and Sherwin (2004) showed that lactate levels increase and glucose levels decrease in 10-min extracellular microdialysis samples taken in the hippocampus. In our recent and current experiments, we used bioprobes with very short sampling times of 1 s; for graphical clarity, the results are displayed in Figure 8 as 10 s averages. These methods permit continual monitoring of lactate and glucose throughout the memory testing procedures via radiofrequency signals sent wirelessly to a computer receiver. Figure 8 shows the changes in lactate and glucose in the hippocampus of rats while they were engaged in the working memory test. First, note that glucose levels decreased early in testing, recovering to baseline at about 10 min after the start of alternation testing. These findings, obtained with bioprobes, replicate in principle findings obtained previously with microdialysis methods. Second, note that increases in lactate levels generally mirrored decreases in glucose levels. The increase in lactate levels is likely generated by glycogenolysis and glycolysis in astrocytes, with release of lactate into the extracellular space (Sampol et al., 2013).
FIGURE 8.
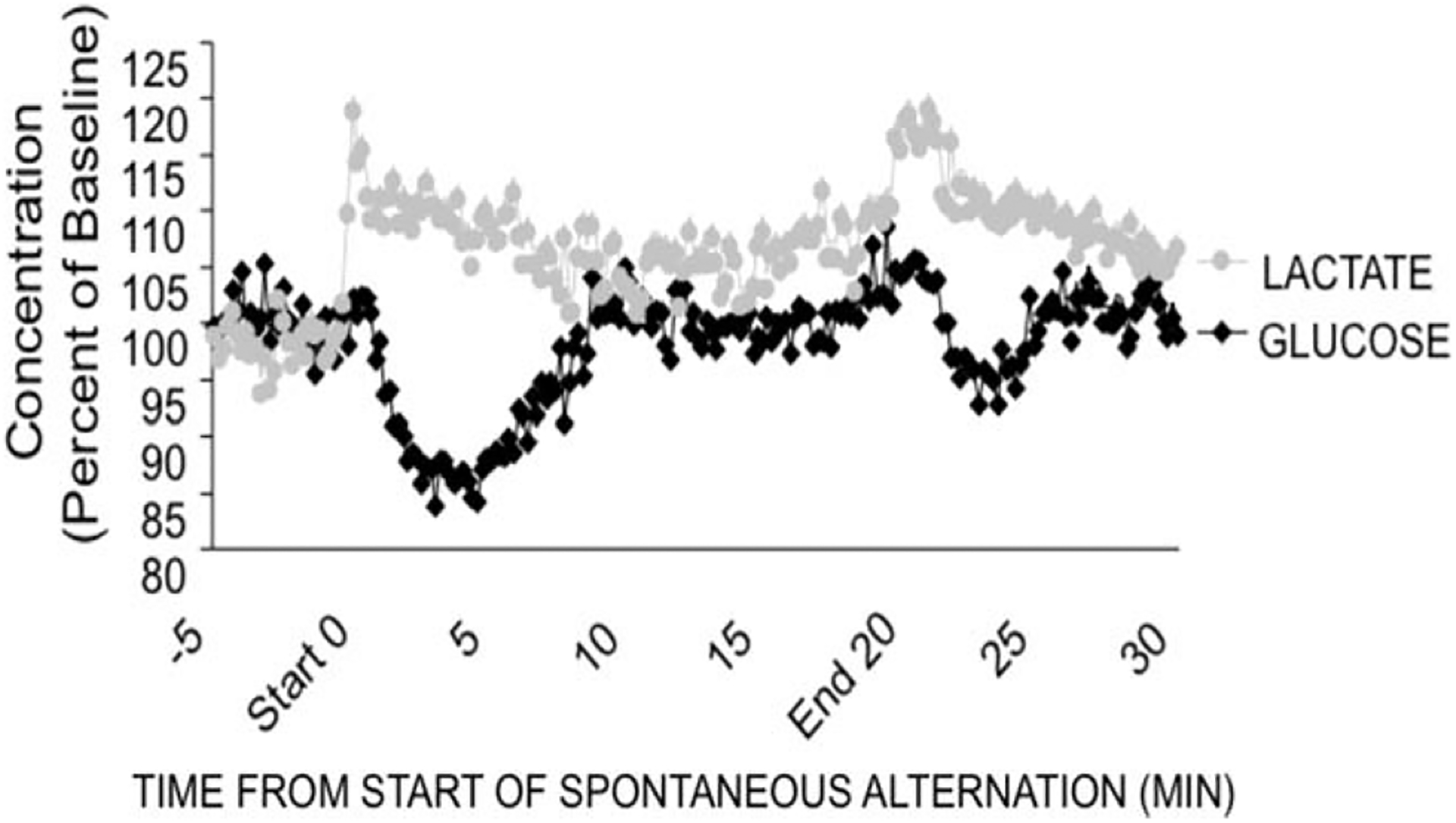
Bioprobe measures of extracellular lactate and glucose levels in the hippocampus during spontaneous alternation testing. Note that lactate increases during early testing. The lactate increase slightly precedes a decrease in extracellular glucose levels. Similar results can be seen when rats were removed from the maze, presumably reflecting arousal and new spatial surroundings. From Newman et al., 2011.
The high temporal resolution (1-s sampling) of lactate and glucose concentrations permitted examination of more precise changes in these concentrations than had been possible with microdialysis methods. Close examination of Figure 8 reveals that the increase in lactate levels precedes the decrease in glucose levels. Thus, it appears that the lactate response anticipates by ~1 min the need for energy supplementation. The rapid increase in lactate levels suggests that neuro-glial signaling initiates glycogenolysis in astrocytes. One candidate signal is norepinephrine, which is released during spontaneous alternation testing (Men et al., 1999) and which can act on β-adrenergic receptors on astrocytes with subsequent production of lactate (Gibbs, 2008; Gibbs and Summers, 2008; Hutchinson et al., 2008). The ability of adrenoreceptors to initiate glycogenolysis in astrocytes is analogous to the established mechanisms that engage glycogen breakdown in the liver (Sutherland and Rall, 1960). Of note, astrocytes have receptors for many neurotransmitters and modulators, including the nicotinic α7 receptors (Sharma and Vijayaraghavan, 2001; Teaktong et al., 2003) discussed above as a mediator of glucose effects on memory (Morris and Gold, 2012). Together with evidence that astrocytes respond functionally to acetylcholine (Perea and Araque, 2005), it is possible that acetylcholine may also influence the synthesis and release of lactate.
The increases in extracellular lactate levels during memory testing are consistent with the model in Figure 7 and led to pharmacological manipulations to test the significance of several aspects of the model as applied to spatial working memory. First, lactate infusions into the hippocampus 5 min before alternation tests enhanced memory in this task, consistent with prior evidence that intrahippocampal injections of glucose also enhance spontaneous alternation scores (Ragozzino et al., 1998; Stefani and Gold, 1998). We also examined the effects on memory of intrahippocampal injections of 1,4-dideoxy-1,4-imino-d-arabinitol (DAB), a drug that inhibits glycogen phosphorylase, thereby blocking glycogenolysis. As shown in Figure 9, inhibition of glycogen breakdown impaired alternation memory in a dose-dependent manner (black bars). This impairment was reversed by coinfusion of either lactate or glucose (gray bars). These findings point to a key role for glycogen breakdown in regulating memory processing. The reversal by lactate suggests that it is the downstream provision of this energy substrate that is important for the effects on memory. The reversal of the impairments seen after block of glycogenolysis has two interpretations, either supporting a direct action of glucose on neurons or providing additional support for a lactate requirement, produced in astrocytes by glycolysis, i.e., bypassing glycogenolysis.
FIGURE 9.

Effects of intrahippocampal infusions of DAB (1,4-dideoxy-1,4-imino-d-aribinitol) on memory in a spontaneous alternation task. DAB blocks the breakdown of glycogen in astrocytes. Consistent with the model in Figure 6, the impairment produced by inhibition of glycogenolysis can be rescued by coinfusion of glucose or lactate, which can act downstream from glycogenolysis to regulate memory processing. From Newman et al., 2011. [*P < 0.05 for comparisons indicated by horizontal lines.]
An additional experiment supports the latter possibility that glucose acts via astrocytic glycolysis to produce lactate. Rats received direct intrahippocampal infusions of a drug, a-cyano-4-hydroxycinnamate (4-CIN) that preferentially blocks the monocarboxylate 2 transporter, thereby blocking uptake of lactate into neurons. Like DAB, 4-CIN also impaired memory in a dose-dependent manner (Fig. 10, black bars). In this case, however, neither lactate nor glucose reversed the impairment. These results provide further evidence that lactate transferred from astrocytes to neurons is a key response in promoting memory processing. In addition, the findings suggest that glucose enhances memory by conversion to lactate in astrocytes.
FIGURE 10.

Effects of intrahippocampal infusions of 4-CIN (α-cyano-4-hydroxycinnamate) on memory in a spontaneous alternation task. The 4-CIN blocks the monocarboxylate transporter, MCT-2, thereby blocking lactate entry into neurons. The 4-CIN impaired memory. In contrast to the results in Figure 7, the impairment of memory produced by 4-CIN was not rescued by coinfusion of either glucose or lactate. The failure of lactate to reverse the impairment suggests entry of lactate into neurons is important for regulating memory processing. Glucose entry into neurons is not blocked by t4-CIN. The failure of glucose to reverse the impairment suggests that it may work through astrocytic glycolysis to lactate to enhance memory. [*P < 0.05 for comparisons indicated by horizontal lines.] From Newman et al., 2011.
The high temporal resolution of measuring lactate concentrations has also permitted evaluation of momentary responses during specific components of behavior. For this microanalysis, videotaped performance was overlaid with the lactate levels as the rat performed a spontaneous alternation task. In the four-arm alternation maze, a rat has three main choices when entering the center. The rat can return to the previous arm (A-D-A), or to an arm three-back (A-D-B-A) or complete an alternation (A-D-B-C). Figure 11 shows the change in extracellular lactate levels measured just after each arm choice based on the rat’s recent behavior. The findings indicate that lactate levels significantly increased in the hippocampus as the rat chose an arm that completed an alternation, defined as completing a set of entries into four different arms. While it is difficult to interpret the meaning of a “correct” alternation in this task, the results open the possibility of identifying chemical fluxes related to energy metabolism while rats are learning in appetitively motivated mazes in which variables like correct/incorrect, anticipation of reward/no-reward or consumption of reward, can be assessed. In this way, the methods open a new window for examining metabolic changes associated with high-order cognitive constructs like decision-making and expectations, in addition to memory processing.
FIGURE 11.
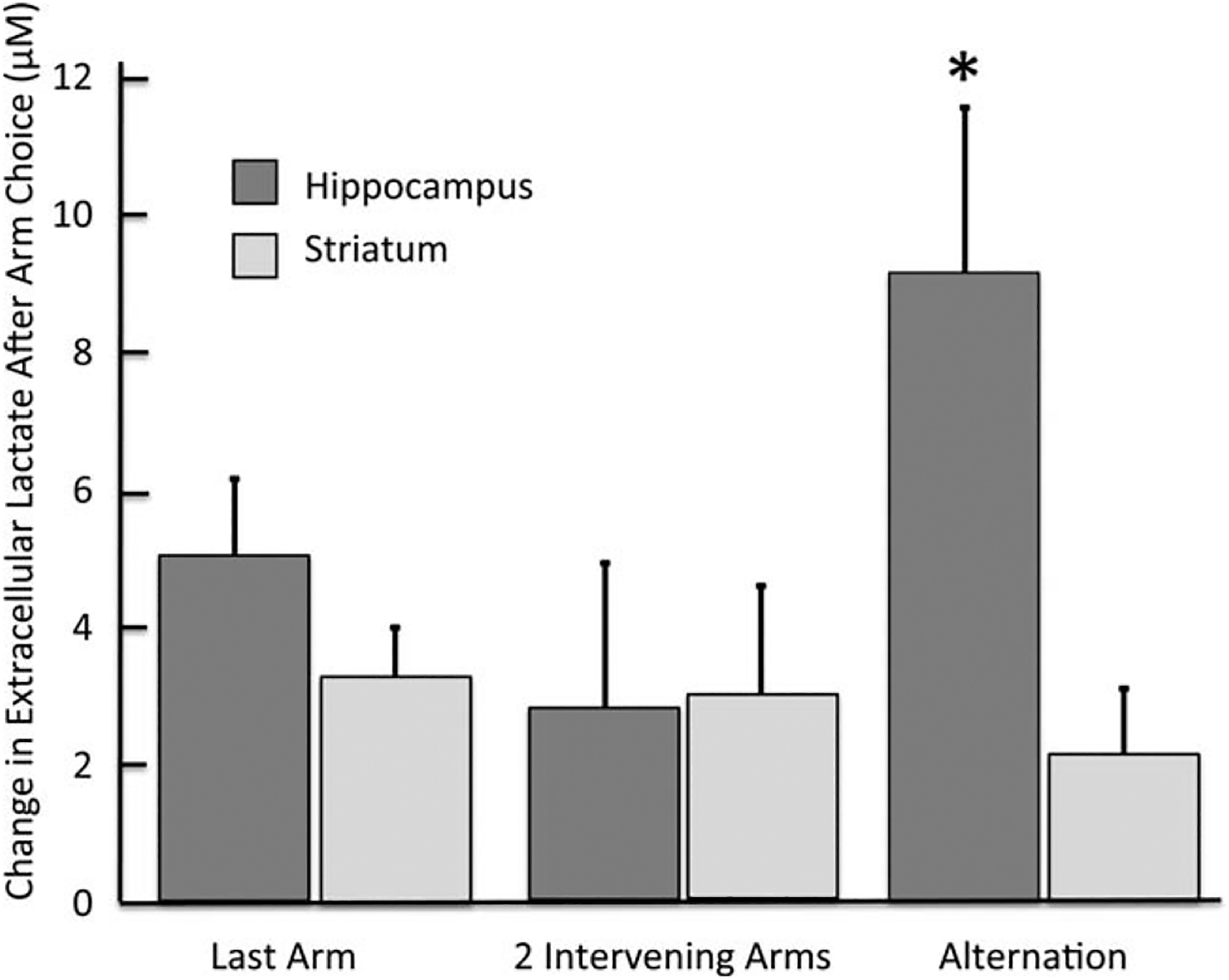
Lactate changes measured in 1-s samples after each arm choice during alternation testing. Lactate levels increased upon completion of an alternation pattern but not after nonalter-nation arm selection. These findings point to the future utility of bioprobes in assessing dynamic changes in the contributions of multiple memory systems during learning. *Ps < 0.05 between hippocampus and striatum after completion of an alternation and between lactate measures in hippocampus after completion of an alternation vs. after two intervening arm choices.
The neurochemical and pharmacological findings in Newman et al. (2011) are generally consistent with those reported by Suzuki et al. (2011) from an experiment that tested memory for inhibitory avoidance training and found that lactate levels increased by 70–90% after training. The methods were microdialysis methods with 10-min sampling; no information is provided about the duration of the increases. Additionally, these authors found that disrupting the expression of MCT2 impaired memory for inhibitory avoidance training, an impairment that was not reversed by either lactate or glucose. These findings are like those of Newman et al. (2011), who found that pharmacological block of the MCT2 transporter impaired memory and that the memory loss was not reversed by lactate or glucose. Suzuki et al. (2011) also found that disruption of the expression of MCT1 impaired memory, but in this case the impairment could be reversed by lactate but not by glucose. These findings are also conceptually similar to those found with in Newman et al. (2011). One difference across the studies needs additional attention. In the Suzuki et al. (2011) paper, inhibitory avoidance memory was impaired at 24 h or more after training but not 1 h after training, interpreted as a sparing of short- but not long-term memory and therefore an effect on memory consolidation. To the extent that working memory in a spontaneous alternation task is based on short-term processing, it seems likely that the provision of lactate by astrocytes is important beyond memory consolidation and will prove to be a more generally important element of learning and memory processing.
As described above, we have found that differential release of acetylcholine in hippocampus and striatum reflected different strategies used to solve food-motivated mazes using place or response bases (Chang and Gold, 2003; Pych et al., 2005a,b), with differences in release in the hippocampus vs. striatum at baseline and during training predicting individual differences in the learning strategy expressed on probe trials (McIntyre et al., 2003). These relationships between glucose, acetylcholine and multiple memory systems led us to begin to examine the role of astrocytes and lactate while rats learned tasks applicable to a multiple memory systems framework. Our initial experiment examined lactate and glucose levels in the hippocampus while rats were trained on a place or response task (Gold et al., 2011). Lactate levels increased substantially in the hippocampus while rats were learning to find food in a place version of the plus-maze, rising well above the response to Cheerio® pieces given to untrained rats that received the food reward on approximately the same schedule (1/4 Cheerio® every 1.5 min) as that of the trained rats (Fig. 12). In contrast, when rats were trained on the response version of the maze, the increase in extracellular lactate in the hippocampus was not very different from that seen in the fed-only, no training condition.
FIGURE 12.
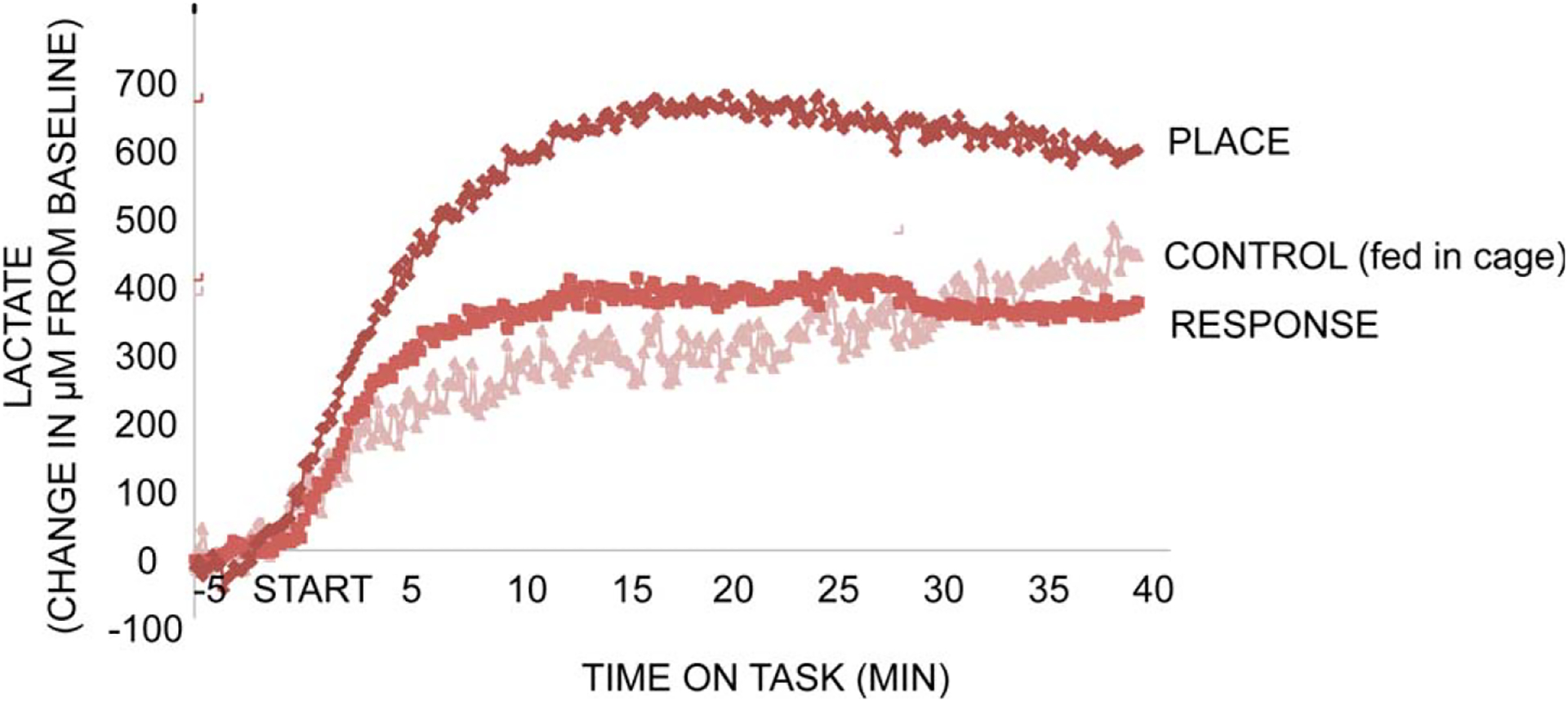
Changes in hippocampal extracellular lactate levels during place and response training for food reward in a four-arm plus-shaped maze. Control rats were food-deprived and received food on a schedule comparable to that of rats during training. First, note that lactate levels increased in all groups, including controls, during feeding. The increase in the hippocampus during response training was not significantly greater than that of controls. However, the increase in lactate levels during place training was significantly greater than that seen in either of the other conditions. Note the rapid—within seconds—rise in lactate levels, particularly during place training.
To evaluate the potential confound of feeding per se vs. training, current experiments are assessing the responses of brain glucose and lactate levels in rats trained on the same mazes but for water reward. Provision of water to control rats does not appear to increase brain glucose or lactate levels, but training on water-motivated versions of place and response tasks still results in increases in these measures (data not shown).
We have also measured changes in extracellular glucose levels while rats were trained on the food-motivated tasks (Fig. 13). Glucose levels in the hippocampus increased above the levels of untrained fed controls beginning about 10 min into training on either task (Fig. 13A, left panel). The increase was somewhat higher in those rats trained on the place maze than in those trained on the response maze, suggesting a directed influx of glucose into the hippocampus to support the increase in lactate utilization. The early responses of glucose to training may be of particular importance. As we had seen during spontaneous alternation testing (Fig. 8), glucose levels in the hippocampus dropped during the first minutes of spontaneous alternation testing. A similar decrease in glucose levels was also evident in the hippocampus of rats trained on the place version of the food-motivated maze but, interestingly, not in rats trained on the response version of the maze. To illustrate these differences, Figure 13B (right panel) shows an expanded view of the hippocampal glucose levels during the first minutes during training. In this graph, it is clear that glucose depletion early in training is evident in the hippocampus when rats are trained on the place version of the maze, for which hippocampal processing is a principal contributor of learning, but not on the response version of the maze, which would be based on striatal functions. Thus, both the increase in lactate levels and the initial decrease in glucose levels in the hippocampus discriminate between the place and response versions of the plus-maze.
FIGURE 13.
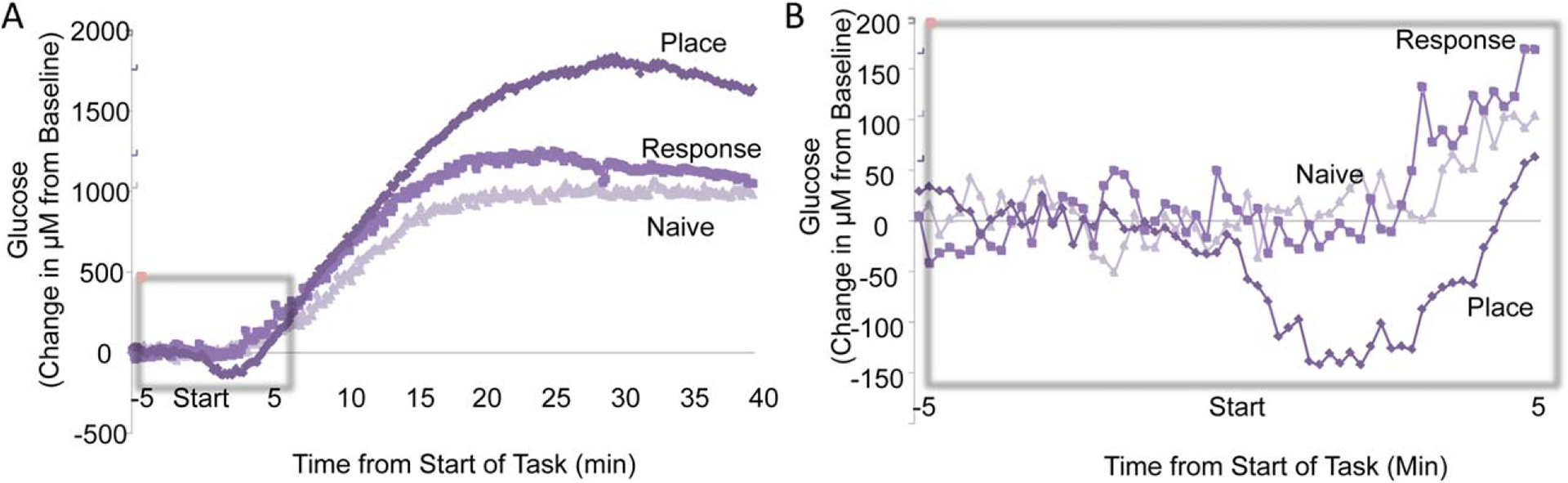
Changes in hippocampal extracellular glucose levels during place and response training for food reward. A. Changes throughout place and response training. Glucose levels increased after about 5 min into training in all groups, likely responding to rises in blood glucose levels after feeding. At about 15 min into training, glucose levels began to differ based on condition, with those rats engaged in learning the place version of the maze attaining significantly higher levels than those of response-trained or control rats. B. Expanded view of glucose changes during the first minutes of training. Note that glucose levels decreased significantly in the hippocampus in those rats trained on the place task but not in those rats trained on the response task or in controls. These findings suggest that hippocampal processing was engaged by place training in a manner that depleted available extracellular glucose, similar to the results obtained previously during spontaneous alternation testing (compare Fig. 8). In addition, note that the decrease in glucose levels was slightly delayed here relative to the increase in lactate shown in Figure 12. Thus, it appears that the lactate rise anticipates the decrease in glucose, possibly by responses to activation by neuroglia transmitters of receptors on astrocytes.
An additional point in these data corresponds to results seen before in the spontaneous alternation study: Increases in lactate levels precede the decline in glucose levels. These findings again suggest that neuromodulator activation of glycogenolysis may anticipate the demand for an increase in energy provision.
CONCLUSIONS
The research described here provides evidence for neurochemical mechanisms that control the balance between multiple learning and memory systems. These mechanisms are subject to pharmacological control, as evidenced by the effects of direct brain injections of glucose into specific brain regions; similar results are also seen with neurotransmitter receptor agonists and antagonists. For acetylcholine, it appears that both hippocampal and striatal tasks result in increases in release of the neurotransmitter, but that the patterns of release change as rats progress through different stages of training reflecting changes in the use of different cognitive strategies and attributes during training.
This review also raises a potentially important role for astrocytes in balancing the relative contributions of different brain areas activated during experiences to be learned and remembered. The release of lactate from astrocytes to neurons appears to be relevant to information processing in the hippocampus; whether similar mechanisms pertain to processing in other brain regions remains to be seen. Importantly, the results obtained in several experiments suggest that glucose, which is a potent enhancer of learning and memory in many tasks, may itself not function directly as an energy substrate via neuronal uptake but may act through utilization of glucose by astrocytes for conversion to lactate. First, results by Chuquet et al. (2010) show selective activation-induced uptake of a glucose analog into astrocytes and not into neurons. Second, Sampol et al. (2013) show similar activation-induced increases in lactate newly synthesized from glucose, likely occurring in astrocytes. Third, data from Newman et al. (2011) show that glucose cannot reverse memory impairments after pharmacologically blocked monocarboxylate transporters (MCT-2) that carry lactate into neurons. Fourth, related findings reported by Suzuki et al. (2011) indicate that disruption of the expression of MCT-2 in mice impairs memory, and that impairment is not reversed by either glucose or lactate.
It is very clear that the understanding of the role of astrocytes and lactate in regulation of memory processing is at a very early stage, one where there are far more questions than answers. However, early evidence indicates that the astrocytes respond to training in a brain area × task manner that is consistent with a multiple memory system view of how brains handle learning and memory processing. The early evidence also indicates that the contribution of astrocytes to the functioning of neural systems is broader than one specifically directed at the formation of long-term memories, but also includes working memory and acquisition phases of learning assessed in different tasks. Even the designation of a role for astrocytes specifically in learning and memory processing is likely to be too constrained and may better be considered within a framework of support of regional brain processing of multiple cognitive and other brain functions. The recent findings showing the importance of astrocytes for normal cognitive functions suggests that dysfunctions of astrocytic activities, particularly those involving energy regulation, may also play a significant role in a wide array of cognitive disorders.
REFERENCES
- Axelrod J, Weil-Malherbe H, Tomchick R. 1959. The physiological disposition of H3-epinephrine and its metabolite metanephrine. J Pharmacol Exp Ther 127:251–256. [PubMed] [Google Scholar]
- Barnes CA, Nadel L, Honig WK. 1980. Spatial memory deficit in senescent rats. Can J Psychol 34:29–39. [DOI] [PubMed] [Google Scholar]
- Bergersen LH. 2007. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience 145:11–19. [DOI] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. 2007. Astrocyte glycogen and brain energy metabolism. Glia 55:1263–1271. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Allaman I, Magistretti PJ, Pellerin L. 2009. Glycogen metabolism as a marker of astrocyte differentiation. J Cereb Blood Flow Metab 30:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Gold PE. 2003. Switching memory systems during learning: Changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci 23:3001–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuquet J, Quilichini P, Nimchinsky EA, Buzs aki G. 2010. Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J Neurosci 30: 15298–15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devan BD, White NM. 1999. Parallel information processing in the dorsal striatum: Relation to hippocampal function. J Neurosci 19: 2789–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. 1993. Glycogen in astrocytes: Possible function as lactate supply for neighboring cells. Brain Res 623:208–214. [DOI] [PubMed] [Google Scholar]
- Gibbs ME. 2008. Memory systems in the chick: Regional and temporal control by noradrenaline. Brain Res Bull 76:170–182. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Summers RJ. 2005. Contrasting roles for β1, β2 and β3-adrenoceptors in memory formation in the chick. Neuroscience 131:31–42. [DOI] [PubMed] [Google Scholar]
- Gold PE. 1991. An integrated memory regulation system: From blood to brain. In: Frederickson RCA, McGaugh JL, Felten DL, editors. Peripheral Signaling of the Brain: Role in Neural-Immune Interactions, Learning and Memory. Toronto: Hogrefe & Huber Publishers. pp 391–419. [Google Scholar]
- Gold PE. 2004. Coordination of multiple memory systems. Neurobiol Learn Mem 82:230–242. [DOI] [PubMed] [Google Scholar]
- Gold PE. 2008. Memory enhancing drugs. In: Eichenbaum H, editor. In: Byrne J, editor. Memory Systems, Vol. 3 of Learning and Memory: A Comprehensive Reference. Oxford: Elsevier Science. pp 555–576. [Google Scholar]
- Gold PE, Korol DL. 2012. Making memories matter. Special issue: The impact of emotion on cognition—Dissociating between enhancing and impairing effects. F. Dolcos, L. Wang, and M. Mather, hosts. Front Int Neurosci 6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, Van Buskirk RB. 1975. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol 13:145–153. [DOI] [PubMed] [Google Scholar]
- Gold PE, Vogt J, Hall JL. 1986. Glucose effects on memory: Behavioral and pharmacological characteristics. Behav Neur Biol 46:145–155. [DOI] [PubMed] [Google Scholar]
- Gold PE, Scavuzzo CJ, Korol DL, Newman LA. 2011. Hippocampal extracellular lactate increases during learning: A role for astrocytes in learning and memory. Presentation abstract 823.14. Society for Neuroscience, 40th Annual Meeting, Washington DC. [Google Scholar]
- Hall JL, Gold PE. 1986. The effects of training, epinephrine, and glucose injections on plasma glucose levels in rats. Behav Neurol Biol 46:156–176. [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Summers RJ, Gibbs ME. 2008. Energy metabolism and memory processing: Role of glucose transport and glycogen in responses to adrenoceptor activation in the chicken. Brain Res Bull 76:224–234. [DOI] [PubMed] [Google Scholar]
- Johnson CT, Olton DS, Gage FH III, Jenko PG. 1977. Damage to hippocampus and hippocampal connections: Effects on DRL and spontaneous alternation. J Comp Physiol Psychol 91:508–522. [DOI] [PubMed] [Google Scholar]
- Kesner RP. 2009. Tapestry of memory. Behav Neurosci 123:1–13. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Bolland BL, Dakis M. 1993. Memory for spatial locations, motor responses, and objects: Triple dissociation among the hippocampus, caudate nucleus, and extrastriate visual cortex. Exp Brain Res 93:462–470. [DOI] [PubMed] [Google Scholar]
- Korol DL. 2004. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem 82:309–323. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE. 2007. Modulation of learning and memory by adrenal and ovarian hormones. In: Kesner RP, Martinez JL, editors. Neurobiology of Learning and Memory. NY: Elsevier Science. p 243–268. [Google Scholar]
- Korol DL, Kolo LL. 2002. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci 116: 411–420. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. 2004. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav 45:330–338. [DOI] [PubMed] [Google Scholar]
- Lalonde R 2002. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26:91–104. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. 2006. Neuron–glia metabolic coupling and plasticity. J Exp Biol 209:2304–2311. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. 1999. Energy on demand. Science 283:496–497. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Ilgen M, White AM, Best PJ. 1999. Acute ethanol administration impairs spatial performance while facilitating non-spatial performance in rats. Neurobiol Learn Mem 72:169–179. [DOI] [PubMed] [Google Scholar]
- Maxwell DS, Kruger L. 1965. The fine structure of astrocytes in the cerebral cortex and their response to focal injury produced by heavy ionizing particles. J Cell Biol 25:141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, White NM. 1993. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav Neurosci 107:3–22. [DOI] [PubMed] [Google Scholar]
- McElroy MW, Korol DL. 2005. Intrahippocampal muscimol shifts learning strategy in gonadally intact young adult female rats. Learn Mem 12:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. 2002. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 12: 205–210. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, Gold PE. 2003. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol Learn Mem 79:177–183. [DOI] [PubMed] [Google Scholar]
- McNay EC, Gold PE. 2001. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. J Gerontol Ser A Biol Sci Med Sci 56:B66–B71. [DOI] [PubMed] [Google Scholar]
- McNay EC, Sherwin RS. 2004. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes 53:418–425. [DOI] [PubMed] [Google Scholar]
- McNay EC, Fries TM, Gold PE. 2000. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci USA 97:2881–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, McCarty RC, Gold PE. 2001. Fluctuations in brain glucose concentration during behavioral testing: Dissociations between brain areas and between brain and blood. Neurobiol Learn Mem 75:325–337. [DOI] [PubMed] [Google Scholar]
- McNay EC, Canal CE, Sherwin RS, Gold PE. 2006. Modulation of memory with septal injections of morphine and glucose: Effects on extracellular glucose levels in the hippocampus. Physiol Behav 87: 298–303. [DOI] [PubMed] [Google Scholar]
- Men D, McCarty R, Gold PE. 1999. Enhanced release of norepinephrine in rat hippocampus during spontaneous alternation tests. Neurobiol Learn Mem 71:289–300. [DOI] [PubMed] [Google Scholar]
- Morris KA, Gold PE. 2013. Epinephrine and glucose modulate training-related CREB phosphorylation in old rats: Relationships to age-related memory impairments. Exp Gerontol 48:115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KA, Chang Q, Mohler EG, Gold PE. 2010. Age-related memory impairments due to reduced blood glucose responses to epinephrine. Neurobiol Aging 31:2136–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KA, Li S, Bui DD, Gold PE. 2012. Glucose attenuates impairments in memory and CREB activation produced by an a4b2 but not an α7 nicotinic receptor antagonist. Neuropharmacology 67: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE. 2011. Lactate produced by glycogenolysis in astrocytes regulates memory. PLoS One 6:e28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG. 2009. Anxiety, cognition, and habit: A multiple memory systems perspective. Brain Res 1293:121–128. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L. 2001. Affective modulation of multiple memory systems. Curr Opin Neurobiol 11:752–756. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. 1996. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem 65:65–72. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. 1991. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav Neurosci 105:295–306. [DOI] [PubMed] [Google Scholar]
- Parent MB, Gold PE. 1997. Intra-septal infusions of glucose potentiate inhibitory avoidance deficits when co-infused with the GABA agonist muscimol. Brain Res 745:317–320. [DOI] [PubMed] [Google Scholar]
- Parent MB, Laurey PT, Wilkniss S, Gold PE. 1997. Intraseptal infusions of muscimol impair spontaneous alternation performance: Infusions of glucose into the hippocampus, but not the medial septum, reverse the deficit. Neurobiol Learn Mem 68:75–85. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. 2007. Activity-dependent regulation of energy metabolism by astrocytes: An update. Glia 55:1251–1262. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. 2005. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci 25:2192–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KU. 1969. Zur Feinstruktur der Neurogliazellen in der Klein-hirnrinde von Säugetieren. Cell Tissue Res 100:616–633. [PubMed] [Google Scholar]
- Pfeiffer-Guglielmi B, Fleckenstein B, Jung G, Hamprecht B. 2003. Immunocytochemical localization of glycogen phosphorylase isozymes in rat nervous tissues by using isozyme-specific antibodies. J Neurochem 85:73–81. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. 2005. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J Neurochem 94:1–14. [DOI] [PubMed] [Google Scholar]
- Pych JC, Chang Q, Colon-Rivera C, Gold PE. 2005a. Acetylcholine release in hippocampus and striatum during testing on a rewarded spontaneous alternation task. Neurobiol Learn Mem 84:93–101. [DOI] [PubMed] [Google Scholar]
- Pych JC, Chang Q, Colon-Rivera C, Haag R, Gold PE. 2005b. Acetylcholine release in the hippocampus and striatum during place and response training. Learn Mem 12:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Gold PE. 1995. Glucose injections into the medial septum reverse the effects of intraseptal morphine infusions on hippocampal acetylcholine output and memory. Neuroscience 68:981–988. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Hellems K, Lennartz RC, Gold PE. 1995. Pyruvate infusions into the septal area attenuate spontaneous alternation impairments induced by intraseptal morphine injections. Behav Neurosci 109:1074–1080. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Unick KE, Gold PE. 1996. Hippocampal acetylcholine release during memory testing in rats: Augmentation by glucose. Proc Natl Acad Sci USA 93:4693–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. 1998. Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J Neurosci 18:1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom B, Behar T, Nedergaard M. 2003. New roles for astrocytes (stars at last). Trends Neurosci 26:520–522. [DOI] [PubMed] [Google Scholar]
- Restle F 1957. Discrimination of cues in mazes: A resolution of the place vs. response controversy. Psychol Rev 64:217–228. [DOI] [PubMed] [Google Scholar]
- Sadowski RN, Jackson GR, Wieczorek L, Gold PE. 2009. Effects of stress, corticosterone, and epinephrine administration on learning in place and response tasks. Behav Brain Res 205:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampol D, Ostrofet E, Jobin M-L, Raffard G, Sanchez S, Bouchaud V, Franconi J-M, Bonvento G, Bouzier-Sore A-|K. 2013. Glucose and lactate metabolism in the awake and stimulated rat: A 13c-nmr study. Front Neuroenerget 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. 2001. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci USA 98:4148–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Gold PE. 1998. Intra-septal injections of glucose and glibenclamide attenuate galanin-induced spontaneous alternation performance deficits in the rat. Brain Res 813:50–56. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Gold PE. 2001. Intrahippocampal infusions of K-ATP channel modulators influence spontaneous alternation performance: Relationships to acetylcholine release in the hippocampus. J Neurosci 21:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Nicholson GM, Gold PE. 1999. ATP-sensitive potassium channel blockade enhances spontaneous alternation performance in the rat: A potential mechanism for glucose-mediated memory enhancement. Neuroscience 93:557–563. [DOI] [PubMed] [Google Scholar]
- Stone WS, Croul CE, Gold PE. 1988. Attenuation of scopolamine-induced amnesia in mice. Psychopharmacology 96:417–420. [DOI] [PubMed] [Google Scholar]
- Stone WS, Rudd RJ, Gold PE. 1995. Glucose attenuation of atropine-induced deficits in paradoxical sleep and memory. Brain Res 694:133–138. [DOI] [PubMed] [Google Scholar]
- Sutherland EW, Rall TW. 1960. The relation of adenosine-3′, 5′-phosphate and phosphorylase to the actions of catecholamines and other hormones. Pharmacol Rev 12:265–299. [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. 2011. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144:810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley CE, Kahn S, Alexander LJ, Gold PE. 2000. Epinephrine fails to enhance performance of food-deprived rats on a delayed spontaneous alternation task. Neurobiol Learn Mem 73:79–86. [DOI] [PubMed] [Google Scholar]
- Teaktong T, Graham A, Perry R, Jaros E, Johnson M, Hall R, Perry E. 2003. Alzheimer’s disease is associated with a selective increase in α7 nicotinic acetylcholine receptor immunoreactivity in astrocytes. Glia 41:207–211. [DOI] [PubMed] [Google Scholar]
- Tolman EC, Ritchie BF, Kalish D. 1946. Studies in spatial learning II. Place versus response learning. J Exp Psychol 36:221–229. [DOI] [PubMed] [Google Scholar]
- White NM. 2008. Multiple memory systems in the brain: Cooperation and competition. In: Eichenbaum HB, editor, Memory Systems, Vol 3 of Byrne J, editor, Learning and Memory: A Comprehensive Reference. Oxford: Elsevier. pp 9–46. [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. 2007. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience 144:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


