Abstract
Acute Kidney Injury (AKI) is the most common cause of organ dysfunction in critically ill adults and prior studies have shown AKI is associated with a significant increase of the mortality risk. Early prediction of the mortality risk for AKI patients can help clinical decision makers better understand the patient condition in time and take appropriate actions. However, AKI is a heterogeneous disease and its cause is complex, which makes such predictions a challenging task. In this paper, we investigate machine learning models for predicting the mortality risk of AKI patients who are stratified according to their AKI stages. With this setup we demonstrate the stratified mortality prediction performance of patients with AKI is better than the results obtained on the mixed population.
Keywords: Acute Kidney Injury, Critical Care, Forecasting
Introduction
Acute Kidney Injury (AKI) is defined as a sudden (within hours) decrease in nephritic function, including both renal structural damage and functional disorder [1]. It has been shown AKI is the most common cause of organ dysfunction in the critical care setting, and AKI is associated with a significant increase on the morbidity and mortality risk [2]. Early prediction of the mortality risk for AKI patients can help clinical decision makers better understand patient conditions in time and take appropriate actions.
There has been prior research on building mortality risk prediction models for AKI patients. For example, Luo et al. [3] created a scoring model based on multivariate logistic regression to predict the 90-day mortality risk of AKI patients. Skarupskienė et al. [4] adopted logistic regression to predict the mortality risk of AKI patients requiring renal replacement therapy after cardiac surgery. Demirjian et al. [5] used logistic regression to predict the 60-day mortality risk of the AKI patients who are enrolled in the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network study. Ohnuma et al. [6] compared the ability of various mortality prediction models in a retrospective, multi-centric cohort of 343 Japanese patients with AKI requiring continuous renal replacement therapy in 14 ICUs captured between January and December 2010.
All of these existing research tried to build a unique predictor for the entire AKI patient cohort. However, it has been demonstrated AKI is a heterogeneous disease with various kinds of causes and clinical manifestations [7]. In this case, it would be challenging to build a single model that is capable of predicting the mortality risk over the entire AKI patient population.
With the above considerations and previous studies [8,9], we propose to perform a stratified prediction in this paper. Specifically, we will first stratify the AKI patients according to their disease stage (I, II, and III). Then, a mortality risk prediction model will be built for each patient strata. In addition to logistic regression, we will also test the performance of other machine learning models including random forest and gradient boosting tree. The Medical Information Mart for Intensive Care III (MIMIC-III) database [10] is used for empirical evaluations. Our results demonstrate the performance of stratified mortality prediction considering different AKI stages is higher than the mortality prediction on the entire mixed population. Additionally, the experiment results show different features play different roles for mortality prediction in patients with different AKI stages.
Methods
Data source
The Medical Information Mart for Intensive Care III (MIMIC-III) database is employed to extract patient data [10], which includes 58,976 admissions of patients. This database has comprehensive information (e.g., patient demographics and vital signs) regarding ICU admissions, which is open-source and freely accessible. The MIMIC-III dataset is passive and de-identified [11], which is in compliance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule and does not make a significant impact on patient privacy.
AKI stages
The criteria of AKI stages has experienced some development progress based on the studies of researchers and clinicians. In particular, there are four AKI criteria: Risk-Injury-Failure-Loss-End (RIFLE) criteria [12], pediatric RIFLE (pRIFLE) criteria [13], Acute Kidney Injury Network (AKIN) criteria [14], and Kidney Disease: Improving Global Outcomes (KDIGO) criteria [15]. KDIGO criteria unified previous criteria in 2012, which improves the sensitivity of AKI diagnostic criteria and has been widely used by researchers and physicians [1]. In this study, we employed the KDIGO criteria to stratify patients into different AKI stages:
Stage 1:
1.5–1.9 times baseline, which is known or presumed to have occurred within the prior 7 days; or not less than 0.3 mg/dL (not less than 26.5 mol/L) absolute increase in serum creatinine (SCr); or urine volume less than 0.5 mL/kg/h for 6–12 hours.
Stage 2:
SCr not less than 2.0–2.9 times baseline; or urine volume less than 0.5 mL/kg/h for not less than 12 hours.
Stage 3:
SCr not less than 3.0 times from baseline; or increase in SCr not less than 4.0 mg/dL (not less than 353.6 mol/L); or initiation of renal replacement therapy; or urine volume less than 0.3 mL/kg/h for not less than 24 hours; or anuria for not less than 12 hours; or renal replacement therapy required.
Patient features
A large number of features from MIMIC-III are extracted, which is shown as follows. Additional details about features can be found at: https://github.com/xuzhenxing2018/amia/blob/master/features_name.xlsx.
Demographics: Gender, age, and ethnicity.
Medications: Medications the patient took from the patients’ ICU admission until prediction time. We focused on the following categories: diuretics, non-steroidal anti-inflammatory drugs (NSAID), radiocontrast agents, and angiotensin.
Comorbidities: We considered all comorbidities (e.g., congestive heart failure, peripheral vascular, hypertension, diabetes, liver disease, myocardial infarction (MI), coronary artery disease (CAD), cirrhosis, and jaundice) that patients already had. There were a total of 31 comorbidities in our dataset.
Chart-events: Vital signs measured at the bedside. We mainly focused on diastolic blood pressure (DiasBP), glucose, heart rate, mean arterial blood pressure (MeanBP), respiration rate, blood oxygen saturation level (SpO2), systolic blood pressure (SysBP), and temperature.
Lab-events: Laboratory test results. We considered bicarbonate, blood urea nitrogen (BUN), calcium, chloride, creatinine, hemoglobin, international normalized ratio (INR), platelet, potassium, prothrombin time (PT), partial thromboplastin time (PTT), and white blood count (WBC).
Data pre-processing
We mainly pre-processed two types of features: time-dependent continuous features and discrete features. For continuous features (e.g., lab and chart-events), we computed statistics including the first, last, average, minimum, maximum, slope, and the count based on observations during the observation window. The final encoding of the continuous features was represented by a vector representation with real numbers. The discrete features (e.g., medication and comorbidities) were encoded as zero-one multi-hot vectors. If there were missing demographics and discrete features for an ICU stay record, we deleted that ICU stay. Finally, each ICU stay was represented by a feature vector with a 224 dimension, which was indexed by icustay_id.
Experimental setting
In this study, we predicted whether a patient with AKI in different stages would die within the next seven days. Specifically, a predictive modeling setting with a rolling observation window design was adopted. We supposed t was the elapsed time (in hours) after a patient was admitted to the ICU, and the patient records in t were used to predict the mortality risk for the next seven days, where t took the value of 24, 48, 72, 96, 120, and 144 hours. An illustration of this rolling window design is demonstrated in Figure 1.
Figure 1 -.
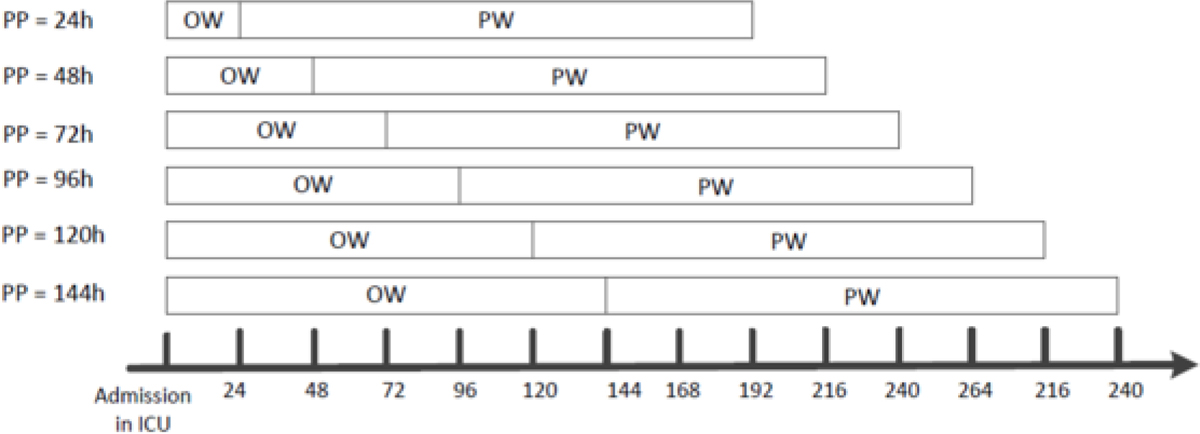
An illustration of mortality prediction for patients with AKI in different stages. PP: Prediction Point; OW: Observation Window; PW: Prediction Window
For each ICU stay, the AKI severity stage was identified based on whether the criteria in KDIGO stages were satisfied in an observation window, and then we put this ICU stay into corresponding cohorts (AKI Stage-I cohort, AKI Stage-II cohort, and AKI Stage-III cohort). If an ICU stay experienced multiple AKI stages during the observation window (from ICU admission to prediction point), we only chose the last AKI stage. Based on the built three cohorts, we predicted whether a patient with AKI in different stages would die within the next seven days. If an ICU stay did not meet one of the three AKI stage criteria in the observation window, we excluded the ICU stay from the prediction.
Predictive models
Several popular machine learning methods including Logistic Regression (LR) [16], L2 norm regularized Logistic Regression (Ridge) [17], Random Forest (RF) [18], and Gradient Boosting Decision Tree (GBDT) [19] were utilized to build predictive models using free and publicly available software. Specifically, for the implementations of LR, Ridge, and RF, we employed the Scikit-learn software library [20]. For the GBDT, we used the XGBoost software library [21]. For each predictive model, we used the 5-fold cross validation to assess the performance of these algorithms, and employed several popular and important metrics (AUC, recall, and precision) to evaluate the performance of these models.
Note that, the number of cases (mortality patient with AKI) and controls (alive patient with AKI) was imbalanced (e.g., an approximate 1:10 case to control ratio in 24 hours for AKI Stage-I) in our dataset. More statistics for all AKI stages are shown in Table 1. With such an imbalanced dataset, most classifiers had a potential to support the majority class (alive patient with AKI) because they were considered to maximize the overall number of correct predictions, thus resulting in poor performance in the minority class (mortality patient with AKI) prediction [22].
Table 1 -.
The number of mortality and alive patients with AKI in different stages
| 24h | 48h | 72h | 96h | 120h | 144h | ||
|---|---|---|---|---|---|---|---|
| Stage-I | Total | 7506 | 8181 | 5801 | 4252 | 3222 | 2416 |
| Mortality | 756 | 816 | 715 | 647 | 567 | 493 | |
| Alive | 6750 | 7365 | 5086 | 3605 | 2655 | 1923 | |
| Mortality Rate | 10.07% | 9.97% | 12.33% | 15.22% | 17.6% | 20.41% | |
| Stage-II | Total | 3216 | 2895 | 2025 | 1576 | 1267 | 1007 |
| Mortality | 514 | 514 | 421 | 386 | 347 | 310 | |
| Alive | 2702 | 2381 | 1604 | 1190 | 920 | 697 | |
| Mortality Rate | 15.98% | 17.75% | 20.79% | 24.49% | 27.39% | 30.78% | |
| Stage-III | Total | 408 | 414 | 404 | 398 | 380 | 367 |
| Mortality | 188 | 167 | 176 | 159 | 158 | 161 | |
| Alive | 220 | 247 | 228 | 239 | 222 | 206 | |
| Mortality Rate | 46.08% | 40.34% | 43.56% | 39.95% | 41.58% | 43.87% |
To address this imbalanced issue, we used case-control matching techniques [23] in this study, which matched each case with a control by considering the APACHE II score [24], Charlson comorbidity index [25], and demographic information. A matched control met two conditions: (1) has the same gender as the case and the age difference is within 5 years; (2) has the highest similarity score with the case measured by Manhattan distance on the basis of APACHE II and Charlson comorbidity index. By this technique, a resampled balanced training set was constructed with matched cases and controls.
Results
Comparison of different methods with all patient features
We tested the performance of different predictive models with different AKI stages using all patient features with varying data observation windows. Figure 2 shows the performance of these methods in terms of AUC. In order to compare the performance of mortality prediction on subpopulations completely, we constructed a mixed cohort, which combined all the patients with different AKI stages together. This mixed cohort is represented as Stage-I-II-III in Figure 2. For the performance in terms of precision and recall, please refer to https://github.com/xuzhenxing2019/MedInfo.
Figure 2 -.

The AUC of different methods with 24–144 hours of data observation window in terms of different AKI stages
From Figure 2, we observed that:
The technique of stratifying patients with AKI into different stages obtained a better performance compared to the mixed cohort. This provided some evidence for our assumption that stratifying patients with AKI into different stages has the potential to improve the performance of mortality prediction because of AKI’s complex etiology and pathophysiology.
The technique of constructing a mortality predictor based on different cohorts has the potential to consider different AKI causes. This predictive modelling strategy can be viewed as a case for local learning [26], which first splits the data space into multiple local areas and constructs different predictors for the areas.
Comparing the Stages, Stage-III shows a higher mortality predictive performance. One implication is the AKI patients in Stage-III who are going to die had more distinctive clinical manifestations compared to the control patients.
Comparing the performance of four predictors, GBDT acquires better results.
Comparison of feature group predictability with GBDT
Five different groups of features were employed to build mortality predictors for patients with different AKI stages. In this section we explored the prediction performance of GDBT with different feature groups within a 24 hour data observation window. The mortality prediction results are demonstrated in Table 2.
Table 2 -.
The performance of different group features in all AKI stages on the basis of 24 hours data
| Demographics | Medications | Comorbidities | Chart-events | Lab-events | |
|---|---|---|---|---|---|
| Stage-I | 0.6823±0.0219 | 0.6971±0.0218 | 0.7027±0.0233 | 0.7031±0.0241 | 0.7237±0.0223 |
| Stage-II | 0.7029±0.0222 | 0.7128±0.0221 | 0.7139±0.0237 | 0.7339±0.0246 | 0.7331±0.0243 |
| Stage-III | 0.7134±0.0238 | 0.7236±0.0247 | 0.7239±0.0251 | 0.7424±0.0255 | 0.7334±0.0249 |
We observed feature groups played different roles when patients were in different AKI stages. For example, the laboratory features acquired better performance than other feature groups when patients were diagnosed with AKI Stage-I. Chart features played a more important role in predicting mortality when patients were in Stage-II and III. One potential reason for this is the chart events had a finer time resolution, and for patients with a later stage of AKI, these events provided more in-time information of the patients’ condition which progressed rapidly. In addition, there were no obvious differences between comorbidities and medications feature groups. The demographic feature group contributed the least to mortality prediction in patients with different stages.
The important features chosen from all feature groups
There were 224 features extracted in total for mortality prediction in different AKI stages. The importance of each factor in mortality prediction was further explored in our experiments. The GBDT model was employed to acquire the importance score of each feature based on 24 hour data. The range of the feature importance score for mortality prediction in AKI Stage-I, Stage-II, and Stage-III were 0.0015–0.0457, 0.0015–0.0556, and 0.0019–0.0316, respectively. The top ten features are shown in Table 3.
Table 3 -.
The top 10 features selected from all feature groups on the basis of importance score
| Stage-I | Stage-II | Stage-III | ||||
|---|---|---|---|---|---|---|
| Features | Importance | Features | Importance | Features | Importance | |
| 1 | POTASSIUM_count | 0.0457 | HEMOGLOBIN_slope | 0.0556 | HeartRate_last | 0.0316 |
| 2 | metastatic_cancer | 0.0365 | HeartRate_last | 0.0278 | MeanBP_last | 0.0247 |
| 3 | CREATININE_avg | 0.0350 | CHLORIDE_slope | 0.0200 | HEMOGLOBIN_slope | 0.0237 |
| 4 | BUN_last | 0.0289 | WBC_min | 0.0185 | RespRate_last | 0.0237 |
| 5 | PTT_avg | 0.0274 | CREATININE_slope | 0.0173 | BICARBONATE_slope | 0.0221 |
| 6 | WBC_min | 0.0243 | CHLORIDE_max | 0.0170 | CREATININE_slope | 0.0217 |
| 7 | PLATELET_last | 0.0223 | SysBP_last | 0.0168 | BUN_slope | 0.0198 |
| 8 | HeartRate_max | 0.0213 | HeartRate_avg | 0.0164 | Glucose_min | 0.0198 |
| 9 | Temp_avg | 0.0213 | Temp_last | 0.0156 | DiasBP_last | 0.0198 |
| 10 | RespRate_avg | 0.0182 | HeartRate_max | 0.0154 | age | 0.0178 |
From the table, we can observe HeartRate, CREATININE, CHLORIDE, WBC, HEMOGLOBIN, and RespRate were more important because there were strong associations between these features and mortality in patients with AKI. These results aligned well with some previous studies [27–32]. For example, the magnitude of increase in serum creatinine levels can determine the severity of AKI [27], which was associated with worse survival rates [28]. Chloride levels are also associated with the severity of AKI [29] and Shaw et al. demonstrated an association between higher intravenous chloride loads and hospital mortality [30].
Discussion and Conclusions
Accurate prediction of the mortality risk for AKI patients is helpful for clinicians to understand the condition of the patients and take appropriate actions. AKI has a complex etiology and pathophysiology, so it is challenging to construct a single model that can accurately predict the mortality risk over the entire AKI patient cohort. This study investigated the impact of different AKI subpopulations on the performance of mortality prediction. We stratified the AKI patients on the basis of their disease stage (I, II, and III), and then a mortality risk predictor was constructed for each patient cohort. Several popular machine learning models (e.g., logistic regression, RF, and GBDT) were built based on these subpopulations for the mortality prediction in patients with AKI. GBDT showed a better performance than other methods for the mortality prediction in this study.
Prior models of risk prediction of mortality in critically ill patients with AKI typically employed mixed cohorts [5,6]. The utilization of stratification mechanisms performed in this study provides a chance to predict mortality in patients with AKI specific to each stage. This technique of constructing a mortality predictor based on different cohorts has the potential to consider different AKI causes. In addition, dynamic real-time data may allow for clinical monitoring of mortality risk beyond a solely static prediction upon ICU arrival. The utilization of dynamic clinical monitoring algorithms for mortality prediction may allow for its incorporation into the electronic medical record [33].
With the ability to identify patients at high risk of mortality by employing real-time data may allow for initiation of earlier interventions to prevent or reduce mortality [34]. It might be useful to incorporate more information (e.g., imaging and genomic biomarkers) to further improve the performance of dynamic clinical mortality risk prediction models. In the future, we will consider some advanced models (e.g., recurrent network) with stronger learning capabilities to improve the performance of prediction.
Acknowledgements
This work was supported in part by NIH Grants 2R01GM105688-06 and 1R21LM012618-01.
References
- [1].Makris K and Spanou L, Acute kidney injury: Definition, pathophysiology and clinical phenotypes, The Clinical Biochemist Reviews 37 (2016) 85–98. [PMC free article] [PubMed] [Google Scholar]
- [2].Doyle JF and Forni LG, Acute kidney injury: Short-term and long-term effects, Critical Care 20 (2016) 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Luo M, Yang Y, Xu J, et al. , A new scoring model for the prediction of mortality in patients with acute kidney injury, Scientific Reports 7 (2017) 7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Skarupskiené I, Adukauskiené D, Kuzminskiené J, et al. , Mortality prediction in patients with acute kidney injury requiring renal repalcement therapy after cardiac surgery, Medicina (Kaunas, Lithuania) 53 (2017) 217–223. [DOI] [PubMed] [Google Scholar]
- [5].Demirjian S, Chertow GM, Zhang JH, et al. , Model to predict mortality in critically ill adults with acute kidney injury, Clin J Am Soc Nephrol 6 (2011) 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ohnuma T, Uchino S, Toki N, et al. , External validation for acute kidney injury severity scores: A multicenter retrospective study in 14 Japense ICUs, American Journal of Nephrology 42 (2015) 57–64. [DOI] [PubMed] [Google Scholar]
- [7].Bellomo R, Kellum JA, and Ronco C, Acute kidney injury, Lancet (London, England) 380 (2012) 756–766. [DOI] [PubMed] [Google Scholar]
- [8].Miller CC, Reardon MJ, and Safi HJ, Risk Stratification: A Practical Guide for Clinicians, 1st ed., Cambridge University Press, Cambridge, 2001. [Google Scholar]
- [9].Kraus VB, Blanco FJ, Englund M, et al. , Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use, Osteoarthritis and Cartilage 23 (2015) 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Johnson AE, Pollard TJ, Shen L, et al. , MIMIC-III, a freely accessible critical care database, Scientific Data 3 (2016) 160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mohamadlou H, Lynn-Palevsky A, Barton C, et al. , Prediction of acute kidney injury with a machine learning algorithm using electronic health record data, Can J Kidney Health Dis 5 (2018) 2054358118776326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bellomo R, Ronco C, Kellum JA, et al. , Acute renal failure - Definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consesnuss conference of the acute dialysis quality initiative (ADQI) group, Critical Care (London, England) 8 (2004) R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Akcan-Arikan A, Zappitelli M, Loftis LL, et al. , Modified RIFLE criteria in critically ill children with acute kidney injury, Kidney Int 71 (2007) 1028–1035. [DOI] [PubMed] [Google Scholar]
- [14].Pickering JW and Endre ZH, GFR shot by RIFLE: Errors in staging acute kidney injury, Lancet (London, England) 373 (2009) 1318–1319. [DOI] [PubMed] [Google Scholar]
- [15].Kellum JA, Lameire N, Aspelin P, et al. , Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury, Kidney International Supplements 2 (2012) 1–138. [Google Scholar]
- [16].Hosmer DW, Lemeshow S, and Sturdivant RX, Applied Logistic Regression, 3rd ed., John Wiley & Sons, Inc., Hoboken, 2013. [Google Scholar]
- [17].Le Cessie S and Van Houwelingen JC, Ridge estimators in logistic regression, Journal of the Royal Statistical Society, Series C (Applied Statistics) 41 (1992) 191–201. [Google Scholar]
- [18].Breiman L, Random forests, Machine Learning 45 (2001) 5–32. [Google Scholar]
- [19].Friedman JH, Stochastic gradient boosting, Computational Statistics & Data Analysis 38 (2002) 367–378. [Google Scholar]
- [20].Pedregosa F, Varaoquaux G, Gramfort A, et al. , Scikitlearn: Machine learning in python, Journal of Machine Learning Research 12 (2011) 2825–2830. [Google Scholar]
- [21].Chen T and Guestrin C, XGBoost: A scalable tree boosting system, In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining (KDD ’16), San Francisco, CA, USA, 2016, 785–794. [Google Scholar]
- [22].Cheng P, Waitman LR, Hu Y, et al. , Predicting inpatient acute kidney injury over different time horizons: How early and accurate?, In AMIA 2017 Annual Symposium Proceedings, Washington, D.C., USA, 2017, 565–574. [PMC free article] [PubMed] [Google Scholar]
- [23].Cologne JB and Shibata Y, Optimal case-control matching in practice, Epidemiology (Cambridge, Massachussetts) 6 (1995) 271–275. [DOI] [PubMed] [Google Scholar]
- [24].Knaus WA, Draper EA, Wagner DP, et al. , APACHE II: A severity of disease classification system, Critical Care Medicine 13 (1985) 818–829. [PubMed] [Google Scholar]
- [25].Charlson ME, Pompei P, Ales KL, et al. , A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation, Journal of Chronic Diseases 40 (1987) 373–383. [DOI] [PubMed] [Google Scholar]
- [26].Bottou L and Vapnik V, Local learning algorithms, Neural Computation 4 (1992) 888–900. [Google Scholar]
- [27].Kellum JA, Sileanu FE, Murugan R, et al. , Classifying AKI by urine output versus serum creatinine level, J Am Soc Nephrol 26 (2015) 2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Linder A, Fjell C, Levin A, et al. , Small acute increases in serum creatinine are associated with decreased long-term survival in the crticially ill, Am J Respir Crit Care Med 189 (2014) 1075–1081. [DOI] [PubMed] [Google Scholar]
- [29].Oh HJ, Kim S, Park JT, et al. , Baseline chloride levels are associated with the incidence of contrast-associated acute kidney injury, Scientific Reports 7 (2017) 17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shaw AD, Raghunathan K, Peyerl FW, et al. , Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS, Intensive Care Medicine 40 (2014) 1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bouchard J, Soroko SB, Chertow GM, et al. , Fluid accumulation, survival and recovery of kidney function in crticially ill patients with acute kidney injury, Kidney International 76 (2009) 422–427. [DOI] [PubMed] [Google Scholar]
- [32].Chen YC, Tsai FC, Chang CH, et al. , Prognosis of patients on extracorporeal membrane oxygenation: The impact of acute kidney injury on mortality, The Annals of Thoracic Surgery 91 (2011) 137–142. [DOI] [PubMed] [Google Scholar]
- [33].Koyner JL, Adhikari R, Edelson DP, et al. , Development of a multicenter ward-based AKI prediction model, Clin J Am Soc Nephrol 11 (2016) 1935–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Keim-Malpass J, Enfield KB, Calland JF, et al. , Dynamic data monitoring improves predictive analytics for failed extubation in the ICU, Physicological Measurement 39 (2018) 075005. [DOI] [PubMed] [Google Scholar]


