Abstract
Since Legionella pneumophila is an intracellular pathogen, entry into and replication within host cells are thought to be critical to its ability to cause disease. L. pneumophila grown in one of its environmental hosts, Acanthamoeba castellanii, is phenotypically different from L. pneumophila grown on standard laboratory medium (BCYE agar). Although amoeba-grown L. pneumophila displays enhanced entry into monocytes compared to BCYE-grown bacteria, the mechanisms of entry used and the effects on virulence have not been examined. To explore whether amoeba-grown L. pneumophila differs from BCYE-grown L. pneumophila in these characteristics, we examined entry into monocytes, replication in activated macrophages, and virulence in mice. Entry of amoeba-grown L. pneumophila into monocytes occurred more frequently by coiling phagocytosis, was less affected by complement opsonization, and was less sensitive to microtubule and microfilament inhibitors than was entry of BCYE-grown bacteria. In addition, amoeba-grown L. pneumophila displays increased replication in monocytes and is more virulent in A/J, C57BL/6 Beige, and C57BL/6 mice. These data demonstrate for the first time that the intra-amoebal growth environment affects the entry mechanisms and virulence of L. pneumophila.
Legionella pneumophila is the causative agent of Legionnaires’ disease, a potentially lethal pneumonia (20), and of Pontiac fever, a self-limiting influenza-like syndrome (28). Growth conditions affect a number of factors that are thought to be related to the virulence of L. pneumophila (1, 2, 8, 9, 33, 39). Although L. pneumophila has been shown to enter and replicate within many different eukaryotic cell types, it is found primarily within monocytes during disease in humans (10, 55) and guinea pigs (11, 15). L. pneumophila grown under different conditions differs in cytotoxicity for, trafficking within, and entry into monocytes (8, 13). Since the ability of L. pneumophila to gain access to and survive within monocytes is an essential pathogenic trait (15, 55), it is likely that growth conditions will affect the ability of this organism to cause disease.
Since growth in protozoa, such as Acanthamoeba castellanii, is thought to reflect the environment growth conditions for L. pneumophila (23, 48), we chose to examine the effects of growth in these amoebae on virulence. Previous studies in our laboratory have demonstrated that growth in environmental amoebae enhances the ability of L. pneumophila to enter epithelial cells (100- to 1,000-fold), murine macrophages (10- to 100-fold), human monocytes (100- to 1,000-fold), and A. castellanii (10- to 100-fold) (13). However, the effects of this growth condition on the mechanism of entry of L. pneumophila into host cells and its role in virulence are not well understood. It has been shown that L. pneumophila enters monocytes through an unusual mechanism, an asymmetric phagocytic event termed coiling phagocytosis (25). Phagocytosis of L. pneumophila is thought to involve the complement receptors CR1 and CR3 and is enhanced by the presence of complement (32, 40). Entry via this mechanism may occur via binding of the major outer membrane protein to complement receptors either directly (30) or through complement (3). Since both conventional and coiling phagocytic events have been observed in L. pneumophila (25, 42) and the frequencies of each have not been quantitated, it is difficult to ascertain the effects of these mechanisms of entry on subsequent intracellular survival. However, recent studies demonstrating that L. pneumophila has the ability to affect very early trafficking events from 5 min (49) to 30 min (52) after entry suggest that entry or other very early events play a role in intracellular survival.
To determine the effects of growing L. pneumophila in amoebae on entry mechanisms, we examined the role of complement opsonization, frequencies of coiling and conventional phagocytosis, and role of cytoskeletal components in entry into monocytes of these bacteria compared to BCYE-grown bacteria. Effects of growth in amoebae on virulence were evaluated by the ability to replicate intracellularly in resting and activated monocytes as well as virulence in the three mouse strains A/J, C57BL/6J Beige, and C57BL/6J. From these data, we conclude that growth in amoebae affects the entry mechanism used for monocytes, enhances intracellular replication in monocytes, and increases virulence in mice.
MATERIALS AND METHODS
Strains and culture conditions.
The L. pneumophila strain used for these studies was the streptomycin-resistant variant (34) of L. pneumophila serogroup 1 (130b) (18). This L. pneumophila strain is virulent in both in vitro and in vivo models of infection (34) and was passaged no more than twice in the laboratory before use in these studies, to prevent loss of virulence. L. pneumophila was grown either on BCYE agar (16) for 3 days or in the amoeba A. castellanii as described previously (13). The preparation of amoeba-grown bacteria has been improved to increase the number of bacteria isolated and reduce contamination with amoebal debris. These improvements were accomplished by using a multiplicity of infection (MOI) of 100 in the amoebae and by allowing intracellular growth to continue for 48 h at 37°C in 5% CO2. Potential remaining amoebae and amoebal debris were removed from the preparation by filtration through a 5-μm-pore-size filter (Costar) after centrifugation. Samples prepared in this manner were examined by light microscopy to confirm the absence of intact amoebae. The Escherichia coli K-12 strain HB101 (ara-14 leuB6 proA2 lacY1 glnV44 glaK2 recA13 rpsL20 xyl-5 mtl-1 thi-1 hsdS29) (Promega) was grown to stationary phase in Lennox broth (GIBCO). Bacterial viability was determined when necessary by using the LIVE-DEAD assay (Molecular Probes, Eugene, Oreg.). All bacterial preparations used in these studies were found to be greater than 99% viable.
Cell lines and culture conditions.
The amoebae used in these experiments were A. castellanii ATCC 30234. They were grown in PYG broth in 75-cm2 tissue culture flasks, and the number of viable cells was determined as described previously (13, 35).
The monocytes used in these experiments were either human peripheral blood monocytes (PBMs) or THP-1 cells (ATCC TIB202). PBMs were isolated from 50 ml of human blood obtained from healthy volunteers. The mononuclear cell fraction was purified by centrifugation in Ficoll at 700 × g for 30 min at room temperature. The PBM-containing band was removed, washed twice in Hanks balanced salt solution (GIBCO), and suspended in RPMI–0.1% heat-inactivated human serum to a concentration of 106 cells/ml. The human serum was heated to 65°C for 1 h to inactivate complement. A 1-ml volume of the resulting suspension was used to seed 24-well microtiter dishes (Falcon) and incubated for 2 h at 37°C. Nonadherent cells were then removed by washing with prewarmed phosphate-buffered saline (PBS), and the wells were refilled with RPMI–5% inactivated human serum. The adherent population was found by microscopy (data not shown) to contain greater than 96% monocytes and greater than 90% viable cells. Viability was determined by measurement of permeability to eosin Y (Sigma). THP-1 cells were used in these studies because they are relatively easy to use, are phagocytic (50), can be fully differentiated into activated macrophages (50), have abundant complement receptors (43), and have no known cell surface receptor mutations that would affect entry. The presence of complement receptors on THP-1 cells was confirmed by immunofluorescence microscopy. THP-1 cells were grown in RPMI–10% fetal calf serum (GIBCO). All tissue culture reagents and media used were guaranteed endotoxin free by the company from which they were purchased. Prior to use in all assays, PBMs and THP-1 cells were washed once with RPMI to remove endogenous cellular products such as complement.
Entry assays.
Entry assays were carried out essentially as described previously (13). Assays with human PBMs and activated THP-1 cells were carried out in 1 ml of RPMI in 24-well microtiter dishes (Falcon), using 106 cells/well. The bacteria to be assayed were mixed with an equal volume of RPMI, complete nonimmune human serum (titer of anti-L. pneumophila antibodies, <1:100 [as measured by enzyme-linked immunosorbent assay]), or heat-inactivated human serum, incubated for 10 min at 37°C, and used to infect the cells for 30 min at 37°C with an MOI of 10 to 100 bacteria/cell. The relative differences in entry between amoeba- and BCYE-grown bacteria remained approximately the same (100- to 1,000-fold) over 5- to 120-min infection periods in entry assays (data not shown). Assays with THP-1 cells that had not been activated were carried out in suspension in 1.5-ml microcentrifuge tubes (Applied Scientific) containing 106 cells in 1 ml of RPMI at 37°C. After infection with bacteria, the cells were washed with PBS once (or three times if the assay was to be carried out in the absence of gentamicin). Treatment with 100 μg of gentamicin per ml for 2 h was used to kill extracellular bacteria when necessary. After the initial washes or gentamicin treatment, the cells were washed once more in PBS and then lysed in 1 ml of sterile distilled water for 10 min at room temperature. For each change of solution for assays in suspension, the cells were pelleted by centrifugation for 1 min at 100 × g. After incubation of the cells with water, they were passed through a 27-gauge syringe three times to ensure lysis and dilutions were plated on BCYE (L. pneumophila) or Lennox broth (E. coli) agar to determine bacterial counts (expressed in CFU). Entry levels were determined by calculating the percentage of the inoculum that became gentamicin resistant over the course of the assay [i.e., % entry = 100 × (CFU of gentamicin-resistant cells/CFU of inoculum)]. To correct for variation in levels of uptake between experiments, entry is reported relative to that of L. pneumophila 130b (AA100) (i.e., relative entry = % entry of test strain/% entry of AA100). Similar to previous studies (13), entry levels of amoeba-grown Legionella were approximately 0.4 and 4% of the inoculum in resting and activated monocytes, respectively, under these conditions.
Inhibitor studies.
To examine the effect of cellular inhibitors on the entry process, the THP-1 cells were incubated with 2 μM cytochalasin D (Sigma) for 1 h, 10 μM colchicine (Sigma) for 30 min, or 20 μM nocodazole (Sigma) for 30 min to 2 h prior to addition of the bacteria. These inhibitors were left in the assay mixture during the 30-min incubation for entry. The subsequent steps in the entry assay were carried out as described above without the addition of gentamicin. Each inhibitor, as well as the solution in which it was solubilized, was tested for cytotoxicity for the bacteria, as assayed by acridine orange staining (58), and for the host cell, as assayed by uptake of 0.1% eosin Y (Sigma). No significant effects of the inhibitors or solubilization buffer on bacterial or host cell viability were observed (data not shown).
Intracellular viability assays.
The THP-1 cells used for viability assays were seeded into 24-well tissue culture dishes (Falcon) containing RPMI plus 10% serum, 5 μg of lipopolysaccharide (E. coli O127:B8; Difco) per ml, and 40 U of human gamma interferon (Boehringer Mannheim) per ml. The cells were incubated for 2 days with this medium, and fresh medium was added for an additional 24 h to allow activation of the monocytes prior to entry. The bacteria were added to the cells and incubated at 37°C for 5 min, washed three times with warm PBS, and suspended in fresh medium with gamma interferon and lipopolysaccharide for various times before lysis with water. Dilutions of the resulting lysates were plated to determined the CFU at each time point. For nonactivated THP-1 cells, the assays were carried out in 1.5-ml microcentrifuge tubes in the same manner as described for entry assays.
Microscopy techniques.
Coiling phagocytosis was evaluated as described previously (13, 25). The bacteria were incubated in the presence of RPMI, heat-inactivated serum, or complete serum in the same manner as for the entry assays prior to addition to the cells. For fluorescence microscopy, the samples were fixed at room temperature for 10 min in PBS containing 3.7% formaldehyde. They were then washed twice with PBS, suspended in ice-cold acetone for 3 min, and washed twice more with PBS. At this point, the distribution of tubulin in the cells was examined by using an anti-α-tubulin antibody (1:200 in PBS [Amersham]) and a secondary anti-mouse immunoglobulin G-fluorescein isothiocyanate-conjugated antibody (1:200 in PBS [Sigma]). Both antibodies were sequentially incubated with the sample for 1 h at room temperature. The cells were then washed three times with PBS and examined by fluorescence microscopy.
Fluorescent staining of THP-1 cells for the presence of the complement receptors CR1 and CR3 was carried out in the same manner as the tubulin staining with the following exceptions: the cells were not permeabilized by the addition of cold acetone, and the primary antibody used was the monoclonal antibody E11 (Serotec) or D12 (Becton Dickinson) against CR1 or CR3, respectively.
Mouse infections.
To examine the virulence of amoeba-grown and BCYE-grown L. pneumophila in mice, we used methods described previously (5, 7). C57BL/6J, C57BL/6JbgJ/bgJ, and A/J mice were infected by intratracheal inoculation with 106 bacteria, and after 2 h and 1, 2, and 4 days the mouse lungs were harvested and the bacteria in the lungs were quantitated as described previously (5, 7). Data presented represent the means and standard deviations of bacterial counts (CFU/gram of lung) for seven mice in each experimental group. Four different bacterial preparations were used: (i) bacteria grown on BCYE agar for 3 days under standard laboratory conditions, (ii) BCYE-grown bacteria mixed with a lysate of A. castellanii, (iii) A. castellanii that had been infected with L. pneumophila for 2 days, and (iv) L. pneumophila that had been previously grown in amoebae for 2 days. Each preparation was equilibrated to 106 bacteria per sample. Preparations i and iv were prepared in the same manner as described for entry assays. Separation of bacteria from amoebae in preparation iv was ensured by passage of the preparation through a 5-μm-pore-size filter (Costar) prior to inoculation. Preparation ii was made by mixing 106 bacteria with a lysate of 106 amoebae. The amoebal lysate was produced by washing 106 amoebae in 1 ml of distilled water and resuspending them in 1 ml of water for 10 min at room temperature. The cells were then vortexed vigorously 10 times for 3 s each. All preparations were suspended in PBS prior to inoculation.
Statistical analyses.
All in vitro experiments were carried out in triplicate and repeated three times. The experiments in vivo were carried out with seven mice per experimental group. The significance of the results was analyzed by analysis of variance. P values of < 0.05 were considered significant.
RESULTS
Effects of complement on entry of amoeba-grown L. pneumophila.
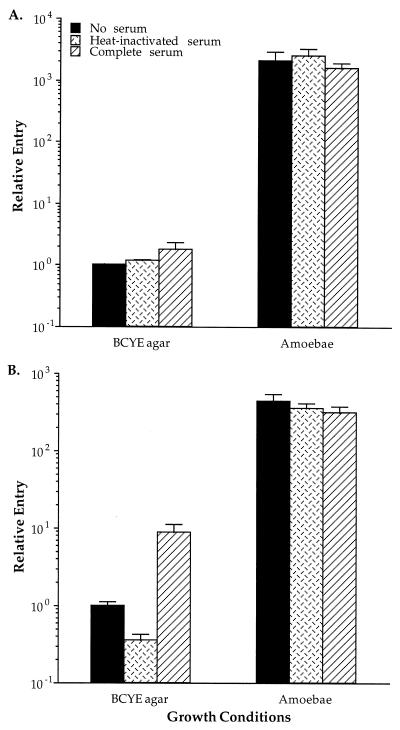
Although L. pneumophila organisms grown in amoebae have been previously shown to enter monocytes at higher levels than do those grown on BCYE (13), the effects of complement on entry of bacteria grown in amoebae were not known. We examined entry of amoeba-grown and BCYE-grown bacteria into PBMs and THP-1 cells by using L. pneumophila that had been preincubated with RPMI, complete human serum, or heat-inactivated serum (Fig. 1). A significant increase in entry into both THP-1 cells (a twofold increase) and PBMs (an eightfold increase) was observed with BCYE-grown bacteria in the presence of complete serum, comparable to results from previous studies (40). In contrast, entry of amoeba-grown bacteria was not affected by the presence of complement. These data suggest that complement opsonization does not play a role in the entry mechanism of amoeba-grown L. pneumophila into monocytes.
FIG. 1.
Effects of human serum on entry of BCYE- and amoeba-grown L. pneumophila into THP-1 cells (A) and human PBMs (B). Entry is expressed relative to BCYE-grown AA100 in the absence of serum and is calculated as described in Materials and Methods. Serum was replaced by RPMI in the no-serum control. Error bars represent the standard deviations for triplicate samples in each experiment. The results shown are from a single representative experiment. All experiments were repeated at least three times.
Quantitation of L. pneumophila entry via coiling phagocytosis.
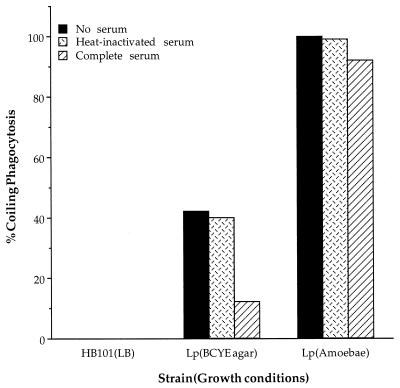
Although both conventional and coiling phagocytic events have been observed previously in BCYE-grown L. pneumophila (25, 42), they have not been quantitated. Thus, to determine the frequencies of coiling phagocytosis and evaluate the role of complement in this event, we compared the structural mechanism of entry by L. pneumophila grown in amoebae and BCYE in the presence and absence of complement. We examined 100 entry events and characterized them as conventional or coiling phagocytosis (Fig. 2). BCYE-grown L. pneumophila enters THP-1 cells by coiling phagocytosis between 40 and 50% of the time, but in the presence of complete serum the frequency of coiling phagocytosis decreases to less than 20%. Amoeba-grown L. pneumophila, however, enters by coiling phagocytosis 90 to 98% of the time in both the presence and absence of complete serum. Apparently, complement has a significant effect upon the mechanism of entry into monocytes by BCYE-grown bacteria, but amoeba-grown bacteria can either overcome the effects of or prevent opsonization.
FIG. 2.
Frequency of coiling phagocytic entry events triggered by BCYE- and amoeba-grown L. pneumophila in THP-1 cells. The percent coiling phagocytosis is the number of coiling phagocytic events in every 100 entry events observed. E. coli HB101 is included as a conventional phagocytosis control. Serum was replaced with RPMI in the no-serum control. The results shown are from a single representative experiment of three independent experiments.
Cellular inhibitors affect the entry of amoeba-grown and BCYE-grown L. pneumophila differently.
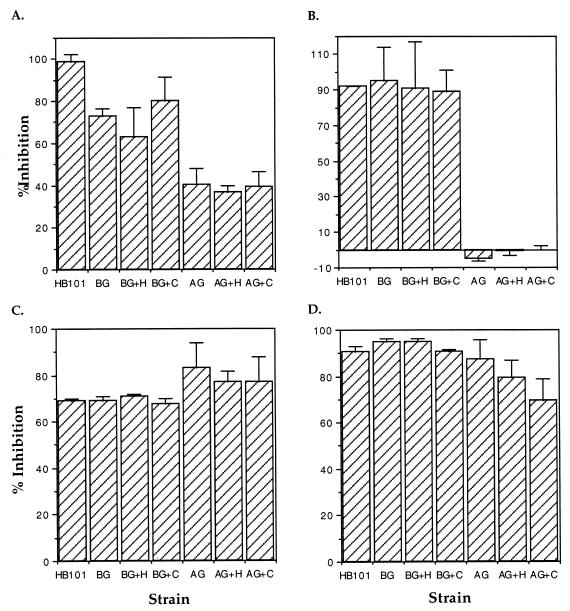
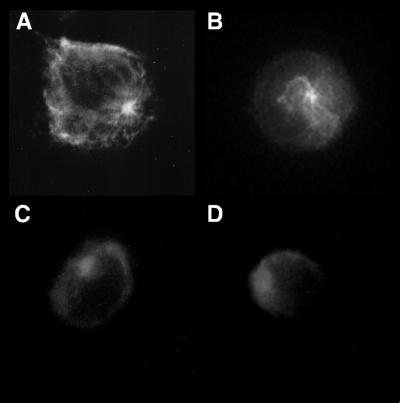
Based on the above results, we now have the ability to compare conditions where 90% of L. pneumophila bacteria enter via conventional phagocytosis (BCYE-grown bacteria in the presence of complement) with conditions where 98% enter via coiling phagocytosis (amoeba-grown bacteria in the absence of complement). In addition, amoeba-grown bacteria enter monocytes at a much higher frequency than do BCYE-grown bacteria. Comparison of the effects of different cytoskeletal inhibitors on the entry of each of these bacterial populations should allow us to dissect the cellular mechanisms of the phagocytic events involved. Entry into and growth in monocytes of BCYE-grown L. pneumophila in the absence of complement are microfilament dependent (17, 29). Thus, we focused our studies on the microfilament polymerization inhibitor cytochalasin D and the microtubule polymerization inhibitors colchicine and nocodazole. THP-1 cells were pretreated with each of these inhibitors, and the subsequent entry of L. pneumophila was examined by using the entry assay (Fig. 3). While cytochalasin D completely inhibited HB101 uptake, it had only a modest effect upon entry of BCYE-grown L. pneumophila and less of an effect upon entry of the amoeba-grown bacteria (Fig. 3A). By contrast, colchicine appeared to inhibit the entry of all bacterial strains to the same level (Fig. 3C). We initially tested nocodazole to confirm the results of the colchicine inhibition studies. Surprisingly, we found that nocodazole pretreatment for 30 min at 37°C affected the entry of HB101 and BCYE-grown L. pneumophila in approximately the same manner as did colchicine but, significantly, that entry of amoeba-grown bacteria was not affected (Fig. 3B). In contrast, when the cells were pretreated for 2 h with nocodazole in the same manner, entry of all bacteria was significantly affected, as had been seen for colchicine (Fig. 3D). The effects of these inhibitors on the distribution of tubulin in THP-1 cells were examined by fluorescence microscopy (Fig. 4). After colchicine (30-min) and nocodazole (2-h) treatment, an anti-α-tubulin antibody gave diffuse labeling. When the cells were treated with nocodazole for only 30 min, however, stable microtubules radiating from the centriole were detected. Thus, microtubule and microfilament polymerization appears to be involved in conventional phagocytic events whereas only stable microtubules and microfilament polymerization are involved in coiling phagocytosis.
FIG. 3.
Comparison of the ability of cytochalasin D (A), 30-min preincubation with nocodazole (B), colchicine (C), and 2-h preincubation with nocodazole (D) to inhibit the entry of BCYE-grown (BG) and amoeba-grown (AG) L. pneumophila into THP-1 cells. The ability of each of these pharmacological agents to inhibit entry is reported as the percentage of CFU that enter in the presence of the inhibitor compared to a control in the absence of the inhibitor. E. coli HB101 was used as a conventional phagocytosis control. Serum was replaced with RPMI in the no-serum controls (BG and AG). The abbreviation +H indicates that heat-inactivated serum was incubated with the bacteria prior to the assay, and +C indicates that complete human serum was used. % Inhibition = 100 − [100 × (cell-associated CFU in the presence of the inhibitor/cell-associated CFU in the absence of the inhibitor)]. Error bars represent the standard deviations of triplicate wells for each experiment. The results shown are from a single representative experiment of at least three independent experiments.
FIG. 4.
Fluorescence microscopy showing the distribution of microtubules in THP-1 cells either untreated (A) or treated with nocodazole for 30 min (B), colchicine for 30 min (C), or nocodazole for 2 h (D) at 37°C.
Growth in amoebae affects intracellular replication.
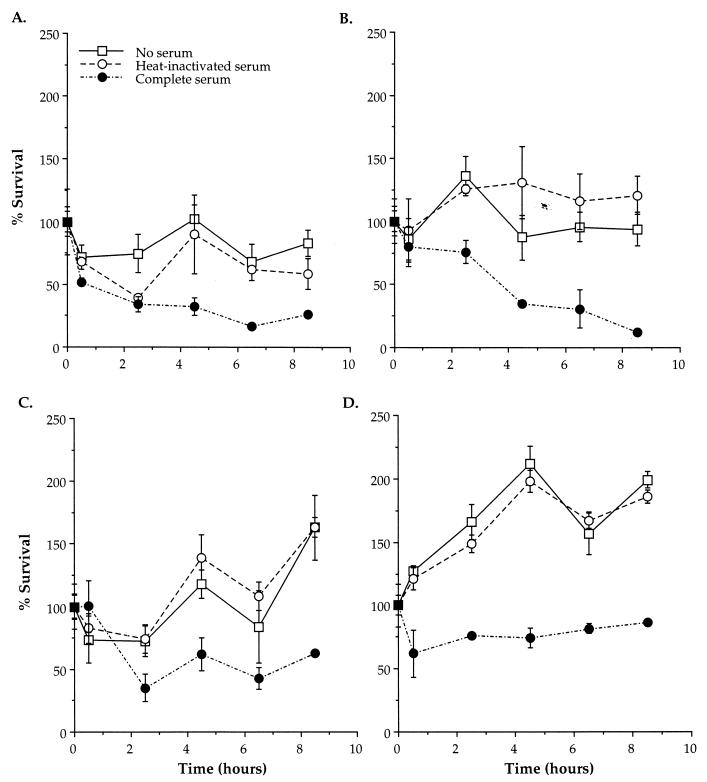
The ability of amoeba-grown and BCYE-grown L. pneumophila to survive and replicate in resting and activated THP-1 cells was examined during the first 8.5 h following entry (Fig. 5). BCYE-grown L. pneumophila had the ability to persist in both resting and activated monocytes. However, the presence of complement decreased intracellular survival in both cell types. Amoeba-grown L. pneumophila displayed intracellular replication during the first 8.5 h in resting and activated monocytes. The presence of complement decreased the intracellular survival of amoeba-grown L. pneumophila initially, and this was followed by slightly slower intracellular growth than in the absence of complement. Thus, growth of L. pneumophila in amoebae enhanced intracellular survival and replication in both resting and activated monocytes compared to growth in BCYE.
FIG. 5.
Viability of BCYE-grown (A and B) and amoeba-grown (C and D) L. pneumophila after entry into resting (A and C) and activated (B and D) macrophages in the presence of RPMI (no serum), heat-inactivated serum, or complete serum. The number of CFU present at the first time point (30 min after entry) was considered 100% survival. Error bars represent the standard deviations of triplicate wells for each experiment. The results shown are from a single representative experiment of at least three independent experiments.
Growth of L. pneumophila in amoebae affects virulence.
Although it is clear that growth in amoebae affects replication in monocytes, this phenotype may not affect virulence in vivo. There are several animal models for L. pneumophila infection, including mice (7, 57), rats (47, 54), and guinea pigs (4, 15, 54). We used the mouse model of infection based on its cost-effectiveness and the availability of a number of different strains that vary in their susceptibility to L. pneumophila. A/J mice are highly susceptible to infection by BCYE-grown L. pneumophila (7). Since infections in a highly susceptible animal model may be very different from those in humans, we also compared the virulence of amoeba- and BCYE-grown bacteria in two other mouse strains, C57BL/6, which is thought to be resistant to infection with L. pneumophila (57), and C57BL/6 Beige, which is commonly used as a model for mycobacterial infection due to its higher susceptibility (5, 21). When each of these mouse strains was infected by the intratracheal route, there was an increased rate of replication in the lungs for amoeba-grown L. pneumophila compared to BCYE-grown L. pneumophila (Table 1). Inoculation of the unpurified bacterium-amoeba 2-day culture resulted in the same bacterial numbers in the lungs as did inoculation of the amoeba-grown bacteria alone. Addition of a lysate of the amoebae to BCYE-grown bacteria prior to inoculation did not affect bacterial replication in the mouse lungs compared to BCYE-grown bacteria alone. Although an increase in bacterial growth of amoeba-grown L. pneumophila was observed in the lungs of all three mouse strains, the infections appeared to be resolving after 4 days in the C57BL/6 and C57BL/6 Beige mouse strains. Based on these data, the C57BL/6 Beige mouse strain is intermediate between A/J and C57BL/6 in its susceptibility to L. pneumophila infection. Overall, these data indicate that amoeba-grown L. pneumophila is more infectious than BCYE-grown bacteria in both susceptible and resistant mice.
TABLE 1.
Replication of L. pneumophila in lungs after intratracheal inoculation
| Mouse strain and experimental groupa | No. of bacteria in lungs (CFU/g)c after:
|
|||
|---|---|---|---|---|
| 2 h | 1 day | 2 days | 4 days | |
| A/J | ||||
| BCYE-grown bacteria | (2.0 ± 0.3) × 105 | (6.2 ± 0.3) × 106 | (9.4 ± 0.3) × 106 | (2.1 ± 0.4) × 107 |
| BCYE-grown bacteria + amoebal lysate | (9.6 ± 0.5) × 104 | (5.8 ± 0.4) × 106 | (8.4 ± 0.3) × 106 | (1.1 ± 0.2) × 107 |
| Amoebae infected with bacteria | (9.1 ± 0.4) × 104 | (6.9 ± 0.3) × 108b | (1.9 ± 0.3) × 108b | (4.3 ± 0.3) × 109b |
| Amoeba-grown bacteria | (1.6 ± 0.1) × 105 | (8.6 ± 0.4) × 108b | (2.7 ± 0.6) × 109b | (4.1 ± 0.5) × 109b |
| C57BL/6 Beige | ||||
| BCYE-grown bacteria | (3.6 ± 0.4) × 105 | (2.9 ± 0.3) × 106 | (6.1 ± 0.1) × 106 | (5.3 ± 0.4) × 106 |
| BCYE-grown bacteria + amoebal lysate | (1.4 ± 0.3) × 105 | (3.3 ± 0.4) × 106 | (7.4 ± 0.2) × 106 | (5.9 ± 0.2) × 106 |
| Amoebae infected with bacteria | (2.4 ± 0.3) × 105 | (5.8 ± 0.3) × 108b | (9.1 ± 0.3) × 108b | (9.9 ± 0.3) × 108b |
| Amoeba-grown bacteria | (1.8 ± 0.5) × 105 | (6.7 ± 0.4) × 108b | (9.7 ± 0.5) × 108b | (9.3 ± 0.4) × 108b |
| C57BL/6 | ||||
| BCYE-grown bacteria | (2.0 ± 0.3) × 105 | (2.1 ± 0.4) × 106 | (4.5 ± 0.4) × 106 | (4.0 ± 0.3) × 106 |
| BCYE-grown bacteria + amoebal lysate | (9.8 ± 0.2) × 104 | (3.0 ± 0.1) × 106 | (5.7 ± 0.3) × 106 | (6.3 ± 0.4) × 106 |
| Amoebae infected with bacteria | (2.1 ± 0.2) × 105 | (5.0 ± 0.3) × 108b | (8.3 ± 0.4) × 108b | (5.1 ± 0.2) × 108b |
| Amoeba-grown bacteria | (1.5 ± 0.5) × 105 | (6.1 ± 0.4) × 108b | (9.4 ± 0.5) × 108b | (6.5 ± 0.3) × 106 |
An inoculum of 106 bacteria was used for all experimental groups.
Significantly different (P < 0.05) from BCYE-grown L. pneumophila alone.
Data represent the means ± standard deviations of duplicate platings from seven mice.
DISCUSSION
We have found that growth of L. pneumophila in amoebae affects entry mechanisms into monocytes. Previous studies performed with BCYE-grown L. pneumophila demonstrated that the presence of complete serum enhances entry and that entry can be blocked by specific antibodies to the complement receptors (32, 40). Our experiments confirmed these studies but also indicated that complement does not affect the frequencies of entry by amoeba-grown L. pneumophila. This result may be explained by the fact that the outer membrane protein profile of bacteria grown under these conditions is very different (13), suggesting that different ligands and host cell receptors may be used. Alternatively, amoeba-grown L. pneumophila may bind to the same receptors but trigger uptake more efficiently. In either case, this is the first demonstration that amoeba-grown L. pneumophila enters monocytes by a complement-independent mechanism. This observation suggests that other growth conditions may also affect the frequencies of entry via the previously described complement-dependent mechanism.
The higher levels of entry and absence of complement involvement suggest that amoeba-grown L. pneumophila enters monocytes via a different mechanism from that of BCYE-grown L. pneumophila. Previous studies have demonstrated that both conventional and coiling phagocytic events occur in amoeba-grown (13) and BCYE-grown (13, 25) bacteria. Quantitation of the frequencies of these phagocytic events was necessary to determine the role of complement and the growth environment. Since the frequencies of coiling phagocytosis are significantly lower in the presence of complete serum, it is likely that complement opsonization enhances conventional phagocytic uptake of L. pneumophila by monocytes. Thus, the increase in the frequency of entry of BCYE-grown bacteria in the presence of complement may be due to an increase in the frequency of conventional phagocytic events. This conclusion is supported by previous observations that complement-opsonized bacterial strains (14, 25) and liposomes (3) enter via conventional phagocytosis. The role of complement receptors in coiling and conventional phagocytic events was not examined in our studies. In future studies it should be possible to determine the role of complement receptors in each of these entry mechanisms by using monoclonal antibodies to block the receptor binding sites followed by quantitation of the structural mechanisms of entry used.
The coiling phagocytic mechanism used by L. pneumophila is unusual and is only rarely seen in other bacteria, with the exception of spirochetes (12, 45, 46). A number of bacteria enter cells via mechanisms that are microfilament dependent (inhibited by cytochalasin D); these include Chlamydia (51), Salmonella (19), Shigella (6, 19), Streptococcus (31), Bordetella (22), Citrobacter (38), Escherichia (37), Ehrlichia (44), and Yersinia (19). Of these, the majority (Escherichia, Streptococcus, Ehrlichia, Bordetella, and Chlamydia) are also microtubule dependent (inhibited by colchicine or nocodazole). In our study, BCYE-grown L. pneumophila entered monocytes by a mechanism involving both microfilaments and microtubules, similar to previous observations for conventional phagocytic mechanisms (41). Our data suggest that microfilaments also play a role in uptake of amoeba-grown L. pneumophila but that this mechanism is not as sensitive to cytochalasin D as is entry by BCYE-grown bacteria. Inhibition of entry of amoeba-grown bacteria in the presence of colchicine and nocodazole indicates that microtubules are required. Interestingly, the 30-min preincubation with nocodazole did not inhibit the entry of amoeba-grown L. pneumophila. This is probably because colchicine causes a rapid breakdown of tubulin polymers to monomers at 37°C (53) whereas nocodazole has little effect on stable microtubules over short periods at this temperature (24). Although breakdown of preformed microtubules by nocodazole is inefficient at 37°C, extended treatment (2 h) at this temperature results in complete breakdown of the microtubular network (27). These data are consistent with the distribution of microtubules in THP-1 cells after treatment with nocodazole observed by fluorescence microscopy in our studies. Taken together, these results suggest that the de novo synthesis of microtubules is required for entry into monocytes by BCYE-grown L. pneumophila but that only stable microtubules are required for entry by amoeba-grown bacteria.
We have found that amoeba-grown L. pneumophila displays increased intracellular survival and replication in macrophages compared to BCYE-grown L. pneumophila. It is possible that this enhanced intracellular survival is related to the different entry mechanisms used by these bacteria, but it may also be due to unrelated factors that are influenced by growth in amoebae. We examined survival at very early time points after entry into monocytes with the assumption that if entry plays a role in intracellular survival, it is likely to affect events soon after entry. As shown in previously published studies, BCYE-grown bacteria will go on to replicate in monocytes even in the presence of complement (26, 32, 40). The conventional phagocytic mechanism is consistent with a complement-mediated mechanism of entry, since complement receptor-mediated uptake involves both microfilaments and microtubules (36). It has been suggested that entry of L. pneumophila into monocytes via the complement-mediated mechanism is advantageous since it prevents activation of the oxidative burst (56). Our data indicate, however, that the complement-mediated mechanism of uptake does not provide a selective advantage in monocytes. In addition, BCYE-grown bacteria were not as successful at replicating in vivo as were amoeba-grown bacteria in all three mouse strains tested. These observations suggest that the differences between the abilities of L. pneumophila grown in amoebae and of that grown in BCYE to replicate in monocytes are significant enough to affect virulence in vivo. Alternatively, other factors unrelated to replication in monocytes could be responsible for this phenotype. Due to the large number of differences between BCYE- and amoeba-grown L. pneumophila, a detailed analysis of the genes involved is necessary to differentiate between these possibilities.
The fact that amoeba-grown L. pneumophila enters monocytes via a somewhat altered mechanism and displays enhanced growth in the lungs of mice suggests that bacteria grown in protozoan hosts present in domestic water supplies are responsible for the production of Legionnaires’ disease in humans. The differences in the ability of L. pneumophila to enter, survive, and replicate in host cells when grown under different conditions underscore the importance of taking bacterial growth conditions into account when carrying out pathogenesis studies. Further examination of the genes induced by growth in amoebae should lead to a better understanding of the bacterial factors required for the production of Legionnaires’ disease.
ACKNOWLEDGMENTS
We thank Raul Barletta for critical review of the manuscript.
This work was supported by grants AI07328, AI30618, and AI40165 from the National Institutes of Health; the Center for Indoor Air Research; and the American Lung Association.
REFERENCES
- 1.Abu Kwaik Y, Eisenstein B I, Engleberg N C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker J, Lambert P A, Brown M R W. Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect Immun. 1993;61:3503–3510. doi: 10.1128/iai.61.8.3503-3510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellinger-Kawahara C, Horwitz M A. Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J Exp Med. 1990;172:1201–1210. doi: 10.1084/jem.172.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berendt R F, Young H W, Allen R G, Knutsen G L. Dose-response of guinea pigs experimentally infected with aerosols of Legionella pneumophila. J Infect Dis. 1980;141:186–192. doi: 10.1093/infdis/141.2.186. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez L E, Petrofsky M, Kolonoski P, Young L S. An animal model of Mycobacterium avium complex disseminated infection after colonization of the intestinal tract. J Infect Dis. 1992;165:75–79. doi: 10.1093/infdis/165.1.75. [DOI] [PubMed] [Google Scholar]
- 6.Bernardini M L, Mounier J, D’Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, Engleberg C. Replicative Legionella pneumophila lung infections in intratracheally inoculated A/J mice: a murine model of human Legionnaires’ disease. Am J Pathol. 1994;145:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 8.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catrenich C E, Johnson W. Characterization of the selective inhibition of growth of virulent Legionella pneumophila by supplemented Mueller-Hinton medium. Infect Immun. 1989;57:1862–1864. doi: 10.1128/iai.57.6.1862-1864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandler F W, Hicklin M D, Blackmon J A. Demonstration of the agent of Legionnaires’ disease in tissue. N Engl J Med. 1977;297:1218–1220. doi: 10.1056/NEJM197712012972206. [DOI] [PubMed] [Google Scholar]
- 11.Chandler F W, McDade J E, Hicklin M D, Blackmon J A, Thomason B M, Ewing E P. Pathologic findings in guinea pigs inoculated intraperitoneally with the Legionnaires’ disease bacterium. Ann Intern Med. 1979;90:671–675. doi: 10.7326/0003-4819-90-4-671. [DOI] [PubMed] [Google Scholar]
- 12.Cheng, X., J. D. Cirillo, and G. E. Duhamel. Coiling phagocytosis is the predominant mechanism for uptake of Serpulina pilosicoli by human monocytes. In P. S. Paul and D. H. Francis (ed.), Mechanisms in the pathogenesis of enteric diseases, 2nd ed., in press. Plenum Publishing Co., New York, N.Y.
- 13.Cirillo J D, Falkow S, Tompkins L S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemens D L, Horwitz M A. Membrane sorting during phagocytosis: selective exclusion of major histocompatibility complex molecules but not complement receptor CR3 during conventional and coiling phagocytosis. J Exp Med. 1992;175:1317–1326. doi: 10.1084/jem.175.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis G S, Winn W C, Jr, Gump D W, Beaty H N. The kinetics of early inflammatory events during experimental pneumonia due to Legionella pneumophila in guinea pigs. J Infect Dis. 1983;148:823–825. doi: 10.1093/infdis/148.5.823. [DOI] [PubMed] [Google Scholar]
- 16.Edelstein P H. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981;14:298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott J A, Winn W C., Jr Treatment of alveolar macrophages with cytochalasin D inhibits uptake and subsequent growth of Legionella pneumophila. Infect Immun. 1986;51:31–36. doi: 10.1128/iai.51.1.31-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engleberg N C, Drutz D J, Eisenstein B I. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect Immun. 1984;44:222–227. doi: 10.1128/iai.44.2.222-227.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlay B B, Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988;70:1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- 20.Fraser D W, Tsai T R, Orenstein W, Parkin W E, Beecham H J, Sharrar R G, Harris J, Mallison G F, Martin S M, McDade J E, Shepard C C, Brachman P S The Field Investigation Team. Legionnaires’ disease: description of an epidemic of pneumonia. N Engl J Med. 1977;297:1189–1196. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- 21.Gangadharam P R, Edwards III C K, Murthy P S, Pratt P F. An acute infection model for Mycobacterium intracellulare disease using Beige mice: preliminary results. Am Rev Respir Dis. 1983;127:648–649. doi: 10.1164/arrd.1983.127.5.648. [DOI] [PubMed] [Google Scholar]
- 22.Guzman C A, Rohde M, Timmis K N. Mechanisms involved in uptake of Bordetella bronchiseptica by mouse dendritic cells. Infect Immun. 1994;62:5538–5544. doi: 10.1128/iai.62.12.5538-5544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henke M, Seidel K M. Association between Legionella pneumophila and amoebae in water. Isr J Med Sci. 1986;22:690–695. [PubMed] [Google Scholar]
- 24.Hoebeke J, Van Nijen G, De Brabander M. Interaction of oncodazole (R 17934), a new anti-tumoral drug, with rat brain tubulin. Biochem Biophys Res Commun. 1976;69:319–324. doi: 10.1016/0006-291x(76)90524-6. [DOI] [PubMed] [Google Scholar]
- 25.Horwitz M A. Phagocytosis of the Legionnaires’ disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz M A, Silverstein S C. Interaction of the Legionnaires’ disease bacterium (Legionella pneumophila) with human phagocytes. II. Antibody promotes binding of L. pneumophila to monocytes but does not inhibit intracellular multiplication. J Exp Med. 1981;153:398–406. doi: 10.1084/jem.153.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin M, Snider M D. Role of microtubules in transferrin receptor transport from the cell surface to endosomes and the Golgi complex. J Biol Chem. 1993;268:18390–18397. [PubMed] [Google Scholar]
- 28.Kaufmann A F, McDade J E, Patton C M, Bennett J V, Skaliy P, Feeley J C, Anderson D C, Potter M E, Newhouse V F, Gregg M B, Brachman P S. Pontiac fever: isolation of the etiological agent (Legionella pneumophila) and demonstration of its mode of transmission. Am J Epidemiol. 1981;114:337–347. doi: 10.1093/oxfordjournals.aje.a113200. [DOI] [PubMed] [Google Scholar]
- 29.King C H, Fields B S, Shotts E B, Jr, White E H. Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoebae and human monocyte-like cells. Infect Immun. 1991;59:758–763. doi: 10.1128/iai.59.3.758-763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krinos C, High A S, Rodgers F G. Role of the 25 kDa major outer membrane protein of Legionella pneumophila in attachment to U-937 cells and its potential as a virulence factor in chick embryos. J Appl Microbiol. 1999;86:237–244. doi: 10.1046/j.1365-2672.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 31.LaPenta D, Rubens C, Chi E, Cleary P P. Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Sci USA. 1994;91:12115–12119. doi: 10.1073/pnas.91.25.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marra A, Horwitz M A, Shuman H A. The HL-60 model for the interaction of human macrophages with the Legionnaires’ disease bacterium. J Immunol. 1990;144:2738–2744. [PubMed] [Google Scholar]
- 33.Mauchline W S, Araujo R, Wait R, Dowsett A B, Dennis P J, Keevil C W. Physiology and morphology of Legionella pneumophila in continuous culture at low oxygen concentration. J Gen Microbiol. 1992;138:2371–2380. doi: 10.1099/00221287-138-11-2371. [DOI] [PubMed] [Google Scholar]
- 34.Moffat J F, Edelstein P H, Regula D P, Jr, Cirillo J D, Tompkins L S. Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig model. Mol Microbiol. 1994;12:693–705. doi: 10.1111/j.1365-2958.1994.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 35.Moffat J F, Tompkins L S. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect Immun. 1992;60:296–301. doi: 10.1128/iai.60.1.296-301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman S L, Mikus L K, Tucci M A. Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J Immunol. 1991;146:967–974. [PubMed] [Google Scholar]
- 37.Oelschlaeger T A, Barret T J, Kopecko D J. Some structures and processes of human epithelial cells involved in uptake of enterohemorrhagic Escherichia coli O157:H7 strains. Infect Immun. 1994;62:5142–5150. doi: 10.1128/iai.62.11.5142-5150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oelschlaeger T A, Guerry P, Kopecko D J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ott M, Messner P, Heesemann J, Marre R, Hacker J. Temperature-dependent expression of flagella in Legionella. J Gen Microbiol. 1991;137:1955–1961. doi: 10.1099/00221287-137-8-1955. [DOI] [PubMed] [Google Scholar]
- 40.Payne N R, Horwitz M A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987;166:1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reaven E P, Axline S G. Subplasmalemmal microfilaments and microtubules in resting and phagocytizing cultivated macrophages. J Cell Biol. 1973;59:12–27. doi: 10.1083/jcb.59.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rechnitzer C, Blom J. Engulfment of the Philadelphia strain of Legionella pneumophila within pseudopod coils in human phagocytes. APMIS. 1989;97:105–114. [PubMed] [Google Scholar]
- 43.Relman D, Tuomanen E, Falkow S, Golenbock D T, Saukkonen K, Wright S D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (αMβ2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 44.Rikihisa Y, Zhang Y, Park J. Inhibition of infection of macrophages with Ehrlichia risticii by cytochalasins, monodansylcadaverine, and taxol. Infect Immun. 1994;62:5125–5132. doi: 10.1128/iai.62.11.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rittig M G, Jacoda J C, Wilske B, Murgia R, Cinco M, Repp R, Burmester G R, Krause A. Coiling phagocytosis discriminates between different spirochetes and is enhanced by phorbol myristate acetate and granulocyte-macrophage colony-stimulating factor. Infect Immun. 1998;66:627–635. doi: 10.1128/iai.66.2.627-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rittig M G, Krause A, Häupl T, Schaible U E, Modolell M, Kramer M D, Lütjen-Drecoll E, Simon M M, Burmester G R. Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi. Infect Immun. 1992;60:4205–4212. doi: 10.1128/iai.60.10.4205-4212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rolstad B, Berdel B P. Immune defenses against Legionella pneumophila in rats. Infect Immun. 1981;32:805–812. doi: 10.1128/iai.32.2.805-812.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowbotham T J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoeba. J Clin Pathol. 1980;33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy C R, Berger K H, Isberg R R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 50.Tsuchiya S, Kobayashi Y, Goto Y, Okamura H, Nakae S, Konno T, Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42:1530–1536. [PubMed] [Google Scholar]
- 51.Ward M E, Murray A. Control mechanisms governing the infectivity of Chlamydia trachomatis for HeLa cells: mechanisms of endocytosis. J Gen Microbiol. 1984;130:1765–1780. doi: 10.1099/00221287-130-7-1765. [DOI] [PubMed] [Google Scholar]
- 52.Wiater L A, Dunn K, Maxfield F R, Shuman H A. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect Immun. 1998;66:4450–4460. doi: 10.1128/iai.66.9.4450-4460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson L. Properties of colchicine binding protein from chick embryo brain. Interactions with vinca alkaloids and podophyllotoxin. Biochemistry. 1970;9:4999–5007. doi: 10.1021/bi00827a026. [DOI] [PubMed] [Google Scholar]
- 54.Winn W C, Jr, Davis G S, Gump D W, Craighead J E, Beaty H N. Legionnaires’ pneumonia after intratracheal inoculation of guinea pigs and rats. Lab Investig. 1982;47:568–578. [PubMed] [Google Scholar]
- 55.Winn W C, Jr, Myerowitz R L. The pathology of Legionella pneumonias. A review of 74 cases and the literature. Hum Pathol. 1981;12:401–422. doi: 10.1016/s0046-8177(81)80021-4. [DOI] [PubMed] [Google Scholar]
- 56.Wright S D, Silverstein S C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983;158:2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida S, Mizuguchi Y. Multiplication of Legionella pneumophila Philadelphia 1 in cultured peritoneal macrophages and its correlation to susceptibility of animals. Can J Microbiol. 1986;32:438–442. doi: 10.1139/m86-083. [DOI] [PubMed] [Google Scholar]
- 58.Zambrano M M, Siegele D A, Almirón M, Tormo A, Kolter R. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science. 1993;259:1757–1760. doi: 10.1126/science.7681219. [DOI] [PubMed] [Google Scholar]