Abstract
Background
Although there has developed an increased interest in the vaccines BNT1622b2 (Pfizer/BioNTech), mRNA-1273 (Moderna/NIAID), and ChAdOx1 nCoV-19 (AstraZeneca/University of Oxford), there are still few reports describing the immune response induced by different vaccine platforms in real-world settings of low-income countries. Here, we proposed to analyse the humoral immune response elicited by the primary vaccines used in Argentina from July-December 2021.
Methods
Anti-SARS-CoV-2-Spike-RBD IgG and neutralising antibodies were assayed by ELISA in a total of 871 serum samples obtained from 376 volunteers from an educational staff. The individuals were vaccinated with BBIBP-CorV (Sinopharm), ChAdOx1 nCoV-19 (AstraZeneca/University of Oxford, AZ), Gam-COVID-Vac (Sputnik V, SpV) or combined vaccines (mostly SpV and mRNA-1273, Moderna). The antibody response was analysed several days after the initial vaccination (20, 40, 120 and 180 days).
Results
After receiving at least one dose of the COVID-19 vaccine, we detected 93.34% of seroprevalence. Previously SARS-CoV-2 infected showed higher antibody concentrations compared with naïve vaccinees. Six months after the initial vaccination, combined vaccination induced higher anti-SARS-CoV-2 antibody levels than the other vaccines in naïve volunteers. However, we did not find differences in the neutralising responses after any vaccine from naïve vaccines or between the naïve and previously infected volunteers on day 120 after vaccination.
Conclusions
Our long-term analysis of volunteers from the educational system provides data in a real-world context, showing the benefits of a boost dose still in previously infected volunteers, and suggesting the advantages of a heterologous prime-boost schedule.
Keywords: BBIBP-CorV, Sinopharm, ChAdOx1nCoV-19, AstraZeneca, Gam-COVID-Vac, Sputnik V
Abbreviations: AZ, AstraZeneca; SpV, Sputnik V
1. Introduction
In the COVID-19 pandemic, vaccines have once again demonstrated to be an effective public health strategy. Since the beginning of the pandemic, there has been a clear political and scientific tendency to develop several vaccines within a short time to control the overwhelming effects of the SARS-CoV-2 infection [1].
The vaccination for COVID-19 in Argentina started on 29 December 2020. As of early February 2022, 76.5 % of the population has been fully vaccinated. To date, seven vaccines have been approved for use in Argentina. Non-replicating adenovirus vector vaccines Gam-COVID-Vac (Sputnik V, SpV), ChAdOx1nCoV-19 (AstraZeneca/University of Oxford, AZ; or AstraZeneca/Serum Institute of India, Covishield), and Ad5-nCoV (CanSino), the inactivated SARS-CoV-2 (whole virus) vaccine (BBIBP-CorV, Sinopharm), and the mRNA vaccines mRNA-1273 (Moderna) and BNT162b2 (Pfizer) [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] have been widely applied. Vaccine efficacy has been demonstrated in many clinical trials. Recently, Rearte et al. (2022) showed that Gam-COVID-Vac and ChAdOx1nCoV-19 used in Argentina effectively reduce infection and death by SARS-CoV-2 and COVID-19 [14]. However, reports in real-world contexts are still necessary. Analyses of the immune response induced by different vaccine platforms are essential to improve the control of the COVID-19 pandemic.
Many studies have explored the waning or persistence of antibodies elicited from other different vaccines platforms [6], [8], [9], [15], [16], [17], [18], [19], [20], [21]. In Argentina, recently, only two works by Chahla et al. [22] and Gonzalez Lopez Ledesma et al. [23] have followed 118 and 602 healthcare workers from Tucumán or Buenos Aires, respectively, after vaccination with Sputnik V. Evaluation of how local populations respond to the different vaccines applied in developing countries such as Argentina is fundamental for improving the public healthcare measures in the pandemic.
San Luis, one of the 23 provinces of Argentina, located west of the country, has been one of the most affected by the pandemic, with more than 145 000 infections and 1527 COVID deaths in a population of 502 003 inhabitants. Its capital, the homonymous San Luis, and Villa Mercedes are the most populous cities in the province. Since the National University of San Luis has its main schools in San Luis and Villa Mercedes, our study included volunteers from these cities.
In the present work, we examined the anti-S/RBD antibody response induced by the primary vaccines used in Argentina in 2021 in a cohort of 376 volunteers from the educational staff of the National University of San Luis, Argentina. Our second objective was to study the IgG antibody response of naïve or previously SARS-CoV-2 infected volunteers at five-time points after the initial vaccination. Since neutralising antibodies are associated with protective immunity, as the third objective, we evaluated the neutralising capacity of the antibodies over time by using a commercial kit that uses a well-established ELISA technology. We hypothesize that different platform COVID-19 vaccines induce similar protective humoral immune responses over time. Our results may contribute to the documentation concerning the humoral response after COVID-19 vaccination in a real-world scenario.
2. Methods
2.1. Population studied
Participants were recruited through advertisements posted on the institutional website of the National University of San Luis. During the early stages of this study, the only vaccines approved for use in Argentina were the Sputnik V, Sinopharm, Covishield and AstraZeneca, while Moderna, CanSino and Pfizer vaccines were approved later in the year for heterologous vaccination. In July 2021, 500 volunteers aged 18 to 70 years old of the educational community, administrative and general service staff immunised with Sinopharm, Sputnik V (SpV), AstraZeneca (AZ) vaccine or combined vaccination were enrolled. Blood samples were obtained at 20, 40, 80, 120 and 180 days after initial vaccination between July and December 2021. All participants provided written Informed Consent prior to collecting data and specimens.
Moreover, the participants completed questionnaires regarding demographic information, medical history, and risk factors for SARS-CoV-2 exposure. Participants self-reported any personal history of immunocompromise, lung disease, cardiovascular disease, diabetes, and symptoms and characteristics of the COVID-19 clinical course. Side effects after vaccination were also screened and classified as mild (pain at injection site, fever, headache, fatigue, general malaise, dizziness, gastrointestinal symptoms) or severe (any effect requiring hospitalisation). Personal data from all volunteers were encrypted. The inclusion criteria were: individuals of the institution enlisted to receive a COVID-19 vaccine and who signed the Informed Consent. The exclusion criterium was: individuals with current COVID-19 diagnosis or symptoms. The Ethical Committee of the San Luis Hospital (San Luis, Argentina) approved this study. A total of 871 serum samples from 376 volunteers (this number from 500 enrolled participants corresponds to a 75.2 % response) were analysed. From the total cohort (N = 376), the number of participants who provided samples for each time point is indicated in Table 1 . Depending on the analysis to be performed, the samples were classified according to the vaccine they received (Sinopham, AZ, SpV or combined vaccination) and subdivided into groups of volunteers without (naïve) or with previous SARS-CoV-2 infection (infected) defined by diagnosis RT-PCR or rapid antigen tests. Individuals infected during the study (N = 17) were excluded from the analysis (Table 1).
Table 1.
Demographics of the volunteer population studied.
 |
 |
1Close Contact: Someone who was within 6 feet of an infected person for a total of 15 min or more over a 24 h period.
2HIV (1), Multiple sclerosis (1), Allergy (1).
3Mild: pain at the injection site, fever, fatigue, headache, muscle pain, chills and diarrhea.
2.2. Blood and serum recollection
Blood was collected by venipuncture into polypropylene tubes with silica particles to accelerate clotting (Tecnon, Buenos Aires, Argentina). For serum recollection, the blood was incubated for 1 h at room temperature and later centrifugated at 3000 rpm. Three aliquots of 200 ul were recollected and stored at −20 °C until antibody determination.
2.3. Anti-SARS-CoV-2 IgG antibodies measurements
The concentration of IgG antibodies specific to the SARS-CoV-2 spike and receptor-binding domain (RBD) was performed with the COVIDAR IgG ELISA kit (Lemos Laboratory SRL, Buenos Aires, Argentina) according to the manufacturer's instructions [24]. Briefly, serum samples were diluted in sample diluent and added to the wells coated with a mixture of spike and RBD and incubated for 1 h at 37 °C. After three washes with a Microplate well washer (BioRad, Hercules, CA, USA), horseradish peroxidase (HRP)-conjugated anti-human IgG antibodies were added to the wells and incubated for 30 min at 37 °C. The peroxidase reaction was visualized by incubating the plates with TMB solution for 30 min at 37 °C. Stop solution was added, and the optical density (OD) was measured at 450 nm in an ELISA microplate reader (EPOCH, BioTek Instruments GmbH, Bad Friedrichshall, Germany). We defined seropositivity using a cut-off value calculated following the manufacturer's instructions. The SARS-CoV-2 antibody concentration of each sample expressed in International Units/ml (IU/ml) was calculated by extrapolating the value on a calibration curve. This calibration curve was made with the OD450 nm of the serial dilutions of a standard of 400 IU/ml adjusted according to the first WHO International Standard for anti-SARS-CoV-2 immunoglobulin and provided by the commercial kit (NIBSC Code 20/136). The linear range used was an OD450 of 0.1 to 2. The sensibility of the assay was 4.03 IU/ml and its specificity was 100 %.
2.4. Determination of neutralising anti-SARS-Cov-2 antibodies
The determination of neutralising antibodies was performed with the commercially available ELISA NeutraLISA SARS-CoV-2 kit (Euroimmun, Lübeck, Germany) on serum samples according to the manufacturer's instructions. Samples were diluted in a sample buffer containing soluble biotinylated angiotensin-converting enzyme 2 (ACE2) and incubated for 1 h at 37 °C in the plate wells coated with recombinant S1/RBD domain of the Spike protein from SARS-CoV-2. During this time, the neutralising antibodies present in the sample compete with the ACE2 receptor for the binding sites of the S1/RBD proteins. The biotinylated ACE2 bound to S1/RBD was detected by incubating the wells with peroxidase-labelled streptavidin for 30 min at room temperature. The peroxidase reaction was visualized with TMB solution for 15 min at room temperature. Finally, a stop solution was added, and OD was measured at 450 nm. The results were calculated as a percentage of inhibition (%IH), using the following equation: %IH = 100 % – (extinction patient sample × 100 %/extinction of blank mean).
2.5. Statistical analysis
All statistical tests and plots were performed using GraphPad Prism 8.0 software (GraphPad, San Diego, CA, USA). Geometric means and 95 % confidence intervals of the antibody levels at each time point were calculated in each vaccine group. The normality of the data distribution was analysed by the Shapiro-Wilk test. One-way ANOVA with Tukey's multiple or Kruskal-Wallis comparisons test was used to assess differences among the vaccines after the first dose, the age groups per vaccine, the kinetic analysis or the neutralising antibodies. At the same time, a two-way ANOVA analysis was used to evaluate differences associated with sex or age. Comparisons of the antibody concentration of naïve vs previously infected volunteers were made using multiple t-tests. Due to little data on 20 days, the statistical analysis could not be performed at this time point; however, the data on day 20 were included in Fig. 4. Comparison of neutralising antibody levels on 40 vs 120 or 180 days after the first dose were made using a paired t-test and between naïve and previously infected volunteers using Mann Whitney U test. Statistical significance is shown in the figure legends with the following notations: asterisks for differences between naïve and previously infected volunteers, or among vaccinations (*p < 0.05; **p < 0.01; ***p < 0.001, ****p < 0.0001); numeral for differences among the time points in each vaccination (##p < 0·01); ns: not significant.
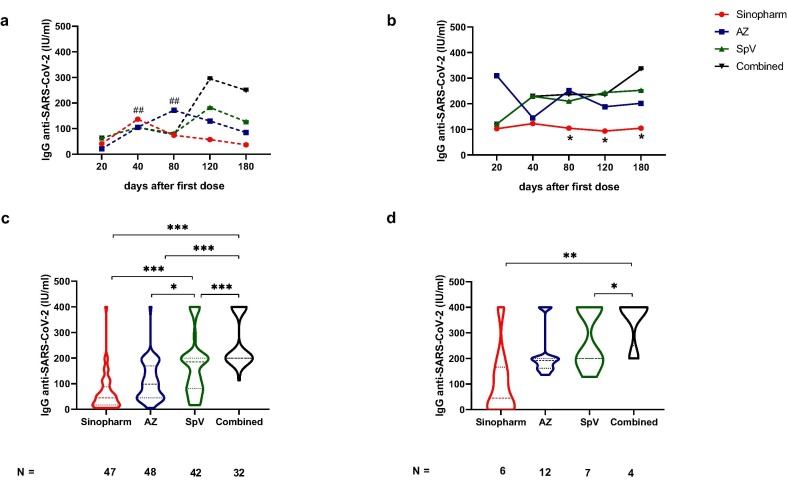
Fig. 4.
Anti-SARS-CoV-2 antibody levels in subjects with different COVID-19 disease statuses and vaccination schemes. Kinetics of IgG anti-SARS-CoV-2 measures in naive (a) and infected (b) vaccinees according to time of examination (20, 40, 80, 120 and 180 days after the first dose of Sinopharm, AstraZeneca (AZ), Sputnik V (SpV) or Combined vaccines. IgG levels on day 180 after initial vaccination in naïve (c) and in previously infected individuals (d). Statistical analysis was performed with one-way ANOVA with Tukey's multiple comparisons tests. Geometric media ± SD are also shown. In Fig. 4 a, numeral, in Sinopharm vaccination kinetic, ##p < 0·01, day 40 vs 120 or 180; in AZ vaccination kinetic, ##p < 0·01, day 80 vs 180. Fig. 4 b-d, asterisk *p < 0·05; **p < 0·01; ***p < 0·001 comparison among vaccines.
3. Results
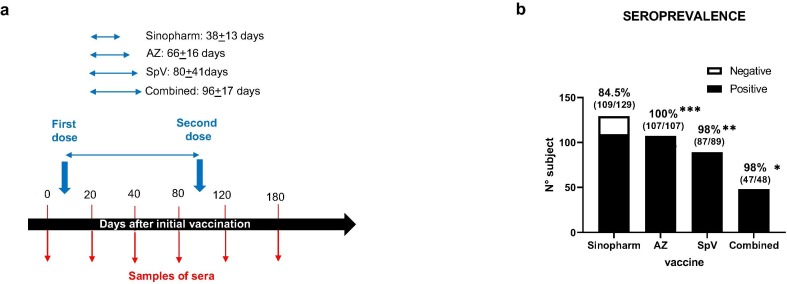
Our study included 376 participants who had received at least one dose of the vaccines used in Argentina during the initial vaccination campaign in 2021. Our population was overrepresented by individuals with high educational levels (65 % had a university education level). Table 1 shows the demographic data of the volunteer population. The distribution across broad age categories was similar in the participants who received the different vaccines. No significant differences were found between the groups concerning the values generated when immunised with the different vaccines and blood types or sex after the second dose. The antibody response was analysed after the initial vaccination on days 20, 40, 80, 120 and 180, as represented in Fig. 1 a.
Fig. 1.
Antibody response to COVID-19 vaccine. (a) Scheme vaccination diagram of the volunteer population studied. We indicate the dosage regimen, the dosing interval for each vaccine studied and the days when serum samples were extracted for antibody determination. (b) Seroprevalence of anti-SARS-CoV-2 antibodies among the population included in this study who had received the indicated vaccines. The graph shows the proportion of antibody-positive (black) and antibody-negative (white) cases at least 20 days after the first dose of each vaccine. Percentages indicate seropositive rate (defined as a positive antibody level any time after vaccination), whereas numbers represent the proportion of subjects with anti-SARS-CoV-2 IgG antibodies in total for each vaccine. *p < 0·05; **p < 0·01; ***p < 0·001.
Anti-SARS-CoV-2 antibodies were detected in 350 out of 376 volunteers receiving at least one dose of COVID-19 vaccine assessed at a time point of more than 20 days (seroprevalence 93.34 %). Overall, 84.5 % (109/129) participants who received Sinopharm, all participants who received AZ (107/107, 100 %), all but two immunised with Sputnik V (87/89, 98 %), and all but one participant with combined vaccination (47/48, 98 %) resulted positive in at least one sample after initial vaccination (Fig. 1b). These prevalence differences were significantly higher for AZ, Sputnik V and combined vaccination compared with Sinopharm (Fig. 1b).
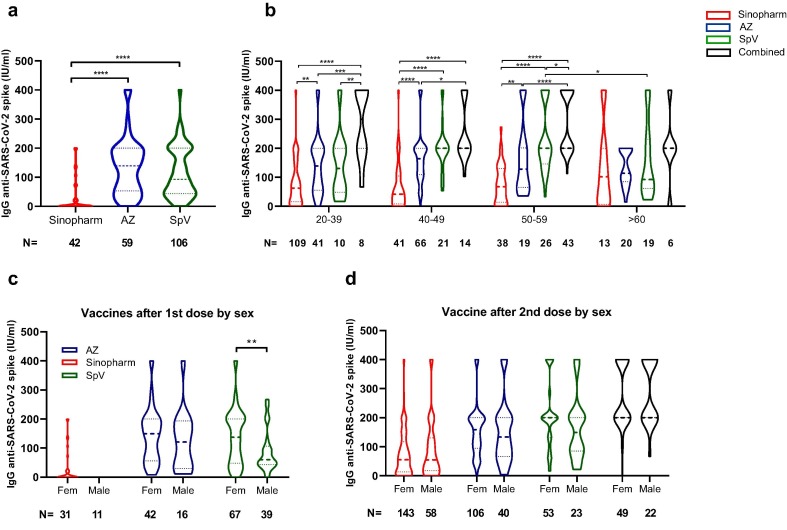
When evaluating the antibodies produced in the volunteers after the first dose of the applied vaccine, we can observe that both AZ and SpV produced higher IgG levels than the volunteers who received Sinopharm (Fig. 2 a). This observation agrees with the seroprevalence value found in volunteers vaccinated with Sinopharm (Fig. 1b).
Fig. 2.
Anti-SARS-Cov2 antibodies after first or second dose. The differences in the level of anti-SARS-Cov2 antibodies found in volunteers who received the first dose of Sinopharm, AstraZeneca (AZ) or Sputnik V (SpV) are plotted (a). The concentration of antibodies detected in the volunteers who received two doses of different vaccines (Sinopharm, AZ, SpV, Combined) is plotted concerning age (b). The differences in the levels of antibodies for each vaccine relating to the sex of the volunteers were also plotted, taking into account the values obtained after the first dose (c) or the second dose (d). Statistical analysis was performed using a one-way ANOVA to evaluate differences among vaccines after the first dose or among age groups per vaccine, and two-way ANOVA for comparisons considering the sex or age of the volunteers for each vaccine. Comparison t-test was used. Asterisk *p < 0·05; **p < 0·01; ***p < 0·001; ****p < 0·0001.
On the other hand, we evaluated the concentration of antibodies produced by the different vaccines (Sinopharm, AZ, SpV and those combined) concerning the age of the volunteers. As a result, we found that the lowest antibody response generated by volunteers vaccinated with Sinopharm is maintained in the age range 20–59 years (Fig. 2b). Besides, the combined vaccines were the ones that produced a higher antibody response in all the ranges studied. Moreover, volunteers who were 60 or older vaccinated with Sputnik V showed a reduced response compared with the group of 50–59 years (Fig. 2b). Notably, when we determined the values produced by volunteers over 60, no significant differences were found between the different vaccines (Fig. 2b). In addition, when the influence of sex on antibody production was evaluated after applying the first dose, we found that women who received Sputnik V generated higher IgG levels than men (Fig. 2c). This difference had not been previously reported for this vaccine, but it should be noted that it is lost after applying the second dose (Fig. 2d).
Among the volunteers who participated in the study, 73 individuals declared having been infected (verified diagnosis with antigen test or PCR). In 52 % of this population, the time elapsed between diagnosis and the first dose of vaccinations was more than 120 days. In addition, 17 contracted the virus during the study: 10 after the first dose (five received AZ, four SpV and one combined vaccination) and seven after the second dose (three Sinopharm, one AZ, two SpV, and one combined vaccination). The data on the antibody level of the infected volunteers after vaccination were removed from the analysis.
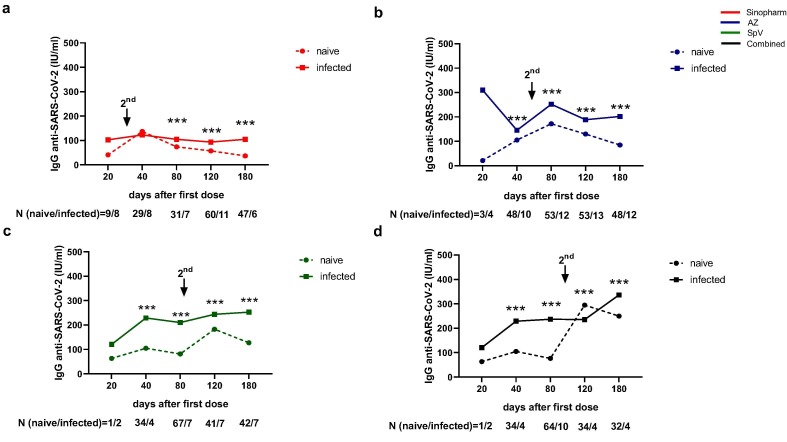
We compared the kinetic of the IgG anti-SARS-CoV-2 levels in naïve and previously infected volunteers of our cohort. We found that previous SARS-CoV-2 exposition increased the antibody concentration after vaccination with the analysed vaccines (Fig. 3 a-d). Interestingly, a second dose was a booster in naïve and previously infected volunteers in all vaccinations except in previously infected participants vaccinated with Sinopharm (Fig. 3a). The anti-SARS-CoV-2 antibody level diminished in naïve volunteers (p < 0·001, compared day 40 vs 120 or 180 after Sinopharm vaccination; p < 0·001 compared day 80 vs 180 after AZ vaccination) (Fig. 4a). In contrast with naïve volunteers (Fig. 4a), when we compared the antibody response in the kinetic analysis in those infected before vaccination, we found that Sinopharm induced significantly lower antibody levels than the other vaccination on days 80, 120 and 180 (Fig. 4b). In the same line, six months after initial vaccination, the anti-SARS-CoV-2 antibody level was significantly lower after Sinopharm immunisation when compared with the other vaccines in naïve (Fig. 4c). Interestingly, on day 180, combined vaccination induced a higher antibody level than all vaccines in naïve volunteers (Fig. 4c) or than Sino and SpV vaccinated participants who had been previously infected (Fig. 4d).
Fig. 3.
Differential kinetics of anti-SARS-CoV-2 antibodies elicited by different COVID-19 vaccines. Graphs show IgG anti-SARS-CoV-2 concentration, measured by ELISA, at 20, 40, 80, 120 and 180 days after the first dose of Sinopharm (a), AstraZeneca (AZ) (b), Sputnik V (SpV) (c) vaccines, or in the case of Combined vaccination (d). Under each graph is indicated the proportion of individuals infected (infected) or not (naive) with SARS-CoV-2 at each time point of antibody determination; it is also represented the time at which the second dose was administered. Statistical analyses were performed with multiple t-tests. *** p < 0·0001.
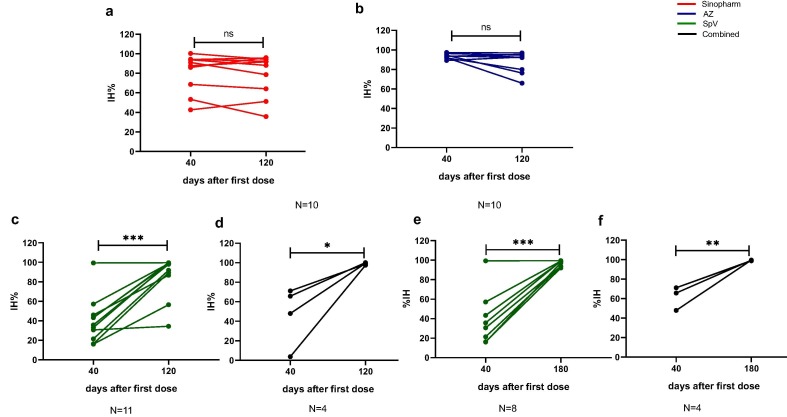
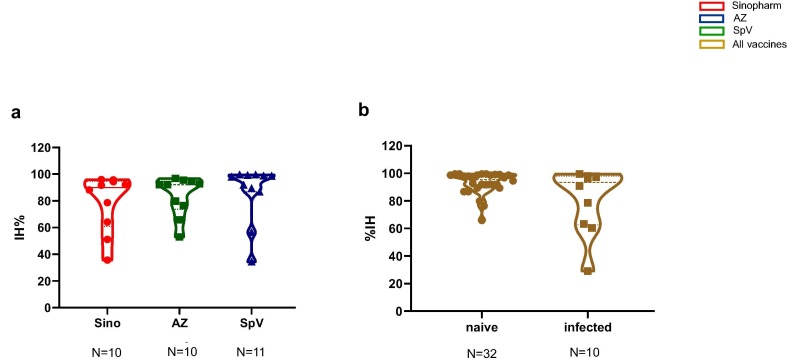
Despite the anti-SARS-CoV antibody waning on day 120 after Sino or AZ vaccination, we noted the persistence of neutralising antibody activity (Fig. 5 a and b). Due to the different intervals between the doses, both SpV and combined vaccination increased both anti-SARS-CoV concentration and neutralising antibodies (Fig. 5c and d). Therefore, we analysed the neutralising activity on day 180, corresponding to three months after the second dose, and surprisingly, we also observed an increase in neutralising antibody activity at this time (Fig. 5e and f). Remarkably, we did not find differences in the neutralising responses after any vaccine from naïve vaccinees or between the naïve and previously infected volunteers on day 120 after vaccination (Fig. 6 a and b).
Fig. 5.
Long-term analysis of neutralising activity of antibody response. SARS-CoV-2 neutralising antibodies of Sinopharm (a), AstraZeneca (AZ, b), Sputnik V (SpV, c and d) and Combined vaccines (e and f). Each circle represents one vaccinee (40 days after the first dose and 120 or 180 days of follow-up). The results were calculated as a percentage of inhibition (%IH), as detailed in the materials and methods section. Asterisk *p < 0·05; **p < 0.01; ***p < 0·001 by paired t-test. ns: not significant.
Fig. 6.
Neutralising activity of antibody response elicited by the vaccine in naïve and previously infected volunteers. SARS-CoV-2 neutralising antibodies of Sinopharm, AstraZeneca (AZ), Sputnik V (SpV) and combined vaccines in naïve (a) or previously infected (b) volunteers. Each circle represents one vaccinee 120 days after the initial vaccination. The results were calculated as a percentage of inhibition (%IH), as detailed in the materials and methods section. One-way ANOVA with Kruskal-Wallis comparisons and Mann Whitney U tests were used in a and b, respectively. No significant differences were found.
4. Discussion
We have studied the anti-SARS-CoV-2 antibody response after immunisation with the vaccines available in 2021 in Argentina in volunteers of an educational cohort in San Luis, Argentina. The high seroprevalence detected for each vaccine (93·34 %) confirms findings from previous studies [2], [3], [4], [5], [6], [7], [8], [9] as well as reports in health workers from Argentina vaccinated with Sputnik V [22], [23], [25]. Strengths of the present study include the long-term analysis (until 180 days) of the humoral immune response after three COVID-19 vaccines, including heterologous prime-boost schedule, in a real-world context. Moreover, the proportion of vaccines used in our population was similar to that of the general population of Argentina in 2021. It is also important to note that no severe side effects were reported in the volunteers (Table 1).
In contrast to other reports that have studied the anti-SARS-CoV-2-antibody response on vaccinees from the public healthcare personnel, we focused our analysis on volunteers from the educational system. We compared the immune response of naïve volunteers and those infected before vaccination. In this regard, 73 (19.4 %) of the 376 recruited participants reported SARS-CoV-2 infection before vaccination, reflecting the pandemićs impact on an educational cohort of Argentina.
After the first dose, we found higher anti-Spike/RBD IgG levels in the volunteers who received AstraZeneca or Sputnik V than those who received Sinopharm (Fig. 2a). In the same line, different immune responses between one dose and two doses, and after the adenoviral-vectored vaccines versus the whole-cell inactivated vaccine have been suggested [26]. Consequently, the 21–28 days interval between doses has been recommended for Sinopharm, and an increased interval has been suggested for the AstraZeneca vaccine [27]. The same strategy was extended to other vaccine platforms.
Our study comprised vaccinated individuals between 20 and 70 years old, mostly female (69.4 %) (Table 1). Interestingly, we found that women who received the first dose of Sputnik V generated higher IgG levels than men (Fig. 2c), but this difference was lost after the second dose (Fig. 2d). Consistent with this result, sex differences following common vaccines have been reported; more precisely, women developed higher antibody responses and more adverse effects than males after, for example, yellow fever virus or seasonal influenza vaccination [28]. On the other hand, in the volunteers segregated by age in groups until 60 years old, we found the lowest and highest antibody response by Sinopharm and combined vaccination, respectively. Nevertheless, there are no differences between the antibodies produced by more than 60 years old volunteers vaccinated with Sputnik V and those who received other vaccines (Fig. 2b), supporting the immune response to vaccines influenced by ageing [29]. Therefore, the continuity with different vaccination strategies depending on each age segment will assure an adequate immune response at the individual level.
The kinetics of anti-S/RBD antibodies up to 180 days after initial vaccination were different in the vaccinees with Sinopharm, AstraZeneca, Sputnik V or combined vaccination (mostly SpV/Moderna). These data are in line with the different vaccine platforms and the timing of the booster dose to ensure protection before protection has waned. However, one limitation of our study is that we did not evaluate cellular immunity. Here, we confirm that previously infected individuals reached higher IgG antibody levels than naïve participants, paralleling regional studies for the Sputnik V vaccine in healthcare personnel [22], [23], [24]. Indeed, in individuals vaccinated with BNT162b2 (Pfizer/BioNTech), it has been reported that the first vaccine dose after previous infection or second vaccine dose in individuals who are SARS-CoV-2 naïve boosts memory B cells [30]. In addition, we observed a boost effect of the second dose in previously infected individuals vaccinated with any vaccines but Sinopharm. Besides, Sinopharm induced significantly lower antibody levels than the other vaccination in previously infected volunteers (Fig. 4b). The natural anti-SARS-CoV-2 immunity might attenuate the response to a booster dose of the Sinopharm vaccine. However, the interval between infection and boosting was more than three months in the majority (11 out of 17, 65 %) in this group of vaccinees, suggesting the natural humoral response waning. Therefore, this finding will require further investigation. Homologous prime-boost with equal viral-vectored vaccines may be limited by anti-vector immunity; however, heterologous prime-boost immunisation plans, such as that used with Sputnik V or based on combinations of different vaccine platforms, overcome this complication [26]. Regarding this issue, we detected that combined vaccination induced the highest antibody level.
In contrast with previously infected volunteers, the kinetics of humoral response elicited by each vaccine showed that anti-S/RBD IgG response in naïve individuals began to drop on day 120 (Fig. 4a). However, 92.1 % of the participants showed positive responses on day 180. This finding agrees with other regional longitudinal works [21], [22].
The majority of the COVID-19 vaccines were designed to induce a protective immune response mediated by neutralising antibodies against the Spike protein. Neutralising antibodies hamper the binding of Spike proteińs receptor-binding domain (RBD) to the ACE2 receptor on the host cell [26]. In this study, to evaluate neutralising antibodies, we used a well-established ELISA commercial kit that imitates the natural inhibitory effect of these antibodies. Interestingly, our longitudinal work showed that neutralising antibodies maintained or increased over time after vaccination of naïve or previously infected individuals vaccinated with any studied vaccine. These results suggest antibody maturation and preservation of the protective capacity of the humoral immune response. Similarly, a recent study showed a temporal increase in the neutralising antibodies after vaccination with Sputnik V [22]. However, one limitation of our study is that neutralising responses against the SARS-CoV-2 new variant were not analysed. It is also important to note that we found no differences in the neutralising responses after any vaccine from naïve vaccinees or between the naïve and previously infected volunteers on day 120 after vaccination. Though, surrogate neutralisation tests were less suitable for detecting specimens with neutralising activity by the assays that work with live viruses, which have been documented in several studies [31], [32], [33]. Therefore, the surrogate neutralisation tests may be less suitable for distinguishing between high and very high neutralising antibodies. Furthermore, future research could subsequently be tested by the reference-standard neutralising SARS-CoV-2 test for quantifying antibodies.
5. Conclusions
Overall, our data provide information in a real-world scenario concerning the persistence of the antibody response after the vaccines were applied in 2021 in naïve and previously infected individuals. Our findings contribute to defining the future routine immunisation schedules in developing countries.
Funding
This work was supported by the National University of San Luis (Res. 986, 2021), Dirección de Obra Social del Personal Universitario (DOSPU), the National Agency for Promotion of Science and Technology (PICT-2017-2828), and the National University of San Luis (PROICO-02-1218).
CRediT authorship contribution statement
Ricardo Javier Eliçabe: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Matías Nicolás Distel: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. Brenda Lucila Jofré: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – review & editing. Marianela Leporati: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. Juan Eduardo Silva: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. José Luis Arias: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. Carolina Virginia Gorlino: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Samanta Celeste Funes: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Marisol Velazquez: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Patricia Vitale: Conceptualization, Methodology, Writing – review & editing. Roberto Carlos Davicino: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. María Silvia Di Genaro: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to the volunteers of the National University of San Luis, San Luis, Argentina. MD, BLJ, ML, SCF, JLA and MV have fellowships of the National Council of Scientific and Technical Investigations (CONICET). RJE, CVG, RCD and MSDG are members of the Scientific Career of CONICET. We are grateful to the Gabinete de Escritura Científica (GAECI) of the Universidad Nacional de San Luis for improving the English language of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.11.019. These data include Google maps of the most important areas described in this article.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
The following KML file contain the Google maps of the most important areas described in this article.
Data availability
All available data of the vaccines and demographics of the volunteer population studied were included in the manuscript.
References
- 1.Pollard A.J., Bijker E.M. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatulin A.I., Shcheblyakov D.V., Dzharullaeva A.S., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA. 2020;324(10):951. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halperin S.A., Ye L., MacKinnon-Cameron D., Smith B., Cahn P.E., Ruiz-Palacios G.M., et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet. 2022;399(10321):237–248. doi: 10.1016/S0140-6736(21)02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. [DOI] [PMC free article] [PubMed]
- 10.Abbasi J. COVID-19 Vaccine Combination Was Superior in Real-world Study. JAMA. 2021;326(22):2250. doi: 10.1001/jama.2021.21715. [DOI] [PubMed] [Google Scholar]
- 11.Shaw R.H., Stuart A., Greenland M., Liu X., Nguyen Van-Tam J.S., Snape M.D. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397(10289):2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X., Shaw R.H., Stuart A.S.V., Greenland M., Aley P.K., Andrews N.J., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillus D., Schwarz T., Tober-Lau P., Vanshylla K., Hastor H., Thibeault C., et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respiratory Medicine. 2021;9(11):1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rearte A., Castelli J.M., Rearte R., Fuentes N., Pennini V., Pesce M., et al. Effectiveness of rAd26-rAd5, ChAdOx1 nCoV-19, and BBIBP-CorV vaccines for risk of infection with SARS-CoV-2 and death due to COVID-19 in people older than 60 years in Argentina: a test-negative, case-control, and retrospective longitudinal study. Lancet. 2022;399(10331):1254–1264. doi: 10.1016/S0140-6736(22)00011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widge A.T., Rouphael N.G., Jackson L.A., Anderson E.J., Roberts P.C., Makhene M., et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med. 2021;384(1):80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naaber P., Tserel L., Kangro K., Sepp E., Jürjenson V., Adamson A., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Regional Health - Europe. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favresse J., Bayart J.-L., Mullier F., Elsen M., Eucher C., Van Eeckhoudt S., et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerging Microbes Infect. 2021;10(1):1495–1498. doi: 10.1080/22221751.2021.1953403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grupel D., Gazit S., Schreiber L., Nadler V., Wolf T., Lazar R., et al. Kinetics of SARS-CoV-2 anti-S IgG after BNT162b2 vaccination. Vaccine. 2021;39(38):5337–5340. doi: 10.1016/j.vaccine.2021.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E., et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398(10298):385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagotto V., Ferloni A., Soriano M.M., Díaz M., Golde M.B., González M.I., et al. Active Surveillance Of The Sputnik V Vaccine In Health Workers. Medicina (B Aires) 2021;81:408–414. [PubMed] [Google Scholar]
- 21.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chahla R.E., Tomas-Grau R.H., Cazorla S.I., Ploper D., Vera Pingitore E., López M.A., et al. Long-term analysis of antibodies elicited by SPUTNIK V: A prospective cohort study in Tucumán, Argentina. Lancet Regional Health - Americas. 2022;6:100123. doi: 10.1016/j.lana.2021.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez Lopez Ledesma MM, Sanchez L, Ojeda DS, Rouco SO, Rossi AH, Varese A, et al. Longitudinal Study after Sputnik V Vaccination Shows Durable SARS-CoV-2 Neutralizing Antibodies and Reduced Viral Variant Escape over Time. mBio 2022;13(1):e0344221. [DOI] [PMC free article] [PubMed]
- 24.Ojeda DS, Gonzalez Lopez Ledesma MM, Pallarés HM, Costa Navarro GS, Sanchez L, Perazzi B, et al. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLOS Pathogens. 2021;17:e1009161. [DOI] [PMC free article] [PubMed]
- 25.Rossi A.H., Ojeda D.S., Varese A., Sanchez L., Gonzalez Lopez Ledesma M.M., Mazzitelli I., et al. Sputnik V vaccine elicits seroconversion and neutralizing capacity to SARS-CoV-2 after a single dose. Cell Reports Med. 2021;2(8):100359. doi: 10.1016/j.xcrm.2021.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischinger S., Boudreau C.M., Butler A.L., Streeck H., Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41(2):239–249. doi: 10.1007/s00281-018-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bajaj V., Gadi N., Spihlman A.P., Wu S.C., Choi C.H., Moulton V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front Physiol. 2021;11 doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination. Sci Immunol. 2021;6(58) doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsoussi W.B., Turner J.S., Case J.B., Zhao H., Schmitz A.J., Zhou J.Q., et al. A Potently Neutralizing Antibody Protects Mice against SARS-CoV-2 Infection. J Immunol. 2020;205(4):915–922. doi: 10.4049/jimmunol.2000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.-T., et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369(6506):956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valcourt E.J., Manguiat K., Robinson A., Chen J.-Y., Dimitrova K., Philipson C., et al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Diagn Microbiol Infect Dis. 2021;99(4):115294. doi: 10.1016/j.diagmicrobio.2020.115294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All available data of the vaccines and demographics of the volunteer population studied were included in the manuscript.