Abstract
Infection of interleukin-10 (IL-10)-nonexpressing (IL-10−/−) mice with Plasmodium chabaudi chabaudi (AS) leads to exacerbated pathology in female mice and death in a proportion of them. Hypoglycemia, hypothermia, and loss in body weight were significantly greater in female IL-10−/− mice than in male knockout mice and all wild-type (WT) mice during the acute phase of infection. At this time, both female and male IL-10−/− mice produced more gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-12p40 mRNA than their respective WT counterparts. Inactivation of IFN-γ in IL-10−/− mice by the injection of anti-IFN-γ antibodies or by the generation of IL-10−/− IFN-γ receptor−/− double-knockout mice resulted in reduced mortality but did not affect body weight, temperature, or blood glucose levels. The data suggest that IFN-γ-independent pathways may be responsible for these pathological features of P. chabaudi malaria and may be due to direct stimulation of TNF-α by the parasite. Since male and female knockout mice both produce more inflammatory cytokines than their WT counterparts, it is likely that the mortality seen in females is due to the nature or magnitude of the response to these cytokines rather than the amount of IFN-γ or TNF-α produced.
Inflammatory cytokines have been implicated in the pathology accompanying Plasmodium infections in humans (18, 32) and in animal models (6, 8, 15, 25). In addition to fever, in Plasmodium falciparum infections particularly, there are several other severe complications of infection such as anemia, hypoglycemia, renal failure, and cerebral malaria (27, 32). Parasite components such as glycophospholipid anchors released at schizont rupture are able to induce macrophages and γδ T cells to produce tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and other cytokines (14, 26, 46). Treatment of infected children with anti-TNF-α antibodies reduces body temperature, suggesting that TNF-α induction following schizont rupture may be responsible for the periodic fever characteristic of a malaria infection (31, 51). In addition, high levels of circulating TNF-α indicate a poor prognosis in cerebral malaria (CM) (18, 32) and are also significantly associated with severe anemia (18, 30, 31, 43).
There is no rodent infection that mimics all the severe symptoms of P. falciparum malaria. In susceptible mouse strains, P. berghei (ANKA) induces a form of CM (45), and development of neurological complications in this model is dependent on TNF-α, IFN-γ, and T cells (15–17). P. chabaudi, P. vinckei, and P. yoelii infections in mice all exhibit other features of malarial disease such as anemia and hypoglycemia (7–9, 50). The exact involvement of inflammatory cytokines in these pathogenic processes is not clear.
Interleukin-10 (IL-10) is important in the down-regulation of inflammatory responses, and it has been shown that a low plasma concentration of IL-10 correlates with the occurrence of CM and anemia in P. falciparum infections (30, 43). In gene-targeted mice in which the IL-10 gene has been inactivated (IL-10−/− mice), there is an excessive production of IFN-γ, TNF-α, and IL-12 (13, 23, 41) in a variety of infections, and there is an increase in mortality rate among female IL-10−/− mice infected with P. chabaudi (35).
In the studies reported here, we examined in detail the effects of an IL-10 defect in mice during a P. chabaudi infection on the production of inflammatory cytokines, body temperature, loss of weight, and development of hypoglycemia. In vivo neutralization of IFN-γ, either by antibody depletion or by inactivation of the IFN-γ receptor (IFN-γR), in the malaria-associated pathology did not ameliorate these symptoms of a P. chabaudi infection but did reduce mortality. Our data therefore suggest that hypoglycemia, loss of body weight, and changes in body temperature may be independent of IFN-γ production.
MATERIALS AND METHODS
Mice and parasites.
IL-10−/− mice (33) on a mixed background of 129sv and C57BL/6 (BL6 × 129sv mice) were obtained from W. Müller (Institut für Genetik, Köln, Germany) and were bred in positive-pressure isolators in the animal facilities at Imperial College, London, United Kingdom. IL-10−/− mice backcrossed six times onto C57BL/6 were purchased from B&K (Hull, United Kingdom), backcrossed further onto a C57/BL6 background (generating N7BL/6 mice), and maintained by interbreeding heterozygous females with heterozygous or homozygous (IL-10+/− or IL-10−/−) males. IL-10−/− IFN-γR−/− double-knockout mice were generated by interbreeding mixed-background (BL6 and 129sv) IL-10−/− and IFN-γR−/− mice (22), obtained from B&K. For experimental work, IL-10−/−, IFN-γR−/−, and wild-type (WT) littermates were used as controls. The defective IL-10 and IFN-γR genes were detected by PCR of tail DNA, using specific primers IL-10 sense (5′-TAGGCGAATGTTCTTCC-3′), IL-10 antisense (5′-CAGGCATAGCATGCTG-3′), neo-antisense (5′-CTTGCGTGCAATCCATCTTG-3′), IFN-γR sense (5′-AGATCCTACATACGAAACATACGG-3′), and IFN-γR antisense (5′-TCATCATGGAAAGGAGGGATACAG-3′), as described previously (22, 33, 35). All mice were maintained in isolators with sterile bedding, food, and water. For experiments with either mixed-background mice or backcrossed mice, heterozygous or WT littermates were used as controls. In the double-knockout experiments, littermate IL-10−/− and IFN-γR−/− single-knockout mice were also used as controls.
P. chabaudi chabaudi (AS) parasites were originally obtained from K. N. Brown (National Institute for Medical Research, London, United Kingdom) and were maintained as described previously (34, 48). Mice 6 to 12 weeks of age were infected with the blood stages of P. chabaudi by injecting 105 infected erythrocytes intraperitoneally (i.p.) or intravenously (i.v.), and the course of infection was monitored by examination of Giemsa-stained blood films every 2 days throughout the experimental period.
Malaria-associated pathology.
Blood glucose, body weight, and temperature were monitored in infected mice every 2 days throughout the experiment. As a control for variation in these parameters not associated with malaria infection, blood glucose, weight, and temperature measurements were taken on day 0 and from uninfected IL-10−/− and WT mice at the same time as the experimental mice during the infection period.
Blood glucose level was measured by using a commercial glucose machine and glucose strips (BM-40; Boehringer Mannheim, East Sussex, United Kingdom). Tail blood (13.5 μl) was placed evenly on a glucose strip and left for 60 s. Excess blood was wiped off before analysis in the machine for a further 60 s. The blood glucose data (means ± standard errors of the means [SEM]) are presented as milligrams per deciliter of blood. Body weight was measured by using a top-pan electronic balance, and the data are presented as the percentage of the weight change with respect to the day 0 value. Body temperature (degrees Celsius) was measured by using a rectal thermoprobe.
IFN-γ and TNF-α in plasma of infected mice.
Blood (100 μl) was removed from tail tips of groups of at least four mice every day until day 10 and then every week until week 4 of the infection, using heparinized sterile pipettes. To ensure that parasite material shed at the time of schizogony did not affect the levels of cytokines in plasma samples from the different mice, blood was taken at the same time each day, shortly after schizont rupture. Plasma samples were obtained after centrifugation at 500 × g for 10 min (4°C) and stored at −70°C until use.
IFN-γ ELISA.
IFN-γ concentration in the plasma was measured by using a sandwich enzyme-linked immunosorbent assay (ELISA) as described previously (48). Mouse recombinant IFN-γ (MG-IFN; Genzyme, Kent, United Kingdom) was used as a standard to calculate the concentration of IFN-γ (nanograms per milliliter) in the plasma samples.
TNF-α ELISA.
TNF-α concentration was measured by using a sandwich ELISA. Briefly, monoclonal antibody TN3 (a kind gift from G. Bancroft, London School of Tropical Medicine and Hygiene, London, United Kingdom) was used as capture antibody, and biotinylated anti-mouse TNF-α (18352D; PharMingen, B&D, London, United Kingdom) was used as detecting antibody. Mouse recombinant TNF-α (TNF-M; Genzyme) was used as a standard to calculate the concentration of TNF-α (picograms per milliliter) in the plasma samples.
In both ELISAs, plasma samples from uninfected IL-10−/− mice (N7BL6) and their WT littermates were included as controls.
TNF-α, IFN-γ and NO production by splenocytes in vitro.
Nitric oxide (NO) production in vitro by splenocytes was measured by the Greiss reaction (19). Spleen cells from uninfected and infected knockout and WT mice (2 × 107 cells) were incubated for 48 h at 37°C and 7% CO2 in 1 ml of complete Iscove’s medium (Gibco, Paisley, United Kingdom) containing 10% fetal calf serum (Global Farm, Surrey, United Kingdom), 1 mM l-glutamine (Gibco), 100 U penicillin and 100 μg of streptomycin per ml (Gibco), 0.05 mM β-mercaptoethanol (Gibco), 5 mM HEPES, and 5 mM sodium pyruvate (Gibco) in a sterile 24-well tissue culture plate. Supernatants were removed after the incubation, and the nitrate concentration was measured as an indicator of NO production. Sodium nitrate solutions at various concentrations (1 to 100 mM) were used as standards to calculate the concentration of nitrate in the supernatant. Medium alone was also used as a negative control. IFN-γ and TNF-α levels in the culture were also measured at the same time by ELISA as described above.
Competitive reverse transcription-PCR (RT-PCR) for cytokine production.
The amounts of mRNA for IFN-γ, TNF-α, IL-4, IL-12p40, and inducible nitric oxide synthase (iNOS) were determined in splenocytes taken from uninfected mice and from mice at various times during a primary P. chabaudi infection. RNA was extracted by a single-step procedure (5) where 107 splenocytes were lysed in 1 ml of solution D (42) containing 4 M guanidine thiocyanate (Fluka, Dorset, United Kingdom), 25 mM sodium citrate (pH 7.0) (Fluka), and 0.5% saccosyl (Fluka) with fresh 0.1 M β-mercaptoethanol (Sigma). Total RNA was extracted in the presence of water-saturated phenol (Gibco), 3 M sodium acetate (Sigma), and chloroform-isoamyl alcohol (Sigma). The RNA was precipitated in 100% ethanol. To remove any genomic DNA contamination, the extracted RNA was treated with 20 U of RNase-free DNase (Boehringer Mannheim) in a cocktail of 5 mM magnesium chloride (Sigma), 10 mM Tris-HCl (pH 7.5) (Fluka), and 40 U of RNasin (Promega, Southampton, United Kingdom). Any degraded DNA was removed by phenol-chloroform extraction.
Extracted RNA was reverse transcribed to cDNA at 37°C for 45 min, using 200 U of Moloney murine leukemia virus polymerase (Gibco) with 0.05 U of random primers (Promega), 1 mM dithiothreitol (Gibco), 100 mM deoxynucleoside triphosphates (equimolar solution of dATP, dCTP, dGTP, and dTTP; Boehringer Mannheim), 40 U of RNasin, and 5× reverse transcription buffer (Gibco). To ensure the completion of the reverse transcription, 100 U of Moloney murine leukemia virus reverse transcriptase was added for a further 45 min of incubation. The resulting cDNA was then diluted to 100 ng input RNA/μl in double-distilled H2O.
Competitive PCR was used to calculate the number of a particular cytokine mRNA molecule present in a test sample with respect to 106 β-2 microglobulin (β2m) molecules. The competitive fragment used was either from pMUS (3) (for β2m, IL-4, IFN-γ, and TNF-α) or pNIL (47) (for IL-12p40 and iNOS). The PCR was performed in a 50-μl reaction volume containing 2 mM dithiothreitol, 10 mM deoxynucleoside triphosphates, 0.2 μM each primer, 1× PCR buffer, and 0.6 U of Taq polymerase (TP05; HT Biotech, Cambridge, United Kingdom). To calculate the number of molecules of a cytokine, a serial fourfold dilution of competitive fragment was amplified with a constant amount of cDNA sample. The PCR products were separated on a 3% agarose gel (Flowgene, Staffs, United Kingdom), and the intensity of each PCR band was measured with the NIH Image 6.0 program. Those bands giving equal intensity indicate the same number of molecules in both the competitive fragment and test samples. The result was then normalized against the number of β2m molecules.
In vitro neutralization of IFN-γ.
Female IL-10−/− and WT mice were treated with a cocktail of R4-6A2 and AN18 (anti-IFN-γ monoclonal antibodies) as described previously (38). Briefly, 0.625 mg of each antibody was given to mice from day 1 postinfection at 4-day intervals until day 17 of infection. Female IL-10−/− and WT mice treated with 1.25 mg of rat immunoglobulin G (rIgG; Sigma) at the same time were used as controls.
Statistic analysis.
Student’s t test was used to calculate the significance of the differences seen in body weight change, temperature change, and glucose levels. The Mann-Whitney test was used to analyze the significance of differences seen in cytokine levels in plasma and mRNA levels in spleens of IL-10−/− and WT mice and in NO, IFN-γ, and TNF-α production by splenocytes in IL-10−/−, IL-10−/− IFN-γR−/−, IFN-γR−/−, and WT mice during the infection.
RESULTS
Erythrocytic-stage infection of P. chabaudi in IL-10−/− mice.
Male and female IL-10−/− mice and sex-matched WT littermates were infected i.v. with 105 P. chabaudi parasites. The course of a primary infection was examined in two strains of mice with a mixed genetic background, BL6 × 129sv and N7BL6 (see Materials and Methods) (Fig. 1A to D). In all groups of mice regardless of genetic background, parasites could be detected 3 to 4 days after inoculation. As described previously for BL6 × 129sv and C57BL/6 mice (35, 49), a peak parasitemia of 20 to 30% infected erythrocytes was observed between 8 and 9 days postinfection. Parasites were then cleared rapidly from the blood and by day 30 were no longer detectable on thin blood films. A patent recrudescence between days 21 and 30 was observed only in female IL-10−/− and WT mice of both backgrounds.
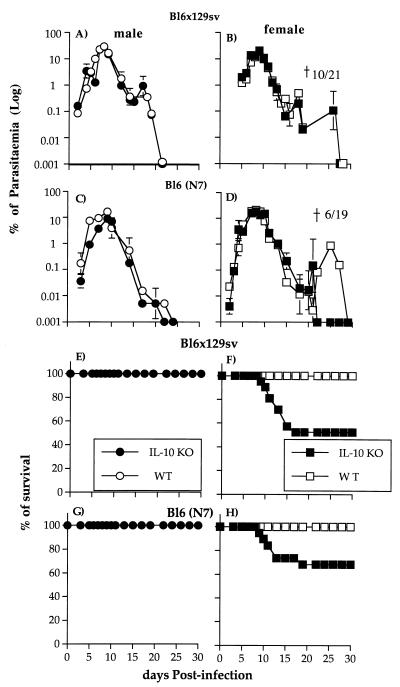
FIG. 1.
Course of a primary erythrocytic-stage P. chabaudi infection (A to D) and survival rate (E to H) in male and female IL-10−/− knockout (KO) (● and ■) and WT (○ and □) mice on BL6 × 129sv and N7BL6 backgrounds. Mice were infected with 105 parasites i.v. and the course of the infection was monitored by examination of Giemsa-stained blood films. Parasitemia values are presented as the geometric means for at least 15 mice, and the error bars represent the SEMs. For clarity, SEMs smaller than 10% of the means are not shown.
As observed previously (35), although the parasitemias were very similar in all groups of male and female mice, the outcomes of infection were different in male and female IL-10−/− mice (Fig. 1E to H). Death occurred in a proportion of female IL-10−/− mice between days 7 and 17. In these experiments, 10 of 21 (50%) of the female BL6 × 129sv IL-10−/− mice died, whereas in N7BL6 IL-10−/− mice the mortality was slightly lower (30%; 6 of 19 mice). However, this difference in mortality was not significant.
All female mice that survived a primary infection were immune to a second challenge infection given 45 days after the primary infection. The course of this secondary infection was similar in mutant and WT mice; parasitemia became patent at day 6, and the peak of infection (less than 1%) was observed on day 10 postchallenge. All mice resolved their infection, and parasitemia became subpatent by day 14 to 25 (data not shown).
Pathology associated with a P. chabaudi infection in IL-10−/− and WT mice during a primary infection.
Although only a proportion of female IL-10−/− mice died during the course of a primary P. chabaudi infection, all female IL-10−/− mice appeared to suffer a more severe disease than WT or male knockout mice. For the first 15 days of infection, defective female mice were significantly less mobile than WT mice and had ruffled fur and hunched backs. A proportion showed hind leg paralysis (data not shown). Several parameters of disease were used to document malarial disease: change in body weight, body temperature, and blood glucose concentration (Fig. 2). Measurements were taken from uninfected IL-10−/− and WT mice at the same time points as for infected mice throughout the infection. There was no significant difference between uninfected IL-10−/− and WT mice.
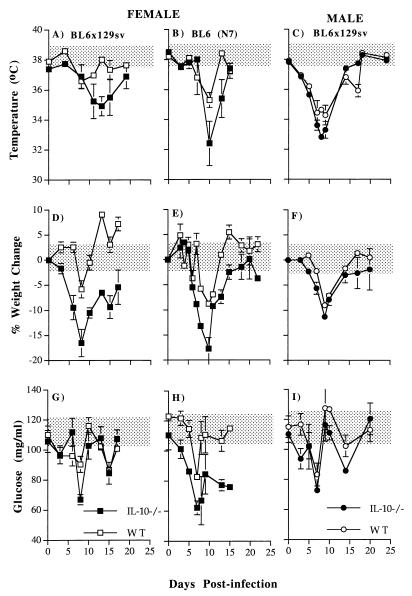
FIG. 2.
Body weight, glucose, and temperature changes during primary P. chabaudi infection in male (five mice in each group) and female IL-10−/− (● and ■) and WT mice (○ and □) on either the BL6 × 129sv or N7BL6 background (15 mice in each group). The error bars represent the SEMs. For clarity, SEMs smaller than 10% of the means are not shown. The shaded areas represent the range in weight, temperature, and glucose level variation of uninfected IL-10−/− and WT animals (five mice in each group) measured at the same time as infected animals throughout the infection.
(i) Body temperature.
All IL-10−/− and WT mice exhibited a transient hypothermia immediately following the peak of parasitemia. However, temperature was significantly lower in female IL-10−/− than in female WT mice on both genetic backgrounds between days 10 and 13 postinfection (P < 0.005; Student’s t test [Fig. 2A and B]).
(ii) Body weight.
In all IL-10−/− and WT mice, there was a transient loss in body weight coinciding with the acute parasitemia (Fig. 2D to F). Weight loss in the female IL-10−/− mice on both genetic backgrounds began earlier and was significantly greater (days 6 to 7, P < 0.005; Student’s t test) than in their WT counterparts. The maximum weight losses of 17 and 20% in IL-10−/− mice, contrasted with 6 and 10% in WT mice, occurred at days 8 and 10 of infection (BL6 × 129sv and N7BL6 mice, respectively). Recovery of normal body weight was more rapid in IL-10−/− N7BL6 mice than in BL6 × 129sv mice, whose weight was significantly less than that of WT mice until day 20 of infection.
(iii) Blood glucose levels.
Hypoglycemia occurred in all IL-10−/− and WT mice during the acute infection and was maximal at 8 days of infection. At this time, female IL-10−/− mice on both genetic backgrounds were significantly more hypoglycemic than their WT controls (P < 0.001; Student’s t test [Fig. 2G and H]). However, blood glucose levels were lower in uninfected N7BL6 IL-10−/− mice than in WT controls, decreased more rapidly, and remained significantly lower than for the controls throughout the 15 days of measurement. The basal (day 0) blood glucose level of uninfected BL6 × 129sv WT mice was lower than that of N7BL6 mice.
There were no significant differences in body weight, temperature, and blood glucose between male IL-10−/− and WT mice and between male and female IL-10−/− mice during the primary infection (Fig. 2C, F, and I).
Cytokine production during the course of a primary P. chabaudi infection in IL-10−/− and WT mice. (i) Plasma IFN-γ and TNF-α in IL-10−/− and WT mice.
IFN-γ was first detectable in the plasma of both male (1 of 7 mice) and female (2 of 12 mice) mice on day 6 postinfection, 2 days before the peak of infection. The highest IFN-γ concentration was measured on day 7 (Fig. 3A and B) in all male and female IL-10−/− and WT mice. From day 9 until the end of the experiment, IFN-γ remained below the detection limit of the assay. Both female and male IL-10−/− mice produced significantly more IFN-γ than their respective WT controls at day 7 postinfection (P < 0.0001 and P < 0.03, respectively; Mann-Whitney test).
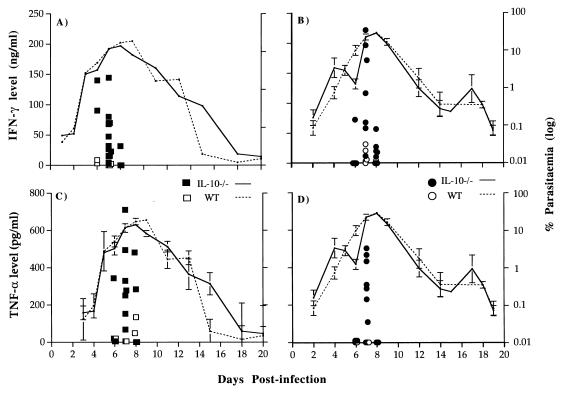
FIG. 3.
Levels of plasma IFN-γ and TNF-α in seven male and at least eight female IL-10−/− (● and ■) and WT (○ and □) N7BL6 mice during primary P. chabaudi chabaudi infection. Mice were bled before and after the infection, and plasma samples were collected. Concentrations of IFN-γ and TNF-α were measured by ELISA. Pooled data from three independent experiments are shown. Each symbol represents one plasma sample. The course of primary infection in IL-10−/− (—) and WT (· · · ·) mice is as indicated. The error bars represent SEMs for seven male and eight female mice. SEMs less than 5% of the means are not shown.
TNF-α was detectable in all of seven male and eight female IL-10−/− mice on day 7 and in two of eight females on day 8 postinfection. By contrast, TNF-α was below the detectable range (less than 60 pg/ml) in seven male and eight female WT mice except on day 8 of infection, when two of eight female WT mice produced less than 200 pg of TNF-α per ml. Both female and male IL-10−/− mice produced higher levels of TNF-α at day 7 of infection (P < 0.0005 and P < 0.03, respectively; Mann-Whitney test).
(ii) Cytokine and iNOS mRNA in splenocytes of P. chabaudi-infected IL-10−/− and WT mice.
Competitive RT-PCR was performed to determine the amount of IFN-γ, TNF-α, IL-4, IL-12 (p40), and iNOS mRNA (Fig. 4). IFN-γ mRNA was already detectable in two of three uninfected female IL-10−/− and WT mice and increased in all mice during the acute infection. By week 2, the level had begun to decline in IL-10−/− mice as well as in WT mice, suggesting that some down-regulatory mechanisms operated despite the lack of IL-10. Female IL-10−/− mice tended to express higher levels of IFN-γ mRNA than their WT controls (P < 0.05; Mann-Whitney test) at 1 week of infection, whereas male IL-10−/− mice expressed higher but not significantly higher levels. There was no significant difference in the level of IFN-γ between male and female mice (Fig. 4A and B).
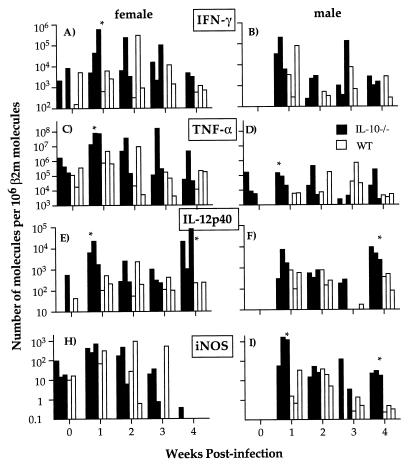
FIG. 4.
Quantification of IFN-γ, TNF-α, IL-12p40, and iNOS mRNA expression in spleens of male and female IL-10−/− (■) and WT (□) mice during primary P. chabaudi infection. Total mRNA was extracted from spleens before and every week after infection. Quantitative RT-PCR was performed to measure the number of molecules of a particular cytokine mRNA present in the sample. The results were then normalized against the number of β2m molecules. The graphs show data for three mice in each group at each time point. ∗, P < 0.05 (Mann-Whitney test).
TNF-α mRNA could be detected in all uninfected female mice and in male IL-10−/− mice (Fig. 4C and D). At 1 and 2 weeks of infection, both male and female IL-10−/− mice expressed more TNF-α than their respective WT controls (P < 0.05; Mann-Whitney test). Female IL-10−/− mice produced at least 100-fold more TNF-α than male IL-10−/− mice from weeks 1 to 3 of infection.
IL-12p40 mRNA (Fig. 4E and F) was present in one of three uninfected female IL-10−/− and WT mice. At 1 week of infection, IL-12p40 increased in all mice, with significantly higher production in female IL-10−/− mice than in WT controls (P < 0.05; Mann-Whitney test). At week 4 of infection, the level of IL-12p40 in male and female IL-10−/− mice increased whereas that of WT mice remained at a low level. This increase coincided with a recrudescence in parasitemia.
Between 10 to 100 molecules of iNOS mRNA (Fig. 4H and I) could be detected in female IL-10−/− and WT mice before infection. At 1 and 4 weeks of infection, male IL-10−/− mice expressed significantly more iNOS mRNA than the corresponding WT controls (P < 0.05; Mann-Whitney test).
IL-4 could not be detected in the spleens of uninfected mice but was detectable in IL-10−/− and WT mice after 1 week of infection. There was no significant difference between the levels of IL-4 in IL-10−/− and WT mice or between male and female mice (data not shown).
(iii) In vitro production of IFN-γ, TNF-α, and NO by spleen cells from infected IL-10−/− and WT mice.
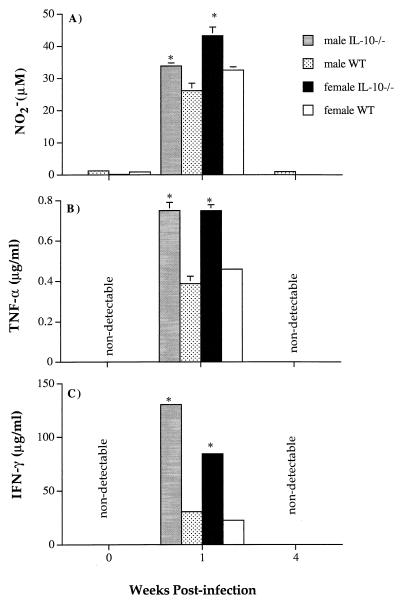
TNF-α, IFN-γ, and NO were measured in the supernatants of IL-10−/− and WT spleen cells from both uninfected and infected mice after 48 h of in vitro culture. Cytokines and NO could be detected only in supernatants from cultures of spleen cells taken from mice at 1 week of infection (Fig. 5A to C). In agreement with plasma TNF-α and IFN-γ levels (Fig. 3), spleen cells from IL-10−/− mice produced significantly greater amounts of cytokines than those from their WT littermates at week 1 of infection (P < 0.01; Mann-Whitney test). Male IL-10−/− cells produced more IFN-γ than female IL-10−/− cells (P < 0.01; Mann-Whitney test), whereas the amounts of TNF-α were equivalent. NO production was greatest in cultures of female IL-10−/− cells (P < 0.01; Mann-Whitney test). Male IL-10−/− and female WT cells produced comparable amounts, and male WT cells produced the smallest amounts (P < 0.01; Mann-Whitney test).
FIG. 5.
NO (A), TNF-α (B), and IFN-γ (C) production by total splenocytes from seven individual male and female IL-10−/− and WT N7BL6 mice during primary P. chabaudi infection. The bars represent the mean data from seven individual spleen samples, and the error bars represent the SEMs. SEMs smaller than 5% are not shown. ∗, P < 0.01 (Mann-Whitney test).
Effects of in vivo inactivation of IFN-γ on P. chabaudi infection in female IL-10−/− mice.
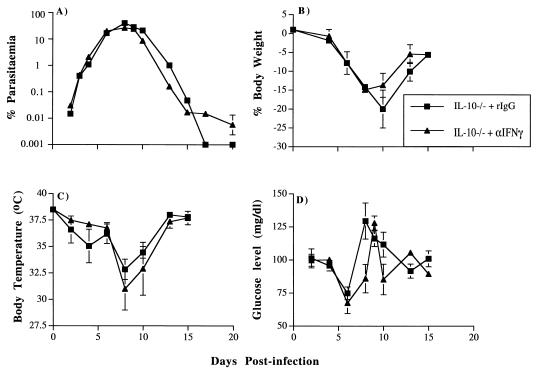
In three independent experiments, P. chabaudi infections in IL-10−/− female BL6 × 129sv mice (one experiment with four mice per group) and N7BL6 mice (two independent experiments with five mice per group each time) treated with control rIgG (a representative experiment on the N7BL6 background is shown in Fig. 6A) were similar to those described above for untreated IL-10−/− mice. In the mice treated with anti-IFN-γ antibodies, recrudescence at days 16 to 18 postinfection was higher than that observed in control rIgG-treated mice. In all cases, parasitemia became subpatent after 26 days of infection. In the three experiments, all IL-10−/− mice (a total 14 of 14 mice in three experiments) given anti-IFN-γ antibodies survived infection, whereas 3 of 14 mice injected with the control rIgG died between days 10 and 15 (20% mortality). The increase in hypothermia, hypoglycemia, and loss of weight observed in female IL-10−/− mice during a primary infection was not dependent on IFN-γ, as administration of anti-IFN-γ antibodies did not significantly ameliorate these symptoms (Fig. 6B to D).
FIG. 6.
Course of primary P. chabaudi infection (A) and pathological changes (B to D) in female N7BL6 IL-10−/− mice treated with anti-IFN-γ antibodies and rIgG. Female IL-10−/− mice were infected with 105 parasites i.p. and treated with antibody as described in Materials and Methods. The course of infection was monitored by examination of Giemsa-stained blood smears (A), and pathological changes were measured during the course of infection (B to D). The error bars represent the SEMs for at least four mice. SEMs smaller than 5% of the means are not shown.
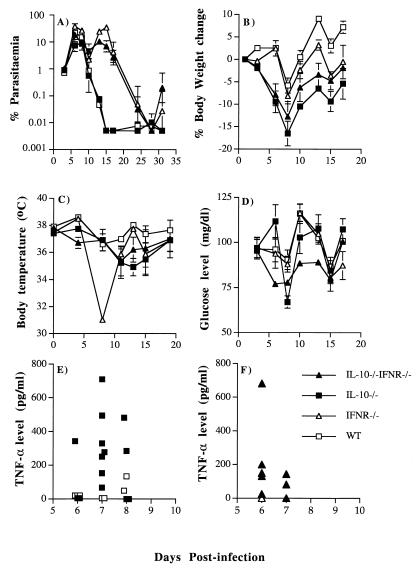
It is possible that in vivo administration of anti-IFN-γ antibodies in this manner is unable to neutralize all IFN-γ. Therefore, P. chabaudi infection was investigated in IL-10−/− IFN-γR−/− double-knockout mice (Fig. 7). The course of infection in the single IL-10−/− mice was similar to that described above. In IFN-γR−/− and IL-10−/− IFN-γR−/− mice, there was a prolonged acute parasitemia with a second peak at day 14 which was resolved more than 10 days later than in IL-10−/− or WT mice. This difference in parasitemia was much more pronounced than that seen in antibody-treated mice and suggested that antibody treatment may not have been completely effective. Although the parasitemia remained at a high level for a longer period in the double-knockout and IFN-γR−/− mice, there was no mortality. By contrast, 2 of 10 IL-10−/− mice died within 15 days. Similar to the depletion of IFN-γ by antibody, the absence of the IFN-γ receptor had no effect on hypoglycemia, hypothermia, or loss in body weight (P > 0.1; Student t test). The greatest decrease in temperature was observed in IFN-γR−/− mice, which also suffered the highest parasitemia.
FIG. 7.
Course of primary P. chabaudi infection in 5 female IL-10−/− IFN-γR−/−, 10 IL-10−/−, 5 IFN-γR−/−, and 7 WT mice. Mice were infected with 105 parasites i.p., and the parasitemia was monitored by examination of Giemsa-stained blood smears (A). Pathological changes (B to D) and plasma TNF-α levels (E and F) were also measured. The error bars in panels A to D represent the SEMs for at least five mice in each group. SEMs smaller than 10% of the means are not shown. For panels E and F, each symbol represents the plasma sample from a single mouse.
Despite the lack of IFN-γ receptor, the five double-knockout mice produced an amount of TNF-α that was not significantly different from that produced by the 10 IL-10−/− mice and significantly more than that produced by the 5 IFN-γR−/− and 7 WT mice (P < 0.05 at day 6; Mann-Whitney test), suggesting that there is an IFN-γ-independent pathway of TNF-α production (Fig. 7E and F).
DISCUSSION
Female IL-10−/− mice suffer a more severe P. chabaudi infection than their female WT counterparts or male IL-10−/− and WT mice. In agreement with earlier studies (35), a proportion of female IL-10−/− mice died between days 10 and 17 of infection, at a time when parasitemia was indistinguishable from that in WT or male IL-10−/− mice. In addition to an increased mortality rate, female IL-10−/− mice, regardless of whether they survived, transiently displayed more pronounced hypoglycemia, hypothermia and loss of body weight than WT mice.
The lack of IL-10 had no significant effect on the course of a primary infection or on resistance to a second infection in surviving mice, suggesting that this cytokine is not crucial in the development of an effective immune response. Rather, it appears that IL-10 may play some role in controlling or down-regulating some of the pathology that accompanies a P. chabaudi infection. Increased susceptibility to infection or enhanced disease associated with infection has also been described for IL-10−/− mice infected with pathogens such as Toxoplasma gondii, Helicobacter hepaticus, and Trypanosoma cruzi (13, 23, 29). In those studies, severity of disease was not related to increased numbers of organisms but was correlated with an excessive production of inflammatory cytokines such as IFN-γ and TNF-α.
IL-10 plays a major role in down-regulating the production of TNF-α, IL-1, and IL-12 by macrophages and thereby T and NK cell proliferation and production of IFN-γ (39). In its absence during a P. chabaudi infection, T cells mount a strong Th1-like response with production of IFN-γ (35). In the present study, we extend these findings and show that IL-10−/− mice infected with P. chabaudi have elevated levels of IFN-γ and TNF-α in the plasma just prior to the peak of infection. Spleen cells produced significantly greater amounts of TNF-α, IFN-γ, and IL-12p40 mRNA and protein and of NO compared with sex-matched WT mice. The coincidence of increased pathology with enhanced inflammatory cytokines suggests that in this malaria infection, IFN-γ and TNF-α may contribute to disease. These findings are in agreement with observations of CM in humans and rodent models. In humans, plasma TNF-α levels are significantly higher in CM cases than in noncomplicated infections (32), and CM is associated with a strong TNF-α promoter (TNF-308A) (36, 37). In rodent models of CM, both TNF-α and IFN-γ have been implicated in pathogenesis and IL-10 has been shown to protect against neurological disease (28).
However, the relationship of these cytokines to other features of severe malaria is less clear. Anemia is probably influenced by different TNF-α-related factors; low plasma TNF-α or an insufficient IL-10 response to TNF-α and a weak TNF-α promoter (TNF-238A) are associated with severe anemia in P. falciparum-infected humans (30, 43). Treatment of uninfected mice with TNF-α does induce transient hypoglycemia, anemia, and hypothermia similar to that seen in P. vinckei and P. yoelii infections (9). However, our previous studies of a P. chabaudi infection in these IL-10−/− mice suggest that the lack of IL-10 and the accompanying increase in the level of TNF-α observed in these studies do not exacerbate anemia (35).
Although IFN-γ was elevated in the infected female IL-10−/− mice, neutralization or inactivation of IFN-γ did not ameliorate hypoglycemia, hypothermia, and loss of weight. However, in the double-knockout mice, plasma levels of TNF-α were relatively unaffected by the lack of an IFN-γ signal. It has been shown that molecules released from Plasmodium are able to stimulate macrophages to produce TNF-α directly (26, 46). Therefore it is possible that these aspects of malarial disease are the result of an IFN-γ-independent TNF-α response. Neutralization of TNF-α in the IL-10−/− mouse would address this. By contrast, there was no mortality among IL-10−/− mice treated with anti-IFN-γ antibody or among IL-10−/− IFN-γR−/− double-knockout mice, suggesting that a pathway involving IFN-γ may contribute to death.
Susceptibility to a lethal infection and the cytokine pattern seen in IL-10−/− mice is different from that seen in naturally susceptible and resistant mice. In those infections with P. chabaudi, males are somewhat more susceptible to lethal infection than females, and susceptibility is associated with a higher parasitemia and a lower and more transient splenic inflammatory or Th1-like response than that observed in resistant mice (10, 24). Sexual dimorphism in susceptibility to infections and in the immune system has been extensively documented (1, 2, 20, 44). Females in general are more prone to autoimmune disease (1), have higher antibody responses to antigen (20), and display greater macrophage activation in response to stimuli (4). The differences in immunoregulation have been ascribed to sex hormones and other steroid hormones (1, 2, 20, 44). For example, estrogen can increase the activity of the IFN-γ promoter (12). In one model of P. chabaudi infection, surgically castrated males revert to a resistant phenotype and administration of testosterone to female mice renders the infection lethal (53).
To reconcile the differences between the greater susceptibility of female IL-10−/− mice and male mice of naturally susceptible strains, we propose that either an insufficient or excessive inflammatory response can lead to disease or death in this rodent malaria infection. In female IL-10−/− mice, the overproduction of inflammatory cytokines results in a pathological macrophage response, whereas this response is lower in males and therefore results in less pathology. By contrast, the reduced early inflammatory response in naturally susceptible mice (24), likely to be more pronounced in males, is insufficient to initiate an effective immune response.
There is clearly not a simple relationship between elevated IFN-γ and TNF-α and the outcome of infection in IL-10−/− mice, since the protein levels of these cytokines were equivalent in spleens and plasma of male and female mice. TNF-α was significantly higher in females only at the mRNA level. Our data are compatible with reports showing that male macrophages respond less to TNF-α and IFN-γ (2). In agreement with observations of others, spleen cells from males, regardless of the status of the IL-10 gene, produce smaller amounts of NO in vitro than do female cells (40).
The association of enhanced pathology with elevated inflammatory cytokines as seen in the female IL-10−/− mouse is in line with the observations in human severe malaria (30, 43), and these experiments suggest that IL-10 regulation may be important in pathogenesis. Similar to findings for the TNF-α gene, polymorphisms within the IL-10 gene promoter have been described (11) and found to influence level of expression of the cytokine (52). It would be of considerable interest to determine whether there is any association of particular promoters with the various forms of severe malaria.
ACKNOWLEDGMENTS
Part of the work described here was supported by the Wellcome Trust, United Kingdom. Inés Corraliza is a recipient of a postdoctoral fellowship from the Spanish Ministry of Education and Science.
We thank Kate Allsopp, Latifu Sanni, and Elsa Seixas for helpful comments and critical review of the manuscript.
REFERENCES
- 1.Ahmed S A, Penhale W J, Talal N. Sex hormones, immune responses and autoimmune diseases—mechanisms of sex hormone action. Am J Pathol. 1985;121:531–551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander J, Stimson W H. Sex hormones and the course of parasitic infection. Parasitol Today. 1988;4:189–193. [Google Scholar]
- 3.Bouaboula M, Legoux P, Pessague B, Delpech B, Dumont X, Piechaczyk M, Casellas P, Shire D. Standardisation of mRNA titration using a polymerase chain reaction method involving co-amplification with a multispecific internal control. J Biol Chem. 1992;267:32442–32448. [PubMed] [Google Scholar]
- 4.Chao T-C, Van Alten P J, Greager J A, Walter R J. Steroid sex hormones regulate the release of tumor necrosis factor by macrophages. Cell Immunol. 1995;160:43–49. doi: 10.1016/0008-8749(95)80007-6. [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1986;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Clark I A, Al Yaman F M, Jacobson L S. The biological basis of malarial disease. Int J Parasitol. 1997;27:1237–1249. doi: 10.1016/s0020-7519(97)00121-5. [DOI] [PubMed] [Google Scholar]
- 7.Clark I A, Chaudhri G. Tumour necrosis factor may contribute to the anaemia of malaria by causing dyserythropoiesis and erythrophagocytosis. Br J Haematol. 1988;70:99–103. doi: 10.1111/j.1365-2141.1988.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 8.Clark I A, Rockett K A, Cowden W B. Proposed link between cytokines, nitric oxide and human cerebral malaria. Parasitol Today. 1991;7:205–207. doi: 10.1016/0169-4758(91)90142-b. [DOI] [PubMed] [Google Scholar]
- 9.Clark I A, MacMicking J D, Gray K M, Rockett K A, Cowden W B. Malaria mimicry with tumor necrosis factor. Contrasts between species of murine malaria and Plasmodium falciparum. Am J Pathol. 1992;140:325–336. [PMC free article] [PubMed] [Google Scholar]
- 10.Cross C E, Langhorne J. Plasmodium chabaudi chabaudi (AS): inflammatory cytokines and pathology in an erythrocytic-stage infection in mice. Exp Parasitol. 1998;90:220–229. doi: 10.1006/expr.1998.4335. [DOI] [PubMed] [Google Scholar]
- 11.Eskdale J, Gallagher G. A polymorphic dinucleotide repeat in human IL-10 promoter. Immunogenetics. 1995;42:444–445. doi: 10.1007/BF00179416. [DOI] [PubMed] [Google Scholar]
- 12.Fox H S, Bond B L, Parslow T G. Estrogen regulates the IFN-γ promoter. J Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R, Müller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 14.Goodier M R, Lundqvist C, Hammarstrom M L, Troye-Blomberg M, Langhorne J. Cytokine profiles for human V gamma 9+ T cells stimulated by Plasmodium falciparum. Parasite Immunol. 1995;17:413–423. doi: 10.1111/j.1365-3024.1995.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 15.Grau E G, Heremans H, Piguet P-F, Pointaire P, Lambert P-H, Billiau A, Vassalli P. Monoclonal antibody against IFN-γ can prevent experimental cerebral malaria and its associated overproduction of TNF-α. Proc Natl Acad Sci USA. 1989;86:5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grau G E, Fajardo L F, Piquet P-F, Allet B, Lambert P-H, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 17.Grau G E, Piguet P-F, Engers H D, Louis J A, Vassalli P, Lambert P-H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986;137:2348–2354. [PubMed] [Google Scholar]
- 18.Grau G E, Taylor T E, Molyneux M E, Wirima J J, Vassalli P, Hommel M, Lambert P H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 19.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 20.Grossman C J. Interaction between the gonadal steroids and the immune system. Science. 1985;227:257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- 21.Ho M, Schollaardt T, Snape S, Looareesuwan S, Suntharasamai P, White N J. Endogenous interleukin-10 modulates proinflammatory response in Plasmodium falciparum malaria. J Infect Dis. 1998;178:520–525. doi: 10.1086/515640. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 23.Hunter C A, Ellis-Neyes L A, Slifer T, Kanaly S, Grung G, Fort M, Rennick D, Araujo F G. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–3316. [PubMed] [Google Scholar]
- 24.Jacobs P, Radzioch D, Stevenson M M. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–541. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakobsen P H, Bate C A, Taverne J, Playfair J H. Malaria: toxins, cytokines and disease. Parasite Immunol. 1995;17:223–231. doi: 10.1111/j.1365-3024.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 26.Jakobsen P H, Moon R, Ridley R G, Bate C A, Taverne J, Hansen M B, Takacs B, Playfair J H, McBride J S. Tumour necrosis factor and interleukin-6 production induced by components associated with merozoite proteins of Plasmodium falciparum. Parasite Immunol. 1993;15:229–237. doi: 10.1111/j.1365-3024.1993.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 27.Kawo N G, Msengi A E, Swai A B, Chuwa L M, Alberti K G, McLarty D G. Specificity of hypoglycaemia for cerebral malaria in children. Lancet. 1990;336:454–457. doi: 10.1016/0140-6736(90)92009-7. [DOI] [PubMed] [Google Scholar]
- 28.Kossodo S, Monso C, Juillard P, Velu T, Goldman M, Grau G E. Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology. 1997;91:536–540. doi: 10.1046/j.1365-2567.1997.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kullberg M C, Ward J M, Gorelick P L, Casper P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurtzhals J A L, Adabayeri V, Goka B Q, Akanmori B D, Oliver-Commey J O, Nkrumah F K, Behr C, Hviid L. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet. 1998;351:1768–1772. doi: 10.1016/S0140-6736(97)09439-7. [DOI] [PubMed] [Google Scholar]
- 31.Kwiatkowski D, Molyneux M E, Stephens S, Curtis N, Klein N, Pointaire P, Smit M, Allan R, Brewster D R, Grau G E, et al. Anti-TNF therapy inhibits fever in cerebral malaria. Q J Med. 1993;86:91–98. [PubMed] [Google Scholar]
- 32.Kwiatkowski D, Hill A V S, Sambou I, Twumasi P, Castracane J, Manogue K R, Cerami A, Brewster D R, Greenwood B M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 33.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 34.Langhorne J, Gillard S, Simon B, Slade S, Eichmann K. Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int Immunol. 1989;1:416–424. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- 35.Linke A, Kühn R, Müller W, Honarvar N, Li C, Langhorne J. Plasmodium chabaudi chabaudi: differential susceptibility of gene-targeted mice deficient in IL-10 to an erythrocytic-stage infection. Exp Parasitol. 1996;84:253–263. doi: 10.1006/expr.1996.0111. [DOI] [PubMed] [Google Scholar]
- 36.McGuire W, Hill A V S, Allsopp C E M, Greenwood B M, Kwiatkowski D. Variation in the TNF-α promoter region associated with susceptibility to cerebral malaria. Nature. 1994;371:508–511. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 37.McGuire W, Knight J C, Hill A V S, Allsopp C E M, Greenwood B M, Kwiatkowski D. Severe malaria anemia and cerebral malaria are associated with different tumor necrosis factor promoter alleles. J Infect Dis. 1999;179:287–290. doi: 10.1086/314533. [DOI] [PubMed] [Google Scholar]
- 38.Meding S J, Cheng S C, Simon-Haarhaus B, Langhorne J. Role of gamma interferon during infection with Plasmodium chabaudi chabaudi. Infect Immun. 1990;58:3671–3678. doi: 10.1128/iai.58.11.3671-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore K W, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin 10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 40.Mossmann H, Benten W P, Galanos C, Freudenberg M, Kuhn-Velten W N, Reinauer H, Wunderlich F. Dietary testosterone suppresses protective responsiveness to Plasmodium chabaudi malaria. Life Sci. 1997;60:839–848. doi: 10.1016/s0024-3205(97)00012-x. [DOI] [PubMed] [Google Scholar]
- 41.Neyer L E, Grunig G, Fort M, Remington J S, Rennick D, Hunter C A. Role of interleukin-10 in regulation and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infect Immun. 1997;65:1675–1682. doi: 10.1128/iai.65.5.1675-1682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nozaki Y, Tanford C. The solubility of amino acids, diglycine, and triglycine in aqueous guanidine hydrochloride solution. J Biol Chem. 1970;247:1648–1652. [PubMed] [Google Scholar]
- 43.Othoro C, Lal A A, Nahlen B, Koech D, Orago A S S, Udhayakumar V. A low interleukin-10 tumor necrosis factor-α ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis. 1999;179:279–282. doi: 10.1086/314548. [DOI] [PubMed] [Google Scholar]
- 44.Paavonen T. Hormonal regulation of immune responses. Ann Med. 1994;26:255–258. doi: 10.3109/07853899409147900. [DOI] [PubMed] [Google Scholar]
- 45.Rest J R. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76:410–415. doi: 10.1016/0035-9203(82)90203-6. [DOI] [PubMed] [Google Scholar]
- 46.Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shakhov A N. New derivative of pMUS for quantitation of mouse IL-12 (p35, p40), IL-10 and IFN-γ receptor mRNA. Eur Cytokine Network. 1994;5:337–338. [PubMed] [Google Scholar]
- 48.Slade S J, Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989;179:353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- 49.Süss G, Eichmann K, Kury E, Linke A, Langhorne J. Roles of CD4- and CD8-bearing T lymphocytes in the immune response to the erythrocytic stages of Plasmodium chabaudi. Infect Immun. 1988;56:3081–3088. doi: 10.1128/iai.56.12.3081-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taverne J, Sheikh N, de Souza J B, Playfair J H, Probert L, Kollias G. Anaemia and resistance to malaria in transgenic mice expressing human tumour necrosis factor. Immunology. 1994;82:397–403. [PMC free article] [PubMed] [Google Scholar]
- 51.Van Hensbroek M B, Palmer A, Onyiorah E, Schneider G, Jaffar S, Dolan G, Memming H, Frenkel J, Enwere G, Bennett S, Kwiatkowski D, Greenwood B. The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. J Infect Dis. 1996;174:1091–1097. doi: 10.1093/infdis/174.5.1091. [DOI] [PubMed] [Google Scholar]
- 52.Westendorp R G, Langerman J A, Huizinga T W, Elouali A H, Verweij C L, Boomsma D I, Vandenbroucke J P. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 53.Wunderlich F, Mossmann H, Helwig M, Schilliger G. Resistance to Plasmodium chabaudi in B10 mice: influence of the H-2 complex and testosterone. Infect Immun. 1988;56:2400–2406. doi: 10.1128/iai.56.9.2400-2406.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]