Highlights
-
•
More than half of oligometastatic cases went through tumor boards at our center.
-
•
The frequency of use of tumor boards for oligometastatic cases is encouraging.
-
•
A recommendation incl. definitive local therapy was fairly common in this series.
-
•
In the absence of phase III study results, decision-making happens case-by-case.
Keywords: Oligometastasis, Multidisciplinary tumor board, Local therapy
Abstract
Background and introduction
Growing evidence supports a combined modality treatment strategy for patients with oligometastatic disease. However, lack of phase III trial data and uncertainties around patient selection highlight the importance of multidisciplinary tumor boards (MDT) in therapeutic decision-making. This study aimed to analyze the recognition of and treatment recommendations for oligometastatic patients by MDTs at a large comprehensive cancer center in order to better understand current treatment patterns of oligometastasis.
Materials and methods
For this retrospective single-center cross-sectional study, oligometastatic patients were identified by screening oncological PET and concurrent brain MRI scans conducted at our center in 2020. MDT discussions and recommendations within four weeks of the imaging diagnosis of oligometastasis were analyzed. Logistic regression analysis was used to identify predictors for the addition of local therapy to standard-of-care.
Results
A total of 787 oligometastatic cases were identified. Lung cancer and mesothelioma, skin cancer, and prostate cancer were the most common histologies with 231 (29 %), 160 (20 %), and 84 (11 %) cases, respectively. Almost half of the cases (46 %) had one distant metastasis on imaging only. More than half (56 %) of all oligometastatic cases were discussed at an MDT. In 47 % of cases, for which a therapeutic recommendation was reached in an MDT, local therapy was part of the therapeutic strategy. On logistic regression analysis, oligometastatic skin cancer was significantly associated with a recommendation for local therapy (p < 0.05), whereas the number of oligometastases was not (p = 0.202).
Conclusion
More than half of oligometastatic cases were discussed in MDTs, of which more than every second received a recommendation including the addition of local therapy. This frequency of MDT use underscores the importance of multidisciplinary decision-making, yet efforts should be increased to standardize reporting and use standard nomenclature on oligometastasis in MDTs to better frame multidisciplinary discussion.
Nomenclature
Glossary
- BASEC
Business Administration System for Ethics Committees
- CCCZ
Comprehensive Cancer Center Zurich
- CUP
Cancer of unknown primary
- DGB
Data governance board
- EMR
Electronic medical records
- FDG-PET/CT
Fluorodeoxyglucose positron emission tomography/computed tomography
- FDG-PET/MRI
Fluorodeoxyglucose positron emission tomography/magnetic resonance imaging
- GIT
Gastrointestinal tract
- HNC
Head & neck cancer
- IQR
Interquartile range
- MDT
Multidisciplinary tumor board
- NSCLC
Non-small-cell lung cancer
- OMD
Oligometastatic disease
- OR
Odds ratio
- PET
Positron emission tomography
- PSMA-PET/CT
Prostate specific membrane antigen positron emission tomography/computed tomography
- PSMA-PET/MRI
Prostate specific membrane antigen positron emission tomography/magnetic resonance imaging
- “reco”
Recommendation
- SBRT
Stereotactic body radiotherapy
- SCLC
Small-cell lung cancer
- STROBE
Strengthening the reporting of observational studies in epidemiology
- USZ
University Hospital Zurich
- UZH
University of Zurich
- w/
With
Introduction and background
Several phase II trials have shown that definitive local therapy in the form of stereotactic body radiotherapy (SBRT) or surgery in combination with standard-of-care (SoC) systemic therapy can benefit selected oligometastatic patients by prolonging progression-free survival (PFS) or overall survival (OS). De Ruysscher et al. (2012) reported long-term PFS in oligometastatic non-small cell lung cancer (NSCLC) patients after definitive local therapy [1]. Gomez et al. (2016; 2019) demonstrated that in patients with oligometastatic NSCLC, who did not progress after front-line systemic therapy, definitive local therapy prolonged PFS and OS compared to maintenance therapy or observation [2], [3]. Iyengar et al. (2017) found that consolidative definitive local therapy prior to maintenance chemotherapy in patients with limited metastatic NSCLC prolonged PFS [4]. Ost et al. (2018) showed that definitive local therapy rather than surveillance alone can prolong systemic therapy free survival in oligorecurrent prostate cancer patients [5]. Palma et al. (2019) reported that definitive local therapy was associated with improved OS in a mixed histology group of patients who received palliative SOC versus SOC plus definitive local therapy to all detected cancerous lesions [6]. Phillips et al. (2020) demonstrated a PFS benefit in oligometastatic prostate cancer patients who had definitive local therapy for up to three distant metastases versus observation [7].
As evidence from randomized phase III trials on the efficacy of definitive local therapy for oligometastatic patients is being awaited, curatively intented definitive local therapy in these patients has already become more widely accepted in the broader oncological community [8], [9]. As the concepts of oligometastatic disease (OMD) and definitive local therapy recommendations are being increasingly implemented in multidisciplinary treatment guidelines [10], [11], [12], [13], [14], many questions remain unanswered. In particular, criteria about the optimal selection of oligometastatic cancer patients, who would benefit most from a multidisciplinary treatment strategy, including definitive local therapy, are still lacking [15], [16], [17].
In the absence of phase III trials, interdisciplinary exchange and decision-making in the form of multidisciplinary tumor boards (MDTs) remains a cornerstone in modern cancer care to provide evidence-based and best-practice recommendations on a case-by-case basis. The regular use of MDTs for cancer patients was shown to improve clinical outcome, increase the adherence to SoC, and lead to more balanced treatment recommendations [18], [19], [20].
In this retrospective single-center cross-sectional case study, we aim to analyze the recognition of and treatment recommendations for oligometastatic patients by MDTs in order to better understand current treatment patterns of patients with oligometastatic disease. Our hypothesis is that in the absence of results from phase III trials addressing the addition of local therapy to SoC for oligometastatic patients, there is ample use of MDTs at a large comprehensive cancer center in order to leverage multidisciplinary expertise and select the subset of oligometastatic patients who are most likely to benefit the addition of local therapy to SoC. Better understanding multidisciplinary therapeutic decision-making for oligometastatic patients will enhance clinical discussions around what subset of oligometastatic patients might profit most from the addition of local therapy to SoC.
Materials and methods
OMD cases
All oncological positron emission tomography (PET) scans and concurrent brain magnetic resonance imaging (MRI) scans conducted consecutively at our comprehensive cancer center (CCC) between January and December 2020 were screened for patients with cancer. Patients were included into our study, if they had (1) an extra-cranial solid organ malignancy, (2) between one and five distant metastases on PET and brain imaging, where available, and (3) were oncologically followed-up at the Comprehensive Cancer Center Zurich (CCCZ). Patients were excluded if they had (1) none or a hematologic malignancy, or (2) evidence of malignant pleural effusion, pleural carcinomatosis, peritoneal carcinomatosis or lymphangitic carcinomatosis on PET imaging [21].
For all cases of OMD, the following variables were recorded: Date of birth, gender (male, female), initial diagnosis (ICD-10), date of primary cancer diagnosis, date of PET scan, treating hospital (CCCZ, other), MDT at CCCZ within four weeks of PET (yes, no), type of MDT (breast cancer and gynecology, dermato-oncology, endocrine and neuro-endocrine oncology, hepatobiliary oncology, head and neck oncology, pituitary gland tumors, sarcomas, thyroid tumors, thoracic oncology, upper and lower gastrointestinal tract (GIT) oncology, uro-oncology), recommended MDT treatment regimen (radiotherapy, surgery, systemic therapy, multimodal), recommendation to treat all tumor manifestations (yes, no), and explicit mention of “oligometastasis” in all variants (e.g., oligoprogression, oligorecurrence [22]) in MDT discussion (yes, no). It was also noted whether, and if so, which further diagnostic work-up was requested before definitely recommending treatment.
OMD definition
OMD is commonly used to refer to a situation, where a solid malignancy has seeded to a limited number of distant metastases [23]. There is yet no agreement on the exact number of distant metastases to differentiate between the OMD state and polymetastasis, with most studies using ranges of 1–3 or 1–5 distant metastases [24]. As 1–5 distant metastases are currently the most commonly used definition, we decided to use maximum 5 metastases (extra- plus intracranial) as a cut-off for defining OMD for our study.
MDT discussions
At CCCZ, a total number of 23 MDTs are organized on a routine basis covering all malignancies: Autologous stem cell transplantation; breast cancer and gynecology; dermato-oncology; endocrine and neuro-endocrine oncology; head & neck oncology; hepatobiliary oncology; leukemia, lymphoma and myeloma; neuro-oncology; pituitary gland tumors; sarcomas; thyroid tumors; thoracic oncology; upper and lower gastro-intestinal tract (GIT) oncology; and uro-oncology (see Supplements 1). In addition, there is a dedicated molecular tumor board for patients beyond SoC. The CCCZ is certified and accredited by the “Deutsche Krebsgesellschaft” (DKG) and MDTs are organized according to DKG standards. Disciplines required to participate in each MDT are predefined and recorded whenever an MDT takes place. MDT recommendations are captured during the MDT, presented to all participants and consent-based. After the MDT, recommendations are shared in written format with all discussants or CCCZ departments and stored in the electronic medical records (EMR) system.
Data collection and statistical analysis
All study data was collected in Microsoft® Excel® (Version 16.0). PET imaging reports and basic patient information were obtained via our Nuclear Medicine imaging repository by one researcher (UJM). Five researchers recorded and subsequently cross-checked PET and brain MR imaging data (SMC, PH, KP, GWT and MG). Cases with diverging assessments were reviewed again by at least two researchers. Data from MDT reports were then manually reviewed in the EMR system by one researcher (PH), before they were subsequently and independently cross-checked by another researcher (SMC). Information on MDTs was obtained from CCCZ administrative staff. Descriptive summary statistics were calculated for all relevant variables under study. Inferential statistical analysis consisted of uni- and multivariable logistic regression analysis in order to identify predictors for the addition of local therapy to SoC. Independent variables were dummy coded based on commonly employed definition and cut-offs: gender (male vs female), age (<70 vs ≥ 70 years of age), OMD state (synchronous vs metachronous), primary histology (skin vs all other), and number of distant metastases (≤1 vs >1). All statistical analysis was conducted using the statistical software package STATA (v.16.0; StataCorp, College Station, Texas, USA). Graphs were created with Microsoft® Powerpoint® (Version 16.0) and GraphPad Prism (v.9).
Ethical approval
This project obtained approval from the Ethics Committee (BASEC ID# 2018–01794). The hospital’s data governance board (DGB) also signed off on the project. This study followed the World Medical Association International Code of Medical Ethics and STROBE checklist (see Supplements 2). The imaging database employed in this study also builds the backbone for other, currently ongoing projects exploring oligo- and polymetastatic disease states.
Results
Imaging repository
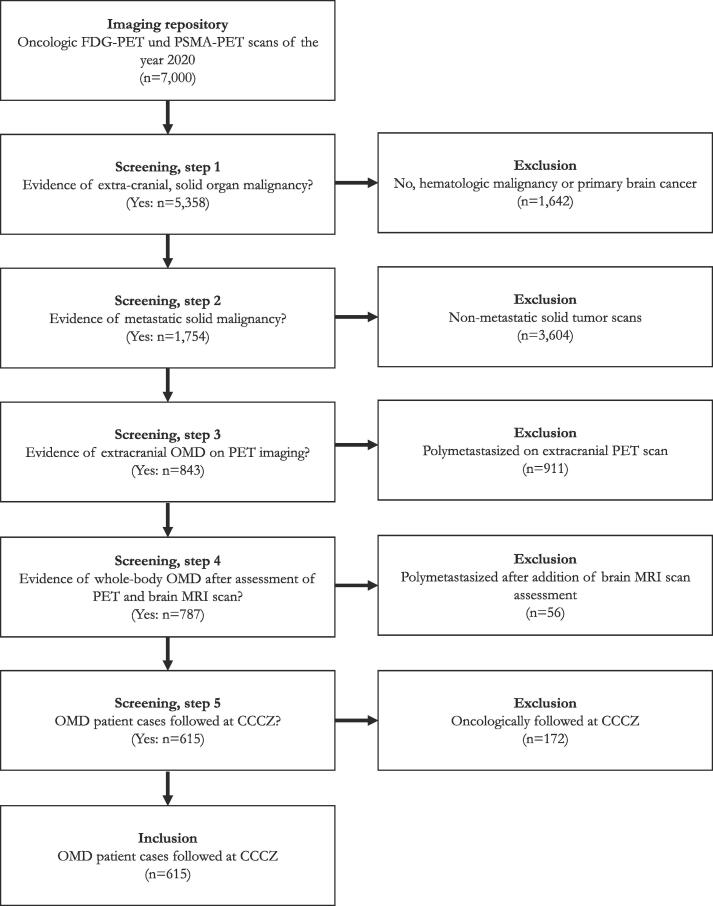
Between January and December 2020, a total of 7,000 oncologic FDG-PET und PSMA-PET scans were acquired at CCCZ, details of which have been reported previously [25]. In summary, cancer was detected on 5,773 (82 %) scans, with 5,358 (93 %) scans originating from patients with solid malignancies. Metastatic disease was present on 1,754 (33 %) scans, 843 (48 %) scans of which harbored between one and five extracranial distant metastases. After assessment of available CT and/or brain MRI scans, 787 cases – incl. repeat scans per patient – of whole-body OMD were identified, which represented the cohort of our case-based study. CCCZ was the treating institution in 615/787 (78 %) of OMD cases. The remaining 22 % of OMD scans (n = 172) originated from patients who had their imaging conducted at CCCZ, but were treated elsewhere (Fig. 1).
Fig. 1.
Patient screening, inclusion and exclusion criteria. Abbreviations: CCCZ = Comprehensive Cancer Center Zurich; MRI = Magnetic resonance imaging; OMD = Oligometastatic disease; PET = Positron emission tomography.
OMD cases
Median age at primary diagnosis of the 787 OMD cases was 65 (interquartile range (IQR), 56–73) years. Thirty-six percent (n = 287) of cases were from female patients. Based on the PET imaging and initial diagnosis dates, 23 % (n = 179) of cases, the OMD state was synchronous, in 74 % (n = 586) metachronous, and in 3 % (n = 20) it remained unknown. Lung cancer and mesothelioma as well as skin cancer patients constituted the largest groups of patients with n = 231 (29 %) and n = 160 (20 %) cases, respectively. In almost half of OMD cases, a single imaging-defined distant metastasis was detected (n = 360, 46 %) (see Table 1).
Table 1.
Basic OMD case characteristics.
| Parameters | Data (n = 787)* |
|---|---|
| Age at primary diagnosis, median (IQR) | 65 (56–73) |
| Female gender, n (%) | 287 (36) |
| Occurrence of OMD, n (%) | |
|
179 (23) |
|
586 (74) |
|
20 (3) |
| Primary tumors, n/n (%) | |
|
231 (29) |
|
160 (20) |
|
84 (11) |
|
68 (9) |
|
63 (8) |
|
48 (6) |
|
44 (6) |
|
35 (4) |
|
16 (2) |
|
10 (1) |
|
28 (4) |
| Frequency of number of distant metastases | |
|
360 (46) |
|
197 (25) |
|
95 (12) |
|
83 (11) |
|
52 (7) |
Abbreviations: CUP = Cancer of unknown primary; GIT = Gastrointestinal tract; IQR = Interquartile range; OMD = Oligometastatic disease.
*We previously reported on 601 individual OMD patients. In the present study, we included repeat OMD scans into our assessment, hence the data on these OMD cases varies slightly from our previous publication [25].
1Includes small-cell lung cancer (SCLC), non-small cell lung cancer (NSCLC) and mesothelioma; 2includes melanoma, squamous cell carcinoma as well as rare skin cancers.
MDT discussions
Of all 23 distinct interdisciplinary boards, 14 (61 %) are for non-pediatric, oncological patients, of which 12 are for solid cancers (52 %). Of the twelve solid organ MDTs, ten (83 %) are organized on a weekly basis, the endocrine and neuro-endocrine oncology as well as the pituitary gland tumors MDTs being the exceptions with a frequency of once a fortnight or once a month, respectively. From January 1st, 2020 to January 31st, 2021, a total of 559 MDTs were collected and analyzed. Due to COVID-19, these MDTs were organized either in-person, fully online or in a hybrid format, depending on room capacity and effective rules at that time. An average of seven (range, 4–11) different medical sub-disciplines were represented at each MDT (Supplements 1).
Of the 615 OMD cases who were oncologically followed at CCCZ, 347 (56 %) were discussed at an MDT within four weeks of their PET scan showing presence of OMD. The absolute number and the proportion of OMD cases discussed varied by MDT. The highest absolute number of OMD cases were discussed in the dermato-oncology (115), thoracic (72) and head & neck (37) oncology MDTs. The highest proportions of OMD cases discussed in an MDT was in the dermato-oncology (78 %), head & neck oncology (55 %), and sarcoma (53 %) MDTs. An overview of OMD case discussions is shown in Supplements 3.
In 100 (29 %) cases, the cumulative number of distant metastases on PET imaging was included verbatim into the MDT documentation. Across all 559 MDTs, the term “oligometastasis” was documented or mentioned verbatim in only 10 cases (3 % of all 347 OMD case discussions). In a single MDT report it was documented that the oral discussion of the case during the MDT session included a “live” correlation of the number of distant metastases with the available imaging scans.
MDT recommendations
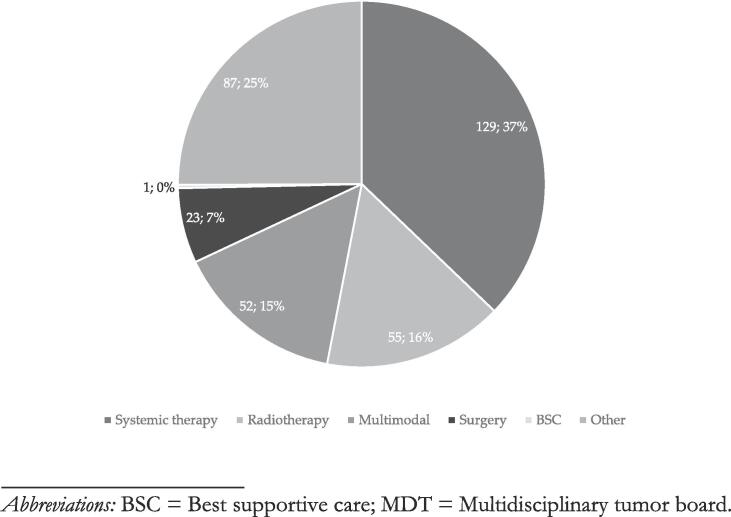
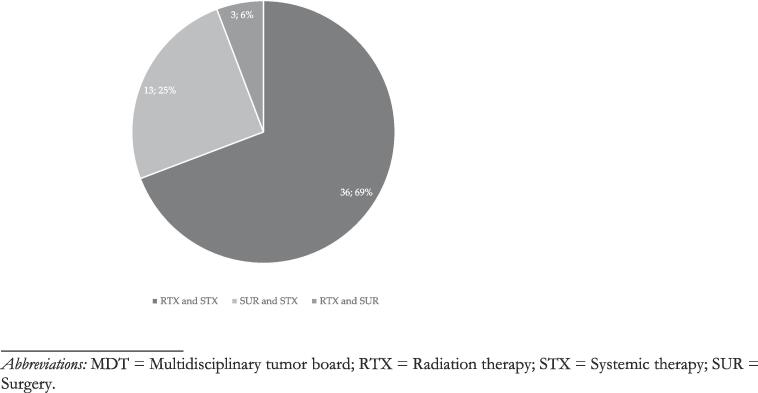
A therapeutic recommendation was made by the MDT in 80 % (n = 278/347) of MDT discussions of OMD cases. In the remaining 20 % (n = 69/347) OMD cases, further diagnostic work-up or watchful waiting was recommended. In 47 % (n = 130/278) of OMD cases with a therapeutic recommendation, local therapy was as a component of the recommended overall treatment strategy. For n = 55/130 (42 %) of OMD cases with recommendation of local therapy, radiotherapy alone was recommended as the sole strategy for local therapy. Surgery alone was recommended for 18 % (n = 23/130) of OMD patients. And in another n = 52/130 (40 %) OMD cases, the recommended multimodal therapeutic algorithm included either radiotherapy or surgery. In nine (3 %) cases, it was explicitly documented that the MDT recommendation entailed local therapy to all PET-positive lesions. The documented MDT recommendation specified in seven (2 %) cases whether the local treatment recommendation referred to the primary or metastatic disease site (metastatic site: 7; primary: 0). Systemic therapy alone was recommended for 50 % (n = 129/260) of discussed OMD cases. Best supportive care (BSC) was the MDT recommendation in a single case of OMD. A summary of MDT recommendations is given in Fig. 2a. In the 52 OMD cases, where the MDT recommended a multimodal approach, radiotherapy was part of the recommendation in 75 % (n = 39/52) of cases, combined with systemic therapy in 36 of 52 (69 %) cases, and with surgery in 3 of 52 (6 %) cases. Surgery combined with systemic therapy was recommended in 13 of 52 (25 %) cases. For a break-down of multimodal treatment recommendations, see Fig. 2b. A more detailed overview of OMD cases, case discussions and treatment recommendations per oncological subdiscipline is given in Supplements 4.
Fig. 2a.
Overview of MDT recommendations. Abbreviations: BSC = Best supportive care; MDT = Multidisciplinary tumor board.
Fig. 2b.
Breakdown of multimodal MDT recommendations by therapeutic combination. Abbreviations: MDT = Multidisciplinary tumor board; RTX = Radiation therapy; STX = Systemic therapy; SUR = Surgery.
In univariable logistic regression analysis, a recommendation of local therapy was significantly associated with two explanatory variables. Patients with a skin cancer diagnosis had higher odds of being recommended local therapy, with an odds ratio (OR) of 1.65 (95 % confidence interval (CI), 1.71–2.53; p-value: 0.023). Also, cases with more than one distant metastasis were less likely to be referred for local therapy (OR: 0.80; 95 % CI, 0.66–0.95; p-value: 0.014). Gender (male vs female), OMD state (synchronous vs metachronous), and age at primary diagnosis (≤70 vs >70 years) were not significantly associated with a recommendation for local therapy in univariable analysis. On multivariable logistic regression analysis, only the effect of primary tumor histology remained significant: Skin cancer OMD cases had an OR of 1.58 (95 % CI, 1.02–2.47; p-value: 0.042) to be recommended for local therapy. For an overview of regression results, see Table 2.
Table 2.
Logistic regression analysis results regarding the recommendation for the addition of local therapy to SoC for OMD cases.
| Explanatory variable | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| OR (95 % CI) | p-value | OR (95 % CI) | p-value | |
| Gender | 0.983 (0.665– 1.455) | 0.935 | 1.055 (0.708–1.572) | 0.790 |
| ||||
| Age (years) | 1.347 (0.909– 1.996) | 0.137 | 1.189 (0.793–1.783) | 0.403 |
| ||||
| OMD state | 0.714 (0.467–1.093) | 0.121 | 0.670 (0.454–1.077) | 0.105 |
| ||||
| Primary histology | 1.646 (1.070–2.533) | 0.023 | 1.584 (1.018–2.466) | 0.042 |
| ||||
| Number of distant metastases | 0.796 (0.664– 0.954) | 0.014 | 0.878 (0.793–1.458) | 0.202 |
| ||||
Abbreviations: CI = Confidence interval; OMD = Oligometastasis; OR = Odds ratio; SoC = Standard-of-care.
Discussion
In this cross-sectional retrospective single-institution assessment, a total 787 imaging-detectable oligometastatic cancer patient cases were identified via PET imaging and the majority of these patients (80 %) cases were treated at our institution. More than half of all patients with an oligometastatic tumor burden (56 %) were discussed at an MDT, with significant differences between the individual MDTs. The MDT recommended local therapy as a component of the treatment strategy in 130 of 278 (47 %) of these OMD cases, radiotherapy was the most frequently recommended local therapy (42 %), followed by surgical resection (18 %). In univariable and multivariable logistic regression analysis, only a skin cancer diagnosis was associated with higher odds for local therapy recommendation, whereas the number of oligometastases did not influence the decision-making process for or against the addition of local therapy to SoC.
More than half of cases with an oligometastatic tumor burden on PET imaging were presented at an MDT discussion (56 %) within 4 weeks of imaging. MDTs are considered the backbone of quality assurance and evidence-based medicine in modern cancer care, especially in areas such as OMD, where evidence from randomized phase III trials about the optimal treatment strategy is lacking. Additionally, multidisciplinary discussion of all patients with a change of the disease status, which might require a change of the multimodal treatment strategy, is a standard operating procedure at our certified CCC. Therefore, the MDT discussion rate of 56 % might be perceived as low; however, the proportion of oligometastatic patients presented at an MDT varied between 25 % and 78 % in our study. In contrast to our expectations, MDT was performed in only 39 % of oligometastatic lung cancer patients, a field where highest evidence in the field of OMD is available [1], [2], [3], [4]. Such an association between available evidence [5], [7] and MDT presentation was indeed observed for oligometastatic genito-urological malignancies, with a high MDT presentation rate of 66 %. Interestingly, oligometastatic skin cancer patients were most frequently discussed in an MDT setting (72 %): these were mostly metachronous OMD patients and RT was the most frequent local therapy, indicating that a potential synergism between RT and immune checkpoint inhibition as SoC systemic therapy in metastatic melanoma patients might have contributed to this finding. For interpretation of the finding that “only” 56 % of OMD cases were discussed in an MDT, it also needs to be considered that patients might have been discussed and presented before the evaluation period of this study, which was not captured by our analysis, which is why we consider the MDT discussion rate of OMD cases at CCCZ to be fairly high, yet not entirely satisfactory. As we are not aware of any other analysis about this question, interpretation of our findings in light of the literature is challenging. With auto-segmentation software under development, refinement and extensive testing, AI-assisted tools might soon be able to identify OMD patients, thus increasing the chance of them being discussed in MDTs and amended to local therapy. Moreover, the use of standard nomenclature and reporting of oligometastatic cases might make multidisciplinary discussion easier and decision-making more transparent.
Across all 278 MDTs which had resulted in a therapeutic recommendation, the MDT recommendation included a referral for local therapy in 47 % of OMD cases. Galata et al. (2018), in an assessment of 1,673 patients discussed in MDTs, identified 151 OMD patients for whom the therapeutic recommendation included local therapy in 69 % [26], which is comparable to our study. In fact, the “true” proportion of oligometastatic patients recommended for local therapy is expected to be higher in our cohort, given that in n = 69 (20 %) of case discussions, no therapeutic recommendation was reached, with further diagnostic tests being requested. In addition, with 46 % of OMD patients not discussed at MDTs after their imaging-based OMD diagnosis, it is unsure whether their treatments might have included local therapy as well. Yet even though MDT recommendations constitute the interdisciplinary consensus, we aim to analyze whether these multidisciplinary recommendations for local therapy were followed and what therapeutic regimens exactly they entailed in a future project.
In our assessment, the factors age, gender, OMD status, and number of distant metastases were not associated with a recommendation for local therapy in univariable and/or multivariable analysis. Especially the fact that the number of distant metastases was not associated with a recommendation of local therapy on regression analysis is contrary to the published evidence, where treated OMD patients mostly presented with one or two distant metastases [1], [2], [4], [6]. However, whenever local therapy was recommended by an MDT, the recommended modality was most likely was radiotherapy in the form of SBRT. This was in line with expectations: Not only is this finding reflective of the clinical and scientific expertise at CCCZ, yet it is also in line with the currently best available evidence for definitively treating OMD patients, which originates from SBRT-based phase II trials. These results also highlight that the decision-making process regarding OMD patients remains highly individual.
It is a strength of our study that all MDT reporting was analyzed manually and comprehensively. Shortcomings of our study include biases arising from its single-institution and retrospective character. However, future collaborative efforts could see the creation of regional or global data repositories collecting data on patients with OMD, in which not only information from large observational studies and prospective trials, but also single-center experiences such as ours could be assembled and analyzed. Moreover, while PET imaging remains the gold standard for OMD detection in the absence of clinically viable biomarkers, it is unclear how representative our sample of OMD cases is for all OMD cases discussed at CCCZ, since cases were selected based on imaging-detectable prevalence of OMD rather than a systematic review of all MDT reports of the year 2020.
In conclusion, more than half of oligometastatic patients treated at our CCC were discussed in MDTs before undergoing treatment and local therapy was recommended for approximately half of OMD patients, for whom a therapeutic recommendation was made. These findings underscore the importance of multidisciplinary decision-making, yet efforts should be increased to standardize reporting and use standard nomenclature on oligometastasis in MDTs to better frame multidisciplinary discussion. Further research is required to better identify patient and disease characteristics of oligometastatic cancer patients who benefit most from a combined modality treatment strategy.
Availability of data and material: Collected patient and imaging data are confidential and not available for publication.
Ethics approval.
This study was approved by the Swiss Cantonal Ethics Committee before the initiation of the project (BASEC ID# 2018–01794).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. PB cited research grants to the institution from ViewRay Inc. (Mountain View, CA, USA). NA has received grants from ViewRay Inc. and BrainLab and personal fees from AstraZeneca, Debiopharm, ViewRay and BrainLab, and non-financial support from ViewRay, all outside of the submitted work. MH has received research support from GE Healthcare, a fund by the Alfred and Annemarie von Sick legacy for translational and clinical cardiac and oncological research, and a grant by the Clinical Research Priority Program (CRRP) “Artificial Intelligence in oncological Imaging” of the University Zurich, all outside of the submitted work.
Funding
Sebastian M. Christ received support through the “Young Talents in Clinical Research” Beginner’s Grant from the Swiss Academy of Medical Sciences (SAMW) and the Bangerter-Rhyner Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2022.11.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.De Ruysscher D., Wanders R., Van Baardwijk A., Dingemans A.M.C., Reymen B., Houben R., et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: Long-term results of a prospective phase II trial (Nct01282450) J Thorac Oncol [Internet] 2012;7(10):1547–1555. doi: 10.1097/JTO.0b013e318262caf6. Available from: [DOI] [PubMed] [Google Scholar]
- 2.Gomez D.R., Tang C., Zhang J., Blumenschein G.R., Hernandez M., Jack Lee J., et al. Local consolidative therapy vs. Maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez D.R., Blumenschein G.R., Lee J.J., Hernandez M., Ye R., Camidge D.R., et al. Local Consolidative Therapy versus Maintenance Therapy/Observation for Patients with Oligometastatic Non-Small Cell Lung Cancer without Progression after Front-Line Systemic Therapy: Results of a Multi-Institutional Phase II Randomized Study. Lancet Oncol [Internet] 2017;17(12):1672–1682. doi: 10.1016/S1470-2045(16)30532-0. http://www.ncbi.nlm.nih.gov/pubmed/27789196%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5143183 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyengar P., Wardak Z., Gerber D.E., Tumati V., Ahn C., Hughes R.S., et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA. Oncol. 2018;4(1) doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ost P., Reynders D., Decaestecker K., Fonteyne V., Lumen N., DeBruycker A., et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- 6.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet [Internet] 2019;393(10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5. Available from: [DOI] [PubMed] [Google Scholar]
- 7.Phillips R., Shi W.Y., Deek M., Radwan N., Lim S.J., Antonarakis E.S., et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650–659. doi: 10.1001/jamaoncol.2020.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lievens Y., Guckenberger M., Gomez D., Hoyer M., Iyengar P., Kindts I., et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol [Internet] 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. Available from: [DOI] [PubMed] [Google Scholar]
- 9.Lewis S.L., Porceddu S., Nakamura N., Palma D.A., Lo S.S., Hoskin P., et al. Definitive Stereotactic Body Radiotherapy (SBRT) for Extracranial Oligometastases. Am J Clin Oncol Cancer Clin Trials. 2017;40(4):418–422. doi: 10.1097/COC.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 10.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Non-Small Cell Lung Cancer Version 3.2022 [Internet]. 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 11.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Prostate Cancer Version 4.2022 [Internet]. 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 12.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Kidney Cancer Version 2.2023 [Internet]. 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf.
- 13.ESMO Clinical Practice Guidelines Metastatic NSCLC [Internet]. 2020. Available from: https://www.esmo.org/content/download/347819/6934778/1/ESMO-CPG-mNSCLC-15SEPT2020.pdf.
- 14.ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer [Internet]. 2016. Available from: https://www.annalsofoncology.org/article/S0923-7534(19)34754-4/pdf. [DOI] [PubMed]
- 15.Tanadini-Lang S., Rieber J., Filippi A.R., Fode M.M., Streblow J., Adebahr S., et al. Nomogram based overall survival prediction in stereotactic body radiotherapy for oligo-metastatic lung disease. Radiother Oncol [Internet] 2017;123(2):182–188. doi: 10.1016/j.radonc.2017.01.003. Available from: [DOI] [PubMed] [Google Scholar]
- 16.Baker S., Bakunina K., Duijm M., Hoogeman M.S., Cornelissen R., Antonisse I., et al. Development and external validation of a nomogram to predict overall survival following stereotactic body radiotherapy for early-stage lung cancer. Radiat Oncol. 2020;15(1):1–11. doi: 10.1186/s13014-020-01537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo L.M., Wang Y., Lin P.X., Su C.H., Huang B.T. The Clinical Outcomes, Prognostic Factors and Nomogram Models for Primary Lung Cancer Patients Treated With Stereotactic Body Radiation Therapy. Front Oncol. 2022:12(March). doi: 10.3389/fonc.2022.863502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brauer D.G., Strand M.S., Sanford D.E., Kushnir V.M., Lim K.H., Mullady D.K., et al. Utility of a multidisciplinary tumor board in the management of pancreatic and upper gastrointestinal diseases: an observational study. Hpb [Internet] 2017;19(2):133–139. doi: 10.1016/j.hpb.2016.11.002. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamburini N., Maniscalco P., Mazzara S., Maietti E., Santini A., Calia N., et al. Multidisciplinary management improves survival at 1 year after surgical treatment for non-small-cell lung cancer: A propensity score-matched study. Eur J Cardio-thoracic Surg. 2018;53(6):1199–1204. doi: 10.1093/ejcts/ezx464. [DOI] [PubMed] [Google Scholar]
- 20.Ioannidis A., Konstantinidis M., Apostolakis S., Koutserimpas C., Machairas N., Konstantinidis K. Impact of multidisciplinary tumor boards on patients with rectal cancer (Review) Mol Clin Oncol. 2018:135–137. doi: 10.3892/mco.2018.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dingemans A.M.C., Hendriks L.E.L., Berghmans T., Levy A., Hasan B., Faivre-Finn C., et al. Definition of Synchronous Oligometastatic Non-Small Cell Lung Cancer—A Consensus Report. J Thorac Oncol [Internet] 2019;14(12):2109–2119. doi: 10.1016/j.jtho.2019.07.025. Available from: [DOI] [PubMed] [Google Scholar]
- 22.Guckenberger M., Lievens Y., Bouma A.B., Collette L., Dekker A., deSouza N.M., et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol [Internet] 2020;21(1):e18–e28. doi: 10.1016/S1470-2045(19)30718-1. Available from: [DOI] [PubMed] [Google Scholar]
- 23.Hellman S., Weichselbaum R.R. Oligometastases. J Clin Oncol. 1995;13(1):8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 24.Palma D.A., Salama J.K., Lo S.S., Senan S., Treasure T., Govindan R., et al. The oligometastatic state-separating truth from wishful thinking. Nat Rev Clin Oncol [Internet] 2014;11(9):549–557. doi: 10.1038/nrclinonc.2014.96. Available from: [DOI] [PubMed] [Google Scholar]
- 25.Christ S.M., Pohl K., Muehlematter U.J., Heesen P., Kühnis A., Willmann J., et al. Imaging-based prevalence of oligometastatic disease: A single-center cross-sectional study. Int J Radiat Oncol Biol Phys [Internet] 2022 doi: 10.1016/j.ijrobp.2022.06.100. S03 . Available from: 10.1016/j.ijrobp.2022.06.100. Epub ahead of print. PMID: 35908582. [DOI] [PubMed] [Google Scholar]
- 26.Galata C., Wimmer E., Kasper B., Wenz F., Reißfelder C., Jakob J. Multidisciplinary Tumor Board Recommendations for Oligometastatic Malignancies: A Prospective Single-Center Analysis. Oncol Res Treat. 2019;42(3):87–94. doi: 10.1159/000495474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.