Abstract
Damage to cartilage tissues is often difficult to repair owing to chronic inflammation and a lack of bioactive factors. Therefore, developing bioactive materials, such as hydrogels acting as extracellular matrix mimics, that can inhibit the inflammatory microenvironment and promote cartilage repair is crucial. Hyaluronic acid, which exists in cartilage and synovial fluid, has been extensively investigated for cartilage tissue engineering because of its promotion of cell adhesion and proliferation, regulation of inflammation, and enhancement of cartilage regeneration. However, hyaluronic acid-based hydrogels have poor degradation rates and unfavorable mechanical properties, limiting their application in cartilage tissue engineering. Recently, various multifunctional hyaluronic acid-based hydrogels, including alkenyl, aldehyde, thiolated, phenolized, hydrazide, and host–guest group-modified hydrogels, have been extensively studied for use in cartilage tissue engineering. In this review, we summarize the recent progress in the multifunctional design of hyaluronic acid-based hydrogels and their application in cartilage tissue engineering. Moreover, we outline the future research prospects and directions in cartilage tissue regeneration. This would provide theoretical guidance for developing hyaluronic acid-based hydrogels with specific properties to satisfy the requirements of cartilage tissue repair.
Keywords: Hyaluronic acid, Hydrogel, Functional modification, Cartilage tissue engineering
Graphical abstract
1. Introduction
Articular cartilage is a special soft tissue composed of chondrocytes and extracellular matrix (ECM) and is located on the surface of bone joints. It plays an important role in maintaining lubrication and joint movement [[1], [2], [3]]. Articular cartilage damage due to trauma, congenital anomalies, and diseases can often cause joint pain and motor dysfunction in patients [[4], [5], [6]]. Owing to the unique tissue structure of the articular cartilage, its repair and treatment after an injury has significant limitations and challenges; the limiting factors include the lack of blood vessels, nerves, and lymph, high ratio of ECM to cells, and elevated expression of proinflammatory factors [[7], [8], [9], [10], [11]]. A continuous provision of bioactive factors as well as the inhibition of the inflammatory response at the articular cartilage injury site is thus required.
Current treatment strategies for articular cartilage injury primarily rely on surgical treatments such as microfractures, subchondral drilling, and autologous chondrocyte transplantation. However, these strategies often have certain limitations, including infliction of trauma, low efficacy, and the formation of postoperative fibrocartilage, which can damage joint function [[12], [13], [14]]. Compared with the aforementioned strategies, biomedical hydrogels can provide a biomimetic ECM similar to natural cartilage. Hydrogels are a class of three-dimensional hydrophilic polymer networks with water in their matrix that can be loaded with cells, drugs, and bioactive molecules and maintain their activity; they have been widely used in cartilage damage repair [[15], [16], [17], [18]]. In cartilage tissue engineering, ideal hydrogels should have a biomimetic cartilage ECM to build an ecological microenvironment and multifunctional properties, including maintenance of cell viability and phenotype, reduction of fibrocartilage generation, suitable degradation rate, excellent biocompatibility, sustained release of drugs and growth factors, and reconstruction of the bone–cartilage interface [19].
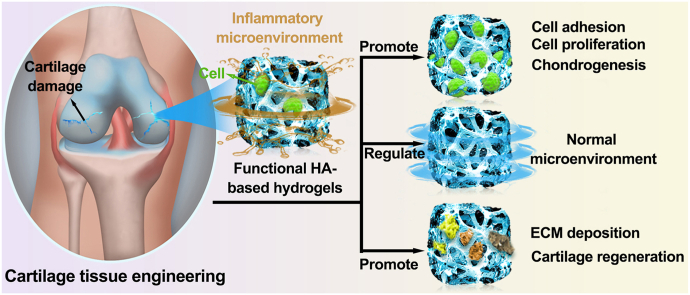
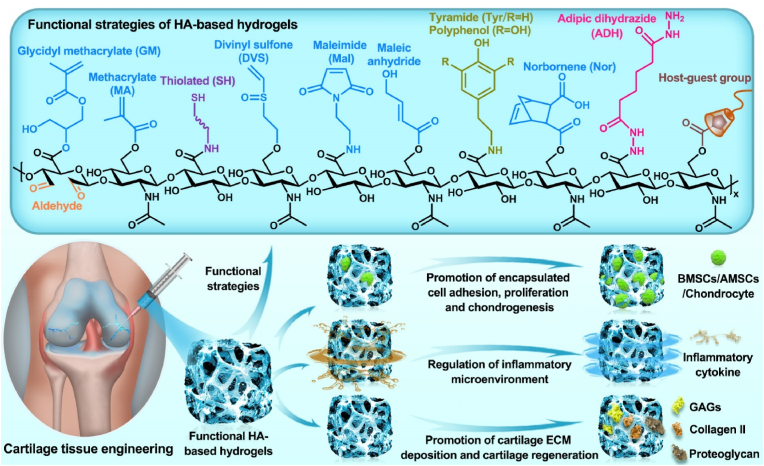
Polysaccharides have significant application potential in cartilage tissue engineering because of their similar structures to that of cartilage ECM components, high rheology and water retention, excellent biocompatibility, and antioxidant and anti-inflammatory properties [20]. Hyaluronic acid (HA) is a polysaccharide composed of alternating d-glucuronic acid and N-acetylglucosamine, which are naturally present in cartilage and synovial fluid [21,22]. Compared with other polysaccharides, HA affects the regulation of cartilage function and repair of cartilage damage in many ways. Previous studies have demonstrated that HA can improve the lubricity of cartilage boundaries, regulate inflammation at cartilage lesions, promote cell adhesion and proliferation, and ameliorate cartilage ECM deposition and cartilage regeneration, all of which have excellent application prospects in cartilage tissue engineering [[23], [24], [25]]. Notably, HA can recognize the HA receptor (cluster of differentiation 44, CD44) to protect cartilage and participate in the regeneration of cartilage ECM during cartilage repair [20]. Therefore, HA-related commercial products, such as Artz®, Cingal®, Durolane®, Hyalgan®, Monovisc®, Orthovisc®, Sinovial®, Supartz®, and Synvisc®, have been widely used for cartilage-related diseases. Currently, most related clinical studies are still in progress (Table 1) (www.clinicaltrials.gov). In addition, functional modification of HA-based hydrogels can effectively promote cartilage repair and regeneration by enhancing the adhesion, proliferation, and chondrogenesis of encapsulated stem cells and chondrocytes, regulating the inflammatory microenvironment at the cartilage injury site, and promoting cartilage ECM deposition, such as glycosaminoglycan (GAG), collagen II, and proteoglycans [20,26] (Fig. 1). In recent years, functional modification based on HA hydrogels in cartilage tissue engineering has primarily focused on the modification of three reactive functional groups in the molecular structure of HA: 1) esterification and acylation of carboxyl groups, 2) esterification and divinyl sulfone (DVS) crosslinking of primary hydroxyl groups, and 3) oxidation reaction of secondary hydroxyl groups [27,28] (Fig. 2).
Table 1.
Clinical trials of HA in cartilage related disease (Source: www.clinicaltrials.gov).
| Gov identifier | Sponsor | Phase | Disease | Interventions | Purpose |
|---|---|---|---|---|---|
| NCT03101163 | KLSMC Stem Cells, Inc. | Phase 2 | Articular Cartilage Disorder | Peripheral blood stem cells combined with HA (PBSCs/HA) | Evaluating the therapeutic effects between PBSCs/HA and standard treatment after subchondral drilling surgery |
| NCT00988091 | Ferring Pharmaceuticals | Phase 3 | Knee osteoarthritis | 1.2% sodium hyaluronate | Evaluating the therapeutic effects of 1.2% sodium hyaluronate through intra-articular injections |
| NCT01319461 | Med Pharma Co., Ltd. | Phase 3 | Knee osteoarthritis | Hyalgan® (sodium hyaluronate); Sterile normal saline | Evaluating the therapeutic effects between Hyalgan® and sterile normal saline through intra-articular injections |
| NCT01295580 | Bioventus LLC | Not Applicable | Knee osteoarthritis | Artz® (HA); Durolane® (HA stabilized) | Evaluating the safety and therapeutic effects between Artz® and Durolane® through intra-articular injections |
| NCT00556608 | IBSA Institut Biochimique SA | Phase 4 | Knee osteoarthritis | Sinovial® (sodium hyaluronate); Synvisc® (Hylan G-F 20) | Evaluating the safety and therapeutic effects between Sinovial® and Synvisc® through intra-articular injections |
| NCT02211521 | Samsung Medical Center | Phase 3 | Knee osteoarthritis | HA; Autologous platelet-rich plasma | Evaluating the therapeutic effects between HA and autologous platelet-rich plasma through intra-articular injections |
| NCT04165902 | Taipei Medical University | Phase 4 | Knee osteoarthritis | Steroid plus HA; Dextrose plus HA | Evaluating the additional therapeutic effects of steroid and dextrose to HA through injection |
| NCT03062787 | Pharmascience Inc. | Not Applicable | Knee osteoarthritis | Cingal® (HA plus triamcinolone hexacetonide); Monovisc® (sodium hyaluronate) | Evaluating the early effects between Cingal® and Monovisc® through intra-articular injections |
| NCT03211650 | University of Milan | Phase 4 | Knee osteoarthritis | HA and platelet-rich plasma (PRP) combination | Evaluating the therapeutic effects of HA and PRP combination through intra-articular injections |
| NCT01557868 | American Orthopaedic Society for Sports Medicine | Phase 4 | Knee osteoarthritis | Synvisc® (Hylan G-F 20); 1% sodium hyaluronate | Developing the algorithm to predict the therapeutic effects of HA products through intra-articular injections |
| NCT02984228 | Hospital for Special Surgery, New York | Phase 4 | Glenohumeral osteoarthritis | HA; Platelet-rich plasma (PRP) | Evaluating the therapeutic effects between HA and PRP through ultrasound-guided injections |
| NCT00918736 | Kaohsiung Veterans General Hospital | Phase 2 | Ankle osteoarthritis | Sodium hyaluronate | Evaluating the therapeutic effects of sodium hyaluronate through three weekly intra-articular injections |
| NCT04204278 | Anika Therapeutics, Inc. | Not Applicable | Ankle osteoarthritis | Monovisc® (sodium hyaluronate) | Evaluating the therapeutic effects of Monovisc® through single injections |
| NCT00436969 | DePuy Mitek | Phase 3 | Shoulder osteoarthritis | Orthovisc® (HA); Corticosteroid | Evaluating the therapeutic effects between Orthovisc® and corticosteroid through injections |
| NCT00479687 | Bioventus LLC | Not Applicable | Shoulder osteoarthritis | Supartz® (HA); Phosphate buffered saline (PBS) | Evaluating the therapeutic effects between Supartz® and PBS through injections |
Fig. 1.
Synthesis and biological function of HA-based hydrogel for cartilage tissue engineering.
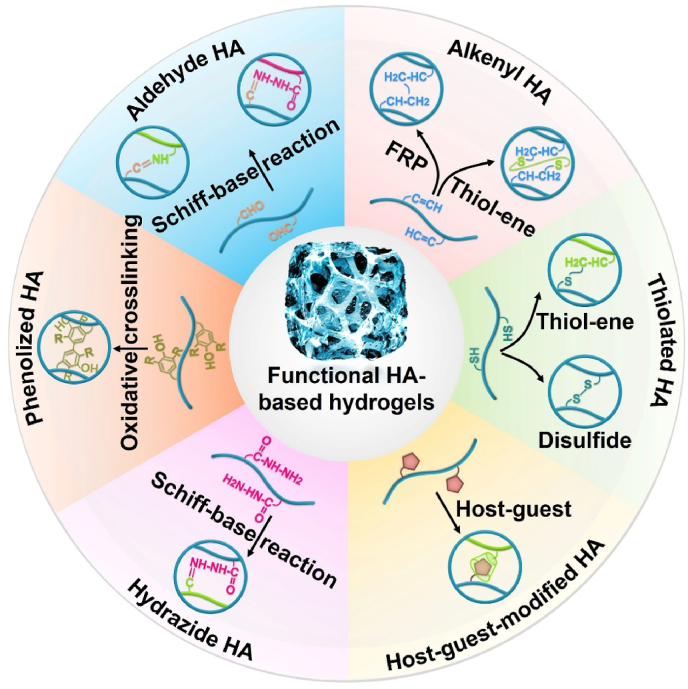
Fig. 2.
Functional modification of HA-based hydrogels.
In this paper, we discuss the functional molecular structure modification of HA-based hydrogels primarily containing alkenyl, aldehyde, thiolated, phenolized, hydrazide, and host–guest groups. We summarize their application in cartilage tissue engineering, and introduce the research status of HA-based hydrogels as 3D printing bioinks in cartilage engineering. We further elucidate the capacities and merits of different functionalized HA-based hydrogels to guide their research in cartilage tissue engineering. Finally, we highlight the advantages and challenges of current HA-based hydrogels for cartilage tissue-engineering applications.
2. Alkenyl HA-based hydrogels for cartilage tissue engineering
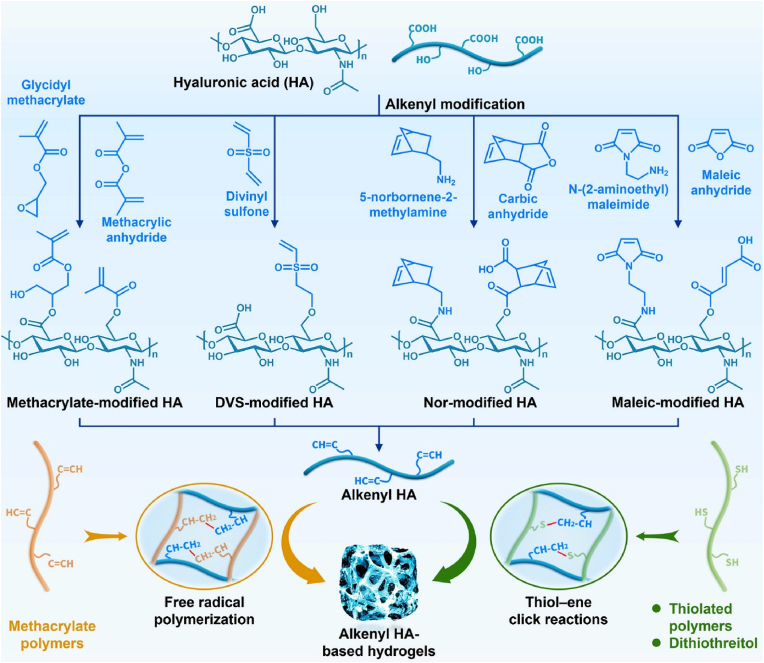
Alkenyl HA-based hydrogels have been widely studied in cartilage tissue engineering because they can be prepared easily by double bonds taking part in simple chemical reactions, such as self-polymerization through free-radical polymerization (FRP) reactions and click chemistry with sulfhydryl groups. The reactions often are fast speeds, occur in mild conditions, and exhibit desirable adaptability and high directional selectivity (Fig. 3 and Table 2).
Fig. 3.
Synthesis of alkenyl HA-based hydrogels for cartilage tissue engineering.
Table 2.
Alkenyl HA-based hydrogels for cartilage tissue engineering.
| Hydrogels | Components | Physicochemical properties | Biofunctions | Ref. |
|---|---|---|---|---|
| Methacrylated HA (HA-MA) | HA; methacrylic anhydride (MA) | Viscoelasticity; controlled pore size and degradation rate | Encapsulating cells; promoting MSCs proliferation, migration and chondrogenesis; promoting ECM deposition | [[30], [31], [32], [33], [34], [35]] |
| HA-MA/MeLAHA | HA, MA, MeLAHA | Hydrolytic degradation and enzymatic degradation | Promoting MSCs chondrogenesis and ECM deposition; ensuring stability of hydrogel scaffold | [36,37] |
| HA-MA/MMP7 | HA, MA, matrix metalloproteinase 7 | Tunable mechanical properties, swelling performance and degradation rates | Promoting MSCs chondrogenesis | [38,39] |
| HA-MA/Gel-MA | HA, MA, gelatin, | Improving the mechanical properties | Enhancing cell proliferation, aggregation and chondrogenesis; maintaining the chondrocyte phenotype; enhancing deposition and distribution of ECM; accelerating cartilage repair | [[40], [41], [42], [43], [44]] |
| HA-MA/Gel-MA/BC | HA, MA, gelatin, bacterial cellulose | Improving the mechanical properties and printing fidelity | Facilitating chondrocyte proliferation and protein expression | [45] |
| HA-MA/Gel-MA/AFnSi | HA, MA, gelatin, acrylate-functionalized nanosilica | Controllable pore sizes, swelling ratios and mechanical properties | Promoting the chondrogenic gene expression and ECM deposition | [46] |
| HA-MA/CNF | HA, MA, methacrylated cellulose nanofibers | Enhanced mechanical properties; decent restorability | Enhancing BMSCs proliferation and chondrogenesis; accelerating full-thickness cartilage repair | [48] |
| HA-MA/CF | HA, MA, chondrogenic fibrinogen | Ameliorating the compressive modulus | Enhancing BMSCs proliferation and early chondrogenesis; | [49] |
| HA-MA/ELP@ZnO | HA, MA, elastin-like polypeptide, zinc oxide | Adjustable mechanical strength, elasticity, and adhesion | Enhancing cell proliferation and migration; negligible inflammatory response; antibacterial ability | [50] |
| HA-MA/ROS-HP/PLGA | HA, MA, ROS-HP, poly (lactide-co-glycolide) | Suitable compressive modulus; good ROS scavenging ability | Regulating inflammation; promoting ECM deposition; accelerating hyaline cartilage regeneration and integration with surrounding tissues | [51] |
| HA-MA/F127DA | HA, MA, pluronic F127 diacrylate | Low swelling ratio; excellent stiffness, toughness and self-recovery | Enhancing thyroid cartilage regeneration of rabbit larynx | [52] |
| HA-MA/PRP | HA, MA, platelet-rich plasma | Suitable mechanical properties | Promoting ECM deposition and hyaline cartilage regeneration | [54] |
| HA-MA/SA/KGN-loaded PLGA | HA, MA, sodium alginate, kartogenin, poly (lactide-co-glycolide) | Microporous; promoting cells and tissues infiltration; sustained release of KNG | Regulating the inflammatory microenvironment; promoting migration and chondrogenesis of BMSCs; accelerating cartilage repair | [55,56] |
| HA-GM | HA, glycidyl methacrylate | Highly interconnected macroporous network; compression-recovery property | Maintaining the activity and phenotype of chondrocytes; promoting chondrocytes proliferation and ECM deposition | [57] |
| HA-DVS | HA, divinyl sulfone | Viscoelasticity; enzymatic degradation | Inducing the chondrogenic differentiation of AMSCs | [61] |
| HA-DVS/Li-BGF | HA, DVS, lithium bioactive glass fibers | Improving the elastic modulus | Promoting chondrocytes proliferation and chondrogenic behavior | [62] |
| HA-DVS/Gel-HS | HA, DVS, thiolated gelatin | Providing bioinspired microenvironment | Inhibiting vascularization and hypertrophy; promoting cartilage repair | [63] |
| HA-Nor/DTT | HA, 5-norbornene-2-methylamine, dithiothreitol | Controllable compressive moduli and CD44 binding | Promoting the deposition and distribution of ECM; promoting the chondrogenic differentiation of BMSCs | [[68], [69], [70], [71]] |
| HA-Nor/DTT | HA, carbic anhydride, dithiothreitol | Simple synthesis; controllable gelation properties, swelling and transmittance | Good cytocompatibility; encapsulating cells | [67] |
| HA-Mal/GelMA | HA, maleimide, gelatin, MA | Adjustable mechanical properties | Supporting the proliferation and chondrogenesis of BMSCs | [74] |
| HA-Mal/DTT | HA, maleimide, dithiothreitol | Adjustable mechanical strength | Maintaining BMSC stemness (lower strength), promoting BMSCs chondrogenesis (higher strength) | [75] |
| HA-Mal/PEG-SH | HA, maleic anhydride, thiol-terminated polyethylene glycol | High substitution degree; rapid gelation; regulatable degradation rate | Good cytocompatibility | [76] |
2.1. Methacrylate HA-based hydrogels
Methacrylic anhydride (MA) has a highly reactive anhydride group and photocrosslinkable C C double bonds, which can be easily grafted to natural polymers through the reaction of anhydride groups with amino groups or hydroxyl groups; moreover, functional polymers can be utilized to prepare hydrogels through photopolymerization [29]. Notably, an in situ photocrosslinkable methacrylate HA-based hydrogel with good viscoelasticity was prepared using esterification between the hydroxyl group of HA and the anhydride group of MA, which not only facilitated the encapsulation of cells but also reduced the mechanical stimulation of surrounding tissues [30]. The pore size, HA concentration, crosslinking density, and degradation rate of HA-MA-based hydrogels impact their application in cartilage tissue engineering [[31], [32], [33], [34], [35]]. Particularly, compared with other pore sizes, HA-MA-based hydrogels with medium pore sizes (200–250 μm) demonstrated the best promotion of endothelial cell proliferation and migration and exhibited the best vascularization behavior for cartilage repair [31]. Moreover, an increased concentration of HA in HA-MA-based hydrogels can enhance chondrogenesis and ECM deposition of encapsulated mesenchymal stem cells (MSCs), but it might cause the uneven distribution of the ECM [32,33]. In addition, the low crosslinking density (1% at 15 min exposure or 5% at 5 min exposure) of HA-MA (∼29% methacrylate modification)-based hydrogels can promote cartilage differentiation, whereas an increase in crosslinking density (5% at 15 min exposure) reduces the deposition and uniform distribution of cartilage ECM and induces hypertrophy and mineralization of MSCs [34]. In addition, the degradation rate of hydrogels plays an important role in cartilage repair. When the degradation rate is high, it is not beneficial for the retention of ECM; whereas when the degradation rate is low, it inhibits the formation of cartilage tissue [[35], [36], [37]]. Compared with collagen-based hydrogels, HA-MA-based hydrogels can maintain the proliferation of bone marrow MSCs (BMSCs) and the production of cartilage-related ECM in the later stages of cartilage tissue engineering because of their relatively stable physical microenvironment. However, the cartilage-inducing activity of HA-MA-based hydrogels cannot compete with that of collagen-based hydrogels in the early stages of cartilage tissue engineering [35]. To optimize the degradation rate of HA-MA-based hydrogels in vivo, Burdick et al. prepared a hybrid hydrogel using HA-MA and a novel HA macromer (MeLAHA, HA macromers with methacrylate modification and ε-caprolactone) with hydrolytic and enzymatic degradation performance, which promoted the chondrogenesis of MSCs by controlling the degradation property of the hydrogel. This is because faster degradation (complete degradation within 7 days) of MeCLHA provided void spaces for the deposition of ECM, and slower degradation (∼40% degradation in 56 days) of HA-MA ensured the stability of the hydrogel scaffold [36,37]. In addition, Tsanaktsidou et al. prepared another HA-MA-based hydrogel with two different crosslinking mechanisms, including FRP reaction and matrix metalloproteinase 7 (MMP7)-peptide-induced thiol-ene click reactions, which have tunable mechanical properties, swelling performance, and degradation rates. Interestingly, HA-MA-based hydrogels prepared using the MMP7-peptide in Dulbecco's modified Eagle's medium (DMEM) are beneficial for the chondrogenesis of human MSCs (hMSCs) because of their appropriate storage modulus of approximately 12 kPa [38]. Tsanaktsidou et al. further prepared a chondroitin sulfate (CS)-biofunctionalized HA-MA hydrogel by crosslinking the MMP7-peptide, which effectively enhanced hyaline cartilage regeneration by upgrading cartilage-related gene expression, GAG deposition, and chondrocyte cluster arrangement [39]. Therefore, the aforementioned reports provide significant guidance for the design and regulation of HA-MA-based hydrogels in cartilage tissue engineering.
In addition to the pore size, HA concentration, crosslinking density, and degradation rate of HA-MA-based hydrogels, mechanical properties and swelling rate influence cartilage tissue engineering. Studies have reported that suitable mechanical properties and a low swelling rate of HA-MA-based hybrid hydrogels can be achieved by incorporating natural polymers, which can effectively promote cartilage repair. For instance, Levett et al. prepared a hybrid hydrogel (HA-MA/Gel-MA) using HA-MA and gelatin (porcine skin) methacrylamide (Gel-MA) via photoinduced polymerization. The HA-MA component significantly improved the mechanical properties of the hydrogel, maintained the chondrocyte phenotype with rounded cell morphologies, and enhanced the deposition and distribution of ECM components, including collagen II and aggrecan, to accelerate cartilage formation. In addition, they incorporated CS methacrylate into an HA-MA/Gel-MA hybrid hydrogel to improve its mechanical properties, which enhanced chondrogenesis as well as the spatial distribution of the ECM [40,41]. Moreover, Lin et al. further demonstrated that the HA-MA/Gel-MA hybrid hydrogel can accelerate cartilage repair in vivo by promoting the chondrogenesis of hMSCs and the production of GAG when the concentration ratio of HA-MA and Gel-MA is 1:9 [42]. Interestingly, Wang et al. used HA-MA/Gel-MA hybrid hydrogels to encapsulate chondrocyte spheroids prepared using the hanging drop method for cartilage repair. Compared with chondrocytes, chondrocyte spheroids can form structures similar to native cartilage tissue, which can enhance cell proliferation and aggregation, maintain the cellular phenotype, and promote the deposition of abundant ECM [43]. Furthermore, compared with porcine-derived Gel-MA, the HA-MA/Gel-MA hybrid hydrogel constructed using bovine-derived Gel-MA and HA-MA through irgacure 2959-induced photocrosslinking (365 nm) exhibited the best similarity to native articular cartilage after 28 days of chondrocyte cultivation [44]. The mechanical properties of HA-MA/Gel-MA hybrid hydrogels can be further optimized by incorporating organic polymers or inorganic nanoparticles [45,46]. For example, Sang et al. incorporated bacterial cellulose (BC) into an HA-MA/Gel-MA hybrid hydrogel, which not only improved the mechanical properties and printing fidelity of the hydrogel but also facilitated chondrocyte proliferation and specific protein expression [45]. Nedunchezian et al. used acrylate-functionalized nanosilica (AFnSi) to crosslink HA-MA/Gel-MA hybrid hydrogels, and the obtained hydrogels exhibited controllable pore sizes, swelling ratios, and mechanical properties. Moreover, the hydrogels could promote the gene expression of chondrogenic markers, including SOX9, proteoglycan, and collagen II, in human adipose-derived MSCs (AMSCs) and increase the formation of sulfated GAG (sGAG) and collagen II [46].
In addition to Gel-MA, the incorporation of other natural polymers into HA-MA-based hydrogel matrices can optimize their mechanical properties and promote the chondrogenesis of cells [47]. For example, Zhao et al. fabricated a nanocomposite hydrogel (HA-MA/CNF) through the co-crosslinking of HA-MA and methacrylated cellulose nanofibers (CNFs); the HA-MA/CNF hydrogel exhibited enhanced mechanical properties with a compressive modulus of ∼0.46 MPa, a compressive strength of ∼0.198 MPa, and satisfactory restorability. Moreover, biological experiments have indicated that the nanocomposite hydrogel can have positive effects on the proliferation and chondrogenesis of BMSCs as well as accelerate full-thickness cartilage repair in vivo [48]. Snyder et al. developed a hybrid hydrogel comprising HA-MA and chondrogenic fibrinogen to ameliorate the compressive modulus of HA-MA-based hydrogels, which provided a suitable microenvironment for the proliferation and delivery of BMSCs as well as increased expression of SOX9 and decreased collagen I, suggesting that it was favorable for early chondrogenesis [49]. Sani et al. prepared a bioactive hydrogel (HA-ELP) using HA-MA and elastin-like polypeptide (ELP), which had adjustable mechanical properties including mechanical strength, elasticity, and adhesion. Meanwhile, the HA-ELP hydrogel supported cell proliferation and migration but had a negligible inflammatory response in vivo. Moreover, the HA-ELP hydrogel exhibited high antibacterial activity against drug-resistant bacteria after doping with zinc oxide (ZnO) nanoparticles, demonstrating good application potential in tissue engineering such as cartilage repair [50]. In addition, compared with natural polymers, synthetic polymers have also been reported to optimize mechanical properties and endow multifunctionality to HA-MA-based hydrogels [51,52]. Wu et al. designed a novel hybrid scaffold by filling an antioxidant hydrogel composed of HA-MA and reactive oxygen species (ROS)-responsive hyperbranched polymers (ROS-HP) into a poly (lactide-co-glycolide) (PLGA) scaffold with radial orientation. The hybrid scaffold had a suitable compressive modulus (1.5 MPa), good ROS-scavenging ability, and ability to regulate inflammation to accelerate hyaline cartilage regeneration. Moreover, the incorporation of hydrogel had a positive impact on the deposition of GAG and collagen II to integrate with surrounding tissues for neocartilage [51]. Ren et al. developed a stiff micelle-crosslinked hydrogel using HA-MA and Pluronic F127 diacrylate (F127DA), which exhibited a low swelling ratio and excellent mechanical properties, including stiffness, toughness, and self-recovery. The built hydrogel was further used in thyroid cartilage defects of rabbit larynx, and animal studies showed that the hydrogel effectively contributed to cartilage regeneration [52].
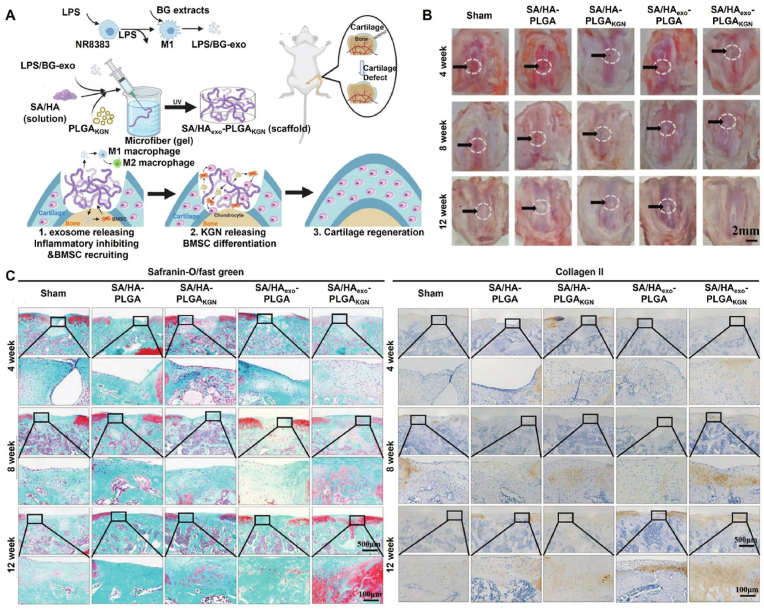
Cartilage can generally be divided into three types: hyaline, elastic, and fibrocartilage. Notably, the regeneration of hyaline cartilage is crucial in articular cartilage repair [53]. Yan et al. encapsulated platelet-rich plasma (PRP) in an HA-MA hydrogel to increase the deposition of GAG and collagen II. In addition, the regeneration of hyaline cartilage was verified without hypertrophic cartilage formation in a porcine femoral condyle cartilage injury model [54]. Shi et al. incorporated small molecule kartogenin (KGN)-loaded nanoparticles into HA-MA hydrogels to recruit and facilitate chondrogenesis of different endogenous cells (BMSCs, synovium-derived MSCs (SMSCs), and chondrocytes). Furthermore, the hydrogel could fill the defect and release KGN continuously to promote the formation of hyaline cartilage in a cartilage defect model of rabbit knee joints [55]. To avoid poor cell and tissue infiltration caused by nanoporous hydrogels, Ma et al. prepared a microporous hydrogel using HA-MA, sodium alginate (SA), and KGN-loaded PLGA microspheres using photopolymerization after crosslinking HA-MA/SA into microfibers to effectively promote the infiltration of cells and tissues. In addition, the hydrogel can gradually release exosomes and KGN-loaded PLGA microspheres, which can regulate the inflammatory microenvironment, enhance the migration of endogenous BMSCs, induce chondrogenesis of BMSCs, and promote cartilage repair (Fig. 4) [56].
Fig. 4.
Schematic illustration of the fabrication of SA/HAexo-PLGAKGN scaffold for cartilage regeneration. (A) Preparation of SA/HAexo-PLGAKGN scaffold and its biological function; (B) gross images of the cartilage defects in different groups at weeks 4, 8, and 12 after surgery; (C) immunohistochemical staining images of cartilage defects in different groups after surgery for 4, 8, and 12 weeks [56]; Copyright 2022, John Wiley and Sons.
In addition to the HA-MA hydrogel, He et al. fabricated a shape-memory-capable cryogel (HA-GM) using HA and glycidyl methacrylate (GM). Compared with HA-GM hydrogels, HA-GM cryogels with a highly interconnected macroporous network structure and compression-recovery property can provide a suitable microenvironment to maintain the activity and phenotype of chondrocytes during injection, as well as promote the proliferation of chondrocytes and the deposition of cartilage ECM [57]. Hsieh et al. developed a biomimetic scaffold using HA-GM, methoxy poly (ethylene glycol)-block-poly (ε-caprolactone) (mPEG-PCL), and hydroxyapatite loaded with transforming growth factor-β1 (TGF-β1) for the regeneration of hyaline cartilage and the ingrowth of bone tissue into the scaffold after implantation [58].
2.2. Divinyl sulfone-modified HA-based hydrogels
Recently, DVS has attracted considerable interest because of its hydrolysis resistance and broad-spectrum reactivity. It can modify biochemical molecules by reacting with sulfhydryl, amino, and hydroxyl groups under certain conditions [59,60]. Mondal et al. prepared an injectable hydrogel (HA-DVS) using HA and different concentrations of DVS via the reaction between hydroxyl and vinyl sulfone groups. The HA-DVS hydrogel exhibited viscoelasticity and enzymatic degradation ability and could induce the chondrogenesis of AMSCs [61]. Riveiro et al. further significantly improved the elastic modulus of HA-DVS hydrogels by doping them with lithium bioactive glass fibers (Li-BGF), which promoted the proliferation and chondrogenic behavior of chondrocytes [62]. Feng et al. developed a BMSC-laden bio-inspired hydrogel using HA-DVS and thiolated gelatin (Gel-HS) to enhance cartilage repair. The obtained hydrogels provided a good microenvironment for cell loading and minimally invasive delivery, protecting and maintaining the viability of BMSCs, and demonstrating positive effects on proliferation, chondrogenesis, and self-assembly into cartilage-like tissues, ultimately through interconnected cells. In addition, hydrogels can inhibit vascularization and hypertrophy, and contribute to cartilage repair [63]. HA-DVS-based hydrogels exhibited promising potential for the treatment of knee osteoarthritis (OA) in clinical trials. Park et al. evaluated the therapeutic effect of a novel HA-DVS hydrogel (YYD302) produced by biological fermentation on human knee OA after intra-articular injection. Compared with Synovian®, an HA-based hydrogel crosslinked with 1,4-butanediol diglycidyl ether, YYD302, could reduce the weight-bearing pain of patients after a single injection for 12 weeks and exhibited a better treatment effect at 2 weeks. In addition, YYD302 demonstrated good biosafety in local and systemic adverse drug reaction (ADR) evaluations [64]. In et al., the efficacy and safety of a single intra-articular injection of YYD302 in patients with knee OA were evaluated. Compared with phosphate-buffered saline, YYD302 effectively reduced pain and improved knee function, and the efficacy of a 2 mL dose was better than that of a 3 mL dose [65].
2.3. Norbornene-modified HA-based hydrogels
Norbornene (Nor)-thiol photocurable hydrogels, another type of material system with high application value in tissue engineering, are typically prepared using Nor-modified macromolecules (HA, gelatin, and polyethylene glycol (PEG)) and sulfhydryl-containing linkers. Such hydrogels have been demonstrated to have tunable biophysical and biochemical properties and good biocompatibility [66]. Compared with the widely studied HA-MA-based hydrogels, nor-modified HA (HA-Nor)-based hydrogels have attracted significant interest in biomedical research because of their low photoinitiator requirements and more stable and tailored structures [67]. Galarraga et al. prepared HA-Nor using HA and 5-norbornene-2-methylamine using a two-step method, including ion-exchange resins and catalytic reaction, and further crosslinked HA-Nor with dithiothreitol (DTT) to prepare hydrogels (HA-Nor-DTT) with different compressive moduli. Compared with stiffer (∼6–60 kPa) hydrogels, softer hydrogels (∼2 kPa) can provide a favorable local microenvironment to promote the deposition and distribution of ECMs, which promotes the chondrogenesis of encapsulated BMSCs. Moreover, the doping of polycaprolactone (PCL) microfibers produced by melt-electrowriting into softer hydrogels can solve the problems of poor initial mechanical properties and poor stability and promote neocartilage regeneration and integration [68]. The HA-Nor-DTT hydrogel has been used as a 3D bioprinting ink for cartilage tissue engineering [69]. Vega et al. further developed a combinatorial hydrogel platform with a biochemical gradient using an HA-Nor-DTT hydrogel and monothiolated peptides, which can be used to analyze early gene expression and long-term cartilage ECM of encapsulated MSCs. This platform can function as a scalable high-throughput technique for screening 3D cellular microenvironments [70]. Subsequently, to elucidate the influence of HA modification on CD44 binding, Kwon et al. evaluated the interaction of HA-Nor or HA-MA with different degrees of modification of CD44 and the effect on chondrogenesis of encapsulated MSCs. The results showed that the degree, type, and site of HA modification affected the binding of CD44. Low (∼10%) and moderately (∼20%) modified HA significantly promoted binding to CD44 and upregulate chondrogenesis of encapsulated MSCs [71]. The abovementioned synthesis of HA-Nor involved multiple synthetic steps, which were time-consuming and caused undesired contamination. To realize the simple synthesis of HA-Nor, Xiao et al. designed a simple and green method to synthesize HA-Nor using HA and carbic anhydride through esterification and further crosslinked HA-Nor with DTT to prepare hydrogels. The hydrogel exhibited controllable gelation properties, swelling, and transmittance and was suitable for cell encapsulation owing to its good cytocompatibility. Notably, the additional carboxyl group in HA-Nor prepared using this method provided a reactive site for further modification [67].
2.4. Maleic-modified HA-based hydrogels
In addition to the abovementioned molecules for grafting double C C bond groups, maleic derivatives are other widely used reagents for modifying polymers with amino or hydroxyl groups [72,73]. Ren et al. prepared a hydrogel with a double network structure crosslinked between maleimide-modified HA (HA-Mal) and Gel-MA to support the proliferation and chondrogenesis of BMSCs, where cells were seeded on the surface or inside the hydrogel [74]. Ren et al. further evaluated the effect of hydrogels with different mechanical strengths prepared using HA-Mal with different molecular weights (4, 10, and 90 kDa) and DTT on the stemness and chondrogenesis of BMSCs. Hydrogels with lower mechanical strength can maintain the stemness properties of BMSCs, whereas hydrogels with higher mechanical strength can promote chondrogenesis of BMSCs by increasing the expression of SOX9 and collagen II [75]. In addition, in contrast to the low methacrylate substitution (<30%) due to the hydrolysis of methacrylic anhydride and sensitivity to pH and temperature, Zhang et al. prepared a photocrosslinked porous hydrogel using maleic anhydride-modified HA with a high substitution degree (2.27) of acrylate groups and thiol-terminated PEG (PEG-SH). The designed hydrogel exhibited a rapid gelation time of only 15 s, regulatable degradation rate, and good cytocompatibility [76].
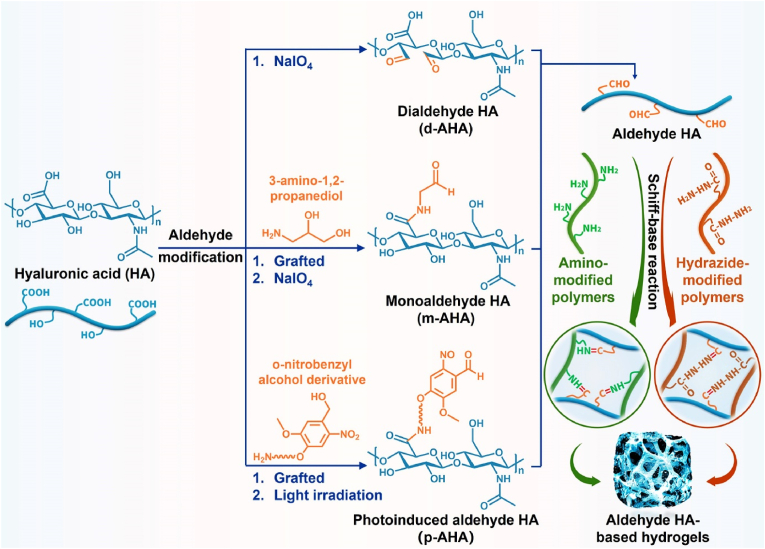
3. Aldehyde HA-based hydrogels for cartilage tissue engineering
Aldehyde HA-based hydrogels have been widely studied in cartilage tissue engineering because the aldehyde group can undergo a Schiff-base reaction with polymers containing amino and hydrazide groups. The reactions often exhibit the advantages of high speed, simple and mild conditions, and no chemicals. Aldehyde HA can be divided into dialdehyde HA (d-AHA), monoaldehyde HA (m-AHA), and photoinduced aldehyde HA (p-AHA) (Fig. 5 and Table 3).
Fig. 5.
Synthesis of aldehyde HA-based hydrogels for cartilage tissue engineering.
Table 3.
Aldehyde HA-based hydrogels for cartilage tissue engineering.
| Hydrogels | Components | Physicochemical properties | Biofunctions | Ref. |
|---|---|---|---|---|
| d-AHA/Chitosan | Dialdehyde HA, chitosan | Controllable stiffness; loading gapmer oligonucleotides | Maintaining chondrocytes spherical morphology and uniform distribution; promoting ECM deposition; inhibiting inflammation | [79,80] |
| d-AHA/Glycol chitosan | Dialdehyde HA, glycol chitosan, cartilage ECM particles | Suitable compression stress; degradability | Promoting ECM deposition; accelerating cartilage regeneration and integration with surrounding tissues | [81] |
| d-AHA/water-soluble chitosan | Dialdehyde HA, succinic anhydride-modified chitosan | Tunable degradation rate and mechanical properties | Supporting the adhesion and proliferation of chondrocytes; maintaining the chondrocytes phenotype | [82] |
| d-AHA/RSV | Dialdehyde HA, amino-functionalized resveratrol | Interconnecting pores | maintaining the chondrocyte phenotype, promoting ECM deposition; reducing inflammation | [84] |
| d-AHA-MA | Dialdehyde HA, methacrylic anhydride | Higher adhesive strength and adhesion | Accelerating cartilage repair | [85] |
| m-AHA/Gel-CDH | Monoaldehyde HA, carbohydrazide-modified gelatin | Higher stability; slower degradation rate | Good biocompatibility | [86,87] |
| m-AHA (aldol crosslinked) | Enolizable monoaldehyde HA, nonenolizable monoaldehyde HA | Fast gelation; excellent hydrolytic stability; good tissue adhesion | Good cytocompatibility | [88] |
| m-AHA/HA-CDH and HA/CSA·dHCl | Monoaldehyde HA, carbohydrazide-modified HA; HA; cysteamine dihydrochloride | Biphasic, porous and dominant elasticity | Layer-specific inducibility; promoting chondrogenesis of BMSCs and ECM deposition; promoting the osteogenesis of BMSCs | [89] |
| HA-NB/PRP | HA, o-nitrobenzyl alcohol, platelet-rich plasma | Tissue adhesion, controlled release property of growth factor | Accelerating hyaline cartilage regeneration | [90] |
d-AHA was synthesized through the oxidation of secondary hydroxyl groups using sodium periodate (NaIO4) and then crosslinked with polymers with amino groups using a Schiff-base reaction to engineer hydrogels [77]. In cartilage tissue engineering, chitosan-based hydrogels with many amino groups can provide a suitable microenvironment for chondrocyte adhesion, proliferation, and differentiation [78]. Thus, Thomas et al. proved that the stiffness of a hydrogel (HA/chitosan) prepared using d-AHA and chitosan through a Schiff-base reaction had important effects on the growth and functionality of encapsulated chondrocytes. The chondrocytes exhibited a spherical morphology and uniform distribution in the HA/chitosan hydrogel group with less stiffness, whereas better deposition of ECM components, such as GAG and collagen II, was observed in the stiffer hydrogel group [79]. Cai et al. further used HA/chitosan hydrogels loaded with gapmer oligonucleotides to inhibit inflammation in OA chondrocytes [80]. Owing to the strong interactions among intermolecular hydrogen bonds, chitosan is almost insoluble in physiological solvents. Liu et al. developed a hydrogel with a compression stress of 90 kPa at 60% compression strain and with degradable ability using d-AHA, water-soluble glycol chitosan, and cartilage ECM particles incorporated with BMSCs for cartilage repair. The encapsulated BMSCs demonstrated positive activity and proliferation, along with better deposition of GAG and collagen II, which could accelerate cartilage regeneration and integration with surrounding tissues [81]. Tan et al. prepared a composite hydrogel with a tunable degradation rate and mechanical properties consisting of d-AHA and water-soluble chitosan modified with succinic anhydride, which could support the adhesion and proliferation of chondrocytes as well as maintain their phenotype [82]. In addition, Heirani-Tabasi et al. prepared a chitosan-functionalized HA hydrogel through acylation between carboxyl and amino groups loaded with chondrocyte extracellular vesicles, and the developed hydrogel could upgrade the chondrogenic gene (SOX9 and COL2A1) expression of encapsulated AMSCs and induce the deposition of collagen II and GAG to accelerate hyaline cartilage regeneration in OA cartilage injury [83]. In addition to the aforementioned hydrogels prepared using HA and chitosan, Sheu et al. used d-AHA and amino-functionalized resveratrol (RSV) to prepare hydrogels to maintain the chondrocyte phenotype, promote the deposition of cartilage ECM, and reduce lipopolysaccharide-induced inflammation and chondrocyte injury, making them ideal candidates for use as chondrocyte carriers [84]. Chen et al. developed a tissue-adhesive one-component hydrogel (d-AHA-MA) that could promote the proliferation and migration of BMSCs. Compared with the adhesive strength of commercial fibrin glue (∼10 kPa) and HA-MA hydrogel (∼20 kPa), the d-AHA-MA hydrogel exhibited higher adhesive strength (more than 40 kPa) and could adhere to native cartilage tissue stably and assist the integration between neo-cartilage and host tissues, facilitating better cartilage regeneration [85].
In addition, unlike d-AHA, m-AHA can be obtained by oxidizing the diol groups grafted onto HA. For example, Hozumi et al. used 3-amino-1,2-propanediol to premodify HA, followed by the oxidation of NaIO4 to fabricate m-AHA, which was reacted with carbohydrazide (CDH)-modified gelatin (Gel-CDH) to prepare a novel injectable hydrogel (m-AHA/Gel-CDH) through a Schiff-base reaction. Compared with the hydrogels formed from d-AHA and Gel-CDH or adipic dihydrazide (ADH)-modified gelatin (Gel-ADH), the m-AHA/Gel-CDH hydrogel exhibited higher stability and a slower degradation rate because the Schiff-base bond formed by CDH and monoaldehyde was more stable, and the ring-opening structure of polysaccharides was susceptible to hydrolysis. Moreover, the m-AHA/Gel-CDH hydrogel had a pore size of 15–55 μm, a shear storage modulus of 0.1–1 kPa, and good biocompatibility, which are appropriate for hydrogel scaffolds for soft tissue engineering [86]. Martinez-Sanz et al. further demonstrated that a hydrazone-crosslinked hydrogel with insignificant cytotoxicity formed from hydrazide-modified HA and m-AHA was stable under physiological conditions and degradable by hyaluronidase. The hydrogel can controllably release the encapsulated protein and maintain its activity, which has good application prospects in tissue engineering [87]. Bermejo-Velasco et al. prepared the first aldol-crosslinked HA-based hydrogel composed of two different m-AHAs: an enolizable m-AHA (HA-Eal) and a nonenolizable m-AHA (HA-Nal). The hydrogel had controllable gelation time, excellent hydrolytic stability, hyaluronidase degradability, good tissue adhesion, and good cytocompatibility. Note that the addition of HA-Nal can improve the crosslinking efficiency of the hydrogel owing to the formation of an intramolecular loop in pure HA-Eal hydrogels [88]. Under cartilage tissue engineering, Chen et al. prepared an HA-based biphasic hydrogel scaffold using different cross-linking methods to promote osteochondral defect regeneration owing to its layer-specific inducibility. The upper layer of the scaffold with stress relaxation was composed of m-AHA and CDH-modified HA, which can significantly promote the chondrogenesis of BMSCs and deposition of cartilage ECM. The lower layer of the scaffold with porous and dominant elasticity was prepared by retaining the gas generated during the gelation process of HA and cysteamine dihydrochloride, which effectively promoted the osteogenesis of BMSCs. The biphasic hydrogel significantly promoted osteochondral regeneration, similar to native osteochondral tissue in vivo [89].
Distinct from the Schiff-base reaction, the photopolymerization reaction is also a typical method for fabricating p-AHA-based hydrogels. For example, Liu et al. developed an in situ photocrosslinkable composite hydrogel consisting of PRP and o-nitrobenzyl alcohol (NB)-modified HA (HA-NB, NB graft ratio: ∼6.0%) that can generate aldehyde groups under light irradiation. The photoinduced crosslinked hydrogel exhibited a tissue adhesive strength of approximately 25 kPa and a controlled release of growth factors for hyaline cartilage regeneration [90].
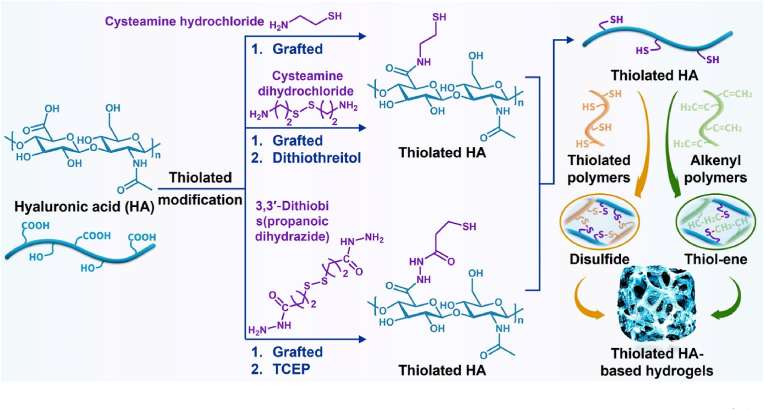
4. Thiolated HA-based hydrogels for cartilage tissue engineering
Thiolated polymers have a high reactive activity because of their thiol groups and can be further used to prepare hydrogels by self-crosslinking intrachain disulfide bonds or thiol-ene click reactions with rapid gelling time, which have been extensively studied in tissue engineering [91]. Therefore, thiolated HA-based hydrogels have been widely studied for cartilage tissue engineering applications (Fig. 6 and Table 4).
Fig. 6.
Synthesis of thiolated HA-based hydrogels for cartilage tissue engineering. TCEP: Tris (2-carboxyethyl)phosphine hydrochloride.
Table 4.
Thiolated HA-based hydrogels for cartilage tissue engineering.
| Hydrogels | Components | Physicochemical properties | Biofunctions | Ref. |
|---|---|---|---|---|
| HA-SH | HA, cysteamine hydrochloride | Regulatable gelling time, mechanical properties, swelling and degradability | Supporting the culture and delivery of chondrocytes | [92] |
| HA-SH/peptides | HA, cysteamine hydrochloride, supramolecular peptides | Excellent mechanical properties; abundant cell adhesion sites | Enhancing cartilage-related gene expression and ECM deposition; inhibiting chondrocyte hypertrophy | [93] |
| HA-SH/TGF-β1 | HA, 3,3′-dithiobis (propanoic dihydrazide), transforming growth factor-β1 | Suitable gelation and swelling behavior, sustained release of TGF-β1 | Promoting chondrogenic gene expression and ECM deposition and distribution | [94,95] |
| HA-SH/PEG-DVS | HA, cysteamine dihydrochloride, 4-arm polyethylene glycol, divinyl sulfone | Adjustable gelling time; enzymatic degradation | Maintaining the viability and proliferation of chondrocytes; promoting ECM deposition | [96] |
| HA-SH/HB-PEG | HA, 3,3′-dithiobis (propanoic dihydrazide), hyperbranched polyethylene glycol diacrylate | Regulatable mechanical properties; appropriate degradation rates | Supporting chondrogenic differentiation; promoting hyaline cartilage regeneration | [[97], [98], [99]] |
| HA-SH/p (HPMAm-lac)-PEG | HA, 3,3′-dithiobis (propanoic dihydrazide), thermosensitive triblock copolymers | Sustained release of HA | Anti-inflammatory ability; inducing chondrogenic differentiation of BMSCs and neo-cartilage formation | [100,101] |
| HA-SH/Col | HA, cysteamine hydrochloride, collagen I | Improving the adhesion of cells | Maintaining chondrocytes phenotype; promoting ECM deposition | [103] |
| HA-SH/Col/BCP | HA, cysteamine hydrochloride, collagen I, biphasic calcium phosphate ceramics | Double-layer structure to mimic the condylar osteochondral | Enhancing the regeneration of fibrocartilage and subchondral bone | [104] |
| HA-SH/Col/icariin-SH | HA, cysteamine hydrochloride, collagen I, thiolated icariin | Controlled-release of bioactive molecules | Inhibiting chondrocyte hypertrophy and dedifferentiation | [105] |
| HA-SH/Col/HA-Mal | HA, cysteamine hydrochloride, collagen I, maleimide | Biomimetic injectable hydrogel; double-crosslinked network | Promoting the adhesion and proliferation of chondrocytes, GAG deposition and hyaline cartilage formation | [106] |
| HA-SH/Gel | HA, cysteamine hydrochloride, gelatin | Different bonding intensities | Promoting chondrocyte proliferation; maintenance of the hyaline cartilage phenotype; (strong bonding strength) | [107] |
Bian et al. fabricated an injectable thiolated HA (HA-SH) hydrogel with regulatable gelling time, mechanical properties, swelling, and degradability through self-crosslinking, which could serve as a 3D scaffold for the culture and delivery of chondrocytes [92]. Wang et al. developed a bionic composite hydrogel based on HA-SH and supramolecular peptides using noncovalent supramolecular self-assembly, and the self-assembled hydrogel exhibited excellent mechanical properties and abundant cell adhesion sites. These peptides had beneficial effects on hyaline cartilage formation and phenotype maintenance by promoting cell adhesion and proliferation, enhancing cartilage-related gene expression and ECM deposition, and inhibiting chondrocyte hypertrophy [93]. In contrast to noncovalent crosslinking, Böck et al. and Hauptstein et al. introduced TGF-β1 into HA-SH based hydrogels using covalent bonds via Traut's reagent. Better chondrogenesis of BMSCs was verified by promoting chondrogenic gene expression and ECM deposition and distribution [94,95].
Hydrogels prepared based on double C C bond-functionalized PEG and HA-SH via the thiol-ene Michael addition reaction have been widely studied in cartilage tissue engineering. For example, Jin et al. prepared an injectable hydrogel with an adjustable gelling time (less than ∼5 min) and enzymatic degradation consisting of HA-SH and 4-arm PEG-DVS, which could be advantageous for the viability and proliferation of bovine chondrocytes and deposition of GAG and collagen II [96]. Li et al. fabricated a rapid in situ crosslinkable hydrogel (HA-SH/HB-PEG) comprising HA-SH and hyperbranched PEG diacrylate with encapsulated arthroscopic flushing fluid-derived MSCs. The obtained hydrogel can support chondrogenesis and hyaline cartilage regeneration in full-thickness cartilage injuries in rats [97]. In addition, the HA-SH/HB-PEG hydrogel can serve as a cellular carrier with an anti-inflammatory ability to aid the viability and proliferation of cartilage-derived progenitor cells (CPCs), together with ECM deposition [98]. Wang et al. designed an injectable drug delivery system based on an HA-SH/HB-PEG hydrogel loaded with RSV-containing PLGA microspheres, which exhibited regulatable mechanical properties and appropriate degradation rates for ECM deposition. The delivery system quickly filled cartilage defects in vivo for hyaline cartilage regeneration through the controlled and sustained release of RSV [99]. Sabbieti et al. and Agas et al. prepared a hybrid hydrogel (HA-p (HPMAm-lac)-PEG) according to HA-SH and thermosensitive triblock copolymers (p (HPMAm-lac)-PEG) (composed of poly (N-(2-hydroxypropyl) methacrylamide lactate) and PEG) by thermal gelation and Michael addition. The hybrid hydrogel can be used as a carrier for cells and proteins and exhibits good biocompatibility and anti-inflammatory ability through the sustained release of HA. In addition, the HA-p (HPMAm-lac)-PEG hydrogel can also induce chondrogenesis of BMSCs and neo-cartilage formation [100,101].
Collagen I has been extensively used in cartilage tissue engineering owing to its high biocompatibility, low immunogenicity, biodegradability, and availability. However, its poor mechanical properties and rapid degradation rate cannot be ignored [102]. To resolve these limitations, Chen et al. fabricated an injectable hybrid hydrogel (HA-SH/Col) by combining collagen I with an HA-SH hydrogel to alleviate the contraction of collagen I and improve the adhesion of cells on the HA-SH hydrogel. The HA-SH/Col hydrogel can be useful for the proliferation and phenotype of chondrocytes as well as the deposition of ECM [103]. Wang et al. prepared a double-layer hybrid scaffold based on HA-SH/Col hydrogel and biphasic calcium phosphate (BCP) ceramics to mimic the structure of rabbit condylar osteochondral bone. In a condylar osteochondral injury model, the scaffold enhanced the regeneration of fibrocartilage and subchondral bone with a similar structure and thickness to host cartilage and seamless integration by loading BMSCs and chondrocytes on the upper and lower layers, respectively [104]. Liu et al. developed an injectable thiolated icariin-functionalized HA-SH/Col hydrogel for chondrocyte proliferation and cartilage ECM secretion and integration while inhibiting chondrocyte hypertrophy and dedifferentiation [105]. Yao et al. prepared a biomimetic injectable hydrogel scaffold combined with collagen I and a double-crosslinked network hydrogel (self-crosslinking of HA-SH and click-crosslinking between HA-SH and HA-Mal) to simulate cartilage ECM and promote the adhesion and proliferation of chondrocytes, GAG deposition, and hyaline cartilage formation [106]. Based on HA-SH and gelatin derivatives, Wang et al. prepared three composite hydrogels with different bonding intensities, including physical mixing, weak, and strong bonds. They further evaluated the influence of bonding strength on chondrogenic phenotypes. Compared with hydrogels with physical mixing and weak bonding strength, hydrogels with strong bonding strength exhibit better physical properties, such as a uniform pore structure, water retention performance, and anti-degradation ability. As a result, they can promote chondrocyte proliferation and phenotypic maintenance of hyaline cartilage [107].
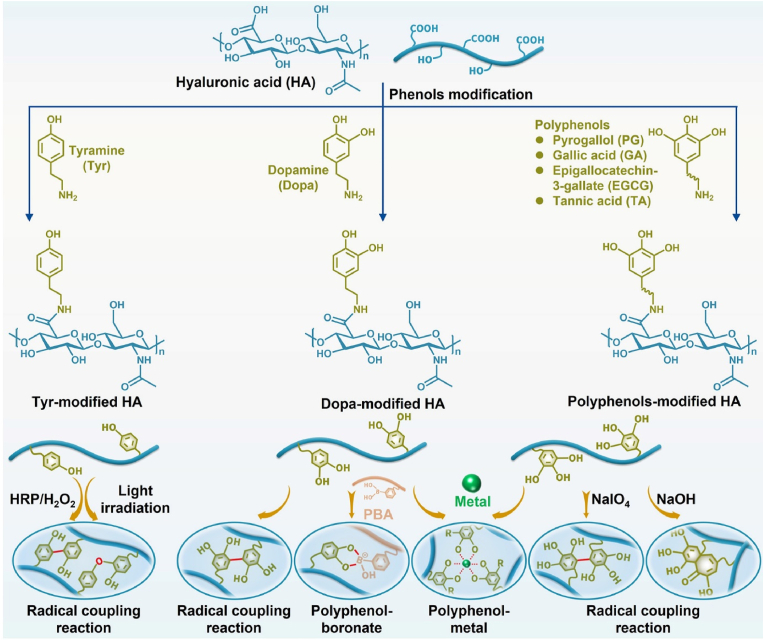
5. Phenolized HA-based hydrogels for cartilage tissue engineering
Polyphenols are widely found in nature and exhibit good biocompatibility, bioadhesion, and antioxidant properties. Owing to their unique polyphenolic structure, polyphenols can bind other molecules through covalent interactions (Michael addition, Schiff-base reaction, radical coupling reaction, and coordination interactions) and noncovalent interactions (hydrogen bonds and electrostatic and π–π interactions). Therefore, research on polyphenol-modified biomaterials, such as dopamine (Dopa), pyrogallol (PG), gallic acid (GA), epigallocatechin-3-gallate (EGCG), and tannic acid (TA), has gained wide interest [108]. Notably, tyramine (Tyr), a phenolic chemical, also undergoes a radical coupling reaction. Therefore, phenolized HA-based hydrogels have been widely studied in cartilage tissue engineering (Fig. 7 and Table 5).
Fig. 7.
Synthesis of phenolized HA-based hydrogels for cartilage tissue engineering.
Table 5.
Phenolied HA-based hydrogels for cartilage tissue engineering.
| Hydrogels | Components | Physicochemical properties | Biofunctions | Ref. |
|---|---|---|---|---|
| HA-Tyr | HA, tyramine | Controllable crosslinking degree; bioadhesive; delivering cells and bioactive molecules | Promoting formation of hyaline cartilage (lower crosslinked) and fibrocartilage (higher crosslinked); anti-inflammatory ability; enhancing ECM deposition; accelerating cartilage repair | [[109], [110], [111], [112], [113]] |
| HA-Tyr/SF | HA, tyramine, silk fibroin | Adjustable mechanical properties; sustained release of the drug | Promoting chondrogenic marker genes expression and ECM deposition | [116,118] |
| HA-Tyr/Gel-Tyr | HA, tyramine, gelatin | Suitable mechanical properties; electrical conductivity | Enhancing the chondrogenic differentiation of BMSCs under electrical stimulation | [119] |
| HA-Dopa | HA, dopamine | Enhanced adhesion | Reducing cartilage friction and wear to protect cartilage | [126] |
| d-AHA-Dopa | Dialdehyde HA, dopamine | Higher tissue bonding strength than HA-Dopa; rapid gel formation | Good cytocompatibility | [127] |
| HA-MA-Dopa | HA, methacrylic anhydride, dopamine | Tissue adhesion; enhanced cell-tissue interaction | Promoting chondrogenesis of hMSCs and cartilage-like matrix formation | [128,129] |
| HA-MA-Dopa/Fe3+ | HA, methacrylic anhydride, dopamine, Fe3+ | Increasing mechanical strength and rapid self-healing performance | As a soft and tough scaffold in the replacement of biological tissues | [130] |
| HA-furan-Dopa/HA-furan-PBA | HA, furfurylamine, dopamine, phenylboronic acid | pH responsiveness; adhesion; anti-degradation and superior mechanical properties | Maintaining the viability and proliferation of cells; reducing the cell death caused by dopamine oxidation | [131] |
| HA/Dopa/Col | HA, dopamine, collagen | ECM-mimicking; double crosslinked network | Inhibiting inflammation, promoting full-thickness cartilage repair | [132] |
| HA-PG | HA, pyrogallol | Different gelation kinetics, mechanical properties, degradability and tissue adhesion | Good biocompatibility | [133] |
| HA-GA | HA, gallic acid | Tissue-adhesive; enhanced stability; antioxidant | Inhibiting inflammation | [134] |
| HA-EGCG | HA, ethylamine-grafted epigallocatechin-3-gallate | Resistance to hyaluronidase-mediated degradation; scavenging of free radicals | Inhibiting inflammation; protecting cartilage | [135,136] |
5.1. Tyramine-modified HA-based hydrogels for cartilage tissue engineering
Tyr-modified HA (HA-Tyr) is synthesized using the acylation reaction between the carboxyl group of HA and the amine group of Tyr, which can be used to prepare HA-Tyr-based hydrogels through catalytic reactions with either hydrogen peroxide (H2O2) and horseradish peroxidase (HRP) or vitamin B2 derivatives and visible-light-induced photocrosslinking [109,110]. Subsequently, Toh et al. indicated that a less crosslinked HA-Tyr-based hydrogel was beneficial to hyaline cartilage formation by enhancing the aggregation and chondrocyte phenotype of BMSCs and increasing the deposition of GAG and collagen II. In contrast, the highly crosslinked HA-Tyr-based hydrogel facilitated the fibrous phenotype of BMSCs and fibrocartilage formation [111]. Behrendt et al. suggested that bioadhesive HA-Tyr-based hydrogels can efficiently load and deliver cells to reduce the potential harm caused by cell invasion. The HA-Tyr-based hydrogel maintains its mechanical properties and activates endogenous TGF-β1 under joint loading to benefit cartilage repair [112]. Moreover, HA-Tyr-based hydrogels were more favorable for the proliferation and pluripotency of human embryonic stem cells (hESCs) than Tyr-modified dextran hydrogels [113]. However, compared with the HA-Tyr hydrogel, the fibrinogen-HA-based hydrogel had a better effect in promoting endogenous BMSCs migration and cartilage formation. The fibrinogen-HA-based hydrogel can further accelerate the chondrogenesis of endogenous BMSCs by inhibiting the expression of miR-221 [114,115].
In addition to single HA-Tyr-based hydrogels, other natural polymers have been reported to be combined with HA-Tyr for cartilage tissue engineering. As a typical example, Ziadlou et al. developed an injectable hybrid hydrogel (HA-Tyr/SF) based on HA-Tyr and silk fibroin (SF) for drug delivery and cartilage tissue engineering. The HA-Tyr/SF hydrogel exhibited adjustable mechanical properties and sustained release of the drug and promoted chondrogenic marker gene expression and ECM deposition [116]. Wang et al. further promoted the recruitment of MSCs and cartilage repair using aptamer-functionalized HA-Tyr/SF-based hydrogels [117]. Weitkamp et al. developed a highly tunable hybrid hydrogel based on HA-Tyr and SF matrices to maintain the long-term viability of encapsulated chondrocytes and upgrade the expression of chondrogenic marker genes, as well as ECM deposition [118]. In addition, Vaca-González et al. prepared an injectable hydrogel with Tyr-functionalized gelatin and HA-Tyr to enhance chondrogenesis of encapsulated BMSCs by promoting the expression of chondrogenic markers and deposition of GAG and collagen II under electrical stimulation [119].
HA-Tyr-based hydrogels have also been extensively investigated for the sustained release of bioactive molecules. For instance, Kim et al. prepared a dexamethasone (DMT)-loaded HA-Tyr-based hydrogel to optimize the therapeutic effect on collagen-induced rheumatoid arthritis (RA) by reducing interleukin-6 (IL-6), prostaglandin E2, and the levels of four cytokines, including IL-8, IL-1β, tumor necrosis factor-α (TNF-α), and CD 14 [120]. Jooybar et al. utilized platelet lysate (PL)-loaded HA-Tyr-based hydrogels to promote the adhesion and spreading of BMSCs and enhance the deposition of cartilage-like ECM, including collagen II and proteoglycans. Notably, the simultaneous degradation of the hydrogel and deposition of the ECM facilitated the formation of a dense and tough matrix [121]. Ren et al. prepared a thermally triggered injectable hydrogel comprising HA-Tyr, HRP-encapsulated thermoresponsive liposomes, and H2O2 to achieve gelation in vivo through the release of HRP because of the phase transition behavior of the liposomes at body temperature. The hydrogel exhibited a controlled gelation rate and desirable activity of encapsulated chondrocytes to support the deposition of GAG and collagen II [122,123].
5.2. Polyphenol-modified HA-based hydrogels for cartilage tissue engineering
As a mussel biomimetic material, Dopa can significantly improve tissue adhesion and cell affinity; therefore, it has been widely studied in tissue engineering [124,125]. To improve the adhesion of HA to the surface of articular cartilage, Ren et al. developed Dopa-conjugated HA (HA-Dopa) using Dopa and HA through an acylation reaction. Compared with HA, HA-Dopa can effectively adhere to the cartilage surface and enhance boundary lubrication, which can reduce cartilage friction and wear to protect cartilage [126]. However, the HA-Dopa hydrogel suffered from insufficient adhesiveness and overfast degradation owing to the low grafting rate of Dopa. To solve these problems, Zhou et al. developed a high-tissue-adhering hydrogel (d-AHA-Dopa) using d-AHA and Dopa via a Schiff-base reaction. The d-AHA-Dopa hydrogel exhibited a higher tissue bonding strength (∼90 kPa) than the HA-Dopa hydrogel (∼10 kPa), rapid gel formation (less than 60 s), degradability, and good cytocompatibility, making it a promising bioadhesive [127]. Salzlechner et al. prepared a Dopa-biofunctionalized HA-MA (HA-MA-Dopa) hydrogel with tissue adhesion at the defect site. As a result, the adhesive hydrogel presented enhanced cell-tissue interaction owing to the presence of the Dopa group, and it had significant positive influences on the chondrogenesis of hMSCs and the formation of the cartilage-like matrix [128,129]. Notably, compared with single-crosslinked hydrogels, double-crosslinked hydrogels exhibit better mechanical properties, stability, and antidegradability. Guo et al. further demonstrated a dynamic reversible double-crosslinked network hydrogel through the catechol of HA-MA-Dopa and Fe3+ and photopolymerization to increase its mechanical strength and rapid self-healing performance, and biological studies further indicated promising application prospects of the obtained hydrogel as a soft and tough scaffold for the replacement of biological tissues such as cartilage [130]. Yu et al. prepared a dual-crosslinked HA-based hydrogel using Dopa/furfurylamine (furan)-modified HA (HA-furan-Dopa) and phenylboronic acid (PBA)/furan-modified HA (HA-furan-PBA) via a Diels–Alder click reaction and phenylboronic ester bond. The hydrogel exhibited injectability, pH responsiveness, adhesion, anti-degradation, and superior mechanical properties. In addition, the hydrogel can maintain the viability and proliferation of encapsulated cells, and the introduced PBA group can reduce cell death caused by Dopa oxidation, which exhibits good application prospects in cartilage tissue engineering [131]. Gan et al. prepared an ECM-mimicking hydrogel using Dopa noncovalently modified HA (HA/Dopa) and collagen with a double-crosslinked network for growth factor-free cartilage regeneration. The hydrogel exhibited a better affinity for cells, inhibition of the expression of inflammatory factors, and M2 polarization of macrophages, which significantly promoted full-thickness cartilage repair of a rabbit knee [132].
In addition to Dopa, other polyphenols have been extensively studied for the functional modification of HA. Similar to the synthesis of HA-Dopa, Cho et al. prepared a fast-forming hydrogel (HA-PG) using HA and PG through dual modes of oxidative crosslinking, including oxidant and pH control. Based on the different crosslinking modes, the HA-PG hydrogels exhibited different gelation kinetics, mechanical properties, degradability, and tissue adhesion. Furthermore, both HA-PG hydrogels exhibited good biocompatibility, providing multifunctional hydrogels for tissue engineering, drug delivery, and stem cell therapy [133]. GA, a small molecule polyphenol, has various biological activities, such as anticarcinogenic, antimutagenic, and anti-inflammatory properties. Samanta et al. designed a tissue-adhesive antioxidant HA-based hydrogel (HA-GA) using HA and GA via covalent crosslinking. The HA-GA hydrogel exhibited enhanced stability owing to the crosslinked GA network. HA-GA hydrogels can polarize macrophages to an immunosuppressive phenotype and inhibit the expression of pro-inflammatory factors, which has significant potential in inflammation-related biomedical applications [134]. EGCG is a polyphenol in green tea with anticancer and anti-inflammatory activities. Jin et al. prepared an injectable hybrid hydrogel based on HA-Tyr and gelatin to load EGCG for cartilage repair in OA patients. The hybrid hydrogel inhibited inflammation and protected the cartilage in surgery-induced OA [135]. Lee et al. prepared an HA-EGCG conjugate using HA and ethylamine-grafted EGCG. Compared with HA, the EGCG-HA conjugate exhibited resistance to hyaluronidase-mediated degradation and scavenging of free radicals, which has good application prospects in arthritis treatment [136]. TA is a large molecular polyphenol with various intrinsic properties, such as antioxidant, metal chelation, and polymerization properties. Gwak et al. and Lee et al. demonstrated that the addition of TA can enhance the adhesion, compression properties, and biostability of HA-based hydrogels owing to strong hydrogen bonding and covalent crosslinking [137,138].
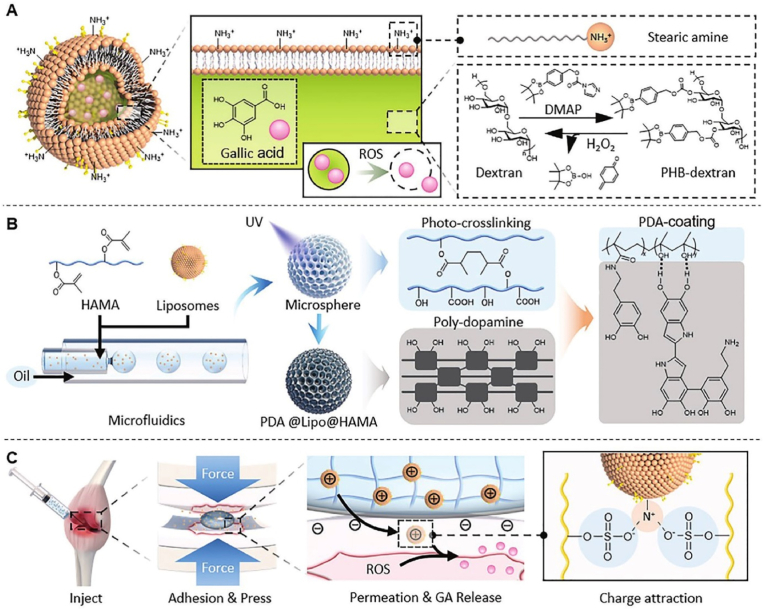
Because the cartilage ECM has a dense structure and a high density of negative charges, the delivery of drugs into cartilage is a challenge. To address this problem, Lin et al. fabricated charge-guided nanohydrogel microspheres through HA-MA, Dopa, and liposomes using microfluidic technology to penetrate the cartilage matrix. The microspheres were surface-modified with Dopa to optimize their adhesion to the cartilage surface. Moreover, the positively charged liposomes prepared from stearyl amine and borate-dextran deeply penetrated the cartilage ECM and achieved ROS-responsive release of GA on chondrocytes. In addition, in a rat OA model, microspheres significantly alleviated OA progression by enhancing the infiltration of cartilage ECM, antioxidant efficacy, and inhibiting oxidative stress-induced chondrocyte apoptosis (Fig. 8) [139].
Fig. 8.
Schematic illustration of the fabrication of charge-guided nanohydrogel microsphere to penetrate the cartilage matrix. (A) Preparation of positive charges liposomes; (B) preparation of PDA@Lipo@HAMA nanohydrogel microsphere; (C) design of charge-guided microsphere for cartilage tissue engineering [139]; Copyright 2021, John Wiley and Sons.
6. Hydrazide HA-based hydrogels for cartilage tissue engineering
Polymers can be modified by hydrazide to endow them with abundant hydrazide groups [140]. Among the various hydrazide compounds, ADH is commonly used to modify HA, and ADH-modified HA-based hydrogels have been widely studied in cartilage tissue engineering (Fig. 9 and Table 6).
Fig. 9.
Synthesis of hydrazide HA-based hydrogels for cartilage tissue engineering.
Table 6.
Hydrazide HA and host-guest group-modified HA-based hydrogels for cartilage tissue engineering.
| Hydrogels | Components | Physicochemical properties | Biofunctions | Ref. |
|---|---|---|---|---|
| HA-ADH/d-AHA | HA, adipic dihydrazide, dialdehyde HA | Shear-responsive; boundary lubricated; anti-inflammatory; injectable; self-healing; controlled-release of bioactive molecules | Reducing friction; alleviating the progression of OA; inhibiting inflammation; protecting chondrocytes | [141,142] |
| HA-ADH/dextran | HA, adipic dihydrazide, dialdehyde dextran | ROS depletion, sustainable drug release; viscosupplementation | Inhibiting inflammation; alleviating the progression of OA; protecting cartilage | [143] |
| HA-ADH/pectin | HA, adipic dihydrazide, oligopeptide-grafted dialdehyde pectin | Short gel times; controllable mechanical properties and degradation capacity | Providing a suitable microenvironment for the phenotype of chondrocytes and chondrogenesis | [144] |
| HA-Fur/CS-Mal/ACS/ADH | Methylfuran-modified HA, maleimide-modified chondroitin sulfate, adipic dihydrazide, dialdehyde chondroitin sulfate | Double-network; controllable viscoelasticity and stress relaxation; good processability; self-healing ability; high swelling capacity and stability | Negligible inflammatory response | [145] |
| HA-Ad/β-CD-Ac | HA, adamantane, monoacrylated β-cyclodextrin | Good self-healing properties, compressibility; sustained release of TGF-β1 | Rapidly integrating with the defect tissue; promoting the chondrogenesis of MSCs and cartilage regeneration | [146] |
| HA-Ad/β-CD-SH/HA-Mal | HA, adamantane, thiolated β-cyclodextrin, maleimide | Tunable viscoelasticity | Maintaining 3D spreading and intracellular interactions of encapsulated MSCs | [147] |
| HA-β-CD/PAA-Fer | HA, β-cyclodextrin, ferrocene, polyacrylic acid | Magnetic navigation; controlled release of glutathione | Promoting the columnar arrangement of chondrocytes and the repair of cartilage damage | [148] |
| HA-CB [6]/HA-PA | HA, cucurbit [6]uril, polyamines | Good mechanical stability; enzymatic degradability | Biocompatibility | [149] |
Lei et al. developed a shear-responsive, boundary-lubricated, anti-inflammatory, injectable, and self-healing HA-based hydrogel (HA-ADH/d-AHA) using ADH-modified HA (HA-ADH) and d-AHA through a Schiff-base reaction to deliver celecoxib (CLX)-loaded liposomes. The hydrogel can be restructured to expose the internal liposome on the outer surface under shear induction and form a stable lubricated boundary layer to reduce friction while alleviating the progression of OA by releasing CLX [141]. Zhou et al. further used an HA-ADH/d-AHA hydrogel to load bovine serum albumin-modified dioxide manganese nanoparticles and PRP. Biological experiments have indicated that the hydrogel can significantly increase the proliferation of chondrocytes and alleviate OA in vivo by inhibiting inflammation and protecting chondrocytes from oxidative stress [142]. Zhou et al. prepared a novel multifunctional complex hydrogel with ROS depletion, sustainable drug release, and viscosupplementation for combinatorial therapy of osteoarthritis. The complex hydrogel was fabricated using HA-ADH and aldehyde dextran through a Schiff base reaction, which can achieve the loading and sustained release of dexamethasone acetate-encapsulated ROS-scavenging micelles (PDMs). The HDH@PDM hydrogels exhibited good biodegradability, similarity to synovial components, and antioxidant and anti-inflammatory properties. Furthermore, in a rat OA model, the HDH@PDM hydrogel inhibited inflammation by polarizing macrophages to the anti-inflammatory M2 phenotype to alleviate OA progression and protect cartilage [143]. Chen et al. used HA-ADH and oligopeptide-grafted dialdehyde pectin to prepare an injectable hydrogel with short gel times (112–399 s), controllable mechanical properties, and degradation capacity, which provided a suitable microenvironment for chondrogenesis and chondrogenesis [144]. In addition, Mihajlovic et al. prepared a double-network hydrogel based on Diels–Alder adducts between methylfuran-modified HA (HA-Fur) and maleimide-modified CS (CS-Mal), and hydrazone bonds between ADH and dialdehyde CS. The hydrogel exhibited controllable viscoelasticity and stress relaxation, good processability and self-healing ability, and a high swelling capacity and stability. In addition, the hydrogel had mechanical properties similar to those of cartilage tissue to support the activity and proliferation of BMSCs. Interestingly, the degradation products of the manufactured hydrogel did not cause an inflammatory response in macrophages, laying the foundation for its further application in cartilage tissue engineering [145].
7. Host–guest group-modified HA-based hydrogels for cartilage tissue engineering
In addition to the abovementioned methods for preparing HA-based hydrogels via covalent bonding, noncovalent crosslinking, such as host–guest interactions, has also been investigated to create HA-based hydrogels for cartilage tissue engineering (Fig. 10 and Table 6).
Fig. 10.
Synthesis of host–guest-modified HA-based hydrogels for cartilage tissue engineering.
As a typical example, Wei et al. prepared a supramolecular hydrogel based on adamantane-functionalized HA (HA-Ad) and monoacrylated β-cyclodextrin (β-CD-Ac) through host–guest interactions and radical polymerization. The hydrogel exhibited excellent self-healing properties, compressibility, and sustained release of TGF-β1. Moreover, the hydrogel could rapidly integrate with the defective tissue and promote chondrogenesis of MSCs and cartilage regeneration in vivo [146]. Jing et al. prepared a highly dynamic network hydrogel with tunable viscoelasticity using HA-Ad, HA-Mal, and thiolated β-CD through host–guest interactions and thiol-ene click reactions. The hydrogel can maintain the 3D spreading and intracellular interactions of encapsulated MSCs [147]. Chiang et al. first developed a smart injectable composite hydrogel using β-CD-modified HA (HA-β-CD) and ferrocene-modified polyacrylic acid through host–guest interactions to encapsulate glutathione (GSH)-loaded magnetic microcapsules. The hydrogel exhibited magnetic navigation and controlled release of GSH, which can effectively promote the columnar arrangement of chondrocytes and the repair of cartilage damage [148]. Park et al. prepared a biocompatible hydrogel using cucurbit [6]uril (CB [6])-conjugated HA and polyamine (1,6-diaminohexane or spermine)-conjugated HA, which achieved in situ gelation through host–guest interaction after subcutaneous injection into nude mice. The hydrogels exhibited excellent mechanical stability, enzymatic degradability, and biocompatibility, and they have application potential in cell culture and various tissue engineering applications [149].
8. HA-based 3D bioprinted hydrogels for cartilage tissue engineering
Articular cartilage is lubricated lamellar tissue with anisotropy and heterogeneity [150]. Artificial cartilage scaffolds prepared using traditional engineering methods have difficulty precisely mimicking the biomechanics and tissue composition of natural cartilage [151]. On one hand, a novel 3D bioprinting method can control the geometry and microstructure of hydrogel scaffolds, which has a prosperous application in regulating the mechanical properties of hydrogel scaffolds and maintaining the viability and function of loaded cells, drugs or cytokines [152,153]. On the other hand, the HA solution has an ideal viscous modulus for 3D printing, but it cannot be used as a bioink for 3D printing alone because of the lack of specific shape-retaining ability. Therefore, HA has been integrated with other components to create bioinks for 3D printing, and HA-based bioinks can be divided into hybrid and chemically modified bioinks [154] (Table 7).
Table 7.
HA-based 3D bioprinted hydrogels for cartilage tissue engineering.
| 3D bioprinted hydrogels | Components | Physicochemical properties | Biofunctions | Ref. |
|---|---|---|---|---|
| HA/PU | HA, polyurethane | High elastic recovery; processability | Promoting self-aggregation and chondrogenesis of MSCs | [155] |
| HA/alginate | HA, alginate | Printability; biodegradability | Promoting chondrogenic genes expression and ECM deposition | [157] |
| HA/elastin/Gel | HA, elastin, gelatin | Printability with reproducible outcomes | Supporting the penetration, proliferation and chondrogenesis of chondrocytes | [158] |
| HA-MA/Gel-MA | HA, gelatin, methacrylic anhydride (MA) | Controllable pore structure; optimum degradation rate | Maintaining chondrocyte phenotype, promoting ECM deposition; accelerating mature cartilage regeneration | [[160], [161], [162]] |
| HA-MA/PCL | HA, MA, polycaprolactone | Biomimetic multiphase structure; sustained release of drugs | Inhibiting inflammation, repairing osteochondral damage; restoring motor function of joints | [163] |
| HA-MA/ECM | HA, MA, ECM | Improving the compressive strength and modulus | Providing a suitable microenvironment to encapsulate and cultivate cells | [164] |
| HA-MA/p (HPMAm-lac)-PEG | HA, MA, triblock copolymers | Thermosensitive, improving printability | Promoting formation of hyaline cartilage (lower concentrations) and fibrocartilage (higher concentrations) | [165] |
| HA-MA-PBA/Gel-MA/HA-Dopa | HA, MA, phenylboronic acid, gelatin, dopamine | Microporosity; injectability; tissue adhesion; sustained release of drug | Promoting chondrogenesis of BMSCs; accelerating articular cartilage repair and regeneration | [166] |
| HA-Ac-PBA/PVA/Gel-HS | Acrylated HA, phenylboronic acid, polyvinyl alcohol, thiolated gelatin | Tunable viscoelasticity; shear thinning property; high structural fidelity | Maintaining chondrocyte phenotype; promoting chondrogenesis and ECM deposition; alleviating chondrocyte damage | [167] |
| HA-GM/Gel-MA | HA, glycidyl methacrylate, gelatin, MA | Excellent stable mechanical properties and printability | Good biocompatibility; providing a suitable microenvironment for the chondrogenesis | [168] |
| HA-SH/P (AGE-co-G) | Thiolated HA, allyl-modified polyglycidol | Improving the stiffness of hydrogel | Promoting the deposition and uniform distribution of ECM | [169,170] |
| hmHA-HS/PEG | Thiolated high molecular weight HA, acrylated PEG, allylated PEG | Better stiffness of the hydrogel | Promoting homogeneous distribution of ECM | [171] |
| HA-SH/ECM | Thiolated HA, ECM particles | Customizable specific shapes; biomimetic mechanical properties | Enhancing the viability of the encapsulated chondrocytes | [173] |
| HA-ADH/SAV and SA/Ca2+ (HBSAC) | biotinylated HA-ADH, streptavidin, sodium alginate, Ca2+ | Better printability and structural integrity | Promoting the chondrogenic marker genes expression and ECM deposition | [174] |
| d-AHA/glycol chitosan/ADH and HAH/Ca2+ | Dialdehyde HA, glycol chitosan, ADH, hyaluronate-alginate hybrid, Ca2+ | improving the mechanical properties and stability | Enhancing the chondrogenesis of chondroprogenitor cells | [175] |
| HA-ADH/d-AHA/Gel-MA | HA-ADH, dialdehyde HA, gelatin, MA | Self-healing; shear-thinning properties; improving mechanical properties | Maintaining the survival and proliferation of BMSCs | [176] |
| HA-Tys | HA, tyramine | Controllable porosity; enhanced the mechanical strength; | Loading and delivering stem cells and chondrocytes with appropriate cell activity for a long time | [177,178] |
| d-AHA/CMC and Gel/PEG-SG | Dialdehyde HA, N-carboxymethyl chitosan, gelatin, PEG succinimidyl glutarate | Viscoelastic; time-sharing structure-supporting; high permeability | Preserving the long-term viability and morphology of chondrocytes | [179] |
8.1. 3D bioprinted hydrogels based on hybrid HA
Hung et al. prepared a 3D printed scaffold based on HA and water-based polyurethane (PU) elastic nanoparticles to control the release of bioactive ingredients or drugs for customized cartilage tissue engineering. The printed scaffold had a high elastic recovery ability and processability for various shapes, which boosted the self-aggregation and chondrogenesis of MSCs while inhibiting cartilage hypertrophy. In addition, cartilage regeneration in rabbit knee-joint defects was obtained [155]. In addition, Shie et al. used HA and light-cured PU to prepare a 3D printed scaffold mimicking the mechanical properties of articular cartilage. The prepared scaffold was favorable for the adhesion, proliferation, and chondrogenesis of MSCs [156]. Antich et al. illustrated a biomimetic 3D printable bioink based on HA and alginate, and chondrogenesis of chondrocytes was observed by promoting chondrogenic gene expression and cartilage ECM deposition [157]. Shokri et al. designed a porous 3D printed scaffold composed of HA, elastin, and gelatin, which supported the penetration, proliferation, and chondrogenesis of chondrocytes, along with the regeneration of nasal septal cartilage defects [158].
8.2. 3D bioprinted hydrogels based on functionally modified HA
Functionalized HA-based hydrogels have application prospects in cartilage tissue engineering [19]. Similarly, they are also elaborately designed and 3D printed to biomimetic the structure and shape of native cartilage [159]. Lam et al. developed two 3D bioprinted hydrogels consisting of HA-MA and Gel-MA using stereolithographic methods to maintain the chondrocyte phenotype and promote the deposition of cartilage-specific ECM, including proteoglycans and collagen II [160]. Xia et al. used HA-MA and Gel-MA to prepare a 3D bioprinted hydrogel by integrating photocuring and lyophilization techniques. The controllable pore structure, desirable mechanical properties, and optimum degradation rate matched those of native cartilage regeneration. In addition, hydrogels loaded with chondrocytes can contribute to mature cartilage regeneration with typical lacunar structures and cartilage-specific ECM [161]. Shopperly et al. also developed a biomimetic 3D bioprinted hydrogel with a structure similar to that of cartilage ECM gradients based on HA-MA and Gel-MA via stereolithographic bioprinting. The increased HA-MA and Gel-MA contents enhanced the stiffness of the scaffolds and cartilage matrix protein formation. In addition, the biomimetic structure of the 3D printed hydrogel could be retained even after ECM formation [162]. Liu et al. developed a biomimetic multiphase composite 3D bioprinted hydrogel combining HA-MA with PCL to simulate the osteochondral structure. The bioprinted hydrogel supported the survival and proliferation of BMSCs and deposition of cartilage ECM. In addition, the hydrogel could inhibit inflammation, repair osteochondral damage, and restore the motor function of OA joints after loading with small-molecule drugs [163]. Li et al. prepared an ECM-doped HA-MA-based 3D bioprinted hydrogel to improve the compressive strength (2.7 times) and modulus (3.1 times) of HA-MA hydrogels. The hydrogels can both satisfy the mechanical requirements of bioprinting and provide a suitable microenvironment for encapsulating and cultivating cells [164]. In addition, Mouser et al. designed a thermosensitive 3D bioprinted hydrogel by incorporating triblock copolymers (p (HPMAm-lac)-PEG) into HA-MA to improve its printability for chondrogenesis. Notably, lower concentrations of HA-MA (0.25%–0.5%) had beneficial effects on the deposition of GAG and collagen II, while higher HA-MA concentrations (1%) could induce fibrocartilage formation [165]. Feng et al. used PBA-grafted HA-MA (HA-MA-PBA) and Gel-MA to fabricate a microgel using microfluidics and photocrosslinking to encapsulate KGN-loaded cyclodextrin nanoparticles and BMSCs and then prepared a nanocomposite microgel through dynamic crosslinking between PBA groups and HA-Dopa. The microgel exhibited microporosity, injectability, tissue adhesion, and sustained drug-release ability. Moreover, the microgel accelerated chondrogenesis of BMSCs and the repair and regeneration of articular cartilage (Fig. 11) [166]. Shi et al. prepared an injectable dynamic double-crosslinked 3D bioprinted hydrogel comprising PBA-grafted acrylated HA (HA-Ac-PBA), polyvinyl alcohol (PVA), and Gel-HS through boronate ester bond and thiol-ene click reactions. The double-crosslinked hydrogels exhibited tunable viscoelasticity, shear thinning, and high structural fidelity. After encapsulating AMSCs, the hydrogel promoted cell adhesion and chondrogenesis, maintained the chondrocyte phenotype, increased the deposition of GAG and collagen II, and alleviated chondrocyte damage from ROS, suggesting decent application prospects of hydrogels in OA cartilage repair [167]. Lee et al. fabricated a photocurable 3D bioprinted hydrogel with HA-GM and Gel-MA, which exhibited excellent stable mechanical properties and printability, enabling the construction of highly complex laryngeal structures (thyroid cartilage, cricoid cartilage, arytenoid cartilage, etc.). Moreover, the hydrogel had good biocompatibility and provided a suitable microenvironment for the chondrogenesis of tonsil-derived mesenchymal stem cells [168].
Fig. 11.
Schematic illustration of the fabrication of dynamic-crosslinked microgel for cartilage regeneration. (A) Microgel with microporosity, injectability, tissue-adhesion, and sustained drug release; (B) immunohistochemical staining images of cartilage defects in different groups after implantation for 4 and 8 weeks [166]; Copyright 2021, John Wiley and Sons.
Stichler et al. engineered a 3D bioprinted hydrogel (HA-SH/P (AGE-co-G)) based on HA-SH and allyl-modified polyglycidol (P (AGE-co-G)), which could encapsulate MSCs and promote the deposition of ECM, although the distribution of ECM was not uniform [169]. Hauptstein et al. further demonstrated that HA-SH/P (AGE-co-G) hydrogel with a low concentration (3%) of HA-SH polymer contributed to the uniform distribution of ECM and significantly improved the stiffness of the hydrogel. In addition, the addition of high-molecular-weight HA (hmHA) further optimized the distribution of ECM and stiffness of the hydrogel [170]. Subsequently, Hauptstein et al. fabricated a 3D bioprinting hydrogel with a loose porous structure based on low concentrations of hmHA-HS, acrylated PEG, and allylated PEG through a two-stage cross-linking method. A homogeneous distribution of proteoglycans and collagen II was obtained, and the improved stiffness of the hydrogel after chondrogenesis was also verified [171]. Mancini et al. developed a 3D bioprinted zonal composite scaffold combining HA-SH/P (AGE-co-G) with PCL to encapsulate articular cartilage progenitor cells (ACPCs) on the top layer and MSCs on the bottom layer to mimic the zonal structure of the native cartilage. In equine osteochondral defects, significant bone repair and integration with a higher compressive modulus of the repaired tissue was observed on the scaffold with a zonal structure. However, the negligible cartilage repair effect might be related to early loss of cells, undesirable degradation behavior of the hydrogel, and failure to provide the biomechanical environment for native cartilage [172]. Barthold et al. fabricated a layered 3D bioprinted hydrogel utilizing HA-SH and ECM particles through disulfide bonds, which exhibited customizable specific shapes and mechanical properties similar to those of the native cartilage. Moreover, the hydrogel enhanced the viability of encapsulated chondrocytes [173].
In addition, HA-ADH-based double-crosslinked network hydrogels have been extensively studied for 3D bioprinting. Nedunchezian et al. prepared a double-cross-linked 3D bioprinted hydrogel (HBSAC) to lade adipose stem cells (ADSCs). The double-crosslinked network of the HBSAC hydrogel included noncovalent crosslinks between biotinylated HA-ADH and streptavidin, and ion transfer crosslinks between SA and Ca2+. Compared with HA hydrogels, HBSAC hydrogels exhibited better printability and structural integrity and effectively promoted the chondrogenesis of ADSCs by promoting the expression of chondrogenic marker genes and the deposition of GAG [174]. Kim et al. prepared an HA-based hydrogel with a double-crosslinked network as 3D printable bioinks. The double-crosslinked network included covalent crosslinks between AHA, glycol chitosan, and ADH, and the physical crosslinks of hyaluronate-alginate hybrid (HAH) polymers with Ca2+. The addition of HAH polymers can significantly improve the mechanical properties and stability of hydrogels and enhance chondrogenesis of chondroprogenitor cells [175]. Wang et al. prepared a dynamic/photocrosslinked HA-Gel dual-network hybrid hydrogel using HA-ADH, AHA, and Gel-MA for 3D bioprinting. The dynamic crosslinking of HA-ADH and AHA through a Schiff-base reaction endowed the hydrogel with self-healing and shear-thinning properties to ensure its smooth extrusion, and the subsequent photopolymerization of Gel-MA improved its mechanical properties and stability. The hydrogel maintained the survival and proliferation of BMSCs [176].
Flégeau et al. reported HA-Tys microgels with controllable porosity for 3D printable bioinks, and the microgels were useful for the activity and chondrogenesis of chondrocytes. Note that the increase in microgel size would induce a more severe inflammatory response [177]. In addition, Petta et al. reported a 3D bioprintable HA-Tys hydrogel with a dual crosslinking mechanism. First, enzymatic reaction-induced crosslinking formed a soft gel suitable for cell encapsulation and extrusion. Second, visible light-induced photocrosslinking enhanced the mechanical strength of hydrogels such that they could preserve their shape and morphology. Printed hydrogels can load and deliver stem cells and chondrocytes with appropriate cell activity for a long time [178]. Chen et al. demonstrated a self-healing viscoelastic 3D bioprintable hydrogel with novel time-sharing structure-supported characteristics and high permeability through fast dynamic crosslinking between d-AHA and N-carboxymethyl chitosan and slow crosslinking between gelatin and 4-arm PEG succinimidyl glutarate. Moreover, hydrogels can preserve the long-term viability, morphology, and remarkable biological characteristics of encapsulated chondrocytes [179].
9. Conclusion and further perspectives
HA has been widely applied in cartilage tissue engineering because of its biocompatibility, biodegradability, promotion of cell adhesion and proliferation, regulation of inflammation, and acceleration of cartilage regeneration. It is an important material for the construction of hydrogels/microgels, and functional groups grafted onto HA can serve as cross-linking and reaction sites to further immobilize bioactive factors, thereby improving the bioactivity of HA hydrogels. With the continuous development of science and technology, numerous functionalized HA-based hydrogels have been elaborately created, and they have exhibited good application prospects in cartilage tissue engineering. This article summarizes the research progress in design strategies (alkenyl, aldehyde, thiolated, phenolized, hydrazide, and host–guest group-modified) for HA-based hydrogels and their applications in cartilage tissue engineering.
Among the above functionalization modifications, alkenyl HA has been extensively and thoroughly studied in cartilage tissue engineering. Alkenyl HA, such as methacrylate, DVS-modified, Nor-modified, and maleic-modified HA, is primarily synthesized through esterification and acylation. Methacrylate HA can self-polymerize to form a hydrogel through the FRP reaction; the pore size, crosslinking density, mechanical properties, and degradation rate of the hydrogel can be effectively controlled by regulating the polymer concentration and polymerization time. Moreover, many polymers (such as gelatin, cellulose, fibrinogen, and F127) have been used to further improve the physicochemical properties and biological activities of hydrogels; the process of hydrogel formation does not depend on the functional groups of polymers because of the self-polymerization of methacrylate HA. In contrast, other alkenyl HA molecules are more complex to synthesize, and related hydrogels are typically fabricated through the thiol-ene click reaction with thiolated polymers. Aldehyde HA can be classified into d-AHA, m-AHA, and p-AHA. Aldehyde HA-based hydrogels have been prepared primarily through the Schiff-base reaction with polymers containing amino groups or hydrazide groups; the preparation process is simple and does not require the addition of other chemicals. These hydrogels exhibit excellent tissue adhesion by reacting with the amino group on the tissue surface and self-healing owing to the presence of dynamic Schiff-base bonds. The synthesis of d-AHA requires only one oxidation step, in which NaIO4 oxidizes the vicinal hydroxyl groups on the second and third carbon atoms of the HA repeat unit. d-AHA have highly reactive dialdehyde groups that have attracted extensive research interest. In contrast to d-AHA, both m-AHA and p-AHA require a two-step preparation process, including the grafting of functional groups initially followed by oxidation/photoinduction. Interestingly, the ring-opening structure of d-AHA is easily hydrolyzed, leading to rapid degradation and instability of the hydrogel, whereas the hydrogel formed by m-AHA and hydrazide has a higher stability and slower degradation rate because of its more stable Schiff-base bond. Compared with d-AHA and m-AHA, p-AHA can effectively avoid the instability of the aldehyde group. Therefore, m-AHA and p-AHA should be investigated comprehensively in cartilage tissue engineering based on the relatively few studies to date. Thiolated HA is prepared primarily through an acylation reaction between the carboxyl group of HA and the amino or hydrazide groups of polymers with disulfide bonds and sulfhydryl groups. Thiolated HA-based hydrogels have been prepared primarily through self-crosslinking of intrachain disulfide bonds or thiol-ene click reactions. The self-crosslinking process of the hydrogels does not require other chemicals, and the disulfide bonds formed can be decomposed by intracellular GSH, which exhibits useful application prospects in the preparation of GSH-responsive hydrogels. Moreover, thiolated HA can effectively improve the cell-adhesion ability of hydrogels when mixed with peptides and collagen. Phenolized HA is synthesized via an acylation reaction using Tyr, Dopa, and polyphenols to graft HA molecules. Phenolized HA-based hydrogels have been prepared primarily through radical coupling reactions, polyphenol-metal coordination, and polyphenol-boronate complexation. Dopa- and polyphenol-modified HA-based hydrogels exhibit good tissue adhesion and cell affinity owing to the presence of catechol groups (mussel biomimetic chemistry), which can reduce cartilage friction and wear to protect cartilage. In addition, many polyphenol-modified HA-based hydrogels can inhibit inflammation owing to their antioxidant and anti-inflammatory properties, which provide useful applications in OA therapy. Hydrazide HA is synthesized primarily using ADH to modify HA through an acylation reaction. Hydrazide HA has abundant hydrazides and can react with polymers containing aldehyde groups through a Schiff-base reaction to develop hydrogels. Host–guest group-modified HA-based hydrogels are prepared using host or guest modification of HA and polymers containing host or guest groups through host–guest interactions. Owing to the dynamic reversibility of both the Schiff-base reaction and host–guest interaction, these two types of hydrogels exhibit the desirable merits of mild preparation conditions, rapid gelation time, good self-healing capacity, and sustained release of bioactive substances.
Furthermore, we reviewed modified HA-based hydrogels for 3D bioprinting in cartilage tissue engineering applications. 3D bioprinted hydrogels generally have double crosslinking networks involving dynamic click chemistry crosslinking and photocrosslinking. Primary dynamic crosslinking endows hydrogels with injectable and self-healing abilities, and further photocrosslinking effectively enhances the mechanical properties and stability of hydrogels. Although tremendous progress has been achieved in HA-based hydrogels for cartilage tissue engineering, current cartilage repair studies are limited to small animals, and only a few reports for large animals are available. In addition, further clinical applications of HA-based hydrogels still encounter significant challenges, such as undetermined molecular weight distribution and inaccurate chemical structure of polymers, inflammatory responses, degeneration effects of proteins, and generation and activation of hyaluronidase. In conclusion, at the intersection of materials science and regenerative medicine, we believe that multifunctional HA-based hydrogels with high biomimicking of the cartilage ECM will play an important role in cartilage tissue engineering. HA-based hydrogels are a class of products with significant clinical application value in the near future.
Author contributions
Min Wang: Investigation, Writing – original draft, Visualization. Zexing Deng: Investigation, Writing – original draft. Yi Guo: Investigation, Writing-editing. Peng Xu: Supervision, Conceptualization.
Funding
We thank the financial support of the China Postdoctoral Science Foundation (grant No. 2021M702644), the Natural Science Foundation of Shaanxi Province (grant No. 2022JQ-384) and the High-level Talents Foundation for Scientific Research of Xi'an University of Science and Technology (grant No. 2050122015).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The authors have obtained figures permission.
References
- 1.Setayeshmehr M., Esfandiari E., Rafieinia M., Hashemibeni B., Taheri-Kafrani A., Samadikuchaksaraei A., Kaplan D.L., Moroni L., Joghataei M.T. Hybrid and composite scaffolds based on extracellular matrices for cartilage tissue engineering. Tissue Eng., Part B. 2019;25(3):202–224. doi: 10.1089/ten.TEB.2018.0245. [DOI] [PubMed] [Google Scholar]
- 2.Wei W.Y., Dai H.L. Articular cartilage and osteochondral tissue engineering techniques: recent advances and challenges. Bioact. Mater. 2021;6(12):4830–4855. doi: 10.1016/j.bioactmat.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin W.F., Klein J. Recent progress in cartilage lubrication. Adv. Mater. 2021;33(18) doi: 10.1002/adma.202005513. [DOI] [PubMed] [Google Scholar]
- 4.Zheng M. Stem cells promote the regeneration of knee joint degenerative bone and articular cartilage. J. Healthcare Eng. 2022;2022 doi: 10.1155/2022/9533211. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Roseti L., Desando G., Cavallo C., Petretta M., Grigolo B. Articular cartilage regeneration in osteoarthritis. Cells. 2019;8(11):1305. doi: 10.3390/cells8111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Bakhshayesh A.R., Babaie S., Nasrabadi H.T., Asadi N., Akbarzadeh A., Abedelahi A. An overview of various treatment strategies, especially tissue engineering for damaged articular cartilage. Artif. Cell Nanomed. Biotechnol. 2020;48(1):1089–1104. doi: 10.1080/21691401.2020.1809439. [DOI] [PubMed] [Google Scholar]
- 7.Li M.Z., Yin H., Yan Z.E., Li H.Y., Wu J., Wang Y., Wei F., Tian G.Z., Ning C., Li H., Gao C.J., Fu L.W., Jiang S.P., Chen M.X., Sui X., Liu S.Y., Chen Z.W., Guo Q.Y. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022;140:23–42. doi: 10.1016/j.actbio.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010;6(11):625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 9.Wang K.Y., Jin X.Y., Ma Y.H., Cai W.J., Xiao W.Y., Li Z.W., Qi X., Ding J. Injectable stress relaxation gelatin-based hydrogels with positive surface charge for adsorption of aggrecan and facile cartilage tissue regeneration. J. Nanobiotechnol. 2021;19(1):1–16. doi: 10.1186/s12951-021-00950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sridhar B.V., Brock J.L., Silver J.S., Leight J.L., Randolph M.A., Anseth K.S. Development of a cellularly degradable peg hydrogel to promote articular cartilage extracellular matrix deposition. Adv. Healthcare Mater. 2015;4(5):702–713. doi: 10.1002/adhm.201400695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huebner K.D., Shrive N.G., Frank C.B. Dexamethasone inhibits inflammation and cartilage damage in a new model of post-traumatic osteoarthritis. J. Orthop. Res. 2014;32(4):566–572. doi: 10.1002/jor.22568. [DOI] [PubMed] [Google Scholar]
- 12.Orth P., Gao L., Madry H. Microfracture for cartilage repair in the knee: a systematic review of the contemporary literature. Knee Surg. Sports Traumatol. Arthrosc. 2020;28(3):670–706. doi: 10.1007/s00167-019-05359-9. [DOI] [PubMed] [Google Scholar]
- 13.Gao L., Goebel L.K.H., Orth P., Cucchiarini M., Madry H. Subchondral drilling for articular cartilage repair: a systematic review of translational research. Dis. Models Mech. 2018;11(6) doi: 10.1242/dmm.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krill M., Early N., Everhart J.S., Flanigan D.C. Autologous chondrocyte implantation (ACI) for knee cartilage defects: a review of indications, technique, and outcomes. JBJS Rev. 2018;6(2):e5. doi: 10.2106/JBJS.RVW.17.00078. [DOI] [PubMed] [Google Scholar]
- 15.Wei W., Ma Y.Z., Yao X.D., Zhou W.Y., Wang X.Z., Li C.L., Lin J.X., He Q.L., Leptihn S., Ouyang H.W. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2021;6(4):998–1011. doi: 10.1016/j.bioactmat.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balakrishnan B., Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 2011;111(8):4453–4474. doi: 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- 17.Wu J.W., Chen Q., Deng C., Xu B.P., Zhang Z.Y., Yang Y., Lu T.L. Exquisite design of injectable hydrogels in cartilage repair. Theranostics. 2020;10(21):9843–9864. doi: 10.7150/thno.46450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang R., Chen F., Guo J.S., Zhou D.F., Luan S.F. Recent advances in polymeric biomaterials-based gene delivery for cartilage repair. Bioact. Mater. 2020;5(4):990–1003. doi: 10.1016/j.bioactmat.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H.R., Qi Z.P., Zheng S., Chang Y.X., Kong W.J., Fu C., Yu Z.Y., Yang X.Y., Pan S. The application of hyaluronic acid-based hydrogels in bone and cartilage tissue engineering. Adv. Mater. Sci. Eng. 2019;2019 [Google Scholar]
- 20.Chen Q.X., Shao X.T., Ling P.X., Liu F., Han G.Y., Wang F.S. Recent advances in polysaccharides for osteoarthritis therapy. Eur. J. Med. Chem. 2017;139:926–935. doi: 10.1016/j.ejmech.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 21.Dovedytis M., Liu Z.J., Bartlett S. Hyaluronic acid and its biomedical applications: a review. Eng. Regend. 2020;1:102–113. [Google Scholar]
- 22.Tsanaktsidou E., Kammona O., Kiparissides C. Recent developments in hyaluronic acid-based hydrogels for cartilage tissue engineering applications. Polymers. 2022;14(4):839. doi: 10.3390/polym14040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin W.F., Liu Z., Kampf N., Klein J. The role of hyaluronic acid in cartilage boundary lubrication. Cells. 2020;9(7):1606. doi: 10.3390/cells9071606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida O., Tanaka Y., Morimoto I., Takigawa M., Eto S. Chondrocytes are regulated by cellular adhesion through CD44 and hyaluronic acid pathway. J. Bone Miner. Res. 1997;12(10):1657–1663. doi: 10.1359/jbmr.1997.12.10.1657. [DOI] [PubMed] [Google Scholar]
- 25.Park H., Choi B., Hu J.L., Lee M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013;9(1):4779–4786. doi: 10.1016/j.actbio.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal G., Agiwal S., Srivastava A. Hyaluronic acid containing scaffolds ameliorate stem cell function for tissue repair and regeneration. Int. J. Biol. Macromol. 2020;165:388–401. doi: 10.1016/j.ijbiomac.2020.09.107. [DOI] [PubMed] [Google Scholar]
- 27.Burdick J.A., Prestwich G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011;23(12):H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuurmans C.C.L., Mihajlovic M., Hiemstra C., Ito K., Hennink W.E., Vermonden T. Hyaluronic acid and chondroitin sulfate (Meth)acrylate-based hydrogels for tissue engineering: synthesis, characteristics and pre-clinical evaluation. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120602. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Zhang S., Wang J. Photo-crosslinkable hydrogel and its biological applications. Chin. Chem. Lett. 2021;32(5):1603–1614. [Google Scholar]
- 30.Smeds K.A., Grinstaff M.W. Photocrosslinkable polysaccharides for in situ hydrogel formation. J. Biomed. Mater. Res. 2001;54(1):115–121. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 31.Lu D.H., Zeng Z.W., Geng Z.J., Guo C.P., Pei D.T., Zhang J., Yu S. Macroporous methacrylated hyaluronic acid hydrogel with different pore sizes for in vitro and in vivo evaluation of vascularization. Biomed. Mater. 2022;17(2) doi: 10.1088/1748-605X/ac494b. [DOI] [PubMed] [Google Scholar]
- 32.Erickson I.E., Huang A.H., Sengupta S., Kestle S., Burdick J.A., Mauck R.L. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17(12):1639–1648. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim M., Erickson I.E., Huang A.H., Garrity S.T., Mauck R.L., Steinberg D.R. Donor variation and optimization of human mesenchymal stem cell chondrogenesis in hyaluronic acid. Tissue Eng. 2018;24(21–22):1693–1703. doi: 10.1089/ten.tea.2017.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bian L.M., Hou C., Tous E., Rai R., Mauck R.L., Burdick J.A. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials. 2013;34(2):413–421. doi: 10.1016/j.biomaterials.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J.R., Tang Z.Z., Liu Y.F., Luo Z.C., Xiao Y.M., Zhang X.D. Comparison of chondro-inductivity between collagen and hyaluronic acid hydrogel based on chemical/physical microenvironment. Int. J. Biol. Macromol. 2021;182:1941–1952. doi: 10.1016/j.ijbiomac.2021.05.188. [DOI] [PubMed] [Google Scholar]
- 36.Sahoo S., Chung C., Khetan S., Burdick J.A. Hydrolytically degradable hyaluronic acid hydrogels with controlled temporal structures. Biomacromolecules. 2008;9(4):1088–1092. doi: 10.1021/bm800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung C., Beecham M., Mauck R.L., Burdick J.A. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30(26):4287–4296. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsanaktsidou E., Kammona O., Kiparissides C. On the synthesis and characterization of biofunctional hyaluronic acid based injectable hydrogels for the repair of cartilage lesions. Eur. Polym. J. 2019;114:47–56. [Google Scholar]
- 39.Tsanaktsidou E., Kammona O., Labude N., Neuss S., Kruger M., Kock L., Kiparissides C. Biomimetic cell-laden meha hydrogels for the regeneration of cartilage tissue. Polymers. 2020;12(7):1598. doi: 10.3390/polym12071598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levett P.A., Hutmacher D.W., Malda J., Klein T.J. Hyaluronic acid enhances the mechanical properties of tissue-engineered cartilage constructs. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levett P.A., Melchels F.P.W., Schrobback K., Hutmacher D.W., Malda J., Klein T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014;10(1):214–223. doi: 10.1016/j.actbio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Lin H., Beck A.M., Shimomura K., Sohn J., Fritch M.R., Deng Y.H., Kilroy E.J., Tang Y., Alexander P.G., Tuan R.S. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. Tissue Eng. Regener. Med. 2019;13(8):1418–1429. doi: 10.1002/term.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang G.H.E., An Y., Zhang X.L., Ding P.B., Bi H.S., Zhao Z.M. Chondrocyte spheroids laden in GelMA/HAMA hybrid hydrogel for tissue-engineered cartilage with enhanced proliferation, better phenotype maintenance, and natural morphological structure. Gels. 2021;7(4):247. doi: 10.3390/gels7040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pahoff S., Meinert C., Bas O., Nguyen L., Klein T.J., Hutmacher D.W. Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. J. Mater. Chem. B. 2019;7(10):1761–1772. doi: 10.1039/c8tb02607f. [DOI] [PubMed] [Google Scholar]
- 45.Sang S.B., Ma Z.W., Cao Y.Y., Shen Z.Z., Duan J.H., Zhang Y.T., Wang L.J., An Y.C., Mao X.J., An Y., Zhang Q. BC enhanced photocurable hydrogel based on 3D bioprinting for nasal cartilage repair. Int. J. Polym. Mater. Polym. Biomater. 2022:1–12. [Google Scholar]
- 46.Nedunchezian S., Wu C.-W., Wu S.-C., Chen C.-H., Chang J.-K., Wang C.-K. Characteristic and chondrogenic differentiation analysis of hybrid hydrogels comprised of hyaluronic acid methacryloyl (HAMA), gelatin methacryloyl (GelMA), and the acrylate-functionalized nano-silica crosslinker. Polymers. 2022;14(10) doi: 10.3390/polym14102003. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao W., Jin X., Cong Y., Liu Y.Y., Fu J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013;88(3):327–339. [Google Scholar]
- 48.Zhao H.B., Zhang Y.J., Liu Y.S., Zheng P.H., Gao T., Cao Y., Liu X.Z., Yin J.B., Pei R.J. In situ forming cellulose nanofibril-reinforced hyaluronic acid hydrogel for cartilage regeneration. Biomacromolecules. 2021;22(12):5097–5107. doi: 10.1021/acs.biomac.1c01063. [DOI] [PubMed] [Google Scholar]
- 49.Snyder T.N., Madhavan K., Intrator M., Dregalla R.C., Park D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014;8(1):1–11. doi: 10.1186/1754-1611-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sani E.S., Portillo-Lara R., Spencer A., Yu W.D., Geilich B.M., Noshadi I., Webster T.J., Annabi N. Engineering adhesive and antimicrobial hyaluronic acid/elastin-like polypeptide hybrid hydrogels for tissue engineering applications. ACS Biomater. Sci. Eng. 2018;4(7):2528–2540. doi: 10.1021/acsbiomaterials.8b00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X.Y., Ding J., Xu P.F., Feng X., Wang Z.Y., Zhou T., Tu C.X., Cao W.B., Xie J.Q., Deng L.W., Shen L.Y., Zhu Y., Gou Z.R., Gao C.Y. A cell-free ROS-responsive hydrogel/oriented poly(lactide-co-glycolide) hybrid scaffold for reducing inflammation and restoring full-thickness cartilage defects in vivo. Biomed. Mater. 2021;16(6) doi: 10.1088/1748-605X/ac21dd. [DOI] [PubMed] [Google Scholar]
- 52.Ren P.G., Zhang H., Dai Z., Ren F., Wu Y.D., Hou R.X., Zhu Y.B., Fu J. Stiff micelle-crosslinked hyaluronate hydrogels with low swelling for potential cartilage repair. J. Mater. Chem. B. 2019;7(36):5490–5501. doi: 10.1039/c9tb01155b. [DOI] [PubMed] [Google Scholar]
- 53.Iwamoto M., Ohta Y., Larmour C., Enomoto-Iwamoto M. Toward regeneration of articular cartilage. Birth Defects Res., Part C. 2013;99(3):192–202. doi: 10.1002/bdrc.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan W., Xu X., Xu Q., Sun Z., Jiang Q., Shi D. Platelet-rich plasma combined with injectable hyaluronic acid hydrogel for porcine cartilage regeneration: a 6-month follow-up. Regener. Biomater. 2020;7(1):77–90. doi: 10.1093/rb/rbz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi D., Xu X., Ye Y., Song K., Cheng Y., Di J., Hu Q., Li J., Ju H., Jiang Q., Gu Z. Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano. 2016;10(1):1292–1299. doi: 10.1021/acsnano.5b06663. [DOI] [PubMed] [Google Scholar]
- 56.Ma Z.J., Song W., He D., Zhang X., He Y.H., Li H.Y. Smart μ-fiber hydrogels with macro-porous structure for sequentially promoting multiple phases of articular cartilage regeneration. Adv. Funct. Mater. 2022;32(22) [Google Scholar]
- 57.He T.F., Li B.T., Colombani T., Joshi-Navare K., Mehta S., Kisiday J., Bencherif S.A., Bajpayee A.G. Hyaluronic acid-based shape-memory cryogel scaffolds for focal cartilage defect repair. Tissue Eng. 2021;27(11–12):748–760. doi: 10.1089/ten.TEA.2020.0264. [DOI] [PubMed] [Google Scholar]
- 58.Hsieh Y.H., Shen B.Y., Wang Y.H., Lin B., Lee H.M., Hsieh M.F. Healing of osteochondral defects implanted with biomimetic scaffolds of poly(epsilon-caprolactone)/hydroxyapatite and glycidyl-methacrylate-modified hyaluronic acid in a minipig. Int. J. Mol. Sci. 2018;19(4):1125. doi: 10.3390/ijms19041125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan K.C., Yu Y., Ng S.H., Mak H.K., Yip Y.W.Y., van der Merwe Y., Ren T.M., Yung J.S.Y., Biswas S., Cao X., Chau Y., Leung C.K.S. Intracameral injection of a chemically cross-linked hydrogel to study chronic neurodegeneration in glaucoma. Acta Biomater. 2019;94:219–231. doi: 10.1016/j.actbio.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pitarresi G., Tripodo G., Triolo D., Fiorica C., Giammona G. Inulin vinyl sulfone derivative cross-linked with bis-amino PEG: new materials for biomedical applications. J. Drug Deliv. Sci. Technol. 2009;19(6):419–423. [Google Scholar]
- 61.Mondal S., Haridas N., Letha S.S., Vijith V., Rajmohan G., Rosemary M.J. Development of injectable high molecular weight hyaluronic acid hydrogels for cartilage regeneration. J. Macromol. Sci., Pure Appl. Chem. 2016;53(8):507–514. [Google Scholar]
- 62.Riveiro A., Amorim S., Solanki A., Costa D.S., Pires R.A., Quintero F., del Val J., Comesana R., Badaoui A., Lusquinos F., Macon A.L.B., Tallia F., Jones J.R., Reis R.L., Pou J. Hyaluronic acid hydrogels reinforced with laser spun bioactive glass micro- and nanofibres doped with lithium. Mater. Sci. Eng., C. 2021;126 doi: 10.1016/j.msec.2021.112124. [DOI] [PubMed] [Google Scholar]
- 63.Feng Q., Li Q.T., Wen H.J., Chen J.X., Liang M.H., Huang H.H., Lan D.X., Dong H., Cao X.D. Injection and self-assembly of bioinspired stem cell-laden gelatin/hyaluronic acid hybrid microgels promote cartilage repair in vivo. Adv. Funct. Mater. 2019;29(50) [Google Scholar]
- 64.Park Y.G., Ha C.W., Yoo J.H., Lee W.S., Lee H.J., In Y., Bae K.C., Shon O.J., Kim Y.M., Seon J.K., Song S.J., Chang C.B., Kim J.M., Kim C.W., Kim D.H., Bae J.H. Intra-articular injection of a novel DVS cross-linked hyaluronic acid manufactured by biological fermentation (YYD302) in patients with knee osteoarthritis: a double-blind, randomized, multicenter, noninferiority study. Clin. Therapeut. 2021;43(11):1843–1860. doi: 10.1016/j.clinthera.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 65.In Y., Ha C.W. A multicenter, randomized, double-blinded, parallel-group, placebo-controlled phase Ⅰ/Ⅱa study to evaluate the efficacy and safety of a single intra-articular injection of YYD302 in patients with knee osteoarthritis. J. Clin. Med. 2022;11:1482. doi: 10.3390/jcm11061482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin C.C., Ki C.S., Shih H. Thiol-norbornene photo-click hydrogels for tissue engineering applications. J. Appl. Polym. Sci. 2015;132(8) doi: 10.1002/app.41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao X., Huang Z., Jiang X., Yang Y., Yang L., Yang S., Niu C., Xu Y., Feng L. Facile synthesize of norbornene-hyaluronic acid to form hydrogel via thiol-norbornene reaction for biomedical application. Polymer. 2022;245 [Google Scholar]
- 68.Galarraga J.H., Locke R.C., Witherel C.E., Stoeckl B.D., Castilho M., Mauck R.L., Malda J., Levato R., Burdick J.A. Fabrication of MSC-laden composites of hyaluronic acid hydrogels reinforced with MEW scaffolds for cartilage repair. Biofabrication. 2021;14(1) doi: 10.1088/1758-5090/ac3acb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galarraga J.H., Kwon M.Y., Burdick J.A. 3D bioprinting via an in situ crosslinking technique towards engineering cartilage tissue. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-56117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vega S.L., Kwon M.Y., Song K.H., Wang C., Mauck R.L., Han L., Burdick J.A. Combinatorial hydrogels with biochemical gradients for screening 3D cellular microenvironments. Nat. Commun. 2018;9(1):614. doi: 10.1038/s41467-018-03021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwon M.Y., Wang C., Galarraga J.H., Pure E., Han L., Burdick J.A. Influence of hyaluronic acid modification on CD44 binding towards the design of hydrogel biomaterials. Biomaterials. 2019;222 doi: 10.1016/j.biomaterials.2019.119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Y.S., Liang K.L., Zhao S.Y., Zhang C., Li J., Yang H.J., Liu X., Yin X.Z., Chen D.Z., Xu W.L., Xiao P. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018;108:383–390. doi: 10.1016/j.ijbiomac.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 73.Ren Y., Zhang H., Qin W.J., Du B., Liu L.R., Yang J. A collagen mimetic peptide-modified hyaluronic acid hydrogel system with enzymatically mediated degradation for mesenchymal stem cell differentiation. Mater. Sci. Eng., C. 2020;108 doi: 10.1016/j.msec.2019.110276. [DOI] [PubMed] [Google Scholar]
- 74.Ren Y., Zhang Y., Zhang H., Wang Y.P., Liu L.R., Zhang Q.Q. A gelatin-hyaluronic acid double cross-linked hydrogel for regulating the growth and dual dimensional cartilage differentiation of bone marrow mesenchymal stem cells. J. Biomed. Nanotechnol. 2021;17(6):1044–1057. doi: 10.1166/jbn.2021.3088. [DOI] [PubMed] [Google Scholar]
- 75.Ren Y., Zhang H., Wang Y., Du B., Yang J., Liu L., Zhang Q. Hyaluronic acid hydrogel with adjustable stiffness for mesenchymal stem cell 3D culture via related molecular mechanisms to maintain stemness and induce cartilage differentiation. ACS Appl. Bio Mater. 2021;4(3):2601–2613. doi: 10.1021/acsabm.0c01591. [DOI] [PubMed] [Google Scholar]
- 76.Zhang C., Dong Q., Liang K., Zhou D., Yang H., Liu X., Xu W., Zhou Y., Xiao P. Photopolymerizable thiol-acrylate maleiated hyaluronic acid/thiol-terminated poly(ethylene glycol) hydrogels as potential in-situ formable scaffolds. Int. J. Biol. Macromol. 2018;119:270–277. doi: 10.1016/j.ijbiomac.2018.07.153. [DOI] [PubMed] [Google Scholar]
- 77.Pandit A.H., Mazumdar N., Ahmad S. Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2019;137:853–869. doi: 10.1016/j.ijbiomac.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 78.Rogina A., Pusic M., Stefan L., Ivkovic A., Urlic I., Ivankovic M., Ivankovic H. Characterization of chitosan-based scaffolds seeded with sheep nasal chondrocytes for cartilage tissue engineering. Ann. Biomed. Eng. 2021;49(6):1572–1586. doi: 10.1007/s10439-020-02712-9. [DOI] [PubMed] [Google Scholar]
- 79.V Thomas L., Vg R., D Nair P. Effect of stiffness of chitosan-hyaluronic acid dialdehyde hydrogels on the viability and growth of encapsulated chondrocytes. Int. J. Biol. Macromol. 2017;104:1925–1935. doi: 10.1016/j.ijbiomac.2017.05.116. [DOI] [PubMed] [Google Scholar]
- 80.Cai Y., Lopez-Ruiz E., Wengel J., Creemers L.B., Howard K.A. A hyaluronic acid-based hydrogel enabling CD44-mediated chondrocyte binding and gapmer oligonucleotide release for modulation of gene expression in osteoarthritis. J. Contr. Release. 2017;253:153–159. doi: 10.1016/j.jconrel.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 81.Liu C., Liu D., Wang Y., Li Y., Li T., Zhou Z., Yang Z., Wang J., Zhang Q. Glycol chitosan/oxidized hyaluronic acid hydrogels functionalized with cartilage extracellular matrix particles and incorporating BMSCs for cartilage repair. Artif. Cell Nanomed. Biotechnol. 2018;46(sup1):721–732. doi: 10.1080/21691401.2018.1434662. [DOI] [PubMed] [Google Scholar]
- 82.Tan H., Chu C.R., Payne K.A., Marra K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30(13):2499–2506. doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heirani-Tabasi A., Hosseinzadeh S., Rabbani S., Ahmadi Tafti S.H., Jamshidi K., Soufizomorrod M., Soleimani M. Cartilage tissue engineering by co-transplantation of chondrocyte extracellular vesicles and mesenchymal stem cells, entrapped in chitosan-hyaluronic acid hydrogel. Biomed. Mater. 2021;16(5) doi: 10.1088/1748-605X/ac0cbf. [DOI] [PubMed] [Google Scholar]
- 84.Sheu S.Y., Chen W.S., Sun J.S., Lin F.H., Wu T. Biological characterization of oxidized hyaluronic acid/resveratrol hydrogel for cartilage tissue engineering. J. Biomed. Mater. Res., Part A. 2013;101(12):3457–3466. doi: 10.1002/jbm.a.34653. [DOI] [PubMed] [Google Scholar]
- 85.Chen J.Q., Yang J.B., Wang L., Zhang X.W., Heng B.C., Wang D.A., Ge Z.G. Modified hyaluronic acid hydrogels with chemical groups that facilitate adhesion to host tissues enhance cartilage regeneration. Bioact. Mater. 2021;6(6):1689–1698. doi: 10.1016/j.bioactmat.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hozumi T., Kageyama T., Ohta S., Fukuda J., Ito T. Injectable hydrogel with slow degradability composed of gelatin and hyaluronic acid cross-linked by schiff's base formation. Biomacromolecules. 2018;19(2):288–297. doi: 10.1021/acs.biomac.7b01133. [DOI] [PubMed] [Google Scholar]
- 87.Martinez-Sanz E., Ossipov D.A., Hilborn J., Larsson S., Jonsson K.B., Varghese O.P. Bone reservoir: injectable hyaluronic acid hydrogel for minimal invasive bone augmentation. J. Contr. Release. 2011;152(2):232–240. doi: 10.1016/j.jconrel.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 88.Bermejo-Velasco D., Kadekar S., Tavares da Costa M.V., Oommen O.P., Gamstedt K., Hilborn J., Varghese O.P. First aldol cross-linked hyaluronic acid hydrogel: fast and hydrolytically stable hydrogel with tissue adhesive properties. ACS Appl. Mater. Interfaces. 2019;11(41):38232–38239. doi: 10.1021/acsami.9b10239. [DOI] [PubMed] [Google Scholar]
- 89.Chen X., Jiang C., Wang T., Zhu T., Li X., Huang J. Hyaluronic acid-based biphasic scaffold with layer-specific induction capacity for osteochondral defect regeneration. Mater. Des. 2022;216 [Google Scholar]
- 90.Liu X.L., Yang Y.L., Niu X., Lin Q.N., Zhao B.Z., Wang Y., Zhu L.Y. An in situ photocrosslinkable platelet rich plasma-complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta Biomater. 2017;62:179–187. doi: 10.1016/j.actbio.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 91.Summonte S., Racaniello G.F., Lopedota A., Denora N., Bernkop-Schnurch A. Thiolated polymeric hydrogels for biomedical application: cross-linking mechanisms. J. Contr. Release. 2021;330:470–482. doi: 10.1016/j.jconrel.2020.12.037. [DOI] [PubMed] [Google Scholar]
- 92.Bian S.Q., He M.M., Sui J.H., Cai H.X., Sun Y., Liang J., Fan Y.J., Zhang X.D. The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture. Colloids Surf., B. 2016;140:392–402. doi: 10.1016/j.colsurfb.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 93.Wang Q., Li X., Wang P.L., Yao Y., Xu Y., Chen Y.F., Sun Y., Jiang Q., Fan Y.J., Zhang X.D. Bionic composite hydrogel with a hybrid covalent/noncovalent network promoting phenotypic maintenance of hyaline cartilage. J. Mater. Chem. B. 2020;8(20):4402–4411. doi: 10.1039/d0tb00253d. [DOI] [PubMed] [Google Scholar]
- 94.Böck T., Schill V., Krahnke M., Steinert A.F., Tessmar J., Blunk T., Groll J. TGF-β1-modified hyaluronic acid/poly(glycidol) hydrogels for chondrogenic differentiation of human mesenchymal stromal cells. Macromol. Biosci. 2018;18(7) doi: 10.1002/mabi.201700390. [DOI] [PubMed] [Google Scholar]
- 95.Hauptstein J., Forster L., Nadernezhad A., Groll J., Tessmar J., Blunk T. Tethered TGF-β1 in a hyaluronic acid-based bioink for bioprinting cartilaginous tissues. Int. J. Mol. Sci. 2022;23(2):924. doi: 10.3390/ijms23020924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin R., Moreira Teixeira L.S., Krouwels A., Dijkstra P.J., van Blitterswijk C.A., Karperien M., Feijen J. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via michael addition: an injectable biomaterial for cartilage repair. Acta Biomater. 2010;6(6):1968–1977. doi: 10.1016/j.actbio.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 97.Li J., Huang Y.C., Song J., Li X.L., Zhang X.T., Zhou Z.Y., Chen D., Ma P.X., Peng W.J., Wang W.X., Zhou G.Q. Cartilage regeneration using arthroscopic flushing fluid-derived mesenchymal stem cells encapsulated in a one-step rapid cross-linked hydrogel. Acta Biomater. 2018;79:202–215. doi: 10.1016/j.actbio.2018.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X., A S., Xu Q., Alshehri F., Zeng M., Zhou D., Li J., Zhou G., Wang W. Cartilage-derived progenitor cell-laden injectable hydrogel-an approach for cartilage tissue regeneration. ACS Appl. Bio Mater. 2020;3(8):4756–4765. doi: 10.1021/acsabm.0c00294. [DOI] [PubMed] [Google Scholar]
- 99.Wang X., Li X., Duffy P., McMahon S., Wang X., Lyu J., Xu Q., A S., Chen N.N., Bi V., Dürig T., Wang W. Resveratrol-loaded poly(D,L-lactide-co-glycolide) microspheres integrated in a hyaluronic acid injectable hydrogel for cartilage regeneration. Adv. NanoBiomed Res. 2021;2(1) [Google Scholar]
- 100.Sabbieti M.G., Dubbini A., Laus F., Paggi E., Marchegiani A., Capitani M., Marchetti L., Dini F., Vermonden T., Di Martino P., Agas D., Censi R. In vivo biocompatibility of p(HPMAm-lac)-PEG hydrogels hybridized with hyaluronan. J. Tissue Eng. Regener. Med. 2017;11(11):3056–3067. doi: 10.1002/term.2207. [DOI] [PubMed] [Google Scholar]
- 101.Agas D., Laus F., Lacava G., Marchegiani A., Deng S.Y., Magnoni F., Silva G.G., Di Martino P., Sabbieti M.G., Censi R. Thermosensitive hybrid hyaluronan/p(HPMAm-lac)-PEG hydrogels enhance cartilage regeneration in a mouse model of osteoarthritis. J. Cell. Physiol. 2019;234(11):20013–20027. doi: 10.1002/jcp.28598. [DOI] [PubMed] [Google Scholar]
- 102.Jiang Y.H., Lou Y.Y., Li T.H., Liu B.Z., Chen K., Zhang D., Li T. Review article cross-linking methods of type I collagen-based scaffolds for cartilage tissue engineering. Am. J. Transl. Res. 2022;14(2):1146–1159. [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Y.F., Sui J.H., Wang Q., Yin Y.J., Liu J., Wang Q.G., Han X.L., Sun Y., Fan Y.J., Zhang X.D. Injectable self-crosslinking HA-SH/Col I blend hydrogels for in vitro construction of engineered cartilage, Carbohydr. Polymer. 2018;190:57–66. doi: 10.1016/j.carbpol.2018.02.057. [DOI] [PubMed] [Google Scholar]
- 104.Wang H.Z., Xu Y., Wang P.L., Ma J.B., Wang P.Q., Han X.L., Fan Y.J., Bai D., Sun Y., Zhang X.D. Cell-mediated injectable blend hydrogel-BCP ceramic scaffold for in situ condylar osteochondral repair. Acta Biomater. 2021;123:364–378. doi: 10.1016/j.actbio.2020.12.056. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y.B., Yang J.R., Luo Z.C., Li D.X., Lu J., Wang Q.G., Xiao Y.M., Zhang X.D. Development of an injectable thiolated icariin functionalized collagen/hyaluronic hydrogel to promote cartilage formation in vitro and in vivo. J. Mater. Chem. B. 2019;7(17):2845–2854. doi: 10.1039/c9tb00211a. [DOI] [PubMed] [Google Scholar]
- 106.Yao Y., Wang P.L., Li X., Xu Y., Lu G.G., Jiang Q., Sun Y., Fan Y.J., Zhang X.D. A di-self-crosslinking hyaluronan-based hydrogel combined with type I collagen to construct a biomimetic injectable cartilage-filling scaffold. Acta Biomater. 2020;111:197–207. doi: 10.1016/j.actbio.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y.X., Chen Y.F., Xu Y., Chen M.Y., Lu Y., Liang J., Sun Y., Fan Y.J., Zhang X.D. Effects of the bonding intensity between hyaluronan and gelatin on chondrogenic phenotypic maintenance. J. Mater. Chem. B. 2020;8(39):9062–9074. doi: 10.1039/d0tb01816c. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X., Li Z., Yang P., Duan G., Liu X., Gu Z., Li Y. Polyphenol scaffolds in tissue engineering. Mater. Horiz. 2021;8(1):145–167. doi: 10.1039/d0mh01317j. [DOI] [PubMed] [Google Scholar]
- 109.Kurisawa M., Chung J.E., Yang Y.Y., Gao S.J., Uyama H. Injectable biodegradable hydrogels composed of hyaluronic acid-tyramine conjugates for drug delivery and tissue engineering. Chem. Commun. 2005;34:4312–4314. doi: 10.1039/b506989k. [DOI] [PubMed] [Google Scholar]
- 110.Hong B.M., Kim H.C., Jeong J.E., Park S.A., Park W.H. Visible-light-induced hyaluronate hydrogel for soft tissue fillers. Int. J. Biol. Macromol. 2020;165:2834–2844. doi: 10.1016/j.ijbiomac.2020.10.155. [DOI] [PubMed] [Google Scholar]
- 111.Toh W.S., Lim T.C., Kurisawa M., Spector M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials. 2012;33(15):3835–3845. doi: 10.1016/j.biomaterials.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 112.Behrendt P., Ladner Y., Stoddart M.J., Lippross S., Alini M., Eglin D., Armiento A.R. Articular joint-simulating mechanical load activates endogenous TGF-β in a highly cellularized bioadhesive hydrogel for cartilage repair. Am. J. Sports Med. 2020;48(1):210–221. doi: 10.1177/0363546519887909. [DOI] [PubMed] [Google Scholar]
- 113.Xu K.M., Narayanan K., Lee F., Bae K.H., Gao S.J., Kurisawa M. Enzyme-mediated hyaluronic acid-tyramine hydrogels for the propagation of human embryonic stem cells in 3D. Acta Biomater. 2015;24:159–171. doi: 10.1016/j.actbio.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 114.Vainieri M.L., Lolli A., Kops N., D'Atri D., Eglin D., Yayon A., Alini M., Grad S., Sivasubramaniyan K., van Osch G.J.V.M. Evaluation of biomimetic hyaluronic-based hydrogels with enhanced endogenous cell recruitment and cartilage matrix formation. Acta Biomater. 2020;101:293–303. doi: 10.1016/j.actbio.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 115.Lolli A., Sivasubramaniyan K., Vainieri M.L., Eglin D., Wexselblatt E., Yayon A., Grad S., Alini M., van Osch G.J. Hyaluronan-based hydrogel delivering antimiR-221 for the guidance of endogenous cartilage repair. Osteoarthritis Cartilage. 2018;26:S163. -S163. [Google Scholar]
- 116.Ziadlou R., Rotman S., Teuschl A., Salzer E., Barbero A., Martin I., Alini M., Eglin D., Grad S. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater. Sci. Eng., C. 2021;120 doi: 10.1016/j.msec.2020.111701. [DOI] [PubMed] [Google Scholar]
- 117.Wang X., Song X.B., Li T., Chen J.J., Cheng G.T., Yang L., Chen C. Aptamer-functionalized bioscaffold enhances cartilage repair by improving stem cell recruitment in osteochondral defects of rabbit knees. Am. J. Sports Med. 2019;47(10):2316–2326. doi: 10.1177/0363546519856355. [DOI] [PubMed] [Google Scholar]
- 118.Weitkamp J.T., Woltje M., Nusspickel B., Schmidt F.N., Aibibu D., Bayer A., Eglin D., Armiento A.R., Arnold P., Cherif C., Lucius R., Smeets R., Kurz B., Behrendt P. Silk fiber-reinforced hyaluronic acid-based hydrogel for cartilage tissue engineering. Int. J. Mol. Sci. 2021;22(7):3635. doi: 10.3390/ijms22073635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vaca-González J.J., Clara-Trujillo S., Guillot-Ferriols M., Rodenas-Rochina J., Sanchis M.J., Ribelles J.L.G., Garzon-Alvarado D.A., Ferrer G.G. Effect of electrical stimulation on chondrogenic differentiation of mesenchymal stem cells cultured in hyaluronic acid-gelatin injectable hydrogels. Bioelectrochemistry. 2020;134 doi: 10.1016/j.bioelechem.2020.107536. [DOI] [PubMed] [Google Scholar]
- 120.Kim K.S., Park S.J., Yang J.A., Jeon J.H., Bhang S.H., Kim B.S., Hahn S.K. Injectable hyaluronic acid-tyramine hydrogels for the treatment of rheumatoid arthritis. Acta Biomater. 2011;7(2):666–674. doi: 10.1016/j.actbio.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 121.Jooybar E., Abdekhodaie M.J., Alvi M., Mousavi A., Karperien M., Dijkstra P.J. An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta Biomater. 2019;83:233–244. doi: 10.1016/j.actbio.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 122.Ren C.D., Kurisawa M., Chung J.E., Ying J.Y. Liposomal delivery of horseradish peroxidase for thermally triggered injectable hyaluronic acid-tyramine hydrogel scaffolds. J. Mater. Chem. B. 2015;3(23):4663–4670. doi: 10.1039/c4tb01832j. [DOI] [PubMed] [Google Scholar]
- 123.Ren C.D., Gao S.J., Kurisawa M., Ying J.Y. Cartilage synthesis in hyaluronic acid-tyramine constructs. J. Mater. Chem. B. 2015;3(9):1942–1956. doi: 10.1039/c4tb01229a. [DOI] [PubMed] [Google Scholar]
- 124.Cheng W., Zeng X.W., Chen H.Z., Li Z.M., Zeng W.F., Mei L., Zhao Y.L. Versatile polydopamine platforms: synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano. 2019;13(8):8537–8565. doi: 10.1021/acsnano.9b04436. [DOI] [PubMed] [Google Scholar]
- 125.Tsai W.B., Chen W.T., Chien H.W., Kuo W.H., Wang M.J. Poly(dopamine) coating of scaffolds for articular cartilage tissue engineering. Acta Biomater. 2011;7(12):4187–4194. doi: 10.1016/j.actbio.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 126.Ren K., Wan H., Kaper H.J., Sharma P.K. Dopamine-conjugated hyaluronic acid delivered via intra-articular injection provides articular cartilage lubrication and protection. J. Colloid Interface Sci. 2022;619:207–218. doi: 10.1016/j.jcis.2022.03.119. [DOI] [PubMed] [Google Scholar]
- 127.Zhou D., Li S., Pei M., Yang H., Gu S., Tao Y., Ye D., Zhou Y., Xu W., Xiao P. Dopamine-modified hyaluronic acid hydrogel adhesives with fast-forming and high tissue adhesion. ACS Appl. Mater. Interfaces. 2020;12(16):18225–18234. doi: 10.1021/acsami.9b22120. [DOI] [PubMed] [Google Scholar]
- 128.Salzlechner C., Haghighi T., Huebscher I., Walther A.R., Schell S., Gardner A., Undt G., da Silva R.M.P., Dreiss C.A., Fan K., Gentleman E. Adhesive hydrogels for maxillofacial tissue regeneration using minimally invasive procedures. Adv. Healthcare Mater. 2020;9(4) doi: 10.1002/adhm.201901134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Salzlechner C., Walther A.R., Schell S., Merrild N.G., Haghighi T., Huebscher I., Undt G., Fan K., Bergholt M.S., Hedegaard M.A.B., Gentleman E. Complementary techniques to analyse pericellular matrix formation by human MSC within hyaluronic acid hydrogels. Mater. Adv. 2020;1(8):2888–2896. [Google Scholar]
- 130.Guo Z., Mi S., Sun W. A facile strategy for preparing tough, self-healing double-network hyaluronic acid hydrogels inspired by mussel cuticles. Macromol. Mater. Eng. 2019;304(4) [Google Scholar]
- 131.Yu C., Gao H., Li Q., Cao X. Injectable dual cross-linked adhesive hyaluronic acid multifunctional hydrogel scaffolds for potential applications in cartilage repair. Polym. Chem. 2020;11(18):3169–3178. [Google Scholar]
- 132.Gan D.L., Jiang Y.A., Hu Y.L., Wang X., Wang Q.G., Wang K.F., Xie C.M., Han L., Lu X. Mussel-inspired extracellular matrix-mimicking hydrogel scaffold with high cell affinity and immunomodulation ability for growth factor-free cartilage regeneration. J. Orthop. Transl. 2022;33:120–131. doi: 10.1016/j.jot.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cho J.H., Lee J.S., Shin J., Jeon E.J., An S., Choi Y.S., Cho S.W. Ascidian-inspired fast-forming hydrogel system for versatile biomedical applications: pyrogallol chemistry for dual modes of crosslinking mechanism. Adv. Funct. Mater. 2017;28(6) [Google Scholar]
- 134.Samanta S., Rangasami V.K., Sarlus H., Samal J.R.K., Evans A.D., Parihar V.S., Varghese O.P., Harris R.A., Oommen O.P. Interpenetrating gallol functionalized tissue adhesive hyaluronic acid hydrogel polarizes macrophages to an immunosuppressive phenotype. Acta Biomater. 2022;142:36–48. doi: 10.1016/j.actbio.2022.01.048. [DOI] [PubMed] [Google Scholar]
- 135.Jin Y.J., Koh R.H., Kim S.H., Kim K.M., Park G.K., Hwang N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater. Sci. Eng., C. 2020;115 doi: 10.1016/j.msec.2020.111096. [DOI] [PubMed] [Google Scholar]
- 136.Lee F., Lim J., Reithofer M.R., Lee S.S., Chung J.E., Hauser C.A.E., Kurisawa M. Synthesis and bioactivity of a conjugate composed of green tea catechins and hyaluronic acid. Polym. Chem. 2015;6(24):4462–4472. [Google Scholar]
- 137.Gwak M.A., Hong B.M., Seok J.M., Park S.A., Park W.H. Effect of tannic acid on the mechanical and adhesive properties of catechol-modified hyaluronic acid hydrogels. Int. J. Biol. Macromol. 2021;191:699–705. doi: 10.1016/j.ijbiomac.2021.09.123. [DOI] [PubMed] [Google Scholar]
- 138.Lee H.Y., Hwang C.H., Kim H.E., Jeong S.H. Enhancement of bio-stability and mechanical properties of hyaluronic acid hydrogels by tannic acid treatment. Carbohydr. Polym. 2018;186:290–298. doi: 10.1016/j.carbpol.2018.01.056. [DOI] [PubMed] [Google Scholar]
- 139.Lin F., Wang Z., Xiang L., Deng L.F., Cui W.G. Charge-guided micro/nano-hydrogel microsphere for penetrating cartilage matrix. Adv. Funct. Mater. 2021;31(49) [Google Scholar]
- 140.Liu L.T., Yu M., Zhang Y., Wang C.C., Lu H.J. Hydrazide functionalized core-shell magnetic nanocomposites for highly specific enrichment of N-glycopeptides. ACS Appl. Mater. Interfaces. 2014;6(10):7823–7832. doi: 10.1021/am501110e. [DOI] [PubMed] [Google Scholar]
- 141.Lei Y., Wang X., Liao J., Shen J., Li Y., Cai Z., Hu N., Luo X., Cui W., Huang W. Shear-responsive boundary-lubricated hydrogels attenuate osteoarthritis. Bioact. Mater. 2022;16:472–484. doi: 10.1016/j.bioactmat.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou T., Ran J.S., Xu P.F., Shen L.Y., He Y.Z., Ye J., Wu L.D., Gao C.Y. A hyaluronic acid/platelet-rich plasma hydrogel containing MnO2 nanozymes efficiently alleviates osteoarthritis in vivo. Carbohydr. Polym. 2022;292 doi: 10.1016/j.carbpol.2022.119667. [DOI] [PubMed] [Google Scholar]
- 143.Zhou T., Xiong H., Wang S.Q., Zhang H.L., Zheng W.W., Gou Z.R., Fan C.Y., Gao C.Y. An injectable hydrogel dotted with dexamethasone acetate-encapsulated reactive oxygen species-scavenging micelles for combinatorial therapy of osteoarthritis, Mater. Today Nano. 2022;17 [Google Scholar]
- 144.Chen F., Ni Y.Z., Liu B., Zhou T.T., Yu C.Y., Su Y., Zhu X.Y., Yu X.W., Zhou Y.F. Self-crosslinking and injectable hyaluronic acid/RGD-functionalized pectin hydrogel for cartilage tissue engineering. Carbohydr. Polym. 2017;166:31–44. doi: 10.1016/j.carbpol.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 145.Mihajlovic M., Rikkers M., Mihajlovic M., Viola M., Schuiringa G., Ilochonwu B.C., Masereeuw R., Vonk L., Malda J., Ito K., Vermonden T. Viscoelastic chondroitin sulfate and hyaluronic acid double-network hydrogels with reversible cross-links. Biomacromolecules. 2022;23(3):1350–1365. doi: 10.1021/acs.biomac.1c01583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wei K.C., Zhu M.L., Sun Y.X., Xu J.B., Feng Q., Lin S., Wu T.Y., Xu J., Tian F., Xia J., Li G., Bian L.M. Robust biopolymeric supramolecular "host-guest macromer" hydrogels reinforced by in situ formed multivalent nanoclusters for cartilage regeneration. Macromolecules. 2016;49(3):866–875. [Google Scholar]
- 147.Jing Y., Yang B., Yuan W., Han S., Song L., Ye M., Zhang Z.-Y., Bian L. Dynamic cell-adaptable hydrogels with a moderate level of elasticity promote 3D development of encapsulated cells. Appl. Mater. Today. 2021;22 [Google Scholar]
- 148.Chiang M.Y., Cheng I.Y., Chou S.H., Tsai J.H., Chen Y.J., Lu H.E., Yang S.W., Chang S.J., Chen S.Y. A smart injectable composite hydrogel with magnetic navigation and controlled glutathione release for promoting in situ chondrocyte array and self-healing in damaged cartilage tissue. J. Mater. Chem. B. 2021;9(45):9370–9382. doi: 10.1039/d1tb02030g. [DOI] [PubMed] [Google Scholar]
- 149.Park K.M., Yang J.A., Jung H., Yeom J., Park J.S., Park K.H., Hoffman A.S., Hahn S.K., Kim K. In situ supramolecular assembly and modular modification of hyaluronic acid hydrogels for 3D cellular engineering. ACS Nano. 2012;6(4):2960–2968. doi: 10.1021/nn204123p. [DOI] [PubMed] [Google Scholar]
- 150.McNary S.M., Athanasiou K.A., Reddi A.H. Engineering lubrication in articular cartilage. Tissue Eng., Part B. 2012;18(2):88–100. doi: 10.1089/ten.teb.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang J.H., Xiong J.Y., Wang D.P., Zhang J., Yang L., Sun S.Q., Liang Y.J. 3D bioprinting of hydrogels for cartilage tissue engineering. Gels. 2021;7(3):144. doi: 10.3390/gels7030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang X., Li S., Ren Y., Qiang L., Liu Y.H., Wang J.W., Dai K.R. 3D printed hydrogel for articular cartilage regeneration. Composites, Part B. 2022;237 [Google Scholar]
- 153.Mei Q.J., Rao J.D., Bei H.P., Liu Y.X., Zhao X. 3D bioprinting photo-crosslinkable hydrogels for bone and cartilage repair. Int. J. Bioprint. 2021;7(3):37–53. doi: 10.18063/ijb.v7i3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Petta D., D'Amora U., Ambrosio L., Grijpma D.W., Eglin D., D'Este M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication. 2020;12(3) doi: 10.1088/1758-5090/ab8752. [DOI] [PubMed] [Google Scholar]
- 155.Hung K.C., Tseng C.S., Dai L.G., Hsu S.H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials. 2016;83:156–168. doi: 10.1016/j.biomaterials.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 156.Shie M.Y., Chang W.C., Wei L.J., Huang Y.H., Chen C.H., Shih C.T., Chen Y.W., Shen Y.F. 3D printing of cytocompatible water-based light-cured polyurethane with hyaluronic acid for cartilage tissue engineering applications. Materials. 2017;10(2):136. doi: 10.3390/ma10020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Antich C., de Vicente J., Jimenez G., Chocarro C., Carrillo E., Montanez E., Galvez-Martin P., Marchal J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020;106:114–123. doi: 10.1016/j.actbio.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 158.Shokri A., Ramezani K., Jamalpour M.R., Mohammadi C., Vahdatinia F., Irani A.D., Sharifi E., Haddadi R., Jamshidi S., Amirabad L.M., Tajik S., Yadegari A., Tayebi L. In vivo efficacy of 3D-printed elastin-gelatin-hyaluronic acid scaffolds for regeneration of nasal septal cartilage defects. J. Biomed. Mater. Res., Part B. 2022;110(3):614–624. doi: 10.1002/jbm.b.34940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abdollahiyan P., Oroojalian F., Mokhtarzadeh A., de la Guardia M. Hydrogel-based 3D bioprinting for bone and cartilage tissue engineering. Biotechnol. J. 2020;15(12) doi: 10.1002/biot.202000095. [DOI] [PubMed] [Google Scholar]
- 160.Lam T., Dehne T., Kruger J.P., Hondke S., Endres M., Thomas A., Lauster R., Sittinger M., Kloke L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res., Part B. 2019;107(8):2649–2657. doi: 10.1002/jbm.b.34354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xia H.T., Zhao D.D., Zhu H.L., Hua Y.J., Xiao K.Y., Xu Y., Liu Y.Q., Chen W.M., Liu Y., Zhang W.J., Liu W., Tang S.J., Cao Y.L., Wang X.Y., Chen H.H., Zhou G.D. Lyophilized scaffolds fabricated from 3D-printed photocurable natural hydrogel for cartilage regeneration. ACS Appl. Mater. Interfaces. 2018;10(37):31704–31715. doi: 10.1021/acsami.8b10926. [DOI] [PubMed] [Google Scholar]
- 162.Shopperly L.K., Spinnen J., Kruger J.P., Endres M., Sittinger M., Lam T., Kloke L., Dehne T. Blends of gelatin and hyaluronic acid stratified by stereolithographic bioprinting approximate cartilaginous matrix gradients. J. Biomed. Mater. Res., Part B. 2022;110(10):2310–2322. doi: 10.1002/jbm.b.35079. [DOI] [PubMed] [Google Scholar]
- 163.Liu Y.Z., Peng L.Q., Li L.L., Huang C.S., Shi K.D., Meng X.B., Wang P.P., Wu M.M., Li L., Cao H.J., Wu K.F., Zeng Q.Q., Pan H.B., Lu W.W., Qin L., Ruan C.S., Wang X.L. 3D-bioprinted BMSC-laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. Biomaterials. 2021;279 doi: 10.1016/j.biomaterials.2021.121216. [DOI] [PubMed] [Google Scholar]
- 164.Li C.R., Zheng Z.Z., Jia J.Y., Zhang W.J., Qin L., Zhang W., Lai Y.X. Preparation and characterization of photocurable composite extracellular matrix-methacrylated hyaluronic acid bioink. J. Mater. Chem. B. 2022;10(22):4242–4253. doi: 10.1039/d2tb00548d. [DOI] [PubMed] [Google Scholar]
- 165.Mouser V.H.M., Abbadessa A., Levato R., Hennink W.E., Vermonden T., Gawlitta D., Malda J. Development of a thermosensitive HAMA-containing bio-ink for the fabrication of composite cartilage repair constructs. Biofabrication. 2017;9(1) doi: 10.1088/1758-5090/aa6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Feng Q., Li D.G., Li Q.T., Li S.X., Huang H.H., Li H.F., Dong H., Cao X.D. Dynamic nanocomposite microgel assembly with microporosity, injectability, tissue-adhesion, and sustained drug release promotes articular cartilage repair and regeneration. Adv. Healthcare Mater. 2022;11(8) doi: 10.1002/adhm.202102395. [DOI] [PubMed] [Google Scholar]
- 167.Shi W., Fang F., Kong Y.F., Greer S.E., Kuss M., Liu B., Xue W., Jiang X.P., Lovell P., Mohs A.M., Dudley A.T., Li T.S., Duan B. Dynamic hyaluronic acid hydrogel with covalent linked gelatin as an anti-oxidative bioink for cartilage tissue engineering. Biofabrication. 2022;14(1) doi: 10.1088/1758-5090/ac42de. [DOI] [PubMed] [Google Scholar]
- 168.Lee J.S., Park H.S., Jung H., Lee H., Hong H., Lee Y.J., Suh Y.J., Lee O.J., Kim S.H., Park C.H. 3D-printable photocurable bioink for cartilage regeneration of tonsil-derived mesenchymal stem cells, Addit. Man. 2020;33 [Google Scholar]
- 169.Stichler S., Bock T., Paxton N., Bertlein S., Levato R., Schill V., Smolan W., Malda J., Tessmar J., Blunk T., Groll J. Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(epsilon-caprolactone) for MSC chondrogenesis. Biofabrication. 2017;9(4) doi: 10.1088/1758-5090/aa8cb7. [DOI] [PubMed] [Google Scholar]
- 170.Hauptstein J., Boeck T., Bartolf-Kopp M., Forster L., Stahlhut P., Nadernezhad A., Blahetek G., Zernecke-Madsen A., Detsch R., Jungst T., Groll J., Tessmar J., Blunk T. Hyaluronic acid-based bioink composition enabling 3D bioprinting and improving quality of deposited cartilaginous extracellular matrix. Adv. Healthcare Mater. 2020;9(15) doi: 10.1002/adhm.202000737. [DOI] [PubMed] [Google Scholar]
- 171.Hauptstein J., Forster L., Nadernezhad A., Horder H., Stahlhut P., Groll J., Blunk T., Tessmar J. Bioink platform utilizing dual-stage crosslinking of hyaluronic acid tailored for chondrogenic differentiation of mesenchymal stromal cells. Macromol. Biosci. 2022;22(2) doi: 10.1002/mabi.202100331. [DOI] [PubMed] [Google Scholar]
- 172.Mancini I.A.D., Schmidt S., Brommer H., Pouran B., Schafer S., Tessmar J., Mensinga A., van Rijen M.H.P., Groll J., Blunk T., Levato R., Malda J., van Weeren P.R. A composite hydrogel-3D printed thermoplast osteochondral anchor as example for a zonal approach to cartilage repair: In vivo performance in a long-term equine model. Biofabrication. 2020;12(3) doi: 10.1088/1758-5090/ab94ce. [DOI] [PubMed] [Google Scholar]
- 173.Barthold J.E., McCreery K.P., Martinez J., Bellerjeau C., Ding Y.F., Bryant S.J., Whiting G.L., Neu C.P. Particulate ecm biomaterial ink is 3D printed and naturally crosslinked to form structurally-layered and lubricated cartilage tissue mimics. Biofabrication. 2022;14(2) doi: 10.1088/1758-5090/ac584c. [DOI] [PubMed] [Google Scholar]
- 174.Nedunchezian S., Banerjee P., Lee C.Y., Lee S.S., Lin C.W., Wu C.W., Wu S.C., Chang J.K., Wang C.K. Generating adipose stem cell-laden hyaluronic acid-based scaffolds using 3D bioprinting via the double crosslinked strategy for chondrogenesis. Mater. Sci. Eng., C. 2021;124 doi: 10.1016/j.msec.2021.112072. [DOI] [PubMed] [Google Scholar]
- 175.Kim H.S., Kim C., Lee K.Y. Three-dimensional bioprinting of polysaccharide-based self-healing hydrogels with dual cross-linking. J. Biomed. Mater. Res., Part A. 2022;110(4):761–772. doi: 10.1002/jbm.a.37325. [DOI] [PubMed] [Google Scholar]
- 176.Wang Y., Chen Y., Zheng J., Liu L., Zhang Q. Three-dimensional printing self-healing dynamic/photocrosslinking gelatin-hyaluronic acid double-network hydrogel for tissue engineering. ACS Omega. 2022;7(14):12076–12088. doi: 10.1021/acsomega.2c00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Flégeau K., Puiggali-Jou A., Zenobi-Wong M. Cartilage tissue engineering by extrusion bioprinting utilizing porous hyaluronic acid microgel bioinks. Biofabrication. 2022;14(3) doi: 10.1088/1758-5090/ac6b58. [DOI] [PubMed] [Google Scholar]
- 178.Petta D., Grijpnia D.W., Alini M., Eglin D., D'Este M. Three-dimensional printing of a tyramine hyaluronan derivative with double gelation mechanism for independent tuning of shear thinning and postprinting curing. ACS Biomater. Sci. Eng. 2018;4(8):3088–3098. doi: 10.1021/acsbiomaterials.8b00416. [DOI] [PubMed] [Google Scholar]
- 179.Chen H.Q., Fei F., Li X.D., Nie Z.G., Zhou D.Z., Liu L.B.A., Zhang J., Zhang H.T., Fei Z., Xu T. A structure-supporting, self-healing, and high permeating hydrogel bioink for establishment of diverse homogeneous tissue-like constructs. Bioact. Mater. 2021;6(10):3580–3595. doi: 10.1016/j.bioactmat.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors have obtained figures permission.