Abstract
Malate dehydrogenases (MDH) serve a critical role in maintaining equilibrium of the NAD+/NADH ratio between the mitochondria and cytosol through the catalysis of the oxidation of L-malate to oxaloacetate in a reversible, NADH-dependent manner. MDH2 encodes the mitochondrial isoform, which is integral to the tricarboxylic acid cycle and thus energy homeostasis. Recently, five patients harboring compound heterozygous MDH2 variants have been described, three with early-onset epileptic encephalopathy, one with a stroke-like episode, and one with dilated cardiomyopathy. Here, we describe an additional seven patients with biallelic variants in MDH2, the largest and most neurodevelopmentally and ethnically diverse cohort to-date, including homozygous variants, a sibling pair, non-European patients, and an adult. From these patients, we learn that MDH2 deficiency results in a biochemical signature including elevations of plasma lactate and the lactate:pyruvate ratio with urinary excretion of malate. It also results in a recognizable constellation of neuroimaging findings of anterior-predominant cerebral atrophy, subependymal cysts with ventricular septations. We also recognize MDH2 deficiency as a cause of Leigh syndrome. Taken with existing patient reports, we conclude that MDH2 deficiency is an emerging and likely under-recognized cause of infantile epileptic encephalopathy and provide a framework for medical evaluation of patients identified with biallelic MDH2 variants.
Keywords: Malate dehydrogenase, MDH2, Mitochondrial malate dehydrogenase, TCA cycle, Epileptic encephalopathy, Leigh syndrome
Highlights
-
•
MDH2 deficiency results from biallelic variants in the MDH2 gene and causes an early-onset epileptic encephalopathy.
-
•
The biochemical signature of MDH2 is high plasma lactate, high plasma lactate:pyruvate ratio, and high urinary malic acid.
-
•
Distinct neuroimaging in MDH2: anterior-predominant cerebral atrophy and subependymal cysts with ventricular septations
-
•
MDH2 care team should include biochemical genetics, neurology, developmental pediatrics, cardiology & ophthalmology
1. Introduction
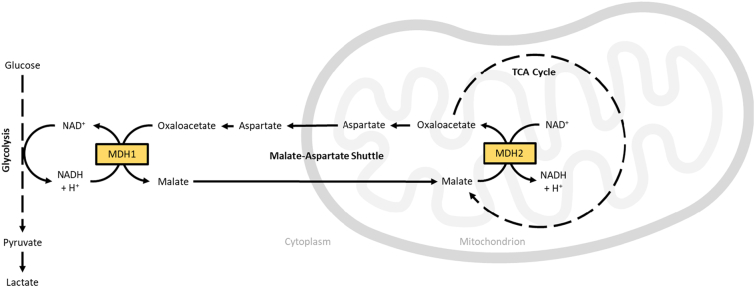
Malate dehydrogenase (MDH) catalyzes the oxidation of L-malate to oxaloacetate in a reversible, NADH-dependent manner. There are two human isoforms existing in distinct intracellular compartments. Cytosolic MDH1 catalyzes the reduction of oxaloacetate to L-malate, oxidizing NADH to NAD+ in the process and driving glycolysis. Mitochondrial MDH2 (MIM: 154100; Enzyme Commission number: 1.1.1.37) catalyzes the oxidation of L-malate to oxaloacetate, regenerating substrate to drive the tricarboxylic acid (TCA) cycle. NAD+ is reduced to NADH in the process, which in turn drives oxidative phosphorylation and energy production. Together, the malate dehydrogenases serve a critical role in maintaining equilibrium of the NAD+/NADH ratio between the mitochondria and cytosol (Fig. 1).
Fig. 1.
A schematic showing the role of cytosolic and mitochondrial malate dehydrogenase isoforms (MDH1 and MDH2, respectively) in cellular energy homeostasis.
Deficiency in mitochondrial malate dehydrogenase due to biallelic variants in MDH2 (PMIM: 617339) was first described in 2017 when Ait-El-Mkadem et al. reported three unrelated male patients [1]. These children presented within the first six months of life with hypotonia and refractory epilepsy followed by severe developmental delays [1]. Biochemical laboratory evaluation showed elevations in plasma lactate, plasma lactate:pyruvate ratio, and urine TCA intermediates, including malate and fumarate [1]. Patient fibroblasts demonstrated reduced MDH2 activity in vitro and yeast complementation studies demonstrated a growth deficit in mutant strains, which collectively supported pathogenicity of the variants [1].
Since then, two additional cases with novel biallelic variants in MDH2 have been published. Laemmle et al. described an 18 month-old female patient with a stroke-like episode and elevated plasma lactate, found to have reduced MDH2 activity in patient fibroblasts [2]. Treatment with the odd-chain triglyceride triheptanoin improved the patient's growth and motor skills and reduced her plasma lactate [2]. Ticci et al. described a two year-old male patient with psychomotor delay and ataxia who developed dilated cardiomyopathy, ultimately requiring orthotopic cardiac transplantation [3]. He, too, had reduced MDH2 activity in patient fibroblasts [3]. Taken together, these cases support that MDH2 deficiency results in an early-onset encephalopathy with a phenotypic spectrum that is incompletely described given the paucity of patient reports and formal natural history studies.
Here, we describe an additional seven patients with biallelic variants in MDH2, the largest and most diverse cohort to-date. These include homozygous variants, a sibling pair, non-European patients, and an adult. Taken with existing patient reports, we conclude that MDH2 deficiency is an emerging and likely under-recognized cause of neurodevelopmental impairment in children and provide a framework for medical evaluation of patients identified with biallelic MDH2 variants.
2. Methods
2.1. Human subject research
This case series did not meet the U.S. Department of Health and Human Services Common Rule definition of human subjects' research per 45 CFR 46.102(d) and thus was not reviewed by an Institutional Review Board (IRB). Investigators from different institutions were connected via GeneMatcher (http://www.genematcher.org) [4]. Written consent was obtained from the patients' parents for inclusion of their child's respective clinical data in this manuscript. Clinical data housed within the medical record was reviewed retrospectively. Culture of patient skin fibroblasts on a research basis was performed under Children's Hospital of Philadelphia (CHOP) IRB 08–6177 (Marni J. Falk, Primary Investigator) with formal informed consent from the patient's parents.
2.2. Biochemical & genetic testing
Biochemical laboratory testing relating to the clinical cases in the U.S. was performed on a clinical basis in Clinical Laboratory Improvement Amendments (CLIA)-certified facilities. In brief, urine organic acids were performed as previously published, using gas chromatography/mass spectrometry [5]. Extracted ion chromatography for select daughter ions of malic acid (233, 245, 335, and 217 m/z) was performed to better evaluate the presence of malic acid.
Molecular genetic testing relating to these cases was also performed on a clinical basis. Selection of testing modality and laboratory occurred at the discretion of the clinical team and thus was not standardized across the cohort. Testing was performed using next-generation sequencing technologies through commercial laboratories including Ambry Genetics (Aliso Viejo, CA, USA), Invitae (San Francisco, CA, USA), MedGenome Labs (Bangalore, India), Molecular Diagnostic Laboratory at the University of Minnesota (Minneapolis, MN, USA), and Prevention Genetics (Marshfield, WI, USA). For exome sequencing performed by Ambry, Invitae, and MedGenome, orthogonal technologies, including Sanger sequencing, are utilized to confirm clinically significant variants. Of note, MDH2 was added to the Invitae epilepsy gene panel in October 2020.
2.3. Malate dehydrogenase enzyme activity
MDH2 enzymatic activity was measured on lysates from cultured patient fibroblasts and two different control fibroblast cell lines using a commercially available kit (ab119693; abcam, Cambridge, UK) and according to manufacturer instructions. Briefly, protein was extracted from cultured fibroblasts and 50 μg was incubated in antibody-coated microplate wells, thereby trapping MDH2 within them. Substrate for the MDH2 reaction was then provided, including a reporter dye that yield a colored product in the presence of NADH at a 1:1 ratio. The production of NADH was monitored through the measurement of absorbance at 450 nm on a BioTek Synergy H1 plate reader (Aligent, Santa Clara, California, USA) using Gen5 3.0 software (Aligent). Graphing and statistical analysis was performed using Excel (Microsoft, Redmond, Washington, USA).
2.4. Variant mapping
The University of California Santa Clara Genome Browser (https://genome.ucsc.edu/) on the human GRCCh38/hg38 genome build was used to map single nucleotide polymorphisms to the MDH2 primary protein structure using the ENST00000315758.10 MDH2 variant transcript. Chimera software (version 1.16, http://www.cgl.ucsf.edu/chimera; University of California San Francisco Computer Graphics Laboratory, San Francisco, CA, USA) was used to map exon-based single nucleotide polymorphisms to the MDH2 (PDB: 2DFD) tertiary structure and generate the resultant fig. [6].
3. Results
3.1. Clinical data
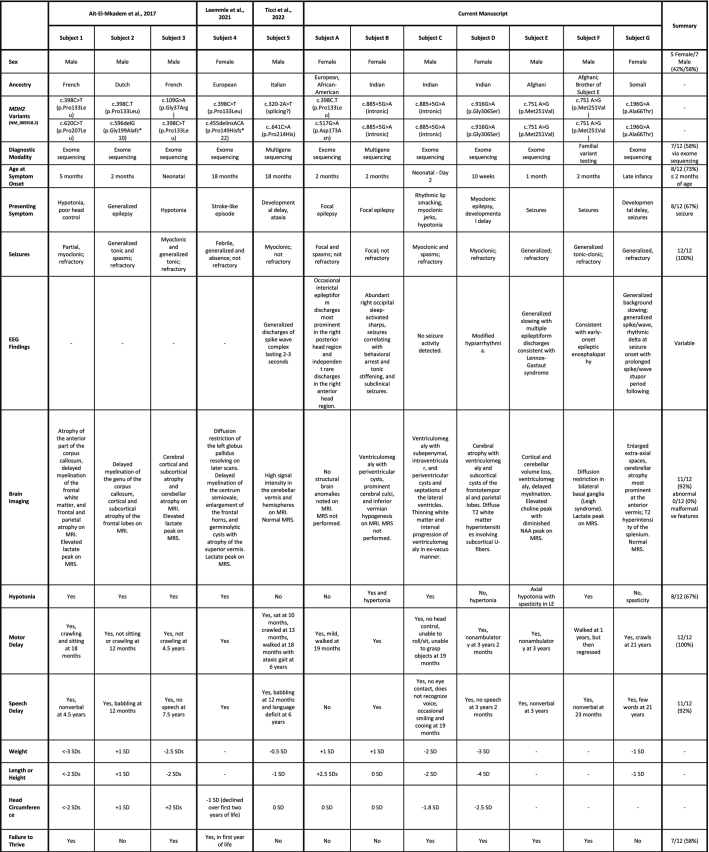
Table 1 summarizes the clinical features of these six patients alongside those of five previously reported patients [[1], [2], [3]]. Narrative descriptions of our six patients' medical histories, clinical presentations, laboratory evaluation, molecular genetic diagnosis, and subsequent management and disease course are available in Appendix A.
Table 1.
Genotype and phenotype data across published accounts of individuals with MDH2 deficiency. CCAM = congenital cystic adenomatoid malformation; GERD = gastroesophageal reflux disorder; MRS = magnetic resonance spectroscopy.
Of the 12 total cases, five (42%) were female. Four patients, all with European ancestry, harbored the c.398C > T variant in trans with an additional MDH2 variant. Six patients, two unrelated children of Indian ancestry, two brothers of Afghani ancestry, and a patient of Somali ancestry harbored homozygous MDH2 variants. All had family histories notable for consanguinity. The two unrelated patients of Indian ancestry, not known to be related, shared the c.885 + 5G > A variant. The average age at symptom onset was five months (median two months). For 8/12 patients (67%) the presenting symptom included seizure. Other presenting symptoms include hypotonia (3/12; 25%) and developmental delay (3/12; 25%). One patient (1/12; 8%) presented with a stroke-like episode. Regardless of presenting symptom, seizures were universally experienced by these patients. Seizure phenotypes were variable and included: absence (1/12; 8%), febrile (1/12; 8%), focal/partial (3/12; 25%), generalized (6/12; 50%), myoclonic (5/12; 42%), and spasms (3/12; 25%). Given differences in seizure phenotypes, EEG abnormalities were variable. Subject A had clinical presentation as well as EEG findings consistent with infantile spasms whereas Subject D had modified hypsarrhythmia. This electroclinical syndrome has not been described in patients with MDH2 deficiency, nor has MDH2 previously been implicated in this phenotype [7]. Subject E and F had a signature consistent with Lennox Gastaut syndrome, which is not surprising in a neurometabolic entity that affects global cerebral energy homeostasis. Eight patients (67%) experienced epilepsy refractory to anti-seizure medications.
Other medical problems for these patients included: growth difficulties/failure to thrive (7/12; 58%), vision/eye problems (7/12; 58%), constipation (3/12; 25%), gastroesophageal reflux (3/12; 25%). One child had dilated cardiomyopathy and resultant heart failure requiring orthotopic transplant. Seven patients (7/11; 64%) had hypotonia noted on physical examination.
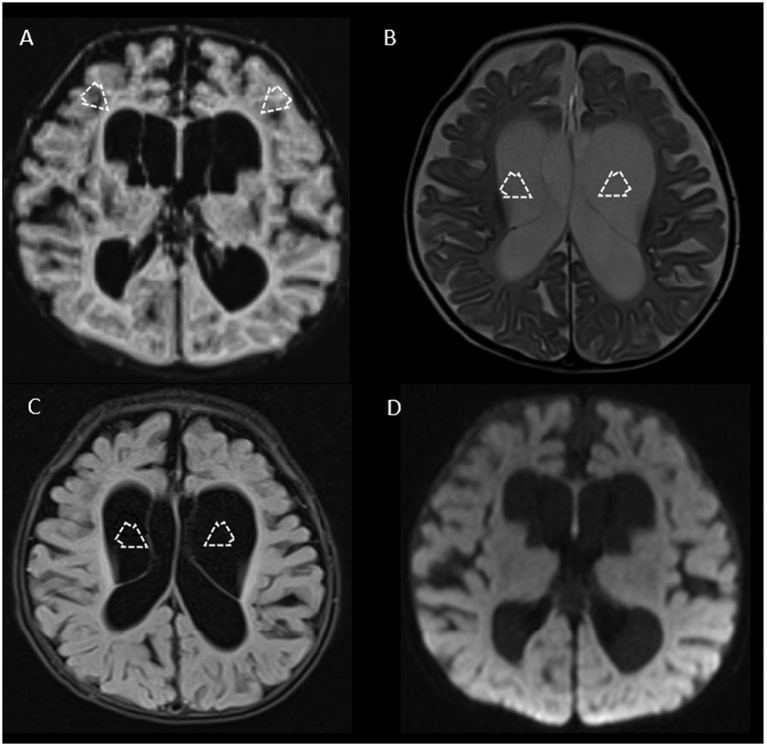
Findings on brain magnetic resonance imaging (MRI) were nearly universally abnormal (11/12 patients; 92%) and commonly presented with similar features, including ventriculomegaly along with subependymal cysts and/or intraventricular septations associated with diffuse volume loss predominantly involving frontoparietal regions (Fig. 2, Fig. 3). Although volume loss was common finding among our patients, no structural brain malformations were noted. Magnetic resonance spectroscopy (MRS) was abnormal in 5/12 patients (42%), most often identifying a lactate peak (4/5; 80%).
Fig. 2.
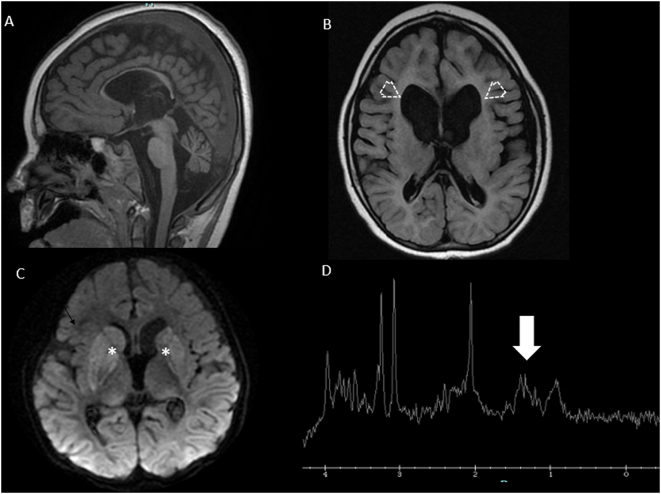
Brain MRI from Subject B at three months of age shows supratentorial ventriculomegaly and large bilateral connatal cysts (arrowheads, panel A) and intraventricular septations (arrowheads, panels B and C), associated with diffuse volume loss particularly involving the white matter of frontoparietal lobes, bilaterally. No restricted diffusion lesions (panel D) or areas of hemorrhage were seen.
Fig. 3.
MRI (panels A-C) and MRS (panel D) from two brothers with biallelic MDH2 variants (Subjects E and F; c.751 A > G; p.Met251Val). In panels A and B, sagittal T1-weighted imaging and axial FLAIR sequences from Patient E at 8 months of age show diffuse volume loss and supratentorial ventriculomegaly with predominant involvement of the frontal horns (open arrows). In panel C, axial diffusion-weighted imaging from Subject F obtained at three years of age shows areas of diffuse and mild restriction in the bilateral basal ganglia (asterisks) confirmed in the apparent diffusion coefficient map (not shown), in keeping with the diagnosis of Leigh syndrome. In panel D, MRS from Subject F obtained at the same time on voxel on left basal ganglia (not shown) demonstrates a small abnormal lactate peak at 1.33 ppm (white arrow).
Developmental delays were universal and often profound. Five patients older than 23 months remained non-verbal. Two patients (Subject 5 and Subject A, Table 1) exhibited milder developmental phenotypes than others. Subject 5 published by Ticci and colleagues was delayed in both motor and language milestones but did go on to acquire those skills. At six years of age, he was able to walk with an ataxic gait and spoke, though with a “language deficit” [3]. Subject A was mildly delayed in acquisition of gross motor skills, with walking occurring at 19 months of age, but has appropriate language milestones at 21 months of age [1]. The average last reported patient age was 5.2 years (median 3 years). The oldest known patient is Subject G from this study, who was 21 years of age at last evaluation.
3.2. Biochemical testing results
Table 2 summarizes available biochemical laboratory testing data for patients with biallelic MDH2 variants. Eleven patients (92%) demonstrated elevated lactate levels, with elevated lactate:pyruvate ratio in all six patients in whom it had been measured. Analysis of urinary excretion of organic acids showed elevations in urinary malic acid in 6/10 patients (60%), fumaric acid in 6/11 patients (55%), and succinic acid in 2/11 patients (18%).
Table 2.
The results of initial plasma and urine biochemical laboratory interrogation of 12 patients found to have MDH2 deficiency. Where no value is provided, testing was not performed. L = lactate, P = pyruvate, Cr = creatinine.
| Ait-El-Mkadem et al., 2017 |
Laemmle et al., 2021 |
Tucci et al., 2022 |
Current manuscript |
Summary (Abnormal) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Subject A | Subject B | Subject C | Subject D | Subject E | Subject F | Subject G | ||
| Plasma Lactate | Elevated (3.0 mmol/L) |
Elevated (5.7 mmol/L) |
Elevated (2.8 mmol/L) |
Elevated (3.0 mmol/L) |
Normal | Elevated (4.26 mmol/L) |
Elevated (8.01 mmol/L) |
Elevated (12.3 mmol/L) |
Elevated (11.1 mmol/L |
Elelvated (3-5 mmol/L) |
Elevated (3–6 mmol/L) |
Elevated (peak 7.5 mmol/L) |
11/12 (92%) |
| Plasma L/P Ratio | Elevated (63) |
Elevated (23) |
Elevated (20) |
– | – | Elevated (28) |
Elevated (40) |
Elevated (25) |
– | – | – | – | 6/6 (100%) |
| Urine Malate | Elevated (56 μmol/mmol Cr) |
Elevated (15–38 μmol/mmol Cr) |
– | Normal | Normal | Elevated (16 μmol/mmol Cr) |
Elevated (28 μmol/mmol Cr) |
Elevated (213 μmol/mmol Cr) |
Elevated (18 μmol/mmol Cr) |
Normal | – | Normal | 6/10 (60%) |
| Urine Fumarate | Elevated (20 μmol/mmol Cr) |
Normal or Elevated (9–55 μmol/mmol Cr) |
Normal | Normal | Elevated (5 μmol/mmol Cr) |
Elevated (27 μmol/mmol Cr) |
Elevated (102 μmol/mmol Cr) |
Elevated (147 μmol/mmol Cr) |
Normal | Normal | – | Normal | 6/11 (55%) |
| Urine Succinate | Normal | Normal | Normal | Normal | Normal | Elevated (57 μmol/mmol Cr) |
Elevated (48 μmol/mmol Cr) |
Normal | Normal | Normal | – | Normal | 2/11 (18%) |
| Other | – | – | – | – | Elevated urinary 3-methylglutaconic acid, plasma C0, C2, C3, and C4 | Elevated urine methylmalonic acid | Elevated urinary 3-methylglutaconic acid, plasma alanine, C2 | Elevated plasma C2, alanine | – | – | – | – | – |
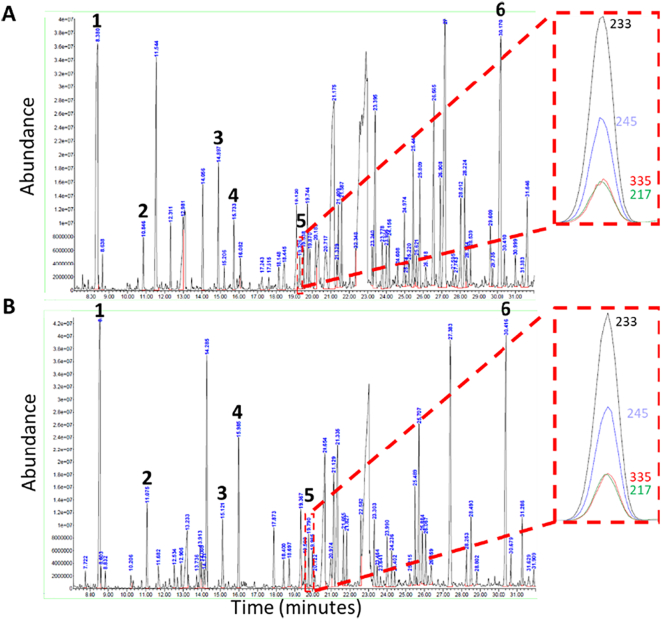
Following the identification of biallelic MDH2 variants in Subject A, spectra from her previous three organic acid analyses were reviewed to help with variant interpretation. Although the interpretation of the total ion chromatograph noted only elevated excretion of lactic acid, post-hoc ion extraction showed the consistent presence of peaks signifying urinary excretion of malic acid, fumaric acid, and succinic acid (Fig. 4A). Fig. 4B shows representative spectra from urinary organic acid analysis for Subject B. Abnormal urinary excretion of lactic acid, malic acid, fumaric acid, and succinic acid was noted.
Fig. 4.
Representative total ion chromatography for patient urine organic acid analysis (gas chromatography/mass spectroscopy) from Subject A (panel A) and Subject B (panel B). Relative abundance is on the vertical axis while time is on the horizontal axis. Selected peaks are indicated for (1) lactic acid, (2) 3-hydroxybutaric acid, (3) succinic acid, (4) fumaric acid, (5) malic acid, and (6) the internal standard (undecanedioic acid). Malic acid eluted at 19.32 min and 19.79 min for Subject A and Subject B, respectively. This is further illustrated in the overlay of specific ions (m/z).
3.3. Malate dehydrogenase enzyme activity
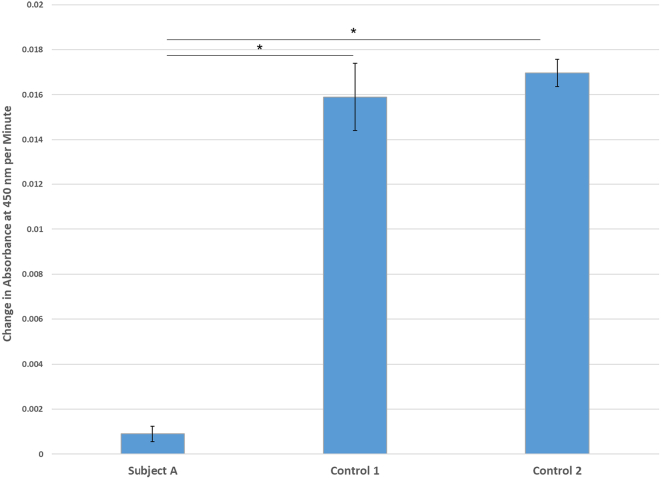
Given the milder neurodevelopmental phenotype of Subject A relative to the other patients in this cohort and others, we sought to better understand the impact of her variants (c.398C > T/p.Pro133Leu and c.517G > A/p.Asp173Asn) on the function of her mitochondrial MDH. The rate of NADH production in fibroblast lysates as measured by the change in absorbance at 450 nm over time was significantly reduced in Subject A relative to two control human fibroblast cell lines (Fig. 5), with the patient having about 5% the NADH production as healthy controls.
Fig. 5.
Mitochondrial malate dehydrogenase enzymatic activity in cultured fibroblasts from Subject A and two control lines. Enzyme activity, quantified as change in absorbance at 450 nm per minute, is substantially decreased in the fibroblasts from Subject A relative to control lines. Samples were performed in triplicate and error bars signify standard deviations. * denotes p < 0.001 by ANOVA and Tukey post hoc testing. There was no statistically significant difference in enzyme activity between the two control samples.
3.4. Relationship between MDH2 variants and primary/secondary protein structure
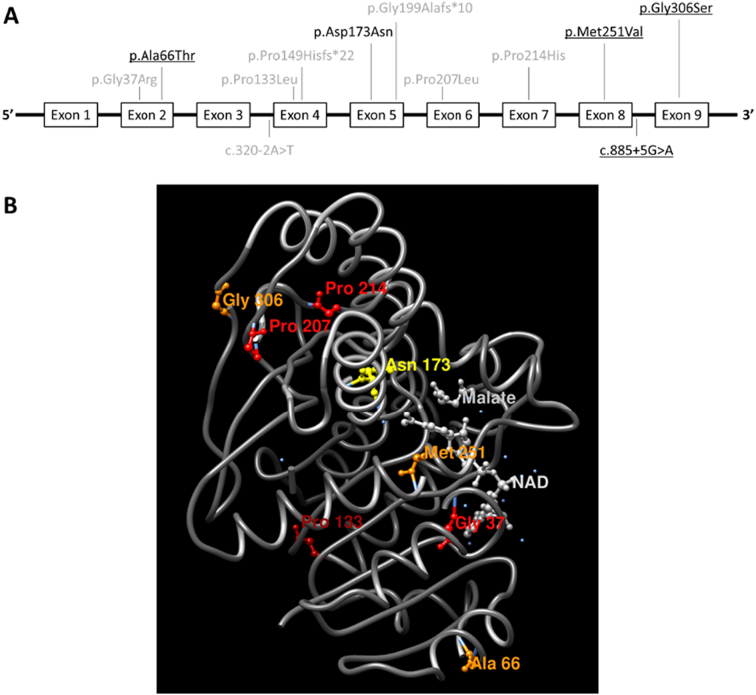
We next wanted to study a potential relationship between the MDH2 variants we observed and MDH2 protein structure. When we mapped the variants to both primary and tertiary protein structure diagrams (Fig. 6), we did not observe a discernable pattern or clustering to suggest an etiology for their pathogenicity or lack thereof.
Fig. 6.
Panel A maps the 12 MDH2 variants harbored by 12 patients with MDH2 deficiency to the primary protein structure where gray text indicates previously reported variants, black text indicates novel variants in this patient cohort, and underlined text indicates variants observed in homozygosity. The variants depicted above the diagram are harbored within exons while the variants depicted below the diagram are harbored within introns. Panel B maps the 10 exonic variants to the tertiary structure of the mitochondrial malate dehydrogenase protein. Binding sites for nicotinamide adenine dinucleotide (NAD) and malate are also shown.
4. Discussion
Inborn errors of the TCA cycle comprise such a small subset of mitochondrial disease that they were once thought to be incompatible with life [8]. Though rare, defects in several TCA cycle enzymes have been described. These include 2-ketoglutarate dehydrogenase complex (KDHC) [9], fumarate hydratase (fumarase) [10,11], and succinate dehydrogenase [12]. Mitochondrial malate dehydrogenase is an emerging inborn error in mitochondrial energy homeostasis manifesting as early onset seizures and neurodevelopmental impairment, brain lesions and volume loss, and variably elevated lactate levels [1]. These data elaborate on the existing phenotypic spectrum and lend ethnic, genetic, and age diversity to the published patient population. The typical patient with MDH2 deficiency demonstrates early-onset seizures and moderate-profound neurodevelopmental delays. A core set of clinical, neuroimaging, and laboratory features are shown in Table 3.
Table 3.
Core clinical, neuroimaging, and laboratory features of MDH2 deficiency resulting from biallelic MDH2 variants using Human Phenotype Ontology (HPO) term name is shown on the left, with its respective term identified on the right. The elbow symbol denotes an HPO term that nests within a more general term within the HPO hierarchy.
| Core clinical features | |
|---|---|
| Neurodevelopmental delay | HP:0012758 |
| └ Motor delay | HP:0001270 |
| └ Delayed speech and language development | HP:0000750 |
| └ Global developmental delay | HP: 0001263 |
| Seizures | HP:0001250 |
| └ Neonatal seizures | HP:0032807 |
| Hypotonia | HP:0001252 |
| └ Neonatal hypotonia | HP:0001319 |
| Failure to thrive | HP:0001508 |
| Abnormality of vision | HP:0000504 |
| └ Visual impairment | HP:0000505 |
| Core neuroimaging features | |
|---|---|
| Cerebral atrophy | HP:0002059 |
| Ventriculomegaly | HP:0002119 |
| Subependymal cysts | HP:0002416 |
| Abnormal brain lactate level by MRS | HP:0025045 |
| └ Elevated brain lactate level by MRS | HP:0012707 |
| Core laboratory features | |
|---|---|
| Lactic acidosis | HP:0003128 |
| Increased serum lactate | HP:0002151 |
| Increased CSF lactate | HP:0002490 |
| Elevated lactate:pyruvate ratio | HP:0032653 |
| Lacticacidura | HP:0003648 |
Neuroimaging abnormalities are a frequent feature of both mitochondrial disorders and TCA cycle defects, including disorders of pyruvate metabolism [[13], [14], [15], [16]]. Mitochondrial malate dehydrogenase deficiency recapitulates both the frequency and variability in MRI findings in related conditions, with abnormalities noted for most patients. From a neuroimaging perspective, the most frequent pattern noted was diffuse atrophy with ventriculomegaly, more evident in the frontal and parietal lobes and with dilatation of the formal horns of the lateral ventricles, along with large subependymal cysts and intraventricular septations (see Fig. 2, Fig. 3). These imaging features overlap with those reported in the context of early disease onset in patients with pyruvate dehydrogenase (PDH) deficiency [15,17]. However, the absence of brain malformations, especially normal morphology of the corpus callosum rather than corpus callosum dysgenesis, which is a hallmark of PDH deficiency, may help distinguish these entities [18]. Subject F is of particular interest, and expands the MDH2 deficiency phenotype to include Leigh syndrome (subacute necrotizing encephalomyelopathy), the constellation of metabolic decompensation, psychomotor regression, and characteristic symmetric necrotic lesions of the basal ganglia [19]. Bilateral lesions in the putamen and basal ganglia nuclei are the sine qua non and most prevalent feature of many mitochondrial syndromes, because these deep nuclei structures are extremely sensitive to deficits in oxidative phosphorylation [20]. The absence of neuroimaging abnormalities in Subject A is also notable and may either portend or be a consequence of her milder overall course. At present, a relationship between MDH2 genotype and neuroimaging phenotype is unclear. One sibling pair share homozygous c.751A > G (p.Met251Val) MDH2 variants while varying in their neuroimaging features. Subject E demonstrated volume loss and ventriculomegaly particularly involving the frontal horns, his brother, Subject F, demonstrated diffusion restriction and edema of bilateral basal ganglia, consistent with Leigh syndrome, as previously discussed (Fig. 3). One non-sibling pair, Subjects B and C, share homozygous c.885 + 5G > A MDH2 variants (which may represent an Indian founder variant), with relatively similar neuroimaging features, including atrophic changes, ventriculomegaly, and anterior subependymal cysts (Table 1). MRS showed abnormalities in 5/7 (71%) of patients, demonstrating abnormal lactate peaks in 4/5 patients (80%) and a disproportional ratio of choline/NAA peaks in 1/5 (20%). One patient also demonstrated an NAA peak (Subject E). Interestingly, Subject G had elevations in plasma lactate but no abnormal lactate peak on MRS.
Variant interpretation in patients with biallelic MDH2 variants remains challenging given the dearth of reported cases and paucity of data on the functional consequences of reported variants. Although previous groups have shown reductions in in vitro MDH2 activity [[1], [2], [3]], such assays are not clinically validated, and not accessible to all patients with identified variants, particularly those who are not cared for at large, academic medical centers. Fibroblasts from one patient in this cohort from whom fibroblasts were available (Subject A) showed a significantly lower NADH production relative to controls, suggesting a reduction in MDH2 quantity or function (Fig. 5). Although the pathogenicity of her maternal c.398C > T/p.Pro133Leu variant has been suggested by previous patient reports and enzyme testing [1,2], this new data suggests pathogenicity of her paternal c.517G > A/p.Asp173Asn variant, at least when observed in heterozygosity. Additionally, mapping variants to the primary and tertiary protein structures did not suggest a clustering of variants that could help with variant interpretation (Fig. 6).
Clinical biochemical testing can provide a valuable, clinically validated, and more widely accessible mechanism for gathering physiological/functional information about the consequences of MDH2 gene changes on lactate and TCA homeostasis. Clinical biochemical testing from patients with MDH2 deficiency reveals a pattern of elevated lactate, high lactate:pyruvate ratio, and urinary excretion of malate and fumarate in most cases. These biochemical findings may fluctuate over time, and in some cases may be absent. However, elevated malate is a relatively specific finding, that can increase clinical suspicion. In children in whom there is suspicion for MDH2 deficiency, careful attention should be paid to malate and fumarate peaks on urine organic acid analysis. For Subject A, it was not until after her molecular diagnosis was available that these species were appreciated, utilizing extracted ion chromatography (Fig. 4). Thus, careful analysis of urinary organic acid excretion and serial collection of these laboratory studies may be helpful for both diagnosis and variant interpretation.
Subject D, with homozygous c.916G > A (p.Gly306Ser) MDH2 variants is particularly notable given the existence of three ostensibly normal adults who are homozygous for this variant in population databases. We speculate that the homozygous subjects identified in gnomAD may demonstrate biochemical changes consistent with MDH2 deficiency (i.e. elevated plasma lactate:pyruvate, elevated urinary excretion of TCA intermediates), and therefore could represent the mildest end of the phenotypic spectrum. Although pathogenicity of this variant has not been established, we argue that Subject D's shared clinical history, biochemical laboratory findings, and neuroimaging findings with other children with MDH2 deficiency warrants her inclusion in this cohort.
Patients in this study were diagnosed via non-targeted gene analysis (exome sequencing or epilepsy panels encompassing hundreds of genes), except for Subject F in whom familial variant analysis was performed. Presently, MDH2 is included on several, but not all, commercially available epilepsy gene panels. We suggest MDH2 deficiency should be a diagnostic consideration for patients with lactatemia in the setting of early-onset seizures, structural brain anomalies, and developmental delays. Careful attention should be paid to organic acid excretion, particularly TCA cycle intermediates. Its biochemical signature has a mix of more specific and non-specific features, partially differentiating it from other conditions with phenotypic overlap. For example, it can be distinguished from disorders of pyruvate metabolism, in which the lactate:pyruvate ratio is not elevated or from fumarase deficiency, which shares urinary excretion of fumarate, but lacks urinary excretion of malate. These distinctions permit a preliminary clinical diagnosis, more targeted molecular testing, and decreased diagnostic delay. Rapid diagnosis of MDH2 is essential, not only for providing families with important answers but also potentially for guiding dietary therapies.
The mechanism by which MDH2 deficiency results in neuropathology is not well understood, although the link between inborn errors of energy homeostasis and pediatric neurodevelopmental disorders is well described [21]. The neurodevelopmental phenotype in MDH2 deficiency is variable and may correlate with structural brain anomalies, seizure burden, or a combination of both. For example, the patient with the best neurodevelopmental phenotype in this cohort (Case A) did not demonstrate any structural brain anomalies and her seizure phenotype was mildest of the reported cases. Recently, two families with gain-of-function MDH2 variants were identified with a hyperglycemia phenotype [22]. In vitro analysis showed reductions in NAD+/NADH ratio and glucose-stimulated insulin secretion [22]. Together with the presumed loss-of-function variants in the present study, these patients suggest a bidirectional role of MDH2 in glucose homeostasis, with diverse phenotypes resulting from different mutational mechanisms.
In addition to the role of MDH2 in the TCA cycle, it also serves as an RNA binding protein [23,24]. Work by Chen and colleagues demonstrated that MDH2 binds the 3′ untranslated region of the SCN1A transcript, downregulating expression of the voltage-gated sodium channel, and potentially contributing to neural excitability in mouse models [24]. That work, however, linked up-regulation of MDH2 to seizure activity. The impact of MDH2 deficiency on neuronal protein expression in humans is unstudied.
Given the multisystemic manifestations apparent in this cohort and previously published cases, we suggest a multidisciplinary approach to care for patients with MDH2 deficiency, including:
-
1.
Biochemical genetic specialists for biochemical characterization, management of potential decompensatory episodes and consideration of triheptanoin therapy; accompanying genetic counseling support
-
2.
Neurologists to guide imaging decision-making and seizure management, including consideration of ketogenic diet
-
3.
Developmental pediatricians and/or rehabilitation physicians as well as supporting disciplines such as physical therapy, occupational therapy, and speech therapy to maximize cognitive and motor development potential
-
4.
Cardiologists to perform baseline echocardiography and monitor for the development of cardiomyopathy, particularly in the setting of metabolic decompensation
-
5.
Ophthalmologists for monitoring for and management of potential vision impairment
Currently, treatment for MDH2 deficiency remains supportive. Although Laemmle and colleagues reported improvements in their patient's motor skills, weight gain, and metabolic decompensations using triheptanoin [2], those results are not yet generalizable and do not address whether triheptanoin may improve other disease features such as seizure burden. The hypothesized mode of action for triheptanoin was generation of acetyl CoA and propionyl CoA to maintain TCA cycle substrates, however this mechanism is not completely understood.
TCA cycle dysfunction is also a well-studied phenomenon in tumorigenesis. Variants in isocitrate dehydrogenase, succinate dehydrogenase, and fumarate hydratase are linked to paraganglioma and pheochromocytoma susceptibility. More recently, loss-of-function germline heterozygous variants in MDH2 have been identified in patients with those tumors, supporting a role for the enzyme in susceptibility to paraganglioma/pheochromocytoma [25,26]. None of the identified MDH2 variants (missense variants p.Arg104Gly, P.Val160Met, and p.Ala256Thr; in-frame deletion variant p.Lys314del, and splice-site variant c.429 + 1G > T) were identified in the present cohort of children with MDH2 deficiency [25,26]. Additionally, no tumors have been reported in the carrier parents of children with MDH2 deficiency or the children themselves [[1], [2], [3]]. The patient reported by Ticci and colleagues (Subject 5 in this manuscript) was noted to have three second-degree relatives with colon cancer and one with breast cancer [3]. One subject in the present cohort had a second-degree relative with colon cancer as well. All relatives received their cancer diagnoses at ages >50 years. Importantly, because variant segregation was not demonstrated in those relatives, any relationship between MDH2 carrier status and increased cancer risk is speculative. At least one group recommends ongoing surveillance and screening for cancer in carrier family members [1]. Until additional data is available, we agree that a careful cancer family history should be obtained for all patients identified with biallelic germline MDH2 variants. Cascade testing for an affected patient's extended family can then guide consideration of paraganglioma/pheochromocytoma surveillance and screening. Referral to a cancer geneticist may be helpful in facilitating discussions about the advantages and disadvantages to screening in this situation.
Mitochondrial malate dehydrogenase deficiency remains an incompletely characterized and understudied entity. The addition of MDH2 to commercially available multigene epilepsy panels may provide a mechanism by which previously unrecognized cases, perhaps those isolated to later-onset or milder epilepsy, are identified. Natural history studies of patients with MDH2 deficiency are important in delineating the full phenotypic spectrum of the condition, any potential genotype-phenotype correlation, and detecting a predisposition to cancer. Larger-scale treatment trials with agents such as triheptanoin are important in providing patients and their families with data-driven options for improved disease outcomes.
Funding
RDG was supported by 5K08DK113250-02 from the NIDDK. This funding source played no part in the design or execution of this work.
Declaration of Competing Interest
None to report.
Acknowledgements
The authors gratefully acknowledge the patients and their families for making this work meaningful and possible. We are also grateful for support from the Herr Family Fund, which defrayed open-assess publication costs for this article.
Contributor Information
Jessica R.C. Priestley, Email: priestleyj@chop.edu.
Lisa M. Pace, Email: lisa.pace@jax.ufl.edu.
Kuntal Sen, Email: ksen2@childrensnational.org.
Anjali Aggarwal, Email: aggar135@umn.edu.
Cesar Augusto P.F. Alves, Email: alvesc@chop.edu.
Ian M. Campbell, Email: campbellim@chop.edu.
Sanmati R. Cuddapah, Email: cuddapahs@chop.edu.
Nicole M. Engelhardt, Email: engelhardn@chop.edu.
Marina Eskandar, Email: meskandar@childrensnational.org.
Andrea Gropman, Email: agropman@childrensnational.org.
Ingo Helbig, Email: hilbigi@chop.edu.
Xinying Hong, Email: hongx@chop.edu.
Laina Lusk, Email: luskl@chop.edu.
Pamela Trapane, Email: pamela.trapane@jax.ufl.edu.
Pim Suwannarat, Email: pim.suwannarat@kp.org.
Rebecca D. Ganetzky, Email: ganetzkyr@chop.edu.
Appendix A. Clinical descriptions of cases
A.1. Subject A
HISTORY & PRESENTATION: Subject A was born via spontaneous vaginal delivery at 39 weeks gestation following an uncomplicated pregnancy to parents of mixed-European and African American ancestry. There was no known family history of tumors. Birth parameters were unremarkable. She was diagnosed with gastroesophageal reflux at two months of age. Shortly thereafter, she developed focal seizures. Initial laboratory studies obtained in the setting of these new-onset seizures showed elevated aminotransferases (aspartate aminotransferase 189 U/L, reference range (RR) 20–64 U/L; alanine aminotransferase 145 U/L, RR 5–45 U/L) and unconjugated hyperbilirubinemia (4.0 mg/dL, RR 0.2–1.0 mg/dL). Screening laboratory studies for a biochemical etiology for her presentation were obtained and showed elevated plasma lactate (4.26 mM, RR 0.8–2.0 mM) with an elevated lactate:pyruvate ratio of 28 (RR 10–20) and urinary excretion of lactic acid (285 mg/g Cr) and methylmalonic acid (26 mg/g Cr) on semi-quantitative urine organic acid analysis (no RR established). Electroencephalography (EEG) showed right-sided epileptiform discharges and a seizure from the right posterior quadrant. There were no structural or signal changes on brain magnetic resonance imaging (MRI). The child was referred to a biochemical genetic specialist and evaluated at three months of age. Repeat urine organic acid analysis showed abnormal excretion of lactic acid (55 mg/g Cr), 3-hydroxybutyratic acid (32 mg/g Cr), methylmalonic acid (35 mg/g Cr), ethylmalonic acid (43 mg/g Cr), and 3-methylglutaconic acid (30 mg/g Cr), consistent with mitochondrial dysfunction.
Her focal seizures were successfully managed with levetiracetam monotherapy until 13 months of age, when her parents noted new head bobbing. A repeat EEG was obtained and showed epochs of disorganized high voltage slow background with embedded multifocal sharps that are at times hypersynchronous consistent with hypsarrhythmia (hypersynchronous hypsarrhythmia variant); near continuous biposterior slowing with embedded sharps; and infantile spasms consisting of head drops occurring in clusters associated with a diffuse EEG pattern. She was treated with a course of corticosteroids.
DIAGNOSIS: Initial genetic testing via the Invitae epilepsy panel was non-diagnostic. MDH2 was not included on the panel at that time. Trio exome sequencing was performed through GeneDx and showed biallelic MDH2 variants: maternally-inherited c.398C > T (p.Pro133Leu) and paternally-inherited c.517G > A (p.Asp173Asn). The former is predicted to be deleterious in silico and was also observed in trans in the initial MDH2 deficiency cohort [1]. It observed at a frequency of 0.0052% (13 in 250,528 alleles) in population databases (gnomAD; http://gnomad.broadinstitute.org) with no homozygous individuals reported. The latter is also predicted to be deleterious in silico and has not previously been reported in patients with MDH2 deficiency. It is observed at a frequency of 0.0028% (8 in 282,824 alleles) in population databases, with no homozygous individuals reported.
Because Subject A had milder seizure and neurodevelopmental phenotypes than other children with biallelic MDH2 variants, we sought to better characterize this patient's biochemistry. Spectra from her previous three urine organic acid analyses were reviewed. Peaks signifying urinary excretion of malic acid, fumaric acid, and succinic acid were appreciated retrospectively (Fig. 4A). Mitochondrial malate dehydrogenase activity in cultured skin fibroblast lysates was markedly lower than enzymatic activity in two different control fibroblast lines (Fig. 5).
MANAGEMENT & EVOLUTION: Subject A was last evaluated at 21 months of age. Her infantile spasms had not recurred since treatment and her focal seizures remained well-controlled on levetiracetam monotherapy. Her development was notable for mild gross motor delay; she walked at 19 months of age. Her language development was appropriate at 21 months of age with a 15–20 word vocabulary and normal receptive language skills. Trihepatoin (Doljovi, Ultragenyx Pharmaceuticals, Novato, CA, USA) therapy was initiated with the goal of maintaining her encouraging developmental trajectory and remains ongoing.
A.2. Subject B
HISTORY & PRESENTATION: Subject B born via spontaneous vaginal delivery at 39 weeks gestation following an uncomplicated pregnancy course to parents who are first cousins of Indian ancestry. The family history was notable for a maternal grandfather who had died of colon cancer at 55 years of age. Birth parameters were unremarkable. She required phototherapy for neonatal hyperbilirubinemia.
Around two months of age, her parents noted fist clenching with upper extremity extension and were concerned for seizures, prompting hospital evaluation. Laboratory studies revealed elevated plasma lactate (peak 19.8 mM, RR 0.8–2.0 mM) with an anion gap metabolic acidosis (bicarbonate 14 mmol/L, RR 21–28 mmol/L; anion gap 20, RR 8–12). Additional metabolic laboratory investigation showed an elevated plasma alanine level (597.3 μmol/L, RR 89–440 μmol/L). There was abnormal urinary excretion of lactic acid, malic acid, fumaric acid, and succinic acid (Fig. 4B). EEG showed broad-based polyphasic epileptiform discharges of high amplitude that spread to adjacent regions present during slow wave sleep, concerning for reduced seizure threshold. Brain MRI showed diffuse volume loss associated with enlarged lateral ventricles with large subependymal cysts and intraventricular septations in the frontal horns (Fig. 2). No malformative features of the brain were noted.
DIAGNOSIS: Genetic testing via the Invitae epilepsy panel performed following inclusion of MDH2 in October 2020 revealed homozygous c.885 + 5G > A variants. This variant falls in intron 8 and is predicted to affect the consensus splice site of that intron in silico. It is observed at a frequency of 0.0044% (5 in 282,686 alleles) in population databases (gnomAD) with no homozygous individuals reported.
MANAGEMENT & EVOLUTION: Her seizures were successfully treated with phenobarbital monotherapy until she experienced increased seizure frequency in the setting of a SARS-CoV-2 viral infection. At that time, she was transitioned to oxcarbazepine monotherapy with reduction in her seizure burden but continued breakthrough seizures. At 13 months of age, her family reported struggles with feeding, irritability, and constipation. Developmentally, she is delayed in both motor and speech milestones, with no ambulation and no speech or social smile.
A.3. Subject C
HISTORY & PRESENTATION: Subject C was born via spontaneous vaginal delivery at 40 weeks gestation to consanguineous parents who are second cousins of Indian ancestry. The pregnancy was complicated by maternal anemia and hypothyroidism. Rhythmic lip smacking and myoclonic jerks were observed on his second day of life, concerning for seizure activity. Laboratory evaluation showed lactic acidosis (lactate 12.3 mM, RR <2.0 mM; bicarbonate 9.0 mEq/L, RR 22–26 mEq/L; anion gap 24 mEq/L, RR <12 mEq/L). Additional biochemical laboratory studies showed elevated acetylcarnitine (C2: 21.4 nmol/L, RR 2.6–15.5 nmol/L), elevated carnitine esters (24 μMol/L, RR 4–12 μMol/L), and elevated plasma alanine (841 μMol/L, RR 119–523 μMol/L). There was increased urinary excretion of lactic acid, malic acid, fumaric acid, and pyruvic acid. Brain MRI showed volume loss and lateral ventriculomegaly with large, thin-walled subependymal cysts within the frontal horns. No malformative features of the brain were noted. An EEG was obtained but did not show seizure activity.
DIAGNOSIS: Genetic testing was performed via exome sequencing through Prevention Genetics and showed homozygous c.885 + 5G > A (intronic) MDH2 variants, see discussion above. As far as we are aware, Subject B and Subject C are unrelated.
MANAGEMENT & EVOLUTION: At 19 months of age, Subject C struggles with generalized hypotonia, poor oral feeding skills, visual impairment with cortical blindness, growth impairment, and severe global developmental delays, including limited social engagement and few/no motor or speech advances since around six months of age. He was treated with lorazepam, clonidine, and methadone with moderate improvement in irritability, posturing and sleep.
A.4. Subject D
HISTORY & PRESENTATION: Subject D was born via spontaneous vaginal delivery at 39 weeks gestation following an uncomplicated pregnancy to parents who are first cousins of Indian ancestry. At 10 weeks of age, she was admitted to the hospital for myoclonic seizures. History taken at that time was concerning for developmental delays in all domains: she demonstrated no neck control, no social smile, and was not cooing/babbling. Laboratory studies showed elevated plasma lactate (11.1 mM, RR 0.4–2.0 mM) and anion gap metabolic acidosis (bicarbonate 17.5 mM, RR 22–26 mM; PCO2 34.4 mmHg, RR 35–45 mmHg). There was urinary excretion of malic acid and fumaric acid. An EEG showed modified hypsarrhythmia. Brain MRI showed volume loss predominantly in the frontoparietal regions with enlarged ventricles, predominantly in the frontal horns, and subcortical cysts in the bilateral frontotemporal and parietal lobes. Diffuse T2 white matter hyperintensities involved the subcortical U-fibers were seen. No malformative features of the brain were noted.
DIAGNOSIS: Genetic testing was performed via exome sequencing through MedGenome Labs. This showed homozygous c.916G > A (p.Gly306Ser) MDH2 variants. This variant is predicted to be deleterious in silico. It is observed at a frequency of 0.32% (141 in 281,492 alleles) in population databases (gnomAD) with three homozygous adults reported. There are conflicting interpretations of pathogenicity in ClinVar. Taken together, the pathogenicity of this variant remains unestablished.
MANAGEMENT & EVOLUTION: At three years of age, Subject D's pharmacologic management includes levetiracetam, clonazepam, valproate, and a mitochondrial cocktail. Her seizures have reduced in frequency, but she has severe developmental delays and is neither verbal nor ambulatory.
A.5. Subject E
HISTORY & PRESENTATION: Subject E is a three-year-old male. He was born via spontaneous vaginal delivery at full term following an uncomplicated pregnancy to consanguineous parents of Afghani ancestry. There was no family history of tumors. During his first month of life, he developed seizures and was diagnosed with epilepsy by a neurologist in Afghanistan; for which he was started on carbamazepine. Initial biochemical screen with ammonia, lactate, plasma amino acids, and urine organic acid analysis were reportedly unremarkable, but not available for review. Brain MRI showed diffuse volume loss and supratentorial ventriculomegaly with predominant involvement of the frontal horns (Fig. 3A and B). No malformative features of the brain were noted. Magnetic resonance spectroscopy (MRS) showed a prominent choline peak with diminished N-acetylaspartate (NAA) peak.
DIAGNOSIS: Genetic testing via the Invitae epilepsy panel that did not include the MDH2 gene was not diagnostic. Subsequently, trio exome sequencing through Ambry Genetics was performed and showed a novel homozygous c.751 A > G (p.Met251Val) MDH2 variant. This variant occurs at a highly conserved amnio acid residue is predicted to be deleterious in silico. It is observed at a frequency of 0.0004% (1 in 251,114 alleles) in population databases.
MANAGEMENT & EVOLUTION: Following diagnosis, Subject E was initiated on co-enzyme Q, citrulline, and biotin therapy for mitochondrial support. Presently, he is three years old and has multiple seizure semiologies including full body stiffening with drooling, atonic and generalized tonic-clonic seizures. His EEG demonstrated generalized slowing with multiple epileptiform discharges, which is consistent with Lennox Gastaut syndrome. His current anti-seizure medications are levetiracetam and clobazam. He has global developmental delay with inability to sit, stand, walk. He is nonverbal. He has spasticity and dystonia in bilateral lower extremities for which he is continued on trihexyphenidyl. He is gastrostomy tube-dependent and has cortical visual impairment.
A.6. Subject F
HISTORY & PRESENTATION: Subject F was born full term and had an uneventful perinatal course. He was the full brother of Subject E. At two months of age, he developed seizures described as episodes of unresponsiveness with upward rolling of eyes. His EEG was consistent with early-onset epileptic encephalopathy. He was developing appropriately until he experienced an acute decompensatory event characterized by lactic acidosis at one year of age. Brain MRI obtained during that episode showed diffusion restriction in bilateral basal ganglia keeping with the syndromic diagnosis of Leigh (Fig. 3C). MRS revealed a small, abnormal lactate peak (Fig. 3D). No malformative features of the brain were noted. Following that decompensation, he was left unable to stand or walk.
DIAGNOSIS: Subject E was diagnosed via targeted testing of the familial MDH2 variant (c.751 A > G; p.Met251Val) identified in his brother, see description above.
MANAGEMENT & EVOLUTION: Following diagnosis, Subject F was also treated with Co-enzyme Q10, citrulline and biotin. Presently, Subject F is 23 months old. He continues to have multiple daily seizures despite being on levetiracetam, clobazam and zonisamide. He has also experienced a few additional decompensatory episodes of mild lactic acidemia.
A.7. Subject G
HISTORY & PRESENTATION: Subject G was born full term after an uneventful perinatal period to consanguineous parents of Somali ancestry. There is no known family history of tumors, although her father died of unclear causes at a young age. Developmental delays and seizures were noted during her first two years of life. Over her pediatric years, she developed spasticity and dystonia.
Diagnosis for Subject G occurred when she was 21 years of age, during a hospitalization for status epilepticus. Biochemical laboratory studies showed elevated plasma lactate (peak 7.5 mmol/L) during hospitalizations for seizures, but normalization during periods of wellness. Plasma and CSF amino acid analysis and plasma acylcarnitine profile were unremarkable. Urine organic acid analysis did not demonstrate increased excretion of malate, fumarate, or succinate. EEG was performed and abnormal due to the presence of moderate generalized slowing of the background activities, generalized spike and wave and rhythmic delta at the onset of the seizure, and prolonged spike and wave stupor period after the generalized seizure. MRI brain at age 21 years revealed volume loss predominantly in the frontoparietal regions. Cerebellar atrophy, most prominently of the anterior vermis but also of the anterior and far lateral hemispheres, was also observed. T2 white matter hyperintensities were most prominent in the splenium. There was no abnormal lactate peak seen on MRS.
DIAGNOSIS: Chromosomal microarray showed multiple regions of homozygosity, consistent with the history of parental consanguinity. Duo exome sequencing utilizing samples from the patient and her mother and performed by the University of Minnesota Molecular Diagnostic Laboratory showed a novel homozygous c.196G > A (p.Ala66Thr) MDH2 variant. In silico prediction models provide conflicting evidence for this variant; however, the accuracy of such models is limited. It is observed at a frequency of (36 in 282,480 alleles). No homozygous individuals were reported. To further understand the implications of this variant of uncertain significance, targeted transcriptome analysis is being performed and is currently in process. Additionally, targeted familial MDH2 variant testing for unaffected siblings is also pending. We note that four other variants of uncertain significance in different genes were also identified via exome sequencing but were not assessed to be clinically significant due to inheritance pattern and/or associated clinical features.
MANAGEMENT & EVOLUTION: Presently, Subject G is 21 years old. She is managed on clobazam, lacosamide, levetiracetam and topiramate. Her epilepsy is poorly controlled, with frequent hospitalizations for breakthrough seizures. From a neurodevelopmental perspective, Subject G demonstrates severe cognitive delays/impairment. She crawls but does not walk. Her expressive language is limited to a few words, but her receptive language is felt to be better, with recognition of her family members, the ability to indicate her needs, and the ability to share interests. She requires assistance with all activities of daily living.
Data availability
Data will be made available on request.
References
- 1.Ait-El-Mkadem S., Dayem-Quere M., Gusic M., Chaussenot A., Bannwarth S., François B., Genin E.C., Fragaki K., Volker-Touw C.L.M., Vasnier C., Serre V., van Gassen K.L.I., Lespinasse F., Richter S., Eisenhofer G., Rouzier C., Mochel F., De Saint-Martin A., Abi Warde M.-T., de Sain-van M.G.M., der Velde J.J.M., Jans J., Amiel Z., Avsec C., Mertes T.B., Haack T., Strom T., Meitinger P.E., Bonnen R.W., Taylor J., van Gagneur P.M., Hasselt A., Rötig A., Delahodde H., Prokisch S.A., Fuchs V. Paquis-Flucklinger. Mutations in MDH2, encoding a Krebs cycle enzyme, cause early-onset severe encephalopathy. Am. J. Hum. Genet. 2017;100:151–159. doi: 10.1016/j.ajhg.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laemmle A., Steck A.L., Schaller A., Kurth S., Perret Hoigné E., Felser A.D., Slavova N., Salvisberg C., Atencio M., Mochel F., Nuoffer J.-M., Gautschi M. Triheptanoin - novel therapeutic approach for the ultra-rare disease mitochondrial malate dehydrogenase deficiency. Mol. Genet. Metab. Rep. 2021;29 doi: 10.1016/j.ymgmr.2021.100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ticci C., Nesti C., Rubegni A., Doccini S., Baldacci J., Dal Canto F., Ragni L., Cordelli D.M., Donati M.A., Santorelli F.M. Bi-allelic variants in MDH2: expanding the clinical phenotype. Clin. Genet. 2022;101:260–264. doi: 10.1111/cge.14088. [DOI] [PubMed] [Google Scholar]
- 4.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett M.J., Ragni M.C., Hood I., Hale D.E. Comparison of post-mortem urinary and vitreous humour organic acids. Ann. Clin. Biochem. 1992;29(Pt 5):541–545. doi: 10.1177/000456329202900509. [DOI] [PubMed] [Google Scholar]
- 6.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 7.Lee S., Baek M.S., Lee Y.M. Lennox-Gastaut syndrome in mitochondrial disease. Yonsei Med. J. 2019;60:106–114. doi: 10.3349/ymj.2019.60.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rustin P., Bourgeron T., Parfait B., Chretien D., Munnich A., Rötig A. Inborn errors of the Krebs cycle: a group of unusual mitochondrial diseases in human. Biochim. Biophys. Acta. 1997;1361:185–197. doi: 10.1016/s0925-4439(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 9.Kohlschütter A., Behbehani A., Langenbeck U., Albani M., Heidemann P., Hoffmann G., Kleineke J., Lehnert W., Wendel U. A familial progressive neurodegenerative disease with 2-oxoglutaric aciduria. Eur. J. Pediatr. 1982;138:32–37. doi: 10.1007/BF00442325. [DOI] [PubMed] [Google Scholar]
- 10.Whelan D.T., Hill R.E., McClorry S. Fumaric aciduria: a new organic aciduria, associated with mental retardation and speech impairment. Clin. Chim. Acta. 1983;132:301–308. doi: 10.1016/0009-8981(83)90008-6. [DOI] [PubMed] [Google Scholar]
- 11.Zinn A.B., Kerr D.S., Hoppel C.L. Fumarase deficiency: a new cause of mitochondrial encephalomyopathy. N. Engl. J. Med. 1986;315:469–475. doi: 10.1056/NEJM198608213150801. [DOI] [PubMed] [Google Scholar]
- 12.Bourgeron T., Rustin P., Chretien D., Birch-Machin M., Bourgeois M., Viegas-Péquignot E., Munnich A., Rötig A. Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat. Genet. 1995;11:144–149. doi: 10.1038/ng1095-144. [DOI] [PubMed] [Google Scholar]
- 13.Baertling F., Klee D., Haack T.B., Prokisch H., Meitinger T., Mayatepek E., Schaper J., Distelmaier F. The many faces of paediatric mitochondrial disease on neuroimaging. Childs Nerv. Syst. 2016;32:2077–2083. doi: 10.1007/s00381-016-3190-3. [DOI] [PubMed] [Google Scholar]
- 14.Mascalchi M., Montomoli M., Guerrini R. Neuroimaging in mitochondrial disorders. Essays Biochem. 2018;62:409–421. doi: 10.1042/EBC20170109. [DOI] [PubMed] [Google Scholar]
- 15.Patel K.P., O’Brien T.W., Subramony S.H., Shuster J., Stacpoole P.W. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol. Genet. Metab. 2012;106:385–394. doi: 10.1016/j.ymgme.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roosendaal S.D., van de Brug T., Alves C.A.P.F., Blaser S., Vanderver A., Wolf N.I., van der Knaap M.S. Imaging patterns characterizing mitochondrial leukodystrophies. AJNR Am. J. Neuroradiol. 2021;42:1334–1340. doi: 10.3174/ajnr.A7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savvidou A., Ivarsson L., Naess K., Eklund E.A., Lundgren J., Dahlin M., Frithiof D., Sofou K., Darin N. Novel imaging findings in pyruvate dehydrogenase complex (PDHc) deficiency—results from a nationwide population-based study. J. Inherit. Metab. Dis. 2022;45:248–263. doi: 10.1002/jimd.12463. [DOI] [PubMed] [Google Scholar]
- 18.Mew N.A., Loewenstein J.B., Kadom N., Lichter-Konecki U., Gropman A.L., Martin J.M., Vanderver A. MRI features of 4 female patients with pyruvate dehydrogenase E1 alpha deficiency. Pediatr. Neurol. 2011;45:57–59. doi: 10.1016/j.pediatrneurol.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alves C.A.P.F., Teixeira S.R., Martin-Saavedra J.S., Guimarães Gonçalves F., Lo Russo F., Muraresku C., McCormick E.M., Falk M.J., Zolkipli-Cunningham Z., Ganetzky R., Vossough A., Goldstein A., Zuccoli G. Pediatric Leigh syndrome: neuroimaging features and genetic correlations. Ann. Neurol. 2020;88:218–232. doi: 10.1002/ana.25789. [DOI] [PubMed] [Google Scholar]
- 20.Gropman A.L. Neuroimaging in mitochondrial disorders. Neurotherapeutics. 2013;10:273–285. doi: 10.1007/s13311-012-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyarzábal A., Musokhranova U., Barros L., García-Cazorla A. Energy metabolism in childhood neurodevelopmental disorders. EBioMedicine. 2021;69 doi: 10.1016/j.ebiom.2021.103474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jungtrakoon Thamtarana P., Marucci A., Pannone L., Bonnefond A., Pezzilli S., Biagini T., Buranasupkajorn P., Hastings T., Mendonca C., Marselli L., Di Paola R., Abubakar Z., Mercuri L., Alberico F., Flex E., Ceròn J., Porta-de-la-Riva M., Ludovico O., Carella M., Martinelli S., Marchetti P., Mazza T., Froguel P., Trischitta V., Doria A., Prudente S. Gain of function of malate dehydrogenase 2 (MDH2) and familial hyperglycemia. J. Clin. Endocrinol. Metab. 2021 doi: 10.1210/clinem/dgab790. dgab790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castello A., Fischer B., Eichelbaum K., Horos R., Beckmann B.M., Strein C., Davey N.E., Humphreys D.T., Preiss T., Steinmetz L.M., Krijgsveld J., Hentze M.W. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y.-H., Liu S.-J., Gao M.-M., Zeng T., Lin G.-W., Tan N.-N., Tang H.-L., Lu P., Su T., Sun W.-W., Xie L.-C., Yi Y.-H., Long Y.-S. MDH2 is an RNA binding protein involved in downregulation of sodium channel Scn1a expression under seizure condition. Biochim. Biophys. Acta Mol. basis Dis. 1863;2017:1492–1499. doi: 10.1016/j.bbadis.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Calsina B., Currás-Freixes M., Buffet A., Pons T., Contreras L., Letón R., Méndez I.C., Remacha L., Calatayud M., Obispo B., Martin A., Cohen R., Richter S., Balmaña J., Korpershoek E., Rapizzi E., Deutschbein T., Vroonen L., Favier J., de Krijger R.R., Fassnacht M., Beuschlein F., Timmers H.J., Eisenhofer G., Mannelli M., Pacak K., Satrústegui J., Rodríguez-Antona C., Amar L., Cascón A., Dölker N., Gimenez-Roqueplo A.-P., Robledo M. Role of MDH2 pathogenic variant in pheochromocytoma and paraganglioma patients. Genet. Med. 2018;20:1652–1662. doi: 10.1038/s41436-018-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cascón A., Comino-Méndez I., Currás-Freixes M., de Cubas A.A., Contreras L., Richter S., Peitzsch M., Mancikova V., Inglada-Pérez L., Pérez-Barrios A., Calatayud M., Azriel S., Villar-Vicente R., Aller J., Setién F., Moran S., Garcia J.F., Río-Machín A., Letón R., Gómez-Graña Á., Apellániz-Ruiz M., Roncador G., Esteller M., Rodríguez-Antona C., Satrústegui J., Eisenhofer G., Urioste M., Robledo M. Whole-exome sequencing identifies MDH2 as a new familial paraganglioma gene. JNCI: J. Natl. Cancer Inst. 2015;107 doi: 10.1093/jnci/djv053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.