Abstract
Background and Purpose
Emerging studies have shown that gut-derived endotoxins might play a role in intestinal and systemic inflammation. Although the significance of intestinal permeability in modulating the pathogenesis of Schizophrenia (SCZ) is recognized, not much data on the specific role of intestinal permeability biomarkers, viz., zonulin, lipopolysaccharide-binding protein (LBP), and intestinal alkaline phosphatase (IAP) in SCZ is available. Therefore, we measured the plasma levels of zonulin, LBP, and IAP and its correlation with neutrophil-to-lymphocyte ratio (NLR); a marker of systemic inflammation in patients with SCZ.
Methods
We recruited 60 individuals, patients with SCZ (n = 40) and healthy controls (n = 20), from a large tertiary neuropsychiatry center. Plasma levels of zonulin, IAP, and LBP were quantified by enzyme-linked immunosorbent assay.
Results
Plasma levels of both LBP and zonulin were significantly increased (P <0.05), whereas the IAP levels (P <0.05) were significantly decreased in patients with SCZ compared to healthy controls. Pearson correlation analysis revealed that zonulin and LBP had a significant positive correlation with NLR, and IAP negatively correlated with NLR. Individuals with SCZ had higher independent odds of zonulin [odds ratio (OR): 10.32, 95% CI: 1.85–57.12], LBP [OR: 1.039, 95% CI: 1.02–1.07], and IAP [OR: 0.643, 95% CI: 0.471–0.879], even after adjusting for potential confounders.
Conclusion
Our study demonstrates an association of zonulin, LBP, and IAP in Asian Indian SCZ patients and correlates with NLR. Our results indicate that low-grade inflammation induced by metabolic endotoxemia might be implicated in the pathoetiology of SCZ.
Keywords: Zonulin, Lipopolysaccharide-binding protein, Intestinal alkaline phosphatase, Intestinal permeability, Inflammation, Schizophrenia
Introduction
Schizophrenia (SCZ) is a complex neuropsychiatric condition with heterogeneous manifestations, which causes substantial morbidity and premature mortality. 1 Although the median incidence is low (15.2 per 100,000), it is considered one of the significant contributors to the global burden of disease. 2 Despite a serious social and economic burden, the underlying mechanism of SCZ pathogenesis is not fully understood. Several environmental risk factors, immune mediators, and genetic determinants are associated with the risk and pathogenesis of SCZ.3–5 Previous studies have reported on analyzing the human genome to identify the pathogenetic risk factors of SCZ. 3 However, the reported associations likely account for only about 4% of the variance in SCZ. 4 The currently available treatments do not completely address all aspects of the disorder. They are associated with considerable side effects, demanding to identify additional etiological factors that are expected to provide new-biology insights into the pathogenesis linked to SCZ and can aid in developing improved treatment strategies.
Gut and metabolic dysbiosis are implicated in the etiology of many nervous system diseases including SCZ mediated through the microbiome-gut-brain axis.6, 7 Both preclinical and clinical studies show potential associations among an altered gut microbiome, increased intestinal permeability, and mucosal damage in SCZ,7, 8 although the precise mechanistic basis still needs to be understood. Changes in the gut microbiota may compromise the integrity of the intestinal tract and subsequently cause a higher translocation of bacterial antigens such as endotoxin lipopolysaccharides (LPS) into the peripheral circulation, referred to as endotoxemia. 9 Endotoxemia has been demonstrated to activate immunoinflammatory signalling and may potentially lead to chronic diseases like SCZ. 10 Genome-wide association study by Ripke et al. substantiated the role of immune dysfunction in SCZ. 5 Some of our earlier studies also suggested a causative role of inflammation in SCZ.11, 12 This emphasizes the need for further studies to investigate the role of intestinal permeability biomarkers and their link with inflammation, as assessed by neutrophil-to-lymphocyte ratio (NLR), an indicator of systemic inflammation, 13 in patients with SCZ.
One of the important noninvasive biomarkers of intestinal permeability is zonulin, 14 a 47-kDa protein, secreted by the intestinal epithelial cells increases intestinal barrier permeability and possibly contributes to intestinal innate immunity. 15 Zonulin was reported to transactivate the epidermal growth factor receptor possibly through the proteinase-activated receptor-2, resulting in disassembly and increased permeability of the tight junction’s function in the cell. 16 In addition, zonulin has shown to be involved in the regulation of blood–brain barrier permeability, resulting in neuroinflammation and neuroimmune activation. 17 Furthermore, recent studies reported a higher level of zonulin in SCZ and its implications for innate immune imbalance.18, 19
Lipopolysaccharide-binding protein (LBP) is an endogenous reactive biomarker produced because of microbial translocation. 20 Studies have reported an association of higher levels of LBP with systemic and intestinal inflammation triggered by LPS and other bacterial products. 21 LBP functions as a lipid transfer protein and is shown to copurify with high-density lipoprotein (HDL) particles. 22 Studies have also demonstrated that LBP can counteract the LPS effects by relocating the LPS to lipoproteins. 23 Despite increasing evidence that LBP plays a vital role in cardiometabolic disorders, 22 no studies so far have reported the association between systemic levels of LBP and SCZ patients who are more prone to develop cardiometabolic abnormalities.
Accumulated literature reports that dysregulation of intestinal alkaline phosphatase (IAP) is linked to altered gut microbial homeostasis. 24 IAP is expressed in the gastrointestinal tract and it is shown to inhibit NF휅B activation and its translocation into the nucleus, thereby inhibiting the expression of proinflammatory cytokines. 25 IAP aids in increasing mucosal tolerance to the resident gut bacteria by preventing LPS-mediated inflammatory response. 26 IAP pretreatment was shown to prevent the deterioration of tight junction protein expression. This effect possibly explains the mechanism of abrogation of the LPS-induced barrier dysfunction in vitro. 27
While zonulin levels are associated with intestinal permeability, LBP and IAP are linked with microbial homeostasis, an integrative hypothesis linking the functional interactions and their relevance in a common central pathway, i.e., inflammation in SCZ is needed. However, no studies in the existing literature have comprehensively studied the biomarker triggers of intestinal permeability/microbial hemostasis that could contribute to the etiopathogenesis of SCZ. Therefore, in this study, we aimed to examine the relationships among zonulin, LBP, and IAP, and to correlate with NLR in healthy controls and SCZ to see whether these biomarkers levels are altered.
Methods
Patients with SCZ (n = 40) were recruited from the clinical services of a large neuropsychiatric hospital, the National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, in South India. For healthy controls (n = 20), we used a commonly used model which has worked well in many previous studies. We approached the staff of NIMHANS working in different departments and explained to them the proposed study and put forth our requirements. We also requested the staff to pass on the study information to their friends/relatives and inform us if anyone were interested in participating in the study. We recruited the South Indian population living in and around Bengaluru. Detailed assessments were done to check if the participant met the eligibility criteria. Detailed medical history (for comorbidities) and PHQ9 (to check the mental health of the participant) were recorded with the help of a psychiatrist during screening and recruitment. Clinical diagnosis of SCZ (DSM-V) was carried out using Mini-International Neuropsychiatric Interview Plus 28 with an independent evaluation and confirmation by two psychiatrists. All the information related to the illness was obtained from a first-degree relative of the patient. Features suggestive of comorbid medical/neurological diagnosis and substance dependence were ruled out by clinical examination. Psychotic symptoms were assessed by the Scale for Assessment of Positive Symptoms (SAPS) 29 and the Scale for the Assessment of Negative Symptoms (SANS). 30 Of the 40 SCZ patients, 15 were drug-naïve and 25 were on the antipsychotic drug (risperidone).
Individuals with any of the following conditions were excluded: gastrointestinal disorders, organic brain disorder, including cerebrovascular accident; traumatic brain injury, epilepsy, loss of consciousness for more than 30 min; chronic diseases including diabetes mellitus, kidney disease, cardiovascular disease, stroke, and those on statins or aspirin treatment; antibiotic or immune therapy within the last 3 months and prebiotic or probiotic treatment within the previous 4 months; and inability to cooperate with study procedures and pregnant or lactating women.
We confirm that all methods were carried out in accordance with relevant guidelines and regulations. Furthermore, we confirm that informed consent was obtained from all the study participants, and all experimental protocols were approved by the Institutional ethical committee [IEC Approval No: NIMHANS/22nd/IEC (BS&NS DIV.)/2019]. Participants responded to interviewer-administered questionnaires, were examined for body measurements, and provided biospecimens.
Based on our pilot study on LBP, the mean ± SD between healthy controls and SCZ were 89.5 ± 25.3 ng/ml and 135.4 ± 60.6 ng/ml, respectively. Based on this, the calculated total sample size was 38 (19 in each group), which achieves the power of 85% with a significance level (α) of 0.05. The total sample size was rounded to 40 (20 in each group). Although the required sample size for cases was 20, we have increased the number of cases because of the availability of samples.
Anthropometric measurements including height, weight, and waist circumference were obtained using standardized methods and body mass index (BMI) was calculated as weight (kg) divided by height (m) squared.
Blood samples were collected from study participants using EDTA vacutainer tubes. 5 ml of blood was drawn from each participant by a trained phlebotomist. Blood tubes were quickly transported to the lab and centrifuged at 3500 rpm for 10 min. Plasma was separated, labeled appropriately, and stored in cryovials at −80ºC until further use. Fasting plasma glucose, serum cholesterol, serum triglycerides, and HDL cholesterol were measured using Cobas c501 Autoanalyser. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula. The intra- and interassay coefficients of variation for the biochemical assays ranged between 5% and 10%. All measurements were performed in a laboratory, certified by the National Accreditation Board for Testing and Calibration of Laboratories, India. Complete blood count was measured by Mindray BC-6200. NLR was calculated as the ratio between neutrophils and total lymphocyte counts (as a percentage) in the studied participants.
Zonulin, LBP, and IAP measurements [ELISA]
Zonulin, LBP, and IAP were measured in plasma samples by commercially available enzyme-linked immunosorbent assay (ELISA) kits. This ELISA kit uses the solid-phase sandwich principle. The experiments were carried out according to the manufacturer’s instructions. Standards and plasma samples were pipetted into the precoated wells with a monoclonal antibody specific for zonulin (CusaBio, Houston, TX, USA), LBP (Elabscience, Houston, TX, USA), and IAP (CusaBio) so that any zonulin, LBP, and IAP present would be bound to the immobilized antibody. Subsequently, after washing any unbound substances, an enzyme-linked polyclonal antibody specific for zonulin, LBP, and IAP was added to the wells, followed by the substrate solution for color development. The intensity of color developed would be in proportion to the amount of zonulin, LBP, and IAP bound in the initial step. The color development was stopped with the stop solution, and the intensity of the color was measured at 450 nm on a TECAN multiplate reader. The intra- and interassay coefficient of variation of the assay were <5 and <10%, respectively. All samples for ELISA estimation were run in duplicates, and the average value was taken for analysis for each biomarker.
Statistical Analysis
We used R software suite (v4.0) with “jmv,” “car,” and “ggplot2” packages for data analysis. Student’s t-test, Chi-square test, or Fisher’s exact test were used to compare groups for continuous variables and proportions. The correlation of metabolic variables with zonulin, LBP, and IAP was done using the Pearson correlation analysis. Multiple logistic regression analysis was used to explore the association of zonulin, LBP, and IAP with SCZ. Any anthropometric, clinical, or biochemical characteristics that were significantly different across groups were adjusted in the regression model. For comparison of zonulin, LBP, and IAP among the study participants, α and P values are set at 0.016 (0.05/3), and for other exploratory analyses, P < 0.05 was considered as the level of significance. Normality was checked by the Kolmogorov–Smirnov test and parametric tests were performed as the data was normally distributed.
Results
The clinical and biochemical parameters of the study participants are reported in Table 1. A total of 60 individuals were included in the study, of whom 50% (n = 30) were males. There were no significant differences in BMI, systolic and diastolic blood pressures, fasting plasma glucose, and lipid profile among the two study groups. NLR, HDL cholesterol, and cholesterol/HDL ratio were higher in patients with SCZ (P <0.05) compared to healthy controls.
Table 1. Clinical and Biochemical Characterization of Study Subjects.
| Variables | Healthy Controls (n = 20) | SCZ (n = 40) | P Value | ||
| Mean | SD | Mean | SD | ||
| Age (years) | 32.5 | 6.8 | 33.3 | 8.1 | 0.70 |
| Male n (%) | 11 (55%) | 19 (47.5%) | 0.79 | ||
| Body mass index (kg/m2) | 25.3 | 5.3 | 23.4 | 5.5 | 0.20 |
| Systolic blood pressure (mmHg) | 122 | 13 | 116 | 14 | 0.08 |
| Diastolic blood pressure (mmHg) | 82 | 8 | 80 | 10 | 0.34 |
| Age at onset (years) | - | - | 31 | 8 | - |
| Duration of untreated illness (in months) | - | - | 17 | 16 | - |
| Total SAPS | - | - | 20 | 24 | - |
| Total SANS | - | - | 24 | 25 | - |
| Fasting plasma glucose (mg/dL) | 88 | 6 | 90 | 8 | 0.16 |
| Total cholesterol (mg/dL) | 160 | 30 | 177 | 46 | 0.16 |
| Serum triglycerides (mg/dL) | 122 | 36 | 153 | 70 | 0.07 |
| Serum HDL cholesterol (mg/dL) | 43 | 8 | 37 | 6 | 0.02* |
| Serum LDL cholesterol (mg/dL) | 101 | 23 | 100 | 32 | 0.83 |
| Serum VLDL cholesterol (mg/dL) | 27 | 9 | 29 | 13 | 0.48 |
| Cholesterol/HDL Ratio | 3.9 | 1.0 | 4.9 | 1.8 | 0.01* |
| Hemoglobin (HB) (g/dl) | 14 | 2.2 | 13 | 2.4 | 0.40 |
| Red blood cells (RBC) (*million/ul) | 5.0 | 0.4 | 5.0 | 0.6 | 0.65 |
| White blood cells (WBC) (*1000/ul) | 7.2 | 1.3 | 8.4 | 1.9 | 0.02* |
| Platelets (*1000/ul) | 293 | 88 | 295 | 82 | 0.94 |
| Lymphocytes (%) | 34 | 6.4 | 27 | 6.1 | 0.00* |
| Monocytes (%) | 6.0 | 1.2 | 5.3 | 1.3 | 0.09 |
| Eosinophils (%) | 3.5 | 2.7 | 3.6 | 3.2 | 0.91 |
| Neutrophils (%) | 55 | 6.2 | 64 | 7.8 | 0.00* |
| Neutrophil lymphocyte ratio (NLR) | 1.7 | 0.5 | 2.6 | 0.9 | 0.00* |
Abbreviations: SAPS: Scale for the Assessment of Positive Symptoms; SANS: Scale for the Assessment of Negative Symptoms; HDL: high density lipoprotein; LDL: low density lipoprotein.
Note: *statistically significant.
Zonulin, LBP, and IAP were measured using ELISA across all the study groups. Compared to mean zonulin levels in healthy controls (1.4 ± 0.58 ng/ml), zonulin levels were approximately 59% higher in SCZ (3.4 ± 1.3 ng/ml, P <0.05) (Figure 1A). LBP levels were significantly (P < 0.01) higher in SCZ (41%) compared to healthy controls (Figure 1B). The mean IAP levels were lower in SCZ (11.4 ± 3.7 ng/ml, P < 0.05) compared to the healthy controls (19 ± 5.8 ng/ml) (Figure 1C). To study the influence of gender on the association of zonulin, LBP, and IAP with SCZ, study subjects were categorized by gender. No significant differences in zonulin, LBP, and IAP levels by gender were found in the present study [data not shown].
Figure 1. Compared to mean zonulin levels in healthy controls (1.4 ± 0.58 ng/ml), zonulin levels were approximately 59% higher in SCZ (3.4 ± 1.3 ng/ml, p<0.05) (1A). LBP levels were significantly (p<0.01) higher in SCZ (41%) compared to healthy controls (1B). The mean IAP levels were lower in SCZ (11.4 ± 3.7 ng/ml, p<0.05) compared to the healthy controls (19 ± 5.8 ng/ml) (1C).
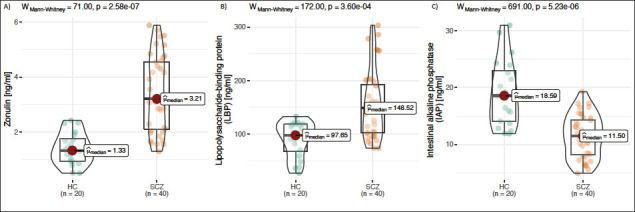
Drug-naïve (n = 15) and risperidone-treated SCZ patients (n = 25) were comparable on demographic and biochemical parameters. A separate analysis of drug-naïve and risperidone-treated SCZ patients revealed a similar trend in zonulin, LBP, and IAP levels as in the total study patients, although it did not reach statistical significance.
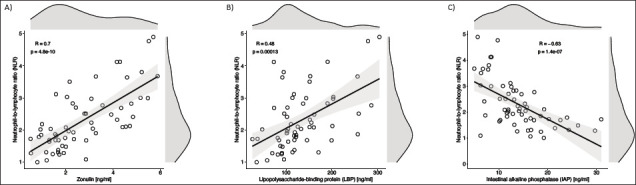
The Pearson correlation analysis revealed that zonulin [r = 0.710, P = 0.00] ( Figure 2a ) and LBP [r = 0.48, P = 0.00] ( Figure 2b ) were positively correlated with NLR, a marker of inflammation, and IAP [r = −0.63, P = 0.01] negatively correlated with NLR ( Figure 2c ). There was no significant correlation between cross-sectional illness severity (SAPS and SANS) and zonulin, LBP, and IAP. Correlation values of the intestinal permeability markers with other clinical and biochemical parameters are given in Supplementary Faniligure 1.
Figure 2. Pearson correlation analysis revealed that zonulin (2a), LBP (2b), were positively correlated with NLR, a marker of inflammation (r=0.710, p=0.00; and r=0.48, p=0.00 respectively), and IAP negatively correlated with NLR (r = - 0.63, p=0.01) (2c).
Multiple logistic regression analysis was carried out using SCZ as the dependent variable and zonulin, LBP, and IAP as independent variables. As shown in Table 2, each standard deviation increases in zonulin and LBP and each standard deviation decrease in IAP were independently associated with SCZ (zonulin: odds ratio (OR): 11.4, 95% confidence interval (CI): 2.74–47.32; LBP: 1.053, 1.02–1.09; IAP: 0.680, 0.551–0.840) (Model 1). Even after adjusting for age, gender, HDL-C, and NLR, higher levels of zonulin and LBP, or lower levels of IAP showed significant associations with SCZ (zonulin: 10.32, 1.85–57.12; LBP: 1.039, 1.02–1.07; IAP: 0.643, 0.471–0.879) (Model 2).
Table 2. Multiple Logistic Regression Analysis Using Schizophrenia (SCZ) as Dependent Variable and Zonulin, Lipopolysaccharide-Binding Protein (LBP), and Intestinal Alkaline Phosphatase (IAP) as Independent Variables.
| Variable | Odds Ratio (OR) | 95% Confidence Interval | P Value |
| Zonulin – Independent Variable | |||
| Zonulin – Unadjusted | 11.40 | 2.74–47.32 | 0.00 |
| Adjusted for age, gender, HDL-C, and NLR | 10.32 | 1.85–57.12 | 0.00 |
| LBP – Independent Variable | |||
| LBP – Unadjusted | 1.053 | 1.02–1.09 | 0.00 |
| Adjusted for age, gender, HDL-C, and NLR | 1.039 | 1.02–1.07 | 0.04 |
| IAP – Independent Variable | |||
| IAP – Unadjusted | 0.680 | 0.551–0.840 | 0.00 |
| Adjusted for age, gender, HDL-C, and NLR | 0.643 | 0.471–0.879 | 0.00 |
NLR: Neutrophil-to-lymphocyte ratio
Discussion
Human gut microbiota offers protection to the host by strengthening the impermeability of the epithelium, establishing a competitive barrier on the mucosal surface, thus maintaining the balanced state of the innate and adaptive immune systems. 31 Interestingly, recent studies have shown a definite role of altered gut microbiota in SCZ.6, 7 The role of increased intestinal permeability and abnormal immune responses in the etiopathogenesis of several brain/nervous system diseases, including SCZ,7, 8, 15 has been previously elucidated. In addition to the already established risk factors, intestinal permeability appears to play a key role in SCZ.14, 15 There is a growing interest in studying the biomarkers of intestinal permeability, particularly the systemic triggers of intestinal integrity and inflammation. In this context, our study has unraveled alterations in a panel of unique “intestinal permeability” biomarkers: (a) SCZ patients exhibited higher levels of zonulin and LBP and lower levels of IAP and (b) increased zonulin and LBP and decreased IAP are associated with low-grade inflammation as evidenced by the increased NLR in SCZ. This study is the first report among Asian Indians to demonstrate an independent association of zonulin, LBP, and IAP with higher odds of SCZ.
Recent studies demonstrate that zonulin is a promising biomarker for evaluating intestinal permeability.16, 18 Dysregulation of the zonulin signaling pathway may disrupt gut barrier function, alter immune responses, and predispose susceptible individuals to inflammatory autoimmune response by increasing the paracellular permeability of the gastrointestinal mucosa. 32 In our study, SCZ patients exhibited increased zonulin circulatory levels, supporting the hypothesis that intestinal permeability increases in SCZ, promoting the progressive entry of microbial factors into the bloodstream. This may trigger the immune system leading to the low-grade systemic inflammation associated with SCZ. Our study findings are in concurrence with the work by Usta et al., 19 in which increased systemic levels of zonulin were reported in patients with SCZ. Our results of higher circulatory levels of zonulin in patients with SCZ appear to increase our understanding of the emerging role of gut metabolites in the pathophysiology of SCZ.
LBP is demonstrated to be a surrogate biomarker of chronic inflammation by activating LPS-induced innate immune responses.21, 33 We observed that the plasma levels of LBP were higher in SCZ patients than in healthy controls. Recent studies indicate that metabolic abnormalities like obesity or type 2 diabetes can increase serum LPS levels, known as metabolic endotoxemia. 34 Interestingly, patients with SCZ are prone to develop cardiometabolic abnormalities. 35 As a biomarker for LPS-induced innate immune responses, our data showed that plasma levels of LBP were correlated with NLR.
IAP is recognized as a gut mucosal defense factor and can detoxify LPS. 26 Recent studies demonstrated that mice deficient in IAP had higher translocation of bacteria to mesenteric lymph nodes. 36 Furthermore, IAP administration was reported to strengthen the barrier function effectively by reserving its integrity and preventing the LPS-induced potential alteration in the tight junction proteins. 27 IAP may represent a novel therapeutic target based on its ability to decrease LPS-mediated inflammation. 37 Lower levels of IAP and its inverse association with zonulin and LBP in patients with SCZ are an important observation in our study. Furthermore, lower levels of IAP may lead to metabolic endotoxemia by triggering low-grade systemic inflammation and this is evident by its association with NLR, as observed in this study.
Several studies have reported a significant correlation of NLR with SCZ. 38 A meta-analysis of ten studies reported that NLR was increased in patients with SCZ, both in first-episode psychosis and chronic disease. 13 In this context, we found a significant association between NLR, zonulin, LBP, and IAP in patients with SCZ, which could provide a greater propensity to an altered intestinal permeability and inflammation. This finding suggests that a chronic state of low-grade endotoxemia exists in patients with SCZ, and all these markers may drive a common central pathway, i.e., inflammation in SCZ. However, these results could be further investigated in future studies. Identifying new biomarkers such as zonulin, LBP, or IAP would open up newer avenues for assessing intestinal permeability and low-grade intestinal inflammation in SCZ. Our work is a proof of concept in this direction.
HDL cholesterol plays a vital role in regulating inflammatory response and has a good binding capacity for LPS. 39 Studies have reported that LBP and specific transfer proteins play an important role in transferring LPS from HDLs to LDLs. 40 Because patients with SCZ are more prone to develop metabolic abnormalities and are characterized by decreased HDL levels, 35 it is conceivable that LPS may get redistributed in circulation and this may induce proinflammatory reactions on several peripheral tissues related to the pathoetiology of SCZ. In this context, the inverse correlation of zonulin levels with HDL cholesterol observed in our study is an important finding. Further studies are required to illuminate whether low HDL cholesterol levels could possibly be one of the pathogenetic links between intestinal permeability and SCZ.
Notwithstanding the limited sample size, this is one of the very few studies simultaneously measuring the systemic levels of zonulin, LBP, and IAP in patients with SCZ. Further studies are needed to correlate these biomarkers with gut microbiome diversity to understand the pathogenesis of SCZ. The strength of the study is that the cases (SCZ) and healthy controls were classified using standard methods and also controlled for a range of potential confounders including biochemical and hematological parameters. The limitations of this study are that being a cross-sectional one, no cause and effect can be drawn, for which prospective studies with serial measurements are needed. It is more vital to replicate the study findings on a larger sample size for robust effects estimation. Another limitation of this study is that the detailed dietary assessments of the study participants were not collected. Future prospective studies would offer more insights on the temporal relationship of intestinal permeability biomarkers at the interface of intestinal permeability and inflammation.
Conclusion
To conclude, our study reinforces the connection between intestinal permeability and inflammation as the important etiological construct of SCZ and adds further novel data on the association of IAP, a gut mucosal defense factor, with SCZ. Further studies are needed to correlate these markers with gut microbiota and understand the mechanisms that contribute to the pathogenesis of SCZ.
Supplementary Material
Acknowledgments
The authors acknowledge research grant supports received from the National Institute of Mental Health and Neurosciences Intramural Research Grant (Grant No. NIMH/DO/ (BS&NS DIV.)/2019). Dr Gokulakrishnan Kuppan is a current recipient of a DBT-Wellcome Trust India Alliance Intermediate Clinical & Public health Fellowship (Grant Number IA/CPHI/18/1/503964). Joyappa Nikhil acknowledges the Indian Council of Medical Research, India for the financial assistance (Junior Research Fellowship). Dr Narasimhan Sandhya acknowledges the Department of Health Research, India for the financial assistance (Women Scientist Fellowship). The authors also acknowledge Dr Hamsa Kumari, Social Scientist for her help in recruiting healthy controls.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge research grant supports received from the National Institute of Mental Health and Neurosciences Intramural Research Grant (Grant No. NIMH/DO/ (BS&NS DIV.)/2019). Dr Gokulakrishnan Kuppan is a current recipient of a DBT-Wellcome Trust India Alliance Intermediate Clinical & Public health Fellowship (Grant Number IA/CPHI/18/1/503964). Joyappa Nikhil acknowledges the Indian Council of Medical Research, India for the financial assistance (Junior Research Fellowship). Dr Narasimhan Sandhya acknowledges the Department of Health Research, India for the financial assistance (Women Scientist Fellowship).
ORCID iDs: Kuppan Gokulakrishnan  https://orcid.org/0000-0003-3167-8239
https://orcid.org/0000-0003-3167-8239
Venkataram Shivakumar  https://orcid.org/0000-0003-0353-8597
https://orcid.org/0000-0003-0353-8597
Shivarama Varambally  https://orcid.org/0000-0003-1420-8606
https://orcid.org/0000-0003-1420-8606
Authors’ Contribution
KG conceptualized the work. KG, MD, GV, and SV, designed and executed the study methods, analyzed data, interpreted the results, and drafted the manuscript. JN and CT performed experiments. SN and HP helped in SANS and SAPS. SVS, BH, and VS helped in recruiting SCZ patients. KG, MD, BH, SN, GV, and SV edited the manuscript and critically reviewed and helped in drafting the final version. KG and JN contributed equally in the research of this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Kuppan Gokulakrishnan), upon reasonable request.
Statement of Ethics
We confirm that all methods were carried out in accordance with relevant guidelines and regulations. Furthermore, we confirm that informed consent was obtained from all the study participants, and all experimental protocols were approved by the Institutional ethical committee.
References
- 1.Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in Schizophrenia. Annu Rev Clin Psychol 2014; 10: 425–448. [DOI] [PubMed] [Google Scholar]
- 2.Charlson FJ, Ferrari AJ, Santomauro DF, et al. Global epidemiology and burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr Bull 2018; 44: 1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new Schizophrenia loci. Nat Genet 2011; 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 Schizophrenia-associated genetic loci. Nature 2014; 511: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 2003; 60: 1187–1192. [DOI] [PubMed] [Google Scholar]
- 6.Lv F, Chen S, Wang L, et al. The role of microbiota in the pathogenesis of Schizophrenia and major depressive disorder and the possibility of targeting microbiota as a treatment option. Oncotarget 2017; 8: 100899–100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with Schizophrenia modulates the glutamate-glutamine-GABA cycle and Schizophrenia-relevant behaviors in mice. Sci Adv 2019; 5: eaau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Julio-Pieper M, Bravo JA, Aliaga E, et al. Review article: Intestinal barrier dysfunction and central nervous system disorders – A controversial association. Aliment Pharmacol Ther 2014; 40: 1187–1201. [DOI] [PubMed] [Google Scholar]
- 9.Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PloS One 2017; 12: e0172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan X, Kang Y, Zhuo C, et al. The gut microbiota promotes the pathogenesis of Schizophrenia via multiple pathways. Biochem Biophys Res Commun 2019; 512: 373–380. [DOI] [PubMed] [Google Scholar]
- 11.Balaji R, Subbanna M, Shivakumar V, et al. Pattern of expression of Toll like receptor (TLR)-3 and -4 genes in drug-naïve and antipsychotic treated patients diagnosed with Schizophrenia. Psychiatry Res 2020; 285: 112727. [DOI] [PubMed] [Google Scholar]
- 12.Subbanna M, Shivakumar V, Venugopal D, et al. Impact of antipsychotic medication on IL-6/STAT3 signaling axis in peripheral blood mononuclear cells of drug-naive schizophrenia patients. Psychiatry Clin Neurosci 2020; 74: 64–69. [DOI] [PubMed] [Google Scholar]
- 13.Karageorgiou V, Milas GP, Michopoulos I. Neutrophil-to-lymphocyte ratio in Schizophrenia: A systematic review and meta-analysis. Schizophr Res 2019; 206: 4–12. [DOI] [PubMed] [Google Scholar]
- 14.Fasano A. Intestinal permeability and its regulation by zonulin: Diagnostic and therapeutic implications. Clin Gastroenterol Hepatol 2012; 10: 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Severance EG, Gressitt KL, Stallings CR, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in Schizophrenia. Schizophr Res 2013; 148: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasano A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011; 91: 151–175. [DOI] [PubMed] [Google Scholar]
- 17.Rahman MT, Ghosh C, Hossain M, et al. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem Biophys Res Commun 2018; 507: 274–279. [DOI] [PubMed] [Google Scholar]
- 18.Barber GS, Sturgeon C, Fasano A, et al. Elevated zonulin, a measure of tight-junction permeability, may be implicated in Schizophrenia. Schizophr Res 2019; 211: 111–112. [DOI] [PubMed] [Google Scholar]
- 19.Usta A, Kılıç F, Demirdaş A, et al. Serum zonulin and claudin-5 levels in patients with Schizophrenia. Eur Arch Psychiatry Clin Neurosci 2021; 271: 767–773. [DOI] [PubMed] [Google Scholar]
- 20.Wan Y, Freeswick PD, Khemlani LS, et al. Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats. Infect Immun 1995; 63: 2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding P-H, Jin LJ. The role of lipopolysaccharide-binding protein in innate immunity: A revisit and its relevance to oral/periodontal health. J Periodontal Res 2014; 49: 1–9. [DOI] [PubMed] [Google Scholar]
- 22.Moreno-Navarrete J, Ortega F, Serino M, et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes 2012; 36: 1442–1449. [DOI] [PubMed] [Google Scholar]
- 23.Wurfel MM, Kunitake ST, Lichenstein H, et al. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med 1994; 180: 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bates JM, Akerlund J, Mittge E, et al. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2007; 2: 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fawley J, Gourlay D. Intestinal alkaline phosphatase: A summary of its role in clinical disease. J Surg Res 2016; 202: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bentala H, Verweij WR, Huizinga-Van der Vlag A, et al. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock 2002; 18: 561–566. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Hu D, Huo H, et al. Intestinal Alkaline Phosphatase regulates tight junction protein levels. J Am Coll Surg 2016; 222: 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59 Suppl 20: 22–33;quiz 34-57. [PubMed] [Google Scholar]
- 29.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and theoretical foundations. Br J Psychiatry Suppl 1989; 49–58. [PubMed] [Google Scholar]
- 30.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) . Iowa City: Univ Iowa; 1984. [Google Scholar]
- 31.Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science 2005; 307: 1915–1920. [DOI] [PubMed] [Google Scholar]
- 32.Maes M, Sirivichayakul S, Kanchanatawan B, et al. Upregulation of the intestinal paracellular pathway with breakdown of tight and adherens junctions in deficit Schizophrenia. Mol Neurobiol 2019; 56: 7056–7073. [DOI] [PubMed] [Google Scholar]
- 33.Kheirandish-Gozal L, Peris E, Wang Y, et al. Lipopolysaccharide-binding protein plasma levels in children: effects of obstructive sleep apnea and obesity. J Clin Endocrinol Metab 2014; 99: 656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007; 56: 1761–1772. [DOI] [PubMed] [Google Scholar]
- 35.Kelly JR, Gounden P, McLoughlin A, et al. Minding metabolism: targeted interventions to improve cardio-metabolic monitoring across early and chronic psychosis. Ir J Med Sci 2021; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldberg RF, Austen WG, Zhang X, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci USA 2008; 105: 3551–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaliannan K, Hamarneh SR, Economopoulos KP, et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Natl Acad Sci USA 2013; 110: 7003–7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Özdin S, Böke Ö. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in different stages of Schizophrenia. Psychiatry Res 2019; 271: 131–135. [DOI] [PubMed] [Google Scholar]
- 39.Levels JH, Abraham PR, van den Ende A, et al. Distribution and kinetics of lipoprotein-bound endotoxin. Infect Immun 2001; 69: 2821–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levels JHM, Marquart JA, Abraham PR, et al. Lipopolysaccharide is transferred from high-density to low-density lipoproteins by lipopolysaccharide-binding protein and phospholipid transfer protein. Infect Immun 2005; 73: 2321–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Kuppan Gokulakrishnan), upon reasonable request.


