Abstract
Background:
In stroke, sensory deficits may affect the motor recovery of the subjects. The evidence for the active sensory intervention to enhance motor recovery is sparsely available.
Purpose:
To systematically review the available evidence from the studies on active sensory therapies augmenting upper limb recovery among poststroke subjects.
Methods:
The following databases were searched for the desired articles: PubMed, the Cochrane Central Register of Trials (CENTRAL), DORIS, PEDro, and OTseeker. The primary search keywords were stroke, sensory, and motor. The articles published in English up to August 2021 were considered for the review. Only investigations that studied active sensory interventions to enhance motor recovery were considered for the review. The studies of robotic training, virtual reality, electrical stimulation, and acupuncture were excluded. Motor recovery and sensory recovery were considered as primary and secondary measures, respectively.
Results:
Out of 3528 screened studies, eight studies were found eligible for the present systematic review. Active sensory interventions in the form of sensory discrimination, mirror therapy, motor imagery, and specific somatosensory training were utilized in the selected studies. The interventions through mirror therapy and mental imaging have some promising roles in enhancing upper limb recovery. However, there is a lack of strong evidence for the effectiveness of the intervention enhancing motor improvement among the stroke subjects.
Conclusion:
A comprehensive active sensory protocol should be developed having components of cognitive, sensory, motor, and functional demand. There is a need to conduct good quality randomized trials to support the existing active sensory therapies.
Keywords: Cerebrovascular accident, Hemiparesis, Hand, Somatosensory, Tactile
Introduction
Poststroke motor paresis is a most common and certainly a challenging manifestation. Consequently, most of the stroke-rehabilitation regimes focus primarily on the motor aspect. 1 The sensory or somatosensory deficits such as impaired light touch and proprioception after stroke insult are also not unusual. 2 Majority of the stroke individuals may experience difficulty in perceiving the sensory modalities. The impairments, especially in the upper limb may affect the motor recovery of the subjects. 3 However, the identification and management of these subtle inabilities have not been effectively considered in clinical and research practice. Rehabilitation interventions ranging from passive to active maneuvers have been investigated. The passive techniques comprise methods such as electrical stimulation, whereas the active methods consist of specific training of the impaired sensory modality. 4 The concept of neuroplasticity is more applicable for active training. 5 The phenomenon of motor control is an outcome of active sensory input. 6
Sensory modalities are an integral component of motor performance. 7 The abilities such as touch, proprioception, and discrimination ability provide decisive information for a flawless and controlled motor output. 3 When these sensory modalities get impaired, the poststroke motor recovery may further get complicated; e.g., a stroke subject with proprioception deficit in the shoulder may not be able to utilize available recovery for the voluntary movements of the upper limb. 8 Sensory retraining, especially the active sensory therapy may be considered an important aspect of stroke rehabilitation. Active sensory interventions usually comprise active involvement of the subject at the motor and cognitive levels. The individual experiences and recognizes sensory modalities to enhance the recovery of that particular sensory deficit; for instance, practice to recognize commonly used objects with vision occluded augmenting the ability of stereognosis.4, 9 Contrary, in passive training, sensory stimulation is provided with negligible involvement of the subject. Active sensory training inevitably provides a context of integrated goal-directed practice involving multiple brain areas, leading to favorable neural reorganization.10, 11 Thus, the active regime has the potential to augment substantial motor recovery of the paretic upper limb. Conversely, the evidence for active sensorimotor therapy is sparsely available.
Four systematic reviews have been found to be conducted on sensory therapy in stroke during the last decade. In a systematic review and meta-analysis 11 on sensory retraining improving sensorimotor function, various types of sensory training such as active, passive, and hybrid were considered. Further, active training was considered for both the upper and lower limbs. In another systematic review, 12 somatosensory discrimination modality was considered as the primary outcome whereas studies using sensory intervention to enhance motor function were excluded. Similarly, the third review 9 also considered the somatosensory measure as a primary outcome. The fourth systematic review 13 grouped the 14 trial-related sensory interventions such as active, stimulation, and thermal to investigate the effect of sensory training on motor function. The result indicated insufficient evidence of the training on upper limb function. No systematic review emphasizing exclusively on the active sensory training enhancing motor recovery of the upper limb in stroke has been found. Thus, there is a need to ascertain the effectiveness of active sensory therapies in stroke. The objective of this study was to systematically review the available studies on active sensory therapies augmenting the upper limb recovery among poststroke subjects. Further objective was to summarize the evidence for clinical practice and future investigation.
Methods
The present systematic review was registered under the PROSPERO database as CRD42020173875. Further, the reporting of this review followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. 14
Data Sources and Searches
The following databases were searched for the necessary articles: PubMed, The Cochrane Central Register of Trials (CENTRAL), DORIS, PEDro, and OTseeker. The studies published in English till August 31, 2021, were considered for the review. The primary keywords for the search were stroke/hemiparesis/cerebrovascular accident and sensory. The detail about the MeSH search terms utilized for the PubMed is provided in Appendix 1. The terms were modified for the other databases.
Study Selection and Data Extraction
The retrieved searches were saved in EndNote X4 and Mendeley reference management software to check and remove the duplicate articles. The studies were initially screened for the titles and abstracts utilizing the search strategy, shared by KNA and AKJ. The article meeting the eligibility criteria (Box 1) were selected for the full text. Further, the eligibility of the studies was carried out by KNA, SP, and AKJ. The other authors (NC or GG) were consulted to resolve the final decision, in case of any disagreement. A data extraction form was formulated for quality appraisal as well as evidence synthesis and analysis. The form primarily comprised the following information: setting, demographics, and baseline characteristics of the subjects, the experimental and control interventions, randomization and allocation process, blinding, outcome measures, mechanisms of intervention, and information related to assessment of the risk of bias. The study authors were contacted for any additional information/data, if required.
Box 1. Eligibility Criteria of the Selected Studies.
| Inclusion Criteria | Exclusion Criteria | |
| Participants/population | Ischemic and hemorrhagic stroke, any age group, both the genders, hemiparesis, sensory deficit in the paretic upper limb, and any phase of recovery | Pure sensory stroke, traumatic head injury, any type of neuropathy, complex regional pain syndrome |
| Intervention(s), exposure(s) | Sensory training of one or more somatosensory modalities having active participation (motor or cognitive) of the subject | Electrical stimulation, noninvasive brain stimulation, thermal, compression, acupuncture, robotics, motor therapies inadvertently providing sensory intervention |
| Study designs | Randomized controlled trial Quasi-experimental studies, |
Cohort studies, single case studies, case series, retrospective studies |
| Outcome measure | Primary outcome: Motor recovery of the upper extremity including hand as assessed by any of the following:
Secondary outcome: Sensory recovery of the upper extremity and/or hand as assessed by any of the following:
|
Quality Assessment
The quality assessment for the methodological aspect of each selected study was carried out using the PEDro scale. 15 The items (except item 1) were scored as either 1 (Yes) or 0 (No/Unclear/Not applicable). Two authors (KNA and SP) independently examined the quality, using the scale. The scores awarded were also verified with that available on www.pedro.org.au. In case of the nonavailability of the study in the PEDro database or any disagreement between the raters, the issue was resolved through discussion with other investigators (NC or GG). The studies with a PEDro score of ≥7 were considered as “high quality,” a score of 5 or 6 as “moderate quality,” and a score of ≤4 as “poor quality” investigations.
Data Synthesis
The findings from the selected studies were reported in the form of a narrative review. In view of limited homogenous studies utilizing a similar design, intervention, and outcome measure, the meta-analysis was not conducted. The key information related to each selected study was also presented in a form of a summary of the findings table.
Results
Study Selection
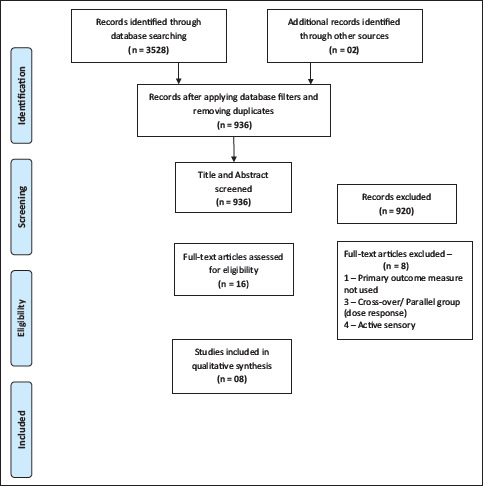
The process of selection of studies is depicted in Figure 1. Initially, 3528 studies were retrieved from the databases. After applying search filters for the databases and removing the duplicates, titles and/or abstracts of 936 studies were screened. Sixteen full-text studies were evaluated in detail for the eligibility criteria. Eight full-text studies were excluded either for the lack of primary measure or active sensorimotor intervention or because of the design (Appendix 2). Finally, eight studies were included for the review.
Figure 1. PRISMA Flow Diagram Showing the Process of Selection of Studies.
Study Characteristics
Out of eight selected studies, two studies16, 17 were conducted in Spain and most of the other studies18–22 were carried out in the Asian continent (two, Iran; one, India; one, Japan; and one, Thailand). Six studies16–20 were randomized trials whereas two studies21, 22 were quasi- and parallel-group randomized investigations. Table 1 shows the summary of the selected studies.
Table 1. Summary of the Basic Characteristics of the Selected Studies (n = 08).
| Authors, Year, and Country of Study | Inclusion Criteria | Design and Sample Size | Experimental Intervention | Control Intervention | Outcome Measures (Motor) | Outcome Measures (Sensory) | Time Points of Assessment | Remarks |
| De Bruyn et al. 2020 23 Belgium |
|
|
|
|
|
|
|
|
| Umeki N et al. 2019
22
Japan |
|
|
|
|
|
|
|
|
| Arya et al. 2018 18 India |
|
|
|
|
|
|
|
|
| Azad A et al. 2018
19
Iran |
|
|
|
|
|
|
|
|
| Salles L et al. 2017
16
Spain |
|
|
|
|
|
|
|
|
| Samaei A et al. 2016
21
Iran |
|
|
|
|
|
|
|
|
| de Diego C et al. 2013
17
Spain |
|
|
|
|
|
|
Experimental group:
|
|
| Chanubol R et al. 2012
20
Thailand |
|
|
|
|
|
– |
|
|
Abbreviations: ARAT, action research arm test; ADL, activities of daily living; FMA-UE, Fugl-Meyer assessment (upper extremity); SULCS, stroke upper limb capacity scale; OT, occupational therapy; PT, physical therapy; SWM, Semmes–Weinstein monofilament; fTORT, functional tactile object recognition test, WMFT, wolf motor function test; CIMT, constraint-induced movement therapy; HDS-R, revised Hasegawa dementia scale; BRS, Brunnstrom recovery stages; MAL, motor activity log; NSA, Nottingham sensory assessment; PTT, perceptual threshold of touch; TDT, texture discrimination test; WPST, wrist position sense test; MESUPES, motor evaluation scale for upper extremity in stroke patients; MI, motricity index; 2PD, two-point discrimination test; RNSA, revised Nottingham sensory assessment; KVIQ, kinesthetic and visual imagery questionnaire; MMSE, mini-mental state examination; Em-NSA, erasmus modified Nottingham sensory assessment; RCT, randomized controlled trial
Participant Characteristics
The number of participants ranged from 8 to 56 with a total of 255 subjects in the selected studies. Although the inclusion criteria for age varied from 18 to 80 years, the average age of the participants ranged from 46 to 76 years across the investigations. Five studies16, 18–20, 22 reported the proportion of participants as per gender (men; 50% to 87.5%) and side of paresis (right side; 37.5% to 95%). The poststroke duration of the recruited subjects ranged from six days to 53 months; five studies16, 19, 20, 22, 23 researched on acute to subacute, whereas three studies17, 18, 21 investigated chronic stroke subjects.
Methodological Quality
Five studies16–18, 20, 23 were found to be of either moderate or high quality. The remaining investigations19, 21, 22 were of poor quality. The internal validity as examined by the PEDro scoring system is provided in Table 2.
Table 2. Internal Validity (PEDro 15 Criteria and Scores) of the Selected Studies (n = 08).
| Study | Random Allocation | Concealed Allocation | Group Similar at Baseline | Participant Blinding | Therapist Blinding | Assessor Blinding | <15% Drop Outs | Intention-to-Treat Analysis | Between Groups Difference Reported | Point Estimate and Variability Reported | Total Score (0 to 10) |
| De Bruyn et al. 2020 23 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Umeki N et al. 2019 22 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 3 |
| Arya et al. 2018 18 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Azad A et al. 2018 19 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 3 |
| Salles L et al. 2017 16 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Samaei A et al. 2016 21 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 4 |
| de Diego C et al. 2013 17 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Chanubol R et al. 2012 20 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
Type of Intervention
The type of active sensory training was not uniform among the selected studies. The intervention utilized as active sensory interventions in the studies were sensor-motor-functional activities, mirror therapy, mental imaging, cognitive sensorimotor therapy, and sensory-constraint therapy. There was variability among these regimes in terms of the basic method of application, underlying mechanism, somatosensory modalities intervened, and associated motor therapy. The components of these active sensory protocols are provided in Table 3. A brief about the regimes is given further.
Table 3. Content of Experimental Intervention in the Selected Studies (n = 08).
| Study | Modalities Intervened by Active Sensory Training | Additional Passive Sensory Training | Integrated Motor Training* | Supplementary Motor/Functional Training |
| De Bruyn et al. 2020 23 |
|
No | Yes | – |
| Umeki N et al. 2019 22 |
|
No | No | – |
| Arya et al. 2018 18 |
|
No | Yes | – |
| Azad A et al.2018 19 |
|
No | No | – |
| Salles L et al. 2017 16 |
|
No | No | – |
| Samaei A et al. 2016 21 |
|
No | Yes | – |
| de Diego C et al. 2013 17 |
|
Yes | No | Yes |
| Chanubol R et al. 2012 20 |
|
No | No | – |
Note. * Other than for proprioception/kinesthesia.
Sensorimotor-Functional Activities
Four studies17, 21–23 imparted sensorimotor-functional activities either as a primary or constituent of the experimental protocol. In one of the studies, 23 investigators imparted sensory discrimination training for texture discrimination (using various materials such as fabric, wallpaper, and sandpaper), limb position sense (for a range of upper limb positions), and tactile object recognition (using different objects with variable characteristics such as shape, size, weight, and hardness). Further, the motor therapy with sensory discrimination tasks was also integrated. In another study, 22 sensory discrimination tasks such as sandpaper, Braille dots, and different types of clothes with or without visual feedback were utilized. Activities such as weight and texture discrimination, clay and dough activities, and puzzle tasks, with or without vision occluded were also imparted in a selected study. 21 A multicomponent intervention such as reaching, grasping, and manipulation using objects of various shapes, sizes, and weights and functional tasks were provided in another investigation. 17
Mirror Therapy
Only one study 18 used the mirror therapy technique to impart active sensory intervention. The affected upper limb was kept behind the reflective wall of the mirror whereas the less-affected place in front of the reflective surface. The subject performed the sensorimotor tasks by the less-affected upper limb to induce illusion for the affected limb. The sensory perception on the less-affected hand was provided for various textures, shapes, and sizes. In addition to this, tasks having sensorimotor demands targeting sensory impairment as well as impaired movements were also provided through mirror therapy.
Mental Imaging
Two studies17, 19 used the concept of mental imaging as a component of their experimental intervention. The first investigation 19 utilized the kinesthetic imagery (internal perspective of the individual’s own movement) and imparted the sensory feedback of the movements. The intervention was targeted specifically for challenging movements such as shoulder abduction and external rotation, elbow extension, forearm supination, wrist extension, and finger flexion. However, the second study 17 considered mental imaging as one of the components of the regime, in which the subjects performed mental imaging of activities of daily living (ADL), sensory perceptions, and normal movement experiences.
Cognitive Sensorimotor Therapy
In one of the selected studies, 20 the experimental protocol was based on cognitive sensorimotor therapy (Perfetti method) emphasizing the proprioceptive training. In this training, the participant practiced judging the different upper limb joint positions (initially passively created by the therapist) with vision occluded. The training proceeded from simple to complex multi-joint practice. At last, actively moving the distal upper limb over an object and perceiving its shape, size, and position was also exercised. In another study, 16 the concept of Perfetti method was utilized to impart training for sensory discrimination tasks ranging from simple to complex, augmented by cognitive techniques such as observation, imagination, and imitation and challenged by occluded vision.
Sensory-Constraint Therapy
The study of a multicomponent regime 17 had a module of constraining the less-affected upper limb (behind the back of the body). The constraint blocked both sensory input and movement of the limb. Unlike the traditional constraint-induced movement therapy, the arrangement did not allow perceiving the less-affected hand by the subject. The sensory-motor interventions such as sensory inputs, sensory perception, and functional activities were imparted in the constraining position.
Outcome Measures
Most of the studies utilized >01 motor recovery measures. In total, 13 types of motor measures were used in the selected studies. Fugl-Meyer assessment–upper extremity (FMA-UE) was used in four studies,17–19, 23 whereas Brunnstrom recovery stages (BRS) of hand were used in one of the investigations. 22 Semmes–Weinstein monofilament (SWM) was the commonly used sensory measure.17, 18, 22
Effectiveness
Out of all the types of active sensorimotor interventions, mirror therapy and mental imaging intervening sensory deficits have shown some promising effects in the recovery of the paretic upper limb. The mirror therapy study 18 exhibited favorable recovery of the wrist and hand among the experimental group subjects. The regime also enhanced the touch-response in the paretic hand and cutaneous threshold of the less-paretic palm. Similarly, the mental imaging investigation 19 demonstrated greater recovery of the entire upper limb and hand dexterity among the intervention subjects as compared to that of the controls.
The other active sensorimotor techniques did not show any superior benefits for the upper limb recovery when compared with the conventional management.
Discussion
Eight studies investigating the role of active sensory therapy augmenting motor recovery were reviewed in this study. Primarily because of variability in the intervention methods, outcome measures, and poststroke duration, the meta-analysis was not computed. There are varied aspects of active sensory therapies in the selected studies. None of the protocols considered all types of somatosensory deficits. The qualitative synthesis of the selected article indicates that active sensory therapy may be considered an important aspect of stroke rehabilitation. However, the evidence for the effectiveness of these regimes for motor recovery could not be deduced because of heterogeneity in the selected investigations. Individually, the studies of mirror therapy 18 and mental imagery 19 demonstrated a favorable change in motor recovery of the upper limb; however, both the investigations did not consider nonsensory mirror therapy or mental imagery for the control group, respectively.
Most of the investigators have researched the single-intervention concept, whereas one study 17 has analyzed multicomponent regime. Elements such as mirror therapy and mental imaging may be considered vital to induce the activation of related neural structures and circuits necessary to perceive somatosensation. 24 Similarly, integrated sensorimotor training would provide feedforward and feedback practice enhancing motor control. 25 Undoubtedly, the cognitive abilities involve in executing a successful movement and thus should also be an ingredient of any sensorimotor regime. 26
Somatosensory modalities and movements are integral components of motor control. Although inadvertently the motor rehabilitation protocols comprise some element of sensory demand, the structured aspect of a specific sensory deficit and its training may be missing in the available motor therapies. During usual mirror therapy, the movement of the less-affected upper limb provides proprioceptive inputs for the affected limb through illusion. However, the therapy may not induce input for light touch unless the related object is introduced in the regime. Similarly, in conventional constraint-induced movement therapy, the task training offers the involvement of sensory modalities such as touch and pressure; but the proprioceptive and stereognosis impairments cannot be engaged without vision occlusion of the subject. Thus, the existing motor therapies need to be restructured in view of the sensory issues in stroke. The inclusion of active-sensory protocol in motor therapies is scientifically and clinically more feasible than that of passive sensory interventions.
The total duration of active sensory therapies varies from 100 min to 1200 min in the selected studies of this review. In view of neuroplasticity principles, the duration plays a crucial role in inducing encouraging changes. The low dosage of intervention could explain the unfavorable response to experimental intervention in certain studies. The importance of dose in somatosensory training was investigated and observed that the gains were substantial among the subjects participating in the high-intensity treatment group of 72 h (4320 min). 27 Thus, the dosage needs to be critically considered for the existing as well as new active sensorimotor protocols.
Numerous motor measures have been used in the selected studies. It is important to note that the FMA or BRS assesses the synergistically linked motor control whereas most of the other measures determine the hand function, dexterity, or grip strength. The Action Research Arm Test, Box and Block Test, Purdue Pegboard Test, and Nine-Hole Test utilized by other authors are applicable to the mild paretic stroke subjects. Similarly, the SWM and two-point discrimination test cannot assess sensory modalities such as proprioception and stereognosis; Erasmus modified Nottingham sensory assessment (Em-NSA) and Nottingham sensory assessment (NSA) comprehensively cover the majority of the deficits. Negligible studies utilized any of these sensory measures. Thus, the measure might be an important factor regarding the findings of the investigations in this review.
Only two studies18, 21 recruited hemiparetic subjects with identified sensory impairments; the remaining investigations selected participants with specified motor deficits. The sensory deficits may not be present among all poststroke hemiparetic subjects. The underlying mechanism for active sensory intervention may be different for a hemiparetic individual with or without sensory deficits. The intervention is justifiable for the stroke subjects having both motor and sensory manifestations.
The limited number of databases and the English language as inclusion criteria for the studies were a few limitations of this review. Lack of homogenous and good-quality trials are other weaknesses that should be considered while generalizing the findings of this review. The varied range of poststroke duration and cumulative intervention-time in the recruited investigations may influence the findings of this review.
Future trials investigating comprehensive active sensorimotor regime using recommended motor and sensory measures in stroke are warranted. The approach may reduce heterogeneity among the studies leading to stronger evidence in future reviews. Various types of somatosensory impairment demand appropriate active sensory interventions. Future investigations should also include poststroke hemiparetic subjects with specific sensory deficits. The existing or novel regimes should be structured in view of the type of somatosensory impairment and associated motor paresis. For instance, proprioceptive deficit and poor shoulder control warrants a particular regime, whereas impaired light touch and nondexterous hand function require another defined protocol. The foundation of active sensory intervention should be utilized to develop a comprehensive protocol. The techniques such as mirror therapy or mental imaging may be considered for the severely upper limb paretic subjects (with associated sensory impairments), whereas techniques to execute active sensory therapy may be targeted for the mild paretic individuals with similar sensory deficits. The principles of cognitive-sensory training shall be utilized across all the stages of motor recovery. The sensory-functional activities should be judiciously executed for the subjects with minimal motor abilities to grossly manipulate the objects. Constraint-sensory therapy may be applied in the later stages of the recovery when learned nonuse leads to deficient exposure to various sensory stimuli. In addition to this, the selection of sensorimotor activities must be emphasized for every type of deficit so that the demand of activity meets the level of impairment and subtly challenge the same. Methodologically, to improve the quality, the prospective trials have to be designed in terms of concealed allocation as well as blinding of the subject and therapist. At the statistical level, the intention-to-treat analysis needs to be preferred enhancing the excellence of potential active sensory rehabilitation trials.
Conclusion
This systematic review suggests that the active sensory therapy may be a vital aspect of poststroke motor rehabilitation. However, the evidence for active sensory training enhancing motor recovery is deficient because of variable types of interventions. A comprehensive active sensory protocol needs to be developed comprising cognitive, somatosensory, motor, and functional abilities. The principles of various evident motor therapies such as mirror therapy, motor imagery, and constraint-induced movement therapy may also be utilized in an integrated sensorimotor regime. Further good-quality randomized trials are warranted to investigate the effectiveness of active sensory therapy. Future trials should utilize the recommended stroke-specific motor and sensory measures to determine the outcome.
Appendix 1. Search Strategy Terms.
| 1. | Stroke |
| 2. | Cerebrovascular accident |
| 3. | CVA |
| 4. | Brain vascular accident |
| 5. | Cerebrovascular stroke |
| 6. | Cerebral stroke |
| 7. | Acute stroke |
| 8. | Acute cerebrovascular accident |
| 9. | Upper extremity paresis |
| 10. | Upper extremity pareses |
| 11. | Hemiparesis |
| 12. | Hemipareses |
| 13. | Hemiplegia |
| 14. | Flaccid hemiplegia |
| 15. | Spastic hemiplegia |
| 16. | 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 |
| 17. | Sensory feedback |
| 18. | Sensorimotor feedback |
| 19. | Proprioceptive feedback |
| 20. | Sensory threshold |
| 21. | Sensation |
| 22. | Sensory function |
| 23. | Somatosensory disorder |
| 24. | Somatic sensation disorder |
| 25. | Position sense disorder |
| 26. | Proprioceptive disorder |
| 27. | Light touch sensation impairment |
| 28. | Pinprick sensation diminished |
| 29. | Proprioception |
| 30. | Position sense |
| 31. | Sense of position |
| 32. | Touch perception |
| 33. | Tactile perception |
| 34. | Touch |
| 35. | Touch sense |
| 36. | Taction |
| 37. | Sense of touch |
| 38. | Tactile sense |
| 39. | Hypesthesia |
| 40. | Hypoesthesia |
| 41. | Numbness |
| 42. | Reduced sensation |
| 43. | Tactile hypesthesia |
| 44. | Impaired sensation |
| 45. | Stereognosis |
| 46. | Kinestheses |
| 47. | Kinesthesia |
| 48. | Movement sensation |
| 49. | Kinesthetic sense |
| 50. | 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47 OR 48 OR 49 |
| 51. | 16 AND 50 |
Appendix 2. Details of the Excluded Studies (Full Text; n = 8).
| Study | Reason |
| Derakhshanfar M, Raji P, Bagheri H, Jalili M, Tarhsaz H. Sensory interventions on motor function, activities of daily living, and spasticity of the upper limb in people with stroke: A randomized clinical trial. J Hand Ther June 18, 2020; S0894–1130(20): 30076–30074. | Intervention: Passive intervention techniques such as weight bearing, joint compression, icing, and brushing. |
| Li YC, Wu CY, Hsieh YW, Lin KC, Yao G, Chen CL, Lee YY. The priming effects of mirror visual feedback on bilateral task practice: A randomized controlled study. Occup Ther Int November 26, 2019; 2019: 3180306. | Intervention: Mirror therapy not designed for specific sensory domains. |
| Hsieh YW, Chang KC, Hung JW, Wu CY, Fu MH, Chen CC. Effects of home-based versus clinic-based rehabilitation combining mirror therapy and task-specific training for patients with stroke: A randomized crossover trial. Arch Phys Med Rehabil December 2018; 99(12): 2399–2407. | Design: Cross-over |
| Wu CY, Huang PC, Chen YT, Lin KC, Yang HW. Effects of mirror therapy on motor and sensory recovery in chronic stroke: A randomized controlled trial. Arch Phys Med Rehabil June 2013; 94(6): 1023–1030. | Intervention: Mirror therapy not designed for specific sensory domains |
| Carey L, Macdonell R, Matyas TA. SENSe: Study of the Effectiveness of neurorehabilitation on sensation: A randomized controlled trial. Neurorehabil Neural Repair May 2011; 25(4): 304–313. | Outcome: No motor recovery measure |
| Byl NN, Pitsch EA, Abrams GM. Functional outcomes can vary by dose: Learning-based sensorimotor training for patients stable poststroke. Neurorehabil Neural Repair September–October 2008; 22(5): 494–504. | Design: Parallel group; dose response |
| Dohle C, Püllen J, Nakaten A, Küst J, Rietz C, Karbe H. Mirror therapy promotes recovery from severe hemiparesis: A randomized controlled trial. Neurorehabil Neural Repair March–April 2009; 23(3): 209–217. | Intervention: Mirror therapy not designed for specific sensory domains |
| Byl N, Roderick J, Mohamed O, Hanny M, Kotler J, Smith A, Tang M, Abrams G. Effectiveness of sensory and motor rehabilitation of the upper limb following the principles of neuroplasticity: Patients stable poststroke. Neurorehabil Neural Repair September 2003; 17(3): 176–191. | Design: Cross-over |
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially assisted by the Indian Council of Medical Research (ICMR), New Delhi, India.
ORCID iD: Kamal Narayan Arya  https://orcid.org/0000-0001-7276-3726
https://orcid.org/0000-0001-7276-3726
Authors’ Contribution
Concept and Design provided by KNA. Methodology provided by KNA, SP, AKJ, NC, and GG. Writing–original draft was taken care by KNA, SP, and AKJ; Writing–critical review and editing by NC and GG. Funding acquisition by KNA.
Statement of Ethics
The study does not require ethical permission.
References
- 1.Stinear CM, Lang CE, Zeiler S. et al. Advances and challenges in stroke rehabilitation. J Neurosci 2020; 19: 348–360. [DOI] [PubMed] [Google Scholar]
- 2.Bolognini N, Russo C, and Edwards DJ. The sensory side of post-stroke motor rehabilitation. J Neurosci 2016; 34: 571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlsson H, Ekstrand E, and Brogårdh C. Sensory function, measured as active discriminative touch, is associated with dexterity after stroke. J Neurosci 2019; 11: 821–827. [DOI] [PubMed] [Google Scholar]
- 4.Schabrun SM, and Hillier S. Evidence for the retraining of sensation after stroke: A systematic review. J Neurosci 2009; 23: 27–39. [DOI] [PubMed] [Google Scholar]
- 5.Borstad AL, Bird T, Choi S. et al. Sensorimotor training and neural reorganization after stroke: A case series. J Neurosci 2013; 37: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Liu F, Yan Z. et al. Therapeutic effects of sensory input training on motor function rehabilitation after stroke. J Neurosci 2018; 97: e13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borich MR, Brodie SM, Gray WA. et al. Understanding the role of the primary somatosensory cortex: Opportunities for rehabilitation. J Neurosci 2015; 79: 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rand D. Proprioception deficits in chronic stroke-upper extremity function and daily living. J Neurosci 2018; 13: e0195043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yilmazer C, Boccuni L, Thijs L. et al. Effectiveness of somatosensory interventions on somatosensory, motor and functional outcomes in the upper limb post-stroke: A systematic review and meta-analysis. J Neurosci 2019; 44: 459–477. [DOI] [PubMed] [Google Scholar]
- 10.Sarasso E, Agosta F, Temporiti F. et al. Brain motor functional changes after somatosensory discrimination training. J Neurosci 2018; 12: 1011–1021. [DOI] [PubMed] [Google Scholar]
- 11.Serrada I, Hordacre B, and Hillier SL. Does sensory retraining improve sensation and sensorimotor function following stroke: A systematic review and meta-analysis. J Neurosci 2019; 13: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turville ML, Cahill LS, Matyas TA. et al. The effectiveness of somatosensory retraining for improving sensory function in the arm following stroke: A systematic review. J Neurosci 2019; 33: 834–846. [DOI] [PubMed] [Google Scholar]
- 13.Grant VM, Gibson A, and Shields N. Somatosensory stimulation to improve hand and upper limb function after stroke-a systematic review with meta-analyses. J Neurosci 2018; 25: 150–160. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. J Neurosci 2009; 6: e1000097. [PMC free article] [PubMed] [Google Scholar]
- 15.Moseley AM, Rahman P, Wells GA. et al. Agreement between the cochrane risk of bias tool and physiotherapy evidence database (pedro) scale: A meta-epidemiological study of randomized controlled trials of physical therapy interventions. J Neurosci 2019; 14: e0222770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallés L, Martín-Casas P, Gironès X. et al. A neurocognitive approach for recovering upper extremity movement following subacute stroke: A randomized controlled pilot study. J Neurosci 2017; 29: 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Diego C, Puig S, and Navarro X. A sensorimotor stimulation program for rehabilitation of chronic stroke patients. J Neurosci 2013; 31: 361–371. [DOI] [PubMed] [Google Scholar]
- 18.Arya KN, Pandian S, Vikas. et al. Mirror illusion for sensori-motor training in stroke: A randomized controlled trial. J Neurosci 2018; 27: 3236–3246. [DOI] [PubMed] [Google Scholar]
- 19.Azad A, Mahmoodi-Manbar A, and Arani-Kashani Z. Effect of motor imagery training with sensory feedback on sensorymotor function of the upper extremity in patients with chronic stroke. J Neurosci 2018; 20: 28–35. [Google Scholar]
- 20.Chanubol R, Wongphaet P, Chavanich N. et al. A randomized controlled trial of cognitive sensory motor training therapy on the recovery of arm function in acute stroke patients. J Neurosci 2012; 26: 1096–1104. [DOI] [PubMed] [Google Scholar]
- 21.Samaei A, Mirshoja MS, and Khalili MA. Comparison of sensorimotor retraining methods by movement therapy based on limitations in upper extremity function in patients with chronic stroke. J Neurosci 2016; 5: 510–517. [Google Scholar]
- 22.Umeki N, Murata J, and Higashijima M. Effects of training for finger perception on functional recovery of hemiplegic upper limbs in acute stroke patients. J Neurosci 2019; 2019: 6508261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Bruyn N, Saenen L, Thijs L. et al. Sensorimotor vs. Motor upper limb therapy for patients with motor and somatosensory deficits: A randomized controlled trial in the early rehabilitation phase after stroke. J Neurosci 2020; 11: 597666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson J. The human imagination: The cognitive neuroscience of visual mental imagery. J Neurosci 2019; 20: 624–634. [DOI] [PubMed] [Google Scholar]
- 25.Scotto CR, Meugnot A, Casiez G. et al. Short-term sensorimotor deprivation impacts feedforward and feedback processes of motor control. J Neurosci 2020; 14: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song JH. The role of attention in motor control and learning. J Neurosci 2019; 29: 261–265. [DOI] [PubMed] [Google Scholar]
- 27.Byl NN, Pitsch EA, and Abrams GM. Functional outcomes can vary by dose: Learning-based sensorimotor training for patients stable poststroke. J Neurosci 2008; 22: 494–504. [DOI] [PubMed] [Google Scholar]


