Abstract
Background:
Meditation is a conscious mental discipline, that has been implicated in the relaxation response. The mechanism behind such a relaxing effect is psychoneuroimmunology (PNI), based on the interaction between mind, physical health, and self-healing; that conceptualizes that stress and an individual’s emotional state led to predisposition to diseases. Research to date suggests that meditation may play an active role in remodeling the imbalance between mind and body by modulating the psychoneuroimmunological effects of stress. However, to date, the multi-dimensional psychoneuroimmune aspects of meditation together have not been completely explicated. An evidence-based mechanism has been framed for the first time in India to explain the psychoneuroimmunology of regular and long-term meditation practice.
Summary:
Present evidence-based mechanism confirms prefrontal cortex (PFC) acts as a ‘Functional Connectome’ where psycho-neuro-immune aspects of meditation function simultaneously to exert positive benefits in the regulation of cognitive and emotional behavior. Also, this mechanism will help us to understand how human augmentation with lifestyle modification fosters brain plasticity to overcome various neuropsychiatric illnesses.
Key Message:
Meditation is a scientific tool against neuro-psychiatric illnesses.
Keywords: Meditation, Psychoneuroimmunology, Evidence-based research, Stress, Diseases, Health
Keypoints
Present evidence-based mechanism has been framed for the first time in India to understand how human augmentation with yoga-based lifestyle modification fosters brain plasticity to overcome various neuropsychiatric illnesses.
The present mechanism confirms that the prefrontal cortex (PFC) acts as a “Functional Connectome” which regulates psychoneuroimmune aspects of meditation simultaneously to exert the positive benefits on health.
Integration of mind-body-associated nonpharmacological interventions with modern medicine should be enhanced for better health outcomes, especially during the COVID-19 pandemic.
Introduction
The perception of a healthy lifestyle is evergreen and everyone’s dream. There are a few who achieve this healthy lifestyle in their lifespan. According to World Health Organization (WHO), “health” is defined as a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity. 1 But in today’s era, life is full of hassles, deadlines, frustrations, and demands, and all these situations lead to stress. Eustress is a physiological, psychological, and physical reaction to positive or negative situations in life whereas distress needs to be controlled to achieve a healthy lifestyle.
Meditation is a well-known Yogic tool to reduce stress and anxiety. 2 The mechanism behind the relaxing response might include the psychoneuroimmunological effects of yoga-meditation. Psychoneuroimmunology (PNI) is based on the interaction between the mind, physical health, and self-healing 3 that conceptualizes that the negative emotional state of an individual lead to susceptibility to diseases. 4 Such an effect is possibly mediated by the nervous system by modulating the behavior and the immune system. Studies to date suggest that meditation might play a dynamic role in remodeling the imbalance between mind and body by modulating the psychoneuroimmunological effects of stress.5–25
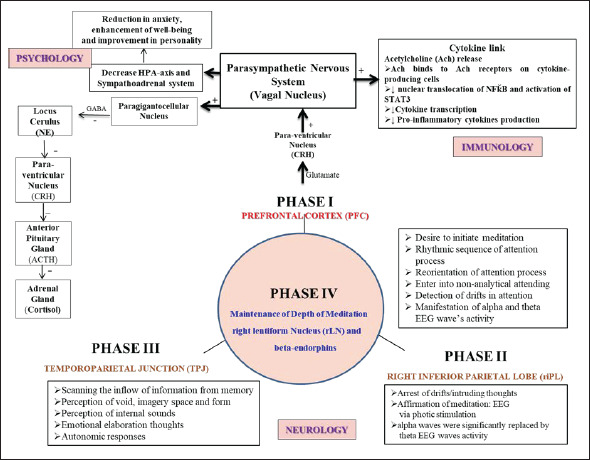
Here, the authors would like to emphasize that in our previous published study, 26 the novel methodology 26 was acquired to scan the human brain during meditation using neuroimaging technique. Therefore, the findings of the author’s psychoneuroimmunological research findings7–10, 26–30 have been used in framing the present mechanism (Figure 1). Based on previous research studies, a few hypothetical mechanisms of meditation had been proposed, but still not completely explicated.28, 31–36 Therefore, the present multidimensional mechanism has been framed for the first time in the Indian scenario to explain the psychoneuroimmunological aspects of meditation. The present mechanism confirms that the prefrontal cortex (PFC) acts as a “Functional Connectome” which regulates psychoneuroimmune aspects of meditation simultaneously to exert positive benefits on health. Also, provides an appropriate nonpharmacological treatment that may ameliorate cognitive and emotional related brain morphological abnormalities in people with neuropsychiatric illness. Before proceeding with an integrated physiological mechanism, the authors would like to give a brief about the psychoneuroimmunological effects of meditation.
Figure 1. Schematic Framework of Psychoneuroimmunological Mechanism of Long-Term Preksha Meditation. The Present Framework is Based on Scientific Evidence to Show Prefrontal Cortex (PFC) Acts as a “Functional Connectome” Where all the Components of Preksha Meditation that Including Attention Control, Emotional Regulation, and Altered Self-Awareness Function Simultaneously to Exert the Positive Benefits in the Maintenance of Neuropsychiatric Illnesses.
Psychoneuroimmunological Effects of Meditation
In the present scenario, mental stress leads to various stress-related neuropsychiatric disorders like depression and anxiety37–40 that affects our immune system. Although antidepressants and anxiolytics are available to overcome neuropsychiatric disorders, they also produce long-term side effects. But the evidence from the existing studies37, 41–47 on nonpharmacological interventions such as yoga and meditation reported as such no side effects and has shown better efficiency in combating various neuropsychiatric disorders. The previous studies5–10 have shown the efficacy of alternative and complementary therapies on various neuropsychiatric disorders and found a significant decrease in the symptoms of anxiety and depression by activating the anti-inflammatory physiological mechanism that significantly improved the quality of life of patients. Studies 48 have also been conducted to show a significant increase in the levels of the alpha and theta electroencephalography (EEG) waves activity in the frontal region of the brain as well as result in extensive changes in the frequency of gamma wave 49 by balancing the effect on the functional activity of participants in the left and right hemisphere.13, 26, 50, 51
Previous neuroimaging studies52, 53 have shown the different brain areas activated including the PFC, amygdala, superior temporal lobe, and hippocampus-strongly support focused attention but no literature has engrossed on the “maintenance of the advance state of meditation.” In a recent neuroimaging study, 26 meditating human brain was scanned with scientific affirmation with activated brain areas including the right mid Frontal gyrus (rGFm), right Broca’s (rBroca) area of right inferior frontal gyrus (rGFi), right inferior Parietal Lobe (riPL), right Superior Lateral Temporal Lobe (rsLT), right parieto-temporal cortex/junction (PTC/PTJ), arcuate nucleus (β-endorphins), right caudate nucleus (rCN), right lentiform nucleus (rLN). These activated brain areas were used in designing the neural model 28 to show the PFC plays an important role in nonanalytical functions 54 of an advanced state of meditation by initiating more synchronizing activity among different right-hemispheric neural correlates. Further, the question arises of how neural signatures 26 of PFC, play an essential role to exert the psychoneuroimmunological effects of meditation to foster brain plasticity to overcome various neuropsychiatric illnesses. On this basis, the authors have designed an integrated framework to understand the brain plasticity of regular meditation practice.
Integrated Psychoneuroimmunological Framework of Long-Term Meditation Practice
Meditation is an important tool of PNI that balances the mental and emotional aspects of the spiritual spectrum. 55 On this concept, studies from our laboratory5–10 served as the evidence base, which highlights the health promotion, disease prevention, and therapeutic role of yoga-meditation in healthy and diseased individuals. Using this evidence-based concept, we propose that PFC acts as a “Functional Connectome” that may aid in improving the health by modulating the psychoneuroimmunological effects of long-term meditation practice; further improve the inflammatory status by reducing inflammatory makers including TNF-α, NFKB, and IL-6 and by upregulating the anti-inflammatory genes expression, decelerating the rate of cellular aging and improving cellular endurance thus promoting positive quality of life and increases lifespan.44, 56–61
In today’s era, many meditation techniques are being practiced but the authors have focused specifically on “preksha meditation” (PM) because of the enhancing properties of attention skills and how these attention skills exert their psychoneuroimmunological effects on understanding the specific physiological pathways that warrant specific treatment interventions. Based on the previous literatures,9, 28, 30, 35, 36 the present evidence-based mechanism of long-term meditation practice is designed to highlight how three components of induction of meditation include efferent attenuation, afferent attenuation, and targeted nonanalytical thinking help in regulating the psychoneuroimmunological effects of depth of meditation.27, 28, 31, 62 These three components represent three Meditative Phases operating in a cyclic pattern within the different brain structures involved in the active preksha meditation process. The PFC plays a vital role in initiating the meditative processes by helping to detect and avoid wandering thoughts (Phase I, Figure 1). This Phase I has a role in a decision-making phase and is revealed by the appearance of both alpha and theta EEG wave’s activity was significantly and consistently higher for experienced meditators, probably reflecting relaxation, awareness, conscious state, and affective processing.13, 63–69 Phase II helps to arrest intruding thoughts; for this, the meditator is encouraged to enhance the capability to focus attention on a focussed target. To detect thought wandering various neurophysiological techniques have been involved that including EEG with photic stimulation,26, 27, 62 button press system, 55 and many more.70, 71 Scientifically, EEG recording with photic stimulation was found to be an appropriate marker to detect the drift-in attention,26, 27, 31, 62 especially in studying the human brain during neuroimaging with a novel methodology. 26 Further, in Phase II i.e., sensory attenuation, alpha EEG waves were significantly substituted by theta EEG waves, proportionately with the depth of the meditation.26, 27, 31, 62 At last, Phase III plays an important role which involves the temporal lobe plays an important role in emotional elaboration and conception of peculiar memory patterns often reported by meditators. The Phase III state is equivalent to samadhi where alpha EEG waves were consistently higher and there was a “shut down” inappropriate network for the maintenance of internalized focused attention.13, 31, 35, 62, 69, 72 and this same was affirmed in the author’s previous study 27 by giving photic stimulation. Hence, all three Meditative Phases may operate in a cyclic pattern and this pattern goes on repeatedly till the meditation process continue. Further, to maintain the cyclic pattern (Phase IV) between all the phases of meditation, it is very important to perceive the inner positive feelings-like experiences that help in the regulation of emotions and the attention process. Further, PFC simultaneously gives excitatory inputs to the arcuate nucleus of the hypothalamus to release β-endorphin, along with subcortical structures including right caudate nucleus (rCN) concerned with emotional and focused attention regulation and right lentiform nucleus (rLN) of basal ganglia, involved in the reward-like experience. Previous studies have also shown an increased level of β-endorphin during various relaxation techniques including meditation and exercise.10, 54, 73, 74
Then involves psychoimmunological aspects of meditation. Here, the authors would like to emphasize that in meditation how precisely activated brain regions of the PFC (rBroca, rGFm) along with the right inferior parietal lobe, and right temporoparietal regions regulates the psychoimmune status. Previous research has shown that stimulation of PFC leads to the stimulation of the hypothalamic-ventromedial nucleus via glutamate excitatory neurotransmitter with subsequent stimulation of the peripheral parasympathetic system,75, 76 which relates to the subjective sensation of relaxation. Further, activation of the parasympathetic system leads to the activation of the paragigantocelluar nucleus of the medulla which ceases to innervate the locus cerulus (LC) of the pons resulting in a decrease in the level of NE.77, 78 This decrease in levels of NE from Locus cerulus during meditation would likely decrease the production of a corticotropin-releasing hormone (CRH) by the PVN which would ultimately decrease cortisol levels.79, 80 Numerous studies have been conducted to show the decrease in plasma cortisol levels during meditation processes.6, 10, 81, 82
Cytokines are implicated in the pathophysiology of various diseases, and a reduction in inflammatory cytokines may play an important role in the prevention of causation and/or progression of such inflammatory diseases. It has been suggested that these changes were because of the activation of the parasympathetic nervous system during meditation.83, 84 It has been shown that stimulation of the vagus nerve leads to the inhibition of inflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-86, 10, 85 through the cholinergic anti-inflammatory pathway which is a neurological mechanism that helps to control cytokines with accuracy and restraint. 86 On the other hand, practicing regular and long-term preksha meditation for five or more than five years, improvement in the levels of anxiety, well-being, and overall personality6, 9, 87 have been observed that might imitate meditators have a relatively stable personality, and there are minimal fluctuations in various psychological aspects over time. 6 Overall, the present mechanism has thrown the light on the power of positive thinking that strengthens the brain neural circuits for experienced meditators, further increasing the ability to fight infection against any diseases. This might reflect not only a better quality of life but also a positive outlook, and improved self-rated health. Hence, from the multidimensional mechanisms, it has been concluded that the PFC acts as a “Functional Connectome,” where psychoneuroimmunological responses interact with each other for the regulation of cognitive and emotional behavior.
Clinical Application
The present evidence-based framework could be a secret tool against stress, which scientifically will help us to understand how a particular pathway fosters brain plasticity to overcome various neuropsychiatric illnesses through a nonpharmacological intervention.
Summary
A simple form of the mental-relaxation technique helps in the regulation of Psychoneuroimmune status by the “Functional Connectome” including the precise activated brain regions of PFC. Further, will provide the scientific platform for clinical research, specifically targeting areas of development in the treatment of various stress-mediated psychoneuroimmunological disorders.
Acknowledgment
The authors were greatly thankful to the Department of Biotechnology (DBT, BT/PR10269/GBD/27/82/2007), New Delhi, for granting the financial support for completing the research study which helps the author’s in designing the evidence-based mechanism.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Department of Biotechnology (DBT, BT/PR10269/GBD/27/82/2007), New Delhi.
ORCID iD: Dipti Magan  https://orcid.org/0000-0003-2982-1294
https://orcid.org/0000-0003-2982-1294
Authors’ Contribution
DM: Implemented the idea for this review, literature search, and drafted initial manuscript; RKY: contributed to review the concept and helped in revising the manuscript.
Statement of Ethics
The study was conducted in accordance with the Declaration of Helsinki, and the protocol of the study was approved by the Institute’s Ethics Committee, All India Institute of Medical Sciences, New Delhi. The study was registered at Clinical Trial Registry India (CTRI), CTRI/2009/091/000727.
References
- 1.Preamble to the Constitution of the World Health Organization as adopted by the International Health Conference, New York: World Health Organization, http://www.who.int/suggestions/faq/en/ (June 19–22, 1946, Accessed March 21, 2017). [Google Scholar]
- 2.Breedvelt JJF, Amanvermez Y, Harrer M, et al. The effects of meditation, yoga, and mindfulness on depression, anxiety, and stress in tertiary education students: A meta-analysis. Front Psychiatry 2019; 10: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irwin M and Vedhara K. Human psychoneuroimmunology . Oxford University Press, 2005. [Google Scholar]
- 4.Ader R, Cohen N, and Felten D. Psychoneuroimmunology: interactions between the nervous system and the immune system. Lancet 1995;345:99–103. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P, Yadav RK, Khadgawat R, et al. A 12-week yoga-based lifestyle intervention might positively modify cellular aging in Indian obese individuals: A randomized-controlled trial. J Altern Complement Med 2022. DOI: 10.1089/jicm.2021.0215. [DOI] [PubMed] [Google Scholar]
- 6.Magan D and Yadav RK. Physiological persona differences based on stress and inflammation between meditators and healthy controls. J Complement Integr Med 2019; 17(2)/j/jcim.2020.17.issue-2/jcim-2019-0106/jcim-2019-0106.xml. [DOI] [PubMed] [Google Scholar]
- 7.Yadav R, Yadav RK, Khadgawat R, et al. Comparative efficacy of 12-week yoga-based lifestyle intervention and dietary intervention on adipokines, inflammation, and oxidative stress in adults with metabolic syndrome: A randomized controlled trial. Transl Behav Med 2019; 9: 594–604. [DOI] [PubMed] [Google Scholar]
- 8.Sarvottam K. Magan D, Yadav RK, et al. Adiponectin, interleukin-6, and cardiovascular disease risk factors are modified by a short-term yoga-based lifestyle intervention in overweight/obese male subjects. J Altern Complement Med 2103; 19: 397–402. [DOI] [PubMed] [Google Scholar]
- 9.Yadav RK, Magan D, Mehta M, et al. A short-term, comprehensive, yoga-based lifestyle intervention is efficacious in reducing anxiety, improving subjective well-being and personality. Int J Yoga 2012; 5: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yadav RK, Magan D, Mehta N, et al. Efficacy of a short-term yoga-based lifestyle intervention in reducing stress and inflammation: Preliminary results. J Altern Complement Med 2012; 18: 662–667. [DOI] [PubMed] [Google Scholar]
- 11.Pullen PR, Thompson WR, Benardot D, et al. Benefits of yoga for African American heart failure patients. Med Sci Sports Exerc 2010; 42: 651–657. [DOI] [PubMed] [Google Scholar]
- 12.Rao MR, Raghuram N, Nagendra HR, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: A randomized controlled trial. http://www.ncbi.nlm.nih.gov/pubmed/19114222 Complement Ther Med 2009; 17: 1–8. [DOI] [PubMed] [Google Scholar]
- 13.Lagopoulos J, Xu J, Rasmussen I, et al. Increased theta and alpha EEG activity during nondirective meditation. J Altern Complement Med 2009; 15: 1187–1192. [DOI] [PubMed] [Google Scholar]
- 14.Upadhyay AK, Balkrishna A, Upadhayay RT.. Effect of pranayama (voluntarily regulated yoga breathing) and yoga asana (yoga postures) in diabetes (DM): A scientific review. J Complement Integr Med 2008; 5: 3. [Google Scholar]
- 15.Pullen PR, Nagamia SH, Mehta PJ, et al. Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. J Card Fail 2008; 14: 407–413. [DOI] [PubMed] [Google Scholar]
- 16.Khatri D, Mathur KC, Gahlot S, et al. Effects of yoga and meditation on clinical and biochemical parameters of metabolic syndrome. Diabetes Res Clin Pract 2007; 78: 9–10. [DOI] [PubMed] [Google Scholar]
- 17.Innes KE, Bourguignon C, Taylor AG.. Risk indices associated with the insulin resistance syndrome, cardiovascular disease, and possible protection with yoga: A systematic review. J Am Board Fam Med 2005; 18: 491–519. [DOI] [PubMed] [Google Scholar]
- 18.Bower J, Woolery A, Sternlieb B, et al. 2Yoga for cancer patients and survivors. Cancer Control 2005; 12: 165–171. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood G, Rampes H, Tuffery V, et al. Yoga for anxiety: A systematic review of the research evidence. Br J Sports Med 2005; 239: 884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aftanas LI, Golosheykin S.. Impact of regular meditation practice on EEG activity at rest and during evoked negative emotions. Int J Neurosci 2005; 115: 893–909. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Murata T, Hamada T, et al. Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. Int J Psychophysiol 2005; 55: 199–207. [DOI] [PubMed] [Google Scholar]
- 22.Lazar SW, Bush G, Gollub RL, et al. Functional brain mapping of the relaxation response and meditation. Neuroreport 2000; 11: 1581–1585. [PubMed] [Google Scholar]
- 23.Kjaer TW, Bertelsen C, Piccini P, et al. Increased dopamine tone during meditation-induced change of consciousness. Cogn Brain Res 2002; 13: 255–259. [DOI] [PubMed] [Google Scholar]
- 24.Lou HC, Kjaer TW, Friberg L, et al. A 15 H2O PET study of meditation and the resting state of normal consciousness. Hum Brain Mapp 1999; 7: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzog H, Lele VR, Kuwert T, et al. Changed pattern of regional glucose metabolism during yoga meditative relaxation. Neuropsychol 1991; 23: 182–187. [DOI] [PubMed] [Google Scholar]
- 26.Magan D, Yadav RK, Bal CS, et al. Brain plasticity and neurophysiological correlates of meditation in long-term meditators: A 18Fluorodeoxyglucose positron emission tomography study based on an innovative methodology. J Altern Complement Med 2019; 25: 1172–1182. [DOI] [PubMed] [Google Scholar]
- 27.Magan D, Yadav RK, Deepak KK.. Comparative analysis of depth of meditation between long-term and short-term meditators using electroencephalography. Int J Clin Exp Physiol 2019; 5: 178–183. [Google Scholar]
- 28.Magan D and Yadav RK. Neural mechanism of attention control in long-term preksha meditation. Med Hypotheses 2020; 143: 109953. [DOI] [PubMed] [Google Scholar]
- 29.Magan D and Yadav RK. Right Broca’s area is hyperactive in right-handed subjects during meditation: Possible clinical implications? Medical Hypotheses 2021; 150: 110556. [DOI] [PubMed] [Google Scholar]
- 30.Magan D, Yadav RK, Mehta M.. Efficacy of a short-term yoga-based lifestyle intervention in improving cognition in overweight/obese subjects. Clin Exp Pharmacol Physiol 2017; 4: 202–206. [Google Scholar]
- 31.Deepak KK. Meditation induces physical relaxation and enhances cognition: A perplexing paradox. Prog Brain Res 2019; 244: 85–99. [DOI] [PubMed] [Google Scholar]
- 32.Nani A, Manuello J, Mancuso L, et al. Neural correlates of consciousness and attention: Two sister processes of the brain. Front Neurosci 2019; 13: 1169. DOI: 10.3389/fnins.2019.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang YY, Hölzel BK, Posner MI.. The neuroscience of mindfulness meditation. Nat Rev Neurosci 2015; 16: 213–225. [DOI] [PubMed] [Google Scholar]
- 34.Malinowski P. Neural mechanisms of attentional control in mindfulness meditation. Front Neurosci 2013; 7. DOI: 10.3389/fnins.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deepak KK. Neurophysiological mechanisms of induction of meditation: A hypothetico-deductive approach. J Physiol Pharmacol 2002; 46: 136–158. [PubMed] [Google Scholar]
- 36.Newberg AB, Iversen G.. The neural basis of the complex mental task of meditation: Neurotransmitter and neurochemical considerations. Med Hypotheses 2003; 61: 282–291. [DOI] [PubMed] [Google Scholar]
- 37.Nagarathna R, Nagendra HR, Majumdar V.. A perspective on yoga as a preventive strategy for coronavirus disease 2019. Int J Yoga 2020; 13: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dantzer R. Cytokine-induced sickness behavior: A neuroimmune response to activation of innate immunity. Eur J Pharmacol 2004; 1: 399–411. [DOI] [PubMed] [Google Scholar]
- 39.Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am 2009; 29: 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butler G. Definitions of stress. Occas Pap R Coll Gen Pract 1993; 61: 1–5. [Google Scholar]
- 41.Thomas R. How meditation improves emotional and physical health. Psychology Today , Sussex Publishers, August 2019. [Google Scholar]
- 42.Abdallah CG, Sanacora G, Duman RS, et al. The neurobiology of depression, ketamine, and rapid-acting antidepressants: Is it glutamate inhibition or activation. Pharmacol Ther 2018; 190: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gałecki P, Mossakowska-W´ojcik J, Talarowska M.. The anti-inflammatory mechanism of antidepressants-SSRIs, SNRIs. Prog Neuro-Psychopharmacol Biol Psychiatry 2018; 80: 291–294. [DOI] [PubMed] [Google Scholar]
- 44.Tyagi A and Cohen M. Yoga, and heart rate variability: A comprehensive review of the literature. Int J Yoga 2016; 9: 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balasubramaniam M, Telles S, Doraiswamy PM.. Yoga on our minds: A systematic review of yoga for neuropsychiatric disorders. Front Psychiatry 2013; 3: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodyard C. Exploring the therapeutic effects of yoga and its ability to increase the quality of life. Int J Yoga 2011; 4: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niu JF, Zhao XF, Hu HT, et al. Should acupuncture, biofeedback, massage, Qi gong, relaxation therapy, device-guided breathing, yoga, and tai chi be used to reduce blood pressure? Recommendations based on high-quality systematic reviews. Complement Ther Med 2019; 42: 322–331. [DOI] [PubMed] [Google Scholar]
- 48.Hata M, Hayashi N, Ishii R, et al. Short-term meditation modulates EEG activity in subjects with post-traumatic residual disabilities. Clin Neurophysiol Pract 2019; 4: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braboszcz C, Rael CB, Levy J, et al. Increased gamma brainwave amplitude compared to control in three different meditation traditions. PLOS One 2017; 12: 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Telles S, Gupta RK, Yadav A, et al. Hemisphere specific EEG related to alternate nostril yoga breathing. BMC Res Notes 2017; 10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baijal S and Srinivasan N. Theta activity and meditative states: Spectral changes during concentrative meditation. Cogn Process 2010; 11: 31–38. [DOI] [PubMed] [Google Scholar]
- 52.Fox KC, Dixon ML, Nijeboer S, et al. 2Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations. Neurosci Biobehav Rev 2016; 65: 208–228. [DOI] [PubMed] [Google Scholar]
- 53.Newberg AB. The neuroscientific study of spiritual practices. Front Psychol 2014; 5: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shrihari TG. Endorphins-a natural healer. J Cancer Prev Curr Res 2018; 9: 223–234. [Google Scholar]
- 55.Dunn BR, Hartigan JA, Mikulas WL.. Concentration and mindfulness meditations: Unique forms of consciousness? Appl Psychophysiol Biofeedback 1999; 24: 147–165. [DOI] [PubMed] [Google Scholar]
- 56.Cotton VA, Low LA, Villemure C, et al. Unique autonomic responses to pain in yoga practitioners. Psychosom Med 2018; 80: 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nijs J, Loggia ML, Polli A, et al. Sleep disturbances and severe stress as glial activators: Key targets for treating central sensitization in chronic pain patients. Expert Opin Ther Targets 2017; 21: 817–826. [DOI] [PubMed] [Google Scholar]
- 58.Deak T, Kudinova A, Lovelock DF, et al. A multispecies approach for understanding neuroimmune mechanisms of stress. Dialogues Clin Neurosci 2017; 19: 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calcia MA, Bonsall DR, Bloomfield PS, et al. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl) 2016; 233: 1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawant HK, Jalali Z.. Detection and classification of EEG waves. Orient J Comput Sci Technol 2010; 3: 207–213. [Google Scholar]
- 61.Liang NY, Saratchandran P, Huang GB, et al. Classification of mental tasks from EEG signals using extreme learning machine. Int J Neural Syst 2006; 16: 29–38. [DOI] [PubMed] [Google Scholar]
- 62.Anand BK. Some aspects of electroencephalographic studies in yogis. Electroencephalogr Clin Neurophysiol 1961; 13: 452–456. [Google Scholar]
- 63.Dafoe T and Stermac L. Mindfulness meditation as an adjunct approach to treatment within the correctional system. J Offender Rehabil 2013; 52: 198–216. [Google Scholar]
- 64.Hagerty MR, Isaacs J, Brasington L, et al. Case study of ecstatic meditation: fMRI and EEG evidence of self-stimulating a reward system. Neural Plast 2013; 2013: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faber PL, Lehmann D, Tei S, et al. EEG source imaging during two Qigong meditations. Cogn Process 2012; 13: 255–265. [DOI] [PubMed] [Google Scholar]
- 66.Hasenkamp W and Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Front Hum Neurosci 2012; 6: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barnhofer T, Chittka T, Nightingale H, et al. State effects of two forms of meditation on prefrontal EEG asymmetry in previously depressed individuals. Mindfulness 2010; 1: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paul J, Kannathal N, Sadasian K, et al. Complex electroencephalogram dynamics during meditation. J Chin Clin Med 2007; 2: 220–230. [Google Scholar]
- 69.Aftanas LI and Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: High-resolution EEG investigation of meditation. Neurosci Lett 2001; 310: 57–60. [DOI] [PubMed] [Google Scholar]
- 70.Soni R and Muniyandi M. Breath rate variability: A novel measure to study the meditation effects. Int J Yoga 2019; 12: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atchley R, Klee D, Memmott T, et al. Event-related potential correlates of mindfulness meditation competence. Neuroscience 2016; 320: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaushik M, Jain A, Agarwal P, et al. Role of yoga and meditation as complimentary therapeutic regime for stress-related neuropsychiatric disorders: Utilization of brain waves activity as novel tool. J Evid Based Integr Med 2020; 25: 2515690X20949451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garland EL, Brintz CE, Hanley AW, et al. Mind-body therapies for opioid-treated pain: A systematic review and meta-analysis. JAMA Intern Med 2020; 180: 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Infante JR, Peran F, Martinez M, et al. ACTH and β-endorphin in transcendental meditation. Physiol Behav 1998; 64: 311–315. [DOI] [PubMed] [Google Scholar]
- 75.Devinsky O. Neurological aspects of the conscious and unconscious mind. Ann NY Acad Sci 1997; 835: 321–329. [DOI] [PubMed] [Google Scholar]
- 76.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci 1992; 15: 353–375. [DOI] [PubMed] [Google Scholar]
- 77.Foote S. Extrathalamic modulation of cortical function. Annu Rev Neurosci 1987; 10: 67–95. [DOI] [PubMed] [Google Scholar]
- 78.Van-Bockstaele EJ, Aston-Jones G.. Integration in the ventral medulla and coordination of sympathetic, pain and arousal functions. Clin Exp Hypertens 1995; 17: 153–165. [DOI] [PubMed] [Google Scholar]
- 79.Ziegler DR, Cass WA, Herman JP.. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J Neuroendocrinol 1999; 11: 361–369. [DOI] [PubMed] [Google Scholar]
- 80.Davies E, Keyon CJ, Fraser R.. The role of calcium ions in the mechanism of ACTH stimulation of cortisol synthesis. Steroids 1985; 45: 543–548. [DOI] [PubMed] [Google Scholar]
- 81.Basso JC, McHale A, Ende V, et al. Brief, daily meditation enhances attention, memory, mood, and emotional regulation in non-experienced meditators. Behav Brain Res 2019; 1: 208–220. [DOI] [PubMed] [Google Scholar]
- 82.Turakitwanakan W, Mekseepralard C, and Busarakumtragul P.. Effects of mindfulness meditation on serum cortisol of medical students. J Med Assoc Thai 2013; 96: 90–95. [PubMed] [Google Scholar]
- 83.D’Aquili EG, Newberg AB.. Religious and mystical states: A neuropsychological model. Zygon 1993; 28: 177–200. [Google Scholar]
- 84.Peng CK, Meitus JE, Liu Y, et al. Exaggerated heart rate oscillations during two meditation techniques. Int J Cardiol 1999; 70: 101–107. [DOI] [PubMed] [Google Scholar]
- 85.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 2004; 10: 1216–1221. [DOI] [PubMed] [Google Scholar]
- 86.Tracey KJ. The inflammatory reflex. Nature 2002; 420: 853–859. [DOI] [PubMed] [Google Scholar]
- 87.Tyrer P, Salkovskis P, Tyrer H, et al. Cognitive-behaviour therapy for health anxiety in medical patients (CHAMP): A randomized controlled trial with outcomes to 5 years. Health Technol Assess 2017; 21: 1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


