Abstract
Objective
The purpose of this study was to explore the possible role of pressure biofeedback in the early activation of quadriceps following lower limb orthopedic surgeries.
Methods
This was a proof of concept clinical trial. A single-blinded randomized controlled study was conducted on 24 patients who underwent lower limb surgeries. The experimental group received standardized physiotherapy treatment in addition to pressure biofeedback for quadriceps retraining. The control group received a standardized physiotherapy treatment developed for an inpatient orthopedic setting. The motor unit action potential (MUAP) duration and amplitude were measured using electromyography on the second and sixth postoperative days.
Results
Twenty-four participants, with a mean age for the control group of 58.67 ± 17.21 and the experimental group of 40.1 ± 6.96, were enrolled. No statistical significance in MUAP amplitude for within the groups (F[5,85] = 1.735, P = .135) was found. However, there was a statistical significance between the control and experimental groups in amplitude measured by electromyography (F[1,17] = 49.09, P < .01). There was no statistical significance in MUAP duration for within the groups (F[5,85] = 1.303, P = .270). However, there was a statistical significance in duration (F[1,17] = 71.84, P < .01).
Conclusion
Pressure biofeedback was more effective in the early activation of quadriceps muscle when coupled with conventional exercises compared with conventional exercises alone following lower limb orthopedic surgeries. Early activation of quadriceps could be a contributing factor to preventing arthrogenic muscle inhibition.
Key Indexing Terms: Muscle Strength; Biofeedback, Psychology; Electromyography
Introduction
According to the World Health Organization, road traffic accidents are the 11th leading cause of death, with the greatest burden on middle- and low-income countries.1 The lower limb is the commonest site of traumatic injury following a road traffic accident. Lower limb involvement (59.91%) was reported to be much higher compared with upper limb involvement (30.66%).2 Surgical intervention or trauma to the lower limb may result in the development of persistent quadriceps weakness, which may be caused by arthrogenic muscle inhibition (AMI) or pain and unwillingness to move.3
Arthrogenic muscle inhibition is the muscle's inability to attain complete contraction even though no damage has occurred to the underlying muscle or its innervating nerve. It leads to quadriceps atrophy and prevents the effective strengthening process. The mechanism for this inhibition includes alteration in muscle resting motor thresholds, changes in discharge of articular sensory receptors, altered spinal reflex excitability, abnormal cortical activity, a requirement of greater frontal cortex theta power in basic movement, and joint position sense tasks.4
Arthrogenic muscle inhibition, though a protective mechanism, becomes a huge hindrance during rehabilitation. 5 It can further lead to extension deficit, gait abnormality, dynamic instability, and persistent pain and can precipitate early osteoarthritis.6, 7, 8, 9, 10 Current joint rehabilitation programs recommend active exercise, which is essential for decreased healing time, increased vascular ingrowth, quicker regeneration of scar tissue, and stronger ligament and tendon healing. This is hindered by the patient's inability to contract surrounding musculature due to AMI.11
Recent clinical studies have recommended therapeutic interventions that improve the voluntary activation of the quadriceps.4,12 Studies have demonstrated the superiority of isometric quadriceps exercises in increasing the strength of the quadriceps muscle. Due to faulty execution, such as compensatory movements using surrounding muscles and faulty techniques, the expected outcome may vary while performing the exercises. Thus, the therapist needs to make sure that it is a tissue-specific procedure.13 One of the methods used to achieve this can be through a biofeedback technique.
Biofeedback is a technique of providing biological information to patients in real-time.14 Electromyography (EMG) biofeedback has been proven to facilitate the recovery of quadriceps by gaining greater voluntary control either by neuromuscular relaxation or by muscle re-education following injury.15 However, due to its high cost and sophisticated nature, it may not be possible in a routine clinical setting.
Pressure biofeedback is simple and inexpensive and may aid in retraining muscle activity and providing visual feedback. Visual feedback plays an important role in gaining the patient's interest and cooperation and shaping their behavior. It helps formulate threshold goals and encourages voluntary control of muscles through active constant visual feedback.16 It has been used effectively for training deep cervical flexor muscles and maintaining neck mobility and endurance.17 Training with biofeedback after total knee arthroplasty has shown remarkable improvements in terms of gait symmetry, pain reduction, and increase in activity level.18 The techniques used with EMG biofeedback are performed passively, where patient participation or motivation is less. For any treatment to be termed as effective, there should be active participation from the patient. Considering the literature available, even though it is an established fact that AMI is a huge hindrance to rehabilitation, measures for early prevention are seldom taken. Studies have demonstrated how quadriceps activation can help overcome AMI. However, there is a dearth of literature on how to prevent AMI. Therefore, the purpose of our study was to assess the role of pressure biofeedback in the early activation of quadriceps to prevent AMI for patients who have had lower limb orthopedic surgeries
Our hypothesis was that pressure biofeedback would be effective in the early activation of quadriceps to prevent AMI following lower limb orthopedic surgeries.
Methodology
Study Design
A proof of concept clinical study was conducted in the orthopedics inpatient ward at a tertiary hospital in Mysuru, India. The study was conducted in 2 parts. Part 1 was further divided into 2 sections. Part 1 consisted of validating the tools and standardization of the procedure before the commencement of the study. Part 2 consisted of a randomized control study. Both parts were conducted between April 2018 and December 2018.
Ethics
The study was approved by the JSS Medical College Institutional Ethical Committee (JSSMC/IEC/3107/10 NCT/2018-19). Patients consented to participating in this study.
Part 1: Validation and Standardization of Procedure
Muscle Activity Using EMG Instrument
Ten participants who were not part of the study were included in the EMG instrument's validation procedure. The participants were asked to lie in a supine position on a plinth. Before placing the electrodes, the participant's limb was exposed with consent, and the overlying skin was cleaned with Sterillium (Paul Hartmann AG, Heidenheim an der Brenz, Germany) to reduce skin resistance. Surface EMG (NeuroStim-NS 2, version 1.1, Medicaid, Mohali Punjab, India) was used for this study. EMG sensors were attached to the skin overlying the muscle belly using adhesive tape. Recording sensors were placed in line with the assumed direction of the underlying muscle fibers according to Surface Electromyography for the Non-Invasive Assessment of Muscles guidelines. An attempt to record the EMG activity of vastus medialis was made with the help of the EMG surface electrodes.
Positioning and Standardization of Pressure Biofeedback Device
Ten participants not included in the study were used for attaining the baseline value of the pressure biofeedback device. The participants were asked to lie in a supine position on the plinth while their knee joints rested on a flat wooden board. The wooden board was placed to prevent pressure absorption by the plinth underneath, following which the pressure biofeedback device was placed under the knee joint. The device was then inflated to 30 mmHg. Following this, a universal goniometer was used to measure if there was any amount of knee flexion. If the flexion angle was measured to be more than 5°, the pressure was reduced, and knee flexion measurement was retaken until it recorded knee flexion to be less than 5°. Participants were then asked to press the knee in the downward direction on the feedback device with a minimal contraction of the muscle where the participant had to press down on the device until there was an increase of pressure up to 8 mmHg tightening the muscle. Following this, they were asked to maintain the same pressure for 10 seconds.
For the second trial, the participant was asked to press the knee joints in a downward direction onto the device with moderate contraction, pressing down and increasing the pressure up to 16 mmHg by pulling the patella upwards without causing the lower limb movement. The maximum contraction was noted if the value was above 16 mmHg from the baseline.
For the third trial, the participant had to press the knee in the downward direction onto the device with maximal contraction where the participant could lift the knee to the terminal extension. This procedure was repeated three times for both the lower limbs. Then the participants were given a rest period of 10 minutes to avoid fatigue that could influence the recordings. Then the procedure was repeated 3 times for both limbs. Two trials were given to the participants before the commencement for a better understanding of the technique. While the participants were undergoing the trial, therapist supervision ensured that compensatory activities like hiking of the pelvis or plantar flexion did not occur. The readings from the pressure biofeedback device were recorded.
Part 2
Randomization
Convenience sampling was employed using specific criteria for this randomized controlled study. Allocation to groups was achieved by the block randomization method. Nine blocks of 8 chits, each with a control and experimental group, were generated and ordered using computer-generated random numbers. The patients were allocated to the groups accordingly. The allocation was performed by a person blinded to the study. Randomized participants received all physiotherapy procedures according to the interventions they were allocated. Study investigators, treating physiotherapists, orthopedic surgeons, consultants, and participants’ families were blinded to treatment allocation.
Study Population
Patients from the inpatient orthopedic ward were screened for inclusion in the study. The participants included in the study were patients who had undergone surgeries of the lower limb (elective and non-elective surgeries of the hip and knee joint. Participants were excluded if they showed signs of cognitive impairments and an inability to understand simple standard instructions. Patients with immobilized knee, associated nerve injuries, external fixators, or an above-knee plaster cast were excluded. The sample size was generated to be 24 participants by using G*power 3.1.9.2 software (Kiel University), with the effect size f = 0.9, α = 0.05, β = 0.66, number of groups = 2, number of measurements = 1, allocation ratio = 1, and noncentrality parameter = 2.0410082.
Study Interventions
The procedure was explained to the patients, and informed consent was obtained, after which the participants were allocated to experimental and control groups as per the randomization. Electromyography of the vastus medialis oblique muscle during isometric quadriceps was recorded by an assessor blinded to group allocation before the commencement of treatment on postoperative day 2 for all participants. Traditional physiotherapy care, which focuses on early range of motion muscle training and strategies to prevent postoperative complications, such as the reduction of edema and chest care, was given to the control group. The standard exercise regimen was the regular physiotherapy intervention after lower limb fracture fixation in acute care, followed by the hospital. The exercises were based on the current practice guidelines (2018), which were framed by the expertise as standard care pathways (Table 1).11 The exercises were as per the “care pathway” specific to the fracture, which has been developed and used as the standard care in the physiotherapy department where the study was conducted. The care pathway was framed by the team of expertise based on the current practice guidelines (2017-2018).11
Table 1.
Standard Exercises Regime of Control and Experimental Groups
| Part and Timeline | Exercise Guidelinesa | Parameters, FITT |
|---|---|---|
| Part 1 Days 0-6 |
|
10 repetitions × 3 sets × 2 times in a day |
|
10 repetitions × 3 sets × 2 times in a day; active assisted isotonic exercises | |
| Part 2 Day 7-21 |
|
10 repetitions × 3 sets × 2 times in a day; active assisted progressed to active |
FITT, frequency, intensity, time, and type.
Protocol and intervention are based on the evaluation, muscle power, and mobility status with the goal to progress to independence (with or without assistance).
The experimental group underwent the established protocol and additional pressure biofeedback-based therapy along with the standardized exercise regime (Table 1). To control for the role of therapy time, the duration and frequency of therapy were kept standard for both groups.
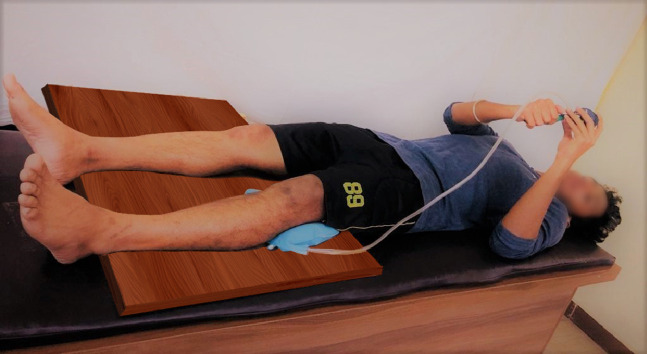
While using the pressure biofeedback device, the participant was made to lie supine on a plinth. A wooden board was placed under the patient's lower limb to maintain the effect of pressure biofeedback and to achieve an optimal force. The pressure biofeedback was positioned under the knee joint, as shown in Figure 1.
Fig 1.
Standardization of pressure biofeedback position.
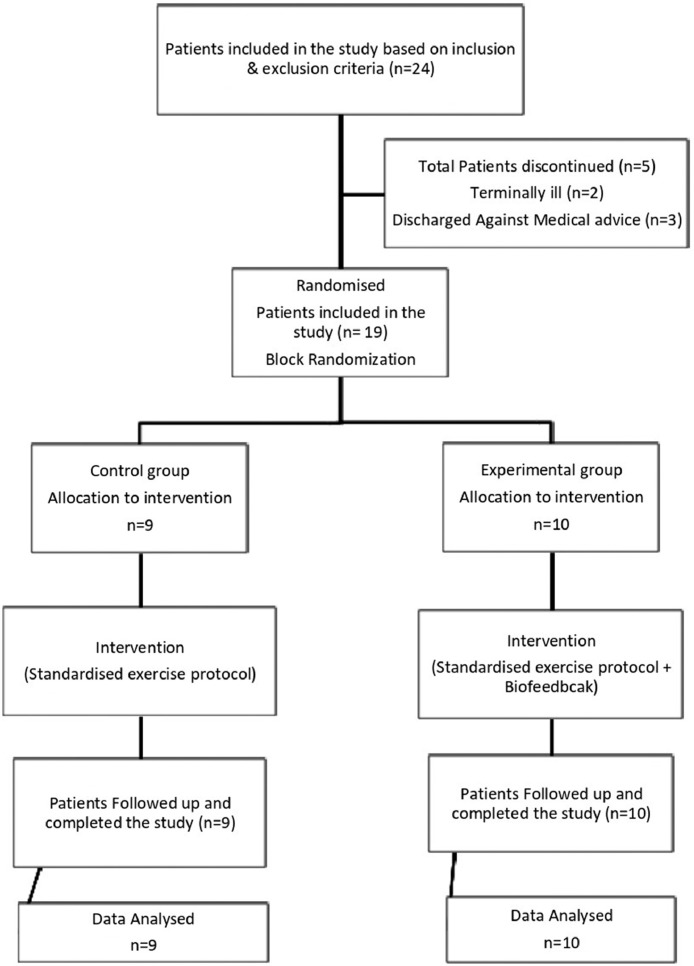
The device was inflated to the baseline value of 30 mmHg. The participant was given 2 trials on the unaffected knee joint for a better understanding of the technique. For the starting position, the participant was instructed to push their knee in a downward direction against the cuff as strongly as possible by contracting the quadriceps muscle. The participants were given feedback to achieve a visible contraction of the quadriceps, including the vastus medialis oblique. The participants were asked to maintain this pressure for 10 seconds before they returned to their initial position. The peak pressure shown on the pressure biofeedback was noted. To prevent the participant from performing the Valsalva maneuver, the technique was instructed to be performed during the expiration phase. The participant was then given a 1-minute break before the next attempt. The training was performed in addition to the daily standard rehabilitation program. The protocol was followed from postoperative day 2 until postoperative day 6 for participants in both groups (Table 2). Electromyography of the vastus medialis muscle during isometric quadriceps recorded by an assessor blinded to group allocation at the cessation of treatment on postoperative day 6 for all participants. The flowchart of participants included in the study is as given in Fig. 2.
Table 2.
Mean Age of Participants
| Men | Women | Mean Age ± Standard Deviationa | Total number, n | |
|---|---|---|---|---|
| Recruitment | 22 | 2 | 47.62 ± 14.49 | 24 |
| Control | 8 | 1 | 58.67 ± 17.21 | 9 |
| Experimental | 9 | 1 | 40.10 ± 6.96 | 10 |
Mean age in years.
Fig 2.
Flowchart of participants in the study.
Data Analysis
Data were analyzed using SPSS Statistics for Windows (version 20.0, IBM Corp, Armonk, NY). Descriptive statistics were used to report the demographic characteristics. Tests of normality were conducted to ascertain data distribution. Surface EMG was analyzed using repeated-measures analysis of variance. The level of significance for all analyses was set at P ≤ .01.
Results
Table 2 depicts the demographics of the participants included in the study. Table 3 mentions details of the participants along with their diagnosis.
Table 3.
Diagnosis and Surgical Management of Participants
| Diagnosis and Surgical Intervention of Participants | Control Group, n = 9 | Experimental Group, n = 10 |
|---|---|---|
| Fractures of femur: ORIF with T buttress plate and screws, CRIF with long PFN | 6 | 4 |
| Fracture around the knee joint: ORIF with tension band wiring | 1 | 2 |
| ACL ligament injuries: ACL reconstruction with semitendinosus graft | 1 | 1 |
| Fractures of tibia: implant removal, ORIF with a condylar plate, CRIF with an intramedullary interlocking nail | 1 | 3 |
ACL, anterior cruciate ligament; CRIF, closed reduction and fixation rotation; ORIF, open reduction and internal fixation; PFN, proximal femur nail.
Part 1
Part 1 included the standardization of muscle activity using EMG. The values of pressure measured through Pressure biofeedback for baseline, mild, moderate, and maximum are depicted in Table 4.
Table 4.
Standardized Pressure Biofeedback Values for Different Strengths of Contraction
Pressure measured in millimeter of mercury (mmHg).
Pressure measured in millimeter of mercury (mm Hg) presented in range.
Part 2
Results of EMG Measurement
The amplitude and duration of the MUAP of the vastus medialis muscle were recorded on postoperative day 2 and postoperative day 6 using surface EMG. A repeated measures analysis of variance with a Mauchly's test for sphericity (0.24) was used in this study for both duration in milliseconds and amplitude in microampere (Table 5, Table 6, Table 7 and 8).
Table 5.
Repeated Measures ANOVA for Amplitude
| Amplitude | Df | Mean Square | F Value | Significance P Value |
|---|---|---|---|---|
| Between-group | 1 17 |
7 726 434.505 | 49.09 | <.001 |
| Within-group | 5 85 |
125 713.466 | 1.735 | .135 |
ANOVA, analysis of variance; Df, degrees of freedom.
Table 6.
Repeated Measures ANOVA for Duration
| Duration | Df | Mean square | F Value | Significance P Value |
|---|---|---|---|---|
| Between-group | 1 17 |
2493.880 | 71.8 | <.001 |
| Within-group | 5 85 |
14.49 | 1.3 | .270 |
ANOVA, analysis of variance.
Table 7.
Comparison of MUAP Amplitude of Groups at Baseline Measurement and on POD 6
| Amplitudea | Group n = 19 | Pre Mean ± Standard Deviation | Post Mean ± Standard Deviation | Mean Difference |
|---|---|---|---|---|
| Mild contraction | Control | 393.4 ± 435.537 | 395.67 ± 421.17 | 2.27 |
| Experimental | 155.5 ± 335.70 | 360.9 ± 229.24 | 205.4 | |
| Moderate contraction | Control | 225.6 ± 287.9 | 221 ± 239.9 | –4 |
| Experimental | 147.5 ± 304.8 | 274.9 ± 236.41 | 127.4 | |
| Maximum contraction | Control | 210 ± 175.6 | 185.1 ± 150.7 | –24.9 |
| Experimental | 125.3 ± 237.6 | 433.4 ± 335.5 | 308.1 |
MUAP, motor unit action potential; POD, postoperative day.
Amplitude measured in microvolts (µV).
Table 8.
Comparison of MUAP Duration of Groups at Baseline Measurement and on POD 6
| Durationa | Group | Pre Mean ± Standard Deviation | Post Mean ± Standard Deviation | Mean Difference |
|---|---|---|---|---|
| Mild contraction | Control | 4.14 ± 4.03 | 7.6 ± 4.7 | 3.46 |
| Experimental | 1.9 ± 3.2 | 5.6 ± 3.2 | 3.7 | |
| Moderate contraction | Control | 4.1 ± 4.15 | 4.69 ± 4.64 | 0.59 |
| Experimental | 1.4 ± 2.73 | 5.5 ± 4.25 | 4.1 | |
| Maximum contraction | Control | 5.6 ± 4.2 | 6.5 ± 3.7 | 0.9 |
| Experimental | 2.03 ± 3.5 | 6.8 ± 3.6 | 4.77 |
MUAP, motor unit action potential; POD, postoperative day.
Duration measured in milliseconds.
There was no statistical significance in MUAP amplitude within the groups (F[5,85] = 1.735, P = .135). However, there was a statistical significance between the control and experimental group in MUAP amplitude measured by EMG (F[1,17] = 49.09, P < .01).
There was a statistical significance in MUAP mean amplitude in the experimental group (mean difference = 205.4) compared to the control group (mean difference = 2.27) postintervention. The experimental group (mean difference = 127.4) showed better mean amplitude for moderate contraction than the control group (mean difference = –4) postintervention. The experimental group (mean difference = 308.1) also exhibited better mean amplitude for maximum contraction than the control group (mean difference = –24.9). Even though there was an improvement in the amplitude clinically within the groups, it was not statistically significant. There was no statistically significant difference in MUAP duration within the groups (F[5,85] = 1.303, P = .270. However, there was a statistical significance between the control and experimental group in duration measured by EMG F[1,17] = 71.84, P < .01. The MUAP mean duration difference was negligible (0.24). The experimental group showed better results in the mean duration (4.1) for moderate contraction than the control group (0.59). The experimental group (4.77) also exhibited a better mean duration for maximum contraction than the control group (0.9). Even though there was an improvement in the duration clinically within the groups, it was not statistically significant. This trend could have occurred since both groups were not equal at the baseline. The control group started off having higher baseline values when compared to the experimental group. Hence, this can be a regression of the mean. No serious complications were reported by any individual following the intervention to either group, and the participants tolerated the intervention well.
Discussion
Arthrogenic muscle inhibition of the quadriceps can severely restrict normal knee functioning and thereby hamper lower extremity biomechanics. The inability to extend the knee often leads to compensatory strategy adoption by the hip and ankle joints to maintain functionality. The motor cortex area that represents the quadriceps may be partially invaded by the hip and ankle musculature. Compensatory strategies thus used can lead to learned nonusage of the quadriceps muscle. This type of compensatory behavior results in maladaptive plasticity, hindering the recovery of quadriceps function.19 To overcome this, our study aimed to explore the effectiveness of pressure biofeedback in the early activation of quadriceps following lower limb orthopedic surgeries. Further, we also attempted to standardize the procedure of using pressure biofeedback for quadriceps training to overcome AMI. The use of pressure biofeedback was focused on enhancing the normal movement by altering involuntary muscle contraction and specifically contracting the targeted muscle—in this case, the vastus medialis muscle.
We found a statistical significance between the control and experimental groups in terms of amplitude and duration of the MUAP. Since the assumption sphericity (0.24) was met, we did not look at the Greenhouse-Geisser correction. The mean difference in duration and amplitude component of EMG recorded in the experimental group showed better outcomes than the control group. This is in line with literature that supports the relationship of MUAP with anatomical phenomena, where an increase in amplitude and duration is a result of an increased number of muscle fibers and grouping.19
One of the major factors that may have helped achieve a significant change in the activation of the vastus medialis muscle of participants enrolled in the experimental group was the pressure biofeedback's real-time information. Although the participant may not have been able to see their quadriceps muscle produce a visible muscle contraction, the use of pressure biofeedback provided them with an alternative method. This method helps the participants quantify their efforts for muscle activation by providing an easy output of numerical origin. This alternative method of quantification helps the participants consciously adjust and monitor their motor output by setting goals that are realistic and achievable.13,20
A combination of exercise and training with pressure biofeedback may have targeted cortical-level mechanisms responsible for muscle force generation. It is well established that when muscular force is produced, neuronal activity in the motor cortex increases.21 When visual feedback is further added to the movement-oriented task, neuronal activity and muscular production of force are collectively increased.22 This may explain the additional effect of the pressure biofeedback in activating the quadriceps muscle obtained in our study.
Additionally, the requirement of active attention brought about by the training may have helped reduce the cortical and neuromuscular changes caused due to AMI by improving the release of modulatory neurotransmitters. This is in line with literature that states that an individual should be attentively engaged, and the exercise performed should be meaningful for them to maximize neuroplasticity.23
Activity-dependent plasticity cannot be maximized with just repetitive exercises. Pressure biofeedback is one such modality when used in conjunction with therapeutic exercise, which boosts activity-dependent plasticity. Providing the participants with a way to observe the quadriceps activation when it is unfelt helps to focus their attention while also providing them with motivation aided by the presence of real-time feedback.24 We assume that the attention-demanding activity-dependent plasticity enhanced by the training may have helped re-establish the topographical representation of quadriceps in the motor cortex, which is assumed to be altered by AMI. This could be why the experimental group had a significant improvement in terms of muscle activation compared to the group that received only conventional exercises.
Strengths of the Study
The study's strength was that the procedure was standardized before the trial's initiation to improve the research rigor and categorize the contraction strength range through pressure biofeedback. The blinding of the assessor to the random allocation and intervention helped to avoid bias.
Limitations and Future Studies
This preliminary study included a small sample size. A larger sample size study should be conducted to affirm these findings further. Another setback faced in the study was that the outcome measures were initially planned to be measured on postoperative days 1 and 5. However, this was not possible since the removal of wound dressing was not feasible due to the chances of infection. Also, the patient's mobilization following the surgery was not initiated equally for the patients since weight-bearing status was not only based on the treating therapist's discretion but also on the orthopedic surgeon.
Future research can be conducted where quadriceps lag is considered along with the EMG recordings to correlate and find the effect of early quadriceps activation using pressure biofeedback to reduce quadriceps lag.
Conclusion
In this proof of concept clinical trial, pressure biofeedback was more effective in the early activation of quadriceps muscle when coupled with conventional exercises compared with conventional exercises alone following lower limb orthopedic surgeries. This early activation of the quadriceps could be a contributing factor in preventing AMI.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): J.K.J., V.S.R., J.T.A.
Design (planned the methods to generate the results): J.K.J., V.S.R.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): J.K.J., V.S.R.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): J.T.A.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): V.S.R., J.T.A.
Literature search (performed the literature search): J.T.A.
Writing (responsible for writing a substantive part of the manuscript): J.T.A.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): V.S.R., J.K.J.
Practical Applications.
-
•
This study explored the possible role of pressure biofeedback in the early activation of quadriceps following lower limb orthopedic surgeries.
-
•
We found a statistical significance between the control and experimental group in terms of amplitude and duration of the motor unit action potential.
-
•
Pressure biofeedback was more effective in the early activation of quadriceps muscle when coupled with conventional exercises compared with conventional exercises alone following lower limb orthopedic surgeries.
Alt-text: Unlabelled box
References
- 1.World Health Organization. Global status report on road safety 2018. (2022). Available at: https://www.who.int/publications-detail-redirect/9789241565684. Accessed July 9, 2022.
- 2.Bhaskara K, Padmanabha TS, Nandini T. Pattern of fractures and dislocations in a tertiary care hospital. Int J Med Res Health Sci. 2014;3(4):847–850. [Google Scholar]
- 3.Hart JM., Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45:87–97. doi: 10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harkey MS, Gribble PA, Pietrosimone BG. Disinhibitory interventions and voluntary quadriceps activation: a systematic review. J Athl Train. 2014;49(3):411–421. doi: 10.4085/1062-6050-49.1.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinzón Ríos ID. Physiotherapy in arthrogenic muscle inhibition: literature review. Rev Med Risaralda. 2019;25(2):115–128. [Google Scholar]
- 6.Guerra-Pinto F, Thaunat M, Daggett M, et al. Hamstring contracture after ACL reconstruction is associated with an increased risk of cyclops syndrome. Orthop J Sports Med. 2017;5(1) doi: 10.1177/2325967116684121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas AC, Wojtys EM, Brandon C, Palmieri-Smith RM. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J Sci Med Sport. 2016;19(1):7–11. doi: 10.1016/j.jsams.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konishi Y, Oda T, Tsukazaki S, Kinugasa R, Fukubayashi T. Relationship between quadriceps femoris muscle volume and muscle torque at least 18 months after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2012;22(6):791–796. doi: 10.1111/j.1600-0838.2011.01332.x. [DOI] [PubMed] [Google Scholar]
- 9.Lindström M, Strandberg S, Wredmark T, Felländer-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431–442. doi: 10.1111/j.1600-0838.2011.01417.x. [DOI] [PubMed] [Google Scholar]
- 10.Segal NA, Glass NA, Torner J, et al. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage. 2010;18(6):769–775. doi: 10.1016/j.joca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnery-Cottet B, Saithna A, Quelard B, et al. Arthrogenic muscle inhibition after ACL reconstruction: a scoping review of the efficacy of interventions. Br J Sports Med. 2019;53(5):289–298. doi: 10.1136/bjsports-2017-098401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum. 2010;40(3):250–266. doi: 10.1016/j.semarthrit.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Anwer S, Alghadir A. Effect of isometric quadriceps exercise on muscle strength, pain, and function in patients with knee osteoarthritis: a randomized controlled study. J Phys Ther Sci. 2014;26(5):745–748. doi: 10.1589/jpts.26.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giggins OM, Persson UM, Caulfield B. Biofeedback in rehabilitation. J Neuroeng Rehabil. 2013;10(1):60. doi: 10.1186/1743-0003-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepley AS, Gribble PA, Pietrosimone BG. Effects of electromyographic biofeedback on quadriceps strength: a systematic review. J Strength Cond Res. 2012;26(3):873–882. doi: 10.1519/JSC.0b013e318225ff75. [DOI] [PubMed] [Google Scholar]
- 16.Robertson V, Ward A, Low J, Reed A. 4th ed. United Kingdom: Butterworth-Heinemann; London: 2006. Electrotherapy Explained: Principles and Practice. [Google Scholar]
- 17.Kang DY. Deep cervical flexor training with a pressure biofeedback unit is an effective method for maintaining neck mobility and muscular endurance in college students with forward head posture. J Phys Ther Sci. 2015;27(10):3207–3210. doi: 10.1589/jpts.27.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeufer D, Gililland J, Böcker W, Kammerlander C, Anderson M, Krähenbühl N, Pelt C. Training with biofeedback devices improves clinical outcome compared to usual care in patients with unilateral TKA: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2019;27(5):1611–1620. doi: 10.1007/s00167-018-5217-7. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Carreño I, Gila-Useros L, Malanda-Trigueros A. In: Advances in Clinical Neurophysiology. Ajeena I, editor. IntechOpen; London, United Kingdom: 2012. Motor unit action potential duration: measurement and significance. [Google Scholar]
- 20.Dursun N, Dursun E, Kiliç Z. Electromyographic biofeedback–controlled exercise versus conservative care for patellofemoral pain syndrome. Arch Phys Med Rehabil. 2001;82(12):1692–1695. doi: 10.1053/apmr.2001.26253. [DOI] [PubMed] [Google Scholar]
- 21.Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136(4):431–438. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- 22.Rearick MP, Johnston JA, Slobounov SM. Feedback-dependent modulation of isometric force control: an EEG study in visuomotor integration. Brain Res Cogn Brain Res. 2001;12(1):117–130. doi: 10.1016/s0926-6410(01)00040-4. [DOI] [PubMed] [Google Scholar]
- 23.Moucha R, Kilgard MP. Cortical plasticity and rehabilitation. Prog Brain Res. 2006;157:111–389. doi: 10.1016/s0079-6123(06)57007-4. [DOI] [PubMed] [Google Scholar]
- 24.Gabler C, Kitzman PH, Mattacola CG. Targeting quadriceps inhibition with electromyographic biofeedback: a neuroplastic approach. Crit Rev Biomed Eng. 2013;41(2):125–135. doi: 10.1615/critrevbiomedeng.2013008373. [DOI] [PubMed] [Google Scholar]