Abstract
Recently, we reported the purification to homogeneity and characterization of Ca2+- and Mg2+-dependent endonuclease P40 produced by Mycoplasma penetrans (M. Bendjennat, A. Blanchard, M. Loutfi, L. Montagnier, and E. Bahraoui, J. Bacteriol. 179; 2210–2220, 1997), a mycoplasma which was isolated for the first time from the urine of human immunodeficiency virus-infected patients. To evaluate how this nuclease could interact with host cells, we tested its effect on CEM and Molt-4 lymphocytic cell lines and on peripheral blood mononuclear cells. We observed that 10−7 to 10−9 M P40 is able to mediate a cytotoxic effect. We found that 100% of cells were killed after 24 h of incubation with 10−7 M P40 while only 40% cytotoxicity was obtained after 72 h of incubation with 10−9 M P40. Phase-contrast microscopy observations of P40-treated cells revealed morphological changes, including pronounced blebbing of the plasma membrane and cytoplasmic shrinkage characteristic of programmed cell death, which is in agreement with the internucleosomal fragmentation of P40-treated cell DNA as shown by agarose gel electrophoresis. We showed that 125I-radiolabeled or fluorescein isothiocyanate-labeled P40 was able to bind specifically in a dose-dependent manner to the cell membrane of CEM cells, which suggested that the cytotoxicity of P40 endonuclease was mediated by its interaction with the cell surface receptor(s). The concentration of unlabeled P40 required to inhibit by 50% the formation of 125I-P40-CEM complexes was about 3 × 10−9 M, indicating a high-affinity interaction. Both P40 interaction and cytotoxicity are Ca2+ dependent. Our results suggest that the cytotoxicity of M. penetrans observed in vitro is mediated at least partially by secreted P40, which, after interaction with host cells, can induce an apoptosis-like death. These results strongly suggest a major role of mycoplasmal nucleases as potential pathogenic determinants.
Mycoplasmas, the smallest self-replicating organisms, are prokaryotes which are parasites of a large variety of animal and plant species (reviewed in reference 19). Several mycoplasma species have been isolated in humans, including Mycoplasma fermentans, M. genitalium, M. hominis, M. pneumoniae, and Ureaplasma urealyticum. Some of these mollicutes are genuinely associated with a pathologic condition, but most seemingly constitute a part of the normal human microflora (reviewed in references 33 and 35). M. penetrans is the latest mycoplasma to be isolated from humans; it was first isolated from the urine of patients infected by human immunodeficiency virus (HIV) (17). This mycoplasma can enter the cells it infects in vitro, hence its name (2, 16), and subsequently can develop a cytopathic effect in the parasitized cells (11, 18). Epidemiological studies by enzyme-linked immunosorbent assay and Western blotting revealed a relatively high frequency of anti-M. penetrans antibodies in HIV-infected homosexual patients. A total of 18% of HIV seropositive patients clinically asymptomatic and at least 35% of those developing AIDS were seropositive for M. penetrans, whereas this prevalence was less than 2% in all persons not infected by HIV (12, 51). There is currently no particular pathology associated with infection by M. penetrans, although an epidemiological study has suggested a link between the presence of anti-M. penetrans antibodies and a faster progression of HIV disease (13). Recently, there was a report describing a female patient infected by M. penetrans who was seronegative for HIV and who presented a primary antiphospholipid syndrome. This is the first case associating a pathological condition with M. penetrans infection; however, a cause-and-effect relationship remains to be shown (53).
The cytopathogenicity of mycoplasmas is believed to involve, at least in vitro, the production of different factors including exotoxins (6, 50), phospholipases (41, 43, 45), immunoglobulin A proteases (38, 48), ureases (26, 42), membrane hemolysins (23), and/or oxidative free radicals (7, 47). However, there are few studies that clearly link a specific activity (other than adherence or adherence-related proteins) to virulence in mycoplasmas, primarily because of the lack of genetic systems and the difficulty in identifying well-defined mutants lacking these activities. Mycoplasma nucleases were first reported by Razin et al. (37). These enzymes have been suggested to be involved in DNA repair and recombination and in restricting foreign DNA. In addition, several authors have reported that the nucleic acids of host cells may be targets for soluble nucleases secreted into the extracellular medium and/or bound to mycoplasma membranes. Indeed, these bacteria are deficient in nucleotide biosynthesis pathways. Their nuclease activities, on the other hand, are developed as a means of producing the nucleic acid precursors required for their metabolism by digesting the DNA and RNA of the cells they parasitize (24, 25, 34, 36). As a consequence, we and others have shown that nucleases produced by contaminating mycoplasmas were responsible for the apparent absence of reverse transcriptase activity in the supernatants from HIV-producing cell lines (31, 44). While many mycoplasmas produce extracellular nucleases, only a limited number of bacteria do (4). Furthermore, these nuclease activities of mycoplasmas were recently implicated in the induction of apoptosis characterized by the internucleosomal fragmentation of the chromatin of infected cells (27, 28, 46).
To determine the potential pathogenic role of mycoplasma nucleases, we studied the effect of M. penetrans endonuclease P40 on lymphocytes. We have recently shown that P40 is synthesized in the form of 50-kDa precursor (P50) and is secreted by the microorganism probably after processing of P50. This precursor is associated with the mycoplasmal membrane and is soluble only in the presence of detergent. P40 was purified to homogeneity, and its characterization showed that it is a Ca2+- and Mg2+-dependent endonuclease. When incubated with native nuclei extracted from human lymphocytes, the enzyme can diffuse into the nuclei and degrade chromatin into oligonucleosomal units similar to those observed in apoptotic cells (3). In this work, we extend our previous studies by presenting direct evidence of the capacity of purified M. penetrans endonuclease P40 to bind specifically to T lymphocytes and alter the cells by inducing physiological changes characteristic of apoptosis.
MATERIALS AND METHODS
Cell cultures.
CEM and Molt-4 lymphocytic cells lines were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (Flow, Irvine, Scotland), 2 mM glutamine, and 1% antibiotic stock solution (Gibco, Paisley, Scotland) in a humidified 5% CO2 atmosphere. Peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll-Hypaque gradient centrifugation of blood from HIV-seronegative donors, and cultured in complete RPMI 1640 medium. All cells were harvested in the exponential growth phase for use in experiments.
M. penetrans GTU-54-6A1 was cultured in PPLO broth containing 10% (vol/vol) heat-inactivated (56°C for 30 min) horse serum, 0.25% (wt/vol) glucose, and 0.002% phenol red (pH 7.8). The cultures were incubated statically at 37°C.
Purification of endonuclease P40.
Active endonuclease P40 was purified from M. penetrans by the method of Bendjennat et al. (3). Briefly, 40-kDa nuclease was extracted in aqueous phase from M. penetrans cells by Triton X-114 phase fractionation. Aqueous P40 was further purified by chromatography on Superdex 75 and chelating Sepharose (Zn2+ form) columns. The purity and activity of the endonuclease preparation were assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (silver nitrate staining) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis nuclease assay, respectively.
Cytotoxicity assay.
The cytotoxic activity of purified M. penetrans endonuclease was evaluated by the trypan blue dye exclusion test. Cells were seeded at a density of 104 cells per well in 96-well flat-bottom microtiter plates and incubated for the desired times at 37°C in culture medium, in the absence or presence of protein P40 (10−9 to 10−7 M). Survival was calculated as the percentage of the unstained cells. Percent cytotoxicity is the difference between control (100%) and the percent survival.
DNA extraction and electrophoresis.
Cells, previously treated or not with P40, were lysed on ice for 20 min in 5 mM Tris-HCl (pH 7.4) containing 0.5% Triton X-100 and 5 mM EDTA. The cells were then centrifuged at 12,000 × g for 30 min at 4°C to separate high-molecular-weight chromatin from nucleosomal DNA fragments. Supernatants were collected, incubated with proteinase K (100 μg/ml for 16 h at 37°C), and brought to 200 mM NaCl. Nucleic acids were precipitated with isopropanol, washed with 70% ethanol, resuspended in 10 mM Tris-HCl (pH 8)–10 mM EDTA, and treated with RNase (50 μg/ml for 4 h at 37°C). The DNA from the final step was separated by electrophoresis on 2% agarose gels in Tris-acetate-EDTA buffer and stained with ethidium bromide.
Iodination of endonuclease P40.
Endonuclease P40 was iodinated by a iodogen standard procedure. A 10-μg quantity of M. penetrans P40 in 100 μl of phosphate-buffered saline (PBS) (pH 7.4) and 0.5 mCi of Na125I (13 to 17 mCi/μg) were added to a tube precoated with 75 nmol of iodogen and incubated for 10 min at room temperature. The reaction was stopped by adding 10 μl of tyrosine (9 mg/ml). The iodinated protein was desalted from free Na125I by filtration through a Sephadex (G-25 PD10 column equilibrated with PBS–0.5% (wt/vol) bovine serum albumin (BSA). The specific radioactivity was about 45 μCi/μg.
Binding of M. penetrans endonuclease to CEM cells determined by the direct radiolabeling assay.
The assay was performed as follows. Cells were incubated with 100 μl of various preparations of iodinated protein in PBS (pH 7.4)–0.5% BSA at 37°C for the desired time. After the cells were washed twice with buffer, bound radioactivity was counted in a gamma counter. Inhibition of 125I-P40 binding to CEM cells by native protein was performed as described above, except that the cells were first incubated with native endonuclease P40 at 37°C for 10 min before the addition of 125I-P40 for the same length of time.
Binding of Endonuclease P40 analyzed by flow cytofluorometry.
P40 protein was solubilized in 250 μl of 100 mM sodium bicarbonate buffer (pH 9.5) and incubated in the dark with fluorescein isothiocyanate (FITC) for 2 h at 25°C. The reaction mixture was then loaded on a Sephadex G-25 PD10 column equilibrated with 0.5 M acetic acid. CEM cells (106) were incubated for 4 h at 37°C with different dilutions of P40 coupled to FITC in 100 μl of PBS (pH 7.4)–0.5% BSA. The cells were washed twice and fixed in 500 μl of 1% paraformaldehyde. The fluorescence intensity was measured with a fluorescence-activated cell sorter (Becton Dickinson). Inhibition of direct binding of P40 to CEM cells was assayed under the same conditions, except that the native P40 protein was first incubated with cells at 37°C for 10 min before the FITC-coupled P40 was added. The same field of cells were visualized by phase-contrast and fluorescence microscopy with the appropriate filters and photographed for presentation.
RESULTS
M. penetrans endonuclease P40-mediated cytotoxicity.
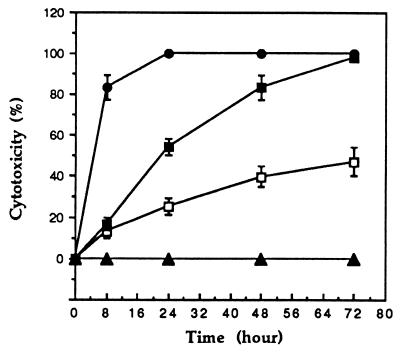
P40 cytotoxicity was tested on CEM and Molt-4 lymphocytic cell lines and on PBMCs by a standard trypan blue exclusion assay. The cells (104) were incubated with 10−9, 10−8, and 10−7 M P40, and the percent cytotoxicity, the difference between control (100%) and percent survival, was determined after 72 h. The results in Fig. 1 show that the cytotoxicity of P40 toward CEM cells is dose and time dependent. At a high P40 concentration (10−7 M), the maximum effect (100% cytotoxicity) was observed after 24 h. At 10−8 M, 100% cytotoxicity was obtained only after 72 h of incubation, whereas at 10−9 M, the percent cytotoxicity never increased beyond 45% even after 72 h of incubation.
FIG. 1.
Cytotoxicity of M. penetrans endonuclease P40 toward CEM cells. CEM cells were washed and seeded in 96-well plates (104 cells/200 μl of culture medium) and exposed at time zero to endonuclease P40 (0 M [▴], 10−9 M [□], 10−8 M [■], or 10−7 M [●]) at 37°C. Percent cytotoxicity was determined at the defined time points and is calculated as the difference between control (100%) and percent survival. Values are means ± standard errors from triplicate experiments.
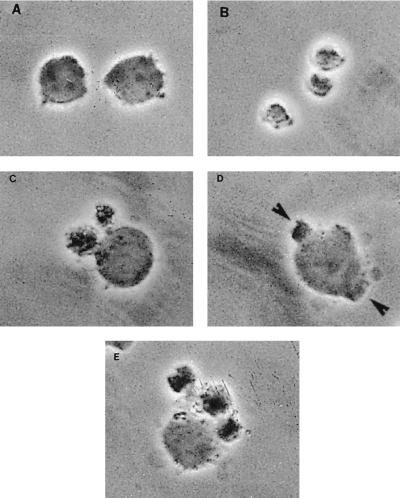
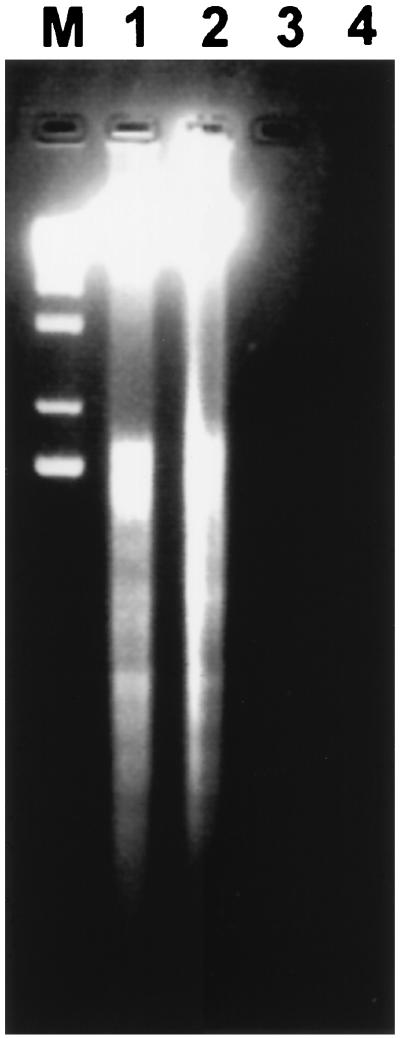
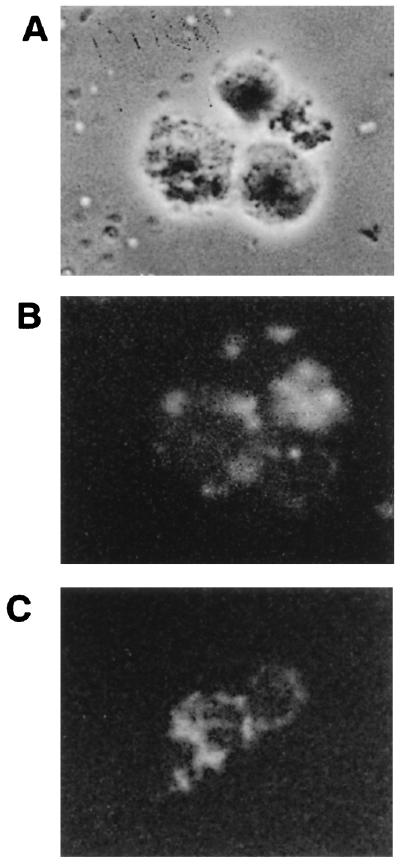
Morphological changes of treated cells were assessed by phase-contrast microscopy observation (Fig. 2). In comparison to untreated cells (Fig. 2A), whose morphology is normal and which have smooth membrane surfaces, phase images of P40-treated cells (Fig. 2B to E) reveal dramatic changes, including pronounced blebbing of the plasma membrane, cytoplasmic shrinkage characteristic of programmed cell death, and a significant loss of refractibility. The release of DNA from nuclei in nonionic detergent is defined as nuclear damage. Therefore, the detergent-soluble DNA released by P40 treatment was analyzed by agarose gel electrophoresis. As shown in Fig. 3 for CEM cells and PBMCs incubated with P40, such detergent-soluble DNA was strikingly fragmented in a characteristic nucleosomal ladder pattern (Fig. 3, lanes 1 [CEM] and 2 [PBMCs]). In cases where no morphological changes were observed (untreated cells), there was no DNA fragmentation. Detergent-insoluble DNA remaining in the pelleted nuclei showed little fragmentation (data not shown).
FIG. 2.
The effects of P40 protein on CEM cells in vitro were assessed by phase-contrast microscopy. CEM cells (104) were cultured in flat-bottom microtiter plates in the absence or presence of 10−8 M P40. After 24 h, photomicrographs were taken. (A) Negative control; (B to E) morphological changes of cells incubated in the presence of P40. Arrows indicate apoptosis-like bodies.
FIG. 3.
Endonuclease P40-induced internucleosomal DNA fragmentation. Small supernatant DNA after centrifugation of detergent-lyzed cells was separated on a 2% agarose gel. CEM cells (lane 1) and PBMCs (lane 2) (106) were incubated with P40 (10−8 M) for 24 h at 37°C in culture medium. Lanes 3 and 4 contain CEM and PBMC negative controls, respectively. Lane M contains the 1-kb DNA ladder used as a standard.
The same cytotoxic effect of P40 on Molt-4 cells and PBMCs was observed. Although there was a slight difference in the optimum concentration for inducing cytopathic effect, both cell alteration and nuclear damage were also dose and time dependent (data not shown).
Binding of P40 to CEM cells as studied by direct radiolabeling assay and direct FITC labeling.
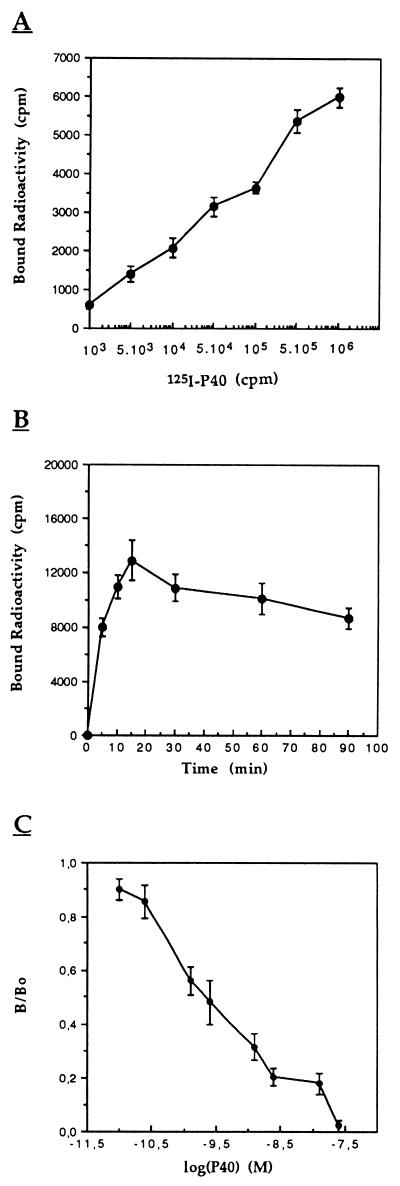
To determine if P40 cytotoxicity was mediated by a cell membrane-P40 interaction, we tested the binding capacity of iodinated P40 (125I-P40) to target cells. The results (Fig. 4A) clearly show that 125I-P40 bound in a dose-dependent manner to CEM cell when the binding assay was carried out with various concentrations of 125I-P40 and a constant cell number. Analysis of the time course and stability of binding indicated a peak of binding capacity at 15 min followed by a significant decrease, finally reaching a steady-state equilibrium (Fig. 4B). This time course of P40 binding was reproducible when different numbers of cells (1 × 106 to 8 × 108 cells) were incubated with a constant quantity of P40 (data not shown).
FIG. 4.
Direct radiolabeling assay analysis of 125I-P40 binding to CEM cells. (A) A 50-μl volume of cells (106) was incubated with 50 μl of various 125I-P40 dilutions (0 to 106 cpm; 0 to 10−9 M). (B) Time course of 125I-P40 (106 cpm; 10−9 M) binding to CEM cells (8 × 106). (C) Inhibition of 125I-P40 binding to CEM cells (106) by native protein at 10−8 M and 37°C. B/B0 corresponds to the binding obtained in the presence of competitor/binding obtained in the absence of competitor. After the cells were washed twice with PBS–BSA buffer, bound radioactivity was counted. Values are means ± standard errors of triplicate experiments.
To test if the 125I-P40 decrease observed after 15 min was due to cell membrane-associated protease activity, the binding assay was performed in the presence of protease inhibitors, including phenylmethylsulfonyl fluoride, pepstatin, leupeptin, and aprotinin. The results were similar to those described above, with dose-dependent curves and maximum binding after 15 min of incubation (data not shown).
The specificity and affinity of the 125I-P40-CEM cell interaction was analyzed in a competitive assay with native P40 (unlabeled) as a competitor. Different concentrations of unlabeled P40 (10−12 to 10−7 M) were incubated with CEM cells, which were then incubated with 125I-P40. The results in Fig. 4C show that unlabeled P40 inhibited 125I-P40 binding in a dose-dependent manner. This inhibition argues for the specificity of this interaction. The concentration of native P40 required to inhibit the formation of 125I-P40-CEM complexes by 50% was about 3 × 10−9 M. This value indicates that P40 binds specifically and with high affinity to at least one potential receptor expressed on CEM lymphocytes.
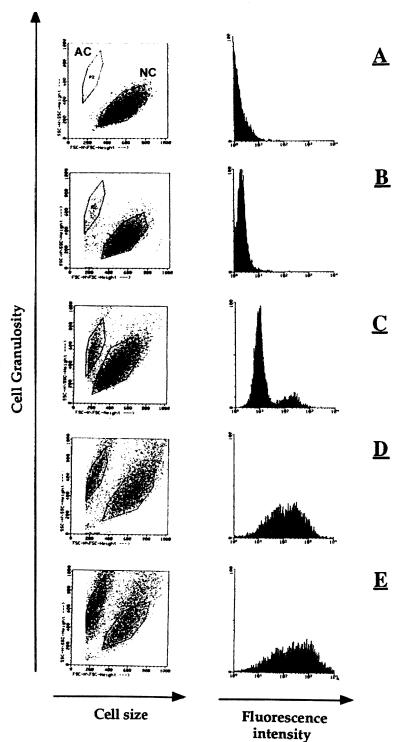
The binding and cytotoxicity of P40 toward CEM cells were further confirmed by FACS analysis with various concentrations of FITC-labeled P40. The results in Fig. 5 show the capacity of P40 to interact with CEM cells in a dose-dependent manner. In addition, we observed the cytotoxic effect of P40 in the form of the appearance of a small and altered CEM cell population. The percentage of this population increased with the quantity of FITC-P40 used. The binding capacity of fluorescent P40 to CEM cells was inhibited by native protein, as was observed in the 125I-P40 experiments (data not shown), confirming the specificity of the P40-CEM cell interaction.
FIG. 5.
Direct fluorescence labeling of P40 bound to CEM cells and inducing a cytopathic effect. A total of 106 cells were incubated with different dilutions of FITC-conjugated P40 (negative control [A] and 1 × 10−9 [B], 1 × 10−8 [C], 5 × 10−7 [D], and 1 × 10−7 M [E], respectively) for 4 h at 37°C, in 100 μl of PBS, BSA buffer. After the cells were washed, they were screened by flow cytometry analysis. NC and AC, normal cells and altered cells, respectively.
The fluorescence microscopic analysis of CEM cells previously incubated with FITC-P40 at 10−7 M (the same cells as those analyzed by fluorescence-activated cell sorting in Fig. 5E) showed both cell membrane and intracellular localization (Fig. 6B and C) of the FITC-P40. We can note from the comparison of the cells observed by phase-contrast and fluorescence microscopy that the labeled protein was mostly associated with altered cells.
FIG. 6.
Distribution of staining on fluorescence analysis of CEM cells from Fig. 5E by fluorescence microscopy. Phase-contrast (A) and fluorescence (B) microscopy observations. (C) Another field of fluorescent cells.
Binding and cytotoxicity-inducing capacities of P40 to CEM cells are Ca2+ dependent.
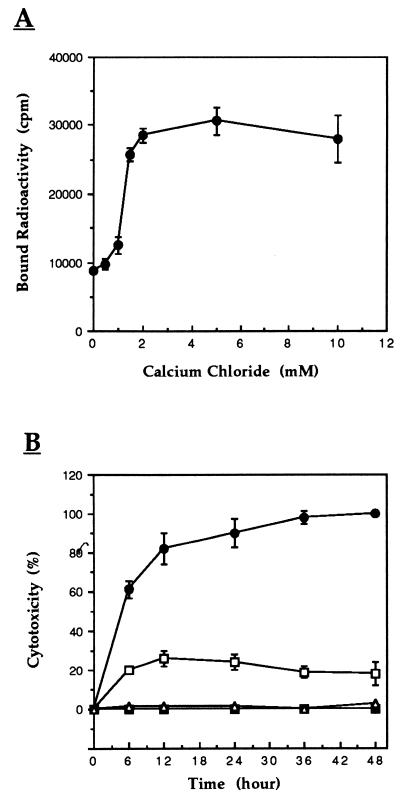
We previously showed that the endonuclease activity of P40 enzyme is Ca2+ and Mg2+ dependent whereas Zn2+ is inhibitory (3). The effect of these divalent ions on the binding capacity of 125I-P40 to CEM cells was therefore tested. While no effect was observed in the presence of Mg2+ and Zn2+, Ca2+ caused an increase by more than threefold in the formation of 125I-P40-cell complexes (Fig. 7A). This effect of Ca2+ started at the concentration of 2 mM CaCl2, and was abolished by the addition of EDTA (data not shown).
FIG. 7.
(A) Effect of Ca2+ on the avidity of 125I-P40 binding. A 50-μl volume of 125I-P40 (2 × 106 cpm, 2 × 10−9 M) in PBS-BSA buffer containing several dilutions of CaCl2 was added to 50 μl of CEM cells (106) and incubated at 37°C for 15 min. After the cells were washed twice with buffer, bound radioactivity was counted. The effect of Ca2+ on 125I-P40 binding activity was abolished in the presence of EDTA and was reproducible in Tris and morpholineproponesulfonic acid (MOPS) buffer instead of PBS. (B) Effect of Ca2+ on cytotoxicity induced by endonuclease P40. The cytopathic effect of P40 was assessed as described in the legend to Fig. 1 (104 cells, 10−8 M P40), except that cells were cultivated in calcium-free medium. Percent cytotoxicity was determined at the defined time points. ■ and ▵, cells cultivated with and without exogenous CaCl2, respectively; □, P40-treated cells; ●, P40-treated cells in the presence of CaCl2. Data are the means ± standard errors.
The effect of Ca2+ on induction of the cytopathic effect of P40 was investigated by first cultivating CEM cells in calcium-free medium. Then protein P40 was added in the absence or presence of 2 mM CaCl2. The results in Fig. 7B show that the cytopathic effect of P40 increased about fivefold in the presence of Ca2+. It is interesting that P40 remained cytopathic even in the absence of Ca2+, although to a lesser extent. Under these conditions, no cytotoxicity was detected with P40-untreated cells cultivated in Ca2+-free medium.
DISCUSSION
In mycoplasmology, there is a need for a better understanding of the mechanisms of virulence, associated pathogenicities, and the effects on host cells. In addition to infecting a large variety of species including humans, recent estimations have shown that between 30 and 70% of eukaryotic cell cultures are contaminated by mycoplasmas (9, 10, 32, 40, 52). The most frequent contaminants are M. hyorhinis, M. orale, M. arginini, M. fermentans, and Acholeplasma laidlawii (21).
Mycoplasmas are generally defective in several metabolic pathways, and their growth requires macromolecular precursors including nucleic acids from the host and/or the surrounding medium (34). They cannot synthesize purine and pyrimidine bases de novo (15, 22, 36, 49). Their nuclease activity has been proposed as a mechanism enabling them to acquire nucleic acids in the form of free bases and/or oligonucleotides (20, 25, 30, 36, 39). The capacity of some species, including M. penetrans, to invade the cells they parasitize suggests that host cell DNA and/or RNA could be a substrate for these nuclease activities.
Using purified M. penetrans endonuclease P40, we observed the effect of the protein on lymphocytes. Compared to untreated controls, cells incubated with the endonuclease exhibited considerable cytopathic effects. The alterations included condensation of the cytoplasm, a loss of surface microvillosities, and the appearance of apoptotic bodies, consistent with the reduction in cell numbers. In addition, the cytopathic effect of high concentrations (>10−7 M) of P40 toward lymphocytes is rapid. The nucleic acids of cells incubated with P40 were analyzed by agarose gel electrophoresis and showed an oligonucleosomal fragmentation of chromatin similar to that observed in apoptotic cells.
Binding assays with 125I-P40 showed a dose-dependent binding of P40 to CEM lymphocytes. Binding exhibits the principal characteristic of a specific interaction since it is inhibited by increasing concentrations of unlabeled P40. The concentration of unlabeled P40 required to inhibit the formation of the 125I-P40-CEM complex by 50% is about 3 × 10−9 M, indicating a high-affinity interaction. The nature and characteristics of the receptor(s) remain to be defined. It is also of interest that 125I-P40 binding to lymphocytes reaches a maximum after 15 min of incubation, decreases thereafter, and finally reaches a new steady-state equilibrium. We have no formal explanation for this decrease in P40 binding by CEM cells, but two hypothesis may be advanced. The first is the degradation of 125I-P40 by cell membrane-bound protease(s). We tested a range of protease inhibitors in incubations of cells with P40, and the results are still inconsistent with this hypothesis. The other hypothesis is the release of receptor-P40 complexes into the media after capping on the surface of the cell. This is commonly observed in lymphocytes during antibody-induced capping of surface proteins. Indeed, before determination of radioactivity, the cells were washed twice by centrifugation.
Characterizing the interaction parameters of 125I-P40-CEM cells showed that among the divalent ions tested, only calcium increased binding and the induction of the cytopathic effect of P40. It was reported that in contrast to Ca2+-independent inducers of apoptosis (etoposide and dexamethasone), Ca2+-dependent inducers (anti-TCR/fas/CD3, ionomycin, and A23187) activate a Ca2+-dependent cellular endonuclease leading to oligonucleosomal fragmentation in lymphocytes (1, 8, 14, 29). Nevertheless, the observed oligonucleosomal fragmentation of lymphocyte chromatin in the presence of P40 appears only partially due to the activity of the Ca2+-dependent cellular endonuclease. Indeed, P40 apparently induces another pathway for activating cell death that is Ca2+ independent. When Ca2+ was absent from the culture medium, P40 was still cytotoxic toward lymphocytes but to a lesser degree. It is interesting that in the absence of Ca2+ and with Mg2+ present, P40 retained 60% of its nuclease activity (3).
M. penetrans is both invasive and attached to the outer surface of cells, as shown by electron microscopic observation (2, 5, 16). The present work shows the cytotoxic effect of P40 toward lymphocytes in vitro that may be susceptible to infection in vivo by M. penetrans. This microorganism can exert two simultaneous effects via P40: (i) a cytotoxic effect caused by P40 secreted by extracellular M. penetrans, and (ii) a second effect caused by P40 secreted by M. penetrans inside the cells. The latter form of endonuclease P40 could rapidly and directly be at the origin of chromatin degradation that causes cell death. We thus propose M. penetrans endonuclease P40 as a potential virulence factor in infection caused by this mycoplasma.
These data suggest that in a large majority of mycoplasmal infections, parasite nuclease activities can participate directly in cell death by apoptosis, in addition to the mechanisms inducing cellular necrosis. The studies of Paddenberg et al. in vitro, using cells contaminated by M. hyorhinis, clearly shows the capacity of mycoplasmal nucleases to cause cell death by apoptosis (27, 28). In a recently published report (46), it was shown that M. bovis infection of cultured cells leads also to an increased sensitivity to various inducers of apoptosis. Interestingly, a mycoplasma nuclease was recovered from nuclear fractions obtained from these infected cells. These results are consistent with our present data and indicate the key role of nucleases in the mycoplasma-induced cytopathic effects. Taken together, our results allowed us to postulate that nucleases secreted by M. penetrans may interact with and then be taken up by parasitized cells. Thus, internalized M. penetrans P40 nuclease may act directly as a cytotoxic factor by cleaving DNA and/or RNA of host cells to induce cells into apoptosis-like death. We believe that this phenomenon should be considered when interpreting apoptosis observed in eukaryotic cells before invoking other mechanisms. Finally, the fact that this endonuclease activity of M. penetrans can be implicated, at least in vitro, as a potential factor in the degradation of the nucleic acids of parasitized cells suggests a potential role of mycoplasmal nucleases as pathogenic factors. The pathophysiological involvement of this phenomenon remains to be shown in vivo.
ACKNOWLEDGMENTS
This work was supported by la Fondation pour la Recherche Medicale and by Ensemble contre le SIDA (SIDACTION).
REFERENCES
- 1.Alnemri E S, Litwack G. Glucocorticoid-induced lymphocytolysis is not mediated by an induced endonuclease. J Biol Chem. 1989;264:4104–4111. [PubMed] [Google Scholar]
- 2.Andreev J, Borovsky Z, Rosenshine I, Rottem S. Invasion of HeLa cells by Mycoplasma penetrans and the induction of tyrosine phosphorylation of a 145 kDa host cell protein. FEMS Microbiol Lett. 1995;132:189–194. doi: 10.1111/j.1574-6968.1995.tb07832.x. [DOI] [PubMed] [Google Scholar]
- 3.Bendjennat M, Blanchard A, Loutfi M, Montagnier L, Bahraoui E. Purification and characterization of Mycoplasma penetrans Ca2+/Mg2+-dependent endonuclease. J Bacteriol. 1997;179:2210–2220. doi: 10.1128/jb.179.7.2210-2220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedik M J, Strych U. Serratia marcescens and its extracellular nuclease. FEMS Microbiol Lett. 1998;165:1–13. doi: 10.1111/j.1574-6968.1998.tb13120.x. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard A, Montagnier L. Aids-associated mycoplasmas. Annu Rev Microbiol. 1994;48:687–712. doi: 10.1146/annurev.mi.48.100194.003351. [DOI] [PubMed] [Google Scholar]
- 6.Bredt W. Pathogenicity factors of mycoplasmas. Infection. 1976;4:9–12. doi: 10.1007/BF01638414. [DOI] [PubMed] [Google Scholar]
- 7.Brown U H, Brightman A H, Fenwick B W, Rider M A. Infections bovine keratoconjunctivitis: a review. J Vet Intern Med. 1998;12:259–266. doi: 10.1111/j.1939-1676.1998.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J J, Duke R C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- 9.Coronato S, Vullo D, Coto C E. A simple method to eliminate mycoplasma from cell cultures. J Virol Methods. 1994;46:85–94. doi: 10.1016/0166-0934(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 10.Fleckenstein E, Uphoff C C, Drexler H G. Effective treatment of mycoplasma contamination in cell lines with enrofloxacin (Baytril) Leukemia. 1994;8:1424–1434. [PubMed] [Google Scholar]
- 11.Giron J A, Lange M, Baseman J B. Adherence, fibronectin binding, and induction of cytoskeleton reorganization in cultured human cells by Mycoplasma penetrans. Infect Immun. 1996;64:197–208. doi: 10.1128/iai.64.1.197-208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grau O, Slizevicz B, Tuppin P, Launay V, Bourgeois E, Sagot N, Lafeuillade A, Bachelez H, Clauvel J P, et al. Association of Mycoplasma penetrans with human immunodeficiency virus infection. J Infect Dis. 1995;172:672–681. doi: 10.1093/infdis/172.3.672. [DOI] [PubMed] [Google Scholar]
- 13.Grau O, Tuppin P, Slizevicz B, Launay V, Goujard C, Bahraoui E, Delfraissy J F, Montagnier L. A longitudinal study of seroreactivity against Mycoplasma penetrans in HIV infected homosexual men: association with disease progression. AIDS Res Hum Retroviruses. 1998;14:664–667. doi: 10.1089/aid.1998.14.661. [DOI] [PubMed] [Google Scholar]
- 14.Lennon S V, Kilfeather S A, Hallett M B, Campbell A K, Cotter T G. Elevations in cytosolic free Ca2+ are not required to trigger apoptosis in human leukaemia cells. Clin Exp Immunol. 1992;87:465–471. doi: 10.1111/j.1365-2249.1992.tb03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liska B, Smith P F. Requirements of Acholeplasma laidlawii A, strain LA 1, for nucleic acid precursors. Folia Microbiol. 1974;19:107–117. doi: 10.1007/BF02872843. [DOI] [PubMed] [Google Scholar]
- 16.Lo S C, Hayes M M, Kotani H, Pierce P F, Wear D J, Newton III P B, Tully J G, Shih J W. Adhesion onto and invasion into mammalian cells by Mycoplasma penetrans: a newly isolated mycoplasma from patients with AIDS. Mod Pathol. 1993;6:276–280. [PubMed] [Google Scholar]
- 17.Lo S C, Hayes M M, Wang R Y, Pierce P F, Kotani H, Shih J W. Newly discovered mycoplasma isolated from patients infected with HIV. Lancet. 1991;338:1415–1418. doi: 10.1016/0140-6736(91)92721-d. [DOI] [PubMed] [Google Scholar]
- 18.Lo S C, Hayes M M, Tully J G, Wang R Y, Kotani H, Pierce P F, Rose D L, Shih J W. Mycoplasma penetrans sp. nov. from the urogenital tract of patients with AIDS. Int J Syst Bacteriol. 1992;42:357–364. doi: 10.1099/00207713-42-3-357. [DOI] [PubMed] [Google Scholar]
- 19.Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: ASM Press; 1992. [Google Scholar]
- 20.Marcus P I, Yoshida I. Mycoplasmas produce double-stranded ribonuclease. J Cell Physiol. 1990;143:416–419. doi: 10.1002/jcp.1041430303. [DOI] [PubMed] [Google Scholar]
- 21.McGarrity G J, Kotani H, Butler G H. Mycoplasmas and tissue culture cells. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: ASM Press; 1992. pp. 445–454. [Google Scholar]
- 22.McIvor R S, Kenny G E. Differences in incorporation of nucleic acid bases by various Mycoplasma and Acholeplasma species. J Bacteriol. 1978;135:483–489. doi: 10.1128/jb.135.2.483-489.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minion F C, Jarvill-Taylor K J. Membrane-associated hemolysin activities in mycoplasmas. FEMS Microbiol Lett. 1994;116:101–106. doi: 10.1111/j.1574-6968.1994.tb06682.x. [DOI] [PubMed] [Google Scholar]
- 24.Minion F C, Jarvill-Taylor K J, Billings D E, Tigges E. Membrane-associated nuclease activities in mycoplasmas. J Bacteriol. 1993;175:7842–7847. doi: 10.1128/jb.175.24.7842-7847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minion F C, Goguen J D. Identification and preliminary characterization of external membrane-bound nuclease activities in Mycoplasma pulmonis. Infect Immun. 1986;51:352–354. doi: 10.1128/iai.51.1.352-354.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neyrolles O, Ferris S, Behbahani N, Montagnier L, Blanchard A. Organization of Ureaplasma urealyticum urease gene cluster and expression in a suppressor strain of Escherichia coli. J Bacteriol. 1996;178:647–655. doi: 10.1128/jb.178.3.647-655.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paddenberg R, Weber A, Wulf S, Mannherz H G. Mycoplasma nucleases able to induce internucleosomal DNA degradation in cultured cells possess many characteristics of eukaryotic apoptotic nucleases. Cell Death Differ. 1998;5:517–528. doi: 10.1038/sj.cdd.4400380. [DOI] [PubMed] [Google Scholar]
- 28.Paddenberg R, Wulf S, Weber A, Heimann P, Beck L A, Mannherz H G. Internucleosomal DNA fragmentation in cultured cells under conditions reported to induce apoptosis may be caused by mycoplasma endonucleases. Eur J Cell Biol. 1996;71:105–119. [PubMed] [Google Scholar]
- 29.Peitsch M C, Polzar B, Stephan H, Crompton T, MacDonald H, Mannherz H G, Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death) EMBO J. 1993;12:371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollack J P, Hoffmann P J. Properties of the nucleases of mollicutes. J Bacteriol. 1982;152:538–541. doi: 10.1128/jb.152.1.538-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quillent C, Grau O, Clavel F, Montagnier L, Blanchard A. Inhibition of HIV type 1 reverse transcriptase assay by nucleases produced by contaminating mycoplasmas. AIDS Res Hum Retroviruses. 1994;10:1251–1257. doi: 10.1089/aid.1994.10.1251. [DOI] [PubMed] [Google Scholar]
- 32.Rawadi G, Dussurget O. Advances in PCR-based detection of mycoplasmas contaminating cell cultures. PCR Methods Appl. 1995;4:199–208. doi: 10.1101/gr.4.4.199. [DOI] [PubMed] [Google Scholar]
- 33.Razin S, Barile M F, editors. Mycoplasma pathogenicity. Vol. 4. New York, N.Y: Academic Press, Inc.; 1985. The Mycoplasmas. [Google Scholar]
- 34.Razin S. The mycoplasmas. Microbiol Rev. 1978;42:414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razin S, editor. The prokaryotes. Vol. 2. 1992. 2nd ed.19371959Springer-VerlagNew York, N.Y. [Google Scholar]
- 36.Razin S, Knight B C J B. The effect of ribonucleic acid and deoxyribonucleic acid on the growth of mycoplasma. J Gen Microbiol. 1960;22:504–519. doi: 10.1099/00221287-22-2-504. [DOI] [PubMed] [Google Scholar]
- 37.Razin S, Knyszynski L, Lifshitz Y. Nucleases of mycoplasmas. J Gen Microbiol. 1964;36:323–331. doi: 10.1099/00221287-36-2-323. [DOI] [PubMed] [Google Scholar]
- 38.Robertson J A, Stemler M E, Stemke G W. Immunoglobulin A protease activity of Ureaplasma urealyticum. J Clin Microbiol. 1984;19:255–258. doi: 10.1128/jcm.19.2.255-258.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roganti F S, Rosenthal A L. DNases of Acholeplasma spp. J Bacteriol. 1983;155:802–805. doi: 10.1128/jb.155.2.802-805.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rottem S, Barile M F. Beware of mycoplasmas. Trends Biotechnol. 1993;11:143–151. doi: 10.1016/0167-7799(93)90089-R. [DOI] [PubMed] [Google Scholar]
- 41.Rottem S, Hasin M, Razin S. Differences in susceptibility to phospholipase C of free and membrane-bound phospholipids of Mycoplasma hominis. Biochim Biophys Acta. 1973;323:520–531. doi: 10.1016/0005-2736(73)90160-0. [DOI] [PubMed] [Google Scholar]
- 42.Ruifu Y, Minli Z, Guo Z, Wang X. Biovar diversity is reflected by variations of genes encoding urease of Ureaplasma urealyticum. Microbiol Immunol. 1997;41:625–627. doi: 10.1111/j.1348-0421.1997.tb01902.x. [DOI] [PubMed] [Google Scholar]
- 43.Salman M, Rottem S. The cell membrane of Mycoplasma penetrans: lipid composition and phospholipase A1 activity. Biochim Biophys Acta. 1995;1235:369–377. doi: 10.1016/0005-2736(95)80026-c. [DOI] [PubMed] [Google Scholar]
- 44.Shang H, Miyakawa Y, Sasaki T, Nakashima H, Ito M. Suppression of HIV-1 reverse transcriptase activity by culture supernatants of mycoplasmas. Microbiol Immunol. 1995;39:987–993. doi: 10.1111/j.1348-0421.1995.tb03302.x. [DOI] [PubMed] [Google Scholar]
- 45.Shibata K, Sasaki T, Watanabe T. AIDS-associated mycoplasmas possess phospholipases C in the membrane. Infect Immun. 1995;63:4174–4177. doi: 10.1128/iai.63.10.4174-4177.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sokolova I A, Vaughan A T, Khodarev N N. Mycoplasma infection can sensitize host cells to apoptosis through contribution of apoptotic-like endonuclease(s) Immunol Cell Biol. 1998;76:526–534. doi: 10.1046/j.1440-1711.1998.00781.x. [DOI] [PubMed] [Google Scholar]
- 47.Somerson N L, Walls B E, Chanock R M. Hemolysin of Mycoplasma pneumoniae: tentative identification as a peroxide. Science. 1965;150:226–228. doi: 10.1126/science.150.3693.226. [DOI] [PubMed] [Google Scholar]
- 48.Spooner R K, Russell W C, Thirkell D. Characterization of the immunoglobulin A protease of Ureaplasma urealyticum. Infect Immun. 1992;60:2544–2546. doi: 10.1128/iai.60.6.2544-2546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanbridge E J, Hayflick L, Perkins F T. Modification of amino-acid concentrations induced by mycoplasmas in cell culture medium. Nat (London) New Biol. 1971;232:242–244. doi: 10.1038/newbio232242a0. [DOI] [PubMed] [Google Scholar]
- 50.Tully J G. Mycoplasmal toxins. Isr J Med Sci. 1981;17:604–607. [PubMed] [Google Scholar]
- 51.Wang R Y, Shih J W, Grandinetti T, Pierce P F, Hayes M M, Wear D J, Alter H J, Lo S C. High frequency of antibodies to Mycoplasma penetrans in HIV-infected patients. Lancet. 1992;340:1312–1316. doi: 10.1016/0140-6736(92)92493-y. [DOI] [PubMed] [Google Scholar]
- 52.Uphoff C C, Gignac S M, Drexler H G. Mycoplasma contamination in human leukemia cell lines. I. Comparison of various detection methods. J Immunol Methods. 1992;149:43–54. doi: 10.1016/s0022-1759(12)80047-0. [DOI] [PubMed] [Google Scholar]
- 53.Yanez A, Cedillo L, Neyrolles O, Alonso E, Prevost M C, Rojas J, Watson H L, Blanchard A, Cassell G H. Mycoplasma penetrans bacteremia and primary antiphospholipid syndrome. Emerg Infect Dis. 1999;5:164–167. doi: 10.3201/eid0501.990122. [DOI] [PMC free article] [PubMed] [Google Scholar]