Highlights
-
•
Provides essential information on the dosage of topical application sodium fluoride varnish and silver diamine fluoride in particular for young children.
-
•
Aids dentists and hygienists in selecting the dental micro-applicators for topical fluoride therapy.
-
•
Choose suitable dental micro-applicators for silver diamine fluoride therapy because not all micro-applicators are compatible with silver diamine fluoride.
Key words: Silver diamine fluoride, Prevention, Caries, Children, Topical fluoride, Sodium fluoride
Abstract
Objectives
The objectives of this study were to compare the amount of fluoride delivered via a topical application of 38% silver diamine fluoride (SDF) solution and 5% sodium fluoride (NaF) varnish as well as to determine the amount of 38% SDF solution delivered using various micro-applicators.
Methods
The weights of 38% SDF (Saforide) and 5% NaF (Duraphat) applied to the occlusal surface of an extracted human upper first premolar with a regular-size (2.50-mm tip diameter) micro-applicator were measured using an electronic-analytical balance. Afterwards, the weight of 38% SDF applied to a premolar using the micro-applicators of 5 common brands (Premium Plus, 3M, Dentsply, Elevate Oral Care, and SDI) were studied. The tip diameter of each micro-applicator was measured under a microscope. The weights of the delivered fluoride and silver were also calculated.
Results
The mean weights of the fluoride delivered via the SDF solution and NaF varnish were 0.25 ± 0.07 mg and 0.49 ± 0.08 mg, respectively (P < .001). In addition, the tip diameters of the micro-applicators ranged from 1.89 ± 0.03 mm to 2.76 ± 0.02 mm. The mean weights of the fluoride delivered per application of 38% SDF using different applicators ranged from 0.13 ± 0.06 mg to 0.30 ± 0.09 mg, whereas the mean weights of the silver ranged from 0.76 ± 0.32 mg to 1.68 ± 0.50 mg. The weights of the delivered 38% SDF solution varied with the brand and with the tip diameters of the dental micro-applicators (P < .001).
Conclusions
The fluoride of the 38% SDF solution delivered topically was significantly less than that of 5% NaF varnish. Moreover, the amount of SDF solution delivered depends on the brand and size of the micro-applicators.
Introduction
Professional fluoride therapy is effective for the prevention of dental caries.1 Common fluoride agents include sodium fluoride (NaF), which can be acidulated and buffered with a phosphate to form acidulated phosphate fluoride (APF); sodium monofluophosphate; and stannous fluoride and silver diamine fluoride (SDF). They contain high concentrations of fluoride ranging from 12,300 ppm (APF) to 44,800 ppm (SDF).
NaF varnish is a commonly used fluoride agent in dental care. Varnish containing 5% NaF contains 22,600 ppm of fluoride. Its use is simple, quick, and well received amongst patients, with negligible side effects. Although the US Food and Drug Administration (FDA) cleared NaF in 1994 as an antihypersensitivity agent, it has become the most commonly used professional fluoride agent for caries management.2 A systematic review concluded that NaF varnish remineralised 64% of enamel caries.3 The American Academy of Pediatrics recommends using 5% NaF varnish at intervals that increase with caries risk status.4 Although use of NaF varnish is considered to be a standard of care for prevention of early childhood caries, a systematic review by the Cochrane Collaboration revealed that use of NaF varnish is inadequate because it reduced only 37% of early childhood caries development.5
Amongst the fluorides that dentists use, 38% SDF contains the highest concentration of fluoride (44,800 ppm). SDF use is noninvasive, simple, and inexpensive.6 Its use has gained wider attention since the FDA cleared it in 2014.7 A review concluded that SDF appears to meet the criteria of both the World Health Organisation Millennium Goals and the Institute of Medicine's criteria for 21st-century medicine in the management of dental caries.8 Along with its high fluoride content, which promotes the remineralisation of enamel and dentine, SDF contains high silver content (253,900 ppm), which inhibits the growth of cariogenic bacteria.9 Studies have also found that SDF inhibits proteolytic peptidases and prevents dentine collagen.10,11 A review concluded that SDF is a more effective caries management reagent than NaF is.12 The American Dental Association supported the use of SDF for caries management in 2020 after conducting a review of evidence-based clinical recommendations.
Clinical studies have found that SDF did not cause acute systemic illness.13, 14, 15 Toothache, gingival pain,1 and swelling were uncommon after SDF therapy.16 The study also concluded that SDF use is safe for young children. However, 38% SDF contains high fluoride and silver content. The excess intake of fluoride can lead to fluorosis, and silver can be absorbed and accumulate in the body.17 In light of this, determining the proper amount of SDF to apply to patients is crucial in clinical care.
SDF is often applied to a tooth using a dental micro-applicator. A micro-applicator is disposable and is designed for a single user; hence, it is often composed of a biodegradable material. The fibre of the micro-applicator's tip is commonly made of nylon and polypropylene. Although most of the commercially available SDF is at 38%, no consensus exists regarding the size of the micro-applicator one should use for an SDF application. The sizes of micro-applicators vary amongst brands, and some brands have micro-applicators at both regular and small/fine sizes. The objectives of the present study were to compare the amount of fluoride delivered in a topical application of 38% SDF and 5% NaF varnish as well as to determine the proper amount of 38% SDF to deliver using different dental micro-applicators.
Materials and methods
Fluoride delivered via 38% SDF and 5% NaF varnish
An extracted sound upper first premolar mounted on a plaster stand was used for the application of fluoride. The micro-applicator used was the Disposable Micro-applicator (Premium Plus, Premium Plus International Limited). An electronic-analytical balance (weighing scale, A&D Corporate) with a precision of 0.01 mg was used for the measurement. The weight of the micro-applicator before soaking with fluoride solutions were recorded. One researcher applied the fluoride agent to the premolar occlusal surface. A single application of freshly opened bottles of 38% SDF (Saforide, Toyo Seiyaku Kasei Co. Ltd.) or 5% NaF varnish (Duraphat, Colgate Palmolive Co.) was topically applied to the occlusal surface of the premolar. We determined the weight of the micro-applicator after soaking with fluoride solutions. We rinsed thoroughly the premolar with water. Afterwards, we dried the tooth with pressurised air and gauze before we performed another application. Twenty applications were performed for each fluoride agent (NaF and SDF), and the mean amounts of fluoride applied were calculated. We assumed that the 38% SDF solution contained 253,900 ppm silver and 44,800 ppm fluoride and the 5% NaF varnish contained 22,600 ppm fluoride to calculate the amount of fluoride and silver delivered to the premolars.
SDF solution delivered via different micro-applicators
This laboratory study involved selecting 5 commercially available dental micro-applicators, namely (1) Disposable Applicators (3M ESPE), (2) Disposable Micro-applicators (Premium Plus, Premium Plus International Limited), (3) Applicator Tips (Dentsply, Dentsply International), (4) Points Disposable Brush Applicators (SDI), and (5) Advantage Arrest Applicators (Elevate Oral Care). Three of them are regular and small/fine in size. Twenty applications of SDF were performed using each type of applicator, and the mean amounts of the applied SDF were calculated.
Tip sizes of different micro-applicators
A digital microscope (Nikon Polarizing Microscope LV100Pol, Nikon Color Cooled Digital Camera Ds-Ri1) was used to scan images of each micro-applicator's tip at different distances, and it generated one image for the measurement of the diameter of the tip of the micro-applicator. A micrometre ruler was used to calibrate the computer software programme (Nikon, Software NIS-Elements AR 3.1) for the measurement of the tip of micro-applicator.18 A trained technician used 20 micro-applicators of each brand and size for the measurement. The measurements were recorded, and the mean diameter of each tip was calculated. Another independent technician verified the measurement to minimise measurement errors.18
Statistical analyses
Two independent researchers performed data entry, and the 2 data sets were compared to ensure accuracy. A statistician performed the data analyses using SPSS software version 26.0 (IBM Corporation). The data were tested for normality using the Shapiro–Wilk test. One-way analysis of variance (ANOVA) with Tukey post hoc tests was used to compare the weights of fluoride delivered using different brands of micro-applicators. The level for statistical significance for all analyses was set at 5%. Separated linear regression analysis was used to explore the relationship between the tip diameter of a micro-applicator and the weight of SDF applied to a premolar.18
Results
Fluoride delivered by 38% SDF solution and 5% NaF varnish
Twenty applications of Saforide solution and Duraphat varnish were performed using the Disposable Micro-applicator. The mean weights (± standard deviation [SD]) of the fluoride delivered via the 38% SDF solution and 5% NaF varnish were 0.25 ± 0.07 mg and 0.49 ± 0.08 mg, respectively (P < .001) (Table 1).
Table 1.
Weight of Saforide and Duraphat applied to tooth with a micro-applicator.*
| Fluoride agent (Brand) | Fluoride agent taken by the micro-applicator (mg), mean ± SD | Fluoride agent applied to a tooth (mg), mean ± SD | Fluoride applied to a tooth (mg), mean ± SD |
|---|---|---|---|
| Silver diamine fluoride 38% solution (Saforide) | 17.89 ± 2.54 | 5.60 ± 1.61 | 0.25 ± 0.07 |
| Sodium fluoride 5% varnish (Duraphat) | 26.48 ± 3.51 | 21.46 ± 3.36 | 0.49 ± 0.08 |
Premium Plus (regular size with tip diameter of 2.5 mm).
SDF solution delivered by different micro-applicators
The mean weights of the SDF delivered via different applicators ranged from 3.00 ± 1.26 mg to 6.60 ± 1.96 mg (Table 2). In addition, the mean weights of the estimation of the applied fluoride of 38% SDF ranged from 0.13 ± 0.06 mg to 0.30 ± 0.09 mg. The mean weights of the estimation of the applied silver of the 38% SDF solution ranged from 0.76 ± 0.32 mg to 1.68 ± 0.50 mg.
Table 2.
Silver diamine fluoride (SDF), fluoride, and silver applied according to the brands and sizes of the dental micro-applicators.
| Brand | Size | Applicator's tip diameter (mm), mean ± SD | SDF applied amount (mg), mean ± SD | Fluoride applied to tooth (mg), mean ± SD | Silver applied to tooth (mg), mean ± SD |
|---|---|---|---|---|---|
| SDI | Regular | 2.76 ± 0.02a | 6.60 ± 1.96a | 0.30 ± 0.09 | 1.68 ± 0.50 |
| 3M | Regular | 2.60 ± 0.07b | 4.50 ± 1.09a, b | 0.20 ± 0.05 | 1.14 ± 0.28 |
| Premium Plus* | Regular | 2.53 ± 0.02c | 5.60 ± 1.61b, c | 0.25 ± 0.07 | 1.42 ± 0.41 |
| Elevate Oral Care | Regular | 2.51 ± 0.01cd | 4.23 ± 0.69 c | 0.19 ± 0.03 | 1.07 ± 0.17 |
| Dentsply | Regular | 2.48 ± 0.02d | 3.78 ± 0.92c, d | 0.17 ± 0.04 | 0.96 ± 0.23 |
| Premium Plus* | Fine | 2.04 ± 0.01e | 3.83 ± 0.57c, d | 0.17 ± 0.03 | 0.97 ± 0.15 |
| Elevate Oral Care | Small | 2.01 ± 0.04e | 3.87 ± 0.77c, d | 0.17 ± 0.03 | 0.98 ± 0.19 |
| SDI | Fine | 1.89 ± 0.02f | 3.00 ± 1.26d | 0.13 ± 0.06 | 0.76 ± 0.32 |
Tukey's post hoc analysis: Each subscript letter denotes a subset of group categories whose column means do not differ significantly from one another at the .05 level (the same column mean was named by a lowercase letter, such as a, b, c, d, e, or f).
The results of the one-way ANOVA determined that significant differences existed amongst different groups for the tip diameter of the dental applicator (P < .001), the weight of the applied SDF solution (P < .001), the weight of the fluoride applied to the tooth (P < 0.001), and the weight of the silver applied to the tooth (P < .001). Although the average tip diameters of the regular-size Premium Plus and Elevate Oral Care were both approximately 2.50 mm, the mean weights of the 38% SDF solution they delivered were 5.60 ± 1.61 mg and 4.23 ± 0.69 mg, respectively (P < .001) (Table 2).
Although most of the micro-applicators’ tips featured no observable changes after being dipped in SDF, one applicator's tip was blackened and discolored (Figure 2). The discolouration of the tip could not be removed by dipping it in water.
Fig. 2.
Discolouration of the tip of a micro-applicator after dipping it into 38% silver diamine fluoride (SDF) solution.
Tip diameters of the micro-applicators
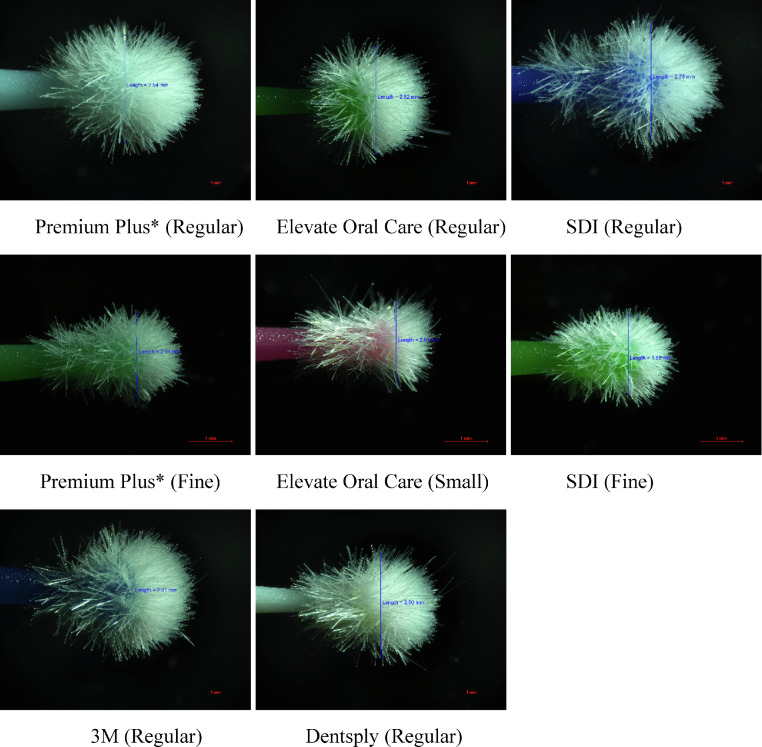
Figure 1 shows micrographs of the 8 micro-applicators. The tip diameter of the micro-applicators ranged from 1.89 ± 0.02 mm to 2.76 ± 0.02 mm (Table 2). The bristles of some of the micro-applicators were not evenly distributed.
Fig. 1.
Typical micrographs of the 8 micro-applicators.
Dental applicator's tip diameter and the weight of 38% SDF solution applied
Linear regression showed that the weight of the 38% SDF solution applied was directly proportional to the dental applicator's tip diameter (R2 = 0.281). The weight of the SDF solution, the fluoride, and the silver applied to the tooth increased 2.767, 0.124, and 0.702, respectively, on average with a unit increase in the dental applicator's tip diameter. In addition, significant variations in the SDF solution were applied via 2 brands of micro-applicators (Elevate Oral Care and Premium Plus) with regular-size (∼ 2.5 mm) tips (R2 = 0.066).
Discussion
This study used an electronic-analytical balance to weigh the fluoride solution and varnish because an electronic-analytical balance is one of the most precise tools for performing measurements in the analytical laboratory setting.19 Because the level of sophistication of the electronic-analytical balance is very high, and because it is sensitive, an error could occur due to dust migration from a dirty environment in particular. Thus, it is essential to perform measurements in a clean environment. We kept the electronic-analytical balance in a translucent plastic cover except for when we put the material on the balance for weighing. We also ensured that the electronic-analytical balance was clean because dust falling into the gap between the pole pieces of the servomotor would affect the calibration and precision of the balance. In this study, we calibrated the electronic-analytical balance before each measurement to ensure the balance's accuracy.
We took a photo of the micro-applicator with digital microscopy to measure the tip diameter and to evaluate the tip shape of the dental micro-applicator. Digital microscopy is an efficient tool for inspecting and analysing small objects, such as the tip of a micro-applicator. It is also more ergonomic than other microscopy is.20 In our study, digital microscopy was used to display the image of a micro-applicator on a monitor. We could therefore view the image immediately and analyse the image using software whilst working comfortably in an upright position. Digital microscopy also shows high sensitivity. A comprehensive slide imaging microscopy system is designed to accurately digitise images and to convert them into high-quality and large images using computer software.21 This study involved scanning the images of a micro-applicator's tip at different distances and generating a single image for measurement. The images at different distances overlapped and possibly affected the measurement results. However, the variations in the observed results were not large and were monitored with 20 measurements of each group of micro-applicators.
This study involved selecting 5 brands of micro-applicators that clinicians commonly use according to unpublished market research. They are designed for the precise placement and easy application of dental materials, such as acid etchant, resin adhesives, cavity liners, and bonding agents, in a limited area. Application of SDF with microbrush alone appears more effective than with Superfloss alone in decreasing incipient approximal lesion progression in permanent teeth.22 The tip fibres should be nonabsorbent and nonlinting fibres. In addition, the fibres should be mechanically rugged material and have high chemical resistance. The fibres should firmly attach to the handle, and their density within the tip should be evenly and uniformly displaced. This study found that SDF did not discolour most of the applicators’ tips. Nylon is a common material for tip fibres, and it is chemically compatible with silver ions. However, SDF did stain one micro-applicator tip brand. SDF contains silver, which might have reacted with the chemical substances in the tip's fibres to form a silver compound, thus discolouring the applicator's tip. Therefore, not all micro-applicators are suitable for topical application. Clinicians should choose the appropriate application for SDF therapy.
Manufacturers have generally produced regular-size micro-applicators with tip diameters of 2.5 mm. In this study, 3 companies produced small/fine micro-applicators with tip diameters of approximately 2 mm. One trained researcher performed fluoride applications in this laboratory study to reduce variations and to minimise errors in the operation process. Through the study, we found that micro-applicators of the same brand and of different brands with similar tip sizes picked up considerably different amounts of the SDF solution.
The results of this study also found variations in the fibre density of the micro-applicator tips of the same brand and also of different brands with similar tip sizes. Apart from the fibre density, an ideal applicator should use nonabsorbent, nonlinting fibres comprising a chemically resistant material. The tips should suspend solution without spilling or dripping. The amount of SDF applied would be more precise if the manufacturers could standardise the tip size and improve the quality of the tip fibres of the micro-applicator. In this study, not all manufacturers provided information on the tip size and the materials used. Studies showed that significant variations exist between the claimed and measured fluoride concentrations amongst the commercially available 38% SDF products.23,24 Therefore, the quality of the SDF products would affect the estimated amount of fluoride and silver applied in this study.
Because 5% sodium fluoride varnish contains 22,600 ppm fluoride and 38% SDF contains 44,800 ppm fluoride, the higher fluoride concentration of SDF may generate a higher risk of dental fluorosis. Some dentists may intuitively consider a topical application of 5% NaF varnish to be preferred when compared with the 38% SDF solution, particularly for young children. This study showed that the fluoride applied per topical application of 5% NaF varnish was almost 50% more than that of the 38% SDF solution. The micro-applicator picked up a larger amount of 5% NaF varnish because it was viscous and gelatinous. Studies found that the small amount of 38% SDF was below the US Environmental Protection Agency oral reference dose.25,26 Although silver can accumulate in the human body over a lifetime, the occasional use of 38% SDF in a minute amount poses little toxicity risk even to young children. In this study, we assumed that a 38% SDF solution contains 253,900 ppm silver and 44,800 ppm fluoride ions. However, studies found variations of silver and fluoride ion concentration that would affect the actual amount of silver and fluoride applied to the tooth.24,26,27
Dental fluorosis is a subsurface enamel hypomineralisation result from a chronic and cumulative intake of excessive fluoride. The risk of dental fluorosis is also extremely low after an one-off topical application of 38% SDF solution. A review concluded that 38% SDF was clinically safe and had a lower absorption rate when the “rinse and spit” procedure was used.28 The potential toxic dose for fluoride is 5 mg/kg.2 The probable toxic dose for a child weighing 20 kg is approximately 100 mg. This study found that 0.25 mg of fluoride is applied to a tooth per topical application of 38% SDF solution. It is still within the safety limit for a young child to receive 400 applications of the 38% SDF solution. In other words, dentists may apply 20 applications of 38% SDF to each of the 20 primary teeth in a young child with a low risk of fluoride toxicity. It is notheworthy that 0.49 mg of fluoride is applied to a tooth per topical application of 5% NaF varnish. This amout is approximate double that of a topical SDF application. Hence, it is incorrect to choose NaF varnish over SDF solution when a dentist wants to minimise fluoride exposure in topical fluoride therapy in patients.
The use of NaF varnish is generally considered to be the standard of care of caries for topical fluoride therapy.2 Studies showed that NaF varnish can reduce caries incidence and remineralise early enamel caries, and SDF can prevent and arrest caries. Although 38% SDF contains a higher fluoride concentration than 5% NaF varnish does, this study showed that an SDF application delivered less flouride per application than NaF varnish did. SDF therapy delivered less fluoride per application than NaF did, and it is more effective than NaF is in caries management. Therefore, it is necessary to consider whether the use of NaF varnish should be the standard of care for caries management.
Conclusions
The fluoride of the 38% SDF solution delivered topically was significantly less than that of 5% NaF varnish. The risk of fluoride toxicity for a 38% SDF application is low even for young children. The brand and size of the micro-applicator determined the amount of the SDF solution applied to a tooth. Not all micro-applicators are compatible with SDF, and clinicians should choose suitable micro-applicators for topical SDF therapy.
Conflict of interest
None disclosed.
Acknowledgements
This study was supported by the General Research Fund (#17100421) of the Research Grant Council of Hong Kong. The authors would like to thank Mr Y.Y. Tsui for technical advice in taking digital photographs and Ms Samantha Li for her statistical advice in the study.
REFERENCES
- 1.Chu CH, Mei ML, Lo ECM. Use of fluorides in dental caries management. Gen Dent. 2010;58:37–43. quiz 4–5, 79–80. [PubMed] [Google Scholar]
- 2.Chu CH, Lo ECM. A review of sodium fluoride varnish. Gen Dent. 2006;54:247–253. [PubMed] [Google Scholar]
- 3.Gao SS, Zhao IS, Hiraishi N, et al. Clinical trials of silver diamine fluoride in arresting caries among children: a systematic review. JDR Clin Trans Res. 2016;1:201–210. doi: 10.1177/2380084416661474. [DOI] [PubMed] [Google Scholar]
- 4.Milgrom P, Taves DM, Kim AS, et al. Pharmacokinetics of fluoride in toddlers after application of 5% sodium fluoride dental varnish. Pediatrics. 2014;134:e870–e874. doi: 10.1542/peds.2013-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marinho VC, Worthington HV, Walsh T, et al. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD002279.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu CH, Lo ECM. Promoting caries arrest in children with silver diamine fluoride: a review. Oral Health Prev Dent. 2008;6:315–321. [PubMed] [Google Scholar]
- 7.Mei ML, Lo ECM, Chu CH. Clinical use of silver diamine fluoride in dental treatment. Compend Contin Educ Dent. 2016;37:93–98. quiz 100. [PubMed] [Google Scholar]
- 8.Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: a caries “silver-fluoride bullet”. J Dent Res. 2009;88:116–125. doi: 10.1177/0022034508329406. [DOI] [PubMed] [Google Scholar]
- 9.Mei ML, Nudelman F, Marzec B, et al. Formation of fluorohydroxyapatite with silver diamine fluoride. J Dent Res. 2017;96:1122–1128. doi: 10.1177/0022034517709738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mei ML, Li QL, Chu CH, et al. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent Mater. 2012;28:903–908. doi: 10.1016/j.dental.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Mei ML, Ito L, Cao Y, et al. The inhibitory effects of silver diamine fluorides on cysteine cathepsins. J Dent. 2014;42:329–335. doi: 10.1016/j.jdent.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Trieu A, Mohamed A, Lynch E. Silver diamine fluoride versus sodium fluoride for arresting dentine caries in children: a systematic review and meta-analysis. Sci Rep. 2019;9:2115. doi: 10.1038/s41598-019-38569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung MHT, Duangthip D, Wong MCM, et al. Randomized clinical trial of 12% and 38% silver diamine fluoride treatment. J Dent Res. 2018;97:171–178. doi: 10.1177/0022034517728496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu CH, Lo ECM, Lin HC. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J Dent Res. 2002;81:767–770. doi: 10.1177/0810767. [DOI] [PubMed] [Google Scholar]
- 15.Gao SS, Chen KJ, Duangthip D, et al. Arresting early childhood caries using silver and fluoride products - a randomised trial. J Dent. 2020;103 doi: 10.1016/j.jdent.2020.103522. [DOI] [PubMed] [Google Scholar]
- 16.Duangthip D, Fung MHT, Wong MCM, et al. Adverse effects of silver diamine fluoride treatment among preschool children. J Dent Res. 2018;97:395–401. doi: 10.1177/0022034517746678. [DOI] [PubMed] [Google Scholar]
- 17.Timms L, Sumner O, Deery C, Rogers HJ. Everyone else is using it, so why isn't the UK? Silver diamine fluoride for children and young people. Community Dent Health. 2020;37:143–149. doi: 10.1922/CDH_00008Timms07. [DOI] [PubMed] [Google Scholar]
- 18.Al Rifaiy MQ. Evaluation of vertical marginal adaptation of provisional crowns by digital microscope. Niger J Clin Pract. 2017;20:1610–1617. doi: 10.4103/1119-3077.196083. [DOI] [PubMed] [Google Scholar]
- 19.Andersen JET. Understanding uncertainty to weighing by electronic-analytical balances. J AOAC Int. 2018;101:1977–1984. doi: 10.5740/jaoacint.17-0428. [DOI] [PubMed] [Google Scholar]
- 20.Rashed B, Iino Y, Ebihara A, Okiji T. Evaluation of crack formation and propagation with ultrasonic root-end preparation and obturation using a digital microscope and optical coherence tomography. Scanning. 2019;2019 doi: 10.1155/2019/5240430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigues-Fernandes CI, Speight PM, Khurram SA, et al. The use of digital microscopy as a teaching method for human pathology: a systematic review. Virchows Arch. 2020;477:475–486. doi: 10.1007/s00428-020-02908-3. [DOI] [PubMed] [Google Scholar]
- 22.Polacek J, Malhi N, Yang YJ, et al. Silver diamine fluoride and progression of incipient approximal caries in permanent teeth: a retrospective study. Pediatr Dent. 2021;43:475–480. [PubMed] [Google Scholar]
- 23.Mei ML, Chu CH, Lo ECM, et al. Fluoride and silver concentrations of silver diammine fluoride solutions for dental use. Int J Paediatr Dent. 2013;23:279–285. doi: 10.1111/ipd.12005. [DOI] [PubMed] [Google Scholar]
- 24.Patel J, Foster D, Smirk M, et al. Acidity, fluoride and silver ion concentrations in silver diamine fluoride solutions: a pilot study. Aust Dent J. 2021;66:188–193. doi: 10.1111/adj.12822. [DOI] [PubMed] [Google Scholar]
- 25.Vasquez E, Zegarra G, Chirinos E, et al. Short term serum pharmacokinetics of diammine silver fluoride after oral application. BMC Oral Health. 2012;12:60. doi: 10.1186/1472-6831-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crystal YO, Rabieh S, Janal MN, et al. Silver and fluoride content and short-term stability of 38% silver diamine fluoride. J Am Dent Assoc. 2019;150:140–146. doi: 10.1016/j.adaj.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mei ML, Chu CH, Lo ECM, et al. Fluoride and silver concentrations of silver diammine fluoride solutions for dental use. Int J Paediatr Dent. 2013;23:279–285. doi: 10.1111/ipd.12005. [DOI] [PubMed] [Google Scholar]
- 28.Peng JY, Tsoi JKH, Matinlinna JP, et al. Silver deposition on demineralized dentine surface dosed by silver diammine fluoride with different saliva. J Investig Clin Dent. 2019;10:e12382. doi: 10.1111/jicd.12382. [DOI] [PubMed] [Google Scholar]