Abstract
Fundamental to the virulence of microbial pathogens is their capacity for adaptation and survival within variable, and often hostile, environments encountered in the host. We describe a novel, extragenomic mechanism of surface modulation which may amplify the adaptive and pathogenic potential of numerous bacterial species, including Staphylococcus, Yersinia, and pathogenic Neisseria species, as well as Helicobacter pylori and Streptococcus pyogenes. The mechanism involves specific bacterial recruitment of heparin, glycosaminoglycans, or related sulfated polysaccharides, which in turn serve as universal binding sites for a diverse array of mammalian heparin binding proteins, including adhesive glycoproteins (vitronectin and fibronectin), inflammatory (MCP-3, PF-4, and MIP-1α) and immunomodulatory (gamma interferon) intermediates, and fibroblast growth factor. This strategy impacts key aspects of microbial pathogenicity as exemplified by increased bacterial invasion of epithelial cells and inhibition of chemokine-induced chemotaxis. Our findings illustrate a previously unrecognized form of parasitism that complements classical virulence strategies encoded within the microbial genome.
Infectious diseases typically involve continuous interplay between microbial pathogenicity factors and host defense systems. Commonly identified bacterial strategies for surviving within the host include exploiting host cell receptors for colonization of mucosal surfaces and tissue invasion, the production of (cyto)toxins and proteases that undermine the host defense, and phenotypic variation intended to evade the immune system. In otherwise-healthy individuals, microbial pathogenic behavior is classically considered to be an intrinsic property of the microorganism, with individual sets of genes providing the necessary tools to direct key events in the infectious process. This concept however, is increasingly challenged by the recognition that host factors may significantly contribute to the intrinsic virulence capabilities of microbial pathogens. It has been demonstrated that bacteria can utilize host matrix constituents, such as fibronectin and vitronectin, to facilitate interactions with mammalian cells (9–12, 15, 17, 23, 28, 38, 40) or use soluble molecules, such as host-derived complement factor H and sialic acid precursors, to protect against being killed by the immune system (29, 34). These observations suggest that by synthesizing specific surface antigens, microbial pathogens can increase their virulence potential by interacting with relevant host molecules.
Recently, we discovered a novel mechanism for microbial exploitation of host molecules in which the human pathogen Neisseria gonorrhoeae binds vitronectin without the involvement of a specific gonococcal vitronectin receptor (10). In this event, vitronectin binding is indirect and mediated through heparin or functionally related sulfated polysaccharides. These molecules form a stable molecular bridge between the bacterial surface adhesin OpaA and vitronectin, both of which are heparin binding proteins (2, 7, 18, 19, 40–42). The relevance of this system was demonstrated by the ability of vitronectin-coated gonococci to invade certain types of epithelial cells that were otherwise not susceptible to gonococcal infection (10).
The ability of proteins to interact with heparin is widespread in both the prokaryotic and eukaryotic worlds. This is due in large part to the fact that heparin binding consensus sites within various proteins are relatively ambiguous, requiring only clusters of six to eight alternating basic and hydropathic amino acid residues (5, 6, 20). These sites interact with exposed sulfate groups present in heparin and other sulfated polysaccharides, such as heparan sulfate and dextran sulfate. Heparin has thus been shown to interact with bacterial, viral, and parasitic pathogens (reviewed in reference 30), as well as with numerous mammalian proteins, including cytokines, adhesive glycoproteins, growth factors, complement components, plasma lipoproteins, and regulators of hemostasis (20). This extensive binding capability led us to hypothesize that binding of heparin and functionally related sulfated polysaccharides is an efficient strategy utilized by microbial pathogens to recruit a diverse array of mammalian heparin binding proteins to their surfaces, bypassing the need to synthesize individual receptors for each of these proteins. In the present study, we tested this concept and evaluated its potential impact on key aspects of the host-pathogen relationship.
MATERIALS AND METHODS
Reagents.
Heparin (sodium salt; 180 U/mg) was obtained from ICN Biomedicals Inc. (Aurora, Ohio). Dextran sulfate (average molecular weight, 500,000) was obtained from Sigma (St. Louis, Mo.). Heparan sulfate was a generous gift from Cornelius Van Gorp (Celsus Laboratories, Cincinatti, Ohio). Vitronectin was purified from adult bovine serum as described previously (45), and the vitronectin-specific polyclonal antiserum was obtained from Calbiochem (San Diego, Calif.). Human fibronectin was obtained from Becton Dickinson (Cockeysville, Md.). The fibronectin-specific monoclonal antibody was purified from culture supernatants of hybridoma cell line HB91 (American Type Culture Collection, Rockville, Md.) as described previously (40). All human cytokines, acidic fibroblast growth factor, and their respective polyclonal antisera were obtained from Peprotech, Inc. (Rocky Hill, N.J.). Human epidermal growth factor (EGF) was obtained from Life Technologies, and the EGF-specific polyclonal antiserum was from Peprotech.
Bacterial strains, cell lines, and growth conditions.
N. gonorrhoeae MS11 (nonpiliated/LOS type b), Neisseria meningitidis B1940siaA (16), staphylococcal strains, Streptococcus pyogenes NZ131, and Yersinia pestis KIM6 and Yersinia enterocolitica “Tacoma” were generously provided by John Swanson (Rocky Mountain Laboratories, Hamilton, Mont.), Matthias Frosch (Institut für Hygiene und Microbiologie, Würzburg, Germany), Michael Minnick (University of Montana, Missoula), Michael Chaussee (Rocky Mountain Laboratories), and Joseph Hinnebusch (Rocky Mountain Laboratories), respectively. Helicobacter pylori MC903 was obtained from the American Type Culture Collection. S. pyogenes and H. pylori were routinely grown on blood agar plates (BBL, Cockeysville, Md.) at 37°C in 5% CO2 or under microaerophilic conditions (using the Campypak Plus system [BBL]), respectively. All other strains were maintained on GC agar plates (composition [per liter]: 3.75 g of Trypticase peptone [BBL], 7.5 g of Thiotone E [BBL], 4 g of K2HPO4, 1 g of KH2PO4, 5 g of NaCl, 1 g of soluble starch [BBL], and 1% Bacto Agar [Difco, Detroit, Mich.], pH 7.2) containing 1% IsoVitaleX (BBL) at 37°C in 5% CO2. CHO-pgs745 cells and HEK293/CCR1 cells were grown in RPMI-1640 and Dulbecco’s modified Eagle’s media, respectively, enriched with 5% fetal bovine serum plus 0.8 mg of geneticin per ml (HEK293/CCR1 cells only).
Binding assays.
For binding experiments, H. pylori was cultured for 3 days in 10 ml of serum-free Brucella Albimi broth (BBL) supplemented with 1% IsoVitaleX without shaking under microaerophilic conditions with the Campypak Plus system, while S. pyogenes was grown to logarithmic phase in 5 ml of Todd-Hewitt–yeast extract broth in 15-ml polystyrene tubes without shaking. All other bacteria were cultured overnight on HEPES agarose plates as previously described (10). The bacteria were suspended in 10 ml of HEPES-buffered saline (HBS; 10 mM HEPES, 145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM glucose, pH 7.2), collected by centrifugation (1,800 × g; 6 min; 20°C), and diluted in HBS to an optical density of 0.13 at 550 nm. Two milliliters (approximately 108 organisms) of this suspension was centrifuged again, and the bacterial pellets were resuspended in 200 μl of HBS containing no sulfated polysaccharide or in HBS containing heparin (5 μg/ml), dextran sulfate (5 μg/ml), or heparan sulfate (15 μg/ml). After incubation for 10 min at 5°C, the bacteria were washed twice by centrifugation (15,000 × g; 2 min; 20°C) with 200 μl of HBS and the pellets were resuspended in 200 μl of HBS containing 5 μg of each purified mammalian protein (Fig. 1 and 2)/ml or 50% pooled normal human serum (Fig. 3) and incubated at 5°C for 10 min. After being washed three times by centrifugation, the pellets were resuspended in 50 μl of deionized water and transferred to new tubes containing 50 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (22), and 5 μl of each sample was subjected to SDS-PAGE. The presence of bacterium-associated mammalian proteins in the lysates was analyzed by immunoblotting with specific antisera or monoclonal antibodies as previously described for vitronectin binding (11). The identity of each protein was confirmed by duplicate Western blots of the purified proteins (data not shown).
FIG. 1.
Sulfated-polysaccharide-mediated binding of mammalian proteins to N. gonorrhoeae producing the heparin binding adhesin OpaA. Gonococci were preincubated with buffer alone (−), heparin (Hp), or dextran sulfate (DS), washed, and subsequently incubated in buffer containing the purified mammalian protein as indicated next to each gel. Bacterium-associated proteins were detected by SDS-PAGE and immunoblotting with polyclonal antisera or monoclonal antibodies specific for each protein, as described in Materials and Methods. Each gel shows the major band(s) recognized by the corresponding antibody for each protein as determined by companion Western blots for each purified protein: vitronectin (Vn), 78 and 68 kDa; fibronectin (Fn), 220 kDa; FGF, 15.8 kDa; EGF, 6.2 kDa; IFN-γ, 16.7 kDa; PF-4, 8 kDa; MIP-1α, 8 kDa; and MCP-3, 9 kDa. αFn, Fn-specific monoclonal antibody; αVn, Vn-specific monoclonal antibody.
FIG. 2.
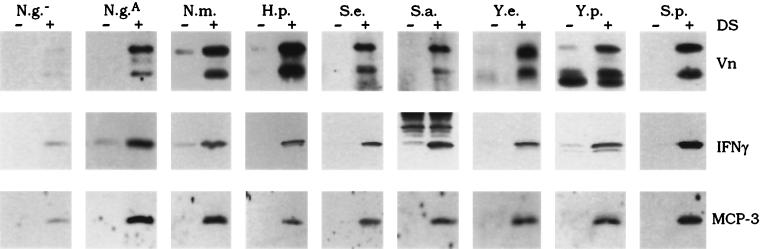
Sulfated-polysaccharide-dependent recruitment of heparin binding proteins by various bacterial pathogens. Each of the bacterial strains was incubated in buffer containing either vitronectin (Vn), IFN-γ, or MCP-3 after preincubation in the absence (−) or presence (+) of dextran sulfate (DS), as described in Materials and Methods. Shown are immunoblots probed with antisera specific for each protein as indicated. N.g.−, N. gonorrhoeae producing no Opa protein; N.g.A, N. gonorrhoeae producing OpaA; N.m., N. meningitidis; H.p., H. pylori; S.e., Staphylococcus epidermidis; S.a., Staphylococcus aureus; Y.e., Y. enterocolitica; Y.p., Y. pestis; S.p., S. pyogenes.
FIG. 3.
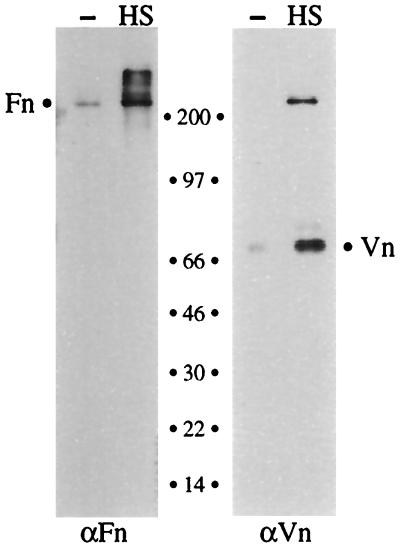
Heparan sulfate-mediated recruitment of heparin binding proteins from human serum. Gonococci were preincubated with buffer alone (−) or with heparan sulfate (HS), washed, and subsequently incubated in buffer containing normal human serum. Bacterium-associated proteins were detected by SDS-PAGE and immunoblotting with vitronectin (Vn)- and fibronectin (Fn)-specific antisera as described in Materials and Methods. The migrations and the sizes of molecular mass standards in kilodaltons are indicated between the gels. The band migrating at >200 kDa may represent cross-reactivity of the anti-vitronectin (αVn) serum with fibronectin. The apparently exclusive binding of the 68-kDa vitronectin band represents the predominance of that band in human serum (26). αFn, anti-fibronectin antibody.
Cell invasion assay.
The CHO-pgs745 cell line was kindly provided by Jeffrey Esko (University of California San Diego, La Jolla). Infection assays with these cells have been described previously (10). Vitronectin was bound to S. pyogenes via dextran sulfate as described above, and approximately 107 bacteria were added to nearly confluent monolayers of CHO-pgs745 cells grown on glass coverslips in 24-well plates. The infections were allowed to proceed for 2 h in serum-free Dulbecco’s modified Eagle’s medium (Life Technologies) at 37°C and 5% CO2. The numbers of extracellular and intracellular streptococci were enumerated microscopically after differential immunogold-silver staining with a polyclonal antiserum specific for group A streptococci (dilution, 1/500; Biodesign, Kennebunk, Maine) as previously described for gonococcal invasion assays (43).
Cell migration assays.
The human embryonic kidney cell line HEK293 transfected with the CCR1 receptor (HEK293/CCR1) was a generous gift from Ji Ming Wang (Frederick Cancer Research and Development Center, Frederick, Md.). Migration of these cells through polyvinylpyrrolidone-free polycarbonate filters with 10-μm pores (Osmonics, Livermore, Calif.) was assayed essentially as described previously (3), except that after trypsinization, the cells were diluted in 10 ml of RPMI medium containing 5% fetal calf serum in 50-ml polypropylene centrifuge tubes (Corning, Corning, N.Y.) and allowed to recover for 2 h at 37°C and 5% CO2. The cells were then washed three times by centrifugation (400 × g; 5 min; 20°C) and resuspended in HBS to a concentration of 3 × 105/ml before addition to the chambers. A total of 108 organisms from logarithmic broth cultures of S. pyogenes or N. gonorrhoeae MS11-OpaA+ grown overnight on HEPES agarose plates were preincubated in HBS alone or in HBS containing 5 μg of dextran sulfate/ml as described for the binding assays above. After being washed by centrifugation (15,000 × g; 2 min; 20°C), the bacteria (3 × 107 streptococci and 108 gonococci) were resuspended in 100 μl of HBS and added to the lower compartment of blind-well chemotaxis chambers (NeuroProbe, Gaithersburg, Md.) with or without MCP-3 (200 ng/ml). The chambers were centrifuged (450 × g; 5 min; 20°C) before assembly and addition of 100 μl of the HEK293/CCR1 cell suspension to the upper chambers. Migration was allowed to proceed for 60 min at 37°C and 5% CO2, after which migrating cells adherent to the underside of the filter were fixed (30 min; 2% paraformaldehyde–0.1% glutaraldehyde), stained for 5 min in 0.05% crystal violet, and counted. The data represent the mean number of cells migrating to the lower side of the filter in 20 high-power fields (final magnification, ×1,000) ± standard errors. The experiments were performed in duplicate and repeated at least three times. Statistical significance was determined by the paired t test.
RESULTS
Sulfated-polysaccharide-mediated binding of bioactive mammalian proteins by N. gonorrhoeae.
The ability of heparin and functionally related molecules to facilitate the recruitment of diverse mammalian heparin binding proteins to bacterial surfaces was initially assayed with N. gonorrhoeae MS11 producing the heparan sulfate-specific adhesin OpaA. For these experiments, gonococci were grown on medium solidified with agarose to avoid interference by agar-derived polysaccharides, which have been shown to bind to OpaA (10, 41). The binding assays involved preincubation of the bacteria in buffer alone or in buffer containing either heparin or the functionally related molecule dextran sulfate in order to saturate OpaA with sulfated polysaccharide. This was followed by incubation in fresh buffer containing one of the following proteins: vitronectin, fibronectin, gamma interferon (IFN-γ), platelet factor 4 (PF-4), macrophage inflammatory protein 1α (MIP-1α), monocyte chemotactic protein 3 (MCP-3), or acidic fibroblast growth factor (FGF). Although these proteins have very different functional and structural characteristics, they all share the ability to bind to heparin. After removal of unbound protein, the bacteria were lysed and associated proteins were detected by electrophoresis and immunoblotting with antibodies specific for each protein.
Figure 1 shows that each of the heparin binding proteins tested bound strongly to gonococci that had been preincubated with dextran sulfate. In contrast, none of the proteins bound to bacteria that had been preincubated in buffer alone, demonstrating that the sulfated polysaccharide was critical in the binding event. Heparin also enabled the binding of proteins to the bacteria, although the interaction was more variable. EGF, a protein which does not exhibit heparin binding capabilities, did not interact with N. gonorrhoeae, even when the bacteria were saturated with sulfated polysaccharides. These data demonstrate that while N. gonorrhoeae does not inherently synthesize cell surface receptors for any of these vastly different mammalian heparin binding proteins, efficient recruitment of all of the proteins could be established after binding of sulfated polysaccharides to the microorganisms.
Binding of mammalian proteins by various bacterial species.
The ability to bind heparin and related sulfated polysaccharides is widespread in the microbial world. Thus, we hypothesized that the sulfated polysaccharide-mediated binding of mammalian proteins observed for N. gonorrhoeae may represent a novel form of phenotypic modulation that can be exploited by any microorganism with heparin binding capabilities. This hypothesis was tested by examining the binding of vitronectin, IFN-γ, or MCP-3 to nine different bacterial species, including H. pylori, S. pyogenes, and Yersinia, Staphylococcus, and pathogenic Neisseria species. For these assays, the microorganisms were cultured in media free of agar-derived sulfated polysaccharides, as described above. Preincubation of every bacterial species with dextran sulfate resulted in efficient binding of all three of these heparin binding proteins (Fig. 2). In contrast, none of the proteins bound significantly to bacteria that had been preincubated without dextran sulfate. Furthermore, Opa− N. gonorrhoeae, which has been shown to exhibit negligible heparin binding capabilities (7, 42), also lacked the ability to bind significant amounts of protein. These data indicate that sulfated polysaccharides are absolutely required for the binding of vitronectin, IFN-γ, and MCP-3 to the tested bacterial species and confirm that the recruitment of mammalian proteins through sulfated-polysaccharide intermediates is a common feature among bacterial pathogens that bind heparin.
Heparan sulfate-mediated recruitment of proteins from a complex biological fluid.
During a natural infection, microbial pathogens encounter highly complex and changing host environments containing various polysaccharides and mixtures of proteins, only some of which may have heparin binding properties. To investigate the efficiency of the microbial sulfated-polysaccharide-mediated recruitment mechanism in a more native biological setting, we tested OpaA+ gonococci for their ability to recruit proteins from human serum, which contains measurable amounts of fibronectin and vitronectin (25, 33). In these experiments, the bacteria were preincubated in buffer with or without heparan sulfate, a natural heparin-like molecule which makes up the side chains of many cell surface proteoglycans and is shed into the environment during tissue damage (21). After incubation of the heparan sulfate-coated bacteria in 50% normal human serum (10 min; 5°C), the bacteria were washed and proteins bound to the gonococcal surface were assayed by Western blotting with vitronectin- and fibronectin-specific antibodies as described for Fig. 1. This procedure demonstrated that gonococci that had been preincubated with heparan sulfate efficiently recruited both fibronectin and vitronectin from human serum (Fig. 3). In contrast, virtually no binding was detected for bacteria that lacked heparan sulfate at their surfaces. These data show that bacterial pathogens can utilize natural glycosaminoglycans (i.e., heparan sulfate) to simultaneously recruit multiple heparin binding proteins from a biological fluid, even though numerous other proteins are present in the mixture.
Polysaccharide-mediated recruitment of vitronectin enhances the invasive potential of bacterial pathogens.
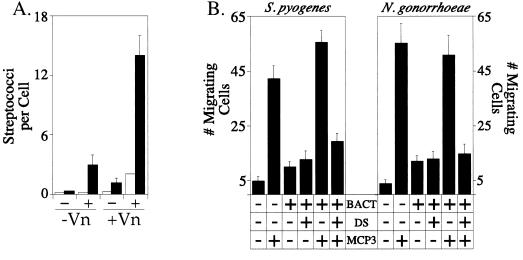
A key question regarding the glycosaminoglycan-mediated interaction of host proteins with microbial pathogens lies in the impact of the event on both bacterial behavior and the host’s physiology. To address these issues, we first analyzed the effect of sulfated polysaccharide-mediated vitronectin binding on the interaction between S. pyogenes and Chinese hamster ovary (CHO) cells, in keeping with the increasingly appreciated role of vitronectin and related adhesive glycoproteins as mediators of bacterial colonization and invasion of eukaryotic cells. In these experiments, epithelial cells deficient in heparan sulfate proteoglycan biosynthesis (CHO-pgs745) were utilized in order to eliminate any influence of proteoglycan molecules that are present at the cell surface or shed into the medium during the assay (33). Preincubation of S. pyogenes in buffer lacking dextran sulfate and vitronectin resulted in virtually no interaction between the bacteria and CHO-pgs745 cells (Fig. 4). After sequential preincubation of the streptococci with dextran sulfate (5 μg/ml) and then with vitronectin (5 μg/ml), however, a mean internalization level of 14.1 bacteria per cell was achieved. These results are fully in line with the observed vitronectin binding under these conditions (Fig. 2). Similar experiments with N. gonorrhoeae producing OpaA gave comparable results (reference 10 and data not shown). Together, these data indicate that sulfated-polysaccharide-dependent binding of vitronectin to bacterial surfaces is an effective bacterial strategy to enhance invasive potential.
FIG. 4.
Biological impact of glycosaminoglycan-mediated interactions between mammalian proteins and pathogenic bacteria. (A) Adherence (open bars) and internalization (solid bars) of S. pyogenes by CHO-pgs745 cells was assayed after preincubation of bacteria in buffer without (−) or with (+) dextran sulfate and subsequent incubation in buffer without (−Vn) or with (+Vn) vitronectin. The experiments were performed in triplicate and repeated four times; the data are presented as the mean number of bacteria per cell ± standard errors. (B) Migration of HEK293 cells transfected with the CCR1 receptor toward MCP-3 in the presence and absence of dextran sulfate (DS)-coated bacteria. The data represent the mean number of migrating cells in 20 high-power fields (final magnification, ×1,000) ± standard errors. The experiments were performed in duplicate and repeated at least three times. Bact, bacteria.
Bacterial recruitment of chemokines inhibits efficient migration of CCR1-expressing cells.
Biological effects resulting from the glycosaminoglycan-mediated bacterial recruitment of chemokines were evaluated by employing the well-documented ability of the chemokine MCP-3 to induce chemotaxis of monocytes and other cells producing the C-C chemokine receptor, CCR1 (1, 3, 39). Through the use of blind-well chemotactic chambers, we assayed the directed migration of human embryonic kidney cells transfected with cDNA encoding CCR1 (HEK293/CCR1) toward MCP-3 in the absence and presence of N. gonorrhoeae or S. pyogenes after preincubation of the bacteria with dextran sulfate. Fig. 4 shows that the presence of MCP-3 alone in the lower chamber stimulated the migration of HEK293/CCR1 cells at levels approximately 8.4-fold (S. pyogenes) and 14.1-fold (N. gonorrhoeae) over migration in buffer alone. When MCP-3 plus bacteria that had been preincubated without dextran sulfate were included in the lower chamber, similar levels of migration were observed. However, when dextran sulfate-coated bacteria were added to the lower chamber along with MCP-3, migration was inhibited by 65% for S. pyogenes (P < 0.002) and 71% for N. gonorrhoeae (P < 0.001) compared to that toward uncoated bacteria plus MCP-3. In the absence of MCP-3, there was no substantial migration toward bacteria present in the lower chamber whether or not they were preincubated with dextran sulfate. These results indicate that bacteria coated with sulfated polysaccharides inhibited migration of CCR1-expressing cells, presumably by sequestering MCP-3 and preventing the formation of an effective chemotactic gradient.
DISCUSSION
In this report, we describe a novel, common bacterial strategy by which pathogens, including H. pylori, S. pyogenes, and Yersinia, Staphylococcus, and pathogenic Neisseria species, may amplify their pathogenic potential without the requirement for complex genetic machineries. The mechanism, schematically presented in Fig. 5, enables bacteria to recruit structurally and functionally diverse bioactive host proteins, including inflammatory mediators, adhesive glycoproteins, growth factors, and cytokines, without producing separate receptors for each protein. The bacteria accomplish this by binding heparin or functionally related sulfated polysaccharides to their surfaces, which in turn serve as secondary binding sites for mammalian heparin binding proteins. Recruitment of these factors profoundly impacts bacterial behavior, as illustrated by observed increases in bacterial invasiveness and inhibition of chemokine-induced chemotaxis.
FIG. 5.
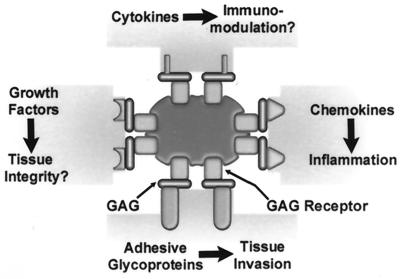
Proposed model for heparin-mediated recruitment of mammalian proteins. Microbes (pictured in the center) with heparin binding activities bind heparin and functionally related glycosaminoglycans (GAG) from the environment through species-specific bacterial glycosaminoglycan receptors. The glycosaminoglycans bound at the bacterial cell surface subsequently serve as universal binding sites for theoretically any mammalian heparin binding protein present in the environment, including adhesive glycoproteins, chemokines, growth factors, and cytokines. The repertoire of surface-bound proteins may affect various aspects of microbial virulence and host defense systems, such as chemotaxis, tissue invasion, tissue integrity, and immunological responses.
A key aspect of the described mechanism lies in the property of single sulfated polysaccharide molecules to interact simultaneously with multiple proteins. This allows the formation of a stable molecular bridge between heparin binding proteins present on the surfaces of most pathogens (7, 8, 24, 27, 32, 37, 41, 42) and mammalian heparin binding proteins (10). Sulfated polysaccharides, such as glycosaminoglycans, appear to be particularly suited for this system, as they contain multiple binding sites that recognize clusters of only six to eight alternating basic and hydropathic amino acid residues that are typical of heparin binding consensus sequences (5, 6, 20). The relatively low level of binding specificity enables promiscuous binding of a number of different microbial and mammalian heparin binding proteins, thus providing the microbes with a tremendous capacity for altering the protein composition of their surfaces (Fig. 1 and 2). Additional variation may be achieved through the variable molecular characteristics of different types of sulfated polysaccharides, which may exhibit slightly different heparin binding properties (10, 31, 36). Our data indicate that heparin, glycosaminoglycans, and dextran sulfate can act as bridging molecules but that they interact with different repertoires of heparin binding proteins (Fig. 1). Thus, under natural conditions, the bacterial phenotype may largely depend on the availability and nature of the sulfated polysaccharides as well as on heparin binding proteins present at the various infection niches.
A remarkable feature of the recruitment strategy is that it provides a rapid and efficient instrument for responding to environmental changes through the production of a single heparin binding protein. This is in contrast to most classical genomic systems of surface variation and adaptation, which often consume considerable amounts of time, energy, and genetic space (13). An additional major difference between the two strategies is that the genomic systems provide programmed bacterial phenotypes while the extragenomic recruitment strategy has a less predictable outcome that largely depends on environmental conditions. This flexibility, however, appears to nicely complement the genetically encoded pathogenic potential and may enable optimal adaptation to the highly dynamic environment encountered in each host.
The potential impact of the recruitment strategy on pathogenesis was substantiated by the observed increased bacterial invasion of epithelial cells in the presence of heparin and vitronectin. Microbial invasion of mammalian cells is considered to be an important virulence trait, providing pathogens with access to deeper tissues and temporary protection against inflammatory and immunological responses. Adhesive glycoproteins, such as vitronectin and fibronectin, have been implicated as intermediates in bacterial interactions with host cell integrin receptors, resulting in bacterial colonization and invasion of epithelial cells (9–12, 15, 17, 23, 28, 38, 40). Our data indicate that pathogens which lack the intrinsic ability to synthesize specific vitronectin receptors can still acquire the invasive capabilities associated with this protein through the glycosaminoglycan bridge mechanism of protein recruitment. This expansion of pathogenic potential was observed for S. pyogenes and N. gonorrhoeae, emphasizing the universality and significance of the recruitment mechanism.
The broad range of mammalian proteins that can be recruited to the bacterial cell surface (Fig. 1), which includes several cytokines and inflammatory mediators, opens the possibility that microorganisms also exploit this system to manipulate the host defense. Support for this concept is provided by the effect of bacterial recruitment of the chemokine MCP-3. MCP-3 is a heparin binding member of the C-C chemokine subfamily that induces the directed migration of cells producing its receptor, CCR1 (1), and stimulates the specific infiltration of monocytes into tissues (39, 44, 46). Our data demonstrate that sulfated polysaccharides mediate MCP-3 binding to N. gonorrhoeae and S. pyogenes and that this recruitment significantly inhibits the migration of CCR1-expressing cells toward MCP-3. This is most likely due to the sequestering of MCP-3 by sulfated polysaccharides bound to the microbial surface, preventing the establishment of an effective chemotactic gradient. The bacterial modulation of chemokine activity is to some extent reminiscent of chemokine sequestering through overexpression of viral C-C chemokine receptor homologs in cytomegalovirus-infected fibroblasts, although chemotaxis was not specifically assayed in these studies (4). Chemokine-directed infiltration of leukocytes during inflammatory responses is a primary host defense mechanism against microbial pathogens (35). Considering the intricate networks of overlapping gradients involving multiple chemoattractants in the control of leukocyte homing into target tissues (14), it is likely that minute disturbances in these gradients caused by microbes coated with sulfated polysaccharides profoundly influence the appropriate infiltration of effector cells to sites of infection and thus the progression of disease.
In mammals, the polysaccharide molecules which participate in the bridge mechanism are unknown. One candidate class of molecules are heparan sulfate glycosaminoglycans. The regulated shedding of heparan sulfate-containing proteoglycans during tissue damage (21), in conjunction with the efficient bacterial recruitment of both vitronectin and fibronectin from serum through heparan sulfate, supports this notion and confirms that the glycosaminoglycan bridge mechanism is functional in a highly complex biological setting. However, it should be noted that bacteria, viruses, and parasites can often bind heparan sulfate and heparin as well as related polysaccharides, indicating that additional undefined sulfated polysaccharides (41) may operate in vivo. This, combined with the fact that many mammalian heparin binding proteins function as adhesive, inflammatory, and immunomodulatory intermediates, led us to suggest that appreciation of the polysaccharide-mediated recruitment mechanism will greatly impact our views regarding microbial pathogenesis and the prospects for the use of glycosaminoglycan derivatives as prophylactic or therapeutic reagents.
ACKNOWLEDGMENTS
We extend our appreciation to Ji Ming Wang for providing the CCR1-transfected cells, Jeffrey Esko for the CHO-pgs745 mutant cell line, and Cornelius Van Gorp (Celsus Laboratories) for the heparan sulfate. We also thank Tom Schwan, Joe Hinnebusch, and Kit Tilly for critical reading of the manuscript.
REFERENCES
- 1.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 2.Barnes D W, Reing J E, Amos B. Heparin-binding properties of human serum spreading factor. J Biol Chem. 1985;260:9117–9122. [PubMed] [Google Scholar]
- 3.Ben-Baruch A, Xu L, Young P, Bengali K, Oppenheim J, Wang J. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors. C-C CKR1, a receptor for macrophage inflammatory protein-1 alpha/Rantes, is also a functional receptor for MCP3. J Biol Chem. 1995;270:22123–22128. doi: 10.1074/jbc.270.38.22123. [DOI] [PubMed] [Google Scholar]
- 4.Bodaghi B, Jones T R, Zipeto D, Vita C, Sun L, Laurent L, Arenzana-Seisdedos F, Virelizier J L, Michelson S. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J Exp Med. 1998;188:855–866. doi: 10.1084/jem.188.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardin A D, Demeter D A, Weintraub H J, Jackson R L. Molecular design and modeling of protein-heparin interactions. Methods Enzymol. 1991;203:556–583. doi: 10.1016/0076-6879(91)03030-k. [DOI] [PubMed] [Google Scholar]
- 6.Cardin A D, Weintraub H J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Chen T, Belland R J, Wilson J, Swanson J. Adherence of pilus−Opa+ gonococci to epithelial cells in vitro involves heparan sulfate. J Exp Med. 1995;182:511–517. doi: 10.1084/jem.182.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries F P, Cole R, Dankert J, Frosch M, van Putten J P. Neisseria meningitidis producing the Opc adhesin binds epithelial cell proteoglycan receptors. Mol Microbiol. 1998;27:1203–1212. doi: 10.1046/j.1365-2958.1998.00763.x. [DOI] [PubMed] [Google Scholar]
- 9.Dehio M, Gomez-Duarte O G, Dehio C, Meyer T F. Vitronectin-dependent invasion of epithelial cells by Neisseria gonorrhoeae involves alpha(v) integrin receptors. FEBS Lett. 1998;424:84–88. doi: 10.1016/s0014-5793(98)00144-6. [DOI] [PubMed] [Google Scholar]
- 10.Duensing T D, van Putten J P M. Vitronectin binds to the gonococcal adhesin OpaA through a glycosaminoglycan molecular bridge. Biochem J. 1998;334:133–139. doi: 10.1042/bj3340133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duensing T D, van Putten J P M. Vitronectin mediates internalization of Neisseria gonorrhoeae by Chinese hamster ovary cells. Infect Immun. 1997;65:964–970. doi: 10.1128/iai.65.3.964-970.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippsen L F, Valentin-Weigand P, Blobel H, Preissner K T, Chhatwal G S. Role of complement S protein (vitronectin) in adherence of Streptococcus dysgalactiae to bovine epithelial cells. Am J Vet Res. 1990;51:861–865. [PubMed] [Google Scholar]
- 13.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foxman E F, Campbell J J, Butcher E C. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Duarte O G, Dehio M, Guzman C A, Chhatwas G S, Dehio C, Meyer T F. Binding of vitronectin to Opa-expressing Neisseria gonorrhoeae mediates invasion of HeLa cells. Infect Immun. 1997;65:3857–3866. doi: 10.1128/iai.65.9.3857-3866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammerschmidt S, Hilse R, van Putten J P, Gerardy-Schahn R, Unkmeir A, Frosch M. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J. 1996;15:192–198. [PMC free article] [PubMed] [Google Scholar]
- 17.Hanski E, Horwitz P A, Caparon M G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992;60:5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi M, Akama T, Kono I, Kashiwagi H. Activation of vitronectin (serum spreading factor) binding of heparin by denaturing agents. J Biochem (Tokyo) 1985;98:1135–1138. doi: 10.1093/oxfordjournals.jbchem.a135363. [DOI] [PubMed] [Google Scholar]
- 19.Hayman E G, Pierschbacher M D, Ohgren Y, Ruoslahti E. Serum spreading factor (vitronectin) is present at the cell surface and in tissues. Proc Natl Acad Sci USA. 1983;80:4003–4007. doi: 10.1073/pnas.80.13.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson R L, Busch S J, Cardin A D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991;71:481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- 21.Kainulainen V, Wang H, Schick C, Bernfield M. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J Biol Chem. 1998;273:11563–11569. doi: 10.1074/jbc.273.19.11563. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Limper A H, Standing J E, Hoffman O A, Castro M, Neese L W. Vitronectin binds to Pneumocystis carinii and mediates organism attachment to cultured lung epithelial cells. Infect Immun. 1993;61:4302–4309. doi: 10.1128/iai.61.10.4302-4309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menozzi F D, Bischoff R, Fort E, Brennan M J, Locht C. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc Natl Acad Sci USA. 1998;95:12625–12630. doi: 10.1073/pnas.95.21.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosesson M W, Umfleet R A. The cold-insoluble globulin of human plasma. J Biol Chem. 1970;245:5728–5736. [PubMed] [Google Scholar]
- 26.Nakashima N, Miyazaki K, Ishikawa M, Yatohgo T, Ogawa H, Uchibori H, Matsumoto I, Seno N, Hayashi M. Vitronectin diversity in evolution but uniformity in ligand binding and size of the core polypeptide. Biochim Biophys Acta. 1992;1120:1–10. doi: 10.1016/0167-4838(92)90417-c. [DOI] [PubMed] [Google Scholar]
- 27.Ortega-Barria E, Pereira M E. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991;67:411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- 28.Patti J M, Allen B L, McGavin M J, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 29.Ram S, McQuillen D P, Gulati S, Elkins C, Pangburn M K, Rice P A. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. 1998;188:671–680. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rostrand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.San Antonio J D, Slover J, Lawler J, Karnovsky M J, Lander A D. Specificity in the interactions of extracellular matrix proteins with subpopulations of the glycosaminoglycan heparin. Biochemistry. 1993;32:4746–4755. doi: 10.1021/bi00069a008. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt K H, Ascencio F, Fransson L A, Kohler W, Wadstrom T. Studies on binding of glycosaminoglycans to Streptococcus pyogenes by using 125I-heparan sulphate as a probe. Int J Med Microbiol Virol Parasitol Infect Dis. 1993;279:472–483. doi: 10.1016/s0934-8840(11)80419-6. [DOI] [PubMed] [Google Scholar]
- 33.Shaffer M C, Foley T P, Barnes D W. Quantitation of spreading factor in human biologic fluids. J Lab Clin Med. 1984;103:783–791. [PubMed] [Google Scholar]
- 34.Smith H, Cole J A, Parsons N J. The sialylation of gonococcal lipopolysaccharide by host factors: a major impact on pathogenicity. FEMS Microbiol Lett. 1992;79:287–292. doi: 10.1111/j.1574-6968.1992.tb14054.x. [DOI] [PubMed] [Google Scholar]
- 35.Standiford T J, Kunkel S L, Strieter R M. Role of chemokines in antibacterial host defense. Methods Enzymol. 1997;288:220–241. doi: 10.1016/s0076-6879(97)88017-2. [DOI] [PubMed] [Google Scholar]
- 36.Sudhalter J, Folkman J, Svahn C M, Bergendal K, D’Amore P A. Importance of size, sulfation, and anticoagulant activity in the potentiation of acidic fibroblast growth factor by heparin. J Biol Chem. 1989;264:6892–6897. [PubMed] [Google Scholar]
- 37.Utt M, Wadstrom T. Identification of heparan sulphate binding surface proteins of Helicobacter pylori: inhibition of heparan sulphate binding with sulphated carbohydrate polymers. J Med Microbiol. 1997;46:541–546. doi: 10.1099/00222615-46-7-541. [DOI] [PubMed] [Google Scholar]
- 38.Valentin-Weigand P, Grulich-Henn J, Chhatwal G S, Muller-Berghaus G, Blobel H, Preissner K T. Mediation of adherence of streptococci to human endothelial cells by complement S protein (vitronectin) Infect Immun. 1988;56:2851–2855. doi: 10.1128/iai.56.11.2851-2855.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Damme J, Proost P, Lenaerts J P, Opdenakker G. Structural and functional identification of two human, tumor-derived monocyte chemotactic proteins (MCP-2 and MCP-3) belonging to the chemokine family. J Exp Med. 1992;176:59–65. doi: 10.1084/jem.176.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Putten J P M, Duensing T D, Cole R L. Entry of OpaA+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol Microbiol. 1998;29:369–380. doi: 10.1046/j.1365-2958.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 41.van Putten J P M, Hayes S F, Duensing T D. Natural proteoglycan receptor analogs determine the dynamics of Opa adhesin-mediated gonococcal infection of Chang epithelial cells. Infect Immun. 1997;65:5028–5034. doi: 10.1128/iai.65.12.5028-5034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Putten J P M, Paul S M. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 1995;14:2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Putten J P M, Weel J F L, Grassme H U C. Measurements of invasion by antibody labeling and electron microscopy. Methods Enzymol. 1994;236:420–437. doi: 10.1016/0076-6879(94)36031-6. [DOI] [PubMed] [Google Scholar]
- 44.Wuyts A, Proost P, Put W, Lenaerts J P, Paemen L, van Damme J. Leukocyte recruitment by monocyte chemotactic proteins (MCPs) secreted by human phagocytes. J Immunol Methods. 1994;174:237–247. doi: 10.1016/0022-1759(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 45.Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988;13:211–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- 46.Zachariae C O, Anderson A O, Thompson H L, Appella E, Mantovani A, Oppenheim J J, Matsushima K. Properties of monocyte chemotactic and activating factor (MCAF) purified from a human fibrosarcoma cell line. J Exp Med. 1990;171:2177–2182. doi: 10.1084/jem.171.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]