Abstract
Banana is an important food crop responsible for ensuring food security, nutrition, and employment for a significant portion of the world population. It has fairly broad genetic diversity and is distributed widely across the globe. Due to its socio-economic importance, there has been growing demand for healthy and improved planting materials of banana. In recent years many companies and organizations are working hard to narrow down the gap between demand and supply of quality planting materials. The other challenges includes its susceptibility to adverse environmental conditions, attack of various pests/pathogens and improvement of nutritional quality of bananas. To address these issues, refinement of existing techniques and introduction of new experimental tools are required. However, the genetic improvement of bananas to a large extent is limited by using conventional methods due to polyploidy, heterozygosity, and sterility of this plant. For rapid multiplication and obtaining disease free and healthy plants, efficient in vitro propagation techniques and fine tuning of the existing protocols are being tried in many laboratories across the globe. Besides, for developing a successful protocol for propagation of different cultivars of bananas, a deeper understanding of the factors associated with various steps of its multiplication till transfer to the land is immensely critical. Similarly, developing biotic and abiotic stress tolerant banana and enhancing its commercial value through biotechnological interventions could be very useful. The key intent of this review is to highlight the research endeavor in this direction, associated challenges and future prospects.
Keywords: Banana, Micropropagation, Regeneration, Genetic improvement, Stress tolerance
Banana; Micropropagation; Regeneration; Genetic improvement; Stress tolerance.
1. Introduction
Banana (Musa spp. L.) is one of the most important cash crops and contributes immensely to global food security (Esan et al., 2022; Tripathi et al., 2020). It belongs to the family Musaceae and is distributed all around the world. With an annual estimated production of 153 million tonnes (FAOSTAT, 2019), the banana is the most popular fruit in terms of international trade (Tripathi et al., 2020). The major producers of bananas include India, China, Indonesia, Brazil, Ecuador, Philippines, Guatemala, Colombia, and Angola, where India is the largest producer with 30.808 million metric tons of production (FAO, 2020). Banana fruit is very popular especially in developing nations due to its low price and high nutritive value. It is a rich source of carbohydrates, proteins, minerals (such as potassium calcium, magnesium, and manganese), and vitamins including vitamins A, C, and B6 (Ranjha et al., 2020). Besides the fruit, the other parts obtained from the banana plant such as peels, pseudo-stem, rhizome, leaves, etc. are utilized in various industries such as agro, food, textiles, etc (Adeniyi et al., 2019, 2021; Ighalo and Adeniyi, 2019; Akatwijuk et al., 2022; Mathew et al., 2021).
The genus Musa is divided into 5 sections viz. Australimusa, Callimusa, Rhodochlamys, Ingentimusa, Eumusa (Lamare et al., 2017). Section Australimusa having basic chromosome number 10 is distributed in Queensland, New Caledonia Philippines, and Australia and is mainly used in the form of fiber, fruit, and vegetables. It includes 5–6 species (M. textilis, M. maclayi, M. lolodensis, M. peekelii, and M. fehi). Section Callimusa with chromosome number 10 is distributed in Indo-China and Indonesia and is mainly used for ornamental purposes. It consists of three species (M. coccinea, M. violascens, and M. gracilis). Section Eumusa having chromosome number 11 is distributed in India and is mainly used in the form of fiber, fruit, vegetables and have medicinal applications. It consists of the following species: M. acuminata, M. balbisiana, M. cheesmani, M. flaviflora, M. itinerans, M. schizocarpa, M. sikkimensis, M. nagensium, M. halabanensisand, M. ochracea. Section Rhodochlamis possessing chromosome number 11 mainly consists of ornamental species (M. aurantiaca, M. laterita, M. sanguinea, M. ornate, M. velutina, M. mannii, M. rosea and, M. rubra) which are distributed throughout India, Indo-China, Philippines, Thailand, and Malaysia (Debnath et al., 2019). There are more than 1000 varieties of banana cultivated all over the world, however, the Cavendish varieties of banana are considered as one of the most important commercial varieties (Tripathi et al., 2019; Thangavelu et al., 2021).
Banana cultivation largely regulates the agri-based global bioeconomy. Hence, understanding the associated challenges in its production and developing appropriate strategies for addressing these concerns are of paramount importance. In recent years, there has been increasing interest to grow healthy and commercially important cultivars of bananas. However, there exists a huge gap between the demand and supply of healthy planting materials (Jacobsen et al., 2019; Nkengla-Asi et al., 2021). Further, in the last few decades, banana production is under threat due to different climatic factors and pathogenic agents such as bacteria, viruses, fungi, and nematodes (Tripathi et al., 2019). To circumvent these challenges and to produce agronomically superior disease-resistant banana crop, various traditional breeding methods have been employed such as diploid breeding (Rowe and Rosales, 1994), 3x/2x strategy (pollination of susceptible triploids with male fertile diploids that are resistant), and 4x/2x strategy which involves the production of a tetraploid parent by chromosome doubling of an ancestral diploid with good agronomic trait followed by the production of triploid hybrids through hybridization of a diploid parent with the tetraploid (Menon, 2016). However, polyploidy in Musa is the biggest challenge in banana breeding which ranges from diploid to tetraploidy (Nansamba et al., 2020). Thus, the development of a new banana cultivar is very exhaustive via breeding because the selection of desirable characters can take more than 12 years (Menon, 2016). A high level of heterozygosity and the requirement of a large population for the selection of individual clones with desirable agronomic traits, make it more cumbersome. Moreover, introgression of desired gene loci from diploid wild cultivars of banana also carries certain undesirable traits such as non-parthenocarpy and low yield (Menon, 2016). Thus, to sustain banana cultivation in the era of changing global climate, the development of new elite banana varieties and preparation of disease-free planting material for its commercial plantations using non-conventional approaches is much warranted. Biotechnology has largely provided solutions to many of these existing problems. The present review focuses on biotechnological interventions in bananas for their mass multiplications and improvement.
2. Micropropagation of banana
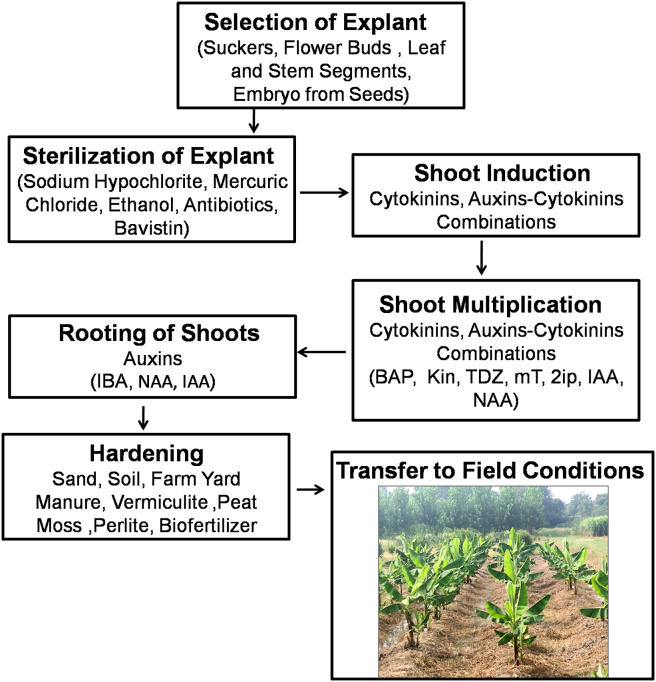
Banana plants are traditionally propagated through vegetative means using suckers (Nkengla-Asi et al., 2021). However, plants produced through suckers have their own limitations as it leads to disease transmission, low productivity, and poor preservation of original plant genetic material (Hussein, 2012). Moreover, there is a huge demand for quality planting materials to narrow the gap between demand and supply. In this scenario, micropropagation techniques have been used in many parts of the world to produce healthy, disease-free banana plants throughout the year that perform better under field conditions (Abdalla et al., 2022). There are several reports available on micropropagation of different cultivars of banana (Table S1). These reports suggest that for successful micropropagation of bananas, optimization of micropropagation protocol is critical. In the present review, we have divided the protocol of micropropagation of bananas into four distinct stages such as (i) initiation of aseptic cultures (ii) shoot multiplication (iii) in vitro rooting of microshoots (iv) hardening of rooted microshoots (Figure 1) and tried to understand the factors that influence each of these stages. A better understanding of these factors will lead to the development of robust micropropagation protocols for large-scale propagation of different cultivars of bananas.
Figure 1.
Different steps involved in the in vitro propagation of banana.
2.1. Factors that influence micropropagation of banana
2.1.1. Stage I: initiation of aseptic cultures
Initiation of aseptic culture is one of the most important steps for the development of a robust micropropagation protocol (Kaur et al., 2022). It involves the identification and selection of the desired explants, and the development of an effective explant sterilization process (Loyola-Vargas and Ochoa-Alejo, 2018) (Figures 2a, 2b). Further, initial troubleshooting involving the browning of explants is critical for the initial culture establishment of banana.
Figure 2.
An overview of micropropagation in banana. (a) Sword sucker from mother plant; (b) Primary inoculation; (c) Shoot induction; (d) Shoot multiplication; (e) Rooting; (f) Primary hardening; (g) Secondary hardening.
2.1.1.1. Choice of explants
Shoot cultures of bananas can be initiated conventionally from mother sword suckers (Hasan et al., 2020) (Figure 2a). However, explant material from mature individuals with known responses to environmental factors and with proclaimed quality traits is preferred. Moreover, larger explants are advantageous because they contain a shoot apex with more lateral buds that develop into shoots, but they are more susceptible to contamination and blackening (Strosse et al., 2004; Kannahi and Buvaneswari, 2019). Among different plant parts of banana, suckers are considered the most suitable explant for micropropagation as they have a vascular connection with the mother plant, are easy to isolate and culture, and produce true to mother type plantlets (Heslop-Harrison and Schwarzacher, 2007).
2.1.1.2. Sterilization
Surface sterilization of explants is a process in which explants are immersed into the appropriate concentration of chemical sterilant to establish contamination-free cultures (Bello et al., 2018). The type of sterilant used primarily depends on the size and type of explants along with the procedure of disinfection (Strosse et al., 2004; Mihaljević et al., 2013). Sodium hypochlorite is the most commonly used agent for surface sterilization. It is very effective against all types of microbial contaminations. It causes biosynthetic alterations in cellular metabolism through phospholipids destruction, fatty acid degradation, formation of chloramines that interfere in cellular metabolism, oxidative damage, and irreversible enzymatic inactivation in bacteria (Estrela et al., 2002). Mercuric chloride (HgCl2) has antimicrobial activity against both fungi and bacteria. It may be used at 0.05–0.1% for 1–5 min according to the type of explant of different plants. It has been observed that a longer duration of 0.1% concentration of mercuric chloride treatment is effective to decrease bacterial contamination in cultures. However, higher concentrations of mercuric chloride can be toxic to plant cells and tissues (Kang et al., 2020; Kapadia and Patel, 2021). Besides, bavistin (0.1%) and 70% (v/v) ethanol also act as a powerful sterilizing agent for banana explants (Bhutani et al., 2021; Prakasha et al., 2019; Yadav et al., 2021). In addition, some antibiotics such as penicillin, ampicillin, and ticarcillin have also been used to curb bacterial contaminants in banana tissue cultures (El-Banna et al., 2021). Liquid cultures containing rifampicin for up to 30 days were able to inhibit the growth of Gram-positive bacteria in banana shoot tips with no effect on plant growth (Van Den Houwe and Swennen, 1999).
2.1.1.3. Browning of the medium
Browning of the medium occurs due to the oxidation of phenolic compounds produced by banana explants resulting in reduced cell division and plant growth (Rodrigues et al., 2022). These exudates form a barrier around the tissue which prevents nutrient uptake (Tabiyeh et al., 2005; Krishna et al., 2008). They further leach into the culture medium and result in the browning of the media. It is for this reason that fresh shoots need to be transferred to new media every now and then (Ahmad et al., 2013). It has been observed that when banana explants were treated with citric acid and ascorbic acid for 30 min, the browning ceased (Ko et al., 2009; De Anicezio, 2012). Cessation of media browning could be attributed to the ascorbic acid's activity to scavenge oxygen radicals produced when the plant is wounded which prevents the cells from further damage. Washing of explants with antioxidant solution {0.125% potassium citrate: citrate (K–C: C) in a ratio of 4:1 w/w} was useful to eliminate browning in Musa spp. cv. Kanthali (Titov et al., 2006). A similar response was observed when explants of banana were presoaked/pre-treated with 0.1–0.5 mg/mL of potassium citrate and citrate (K–C: C) (Onuoha et al., 2011). Pre-soaking of explants in 1.2 g/l of ascorbic acid solution prevented lethal browning in local Musa spp. cv. Mzuzu (Ngomuo et al., 2014). For genotypes like Musa spp. ABB and BB groups, which produce compact proliferating masses of buds, the addition of activated charcoal to the medium minimized the phenol exudation from explants (Strosse et al., 2004). Other anti-browning agents that have been used in controlling phenolics in bananas include L-cysteine, polyvinylpyrrolidone (Onuoha et al., 2011; Oliveira et al., 2011).
2.1.2. Stage II: shoot multiplication
Shoot multiplication is the most crucial step in the development of an efficient micropropagation protocol that directly affects the success of the given protocol (Pati et al., 2006; Nowakowska et al., 2022) (Figures 2c, 2d). Various factors that greatly influence the shoot multiplication in bananas are highlighted in this section.
2.1.2.1. Genotype
Genotype of the given cultivar is one of the key factors that influence the shoot proliferation rate. In comparison to other banana genotypes, the B genotype exhibits a higher shoot proliferation rate under in vitro conditions (Vuylsteke et al., 1996). This could be due to the variation in the activity of cytokinins in different genotypes that can be explained by their different uptake rates (Blakesley, 1991), varied translocation rates to meristematic regions, and metabolic processes, in which the cytokinin may be degraded or conjugated with amino acids or sugars to form biologically inactive compounds (Tran Thanh Van and Trinh, 1990; Kaminek, 1992). The presence of less endogenous cytokinin prompts the requirement of a higher concentration of exogenous cytokinin for multiplication in recalcitrant cultivars (Makara et al., 2010). Diploid and triploid banana cultivars showed different micropropagation responses to exogenous cytokinin treatment concerning their genotype and ploidy (Resmi and Nair, 2011).
2.1.2.2. Media
Tissue culture media plays a vital role in the growth and development of shoot tips. The choice of nutrient media, chemical composition, and concentration of the salt largely determine the success of micropropagation (Suman, 2017; Park et al., 2020) (Table S1). Several media have been used for shoot multiplication of bananas including Murashige and Skoog (MS) media, SH (Schenk and Hildebrant, 1972), Linsmaier and Skoog (LS) (Linsmaier and Skoog, 1965), N6 (Chu et al., 1975) and B5 (Gamborg et al., 1968) media (Table S1). Among all the different types of media, MS medium (supplemented with specific growth regulators) was reported to be the most efficient for shoot multiplication (Shirani et al., 2009; Hui et al., 2012; Ferdous et al., 2015; Hossain et al., 2016). Modified MS mediums have also been used to study the effect of media substitution with foliar fertilizers and coconut water. Three different types of media were prepared by modification of MS media such as full MS media, ½ MS, ½ MS + ½ foliar fertilizer, and fully foliar fertilizer each containing different concentrations of coconut. However, these substitute media could not compete with the full MS media supplemented with coconut water (50–100 ml l−1) water (Mardhikasari et al., 2020).
2.1.2.3. Growth regulators
For the shoot multiplication of banana, various plant growth regulators (PGRs) such as abscisic acid, auxins, cytokinins, and gibberellins are used (Table S1). Cytokinins such as BAP, zeatin, thiadizuron (TDZ), and kinetin (KN) are used for the growth of axillary buds, and shoot multiplication, while auxins such as indole acetic acid (IAA), indole butyric acid (IBA) and naphthalene acetic acid (NAA) promote root development (Gupta et al., 2020; Kaur et al., 2022). BAP is the most preferred cytokinin to enhance shoot multiplication in Musa spp. due to its high cytokinin activity, accessibility, and low cost (Shirani et al., 2009; Singh et al., 2017). It has been reported to be the optimum cytokinin for shoot multiplication in bananas either alone or in combination with different auxins such as IAA and NAA (Huq et al., 2012; Shirani et al., 2009; Ahmed et al., 2014; Mahdi et al., 2014; Shankar et al., 2014; Ferdous et al., 2015; Qamar et al., 2015; Suman and Kumar 2015; Uzaribara et al., 2015; Hossain et al., 2016; Devi et al., 2017; Khatun et al., 2017; Safarpour et al., 2017; Khatab et al., 2017; Hoque et al., 2018; Rajoriya et al., 2018; Selvakumar and Parasurama, 2020) (Table S1). MS medium fortified with BAP (20.0 μM) in combination with NAA (1.0 μM) was reported to give the best proliferation for banana cultivar Grand Nain (Safarpour et al., 2017). Moreover, a higher multiplication rate in Dwarf Cavendish bananas on media supplemented with BAP (13.31 μM) and IAA (2.28 μM) was observed in the five subcultures which then declined after the 5th cycle (Dagnew et al., 2012). However, the proliferation of shoots was lesser at lower concentrations of BAP (10 μM) compared to a higher concentration of BAP (30.0 μM). For the banana cultivars ‘SH3362’, “-‘Basrai’, ‘William’, ‘GN60A’ and ‘High gate', MS + BAP (10.0 μM) + IAA (5.0 μM) + 40 mg/l cysteine HCl + 4% sucrose was used for initiation of cultures, and MS + BAP (20.0 μM) + 40 mg/l cystein HCl + 4% sucrose for the shoot proliferation (Khatri et al., 1997). In addition, other cytokinins like TDZ and KN have been used for in vitro shoot proliferation in bananas (Gubbuk and Pekmezci, 2009; Farahani et al., 2008; Gubbuk, and Pekmezci 2009; Roy et al., 2010; Hrahsel et al., 2014). TDZ at a lower concentration (2.0 μM) promoted shoot proliferation (Shirani et al., 2009; Manjula et al., 2015), however, a higher concentration of TDZ (5.0 μM) was reported to cause a high abnormality index of shoots in bananas (Shirani et al., 2009). Besides, a new compound with cytokinin-like activity, named meta-Topolin (N6-(3-hydroxy-benzyladenine) (mT) has been used in place of BAP for the micropropagation of certain banana genotypes (Aremu et al., 2012; Escalona et al., 2003; Bairu et al., 2008).
2.1.2.4. Status of the medium
Besides media composition and plant growth regulators, the type of gelling agent used in a tissue culture medium is also critical (Thorpe et al., 2008). Gelling agents such as agar and phytagel are commonly used in plant tissue culture because of their higher gelling capabilities. Agar is routinely used because of its inertness, stability, and clarity (Palanyandy et al., 2020). However, due to the high cost of tissue-culture-grade agar, the use of alternative cost-effective gelling agents including phytagel, gelrite, isabagol, etc is being explored (Ayenew et al., 2021; Dhawale et al., 2021). During the in vitro propagation of banana Grand Nain, when the gelrite in the medium was replaced with a mixture of gelrite/starch, the micropropagation rates were found to be relatively equal (Kodym and Zapata-Arias, 2001). When isabgol was used as an alternate gelling agent to phytagel and agar for in vitro propagation of banana cv. Karpura Chakkarakeli (AAB; Mysore subgroup), there was no significant difference in the number of shoots produced, although the survival rate of the shoots was higher with a slower multiplication rate in isagbol media than in phytagel and agar supplemented media. This could be ascribed to the low availability of water and a hence slower rate of absorption of nutrients from the isabgol matrix to the plantlets than that of other media tested (Agrawal et al., 2010). Use of phytagel in micropropagation of banana cultivar Dwarf Cavendish resulted in a higher shoot number and shoot weight than agar-agar, agargel, and plant agar (Kacar et al., 2010). Besides, a higher shoot multiplication rate and fresh weight were also observed in medium solidified with 0.9 g/l gelrite in comparison to medium gelled with 2.6 g/l gellan gum and 4–8 g/l agar in Shima Banana (AAA) (Buah et al., 1999). In addition, when sago + Isabgol were used, the maximum number of shoots was observed in ‘Udhayam’ and ‘Rasthali’ cultivars compared to the control (Saraswathi et al., 2016). Nevertheless, till now, there are only a fewer reports on the use of low-cost gelling agents for banana micropropagation and thus more research is necessary to optimize their use in banana in vitro propagation.
Total elimination of gelling agents (liquid medium) has also been tried for banana micro propagation by different researchers (Alvard et al., 1993). It has been observed that the rate of shoot multiplication and dry weight of shoots was higher on liquid media when compared with gelled media (Alvard et al., 1993). However, these experiments required specialized culture vessels for highly controlled intermittent submergence of cultures in the medium. A simple polypropylene container with cotton fiber support was found more effective than that of an agar-gelled medium for micropropagation in banana (Musa acuminata) cv. Grand Nain (Prabhuling and Sathyanarayana, 2017). The plantlets produced were also sturdier and were of better quality in comparison with the agar-gelled medium. Liquid medium not only reduces the cost of propagation but also facilitates better availability of nutrients and plant growth regulators leading to higher shoot multiplication. Moreover, the liquid medium promotes proper aeration of cultures and dilutes any exude from the explant which might inhibit the growth of the culture (Ziv and Halevy, 1983; Abdulmalik et al., 2020. However, in general, many in vitro propagated plants respond poorly to liquid culture medium due to hyperhydricity that is a result of prolonged contact between the explants and liquid culture (Ziv, 2005; Snyman et al., 2011). To circumvent this challenge, a partial immersion system is utilized to make sure explants are properly aerated. Materials such as rockwool, coconut coir, filter paper, luffa sponge, cotton fiber, glass wool, polystyrene foam, nylon cloth, polyester screen raft, and polypropylene membrane raft are utilized to ensure there is contact between the lower portion of the explant and the culture medium (Gupta and Prasad, 2006). Filter sterilized air can also be bubbled through the medium for micropropagation (Preil, 1991). In bananas, four different liquid medium systems (solid medium (A), liquid medium with the immersion of plants (B), liquid medium with cotton culture support (C), and liquid medium aerated by bubbling (D) were compared for micropropagation. In this study, maximum shoot number in a temporary immersion bioreactor was observed in banana cultivar Musa cv. Dwarf Cavendish and hyperhydricity were observed in shoots that were cultured in a continuously aerated liquid media setup (Farahani and Majd, 2012). Thus, temporary immersion bioreactors can help to overcome the hyperhydricity deformities through better aeration, and intermittent or partial submergence of the cultures in the medium (Sajid and Parvaiz, 2008; Farahani and Majd, 2012).
2.1.2.5. Physical factors
Temperature plays a critical role in the shoot multiplication of bananas. For banana shoot proliferation, a temperature in the range of 24 °C–26 °C has been reported by various workers to be optimum (Safarpour et al., 2017; Bello-Bello et al., 2019). However, there are few reports where a temperature up to 28 ± 2 °C is recommended for banana micropropagation (Alvard et al., 1993; Gebeyehu 2015; Bohra et al., 2016). Along with temperature, light intensity and photoperiod also depict an important role in plant tissue culture (Kaur et al., 2021). In bananas, most workers have reportedly used a light intensity in the range of 30 and 100 μmol/m2/s (El-Mahdy and Youssef 2019; Mekonen et al., 2021; Subrahmanyeswari and Gantait, 2022). However, optimum banana shoot proliferation was found at 40 μmol/m2/s (Wilken et al., 2014). Exposure to higher light intensity during later stages led to the improved survival rate of banana plantlets upon subsequent transfer to soil (Suman, 2017). Fluorescent lamps are currently the most common light for photosynthesis; however they are expensive and produce unnecessary wavelengths and radiations (Yeh et al., 2009; Sonthisut et al., 2022). Thus, the use of light-emitting diodes (LEDs) (combining LEDs emitting in far red, red, and blue colors) is encouraged these days for better plant growth under in vitro conditions (Nhut and Nam, 2010). It was reported that blue and red LED lights (B:R = 1:1) are more suitable for higher fresh and dry weight in bananas (Duong et al., 2003). Moreover, a maximum number of shoots was observed under the LED lamps as compared to the leaves under the white fluorescent lamps (Bhaya and Al-RazzaqSalim, 2019). Besides, high chlorophyll content was also observed in the case of two ornamental banana varieties using LED illumination (Vendrame et al., 2022).
2.1.3. Stage III: rooting of microshoots
For an efficient micropropagation protocol, rooting of microshoots is necessary for the successful transfer of in vitro propagated shoots to the field (Waman et al., 2015) (Figure 2e). MS basal medium was found to be the best suitable medium for inducing rooting from shoot tips in Musa spp. Cv. Sirumalai (Mahadev et al., 2011)). 1/2 MS, MS0 media alone or supplemented with activated charcoal and other additives have also been reported to promote rooting of in vitro banana microshoots (Mahadev et al., 2011; Hrahsel et al., 2014; Thanakronpaisan et al., 2019; Selvakumar and Parasurama, 2020). Moreover, rooting in micropropagated shoots was successfully induced in the banana cultivar Elaki using ½ MS medium augmented with 20 mg/l adenine sulfate + 200 mg/l activated charcoal (AC) + 3% sucrose (Selvakumar and Parasurama, 2020). Further, MS medium supplemented with chitosan (20 mg l−1) showed maximum rooting in shoots obtained from shoot tips (Thanakronpaisan et al., 2019).
MS media augmented with different auxins such as IBA, IAA and NAA had also been used for in vitro rooting of banana microshoots (Table S1). Auxins stimulate lateral root initiation and primordium growth by stimulating cell division, differentiation, and expansion (Kaur et al., 2021). Among different auxins, IBA has been reported by most workers as the most ideal auxin for rooting of in vitro developed banana shoots (Suman et al., 2013; Safarpour et al., 2017; Kavitha et al., 2021; Quiñonez et al., 2021). MS medium with 3% sucrose supplemented with IBA (1.47 μM) + 1 g/l activated charcoal was able to elicit rooting in vitro propagated shoots of Musa (AAB) Curare (Quiñonez et al., 2021). It was also observed that MS medium fortified with BAP (8.87 μM) + IAA (11.42 μM) + 0.1% activated charcoal is suitable for root induction in shoot tip culture (Vani et al., 1999). Further, MS media supplemented with NAA (2.65 μM) + 0.2% activated charcoal successfully induced rooting in bananas (Shashikumar et al., 2017). Besides, IAA (2.8 μM) in combination with BAP (22.19 μM) has also been widely used by different groups for in vitro rooting of banana shoots (Khan et al., 2021; Quiñonez et al., 2021).
2.1.4. Stage IV: hardening of rooted microshoots
Successful hardening of in vitro propagated plantlets is a prerequisite for an efficient lab-to-land transfer protocol (Khatik and Joshi, 2016) (Figures 2f, 2g). It is dependent on factors such as the composition of potting mixture, genotype of the plant, temperature, and humidity, etc (Twaij et al., 2020; Waman et al., 2015). Soil mixtures containing different ratios of vermiculite, peat moss, perlite, sand, and vermicompost have been used for the successful hardening of banana cultivars (Robinson and Sauco, 2009; Safarpour et al., 2017; Chamling et al., 2021). Besides combinations of bio-fertilizers (including VAM), peat fortified with nitrogen fixing and phosphate solubilizing microbes (each 1 g/plantlet) has been used for hardening in banana plantlets (Vasane et al., 2008). For hardening of banana cultivar Grand Nain (cocopeat: red soil: sand (1:1:1) was found to be the optimum potting mix for vigorous growth with a 96.5% survival rate (Selvakumar and Parasurama, 2020). In a similar study for Grand Nain banana, cocopeat (temperature 25 ± 2 °C) was used for hardening in the green house and further acclimatization was carried out using soil and vermicompost (1:1) (Manokari et al., 2022). Sand and farmyard manure (FYM) have also been used for acclimatization and hardening of banana cv. Malbhog and B. B. Battisa, with a survival rate of 95 % and 80%, respectively (Suman et al., 2013; Suman and Kumar, 2015).
3. Somatic embryogenesis system
The establishment of a high-frequency regeneration protocol is an important prerequisite for direct regeneration and genetic transformation (Rajput et al., 2022). It relies on the utilization of PGRs to induce tissue differentiation thus forming embryogenic callus (EC). Embryogenic callus has been found to have a high competence for embryogenesis (Rustagi et al., 2019). Moreover, the embryogenic callus also provides the starting material for the formation of embryogenic cell suspensions (ECS). From these cell suspensions, somatic embryos are produced and plants are regenerated. The first successful protocol on somatic embryogenesis in bananas was reported by Cronauer-Mitra and Krikorian (1988) followed by Escalant Teision (1989). However, despite their high regeneration potential, they are not widely used for propagation because of the frequent occurrence of somaclonal variations (Kavitha et al., 2021). Banana regeneration has been successfully achieved using different types of explants as discussed in the following sections (Table S2).
3.1. Embryogenesis from leaf sheath and rhizome
Modified Schenk and Hildebrandt medium fortified with TDZ (5.0 μM) and 20.0 μM Dicamba (3,6-dichloro-2- methoxy benzoic acid) was used for inducing callus from rhizome slices and leaf bases of cooking banana (ABB) and dessert banana (AAA). Later, somatic embryos were obtained from the induced calli in cell suspension after one month in media augmented with zeatin (5.0 μM) in dessert (AA and AAA) and cooking (ABB) bananas (Musa spp.) (Novak et al., 1989). Leaf sheath disks of 'Nanico' banana (Musa sp., AÁA group, Cavendish subgroup) were used to induce embryogenic calli on MS medium supplemented with activated charcoal (0.2 %), MES (2 [N-morpholino]ethanesulfonic acid) (15.3 mM), Picloram (414.0 μM), 2-iP (492.0 μM) and arginine (300 mM) (DA- Silva et al., 1998). Besides, MS medium supplemented with picloram (16.56 μM) induced embryogenic callus in Musa acuminata cv. Njalipoovan (AB). Further, subculturing of the callus in dark on MS basal media resulted in the development of shoots and roots (Smitha and Nair, 2011).
3.2. Embryogenesis from zygotic embryos
Somatic embryogenesis was induced in ornamental banana (Musa ornata Roxb.) using zygotic embryos propagated on semi-solid MS medium supplemented with 2,4–D (2.25 μM, 4.52 μM, 9.04 μM) + coconut water (5%) + 3% sucrose (Cronauer and Krikorian, 1988). Embryo germination and growth were achieved through the elimination of 2,4-D and subsequent transfer of the somatic embryos to Schenk and Hildebrandt media. Similarly, somatic embryos were obtained in Musa balbisiana (BB) and Musa acuminata (AA) from zygotic embryos using MS medium containing picloram (7.5 μM) or NAA (5.5 μM). Plant regeneration was achieved on MS media augmented with NAA (5.3 μM) (Eschalant and Teisson, 1989). Immature zygotic embryos of Musa acuminata ssp. burmannicoides and Musa acuminata ssp. malaccensis were used to induce embryogenic calli on MS medium containing picloram (7.5 μM). These calli were used to prepare embryogenic cell suspensions (ECS) in a liquid MS medium. ECS produced somatic embryos that germinated on an MS medium supplemented with BAP (0.22 μM) + IAA (1.14 μM) (Morroquin et al., 1993). However, regeneration of plantlets from immature embryos was higher than mature embryos (Uma et al., 2021).
3.3. Embryogenesis from immature male/female flowers
Immature floral tissues have been reported to have excellent embryogenic potential and are thus widely used in in vitro cultures (Ammirato, 1983). MS medium supplemented with 2,4-D (1.0 μM) + NAA (5.7 μM) + IAA (5.4 μM) + 1 mg/l biotin + 100 mg/l glutamine + 100 mg/l malt extract + 3% sucrose and gelled with 2.6 g/l phytagel was used for induction of embryogenic callus and regeneration of Musa acuminata cv. Mas (AA) using immature male flowers as the starting material (Jalil et al., 2003). Callus was obtained in MS medium supplemented with 2,4-D (4.52 μM). However, embryo development occurred on MS medium supplemented with 2,4-D (0.23 μM). For embryo-to-plant retrieval, MS medium with BAP (9.76 μM) was used. 2,4-D has been used in many studies to induce somatic embryos from immature male flower buds of different cultivars of banana (Wei et al., 2005; Kulkarni et al., 2006; Sidha et al., 2006; Ali et al., 2013). Besides, embryogenic calli of Musa acuminata cv. Matti was obtained through inoculation of bract explants on MS medium supplemented with TDZ (0.45 μM) + 3% sucrose (Divakaran and Nair, 2011). Embryo development was attained on MS + 8.18 μM biotin. Similar results were obtained in Musa acuminata cv. Njalipoovan using MS medium with TDZ (4.5 μM) + 3% sucrose for initiation of embryogenic callus and MS medium supplemented with 16.37 μM biotin for embryo development. In addition, embryogenic callus in Musa spp. Rasthali (AAB) was established using shoot tips as explants on MS medium augmented with 2, 4-D (9.05 μM) + zeatin (1.0 μM) and 1 mg/1 D-biotin and MS medium supplemented with 2,4-D (4.5 μM) + 1 mg/l D biotin +100 mg/l glutamine + 100 mg/l malt extract for induction of embryogenic cell suspension. Embryo development was finally achieved on ½ MS medium supplemented with Zeatin (10.0 μM) + 3% sucrose (Ganapathi et al., 2001). In a recent study, somatic embryos were induced in three ornamental bananas using immature male flower buds as the starting material. In this study, it was observed that embryogenic calli desiccated up to 2 h at 25 ± 1 °C resulted in higher frequencies of embryo induction and maturation in comparison with non-desiccated embryos (Natarajan et al., 2020).
3.4. Embryogenesis from scalps
Liquid ½ MS medium supplemented with zeatin (1.0 μM) and 2, 4-D (5.0 μM) was used to induce embryogenic cell suspensions of cooking banana cv. `Bluggoes' (Musa spp. ABB group) using scalps as the explant. Embryo maturation was achieved in MS basal medium and further plant regeneration in ½ MS medium supplemented with BAP (10.0 μM) (Dhed'a et al., 1991). Shoot tips of Musa sp. cavendish were inoculated in ½ MS medium supplemented with BAP (22.19 μM) and IAA (1.14 μM). Explants were subsequently transferred to a medium composed of ½ MS salts augmented with BAP (1.02 μM) and NAA (1.08 μM). The explants were further transferred to MS medium supplemented with BAP (0.88 μM) + 2,4-D (9.04 μM) + 1 mg/l biotin,whereby somatic embryos were observed after two weeks. Moreover, somatic embryos of hybrid banana FHIA-18 were obtained using the liquid medium in a bioreactor (Koskyet al., 2002).
3.5. Embryogenesis from shoot tips obtained from in vitro cultures
Direct somatic embryos in banana (Musa acuminata AAA cv. Grand Nain) were induced from split shoot tips obtained from 4 weeks old in vitro multiple shoot cultures on MS medium supplemented with picloram (4.14 μM) and BAP (0.22 μM). These somatic embryos germinated into plantlets on MS medium supplemented with NAA (0.53–2.68 μM) together with BAP (2.22–44.39 μM), or thidiazuron (4.54 μM) plus glutamine (200 mg/l) (Remakanthan et al., 2014).
3.6. Plant regeneration from protoplasts
The isolation of protoplasts in a banana for the very first time was reported in 1984 from inflorescence–derived callus of Cavendish (Musa AAA) (Bakry, 1984). However, no regeneration was obtained from the protoplasts. Later, successful plant regeneration from protoplasts in wild banana Long Tavoy (AA) was obtained using immature seeds (Megia et al., 1993). Embryogenic cell suspensions were established from the upper meristematic part of proliferating shoot-tips in cv. 'Bluggoe' (Musa spp., ABB subgroup). Protoplasts were isolated from ECS and they directly formed somatic embryos without undergoing any callus phase on ½ MS salt solution, mannitol (5%), 2,4-D (5.0 μM), and agarose (0.8%) or Gelrite (0.2%) (Panis et al., 1993). Moreover, protoplast regeneration through somatic embryogenesis using immature male flowers of seven banana cultivars viz. Dominico and curare Enano (subgroup plantain AAB), IRFA 903, SF 265 and Col 49 (AA); Grand Nain and Gros Michel (subgroup Cavendish AAA) were achieved (Assani et al., 2001). The conversion rate of protoplast into somatic embryos was 2% on MS medium supplemented with BAP (2.2 μM) and IAA (11.4 μM), vitamins of Morel + 3% sucrose, and solidified with 0.75% agarose. Of all the embryos, 43% were able to germinate and develop into plantlets (Assani et al., 2001).
4. Genetic transformation of banana
In the current scenario of global climate change and threatened food security, genetic improvement of bananas using biotechnological tools has fascinated the researchers (Ganapathi et al., 2021b, Ganapathi et al., 2021a; Subrahmanyeswari and Gantait, 2022). There are several methods available for the genetic transformation of bananas including electroporation of protoplasts, Agrobacterium-mediated transformation, and particle bombardment using embryogenic cells (Ganapathi et al., 2021a, Ganapathi et al., 2021b; Tripathi et al., 2015). Nevertheless, Agrobacterium-mediated transformation is the most sought-after approach for the genetic improvement of bananas with the advent of biotechnology. This method has an edge over other techniques as it relies on the utilization of differentiated tissues that can be regenerated into the complete plant using routine protocols (Tripathi et al., 2015). Besides, it offers integration of the transgene in low copy number and can transform larger stretches of DNA as compared to other direct methods of transformation (Tripathi et al., 2015). In recent decades, banana has been successfully transformed using several genes that have resulted in improved stress endurance, introduction of novel attributes, and value addition into the Musa spp. as discussed below:
4.1. Genetic transformation for production of transgenic banana resistant to biotic and abiotic stresses
Banana plants experience different biotic and abiotic stresses through their production cycle that significantly hampers their overall productivity (Li et al., 2021; Wang et al., 2021). Among biotic factors, the major diseases that are causing serious concerns and losses to banana yield currently include black Sigatoka, Fusarium wilt tropical race 4 (TR4), banana Xanthomonas wilt (BXW), and banana bunchy top disease (De souza-pollo and de Goes , 2020). Among these, Fusarium wilt (Panama disease) caused by Fusarium oxysproum f. sp. cubense (Foc) was the first major calamity of banana (Gros Michel). There are four different races of Foc fungus, and all of these except race 3 are pathogenic (Ganapathi et al., 2021). Fusarium wilt tropical race-4 (TR-4) imposes the sternest threat on the cultivation of bananas as it can destroy the banana plantations and result in global pandemics (Rocha et al., 2021). As control of Foc using different physical, chemical, or biological methods is practically impossible, thus, in the past few decades, sufficient attention has been given to the development of Foc-resistant genetically engineered banana varieties (Wang et al., 2021). Foc-resistant banana varieties have been generated by overexpressing the genes encoding for antifungal proteins like ferredoxin-like proteins, defensins, chitinase, lysozyme, anti-apoptosis proteins, etc from other organisms (Table S3). Another yield-limiting fungal pathogen that affects banana production is Mycosphaerella fijiensis which results in black Sigatoka disease. This disease can be managed by the use of certain chemicals, however, the arbitrarily use of such chemicals imposes a serious hazard on mankind and the environment (Sowmya et al., 2016; Soares et al., 2021). Thus, the development of Sigatoka-resistant banana varieties by employing genetic engineering approach has provided a most suitable option to protect the plants from this harmful fungus (Sowmya et al., 2016). Sigatoka-resistant banana has been developed by overexpressing antifungal proteins including chitinase, glucanase, RCC2, RCG3, etc (Table. S3) (Kosky et al., 2010; Vishnevetsky et al., 2011; Kovács et al., 2013). Apart from fungal pathogens, the transgenics approach has been effectively used for the management of the bacterial disease Xanthomonas wilt which can devastate banana production (Ocimati et al., 2020). The transgenic banana plants developed using sweet pepper Pflpor, Hrap genes showed high resistance to Xanthomonas campestris pv. Musa cearum that causes Xanthomonas wilt disease (Namukwaya et al., 2012; Tripathi et al., 2014a, Tripathi et al., 2010). Similarly, transgenic banana plants expressing the rice Xa21 gene showed increased resistance towards the Xanthomonas wilt pathogen (Tripathi et al., 2014b).
Apart from bacterial and fungal pathogens, infestation by nematodes and weevils is another persistent challenge for banana cultivation. Nematodes account for almost 20% loss in global banana production that may rise to even 40% in tropical storms-prone areas (Roderick et al., 2016). Nematodes are conventionally controlled with the use of harmful pesticides that contaminate the environment (Tripathi et al., 2019). Thus, to circumvent this problem, nematode-resistant transgenic banana cultivars were developed using cysteine proteinase inhibitors (cystatins) and nicotinic acetylcholine receptors (naCHR) inhibiting peptides encoding genes. Cystatins inhibit the dietary protein's intestinal digestion, whereas naCHR inhibiting peptides interfere with the nervous system of pests (Roderick et al., 2016) (Table S3). Rice cystatin (OcIΔD86) transformed banana plants have been developed that show almost 69–70% resistance towards Radopholus similis (Atkinson et al., 2004). In another attempt, genetic transformation of banana using maize naCHR inhibiting peptide or cystatin or both of these used together conferred resistance against Helicotylenchus multicinctus, and Radopholus similis under field trials conducted in a confined area in Uganda (Roderick et al., 2012). Moreover, transgenic banana ‘Sukali Ndiizi’ (ABB) has been developed using the cystatin (CpCYS-Mut89) gene from papaya for their potential resistance against common pests that infest banana. However, a precise evaluation of such plants for resistance against nematodes and weevils is yet to be conducted (Namuddu et al., 2013).
Besides different biotic agents, abiotic stresses including salinity, sub-optimum temperatures, and drought also act as a major impediment to banana cultivation (Ganapathi et al., 2021). Thus, researchers have undertaken profound efforts to improve the abiotic stress tolerance window of banana plants using a transgenic approach. Genetically modified banana plants with enhanced tolerance against salinity and drought stress were generated by overexpression of genes that belong to different families including WRKY transcription factors, late embryogenesis abundant (LEA) proteins, aquaporin coding genes, pathogenesis-related (PR) proteins, MYB transcription factors, NAC transcription factors, and several other stress-associated proteins, etc (Table S4) (Sreedharan et al., 2012, 2013, 2015; Shekhawat and Ganapathi 2013; Rustagi et al., 2015; Dou et al., 2016). However, limited attention has been given to the generation of genetically engineered banana cultivars that can resist changes in their optimum growth temperature.
Although, appreciable efforts have been undertaken by the researchers to develop stress-tolerant genetically modified banana cultivars, yet there is no report so far on the commercialization of such varieties. A major portion of such studies are greenhouse restricted and are not extended to field conditions. However, recent field trials conducted for over 3 years using the RGA2 gene (resistance gene analogue2) expressing TR4-resistant Cavendish transgenic plants showed promising results of resistance towards TR4 Foc strain (Dale et al., 2017). Similarly, field trials are underway for the transgenic banana plants resistant to Xanthomonas wilt disease and nematodes. Moreover, researchers have identified several candidate genes that are associated with the stress-induced senescence and decreased productivity of banana plants. The manipulation of such genes using modern genomics approaches can enhance the stress endurance and production of banana plants in the coming years (Lira et al., 2017; Tak et al., 2018; Ma et al., 2018).
4.2. Genetic transformation of banana for crop improvement
The improvement of banana cultivars for better agronomic traits to enhance their growth, yield and nutritional value are one of the prime agendas of researchers for long time (Sipen et al., 2011; Ganapathi et al., 2021; Wang et al., 2021). A Global Programme for Musa Improvement named ‘PROMUSA’ has been initiated by the World Bank and INIBAP since 1997. The major aim of this program is to develop novel banana varieties using conventional breeding approaches in conjunction with genetic engineering to address the needs and challenges faced by banana farmers in different regions of the world (Frison et al., 1998; Eksoy, 2018). It has been realized that semi-dwarf banana varieties with thick/sturdy stems and root system that shows better hydrotropism are ideal for increasing the production of banana (Wang et al., 2021). However, limited genetic engineering efforts have been made to impart these traits in banana varieties. In one such investigation, the overexpression of the NAC domain-containing MaVND1, MaVND2, and MaVND3 genes in bananas has been observed to modulate the secondary wall deposition that may influence the stem thickness (Negi et al., 2015, 2016). In addition, the biofortification of bananas is one of the key components of the global mission of banana improvement, especially in the African continent (Paul et al., 2018). Banana is one of the staple fruits for African populations; however, it has a lower content of iron, vitamin A, and protein (Paul et al., 2018). As people in African nations especially younger women and children are mostly deficient in micronutrients; thus reasonable efforts have been undertaken for the biofortification of bananas to enhance the content of iron and vitamin A (Amah et al., 2019; Ganapathi et al., 2021; Yadav et al., 2017). Iron fortification of bananas has been done using IRT1, FRO2, SFER, NAS1, NAS2, FER1, and YSL2 genes from different sources (Kumar et al., 2011; Moses et al., 2016; Yadav et al., 2017). Similarly, PSY2a, PSY1, and CRTL genes have been used to generate pro-vitamin A-rich transgenic banana plants (Paul et al., 2017). Moreover, a banana project named Banana-21 has been initiated by the National Banana Research Program of the National Agricultural Research Organisation (NARO) of Uganda in collaboration with the Centre for Tropical Crops and Biocommodities at Queensland University of Technology (QUT) in Australia since 2005 for the development of vitamin A-rich transgenic banana by stacking and overexpressing various genes that participate in the pro-vitamin A synthesis (Paul et al., 2017, 2018).
Along with biofortification, the genetic transformation of banana plants for ‘molecular farming’ has come into the limelight in recent years (Tak et al., 2016). Molecular farming involves the production of pharmaceutically important products including antibodies, hormones, enzymes, and vaccines using plants (Schillberg and Finnern, 2021). Bananas can serve as the ideal system for molecular farming owing to their year-round availability, large-scale cultivation, easy digestibility especially by infants, and availability of an efficient genetic transformation protocol, etc (Tak et al., 2016). Earlier, embryogenic cells of bananas have been successfully transformed with hepatitis B surface antigen (HBsAg) for the generation of edible banana vaccines against hepatitis B (Kumar et al., 2005). Thus, in the future banana can provide a suitable platform for the large-scale production of a range of pharmaceuticals.
5. Genome editing system
Genome editing is the latest approach that is in limelight for improving the different traits in crop plants (Alvarez et al., 2021; Hüdig et al., 2022; Kaur et al., 2019, 2022). Although, there are not many reports on exploring the potential of genome editing for the genetic improvement of bananas, yet, in the forthcoming era, genome editing may act as a saviour for sustaining banana cultivation. The initial experiments of genome editing in bananas were conducted on the Phytoene desaturase (PDS) gene. The PDS enzyme participates in the carotene biosynthetic pathway and its silencing results in the albino phenotype (Tripathi et al., 2019). The mutations induced in this gene using single gRNA resulted in the silencing of the PDS gene with 59% efficiency in the “Rasthali” cultivar (Kaur et al., 2018). Later, the same gene was mutated in the “Cavendish Williams” banana cultivar with the advent of polycistronic gRNAs (Naim et al., 2018). In the year 2020, the PDS gene was silenced in “Sukali Ndiizi” and “Gonja Manjaya” cultivars by employing multiple gRNAs with an efficiency of almost 100% (Ntui et al., 2020). All these efforts paved the way for the successful generation of genome-edited banana plants with specific elite traits. In 2019, endogenous banana streak virus (eBSV) that integrates into the B genome of Musa balbisiana was mutated using CRISPR/Cas. eBSV can reactivate upon exposure to stress conditions and due to this reason, the banana cultivars that comprise of atleast one B genome are not selected as a parent for genetic improvement of banana. Upon disruption of eBSV sequence in the B genome of banana using multiple gRNAs, approximately 75% of the mutated plants were observed to be asymptomatic in response to water stress thus affirming the successful deactivation of eBSV (Tripathi et al., 2019). Moreover, the transgenic banana plants that carried the small interfering RNA (siRNA) designed to target the Fusarium transcription factor 1 (ftf1) and the Foc velvet genes showed increased resistance towards Foc Race 1 (Ghag et al., 2014). Similarly, silencing of ERG6 and ERG11 genes involved in ergosterol biosynthesis genes enhanced the resistance of banana plants towards Foc Race 4 (Dou et al., 2020). The introduction of RNAi targets designed against replicase-associated protein (Rep) gene of viral origin was found to successfully resist BBTV infection in banana plants (Table S3) (Shekhawat et al., 2012; Elayabalan et al., 2013). In another report, semi-dwarf plants of “Gros Michel” have been developed by mutating the gibberellin 20ox2 (MaGA20ox2) gene involved in gibberellin biosynthesis (Shao et al., 2020). Besides, the shelf life of banana fruits has been improved through the modulation of the MaACO1 gene that plays a key role in the ripening of banana fruits using CRISPR/Cas (Hu et al., 2021). In a recent report, the role of the carotenoid cleavage dioxygenases4 (CCDs4) gene in the regulation of carotenoid accumulation was unravelled using CRISPR/Cas. In this investigation, transgene-free editing of the banana genome was successfully done by transfecting the embryogenic cells and protoplasts of the banana (Awasthi et al., 2022).
Till now, in the majority of the reports, the agrobacterium-mediated transformation method has been used for transforming the genome editing cassette to the different banana cultivars. But, as the banana is a sterile plant, the elimination of foreign DNA sequences derived from plasmids including the selection marker is a major hurdle (Tripathi et al., 2021). To overcome this issue, researchers have suggested that preassembled Cas9 protein-gRNA ribonucleoproteins (RNPs) may be designed with specific gene targets (Awasthi et al., 2022; Ntui et al., 2020). These RNPs could be coated on gold particles and can be transferred to either embryonic cells or protoplast through particle bombardment, etc. thus escaping the GM legislation and to further easing the commercialization of genome-edited bananas (Awasthi et al., 2022; Ntui et al., 2020).
6. Integration of omics studies for banana improvement
An in-depth understanding of the structure and evolution of the banana genome is critical in overcoming the challenges faced by the banana breeders (Sampangi and Ravishanker, 2016). Further, the precise identification and elucidation of the mode of action of key genes regulating different features of the banana plant are much warranted for the implementation of modern genomics approaches including genetic engineering and genome editing for banana crop improvement (Tripathi et al., 2019). In this scenario, different omics-based methods involving metabolomics, proteomics, transcriptomics, and genomics have opened new avenues to improve banana varieties for ensuring their sustainable and eco-friendly cultivation (Mohanty et al., 2017; Kissel and Carpentier, 2016). The size of the banana haploid genome was estimated to be 600 Mbp in M. acuminata and 550 Mbp in M. balbisiana using flow cytometry (Dolezel et al., 1994). The genome sequence of bananas was decoded by researchers working in association with the Global Musa Genomics Consortium in 2012 (D'Hont et al., 2012). This unravelling of the blueprint of the banana genome provided the necessary impetus to the research efforts focused on the genetic improvement of bananas. The draft sequence of the banana genome suggests that there are almost 36000 protein-coding regions, 37 Micro RNA (MIR) families and around half of the genome is comprised of transposable elements (Sampangi and Ravishanker, 2016). Besides, transcriptomics studies conducted using different parts of the plant including the flower, rhizome root, leaf, etc. aided in the precise functional interpretation of the critical elements of the banana genome (Ibrahim and Thangjam, 2015; Singh et al., 2021). The comparative transcriptome analysis of different banana varieties assisted in the identification of key genes involved in the regulation of vital processes of the banana life cycle including growth, development, and disease resistance, etc (Hu et al., 2017; Li et al., 2013; Sun et al., 2019; Dong et al., 2020; Kaushal et al., 2021; Yumbya et al., 2021). Moreover, transcriptomics studies generated useful resources to identify the critical genes that were not annotated in the banana draft genome sequence (Li et al., 2013). The deep sequencing of the transcriptome of Foc 1 and Foc race 4 infected banana plants using Illumina unravelled almost 842 genes that were not annotated in the banana draft genome sequence (Li et al., 2013). In addition to transcriptomics, several studies have been conducted to study the proteome of banana plants for the characterization and analysis of genetic variations observed in various banana varieties. Proteome analysis provided insights into the molecular dynamics of key proteins and their modulations at the post-transcriptional and post-translational levels during the vital processes of Foc resistance, drought tolerance, cold stress tolerance, fruit ripening, identification of banana allergens, etc (Vanhove et al., 2012; Li et al., 2015; Nikolic et al., 2018; Dong et al., 2019; Bhuyian et al., 2020). Thus, understanding banana genetics through an integrative approach that involves comparative genomics in conjunction with transcriptomics and proteomics may provide better access to the genes and their cis-regulatory elements for the domestication of banana ‘new generations’ through breeding and for the development of genetically modified banana with elite traits.
Apart from the analysis of DNA, RNA, and proteins, the investigation of plant metabolomis has emerged as a powerful technology in recent years (Price et al., 2020; Li et al., 2021). The metabolic profiling of bananas holds promise for the precise interpretation of biochemical diversity that exists within banana germplasm (Drapal et al., 2016). Researchers have generated a database of metabolic fingerprinting of 20 different accessions of bananas using their vegetative parts. The varieties included in this collection comprised both diploid and triploid along with Musa acuminata, Musa balbisiana, and distant wild species (Musa ornata). These varieties were then categorized based on their differential metabolites and genotypes (Drapal et al., 2016). As banana is a nutritionally rich plant, metabolic profiling in combination with other omics approaches can help the researchers to choose the banana varieties as per the consumer demands and elite agronomic traits for future crop improvement programs (Price et al., 2020).
7. Nano-biotechnology for banana
The use of nano-biotechnology in the field plant science is emerging rapidly (Arab et al., 2014). Lately, the exploration of the potential of nanoparticles (NPs) in improving the different traits of plants has grasped the attention of researchers (Sanzari et al., 2019; Zahedi et al., 2020). For in vitro propagation and regeneration of bananas, the addition of NPs in the routine media has given promising preliminary results. The zinc (Zn) and zinc oxide (ZnO) NPs were effective in eliminating the 9 different types of bacterial and four types of fungal contaminants from in vitro grown cultures of bananas. They further positively influenced the rate of regeneration and rooting of plantlets (Helaly et al., 2014). Moreover, augmentation of Ag NPs promoted the shoot proliferation rate in bananas. They also increased the shoot length, the number of roots per explant, root length, fresh weight, and the number of leaves (Do et al., 2018). In addition, Ag NPs promoted callus induction, shoot regeneration, rooting, and secondary hardening during in vitro propagation of bananas (Huong et al., 2021). Besides, the treatment of AgNPs was also effective in combating the spread of banana bunchy top virus (BBTV) in the field-grown Grand Nain banana. The treated plants showed an increase in leaf area, and dry weight compared to the control plants (Mahfouze et al., 2020). The foliar application of chitosan nanoparticles (CH-NPs) increased the fresh weight and dry weight of hardened Musa acuminata var. Baxi. Further, the application of CH-NPs resulted in a decrease in reactive oxygen species (ROS) levels and malondialdehyde (MDA) content in response to cold stress (Wang et al., 2021). Silicon oxide (SiO2-NPs) promoted shoot growth and proliferation, and also improved the photosynthesis in banana plants exposed to drought stress conditions. Moreover, a significant enhancement in phenolic secretion and a reduction in lipid peroxidation rate were observed at low concentrations of SiO2 (50 mg/l) (Subrahmanyeswari and Gantait 2022). Further, silicon NPs were helpful in the mitigation of the salinity and drought stress-induced oxidative stress in banana plants. The treated plants also exhibited improved chlorophyll levels, and Na+/K+ homeostasis under stress conditions (Mahmoud et al., 2020).
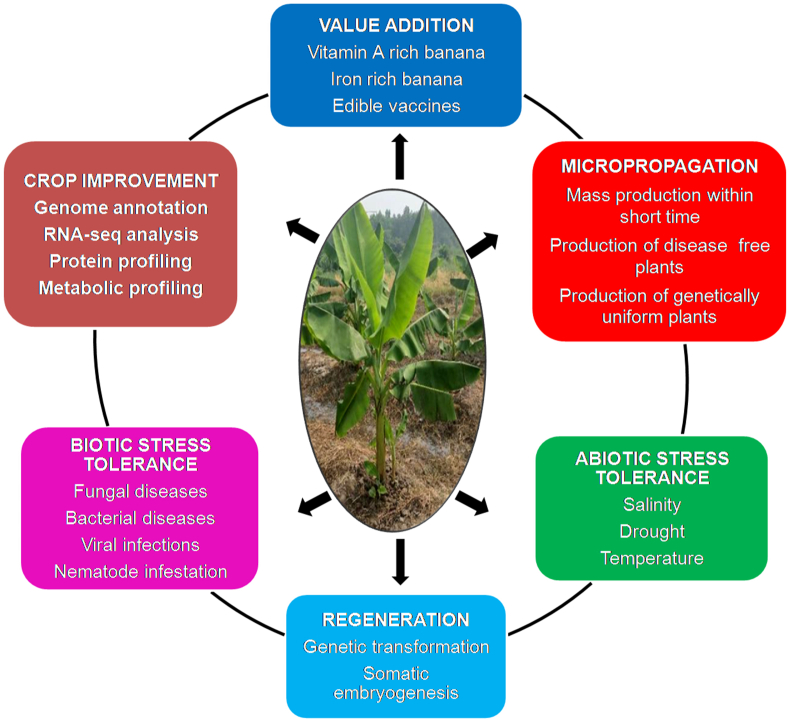
8. Conclusion and future prospects
Banana is one of the most loved food crops across the globe owing to its taste, easy digestibility, and nutritional value. The use of biotechnology has provided ample opportunities to solve the different problems associated with the conventional cultivation of banana (Figure 3). Traditionally, banana is cultivated using suckers as the planting material. However, these suckers often act as the reservoirs for different pests/pathogens that limit the productivity of the crop. Moreover, the age, size, and uneven maturity of suckers extend the crop duration. The establishment of various protocols for in vitro propagation of bananas has assured the availability of disease-free planting material throughout the year. However, still, an efficient and cost-effective protocol to propagate various nutritionally rich, recalcitrant banana varieties that can thrive in the changing global climate needs to be developed. In addition to micropropagation, the genetic transformations, biofortification and use of the banana in the production of edible vaccines have resulted in value addition to the crop. Although, many success stories have emerged in the past where biotechnology has served as a saviour for banana cultivation, still many hurdles limit the use of this approach to its full potential in banana improvement. The most important among these are the strict guidelines that restrict the field trials and commercialization of genetically modified banana plants. Moreover, the lack of public acceptance of genetically modified crops is another censorious issue. In this scenario, the recent breakthrough biotechnological tool CRISPR/Cas seems to be the samaritan option that can help in sustaining banana cultivation in near future. Researchers have identified several key candidate genes that regulate the agronomic and stress ameliorative traits in bananas. The exploitation of such genes using genome editing can assist in designing next-generation banana varieties that will be more palatable to the regulatory authorities and the public. Besides, considering the public's concern about genetically modified crops, the development of transgene-free or DNA-free genetics transformation and genome editing technology like the use of RNPs should be considered in the future.
Figure 3.
Biotechnological interventions for the improvement of banana.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors acknowledge the support from the Rashtriya Uchchattar Shiksha Abhiyan (RUSA-III) Program, Ministry of Human Resource Development (MHRD), Government of India, New Delhi; the Department of Biotechnology, Government of India, New Delhi (DBT-NER/AGRI/33/2016); and Centre for Agricultural Research and Innovation (CARI), Guru Nanak Dev University, Amritsar, Punjab, India.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abdalla N., El-Ramady H., Seliem M.K., El-Mahrouk M.E., Taha N., Bayoumi Y., Dobránszki J. An academic and technical overview on plant micropropagation challenges. Horticulturae. 2022;8(8):677. [Google Scholar]
- Abdulmalik M.M., Usman I.S., Nasir A.U., Sani L.A. Micropropagation of banana (Musa spp) using temporary immersion bioreactor system. Bayero j. pure appl. sci. 2020;12(2):197–200. [Google Scholar]
- Adeniyi A.G., Adeoye A.S., Ighalo J.O., Onifade D.V. FEA of effective elastic properties of banana fiber-reinforced polystyrene composite. Mech. Adv. Mater. Struct. 2021;28(18):1869–1877. [Google Scholar]
- Adeniyi A.G., Ighalo J.O., Onifade D.V. Banana and plantain fiber-reinforced polymer composites. J. Polym. Eng. 2019;39(7):597–611. [Google Scholar]
- Agrawal A., Sanayaima R., Tandon R., Tyagi R.K. Cost-effective in vitro conservation of banana using alternatives of gelling agent (isabgol) and carbon source (market sugar) Acta Physiol. Plant. 2010;32(4):703–711. [Google Scholar]
- Ahmad I., Hussain T., Ashraf I., Nafees M., Maryam R.M., Iqbal M. Lethal effects of secondary metabolites on plant tissue culture. Am.-Eurasian J. Agric. Environ. Sci. 2013;13(4):539–547. [Google Scholar]
- Ahmed S., Sharma A., Bhushan B., Wali V.K., Bakshi P., Singh A.K. Studies on hardening and acclimatization of micropropagated plantlets of banana cv. Grand Naine. Bioscan. 2014;9(3):965–967. [Google Scholar]
- Akatwijuka O., Gepreel M.A.H., Abdel-Mawgood A., Yamamoto M., Saito Y., Hassanin A.H. Overview of banana cellulosic fibers: agro-biomass potential, fiber extraction, properties, and sustainable applications. Biomass Convers. Biorefin. 2022:1–17. [Google Scholar]
- Alvard D., Cote F., Teisson C. Comparison of methods of liquid medium culture for banana micropropagation: effects of temporary immersion of explants. PCTOC. 1993;32:55–60. [Google Scholar]
- Alvarez D., Cerda-Bennasser P., Stowe E., Ramirez-Torres F., Capell T., Dhingra A., Christou P. Fruit crops in the era of genome editing: closing the regulatory gap. Plant Cell Rep. 2021;40(6):915–930. doi: 10.1007/s00299-021-02664-x. [DOI] [PubMed] [Google Scholar]
- Amah D., Van Biljon A., Brown A., Perkins-Veazie P., Swennen R., Labuschagne M. Recent advances in banana (Musa spp.) biofortification to alleviate vitamin A deficiency. Crit. Rev. Food Sci. Nutr. 2019;59(21):3498–3510. doi: 10.1080/10408398.2018.1495175. [DOI] [PubMed] [Google Scholar]
- Ammirato P.V. The regulation of somatic embryo development in plant cell cultures: suspension culture techniques and hormone requirements. Biotechnol. 1983;1(1):68–73. [Google Scholar]
- Arab M.M., Yadollahi A., Hosseini-Mazinani M., Bagheri S. Effects of antimicrobial activity of silver nanoparticles on in vitro establishment of G× N15 (hybrid of almond× peach) rootstock. J. Genet Eng. Biotechnol. 2014;12(2):103–110. [Google Scholar]
- Aremu A.O., Bairu M.W., Szüčová l., Doležal K., Finnie J.F., Staden J.V. Shoot and root proliferation in ‘Williams’ banana: are the topolins better cytokinins. PCTOC. 2012;111.2:209–218. [Google Scholar]
- Assani A., Haicour R., Wenzel G., Cote F., Bakry F., Foroughi-Wehr B., Grapin A. Plant regeneration from protoplasts of dessert banana cv. Grande Naine (Musa spp., Cavendish sub-group AAA) via somatic embryogenesis. Plant Cell Rep. 2001;20(6):482–488. [Google Scholar]
- Atkinson H.J., Grimwood S., Johnston K., Green J. Prototype demonstration of transgenic resistance to the nematode Radopholussimilis conferred on banana by a cystatin. Transgenic Res. 2004;13(2):135–142. doi: 10.1023/b:trag.0000026070.15253.88. [DOI] [PubMed] [Google Scholar]
- Awasthi P., Khan S., Lakhani H., Chaturvedi S., Kaur N., Singh J., Tiwari S. Transgene-free genome editing supports the role of carotenoid cleavage dioxygenase 4 as a negative regulator of β--carotene in banana. J. Exp. Bot. 2022;73(11):3401–3416. doi: 10.1093/jxb/erac042. [DOI] [PubMed] [Google Scholar]
- Ayenew B., Mengesha A., Tadesse T., GebreMariam E. Ensete ventricosum (Welw.) Cheesman: a cheap and alternative gelling agent for pineapple (Ananas comosus var. smooth cayenne) in vitro propagation. J. Microbiol. Biotechnol. Food Sci. 2021:640–652. [Google Scholar]
- Bairu M.W., Stirk W.A., Doležal K., Van Staden J. The role of topolins in micropropagation and somaclonal variation of banana cultivars ‘Williams’ and ‘Grand Naine’(Musa spp. AAA) PCTOC. 2008;95(3):373–379. [Google Scholar]
- Bakry F. Choix du mateáriel a` utiliser pour I’solement de protoplasts de bananier (Musa sp.) Fruits. 1984;39:449–452. [Google Scholar]
- Bello O.A., Esan E.B., Obembe O.O. Establishing surface sterilization protocol for nodal culture of Solanecio biafrae. IOP Conf. Ser. Earth Environ. Sci. 2018;210(1) [Google Scholar]
- Bello-Bello J.J., Cruz-Cruz C.A., Pérez-Guerra J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine). In Vitro Cell. Dev. Biol. 2019;55(3):313–320. [Google Scholar]
- Bhaya M.H.M., Al-Razzaq Salim S.A. Impacts of plant growth regulators and light quality on banana (Musa spp) micropropagation. Plant Arch. 2019;19(1):1379–1385. [Google Scholar]
- Bhuiyan F., Campos N.A., Swennen R., Carpentier S. Characterizing fruit ripening in plantain and Cavendish bananas: a proteomics approach. J. Proteonomics. 2020;214 doi: 10.1016/j.jprot.2019.103632. [DOI] [PubMed] [Google Scholar]
- Bhutani R., Shukla S., Shukla S. Impact of sterilants on culture establishment of indigenous Musa L. varieties: a step forward for conservation. Environ. Sci. Pollut. Res. 2021;28(4):3913–3919. doi: 10.1007/s11356-020-10059-w. [DOI] [PubMed] [Google Scholar]
- Blakesley D. Uptake and metabolism of 6-benzyladenine in shoot proliferation of Musa and Rhododendron. PCTOC. 1991;25:69–74. [Google Scholar]
- Bohra P., Waman A.A., Sathyanarayana B.N., Umesha K., Gowda B. Influence of different growth regulators on in vitro multiplication of mixed diploid banana (Musa AB) Proc. Natl. Acad. Sci. India B Biol. Sci. 2016;86(1):179–185. [Google Scholar]
- Buah J.N., Kawamitsu Y., Sato S., Murayama S. Effects of different types and concentrations of gelling agents on the physical and chemical properties of media and the growth of banana (Musa spp.) in vitro. Plant Prod. Sci. 1999;2(2):138–145. [Google Scholar]
- Chamling N., Bhowmick N. Effect of secondary hardening media on the performance of in-vitro raised banana plantlets cv. Grand Naine. J. crop weed. 2021;17(1):93–98. [Google Scholar]
- Chu C.C., Wang C.C., Sun C.S., Hsu C., Yin K.C., Chu C.Y., Bi F.Y. Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci. Sin. 1975;18:659–668. [Google Scholar]
- Cronauer-Mitra S.S., Krikorian A.D. Plant regeneration via somatic embryogenesis in the seeded diploid banana Musa ornata Roxb. Plant Cell Rep. 1988;7(1):23–25. doi: 10.1007/BF00272970. [DOI] [PubMed] [Google Scholar]
- Dagnew A., Shibru S., Debebe A., Lemma A., Dessalegn L., Berhanu B., Sierra Y.M. Micropropagation of banana varieties (Musa spp.) using shoot-tip culture. Ethiop. J. Agric. Sci. 2012;22(1):14–25. [Google Scholar]
- Dale J., Paul J.Y., Dugdale B., Harding R. Modifying bananas: from transgenics to organics. Sustainability. 2017;9(3):333. [Google Scholar]
- De Anicezio L.C. Efeito de Antioxidantes e Descontaminantes no Estabelecimento de Explantes de Bananeira (Musa spp) in vitro. Uniciencia. 2012;16(1) [Google Scholar]
- De Souza-Pollo A., De Goes A. Handbook of Banana Production, Postharvest Science, Processing Technology, and Nutrition. 2020. Banana pathology and diseases; pp. 45–59. [Google Scholar]
- Debnath S., Khan A.A., Das A., Murmu I., Khan A., Mandal K.K. Genetic Diversity in Horticultural Plants. 2019. Genetic diversity in banana; pp. 217–241. [Google Scholar]
- Devi K., Gogoi M.B., Singh S., Sarmah B.K., Modi M.K., Sen P. In vitro regeneration of banana and assessment of genetic fidelity in the regenerated plantlets through RAPD. Annu. res. rev. 2017:1–11. [Google Scholar]
- Dhawale R., Patwari L., Sharma K., Bharose A. A mixture of Isubgol Husk together with agar as gelling agent for sugarcane callus induction. J. Pharm. Innov. 2021;10:657–680. [Google Scholar]
- Dhed'a D.B., Dumortier F., Panis B., Vuylsteke D. Plant regeneration in cell suspension cultures of the cooking banana cv. ‘Bluggoes’ (Musa spp. ABB group) Fruits. 1991;46(2):125–135. [Google Scholar]
- D'Hont A., Wincker P. 2012. The Sequence of the Banana (Musa Acuminata) Genome; p. W068. [Google Scholar]
- Divakaran S.P., Nair A.S. Somatic embryogenesis from bract cultures in diploid Musa acuminata cultivars from South India. Sci. Hortic. 2011;131:99–102. [Google Scholar]
- Do D.G., Dang T.K.T., Nguyen T.H.T., Nguyen T.D., Tran T.T., Hieu D.D. Effects of nano silver on the growth of banana (Musa spp.) cultured in vitro. J. Vietnam. environ. 2018;10(2):92–98. [Google Scholar]
- Doležel J., Doleželová M., Novák F.J. Flow cytometric estimation of nuclear DNAamountin diploid bananas (Musa acuminata and M. balbisiana) Biol. Plant. (Prague) 1994;36(3):351–357. [Google Scholar]
- Dong H., Li Y., Fan H., Zhou D., Li H. Quantitative proteomics analysis reveals resistance differences of banana cultivar ‘Brazilian’ to Fusarium oxysporum f. sp. cubense races 1 and 4. J. Proteonomics. 2019;203 doi: 10.1016/j.jprot.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Dong H., Ye Y., Guo Y., Li H. Comparative transcriptome analysis revealed resistance differences of Cavendish bananas to Fusarium oxysporum f. sp. cubense race 1 and race 4. BMC Genet. 2020;21(1):1–17. doi: 10.1186/s12863-020-00926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou T.X., Hu C.H., Sun X.X., Shao X.H., Wu J.H., Ding L.J., Yi G.J. MpMYBS3 as a crucial transcription factor of cold signaling confers the cold tolerance of banana. PCTOC. 2016;125(1):93–106. [Google Scholar]
- Dou T., Shao X., Hu C., Liu S., Sheng O., Bi F., Yi G. Host-induced gene silencing of Foc TR4 ERG6/11 genes exhibits superior resistance to Fusarium wilt of banana. Plant Biotechnol. J. 2020;18(1):11. doi: 10.1111/pbi.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapal M., Carvalho E., Houwe I., Rouard M., Sardos J., Amah D., Fraser P. XXIV Plant and Animal Genome Conference. San Diego, CA (USA) 2016. Metabolomics Approach to the Assessment of Banana Diversity and Traits. [Google Scholar]
- Duong T.N., Hong L.T.A., Watanabe H., Goi M., Tanaka M. Efficiency of a novel culture system by using light-emitting diode (LED) on in vitro and subsequent growth of micropropagated banana plantlets. Acta Hortic. 2003;616:121–127. [Google Scholar]
- Elayabalan S., Kalaiponmani K., Subramaniam S., Selvarajan R., Panchanathan R., Muthuvelayoutham R., Balasubramanian P. Developmentof Agrobacterium-mediated transformation of highly valued hill banana cultivar Virupakshi(AAB) for resistance to BBTV disease. World J. Microbiol. Biotechnol. 2013;29(4):589–596. doi: 10.1007/s11274-012-1214-z. [DOI] [PubMed] [Google Scholar]
- El-Banna A.N., El-Mahrouk M.E., Dewir Y.H., Farid M.A., Abou Elyazid D.M., Schumacher H.M. Endophytic bacteria in banana in vitro cultures: molecular identification, antibiotic susceptibility, and plant survival. Horticulturae. 2021;7(12):526. [Google Scholar]
- El-Mahdy M.T., Youssef M. Genetic homogeneity and high shoot proliferation in banana (Musa acuminata Colla) by altering medium thiamine level and sugar typeIn Vitro Cell. Dev. Biol. Plant . 2019;55(6):668–677. [Google Scholar]
- Esan A.M., Akeredolu O.E., Adeniji A.O., Olaiya C.O. Comparative effects of gibberellic acid, salicylic acid and Bacillus subtilis on oxidative stress marker and antioxidant potential of Musa sapientum Linn. Arch. Phytopathol. Pflanzenschutz. 2022;55(5):549–563. [Google Scholar]
- Escalant J.V., Teisson C. Somatic embryogenesis and plants from immature zygotic embryos of the species Musa acuminata and Musa balbisiana. Plant Cell Rep. 1989;7(8):665–668. doi: 10.1007/BF00272056. [DOI] [PubMed] [Google Scholar]
- Escalona M., Cejas I., Gonzalez-Olmedo J., Capote I., Roels S., Canal M.J., Rodreguez R., Sandoval J., Debergh P. The effect of meta-topolin on plantain propagation using a temporary immersion bioreactor. Info. 2003;12:28–30. [Google Scholar]
- Estrela C., Estrela C.R., Barbin E.L., Spanó J.C.E., Marchesan M.A., Pécora J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002;13:113–117. doi: 10.1590/s0103-64402002000200007. [DOI] [PubMed] [Google Scholar]
- FAOSTAT. 2020. www.fao.org [Google Scholar]
- Farahani F., Aminpoor H., Sheidai M., Noormohammad i Z., Mazinani M.H. An improved system for in vitro propagation of banana (Musa acuminate L.) cultivars. Asian J. Plant Sci. 2008;7(1):116–118. [Google Scholar]
- Farahani F., Majd A. Comparison of liquid culture methods and effect of temporary immersion bioreactor on growth and multiplication of banana (Musa, cv. Dwarf Cavendish) Afr. J. Biotechnol. 2012;11(33):8302–8308. [Google Scholar]
- Ferdous M.H., Billah A.A.M., Mehraj H., Taufique T., Uddin A.F.M.J. BAP and IBA pulsing for in vitro multiplication of banana cultivars through shoot-tip culture. J. biosci. agric. res. 2015;3(2):87–95. [Google Scholar]
- Food and Agriculture Organization of the United Nations . 2019. FAOSTAT Crops.http://www.fao.org/faostat/en/#data/QC Accessed on 13 November 2019. [Google Scholar]
- Frison E.A., Orjeda G., Sharrock S. 1998. Pro Musa: the Global Programme for Musa Improvement; pp. 6–8. [Google Scholar]
- Gamborg O.L., Miller R.A., Ojima K. Plant cell cultures. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Ganapathi T.R., Negi S., Tak H., Bapat V.A. Transgenic banana: current status, opportunities and challenges. Genetically Modified Crops. 2021:111–128. [Google Scholar]
- Ganapathi T.R., Higgs N.S., Balint-Kurti P.J., Arntzen C.J., May G.D., Van Eck J.M. Agrobacterium-mediated transformation of embryogenic cell suspensions of the banana cultivar Rasthali (AAB) Plant Cell Rep. 2001;20(2):157–162. doi: 10.1007/s002990000287. [DOI] [PubMed] [Google Scholar]
- Ganapathi T.R., Srinivas L., Suprasanna P., Bapat V.A. Regeneration of plants from alginate-encapsulated somatic embryos of banana cv. Rasthali (Musa spp. AAB group) Vitro Cell Dev. Biol. 2001;37(2):178–181. [Google Scholar]
- Ganapathi T.R., Negi S., Tak H., Bapat V.A. TransgenicBanana: current status, opportunities and challenges. Genetically Modified Crops. 2021:111–128. [Google Scholar]
- Gebeyehu A. Effects of different concentrations of BAP (6-Benzyl amino purine) and NAA (Naphthalene acetic acid) on Banana (Musa spp.) cv. giant Cavendish shoot proliferation. International Journal of Plant Science and Ecology. 2015;1(2):36–43. [Google Scholar]
- Ghag S.B., Shekhawat U.K., Ganapathi T.R. Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol. J. 2014;12(5):541–553. doi: 10.1111/pbi.12158. [DOI] [PubMed] [Google Scholar]
- Gubbuk H., Pekmezci M. The effect of different thidiazuron and indole butyric acid concentrations on in vitro propagation of 'grand nain' and 'basrai' banana cultivars. Acta Hortic. 2009;829:295–298. [Google Scholar]
- Gupta S.D., Prasad V.S.S. Vol. 2. 2006. Matrix-supported liquid culture systems for efficient micropropagation of floricultural plants; pp. 488–495. (Floriculture, ornamental and plant biotechnology: advances and topical issues). [Google Scholar]
- Gupta V., Guleri R., Gupta M., Kaur N., Kaur K., Kumar P., Anand M., Kaur G., Pati P.K. Anti-neuroinflammatory potential of Tylophora indica (Burm.f) Merrill and development of an efficient in vitro propagation system for its clinical use. PLoS One. 2020;15(3):e0230142. doi: 10.1371/journal.pone.0230142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A.S., Khasim S.M., Ramudu J. Medicinal Plants: Biodiversity, Sustainable Utilization and Conservation. Springer; Singapore: 2020. Development of standard protocols for in vitro regeneration of some selected banana cultivars (Musa spp.) from India; pp. 743–759. [Google Scholar]
- Helaly M.N., El-Metwally M.A., El-Hoseiny H., Omar S.A., El-Sheery N.I. Effect of nanoparticles on biological contamination of'in vitro cultures and organogenic regeneration of banana. Aust. J. Crop. Sci. 2014;8(4):612–624. [Google Scholar]
- Heslop-Harrison J.S., Schwarzacher T. Domestication, genomics and the future for banana. Ann. Bot. 2007;100(5):1073–1084. doi: 10.1093/aob/mcm191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque F., Robbani M., Hasan M.F., Parvin J. Standardization of protocol for in vitro propagation of banana (Musa sapientum) J. Bangladesh Agric. Univ. 2018;16(1):27–30. [Google Scholar]
- Hossain M.A., Rubel M.H., Nasiruddin K.M., Evamoni F.Z. Influence of BAP and NAA on in vitro plantlet regeneration of local and exotic banana cultivars. J. Biosci. Agric. Res. 2016;6(2):553–564. [Google Scholar]
- Hrahsel L., Basu A., Sahoo L., Thangjam R. In vitro propagation and assessment of the genetic fidelity of Musa acuminata (AAA) cv. Vaibalhla derived from immature male flowers. Appl. Biochem. Biotechnol. 2014;172(3):1530–1539. doi: 10.1007/s12010-013-0637-9. [DOI] [PubMed] [Google Scholar]
- Hu C., Sheng O., Deng G., He W., Dong T., Yang Q., Bi F. CRISPR/Cas9-mediated genome editing of MaACO1 (aminocyclopropane-1-carboxylate oxidase 1) promotes the shelf life of banana fruit. Plant Biotechnol. J. 2021;19(4):654. doi: 10.1111/pbi.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Ding Z., Tie W., Yan Y., Liu Y., Wu C., Jin Z. Comparative physiological and transcriptomic analyses provide integrated insight into osmotic, cold, and salt stress tolerance mechanisms in banana. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/srep43007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüdig M., Laibach N., Hein A.C. Genome editing in crop plant research— alignment of expectations and current developments. Plants. 2022;11(2):212. doi: 10.3390/plants11020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui A.V., Bhatt A., Keng C.L. Micropropagation of Musa acuminata x M. balbisiana cv. Pisang Awak (ABB genome) and three other cultivars. Pakistan J. Bot. 2012;44(2):777–780. [Google Scholar]
- Huong B.T.T., Xuan T.D., Trung K.H., Ha T.T.T., Duong V.X., Khanh T.D., Gioi D.H. Influences of silver nanoparticles in vitro morphogenesis of specialty king banana (Musa ssp.) in Vietnam. Plant Cell Biotechnol. Mol. Biol. 2021;22:163–175. [Google Scholar]
- Huq A., Akter S., Islam S., Khan S. In vitro plant regeneration in Banana (Musa sp.)cv. Sabri.Bangladesh J. Sci.Ind. Res. 2012;47(2):143–146. [Google Scholar]
- Hussein N. Effects of nutrient media constituents on growth and development of banana (Musa spp.) shoot tips cultured in vitro. Afr. J. Biotechnol. 2012;11(37):9001–9006. [Google Scholar]
- Ibrahim K.S., Kumar N.S., Thangjam R. Transcriptome Analysis in Banana. Transcriptomics 3: 117, 2 cultivars from northeast India as revealed by ITS and IRAP markers. Appl. Biochem. Biotechnol. 2015;172:3939–3948. doi: 10.1007/s12010-014-0827-0. [DOI] [PubMed] [Google Scholar]
- Ighalo J.O., Adeniyi A.G. Thermodynamic modelling and temperature sensitivity analysis of banana (Musa spp.) waste pyrolysis. SN Appl. Sci. 2019;1(9):1–9. [Google Scholar]
- Jacobsen K., Omondi B.A., Almekinders C., Alvarez E., Blomme G., Dita M., Staver C. Seed degeneration of banana planting materials: strategies for improved farmer access to healthy seed. Plant Pathol. 2019;68(2):207–228. [Google Scholar]
- Jalil M., Khalid N., Yasmin O.R. Plant regeneration from embryogenic suspension cultures of Musa acuminata cv. Mas (AA) PCTOC. 2003;75(3):209–214. [Google Scholar]
- Kacar Y.A., Biçen B., Varol I., Mendi Y.Y., Serçe S., Çetiner S. Gelling agents and culture vessels affect in vitro multiplication of banana plantlets. Genet. Mol. Res. 2010;9(1):416–424. doi: 10.4238/vol9-1gmr744. [DOI] [PubMed] [Google Scholar]
- Kaminek M. Progress in cytokinin research. TIBTECH. 1992;10:159–162. [Google Scholar]
- Kang L., Zheng K., Xie Y., Deng Y., Yu Y., Zhu M., Deng X. Efficient tissue culture protocol for Magnolia lucida (Magnoliaceae) and confirmation of genetic stability of the regenerated plants. Plants. 2020;9(8):997. doi: 10.3390/plants9080997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannahi M., Buvaneswari R. Review on micropropagation of musa accuminata l. AJMR (Am. J. Ment. Retard.) 2019;8(3):147–163. [Google Scholar]
- Kapadia C., Patel N. Sequential sterilization of banana (musa spp.) sucker tip reducing microbial contamination with highest establishment percentage. Bangladesh J. Bot. 2021;50(4):1151–1158. [Google Scholar]
- Kaur K., Dolker D., Behera S., Pati P.K. Critical factors influencing in vitro propagation and modulation of important secondary metabolites in Withania somnifera (L.) dunal. PCTOC. 2022:1–20. doi: 10.1007/s11240-021-02225-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K., Singh P., Kaur K., Bhandawat A., Nogia P., Pati P.K. Development of robust in vitro culture protocol for the propagation of genetically and phytochemically stable plants of Withania somnifera (L.) Dunal (Ashwagandha) Ind. Crop. Prod. 2021;166 [Google Scholar]
- Kaur N., Alok A., Kaur N., Pandey P., Awasthi P., Tiwari S. CRISPR/Cas9-mediated efficient editing in phytoene desaturase (PDS) demonstrates precise manipulation in banana cv. Rasthali genome. Funct. Integr. Genomics. 2018;18(1):89–99. doi: 10.1007/s10142-017-0577-5. [DOI] [PubMed] [Google Scholar]
- Kaur N., Kaur G., Pati P.K. Advances in Rice Research for Abiotic Stress Tolerance. Woodhead Publishing; 2019. Deciphering strategies for salt stress tolerance in rice in the context of climate change; pp. 113–132. [Google Scholar]
- Kaushal M., Mahuku G., Swennen R. Comparative transcriptome and expression profiling of resistant and susceptible banana cultivars during infection by Fusarium oxysporum. Int. J. Mol. Sci. 2021;22(6):3002. doi: 10.3390/ijms22063002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavitha N., Saraswathi M.S., Kannan G., Bathrinath M., Backiyarani S., Uma S. Development of direct regeneration protocol for mass multiplication of Musa spp. variety Udhayam (Pisang Awak, ABB) using different explants. Sci. Hortic. 2021;290 [Google Scholar]
- Khan A., Bashir A., Erum S., Khatak J.Z.K., Muhammad A. Effects of 6-Benzylaminopurine and Indole-3-acetic acid on growth and root development of banana explants in micropropagation. Sarhad J. Agric. 2021;37(1):9–13. [Google Scholar]
- Khatik N., Joshi R. In vitro adventitious shoot regeneration from cotyledon and hypocotyl explants of Murraya koenigii (l) spreng. J. Phytol. Res. 2016;29(1):7–16. [Google Scholar]
- Khatri A., Khan I.A., Siddiqui S.H., Ahmad M., Siddiqui K.A. In vitro culture of indigenous and exotic banana clones for maximising multiplication. Pakistan J. Bot. 1997;29:143–150. [Google Scholar]
- Khatun F., Hoque M.E., Huq H., Adil M., Ashraf-Uz-Zaman K., Rabin M.H. Effect of BAP and IBA on in vitro regeneration of local banana variety of sabri. Biotechnol. J. Int. 2017;18(1):1–10. [Google Scholar]
- Kissel E., Carpentier S.C. Banana. Genomics And Transgenic Approaches For Genetic Improvement. Springer; Singapore: 2016. Abiotic stress tolerance research using-omics approaches; pp. 77–91. [Google Scholar]
- Ko W.H., Su C.C., Chen C.L., Chao C.P. Control of lethal browning of tissue culture plantlets of Cavendish banana cv. Formosana with ascorbic acid. PCTOC. 2009;96(2):137–141. [Google Scholar]
- Kodym A., Zapata-Arias F.J. Low-cost alternatives for the micropropagation of banana. PCTOC. 2001;66(1):67–71. [Google Scholar]
- Kosky R.G., Chong-Pérez B., López-Torres J., Reyes M., Bermúdez-Caraballoso I., Martín N.M., Hernández L. Plantain (Musa spp. cv.‘Navolean’AAB) transgenic plants from Agrobacterium tumefaciens-mediated transformation of embryogenic cell suspensions. Biotecnol. Veg. 2010;10(4):209–218. [Google Scholar]
- Kosky R.G., Silva M.deF.., Perez L.P., Gilliard T., Martínez F.,B., Vega M.R., Milian M.C., Mendoza E Q. Somatic embryogenesis of the banana hybrid cultivar FHIA-18 (AAAB) in liquid medium and scaled-up in a bioreactor. PCTOC. 2002;68(1):21–26. [Google Scholar]
- Kovács G., Sági L., Jacon G., Arinaitwe G., Busogoro J.P., Thiry E., Remy S. Expression of a rice chitinase gene in transgenic banana (‘Gros Michel’, AAA genome group) confers resistance to black leaf streak disease. Transgenic Res. 2013;22(1):117–130. doi: 10.1007/s11248-012-9631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna H., Sairam R.K., Singh S.K., Patel V.B., Sharma R.R., Grover M.N.L., Sachdev A. Mango explants browning: effect of ontogenic age, mycorrhization and pre- treatments. Sci. Hortic. 2008;118(2):132–138. [Google Scholar]
- Kumar G.B.S., Srinivas L., Ganapathi T.R. Iron fortification of banana by the expression of soybean ferritin. Biol. Trace Elem. Res. 2011;142(2):232–241. doi: 10.1007/s12011-010-8754-6. [DOI] [PubMed] [Google Scholar]
- Kumar G.S., Ganapathi T.R., Revathi C.J., Srinivas L., Bapat V.A. Expression of hepatitis B surface antigen in transgenic banana plants. Planta. 2005;222(3):484–493. doi: 10.1007/s00425-005-1556-y. [DOI] [PubMed] [Google Scholar]
- Lamare A., Otaghvari A.M., Rao S.R. Phylogenetic implications of the internal transcribed spacers of nrDNA and chloroplast DNA fragments of Musa in deciphering the ambiguities related to the sectional classification of the genus. Genet. Resour. Crop Evol. 2017;64(6):1241–1251. [Google Scholar]
- Li C., Shao J., Wang Y., Li W., Guo D., Yan B., Peng M. Analysis of banana transcriptome and global gene expression profiles in banana roots in response to infection by race 1 and tropical race 4 of Fusarium oxysporum f. sp. cubense. BMC Genet. 2013;14(1):1–16. doi: 10.1186/1471-2164-14-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Li M., Jiang Y., Duan X. Genome-wide identification, characterization and expression profile of glutaredoxin gene family in relation to fruit ripening and response to abiotic and biotic stresses in banana (Musaacuminata) Int. J. Biol. Macromol. 2021;170:636–651. doi: 10.1016/j.ijbiomac.2020.12.167. [DOI] [PubMed] [Google Scholar]
- Li T., Yun Z., Zhang D., Yang C., Zhu H., Jiang Y., Duan X. Proteomicanalysisof differentially expressed proteins involved in ethylene-induced chilling tolerance in harvested banana fruit. Front. Plant Sci. 2015;6:845. doi: 10.3389/fpls.2015.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsmaier E.M., Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plantarum. 1965;18:100–127. [Google Scholar]
- Lira B.S., Gramegna G., Trench B.A., Alves F.R., Silva E.M., Silva G.F., Rossi M. Manipulation of a senescence-associated gene improves fleshy fruit yield. Plant Physiol. 2017;175(1):77–91. doi: 10.1104/pp.17.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyola-Vargas V.M., Ochoa-Alejo N. An introduction to plant tissue culture: advances and perspectives. Plant cell culture protocols. 2018:3–13. doi: 10.1007/978-1-4939-8594-4_1. [DOI] [PubMed] [Google Scholar]
- Ma X., Zhang Y., Turečková V., Xue G.P., Fernie A.R., Mueller-Roeber B., Balazadeh S. The NAC transcription factor SlNAP2 regulates leaf senescence and fruit yield in tomato. Plant Physiol. 2018;177(3):1286–1302. doi: 10.1104/pp.18.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadev S.R., Kathithachalam A., Marimuthu M. An efficient protocol for large-scale plantlet production from male floral meristems of Musa spp. cultivars Virupakshi and Sirumalai. In Vitro Cell. Dev. Biol. 2011;47(5):611–617. [Google Scholar]
- Mahdi R., Islam M.J., Rahman M.A., Biswas A., Azam F.S., Rahmatullah M. In vitro regeneration protocol for anupam and chini champa: two banana (Musa sapientum) cultivars of Bangladesh. Am.-Eurasian J. Sustain. Agric. (AEJSA) 2014;8:28–33. [Google Scholar]
- Mahfouze H.A., El-Dougdoug N.K., Mahfouze S.A. Virucidal activity of silver nanoparticles against Banana bunchy top virus (BBTV) in banana plants. Bull. Natl. Res. Cent. 2020;44(1):1–11. [Google Scholar]
- Mahmoud L.M., Dutt M., Shalan A.M., El-Kady M.E., El-Boray M.S., Shabana Y.M., Grosser J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. South Afr. J. Bot. 2020;132:155–163. [Google Scholar]
- Makara A.M., Rubaihayo P.R., Magambo M.J.S. Carry-over effect of Thidiazuron on banana in vitro proliferation at different culture cycles and light incubation conditions. Afr. J. Biotechnol. 2010;9(21):3079–3085. [Google Scholar]
- Manjula R., Praveen J., Sunnaiah K.V., Swamy G.S.K., Prabhuling G. Enhancement of in vitro shoot multiplication in banana cv. Rajapuri (AAB) using TDZ. Res. J. Biotechnol. 2015;10(5):5–10. [Google Scholar]
- Manokari M., Badhepuri M.K., Cokulraj M., Rajput B.S., Dey A., Faisal M., Shekhawat M.S. High-throughput in vitro propagation and evaluation of foliar micro- morpho-anatomical stability in Musa acuminata cv.‘Grand Nain’using 6-benzoyladenine (BOA) in the nutrient medium. Sci. Hortic. 2022;304 [Google Scholar]
- Mardhikasari S., Yunus A. Modification of media for banana in vitro propagation with foliar fertilizer and coconut water in cv. Rajabulu. Caraka Tani: J. Sustain. Agric. 2020;35(1):23–32. [Google Scholar]
- Mathew N.S., Kurrey N.K., Bettadaiah B.K., Negi P.S. Anti-proliferative activity of Ensete superbum Roxb. Cheesman extract and its active principles on human colorectal cancer cell lines. J. Food Sci. 2021;86(11):5026–5040. doi: 10.1111/1750-3841.15927. [DOI] [PubMed] [Google Scholar]
- Megia R., Haicour R., Tizroutine S., Trang V.B., Rossignol L., Sihachakr D., Schwendiman J. Plant regeneration from cultured protoplasts of the cooking banana cv. Bluggoe (Musa spp., ABB group) Plant Cell Rep. 1993;13(1):41–44. doi: 10.1007/BF00232313. [DOI] [PubMed] [Google Scholar]
- Mekonen G., Egigu M.C., Muthsuwamy M. In vitro propagation of banana (Musa paradisiaca L.) plant using shoot tip explant. TURJAF) 2021;9(12):2339–2346. [Google Scholar]
- Menon R. Banana: Genomics and Transgenic Approaches for Genetic Improvement. Springer; Singapore: 2016. Banana breeding; pp. 13–34. [Google Scholar]
- Mihaljević I., Dugalić K., Tomaš V., Viljevac M., Pranjić A., Čmelik Z., Jurković Z. In vitro sterilization procedures for micropropagation of ‘Oblačinska’sour cherry. J. Agric. Sci. 2013;58(2):117–126. [Google Scholar]
- Mohanty S., Das M.P., Surabhi G.K. ‘OMICS’-approach to regulate ripening and enhance fruit shelf-life in banana: an important fruit crop for food security. Can J Biotech. 2017;1:289. [Google Scholar]
- Moses M. Queensland University of Technology at Brisbane; Queensland, Australia): 2016. Molecular and Biochemical Characterisation of Transgenic Banana Lines Containing Iron Uptake and Storage Enhancing Genes (Doctoral Dissertation, PhD Thesis. [Google Scholar]
- Naim F., Dugdale B., Kleidon J., Brinin A., Shand K., Waterhouse P., Dale J. Gene editing the phytoene desaturase alleles of Cavendish banana using CRISPR/Cas9. Transgenic Res. 2018;27(5):451–460. doi: 10.1007/s11248-018-0083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namuddu A., Kiggundu A., Mukasa S.B., Kurnet K., Karamura E., Tushemereirwe W. Agrobacterium mediated transformation of banana (Musa sp.) cv. Sukali Ndiizi (ABB) with a modified Carica papaya cystatin (CpCYS) gene. Afr. J. Biotechnol. 2013;12(15) [Google Scholar]
- Namukwaya B., Tripathi L., Tripathi J.N., Arinaitwe G., Mukasa S.B., Tushemereirwe W.K. Transgenic banana expressing Pflp gene confers enhanced resistance to Xanthomonas wilt disease. Transgenic Res. 2012;21(4):855–865. doi: 10.1007/s11248-011-9574-y. [DOI] [PubMed] [Google Scholar]
- Nansamba M., Sibiya J., Tumuhimbise R., Karamura D., Kubiriba J., Karamura E. Breeding banana (Musa spp.) for drought tolerance: a review. Plant Breed. 2020;139(4):685–696. [Google Scholar]
- Natarajan N., Sundararajan S., Ramalingam S., Chellakan P.S. Efficient and rapid in- vitro plantlet regeneration via somatic embryogenesis in ornamental bananas (Musa spp.) Biologia. 2020;75(2):317–326. [Google Scholar]
- Negi S., Tak H., Ganapathi T.R. Cloning and functional characterization of Musa VND1 using transgenic banana plants. Transgenic Res. 2015;24(3):571–585. doi: 10.1007/s11248-014-9860-6. [DOI] [PubMed] [Google Scholar]
- Negi S., Tak H., Ganapathi T.R. Functional characterization of secondary wall deposition regulating transcription factors MusaVND2 and MusaVND3 in transgenic banana plants. Protoplasma. 2016;253(2):431–446. doi: 10.1007/s00709-015-0822-5. [DOI] [PubMed] [Google Scholar]
- Ngomuo M., Mneney E., Ndakidemi P. Control of lethal browning by using ascorbic acid on shoot tip cultures of a local Musa spp.(Banana) cv. Mzuzu in Tanzania. Afr. J. Biotechnol.1. 2014;3(16) [Google Scholar]
- Nhut D.T., Nam N.B. The Third International Conference on the Development of Biomedical Engineering in Vietnam. Springer; Berlin, Heidelberg: 2010. Light-emitting diodes (LEDs): an artificial lighting source for biological studies; pp. 134–139. [Google Scholar]
- Nikolić J., Nešić A.N., Kull S., Schocker F., Jappe U., Gavrović-Jankulović M., Supplementary Material for the Article. Nikolić J., Nešić A., Kull S., Schocker F., Jappe U., Gavrović-Jankulović M. Employment of proteomic and immunological based methods for the identification of catalase as novel allergen from banana. J. Proteonomics. 2018;175:87–94. doi: 10.1016/j.jprot.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Nkengla-Asi L., Eforuoku F., Olaosebikan O., Adejoju Ladigbolu T., Amah D., Hanna R., Kumar P.L. Gender roles in sourcing and sharing of banana planting material in communities with and without banana bunchy top disease in Nigeria. Sustainability. 2021;13(6):3310. [Google Scholar]
- Novak F.J., Afza R., Van Duren M., Perea-Dallos M., Conger B.V., Xiaolang T. Somatic embryogenesis and plant regeneration in suspension cultures of dessert (AA and AAA) and cooking (ABB) bananas (Musa spp.) Biotechnol. 1989;7(2):154–159. [Google Scholar]
- Nowakowska K., Pińkowska A., Siedlecka E., Pacholczak A. The effect of cytokinins on shoot proliferation, biochemical changes and genetic stability of Rhododendron ‘Kazimierz Odnowiciel’in the in vitro cultures. PCTOC. 2022;149(3):675–684. [Google Scholar]
- Ntui V.O., Tripathi J.N., Tripathi L. Robust CRISPR/Cas9 mediated genome editing tool for banana and plantain (Musa spp.) Curr. Plant Biol. 2020;21 [Google Scholar]
- Ocimati W., Groot J.J., Tittonell P., Taulya G., Ntamwira J., Amato S., Blomme G. Xanthomonas wilt of banana drives changes in land-use and ecosystem services across infected landscapes. Sustainability. 2020;12(8):3178. of banana using alternatives of gelling agent (isabgol) and carbon source (market sugar). Acta. [Google Scholar]
- Oliveira H.S.D., Lemos O.F.D., Miranda V.S., Moura H.C.D.P., Campelo M.F., Santos L.R.R.D. Estabelecimento e multiplicação in vitro de brotos no processo de micropropagação de cultivares de bananeira (Musa spp.) Acta Amazonica. 2011;(41):369–376. [Google Scholar]
- Onuoha I.C., Eze C.J., Unamba C.I.N. In vitro prevention of browning in plantain culture. Online J. Biol. Sci. 2011;11(1):13–17. [Google Scholar]
- Palanyandy S.R., Gantait S., Sinniah U.R. Effects of some gelling agents and their concentrations on conversion of oil palm polyembryoids into plantlets. J. Genet. Eng. Biotechnol. 2020;18(1):1–5. doi: 10.1186/s43141-019-0018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Ho T.T., Paek K.Y. Orchid Biology: Recent Trends & Challenges. Springer; Singapore: 2020. Medicinal orchids: production of bioactive compounds and biomass; pp. 439–450. [Google Scholar]
- Pati P.K., Rath S.P., Sharma M., Sood A., Ahuja P.S. In vitro propagation of rose- a review. Biotechnol. Adv. 2006;24(1):94–114. doi: 10.1016/j.biotechadv.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Paul J.Y., Harding R., Tushemereirwe W., Dale J. Banana 21: from gene discovery to deregulated golden bananas. Front. Plant Sci. 2018;9:558. doi: 10.3389/fpls.2018.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J.Y., Khanna H., Kleidon J., Hoang P., Geijskes J., Daniells J., Dale J. Golden bananas in the field: elevated fruit pro-vitamin A from the expression of a single banana transgene. Plant Biotechnol. J. 2017;15(4):520–532. doi: 10.1111/pbi.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhuling G., Sathyanarayana B.N. Liquid medium culture method for rapid multiplication of banana (Musa acuminata) cv.‘Grand Naine’through tissue culture. Int. J. Plant Sci. 2017;12(1):85–89. [Google Scholar]
- Prakasha D., Ramya G., Shivayogi R., Gajanana K., Kattimani K. Kamalapur red (AAA); 2019. In Vitro Mass Propagation System in Banana Cv. [Google Scholar]
- Preil W. Micropropagation. Springer; Dordrecht: 1991. Application of bioreactors in plant propagation; pp. 425–445. [Google Scholar]
- Price E.J., Drapal M., Perez-Fons L., Amah D., Bhattacharjee R., Heider B., Fraser P.D. Metabolite database for root, tuber, and banana crops to facilitate modern breeding in understudied crops. Plant J. 2020;101(6):1258–1268. doi: 10.1111/tpj.14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamar M., Qureshi S.T., Khan I.A., Raza S. Optimization of in vitro multiplication for exotic banana (Musa spp.) in Pakistan. Afr. J. Biotechnol. 2015;14(24):1989–1995. [Google Scholar]
- Quiñonez L., Ponce F.M., Moreira M.D.V., Mieles A.M., Gavilanes F.Z., Alcívar F.A., Cedeño-García G., Vélez-Olmedo J., Jaimez R.E. Effect of auxins, cytokinin and activated charcoal on in vitro propagation of plantains barraganete and curare (musa AAB). Proc.Natl. Acad. Sci. India B: biological sciences. Proc. Biol. Sci. 2021;91(2):431–440. [Google Scholar]
- Rajoriya P., Singh V.K., Lall N.J.R. Optimizing the effect of plant growth regulators on in vitro micro propagation of Indian red banana (Musa acuminata) J. Pharmacogn. Phytochem. 2018;8:628–634. [Google Scholar]
- Rajput P., Agarwal P., Gangapur D.R., Agarwal P.K. Development of a high-frequency adventitious shoot regeneration using cotyledon explants of an important oilseed crop Sesamum indicum L. In Vitro Cell. Dev. Biol. 2022:1–9. [Google Scholar]
- Ranjha M.M.A.N., Irfan S., Nadeem M., Mahmood S. A comprehensive review on nutritional value, medicinal uses, and processing of banana. Food Rev. Int. 2020:1–27. [Google Scholar]
- Remakanthan A., Menon T.G., Soniya E.V. Somatic embryogenesis in banana (Musa acuminata AAA cv. Grand Naine): effect of explant and culture conditions. In Vitro Cell. Dev. Biol. 2014;50(1):127–136. [Google Scholar]
- Resmi L., Nair A.S. Differential effect of cytokinins in the micropropagation of diploid and triploid Musa cultivars. Int. J. Integr. Biol. 2011;11(1):35–38. [Google Scholar]
- Robinson J.C., Sáuco V.G. Weaning (acclimatization) of in vitro-produced banana plants. Fruits. 2009;64(5):325–332. [Google Scholar]
- Rocha A.D.J., Soares J.M.D.S., Nascimento F.D.S., Santos A.S., Amorim V.B.D.O., Ferreira C., Haddad F., Santos-Serejo J.A.D.F., Amorim E.P. Improvements in the resistance of the banana species to Fusarium wilt: a systematic review of methods and perspectives. J. Fungi. 2021;7(4):249. doi: 10.3390/jof7040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick H., Tripathi L., Babirye A., Wang D., Tripathi J., Urwin P.E., Atkinson H.J. Generation of transgenic plantain (Musa spp.) with resistance to plant pathogenic nematodes. Mol. Plant Pathol. 2012;13(8):842–851. doi: 10.1111/j.1364-3703.2012.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick H., Tripathi L., Poovarasan S. Banana: Genomics and Transgenic Approaches for Genetic Improvement. Springer; Singapore: 2016. Transgenic approaches to improve resistance to nematodes and weevils; pp. 247–260. [Google Scholar]
- Rodrigues P.H.V., Oliveira E.L., Demetrio C.A., Ambrosano G.B., Piedade S.M.S. Effects of different light spectra on the slow-grown in vitro storage and quality of banana plantlets cv. Prata Catarina (AAB) PCTOC. 2022:1–7. doi: 10.1007/s11240-022-02280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe P., Rosales F. INIBAP; Montpellier, France: 1994. Musa Breeding at FHIA. The Improvement and Testing of Musa: a Global Partnership; pp. 117–129. [Google Scholar]
- Roy O.S., Bantawa P., Ghosh S.K., da Silva J.A.T., Ghosh P.D., Mondal T.K. Micropropagation and field performance of ‘Malbhog’(Musa paradisiaca, AAB group): a popular banana cultivar with high keeping quality of North East India. Tree For. Sci. Biotech. 2010;4(1):52–58. [Google Scholar]
- Rustagi A., Jain S., Kumar D., Shekhar S., Jain M., Bhat V., Sarin N.B. High efficiency transformation of banana [Musa acuminata L.cv. Matti (AA)] for enhanced tolerance to salt and drought stress through overexpression of a peanut salinity-induced pathogenesis-related class 10 protein. Mol. Biotechnol. 2015;57(1):27–35. doi: 10.1007/s12033-014-9798-1. [DOI] [PubMed] [Google Scholar]
- Rustagi A., Shekhar S., Kumar D., Lawrence K., Bhat V., Sarin N.B. High speed regeneration via somatic embryogenesis in elite Indian banana cv. Somrani monthan (ABB) Vegetos. 2019;32(1):39–47. [Google Scholar]
- Safarpour M., Sinniah U.R., Subramaniam S., Swamy M.K. A novel technique for Musa acuminata Colla ‘Grand Naine’(AAA) micropropagation through transverse sectioning of the shoot apex. In Vitro Cell. Dev. Biol. 2017;53(3):226–238. [Google Scholar]
- Sajid G.M., Pervaiz S. Bioreactor mediated growth, culture ventilation, stationary and shake culture effects on in vitro growth of sugarcane. Pakistan J. Bot. 2008;40(5):1949–1956. [Google Scholar]
- Sampangi-Ramaiah M.H., Ravishankar K.V. Banana: Genomics and Transgenic Approaches for Genetic Improvement. Springer; Singapore: 2016. Current status of banana genome in the age of next generation sequencing; pp. 51–59. [Google Scholar]
- Sanzari I., Leone A., Ambrosone A. Nanotechnology in plant science: to make a long story short. Front. Bioeng. Biotechnol. 2019;7:120. doi: 10.3389/fbioe.2019.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswathi M.S., Uma S., Kannan G., Selvasumathi M., Mustaffa M.M., Backiyarani S. Cost-effective tissue culture media for large-scale propagation of three commercial banana (Musa spp.) varieties. J. Hortic. Sci. Biotechnol. 2016;91(1):23–29. [Google Scholar]
- Schenk R.U., Hildebrand t H.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell culture. Can. J. Bot. 1972;50:199–204. [Google Scholar]
- Schillberg S., Finnern R. Plant molecular farming for the production of valuable proteins–Critical evaluation of achievements and future challenges. J. Plant Physiol. 2021;258 doi: 10.1016/j.jplph.2020.153359. [DOI] [PubMed] [Google Scholar]
- Selvakumar S., Parasurama D.S. Maximization of micropropagule production in banana cultivars Grand naine (AAA) and Elakki (AB). In Vitro Cell. Dev. Biol. 2020;56(4):515–525. [Google Scholar]
- Shankar C.S., Balaji P., Sekar D.S. Mass propagation of banana (Musa sp.) cv. grand naine through direct organogenesis by benzyl adenine purine and kinetin. J. Acad. ind. res. 2014;3(2):92–97. [Google Scholar]
- Shao X., Wu S., Dou T., Zhu H., Hu C., Huo H., Yi G. Using CRISPR/Cas9 genome editing system to create MaGA20ox2 gene-modified semi-dwarf banana. Plant Biotechnol. J. 2020;18(1):17. doi: 10.1111/pbi.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashikumar R., Krishna V., Lokesha S. Analysis of genetic variations in Musa paradisiaca cv Karibale-Monthan using AFLP markers. Indian J. Agric. Res. 2017;51(6):543–549. [Google Scholar]
- Shekhawat U.K., Ganapathi T.R. MusaWRKY71 overexpression in banana plants leads to altered abiotic and biotic stress responses. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0075506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhawat U.K., Ganapathi T.R., Hadapad A.B. Transgenic banana plants expressing small interfering RNAs targeted against viral replication initiation genedisplay high-level resistance to banana bunchy top virus infection. J. Gen. Virol. 2012;93(8):1804–1813. doi: 10.1099/vir.0.041871-0. [DOI] [PubMed] [Google Scholar]
- Shirani S., Mahdavi F., Maziah M. Morphological abnormality among regenerated shoots of banana and plantain (Musa spp.) after in vitro multiplication with TDZ and BAP from excised shoot tips. Afr. J. Biotechnol. 2009;8(21) [Google Scholar]
- Singh P., Guleri R., Angurala A., Kaur K., Kaur K., Kaul S.C., Wadhwa R., Pati P.K. Addressing challenges to enhance the bioactives of Withania somnifera through organ, tissue, and cell culture based approaches. BioMed Res. Int. 2017;2017:1–15. doi: 10.1155/2017/3278494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Thangjam R., Harish G.D., Singh H., Kumar R., Meena D.P.S., Agrawal A. Conservation protocols for Ensete glaucum, a crop wild relative of banana, using plant tissue culture and cryopreservation techniques on seeds and zygotic embryos. PCTOC. 2021;144(1):195–209. [Google Scholar]
- Sipen P., Chubo J.K., King P.J., Huat O.K., Davey M.R. Genetic improvementof banana using conventional and in vitro technologies. J. Crop Improv. 2011;25(6):697–727. [Google Scholar]
- Smitha P.D., Nair A.S. Effect of picloram on somatic embryogenesis from leaf-sheath explants in diploid Musa acuminata cv. Njalipoovan. Plant Tissue Cult. Biotechnol. 2011;21(1):83–87. [Google Scholar]
- Snyman S.J., Nkwanyana P.D., Watt M.P. Alleviation of hyperhydricity of sugarcane plantlets produced in RITA® vessels and genotypic and phenotypic characterization of acclimated plants. South Afr. J. Bot. 2011;77(3):685–692. [Google Scholar]
- Soares J.M., Rocha A.J., Nascimento F.S., Santos A.S., Miller R.N., Ferreira C.F., Amorim E.P. Genetic improvement for resistance to Black Sigatoka in bananas: a systematic review. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.657916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonthisut M., Wongpanya R., Phonphoem A., Phonphoem W.P. Enhancement of 1-deoxynojirimycin production in mulberry (Morus spp.) using LED irradiation. PCTOC. 2022;148(1):167–176. [Google Scholar]
- Sowmya H.D., Usharani T.R., Sunisha C., Mohandas S. Banana:Genomics and Transgenic Approaches for Genetic Improvement. Springer; Singapore: 2016. Engineering resistance to Sigatoka; pp. 227–236. [Google Scholar]
- Sreedharan S., Shekhawat U.K.S., Ganapathi T.R. Constitutive and stress -inducible overexpression of a native aquaporin gene (MusaPIP2; 6) in transgenic banana plants signals its pivotal role in salt tolerance. Plant Mol. Biol. 2015;88(1-2):41–52. doi: 10.1007/s11103-015-0305-2. [DOI] [PubMed] [Google Scholar]
- Sreedharan S., Shekhawat U.K.S., Ganapathi T.R. MusaSAP1, a A20/AN1 zinc finger gene from banana functions as a positive regulator in different stress responses. Plant Mol. Biol. 2012;80(4-5):503–517. doi: 10.1007/s11103-012-9964-4. [DOI] [PubMed] [Google Scholar]
- Sreedharan S., Shekhawat U.K., Ganapathi T.R. Transgenic banana plants overexpressing a native plasma membrane aquaporin M USA PIP 1; 2 display high tolerance levels to different abiotic stresses. Plant Biotechnol. J. 2013;11(8):942–952. doi: 10.1111/pbi.12086. [DOI] [PubMed] [Google Scholar]
- Strosse H., Van den Houwe I., Panis B. 2004. Banana Cell and Tissue Culture-Review. Banana Improvement: Cellular, Molecular Biology, and Induced Mutations; pp. 1–12. [Google Scholar]
- Subrahmanyeswari T., Gantait S. Biotechnology of banana (Musa spp.): multi-dimensional progress and prospect of in vitro–mediated system. Appl. Microbiol. Biotechnol. 2022:1–25. doi: 10.1007/s00253-022-11973-4. [DOI] [PubMed] [Google Scholar]
- Suman S. Plant tissue culture: a promising tool of quality material production with special reference to micropropagation of banana. Biochem. Cell. Arch. 2017;17(1):1–26. [Google Scholar]
- Suman S., Rajak K.K., Kumar H. Micropropagation of banana cv. BB Battisa. Biochem. Cell. Arch. 2013;13:249–254. [Google Scholar]
- Suman ., Kumar H. Micropropagation of banana cv. Malbhog. Bioscan. 2015;10(2):647–650. [Google Scholar]
- Sun J., Zhang J., Fang H., Peng L., Wei S., Li C., Lu J. Comparative transcriptome analysis reveals resistance-related genes and pathways in Musa acuminate banana'Guijiao 9' in response to Fusarium wilt. Plant Physiol. Biochem. 2019;141:83–94. doi: 10.1016/j.plaphy.2019.05.022. [DOI] [PubMed] [Google Scholar]
- Tabiyeh D., Bernard F., Shacker H. Investigation of glutathione, salicylic acid and GA3 effects on browning in Pistacia vera shoot tips culture. Acta Hortic. 2005;726:201–204. [Google Scholar]
- Tak H., Negi S., Ganapathi T.R., Bapat V.A. 2016. Molecular Farming: Prospects and Limitation. Banana: Genomics and Transgenic Approaches for Genetic Improvement; pp. 261–275. [Google Scholar]
- Tak H., Negi S., Gupta A., Ganapathi T.R. A stress associated NAC transcription factor MpSNAC67 from banana (Musa x paradisiaca) is involved in regulation of chlorophyll catabolic pathway. Plant Physiol. Biochem. 2018;132:61–71. doi: 10.1016/j.plaphy.2018.08.020. [DOI] [PubMed] [Google Scholar]
- Thanakronpaisan K., Kongbangkerd A., Premjet S. Effect of BA and chitosan on in vitro growth of musa (ABB Group)‘Kluai namwa Mali-ong’. Asia-pac. J. Sci. Technol. 2019;24(1) [Google Scholar]
- Thangavelu R., Saraswathi M.S., Uma S., Loganathan M., Backiyarani S., Durai P., Swennen R. Identification of sources resistant to a virulent Fusarium wilt strain (VCG 0124) infecting Cavendish bananas. Sci. Rep. 2021;11(1):1–14. doi: 10.1038/s41598-021-82666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe T., Stasolla C., Yeung E.C., de Klerk G.J., Roberts A., George E.F. In: Plant Propagation by Tissue Culture. third ed. George E.F., Hall M.A., de Klerk G.J., editors. Springer; Dordrecht: 2008. The components of plant tissue culture media II: organic additions, osmotic and pH effects, and support systems; pp. 115–173. [Google Scholar]
- Titov S., Bhowmik S.K., Mandal A., Alam M.S., Udin S.N. Control of phenolic compound secretion and effect of growth regulators for organ formation from Musa spp. cv. kanthali floral bud explants. Am. J. Biochem. Biotechnol. 2006;2(3):97–104. [Google Scholar]
- Tran Thanh Van K., Trinh . In: Plant Tissue Culture, Application and Limitations. Bhojwani S.S., editor. Elservier; Amsterdam: 1990. Organogenic differentiation. [Google Scholar]
- Tripathi J.N., Ntui V.O., Ron M., Muiruri S.K., Britt A., Tripathi L. CRISPR/Cas9 editing of endogenous banana streak virus in the B genome of Musa spp. overcomes a major challenge in banana breeding. Commun. Biol. 2019;2(1):1–11. doi: 10.1038/s42003-019-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi J.N., Oduor R.O., Tripathi L. A high-throughput regeneration and transformation platform for production of genetically modified banana. Front. Plant Sci. 2015;6:1025. doi: 10.3389/fpls.2015.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi L., Tripathi J.N., Kiggundu A., Korie S., Shotkoski F., Tushemereirwe W.K. Field trial of Xanthomonas wilt disease-resistant bananas in East Africa. Nat. Biotechnol. 2014;32(9):868–870. doi: 10.1038/nbt.3007. [DOI] [PubMed] [Google Scholar]
- Tripathi L., Mwaka H., Tripathi J.N., Tushemereirwe W.K. Expression of sweet pepper Hrap gene in banana enhances resistance to Xanthomonas campestris pv. musacearum. Mol. Plant Pathol. 2010;11(6):721–731. doi: 10.1111/j.1364-3703.2010.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi L., Ntui V.O., Tripathi J.N. CRISPR/Cas9-based genome editing of banana for disease resistance. Curr. Opin. Plant Biol. 2020;56:118–126. doi: 10.1016/j.pbi.2020.05.003. [DOI] [PubMed] [Google Scholar]
- Tripathi L., Ntui V.O., Tripathi J.N., Kumar P. Application of CRISPR/Cas for diagnosis and management of viral diseases of banana. Front. Microbiol. 2021:3622. doi: 10.3389/fmicb.2020.609784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaij B.M., Jazar Z.H., Hasan M. Trends in the use of tissue culture, applications and future aspects. Int. J. Plant Biol. 2020;11(1):8385. [Google Scholar]
- Uma S., KumaraveL M., Backiyarani S., Saraswathi M.S., Durai P., Karthic R. Somatic embryogenesis as a tool for reproduction of genetically stable plants in banana and confirmatory field trials. PCTOC. 2021;147(1):181–188. [Google Scholar]
- Uzaribara E., Nachegowda V., Ansar H., Sathyanarayana B.N., Taj A. In vitro propagation of red banana (Musa acuminata) Bioscan. 2015;10:125–129. [Google Scholar]
- Van den Houwe I., Swennen R. Vol. 530. 1999. Characterization and control of bacterial contaminants in in vitro cultures of banana (Musa spp.) pp. 69–79. (International Symposium on Methods and Markers for Quality Assurance in Micropropagation). [Google Scholar]
- Vanhove A.C., Vermaelen W., Panis B., Swennen R., Carpentier S. Screening the banana biodiversity for drought tolerance: can an in vitro growth model and proteomics be used as a tool to discover tolerant varieties and understand homeostasis. Front. Plant Sci. 2012;3:176. doi: 10.3389/fpls.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vani A.K.S., Reddy G.M. Novel techniques in efficient micropropagation of certain popular banana cultivars. Indian J. Genet. Plant Breed. 1999;53:247–250. [Google Scholar]
- Vasane S.R., Patil A.B., Kothari R.M. Vol. 865. 2008. Bio-Acclimatization of in vitro propagated banana plantlets'GRAND NAIN'; pp. 217–224. (IV International Symposium on Acclimatization and Establishment of Micropropagated Plants). [Google Scholar]
- Vendrame W.A., Feuille C., Beleski D., Bueno P.M.C. In vitro growth responses of ornamental bananas (musa sp.) as affected by light sources. Horticulturae. 2022;8(2):92. [Google Scholar]
- Vishnevetsky J., White T.L., Palmateer A.J., Flaishman M., Cohen Y., Elad Y., Perl A. Improved tolerance toward fungal diseases in transgenic Cavendish banana (Musa spp. AAA group) cv. Grand Nain. Transgenic Res. 2011;20(1):61–72. doi: 10.1007/s11248-010-9392-7. [DOI] [PubMed] [Google Scholar]
- Vuylsteke D., Ortiz R., Ferris R.S.B., Crouch J.H. Vol. 14. John Wiley & Sons; New York, NY: 1996. Plantain improvement; pp. 267–273. (Plant Breeding Reviews). [Google Scholar]
- Waman A.A., Bohra P., Sathyanarayana B.N., Umesha K., Mukunda G.K., Ashok T.H., Gowda B. Optimization of factors affecting in vitro establishment, ex vitro rooting and hardening for commercial scale multiplication of silk banana (Musa AAB) Erwerbsobstbau. 2015;57(3):153–164. [Google Scholar]
- Wang A., Li J., Al-Huqail A.A., Al-Harbi M.S., Ali E.F., Wang J., Eissa M.A. Mechanisms of chitosan nanoparticles in the regulation of cold stress resistance in banana plants. Nanomaterials. 2021;11(10):2670. doi: 10.3390/nano11102670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken D., Jiménez Gonzalez E., Gerth A., Gómez-Kosky R., Schumann A., Claus D. Effect of immersion systems, lighting, and TIS designs on biomass increase in micropropagating banana (Musa spp. cv.'Grande naine'AAA). In Vitro Cell. Dev. Biol. Plant. 2014;50(5):582–589. [Google Scholar]
- Yadav A., Prasad Y., Kumar M., Pandey S., Maurya R., Pandey P. Effects of Mercuric chloride and Ethanol for surface sterilization under in vitro plant growth in banana (Musa paradisiaca L.) variety “Udhayam”. J. Pharmacogn. Phytochem. 2021;10(1):2281–2283. [Google Scholar]
- Yadav K., Patel P., Srivastava A.K., Ganapathi T.R. Overexpression of native ferritin gene MusaFer1 enhances iron content and oxidative stress tolerance in transgenic banana plants. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh N., Chung J.P. High-brightness LEDs—Energy efficient lighting sources and their potential in indoor plant cultivation. Renew. Sustain. Energy Rev. 2009;13(8):2175–2180. [Google Scholar]
- Yumbya P., Ambuko J., Hutchinson M., Owino W., Juma J., Machuka E., Mutuku J.M. Transcriptome analysis to elucidate hexanal's mode of action in preserving the post-harvest shelf life and quality of banana fruits (Musa acuminata) J. Agric. Food Inf. 2021;3 [Google Scholar]
- Zahedi S.M., Karimi M., Teixeira da Silva J.A. The use of nanotechnology to increase quality and yield of fruit crops. J. Sci. Food Agric. 2020;100(1):25–31. doi: 10.1002/jsfa.10004. [DOI] [PubMed] [Google Scholar]
- Ziv M., Halevy A.H. Control of oxidative browning and in vitro propagation of Strelitzia reginae. Hortic. Sci. (Stuttg.) 1983;18(4):434–436. [Google Scholar]
- Ziv M. Liquid Culture Systems for in Vitro Plant Propagation. Springer; Dordrecht: 2005. Simple bioreactors for mass propagation of plants; pp. 79–93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.