Abstract
Objective:
To evaluate the comparative influence of NPT and standard surgical dressing administration on incidence risk for surgical site infections, complications, and hospital re-admission after hepatopancreatobiliary surgery.
Methods:
Five databases were systematically searched according to PRISMA guidelines. These databases included Web of Science, MEDLINE, CENTRAL, EMBASE, and Scopus for eligible studies published prior to March 2021. With eligible studies, we conducted a random-effects meta-analysis to evaluate comparative outcomes such as superficial surgical infection, deep surgical infection, seroma incidence, hematoma incidence, and hospital re-admission in patients receiving NPT or standard surgical dressings after hepatopancreatobiliary surgery.
Results:
The search strategy yielded 963 studies, with six studies meeting inclusion criteria. Odds of superficial surgical site infection (OR: 1.58), deep surgical site infection (1.43), seroma complication (1.64), hematoma complication (0.40) were insignificantly different between patients receiving NPT and standard surgical dressing. The odds of hospital re-admission rate (2.37), however, were elevated in patients receiving standard surgical dressing relative to those receiving NPT.
Conclusion:
This meta-analysis shows that NPT usage slightly reduces risk of hospital readmission as compared to standard surgical dressing. We did not observe any significant effect of NPT on superficial, deep surgical infections, seroma, and haematoma outcomes following hepatopancreatobiliary surgery. These findings may aid clinicians in stratifying risk and selecting treatment strategy in patients undergoing hepatopancreatobiliary surgery.
Keywords: Surgical site infection, Seroma, hematoma, Negative pressure wound therapy, Morbidity, Hospital re-admission
INTRODUCTION
Surgical site infection incidence for patients undergoing abdominal surgeries such as hepatopancreatobiliary surgery has been reported to be as high as 40%.1–3 Although medical advances have limited mortality in patients undergoing hepatopancreatobiliary surgery, recent studies indicate that morbidity remains high.4 Surgical site infections increase patient discomfort, delay adjuvant treatments, impose higher financial burdens, and lowers quality of life.5
Surgical site infection in hepatopancreatobiliary surgery patients typically occurs due to several pre-, intra-, and post-operative factors.1,6 Ceppa et al.1 reported discrepancies in surgical technique, execution, and wound management to be the most critical risk factor impacting wound soilage. They also noted that surgical site infections can develop either as superficial (i.e. skin and subcutaneous tissue), deep (i.e. fascia and muscle), or organ space infections.
Negative pressure wound therapy (NPT) has attracted much attention for facilitating wound recovery.7–10 NPT applies sub-atmospheric pressure on wound sites, leading to improved wound perfusion, granular tissue formation, exudate removal, and reduced microbial colonization.11–13 Specifically, studies suggest that the reverse-tissue expansion effect generated by NPT exploits the viscoelastic properties of the skin (i.e. the crinkle effect) to facilitate vascularity and mitotic activity at the wound site.14–16
Several studies have attempted to compare NPT and standard surgical dressing practices in terms of morbidity-related outcomes in patients undergoing hepatopancreatobiliary surgery.7–10,17,18 Nonetheless, no consensus currently exists in the literature regarding the impact of these different wound therapies on superficial and deep surgical site infections in hepatopancreatobiliary surgery patients. While some studies reported fewer superficial surgical site infections in patients receiving NPT,8,10,17 others noted either negligible differences or the opposite trend.7,18 Similarly, some studies have fewer deep surgical site infections in patients receiving NPT,7,17 while others have noted the opposite effect.8,9,18
To the best of our knowledge, no study has attempted to evaluate comparative morbidity when using NPT or standard surgical dressings after hepatopancreatobiliary surgery. We therefore sought to perform a meta-analysis of the available body of evidence on this subject. We attempted to evaluate the comparative impact of NPT and standard surgical dressing strategies on superficial surgical infection, deep surgical infection, seroma complication, hematoma complication, and hospital re-admission rates in patients undergoing hepatopancreatobiliary surgery. The present study aims to increase clinical awareness among surgeons concerning how wound therapy impacts patients undergoing hepatopancreatobiliary surgery.
METHODS
This meta-analysis was conducted according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.19
Data search strategy:
We searched five scientific databases (Web of Science, MEDLINE, CENTRAL, EMBASE, and Scopus) for eligible studies published prior to March 2021. A number of MeSH keywords were used in combination, including “hepatopancreatobiliary”, “hepatic resection”, “pancreatic resection”, “negative pressure wound therapy”, “standard surgical dressing”, “surgical infection site, superficial”, “surgical infection site, deep”, “complications”, “seroma”, and “hematoma”. References cited by included studies were manually scanned to identify additional relevant studies. The inclusion criteria were as follows:
Studies comparing superficial or deep surgical infection rates between patients receiving NPT and standard surgical dressing strategies after hepatopancreatobiliary surgery.
Studies comparing post-surgical complications, including seroma, hematoma, and re-admission rates, between patients receiving NPT and standard surgical dressing strategies after hepatopancreatobiliary surgery.
Studies involving human participants.
Studies conducted as randomized controlled trials, controlled clinical trials, or cohort trials.
Studies published in peer-reviewed scientific journals.
Studies published in English.
Study screening was performed independently by two reviewers. Disagreements were resolved through discussions with a third reviewer.
Quality assessment:
Bias risk within included randomized controlled trials was assessed using Cochrane’s risk of bias assessment tool (Sterne et al., 2016). Bias risk within included cohort trials was appraised using the ROBINS-I tool.20 Methodological quality appraisal was performed independently by two reviewers. Again, a third reviewer arbitrated any disputes.
Data analysis:
A within-group meta-analysis was performed using Comprehensive Meta-analysis software version 2.0.21 The meta-analysis was conducted based on a random-effects model.22 We calculated odds ratios to evaluate the odds of superficial surgical infection, deep surgical infection, seroma complications, hematoma complications, and re-admission rates between patients receiving negative pressure wound therapy and standard surgical dressings after hepatopancreatobiliary surgery. I2 values were computed to assess heterogeneity (0-25%: negligible, 25%-75%: moderate, ≥75%: substantial.23 When studies provided medians and ranges, we used a previously established method by24 to convert these values into means and standard deviations. Publication bias was evaluated using Duval and Tweedy’s trim and fill procedure.25 The significance level for this study was determined to be 5%.
RESULTS
Our literature search identified 950 studies for inclusion. Reference section screening yielded an additional 13 studies. After applying inclusion criteria, a total of six studies remained (Fig.1). Five of these were randomized controlled trials7–10,18 while the other one was a retrospective cohort trial.17 Data extracted from these studies can be found in Table-I. The six included studies contained data detailing 657 (345F, 311M) patients. A total of 336 (127F, 153M) patients received standard surgical dressing treatment, while the other 321 (118F, 158M) patients received NPT. Two studies did not define the gender distribution of their patient sample.10,17
Fig.1.
PRISMA flowchart detailing literature search and screening strategy.
Table-I.
Relevant information from included studies.
| Study | Country | Study type | Sample size | Age (M ± S.D years) | Superficial surgical site infections | Deep surgical site infections | Seroma formation | Hematoma formation | Re-admission due to complications |
|---|---|---|---|---|---|---|---|---|---|
| O’Neill and Martin (2020) | USA | Randomized controlled trials | SSD: 20 NPT: 20 |
SSD: 61.2 NPT: 59.6 |
SSD: 2 NPT: 1 |
- | - | - | - |
| Andrianello et al. (2020) | Italy | Randomized controlled trials | SSD: 49 (19F, 30M) NPT: 46 (19F, 27M) | SSD: 64 ± 17 NPT: 69 ± 12 | SSD: 3 NPT: 4 | SSD: 3 NPT: 1 | SSD: 6 NPT: 0 | SSD: 1 NPT: 2 | - |
| Javed et al. (2019) | USA | Randomized controlled trials | SSD: 61 (27F, 34M) NPT: 62 (31F, 31M) |
SSD: 66.1 ± 9 NPT: 66.4 ± 9.3 |
SSD: 17 NPT: 4 |
SSD: 2 NPT: 2 |
- | - | SSD: 12 NPT: 5 |
| Kuncewitch et al. (2019) | USA | Randomized controlled trials | SSD: 37 (17F, 20M) NPT: 36 (13F, 23M) |
SSD: 65 NPT: 65.5 |
SSD: 6 NPT: 5 |
SSD: 2 NPT: 3 |
SSD: 6 NPT: 4 |
- | SSD: 6 NPT: 3 |
| Shen et al. (2017) | USA | Randomized controlled trials | SSD: 133 (64F, 69M) NPT: 132 (55F, 77M) |
SSD: 62 NPT: 59.5 |
SSD: 17 NPT: 17 |
SSD: 4 NPT: 4 |
SSD: 8 NPT: 7 |
SSD: 0 NPT: 1 |
SSD: 6 (119) NPT: 3 (118) |
| Gupta et al. (2017) | USA | Retrospective cohort study | SSD: 36 NPT: 25 |
- | SSD: 2 NPT: 0 |
SSD: 9 NPT: 1 |
- | - | - |
Legends: M: Mean: S.D: Standard deviation, F: Female, M: Male, SSD: Standard surgical dressing, NPT: Negative pressure wound therapy.
Average patient age was 63.8 ± 3.15 years. The average age of patients receiving standard surgical dressings was 63.6 ± 2.0 years, while the average age of patients receiving NPT was 64 ± 4.2 years. One study did not report the age of their patient sample.17
Quality assessment for included randomized controlled trials:
Risk of methodological bias in RCTs was evaluated using Cochrane’s risk of bias assessment tool for randomized controlled trials (Supplementary Table-I). Overall risk of bias was found to be low in the included studies. We observed that allocation of concealment, blinding of participants, and other biases were the most common areas of bias (Supplementary Fig.1).
Supplementary Table-I.
Demonstrates the risk of bias according to Cochrane’s risk of bias assessment tool for randomized controlled trials (+: low risk, -: high risk, ?: unclear).
| Study | Random sequence generation | Allocation concealment | Selective reporting | Other bias | Blinding of participants & personnel | Blinding of outcome assessment | Incomplete outcome data |
|---|---|---|---|---|---|---|---|
| O’Neill and Martin (2020) | + | ? | + | - | + | ? | + |
| Andrianello et al. (2020) | + | ? | + | - | + | ? | + |
| Javed et al. (2019) | + | ? | + | - | + | ? | + |
| Kuncewitch et al. (2019) | + | ? | + | - | + | ? | + |
| Shen et al. (2017) | + | + | + | + | + | + | + |
Supplementary Fig.1.
Risk of bias according to Cochrane’s risk of bias assessment tool for randomized controlled trials.
Quality assessment for included cohort studies:
Risk of methodological bias in retrospective cohort studies were assessed with the ROBINS-I tool (Supplementary Table-II) The overall risk was found to be high in the one included cohort study. We observed that missing data, selection bias, and selective reporting of results were the main biased areas.
Supplementary Table-II.
Demonstrates the risk of bias according to ROBINS-I tool (+: low risk, -: high risk, ?: unclear)
| Study | Confounding bias | Selection bias | Deviation from intended intervention | Missing data | Measurement in outcome | Selection of reported result | Classification of intervention |
|---|---|---|---|---|---|---|---|
| Gupta et al. (2017) | + | ? | + | - | + | ? | + |
Publication bias:
We used Duval and Tweedy’s trim and fill method to determine missing studies according to the random effects model on either side of the mean effect of the funnel plot (Supplementary Fig.2). Three studies were missing on the left side of the mean effect. The overall random effects model determined the point estimates and the 95% confidence intervals for all the combined studies as 1.50 (0.91 to 2.47). After using the trim and fill, the imputed point estimates were estimated as 1.05 (0.56 to 1.97).
Supplementary Fig.2.

Demonstrates the publication bias by Duval & Tweedy’s trim and fill method.
Meta-analysis report
Superficial surgical site infection incidence:
Six studies reported on superficial surgical infection incidence in patients receiving standard surgical dressing or negative pressure wound therapy. Patients receiving standard surgical dressings presented increased odds of developing superficial surgical infections (Fig.2) (Odds ratio: 1.58, 95% C.I: 0.78 to 3.22, p=0.20). No study heterogeneity was noted (I2: 0%).
Fig.2.
Forest plot for studies evaluating the odds of superficial surgical infection in patients receiving a standard surgical dressing or negative pressure wound therapy.
Deep surgical site infection:
Five studies reported deep surgical infection incidence in patients receiving standard surgical dressing or negative pressure wound therapy. Patients receiving standard surgical dressing presented increased odds of deep surgical infection (Fig.3) (Odds ratio: 1.43, 95% C.I: 0.62 to 3.28, p=0.39). No study heterogeneity was noted (I2: 0%).
Fig.3.
Forest plot for studies evaluating the odds of deep surgical infection in patients receiving a standard surgical dressing or negative pressure wound therapy.
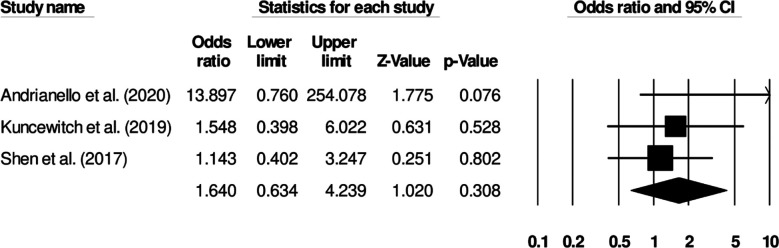
Seroma formation:
Three studies investigated seroma incidence in patients receiving standard surgical dressing or negative pressure wound therapy. Patients receiving standard surgical dressing showed increased odds of seroma incidence (Fig.4) (Odds ratio: 1.64, 95% C.I: 0.63 to 4.23, p=0.30). Study heterogeneity was negligible (I2: 10.6%).
Fig.4.
Forest plot for studies evaluating the odds of seroma in patients receiving a standard surgical dressing or negative pressure wound therapy.
Hematoma formation:
Two studies reported hematoma incidence in patients receiving standard surgical dressing or negative pressure wound therapy. Patients receiving negative pressure wound therapy showed increased odds of hematoma incidence (Fig.5) (Odds ratio: 0.40, 95% C.I: 0.05 to 2.82, p=0.36). No study heterogeneity was noted (I2: 0%).
Fig.5.
Forest plot for studies evaluating the odds of hematoma in patients receiving a standard surgical dressing or negative pressure wound therapy.
Hospital re-admission:
Three studies investigated hospital re-admission rates in patients receiving standard surgical dressing or negative pressure wound therapy Patients receiving standard surgical dressing showed increased odds of hospital re-admission (Fig.6) (Odds ratio: 2.37, 95% C.I: 1.12 to 5.03, p=0.024). No study heterogeneity was noted (I2: 0%).
Fig.6.
Forest plot for studies evaluating the odds of hospital re-admission in patients receiving a standard surgical dressing or negative pressure wound therapy.
DISCUSSION
This systematic review and meta-analysis shows increased risk of superficial and deep surgical site infection in patients receiving standard surgical dressings after hepatopancreatobiliary surgery compared to those receiving NPT. We also find elevated risk of seroma and hospital re-admission for patients receiving standard surgical dressings, but an elevated risk for hematoma in patients receiving NPT.
Surgical site infection management is challenging, and patients with surgical site infections after hepatopancreatobiliary surgery exhibit poorer morbidity and mortality-related outcomes.1,2,4 Many researchers have recommended the administration of specialized NPT to avoid or ameliorate these adverse outcomes,26,27 suggesting that NPT can not only improve morbidity outcomes, but also result in better patient adherence to treatment, treatment cost-effectiveness, and overall quality of life.28,29 However, while the consensus indicates NPT’s superiority over standard medical dressing for managing normal wounds, no consensus regarding NPT’s efficacy after hepatopancreatobiliary surgery currently exists.
Here, although there was a collective trend towards decreased risk in patients receiving NPT, not all of the individual studies concurred. While Andrianello et al.7 reported higher superficial surgical site infection incidence in patients receiving NPT, others Javed et al. 2019, O’Neill and Martin, 2020, Gupta et al.8,10,17 showed the opposite. Andrianello et al.7 also found increased rates of organ space infection and post-pancreatectomy hemorrhage in the NPT group. Cohort variation (i.e. the presence of higher body mass index values and more co-morbidities) may account for this discrepancy by affecting surgical procedure and wound management complexity.
Similarly, a lack of consensus also existed for deep surgical site infections between examined studies. Shen et al.18 reported no differences in the rate of deep surgical site infection between wound treatment groups. However, Gupta et al.17 reported the opposite. The actual nature of the surgical procedure being examined may be the cause of this discrepancy. While NPT can diminish wound surface inflammation, a sealed incision may prevent NPT’s ability to remove excess fluid. Nonetheless, collectively, the risks of both superficial surgical site infection and deep surgical site infections were higher in patients receiving standard surgical dressing as compared to NPT after hepatopancreatobiliary surgery. Furthermore, NPT also directly influences healthcare costs8,17 by reducing hospital re-admission—something that the present meta-analysis supports.
The present meta-analysis also attempted to reach a consensus concerning the impact of wound treatment approach on seroma and hematoma incidence. Andrianello et al. (2020)7 noted that while NPT was successful in preventing seroma onset, it was not successful in limiting hematoma-based complications. Shen et al.18 also reported a similar pattern. It is possible that reduced seroma incidence in NPT-receiving patients results from enhanced lymphatic circulation induced by NPT.30,31 However, it is unclear why NPT is ineffective in limiting hematoma.
Limitations of the study:
First and foremost, this study was not pre-registered in a systematic review repository such as PROSPERO York or Joanna Briggs Institute owing to logistical issues raised by the current COVID-19 pandemic crisis. Second, we acknowledge the relative scarcity of available data may bias our understanding of the comparative impact of NPT and standard surgical dressing on hematoma complications. As only two studies (featuring small sample sizes) investigated this outcome, incurring a type II error cannot be ruled out.32 We therefore strongly recommend future studies to address these limitations by improving the amount of data available on this subject.
CONCLUSION
This meta-analysis provides preliminary evidence showing that NPT is superior to standard surgical dressing practices for reducing the risks of hospital readmission in patients undergoing hepatopancreatobiliary surgery. We did not observe any significant effect of NPT as compared to standard surgical dressing on superficial, deep surgical infections, seroma, and haematoma outcomes following hepatopancreatobiliary surgery. The findings from the present study cautiously recommend the administration of NPT for managing wound recovery in patients undergoing hepatopancreatobiliary surgery.
Authors’ contributions:
BR conceived and designed the study.
XJ, JC and JM collected the data and performed the analysis.
BR was involved in the writing of the manuscript and the integrity of the study.
All authors have read and approved the final manuscript.
REFERENCES
- 1.Ceppa EP, Pitt HA, House MG, Kilbane EM, Nakeeb A, Schmidt CM. Reducing surgical site infections in hepatopancreatobiliary surgery. HPB. 2013;15(5):384–391. doi: 10.1111/j.1477-2574.2012.00604.x. doi:10.1111/j.1477-2574.2012.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isik O, Kaya E, Sarkut P, Dundar HZ. Factors Affecting Surgical Site Infection Rates in Hepatobiliary Surgery. Surg Infect. 2015;16(3):281–286. doi: 10.1089/sur.2013.195. doi:10.1089/sur.2013.195. [DOI] [PubMed] [Google Scholar]
- 3.Morikane K. Epidemiology and risk factors associated with surgical site infection after different types of hepatobiliary and pancreatic surgery. Surg Today. 2017;47(10):1208–1214. doi: 10.1007/s00595-017-1503-0. doi:10.1007/s00595-017-1503-0. [DOI] [PubMed] [Google Scholar]
- 4.Parikh P, Shiloach M, Cohen ME, Bilimoria KY, Ko CY, Hall BL, et al. Pancreatectomy risk calculator:An ACS-NSQIP resource. HPB. 2010;12(7):488–497. doi: 10.1111/j.1477-2574.2010.00216.x. doi:10.1111/j.1477-2574.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennfleck FW, Bachmeier T, Simet W, Zeman F, Junger HHG, Schlitt HJ, et al. Surgical Site Infections and their economic significance in hepatopancreatobiliary surgery:A retrospective incidence, cost, and reimbursement analysis in a German centre of the highest level of care. Int Wound J. 2021;18(1):17–23. doi: 10.1111/iwj.13511. doi:10.1111/iwj.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ejaz A, Schmidt C, Johnston FM, Frank SM, Pawlik TM. Risk factors and prediction model for inpatient surgical site infection after major abdominal surgery. J Surg Res. 2017;217:153–159. doi: 10.1016/j.jss.2017.05.018. doi:10.1016/j.jss.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Andrianello S, Landoni L, Bortolato C, Iudici L, Tuveri M, Pea A, et al. Negative pressure wound therapy for prevention of surgical site infection in patients at high risk after clean-contaminated major pancreatic resections:A single-center, phase 3, randomized clinical trial. Surgery. 2021;169(5):1069–1075. doi: 10.1016/j.surg.2020.10.029. doi:10.1016/j.surg.2020.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Javed AA, Teinor J, Wright M, Ding D, Burkhart RA, Hundt J, et al. Negative Pressure Wound Therapy for Surgical-site Infections:A Randomized Trial. Ann Surg. 2019;269(6):1034–1040. doi: 10.1097/SLA.0000000000003056. doi:10.1097/SLA.0000000000003056. [DOI] [PubMed] [Google Scholar]
- 9.Kuncewitch MP, Blackham AU, Clark CJ, Dodson RM, Russell GB, Levine EA, et al. Effect of Negative Pressure Wound Therapy on Wound Complications Post-Pancreatectomy. Am Surg. 2019;85(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 10.O'Neill CH, Martin RCG. Negative-pressure wound therapy does not reduce superficial SSI in pancreatectomy and hepatectomy procedures. J Surg Oncol. 2020;122(3):480–486. doi: 10.1002/jso.25980. doi:10.1002/jso.25980. [DOI] [PubMed] [Google Scholar]
- 11.Banwell PE, Musgrave M. Topical negative pressure therapy:mechanisms and indications. Int Wound J. 2004;1(2):95–106. doi: 10.1111/j.1742-4801.2004.00031.x. doi:10.1111/j.1742-4801.2004.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lima RVKS, Coltro PS, Farina JA. Negative pressure therapy for the treatment of complex wounds. Rev Col Bras Cir. 2017;44(1):81–93. doi: 10.1590/0100-69912017001001. doi:10.1590/0100-69912017001001. [DOI] [PubMed] [Google Scholar]
- 13.Arti H, Khorami M, Ebrahimi-Nejad V. Comparison of negative pressure wound therapy (NPWT) &conventional wound dressings in the open fracture wounds. Pak J Med Sci. 2016;32(1):65–69. doi: 10.12669/pjms.321.8568. doi:10.12669/pjms.321.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borgquist O, Ingemansson R, Malmsjö M. The influence of low and high pressure levels during negative-pressure wound therapy on wound contraction and fluid evacuation. Plast Reconstr Surg. 2011;127(2):551–559. doi: 10.1097/PRS.0b013e3181fed52a. doi:10.1097/PRS.0b013e3181fed52a. [DOI] [PubMed] [Google Scholar]
- 15.Brace JA. Negative Pressure Wound Therapy for Abdominal Wounds. J Wound Ostomy Continence Nurs. 2007;34(4):428–430. doi: 10.1097/01.WON.0000281661.77086.d1. doi:10.1097/01.WON.0000281661.77086.d1. [DOI] [PubMed] [Google Scholar]
- 16.Olenius M, Dalsgaard CJ, Wickman M. Mitotic activity in expanded human skin. Plast Reconstr Surg. 1993;91(2):213–216. doi: 10.1097/00006534-199302000-00001. doi:10.1097/00006534-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R, Darby GC, Imagawa DK. Efficacy of negative pressure wound treatment in preventing surgical site infections after whipple procedures. Am Surg. 2017;83(10):1166–1169. [PubMed] [Google Scholar]
- 18.Shen Y, Cai R, Sun J, Dong X, Huang R, Tian S, et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men:a systematic review and meta-analysis. Endocrine. 2017;55(1):66–76. doi: 10.1007/s12020-016-1014-6. doi:10.1007/s12020-016-1014-6. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. doi:10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Hernán MA, Reeves BC, SavovićJ , Berkman ND, Viswanathan M, Henry D, et al. ROBINS-I:a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. doi:10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bax L, Yu LM, Ikeda N, Moons KGM. A systematic comparison of software dedicated to meta-analysis of causal studies. BMC Med Res Methodol. 2007;7:40. doi: 10.1186/1471-2288-7-40. doi:10.1186/1471-2288-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–159. doi: 10.1111/j.1467-985X.2008.00552.x. doi:10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. doi:10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. doi:10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duval S, Tweedie R. Trim and fill:A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. doi:10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 26.de Laat EHEW, van den Boogaard MHWA, Spauwen PHM, van Kuppevelt DHJM, van Goor H, Schoonhoven L. Faster wound healing with topical negative pressure therapy in difficult-to-heal wounds:a prospective randomized controlled trial. Ann Plast Surg. 2011;67(6):626–631. doi: 10.1097/SAP.0b013e31820b3ac1. doi:10.1097/SAP.0b013e31820b3ac1. [DOI] [PubMed] [Google Scholar]
- 27.Suissa D, Danino A, Nikolis A. Negative-pressure therapy versus standard wound care:a meta-analysis of randomized trials. Plast Reconstr Surg. 2011;128(5):498e–503e. doi: 10.1097/PRS.0b013e31822b675c. doi:10.1097/PRS.0b013e31822b675c. [DOI] [PubMed] [Google Scholar]
- 28.Moffatt CJ, Murray S, Aubeeluck A, Quere I. Communication with patients using negative wound pressure therapy and their adherence to treatment. J Wound Care. 2019;28(11):738–756. doi: 10.12968/jowc.2019.28.11.738. doi:10.12968/jowc.2019.28.11.738. [DOI] [PubMed] [Google Scholar]
- 29.Othman D. Negative pressure wound therapy literature review of efficacy, cost effectiveness, and impact on patients'quality of life in chronic wound management and its implementation in the United kingdom. Plast Surg Int. 2012;2012:374398. doi: 10.1155/2012/374398. doi:10.1155/2012/374398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM):hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2011;19(5):588–596. doi: 10.1111/j.1524-475X.2011.00714.x. doi:10.1111/j.1524-475X.2011.00714.x. [DOI] [PubMed] [Google Scholar]
- 31.Wilkes RP, Kilpad DV, Zhao Y, Kazala R, McNulty A. Closed incision management with negative pressure wound therapy (CIM):biomechanics. Surg Innov. 2012;19(1):67–75. doi: 10.1177/1553350611414920. doi:10.1177/1553350611414920. [DOI] [PubMed] [Google Scholar]
- 32.Harmon LJ, Losos JB. The effect of intraspecific sample size on type I and type II error rates in comparative studies. Evol Int J Org Evol. 2005;59(12):2705–2710. [PubMed] [Google Scholar]