Abstract
Introduction
Cerebral venous sinus thrombosis (CVST) is a rare neurovascular emergency that has been observed following COVID-19 infection, as well as following the use of non-mRNA COVID-19 vaccines.
Case Presentation
The authors report a case of CVST in a 67-year-old woman, unvaccinated for COVID-19, who presented with acute otitis externa. It remains unclear whether the CVST was a following COVID-19 infection complication, otogenic CVST, or a combination of both.
Conclusion
This case demonstrates the diagnostic and therapeutic dilemmas in managing this patient’s challenging anticoagulation and antibiotic duration, as well as subsequent COVID-19 vaccination recommendations.
Keywords: acute otitis externa, cerebral venous sinus thrombosis, COVID-19, vaccine-induced thrombotic thrombocytopenia, case report
Introduction
Cerebral venous thrombosis (CVST) is a rare (0.22–1.57 per 100,000) neurovascular emergency that typically presents with headache, visual disturbances, focal deficits, seizures, and encephalopathy. CVST is more common in females aged less than 65 years old. 1–3 CVST has been reported following COVID-19 infection, as well as following the use of non-mRNA COVID-19 vaccines, and most recently following mRNA COVID-19 vaccines. The authors present a case of CVST diagnosed incidentally during acute otitis externa workup. The anticoagulation, antimicrobial, and COVID-19 vaccination considerations following CVST are described. This case report was prepared following the CAse REport Statement and Checklist Guidelines. 4
Case Presentation
Presenting concerns
A 67-year-old Black woman with a history of diabetes mellitus type 2 and a single provoked postoperative deep venous thrombosis and pulmonary embolism 18 years prior that required anticoagulation presented with 1 week of severe redness, induration, and swelling of the left ear that extended downward to the lower mandible. Spontaneous drainage of pustular and serosanguinous fluid from the ear was also present. Of note, she was diagnosed with COVID-19, presenting with severe headache and mild respiratory symptoms 42 days prior. The patient had not yet received her COVID-19 vaccination.
Therapeutic Intervention and Treatment
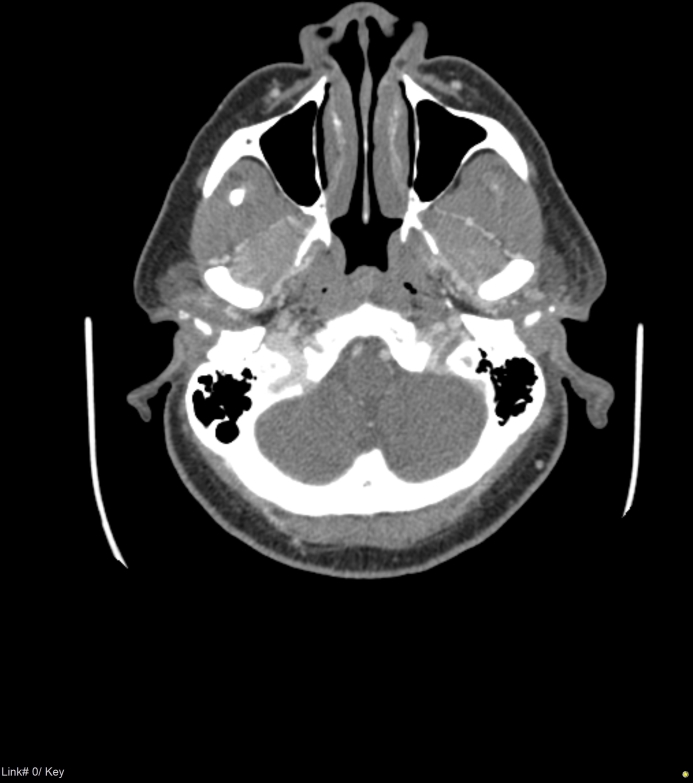
A computed tomography (CT) scan was performed to evaluate for extension of possible malignant otitis externa and demonstrated significant superficial subcutaneous inflammatory stranding and reticulation in the left periauricular region and mild borderline left upper cervical adenopathy, with no abscess. However, the CT scan revealed unexpected ipsilateral left CVST in the left sigmoid sinus and left jugular foramen. There was no mastoid or bony involvement. Subsequent magnetic resonance imaging (MRI) of the brain (Figure 1) found no evidence of acute infarct or intracranial hemorrhage. The patient’s vitals and basic metabolic panel were normal except for elevated blood glucose (222 mg/dL). Hypercoagulable workup was within normal limits, with the exception of elevated Factor VIII activity. She was SARS-COV-2 negative.
Figure 1:
Magnetic resonance imaging of brain.
Intravenous cefepime 2 g and vancomycin 1.5 g were initiated for acute otitis externa for 2 days, then converted to an outpatient regimen of ciprofloxacin 500 mg twice daily and linezolid 600 mg twice daily for a duration of 7 days. The patient was also prescribed ciprofloxacin/dexamethasone otic drops for a duration of 10 days. The infectious disease department was consulted and recommended extending duration of therapy up to 4–6 weeks based on clinical status and monitoring of inflammatory markers, specifically white blood cells and C-reactive protein. She improved significantly at 2 weeks with mild residual auricular erythema and pain. However, during an otolaryngology follow-up 1 week later, the patient was felt to have a relapse of her otitis externa (erythema and swelling of the lobule, but normal mastoid, external auditory canal, and tympanic membrane landmarks). A wick was placed, and wound cultures were positive for Klebsiella pneumoniae. The patient reported compliance to oral linezolid and ciprofloxacin but nonadherence to ciprofloxacin/dexamethasone otic drops. The international normalized ratio (INR) was elevated at 4.8, and there was concern that the antibiotics were contributing to the INR elevation.
Follow-Up and Outcomes
A midline catheter was inserted, and intravenous cefepime 2 g every 8 h was administered. The patient continued to receive care through the virtual home monitoring program. The total duration of antibiotics was 6 weeks. Anticoagulation with enoxaparin dosed 1 mg/kg was initiated at CVST diagnosis, followed by warfarin for a total duration of 6 months. A repeat magnetic resonance venography at the conclusion of anticoagulation to ensure resolution of thrombosis was performed.
The Centers for Disease Control and Prevention (CDC) was consulted regarding COVID-19 vaccination recommendations. The non-mRNA vaccine, Johnson & Johnson’s Janssen COVID-19 vaccine, was not considered because of rare case reports of CVST in recipients. The CDC recommended the mRNA Pfizer COVID-19 vaccination. The neurologist, infectious disease specialist, and hematologist all agreed that the best time to provide COVID-19 vaccination would be during anticoagulation to eliminate any theoretic risks of thrombosis. The patient received her first vaccination 3 weeks after her initial diagnosis of CVST. Her second vaccination was performed 3 weeks later without complications.
Discussion
This case describes a patient who was diagnosed incidentally with a CVST, which could have arisen from an otogenic source, a post COVID-19 complication, both, or other hypercoagulable state due to her history of remote provoked pulmonary embolism. This diagnostic uncertainty led to dilemmas regarding the duration of the antimicrobial course, the duration of anticoagulation, and, ultimately, which COVID-19 vaccination to use. Although the onset of the CVST and the ipsilateral side affected favored a diagnosis of an otogenic CVST, there was lack of mastoid involvement. Moreover, post COVID-19 CVST has been reported in the literature to generally occur within 2 weeks of infection, and our patient presented nearly 6 weeks following COVID-19 infection. Determining the source of CVST also influenced antimicrobial duration because otogenic CVST is suggested to be treated in the medical literature for a duration of 4–6 weeks. To our knowledge there is no literature or algorithm regarding how to proceed with COVID-19 vaccination in patients recovering from CVST, which vaccine is preferred given reports of thrombosis with all currently available COVID-19 vaccines in the United States, and whether continued anticoagulation is necessary during the vaccination series.
CVST Before COVID-19
CVST is a rare, but potentially devastating, neurovascular emergency that can be caused by heterogeneous systemic and local risk factors. These include hematologic prothrombotic states, infection, malignancy, pregnancy, immobility, autoimmune disorders, hormonal contraceptives, dehydration, and trauma. Infections associated with hypercoagulability include herpes simplex, HIV, cytomegalovirus, Middle East respiratory syndrome, and systemic acute respiratory syndrome. Clinical manifestations of CVST are variable and may encompass acute-to-subacute headache, seizures, focal deficits, or encephalopathy. CVST is a subtype of stroke with an annual incidence of 2–5 cases per million persons and has a predilection for younger patients, especially women. Headache is most frequently described, followed by seizures, and then focal neurologic deficits. MRI venography is the best imaging modality because of its high sensitivity, while the gold standard is cerebral angiogram. CT is used when MRI is contraindicated or not accessible. Proper management is therapeutic anticoagulation, and survival reaches 60% with early therapy. 1–6
CVST and COVID-19 Infection
Venous thromboembolism (VTE) is well described in patients with COVID-19; however, there is a paucity of literature regarding COVID-19–associated CVST. The most common cerebrovascular complication following COVID-19 infection is ischemic stroke; even so, there has been an increase in reported cases of CVST, a rare type of stroke. The exact mechanism of cerebrovascular manifestations following COVID-19 infection is unclear. Direct viral action from the olfactory nerve or an indirect inflammatory response leading to cytokine storm and inducing a hypercoagulable state are some possible mechanisms. 5–9 A systematic review of 1210 articles revealed 226 cases of ischemic stroke, 35 cases of intracranial bleeding, and 14 cases of CVST in patients with COVID-19. Of these 14 cases, half were female with a median age of 49 years old. Three patients had obesity and 2, like our patient, had diabetes. COVID-19 infection preceded CVST diagnoses by 4 days to 2 weeks in most cases. 10 One case reported CVST 4 months following COVID-19 infection; our patient was diagnosed 42 days following her positive COVID diagnosis.
Although hypercoagulability is well documented in more severe cases of SARS-CoV 2 infection, such as those admitted to the Intensive Care Unit, thromboembolism may also occur in those with asymptomatic or mild respiratory or systemic infections, such as occurred in our patient. COVID-19 infection is associated with increases in prothrombotic markers, such as fibrinogen and D-dimer, and inflammatory markers, such as C-reactive protein and interleukin 6. 5–8 Because headache occurs with many viral infections, a high index of suspicion for CVST should be maintained. Venography should be considered in patients with COVID-19 infection who present with atypical infarct or hemorrhage or unexpected elevations in intracranial pressure to look for CVST. 5–8
CVST has been described concomitantly with immune thrombocytopenia purpura (ITP) in a patient who presented with a history of COVID-19, followed by severe occipital headache and right homonymous hemianopia. 11 These authors proposed that COVID-19 caused ITP, and ITP lead to CVST, or that COVID-19 complications resulted in ITP and CVST independently and simultaneously. To date, nearly all cases of CVST have occurred with active COVID-19 infection. There are isolated case reports of patients with negative polymerase chain reaction (PCR) and positive IgG antibodies who have developed CVST. 11
Some guidance or position papers support VTE prophylaxis in patients treated with home and post-discharge prophylaxis if additional risk factors for VTE are present, including obesity, old age, reduced mobility, previous VTE, or active cancer. In these patients, novel oral anticoagulants may have advantages over vitamin K antagonists and low molecular weight heparin. 12
Otogenic CVST
Otitis externa is characterized by otorrhea, everted auricular pinna, retroauricular swelling and erythema, and pain on palpation of the mastoid region. It may progress to malignant otitis externa (inflammation of the temporal bone, the mastoid bone, and adjacent structures) that can lead to CVST and internal jugular vein thrombosis. The proximity of the sigmoid sinus to the mastoid is a conduit for adjacent inflammation, activation of platelets and fibrin, and formation of a mural thrombus. This often occurs in the setting of uncontrolled diabetes due to microangiography, hypoperfusion, and immunodeficiency. 13 The main infectious organism is Pseudomonas aeruginosa. CT is the most effective modality for diagnosis, whereas MRI is better for enhanced visualization of soft tissue and intracranial extension. Clinically improved physical examination and normalization of white blood cell count and C-reactive protein/erythrocyte sedimentation rate occur with proper therapy. Untreated otogenic CVST is associated with neurologic complications and fatality. Our patient presented with the concurrent diagnosis of left otitis externa and ipsilateral CVST. Although there was no mastoid infection present in our case, the patient was treated for an extended course of 4–6 weeks of antibiotics because of concern about otogenic CVST and persistence of otitis externa. The duration of antibiotic therapy is uncertain in the medical literature. 13 Anticoagulation therapy for otogenic CVST is an area of debate as well. All patients with a diagnosis of otogenic CVST should be screened for additional thrombophilia to evaluate for risk of recurrence and anticoagulation treatment duration. 13
Uncertainty of COVID-19 vs Otogenic Infection as Underlying Etiology for CVST
A limitation in our study was the delayed CVST diagnosis occurring 6 weeks following COVID-19 infection, as well as the existence of a possible alternative trigger: otogenic CVST. However, the underlying etiology is not conclusive for either COVID-19 or otogenic infection. Different aspects of the presentation both favored and opposed otogenic CVST. The concurrent presentation of CVST with otogenic infection and ipsilateral involvement supported diagnosis of otogenic CVST. Acute otitis media and associated mastoiditis have been described with CVST. However, to our knowledge CVST has not been reported in association with otitis externa without mastoiditis. The patient had positive Griesinger sign (edema and tenderness over the mastoid), but no radiographic evidence of mastoiditis.
Delayed Presentations of CVST in COVID-19 Recovery
Hence, the authors considered COVID-19–associated delayed CVST as a potential etiology owing to case reports of patients with IgG antibodies and negative COVID-19 PCR. Chakir et al described a case of an asymptomatic patient who had never been tested for COVID-19 and was diagnosed with simultaneous myocardial infarction, pulmonary embolisms, and ischemic cerebral stroke. 14 The patient tested positive for IgG SARS/COV2 antibodies but negative for IgM antibodies and PCR swabs. Alternate etiologies for multisystemic thromboembolism were excluded. Lim et al described a case of intracardiac thrombus in a patient presenting 4 weeks following COVID-19 infection. 15 Kaur et al reported CVST in a patient with IgG antibodies and negative PCR, suggesting that a hypercoagulable state can be a late manifestation in immune patients following asymptomatic COVID-19 infection. 16
Coagulability of COVID-19 vs Other Infections
COVID-19 appears to cause thromboembolism through unique mechanisms vs other infections. COVID-19 coagulopathy is complex, centering around bidirectional interactions of inflammation and thrombosis. Cytokine storm, endothelial damage, and platelet activation are postulated. COVID-19 hypercoagulability implicates increased prothrombotic factors, such as Factor VIII, fibrinogen, and neutrophil extracellular traps. 5,6 Factor VIII levels are increased in both COVID-19 and otogenic infection. However, platelet counts were markedly elevated in a series of patients with otogenic CVST (100% had thrombocytosis, with a mean of 712 k/µL), while patients with COVID-19–associated CVST have normal platelet counts, like our patient. 13
CVST and Non-mRNA COVID-19 Vaccines
Cases of CVST have been reported following adenovirus vector COVID-19 vaccines, including Ad26.COV2.S (Johnson & Johnson’s Janssen), which was paused for 10 days due to 6 reports of CVST with thrombocytopenia and ChadOx1 (AstraZeneca), which is not available for use in the United States. 17 The specific mechanism causing CVST is vaccine-induced immune thrombotic thrombocytopenia (VITT). A summary of VITT can be found in Table 1. Given the known risk of VITT following non-mRNA vaccination, it was decided to use an mRNA-based vaccine in our patient. The majority of cases of these patients with VITT were female. Headache was the most common symptom, and intracerebral hemorrhage and subarachnoid hemorrhage have occurred.
Table 1:
Vaccine-induced immune thrombotic thrombocytopenia
| Clinical features | Findings |
|---|---|
| COVID-19 vaccines associated with VITT | ChadOx1 (AstraZeneca), Ad26.COV2.S (Johnson & Johnson) |
| Incidence | Rare, exact incidence unknown |
| Possible risk factors | Female sex, age less than 60 years |
| Onset | 5–48 days post vaccination |
| Typical laboratory findings | Thrombocytopenia, platelet range 6000–344,000/µL, low fibrinogen levels (range 0.3–4.4 mg/dL), elevated D-dimer (range 5000–80,000), positive anti-PF4 antibodies, normal or mildly increased PT, INR, aPTT |
| Sites of thrombosis | Cerebral veins, deep veins of the leg, pulmonary arteries, and splanchnic vessels |
| Treatment | Non-heparin anticoagulant agents (argatroban, danaparoid, fondaparinux, or direct oral anticoagulants), IVIG, plasma exchange with plasma but not albumin, high-dose corticosteroids. Preliminary evidence suggest heparin is not harmful and may be used. Avoid platelet transfusions |
| Proposed mechanism | IgG antibodies bind to platelet Factor 4 (positively charged tetrameric protein), inducing platelet activation and subsequent thromboembolic complications |
aPTT, activated partial thromboplastin time; INR, international normalized ratio; IVIG, intravenous immunoglobulin; PT, prothrombin time; VITT, vaccine-induced immune thrombotic thrombocytopenia.
CVST and mRNA COVID-19 Vaccines
There have been reports of a possible correlation between BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine and thromboembolism. 18,19 Two case reports recently reported CVST 6- and 3-days post Pfizer vaccination. Both women were COVID negative, did not have thrombocytopenia nor antiplatelet antibodies, suggesting the mechanism is not similar to VITT as seen with non-mRNA COVID-19 vaccines but, rather, was pathologically similar to regular CVST. 20 Andrasaka et al reported 3 cases of women who developed other venous thromboembolic events following mRNA Moderna vaccination including pulmonary embolism and deep venous thrombosis. 21 To date, no CVST cases have been reported following the use of mRNA-1273 (Moderna). 21 It has been postulated that mRNA may bind to pattern recognition receptors, thus inducing pro-inflammatory cascades, which may be a trigger for thromboembolic events. 10,12 In addition, VITT-induced antibodies against PF4 do not cross-react with the spike protein of SARS-CoV-2, which the mRNA-based vaccine uses for immune recognition and antibody production. This further suggests that VITT is not the mechanism causing CVST with mRNA COVID-19 vaccines. 22 With this information in mind, the CDC’s recommendations to vaccinate our patient with an mRNA-based vaccine was appropriate, and patients needing to be vaccinated against COVID-19 with VTE or CVST risk factors should also consider mRNA-based vaccine options in favor of viral vector COVID-19 vaccines.
Conclusion
Because of the lack of guidelines and evidence, it was difficult to determine appropriate therapy durations for anticoagulation and antibiotic therapy in this patient. Patients presenting with VTE or CVST following COVID-19 infection should be anticoagulated until COVID-19 vaccination, which has its own risk for VTE and CVST, or 6 months for those without additional risk factors. Patients with risk factors for VTE and/or CVST should be vaccinated with an mRNA COVID-19 vaccine in favor of a viral vector vaccine to reduce risk of further thromboembolic complications. It is interesting that CVST prepandemic has a female predilection, just like VITT associated with non-mRNA vaccinations, whereas COVID-19–associated CVST has an equal distribution between male and female patients. This case is limited by the delayed onset of CVST in a patient recovered from COVID-19 infection and an alternative etiology. However, delayed presentations of COVID-19–associated CVST have been described. Providers should be alert to this possibility, even in patients who have recovered from COVID-19.
Footnotes
Author Contributions: All authors conceptualized this paper, drafted the initial manuscript, and revised the final submission. All authors have given final approval to the manuscript.
Conflicts of Interest: None declared
Funding: None declared
Consent: Informed consent was received from the case patient.
References
- 1.Ferro JM, Correia M, Pontes C, Baptista MV, Pita F. Cerebral vein and dural sinus thrombosis in Portugal: 1980-1998. Cerebrovasc Dis. 2001;11(3):177–182. 10.1159/000047635 [DOI] [PubMed] [Google Scholar]
- 2.Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: A cross-sectional study. Stroke. 2012;43(12):3375–3377. 10.1161/STROKEAHA.112.671453 [DOI] [PubMed] [Google Scholar]
- 3.Devasagayam S, Wyatt B, Leyden J, Kleinig T. Cerebral venous sinus thrombosis incidence is higher than previously thought: A retrospective population-based study. Stroke. 2016;47(9):2180–2182. 10.1161/STROKEAHA.116.013617 [DOI] [PubMed] [Google Scholar]
- 4.Riley DS, Barber MS, Kienle GS, Aronson JK, von SchoenAngerer T, et al. . CARE 2013 explanation and elaborations: Reporting guidelines for case reports. JClinEpi. 2017;89:218–235. 10.1016/j.jclinepi.2017.04.026 [DOI] [PubMed] [Google Scholar]
- 5.Almarcha-Menargues ML, Martínez-Martínez MM, Fernández-Travieso J. SARS-CoV-2 infection: Possible underdiagnosis of cerebral venous sinus thrombosis. Neurologia (Engl Ed). 2021;36(7):575–576. 10.1016/j.nrleng.2021.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dakay K, Cooper J, Bloomfield J, et al. . Cerebral venous sinus thrombosis in COVID-19 infection: A case series and review of the literature. J Stroke Cerebrovasc Dis. 2021;30(1):105434. 10.1016/j.jstrokecerebrovasdis.2020.105434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta P, McAuley DF, Brown M, et al. . COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraiman P, Godeiro Junior C, Moro E, Cavallieri F, Zedde M. COVID-19 and cerebrovascular diseases: A systematic review and perspectives for stroke management. Front Neurol. 2020;11. 10.3389/fneur.2020.574694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nwajei F, Anand P, Abdalkader M, et al. . Cerebral venous sinus thromboses in patients with SARS-CoV-2 infection: Three cases and a review of the literature. J Stroke Cerebrovasc Dis. 2020;29(12):105412. 10.1016/j.jstrokecerebrovasdis.2020.105412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahadorizadeh L, Emamikhah M, Pour Mohammad A, Gholizadeh Mesgarha M. Simultaneous occurrence of cerebral venous sinus thrombosis and immune thrombocytopenic purpura in a patient with a history of COVID-19 infection. Neurol Ther. 2022;11(1):491–497. 10.1007/s40120-021-00294-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulman S, Hu Y, Konstantinides S. Venous thromboembolism in COVID-19. Thromb Haemost. 2020;120(12):1642–1653. 10.1055/s-0040-1718532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellazzi ML, di Pietro GM, Gaffuri M, et al. . Pediatric otogenic cerebral venous sinus thrombosis: A case report and A literature review. Ital J Pediatr. 2020;46(1):122. 10.1186/s13052-020-00882-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakir M, El Jamili M, Boudhar Z, El Hattaoui M. Simultaneous acute myocardial infarction, bilateral pulmonary embolism, and acute ischaemic cerebral stroke, a delayed complication in a patient with COVID-19 infection: Case report. Eur Heart J Case Rep. 2021;5(6). 10.1093/ehjcr/ytab218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim W, Tin S, Breitling M, Grodman R, Diaz K. A case of intracardiac thrombus in a recovery coronavirus disease 2019 (COVID-19) patient. Cureus. 2022;14(1). 10.7759/cureus.20972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur I, Vyas C, Mughal M, Gandhi H, Du D. Cerebral venous sinus thrombosis in COVID-19: An unusual presentation. Cureus. 2021;13(3):e13767. 10.7759/cureus.13767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimabukuro T. Reports of cerebral venous sinus thrombosis with thrombocytopenia after Janssen COVID-19 vaccine. Accessed July 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html
- 18.Al-Maqbali JS, Al Rasbi S, Kashoub MS, et al. . A 59-year-old woman with extensive deep vein thrombosis and pulmonary thromboembolism 7 days following a first dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine. Am J Case Rep. 2021;22. 10.12659/AJCR.932946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias L, Soares-Dos-Reis R, Meira J, et al. . Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J Stroke Cerebrovasc Dis. 2021;30(8):105906. 10.1016/j.jstrokecerebrovasdis.2021.105906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases.” Clin Immunol. 2021;224:108665. 10.1016/j.clim.2021.108665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andraska EA, Kulkarni R, Chaudhary M, Sachdev U. Three cases of acute venous thromboembolism in females after vaccination for coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord. 2022;10(1):14–17. 10.1016/j.jvsv.2021.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reetz K, Dogan I, Hilgers R-D, et al. . Progression characteristics of the European Friedreich’s Ataxia Consortium for Translational Studies (EFACTS): A 4-year cohort study. Lancet Neurol. 2021;20(5):362–372. 10.1016/S1474-4422(21)00027-2 [DOI] [PubMed] [Google Scholar]