Abstract
The most abundant protein on the surface of the promastigote form of the protozoan parasites Leishmania spp. is a 63-kDa molecule, designated gp63 or leishmanolysin. Because gp63 has been shown to possess fibronectin-like properties, we examined the interaction of gp63 with the cellular receptors for fibronectin. We measured the direct binding of Leishmania to human macrophages or to transfected mammalian cells expressing human fibronectin receptors. Leishmania expressing gp63 exhibited modest but reproducible adhesion to human macrophages and to transfected CHO cells expressing α4/β1 fibronectin receptors. In both cases, this interaction depended on gp63 but occurred independently of the SRYD sequence of gp63, because parasites expressing gp63 with a mutated SRYD sequence bound to macrophages and α4/β1 receptor-expressing cells as well as did wild-type parasites. The contribution of gp63 to parasite adhesion was more pronounced when the assays were performed in the presence of complement, suggesting that the receptors for complement and fibronectin may cooperate to mediate the efficient adhesion of parasites to macrophages. The interaction of gp63 with fibronectin receptors may also play an important role in parasite internalization by macrophages. Erythrocytes to which gp63 was cross-linked were efficiently phagocytized by macrophages, whereas control erythrocytes opsonized with complement alone bound to macrophages but remained peripherally attached to the outside of the cell. Similarly, parasites expressing wild-type gp63 were rapidly and efficiently phagocytized by resting macrophages, whereas parasites lacking gp63 were internalized more slowly. This rapid internalization of gp63-expressing parasites was dependent on the β1 integrins, because pretreatment of macrophages with monoclonal antibodies to the β1 integrins decreased the internalization of gp63-expressing parasites. These observations indicate that complement receptors are the primary mediators of parasite adhesion; however, maximal parasite adhesion and internalization may require the participation of the β1 integrins, which recognize fibronectin-like molecules such as gp63 on the surface of the parasite.
gp63 is the major surface protein on Leishmania promastigotes (3). Due to its abundance and its characterization as a zinc-metalloproteinase (9), much work has been done to define a role for gp63 in Leishmania virulence (4, 7). Previous studies have implicated gp63 as a ligand for multiple macrophage receptors, including Mac-1 (CD11b/CD18) (26, 33) and the cellular receptors for fibronectin (22). The gp63 molecule is highly conserved among all species of Leishmania (16); this conservation includes the amino acid sequence SRYD, which is found at amino acids 252 to 255 in Leishmania major (5). Soteriadou et al. (29) demonstrated that the SRYD region of gp63 was antigenically similar to the RGDS region of fibronectin. Antibodies against fibronectin have been shown to cross-react with gp63 (22, 29) and to inhibit the binding of promastigotes to macrophages (22, 37). Wyler et al. (37) demonstrated that RGDS-containing peptides could inhibit the immunoprecipitation of gp63 by antibodies to fibronectin.
Two of the most abundant cellular receptors for fibronectin are members of the β1 integrin family, VLA-4 (CD49d/CD29) and VLA-5 (CD49e/CD29) (24, 34). VLA-5, like many of the cellular receptors for fibronectin, recognizes the Arg-Gly-Asp-Ser (RGDS) sequence of fibronectin (20). VLA-4, in contrast, recognizes the CS-1 domain of fibronectin, which contains the amino acid sequence Glu-Ile-Leu-Asp-Val (EILDV) (12). VLA-4 and VLA-5 are both expressed on a wide range of tissues and cell types (32, 34), with VLA-4 appearing primarily on hematopoietic cells and being prominently expressed on macrophages and activated lymphocytes (34). The cooperation between fibronectin and complement receptors has been suggested by several groups, who have observed that the presence of fibronectin can enhance the phagocytosis of complement-opsonized particles (2, 21, 27). These observations have been extended to microbial phagocytosis. The FimD molecule on Bordetella pertussis can enhance the complement-dependent phagocytosis of that organism by interacting with fibronectin receptors (11). Similarly, the interaction of Mycobacterium avium with β3 integrins on macrophages enhances complement receptor expression and augments phagocytosis (10).
It has previously been demonstrated that the presence of gp63 on the surface of promastigotes can enhance their interaction with murine macrophages (6, 13, 14). Here we examine the importance of gp63 and specifically the SRYD region of gp63 in the interaction of promastigotes with human macrophages. We demonstrate that gp63 on Leishmania can bind specifically to human α4/β1 fibronectin receptors. This is the first identification of a specific fibronectin receptor with which Leishmania can interact. We also demonstrate that the SRYD region of gp63, a domain previously implicated in cell adhesion (26, 29), is not required for this interaction. Finally, although gp63 can bind directly to α4/β1 receptors, the direct binding of parasites to human macrophages is minimal unless complement is also present. In the presence of complement, however, the interaction of gp63 with fibronectin receptors may cooperate with complement receptor-dependent adhesion to mediate the efficient attachment to and entry of parasites into macrophages.
MATERIALS AND METHODS
Macrophages.
Mononuclear cells were isolated from human peripheral blood with Lymphoprep (Nycomed Pharma, Oslo, Norway) as specified by the manufacturer. To obtain monocyte-derived macrophages, monocytes were cultured for 4 to 5 days in Teflon beakers (Savilex, Minnetonka, Minn.) containing RPMI 1640 supplemented with glutamine, penicillin-streptomycin (Mediatech, Herndon, Va.), and 5% autologous serum. Cells were removed from beakers with cold cation-free Dulbecco’s phosphate-buffered saline (PBS) (GIBCO, Grand Island, N.Y.) and washed in RPMI 1640. Cells were then allowed to adhere to 13-mm-diameter round glass coverslips overnight in the presence of 5% autologous serum. Nonadherent cells were removed on the following day by washing, and monolayers were used that day for Leishmania-binding assays.
Murine bone marrow-derived macrophages were established as previously described, with minor modifications (30). Briefly, femurs were flushed with cation-free Dulbecco’s PBS by use of a 23-gauge needle. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM [Mediatech]) containing 20% L929 cell-conditioned medium, 10% heat-inactivated (HI) fetal calf serum (FCS), 2 mM l-glutamine, 100 U of penicillin G per ml, and 100 μg of streptomycin per ml. Cells were incubated at 37°C in 5% CO2 for 5 to 7 days until uniform monolayers of macrophages were established. Twelve hours before use, cells were removed from the original plastic petri dishes with EDTA; a total of 105 cells in DMEM containing 10% HI FCS, 2 mM l-glutamine, 100 U of penicillin G per ml, and 100 μg of streptomycin per ml (complete medium) were plated on 13-mm-diameter round glass coverslips in 24-well plates (Nunc, Naperville, Ill.).
Transfected CHO cells.
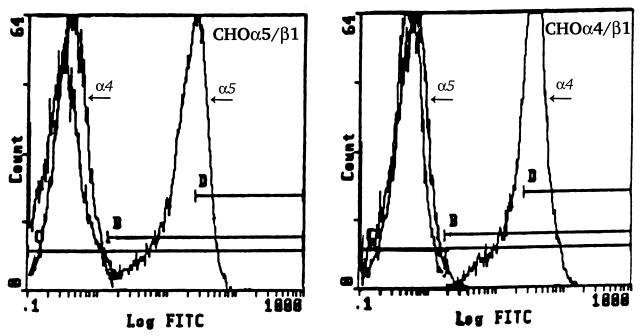
Clonal CHO cell lines stably expressing wild-type human Mac-1 (8) and complement receptor type 1 (CR1) (23) have been described previously. Mutant CHO cells deficient in hamster β1 integrin expression (CHO-B2) or transfected with constructs encoding either the human α4 (CHO-B2/α4) or the human α5 (CHO-B2/α5) subunit were kindly provided by Rudolph Juliano, University of North Carolina (1, 28). Cells were maintained in alpha minimal essential medium containing 10% HI FCS, 2 mM l-glutamine, 100 U of penicillin G per ml, and 100 μg of streptomycin per ml. G418 was added to transfected cell cultures at a final concentration of 200 μg/ml. To confirm integrin expression, transfected cells were stained with monoclonal antibodies (MAb) to α4/β1 (HP2/1) or α5/β1 (SAM1) receptors. These antibodies were generously provided to us by Stefan Niewiarowski, Temple University School of Medicine. Flow cytometry confirmed β1 integrin expression and demonstrated that the α4 subunit-transfected cells expressed α4/β1 but not α5/β1 on their surface and that the α5 subunit-transfected cells expressed α5/β1 but not α4/β1 on their surface (Fig. 1).
FIG. 1.
Cell surface expression of β1 integrins on transfected CHO-B2 cells. CHO-B2/α5 cells (left panel) or CHO-B2/α4 cells (right panel) were incubated individually with MAb to detect either α4 (HP2/1) or α5 (SAM1) receptor expression on their surface. Cells were stained with FITC-conjugated goat anti-mouse immunoglobulin G (Jackson ImmunoResearch) and analyzed by flow cytometry. Profiles of cells stained with each antibody were overlaid and compared to those of cells stained with secondary antibody alone.
Parasites.
The C1250 variant of Leishmania amazonensis (LV78), expressing markedly reduced levels of gp63, and the transfection of C1250 with the gene encoding wild-type gp63 from L. major and with the gp63 gene containing a point mutation resulting in conversion of the SRYD sequence to SRDD have been previously described (14, 15). Briefly, plasmids encoding wild-type gp63 and mutant gp63 were prepared by use of Escherichia coli and isolated by cesium chloride-ethidium bromide density gradient centrifugation. They were transfected into the gp63-deficient variant of L. amazonensis by electroporation at 400 V with a capacitance of 450 μF. After a 5- to 8-h recovery in drug-free medium, transfectants were selected with G418 at 20 μg/ml. Transfectants which emerged in 1 to 2 weeks were further selected with 200 μg of G418 per ml. Stationary-phase organisms were used for all experiments unless otherwise stated.
gp63-coated erythrocytes.
Recombinant gp63 was generously provided by Robert McMaster, University of British Columbia, Vancouver, British Columbia, Canada. Erythrocytes coated with Leishmania gp63 were generated as follows. 2-Iminothiolane (Pierce, Rockford, Ill.) was used to introduce sulfhydryl residues into gp63 according to the manufacturer’s instructions. Briefly, gp63 (0.5 mg/ml) was incubated with 0.3 mM 2-iminothiolane in PBS containing 1 mM EDTA (pH 8.0) for 45 min at room temperature. Unreacted 2-iminothiolane was separated on a PD-10 column (Pharmacia, Piscataway, N.J.). gp63 containing sulfhydryl groups was coupled to sheep erythrocytes (SRBC) by a modification of a method described previously (31). Sulfosuccinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (Pierce) at a final concentration of 0.5 mM was incubated with SRBC (109 cells/ml) in Hanks’ balanced salt solution (HBSS) for 60 min at room temperature with continuous rotation. The thiol-activated SRBC were washed four times with HBSS, resuspended in gp63 containing sulfhydryl groups (0.4 mg/ml) to a concentration of 5 × 108 cells/ml, and incubated at room temperature with continuous rotation for 60 min. The resulting gp63-coated erythrocytes were washed three times with HBSS and resuspended to a concentration of 108 cells/ml.
Parasite-binding assays.
For radiolabeling, promastigotes were cultivated in the presence of [3H]uracil (18). Radiolabeled parasites were washed and resuspended in phagocytosis buffer, which consists of equal parts of DMEM and medium 199 buffered with 25 mM HEPES (Mediatech) and supplemented with 1% bovine serum albumin. Parasites were added to 24-well plates containing either monocyte-derived macrophages on 13-mm-diameter round coverslips or transfected CHO cells plated directly on the plastic wells the night before. This overnight incubation allowed transfected cells to adhere tightly to the plastic, but this amount of time did not allow for significant cellular proliferation in the wells. For assays performed in the presence of serum, a final concentration of 4% serum from a patient with a deficiency in the eighth component of complement, C8D serum (19), was added to each well. Following 45 min of incubation at 35°C, the wells were washed thoroughly to remove unbound organisms. Monolayers and bound parasites were solubilized in 0.5% Triton X-100, and the amount of radioactivity associated with the cell lysates was determined with a Beckman LS6500 liquid scintillation counter. The absolute number of parasites in each well was determined by comparing the amount of radioactivity in the lysate to that of a standard curve of known numbers of radiolabeled parasites as previously described (18). All assays were performed in triplicate.
For Leishmania phagocytosis assays, unlabeled promastigotes were added to monolayers of murine bone marrow-derived macrophages in the presence of 4% C8D as a source of complement. After 10 min, monolayers were washed and immediately fixed in methanol. Parasites were stained by immunofluorescence with a murine polyclonal antiserum to L. amazonensis followed by fluorescein isothiocyanate (FITC)-conjugated antibody to mouse immunoglobulin G (Jackson ImmunoResearch, West Grove, Pa.). In contrast to peripherally attached promastigotes, internalized organisms were oval and were enclosed within phagocytic vacuoles.
Binding of 125I-C3 to promastigotes.
Purified human C3 was generously provided by John Lambris, University of Pennsylvania, Philadelphia. It was radiolabeled to a specific activity of 1.6 × 106 cpm/μg with IODO-BEADS (Pierce) as previously described (17). Leishmania promastigotes were resuspended to a concentration of 108 cells/ml in dextrose-Veronal buffer with 0.2% gelatin (dVBG). One hundred microliters of this suspension was mixed with 15 μl of C8D and 5 μl of 125I-C3, bringing the final concentration of radiolabeled C3 in the reaction mixture to 2.5 μg/ml. Samples were incubated for 15 min at 37°C and pelleted in a microcentrifuge for 3 min at room temperature. Following two washes with dVBG, parasites were resuspended in 100 μl of dVBG and washed with HBSS containing 1% bovine serum albumin. Following centrifugation, the supernatant was discarded, the contents in the bottom of the microcentrifuge tube, containing the pellet, were removed, and the amount of radiolabel associated with the cell pellet was determined on a Tracor Analytic Gamma Trac 1191 gamma counter. Nonspecific binding, the amount of radiolabel bound in the presence of serum which had been heat inactivated (30 min, 56°C) and treated with EDTA (10 mM), was subtracted from each value. The percentage of total radioactivity in the reaction mixture that remained associated with the cell pellet was multiplied by the total number of C3 molecules in the reaction mixture, assuming the C3 concentration in C8D to be 1.1 mg/ml (17). This product was then divided by the total number of cells in the reaction mixture to determine the number of C3 molecules bound per cell.
Erythrocyte binding and phagocytosis.
Murine bone marrow-derived macrophages were allowed to adhere to 13-mm-diameter glass coverslips and were washed twice with phagocytosis buffer. Erythrocytes were opsonized with 10% C8D serum and added to the cells at a ratio of 50:1. Macrophages were incubated with the opsonized erythrocytes for 1 h at 37°C. Wells were washed with phagocytosis buffer in order to remove unbound erythrocytes. In order to differentiate surface-bound from internalized erythrocytes, parallel macrophage monolayers were subjected to hypotonic erythrocyte lysis with H2O for 15 s to lyse surface-bound erythrocytes. Monolayers were then fixed with 2.5% glutaraldehyde and stained with Giemsa stain (Accura Labs, Bridgeport, N.J.). The number of erythrocytes per macrophage was quantitated by light microscopy by examining 200 macrophages per coverslip.
Analysis of data.
Statistical analysis of all data was performed with Student’s t test, with statistical significance defined as a P value of ≤0.05.
RESULTS
Direct binding of Leishmania to human monocyte-derived macrophages.
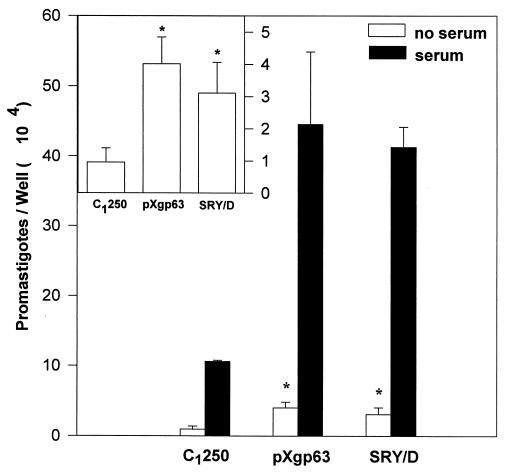
We examined the interaction of Leishmania promastigotes with human monocyte-derived macrophages, with a particular interest in determining the relative contribution of gp63 to parasite adhesion. Three variants of Leishmania differing in gp63 expression were used for these studies. The gp63-deficient variant of L. amazonensis, designated C1250, and transfectants of C1250 expressing either wild-type L. major gp63 or gp63 containing a Y-254-D point mutation (SRY/D) have been previously described (14, 15). These organisms were added to human monocyte-derived macrophages in the presence (Fig. 2) or absence of fresh serum as a source of complement. In the absence of exogenous complement, there was only a modest degree of direct binding of parasites to human macrophages. Despite this modest degree of direct binding, parasites expressing gp63 on their surface bound significantly better to macrophages than did those lacking gp63 (Fig. 2, inset). This direct binding of parasites to macrophages was not dependent on the SRYD sequence of gp63, because an L. amazonensis variant expressing gp63 with the SRY/D mutation on the surface bound directly to macrophages as well as did parasites expressing wild-type gp63. Thus, parasites expressing gp63 on their surface exhibit a modest degree of direct binding to human macrophages.
FIG. 2.
Binding of radiolabeled L. amazonensis gp63 variants to human monocyte-derived macrophages. Radiolabeled promastigotes were added to monolayers of human monocyte-derived macrophages at a 10:1 ratio in the presence (filled bars) or absence (open bars) of 4% C8D serum as a source of opsonic complement. Following 45 min of incubation and extensive washing, the number of promastigotes associated with human monocyte-derived macrophages was determined as described in Materials and Methods. Values represent the mean of four experiments, each performed in triplicate, ± the standard error. Statistical significance at the 95% confidence level (P, ≤0.05), determined with the Student’s t test, is denoted by an asterisk. The inset is an enlargement of the binding of promastigotes to macrophages in the absence of serum.
Binding of Leishmania to transfected cells expressing human fibronectin receptors.
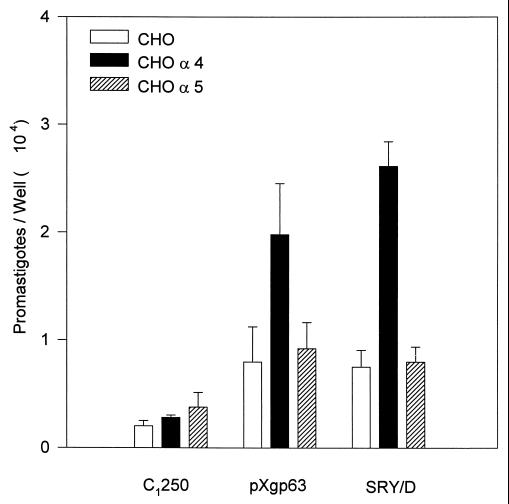
Several groups have demonstrated that gp63 has fibronectin-like properties (22, 29). Consequently, we examined the interaction of Leishmania promastigotes with cellular receptors for fibronectin. Leishmania parasites were added to parallel monolayers of transfected CHO cells varying only in their expression of recombinant human fibronectin receptors. Exogenous opsonins, such as antibody or complement, were omitted from the assay to examine the direct adhesion of parasites to these cells. Parasites lacking gp63 (C1250) bound poorly to all three of the cell types examined (Fig. 3). Parasites expressing wild-type gp63 (pXgp63), however, exhibited a significant degree of binding to transfected cells expressing the α4 fibronectin receptor (VLA-4). This binding was specific, because these parasites failed to bind to transfected cells expressing the α5/β1 fibronectin receptor (VLA-5). Because of the antigenic cross-reactivity of the SRYD region of gp63 with the RGDS region of fibronectin (29), we also examined parasites expressing recombinant gp63 lacking the intact SRYD sequence of gp63. Like parasites expressing wild-type gp63, a variant of L. amazonensis (SRY/D) expressing gp63 containing a point mutation converting SRYD to SRDD on the surface bound to cells expressing the α4/β1 receptor but not to cells lacking fibronectin receptors or to those expressing the α5/β1 receptor (Fig. 3). Thus, parasites expressing gp63 exhibit modest direct adhesion to cellular receptors for fibronectin. The specific fibronectin receptor which mediates this interaction is the α4/β1 fibronectin receptor. Neither the SRYD region of gp63 nor the α5/β1 receptor, which recognize the RGDS region of fibronectin, is involved in this interaction.
FIG. 3.
Binding of L. amazonensis gp63 variants to CHO cells expressing human fibronectin receptors. Radiolabeled promastigotes were added to monolayers of CHO cells at a 50:1 ratio for 1 h at 37°C. The number of promastigotes associated with fibronectin receptor-deficient CHO cells (open bars) and CHO cells transfected with cDNA for the human α4 receptor (CHO α4) (solid bars) or the human α5 receptor (CHO α5) (hatched bars) was determined as described in Materials and Methods. Determinations were performed in triplicate, and values are expressed as the mean ± standard deviation. This experiment is representative of three experiments.
Complement-dependent binding of Leishmania to human monocyte-derived macrophages.
Parasite adhesion to macrophages in the presence of complement was studied in order to determine the significance of fibronectin-dependent binding relative to that mediated by complement. In the presence of complement, parasites bound efficiently to macrophages, as previously described (18). Fresh serum improved the binding of all three of the variants (Fig. 2) relative to their binding in the absence of serum. Surprisingly, however, complement opsonization did not improve binding for parasites lacking gp63 as well as it did for parasites expressing gp63. The two variants expressing gp63 bound significantly better to macrophages than did the gp63-deficient variant C1250 (Fig. 2). Thus, although parasites expressing gp63 on their surface exhibited only a modest increase in their direct binding to macrophages relative to the binding of the gp63-deficient variant, the presence of complement in the assay accentuated this difference and resulted in improved binding of gp63-expressing parasites to macrophages.
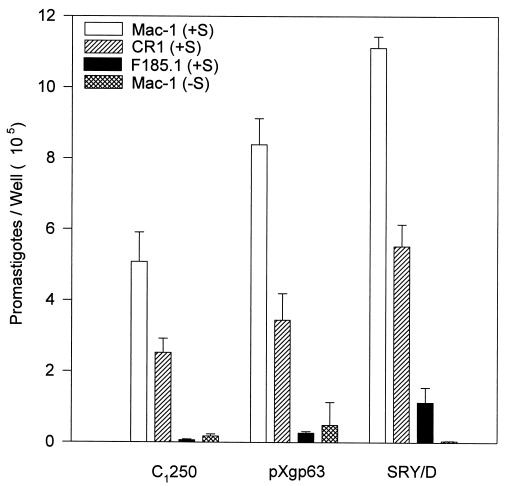
To examine the mechanism by which gp63 increased complement-dependent adhesion, the fixation of radiolabeled C3 to the three variants was measured. In the presence of complement, all three of the organisms fixed comparable amounts of radiolabeled C3, indicating that the reduced binding of the C1250 variant was not due to a failure to fix complement. In a single experiment that was performed in triplicate and that is representative of four experiments, the mean ± standard deviation values were (2.2 ± 0.19) × 10−5, (1.5 ± 0.20) × 10−5, and (1.5 ± 0.09) × 10−5 molecules of 125I-C3 bound by C1250, pXgp63, and SRY/D, respectively. To confirm that the C3 fixed to the parasites was opsonic, the three variants of L. amazonensis were added to transfected cells expressing human complement receptors in the presence or absence of complement (Fig. 4). All three of the organisms exhibited substantial amounts of complement-dependent binding to transfected cells expressing either of the two human complement receptors, CR1 or Mac-1 (Fig. 4). The degree of complement-dependent adhesion of parasites to complement receptors on transfected CHO cells was far in excess of the degree of direct adhesion of parasites to β1 receptor-transfected cells (note differences in the ordinates in Fig. 3 and 4). These data demonstrate that all three of the variants studied can fix comparable amounts of opsonic complement and bind to transfected cells expressing human complement receptors. These results suggest that the efficient complement-dependent binding of parasites expressing gp63 to human macrophages (Fig. 2) is not due to differences in complement fixation but rather is due to cooperation between fibronectin and complement receptors.
FIG. 4.
Binding of L. amazonensis gp63 variants to transfected CHO cells expressing human complement receptors. Radiolabeled promastigotes were added to monolayers of transfected CHO cells expressing Mac-1 (open bars) or CR1 (hatched bars) or control transfected cells (F185.1) expressing a truncated form of intracellular adhesion molecule 1 (solid bars), at a 50:1 ratio, in either the presence (+S) or the absence (−S) of serum. The number of promastigotes associated with each monolayer is expressed as the mean ± standard deviation of triplicate determinations. This experiment is representative of three experiments.
Role of gp63 in promoting complement-dependent phagocytosis by macrophages.
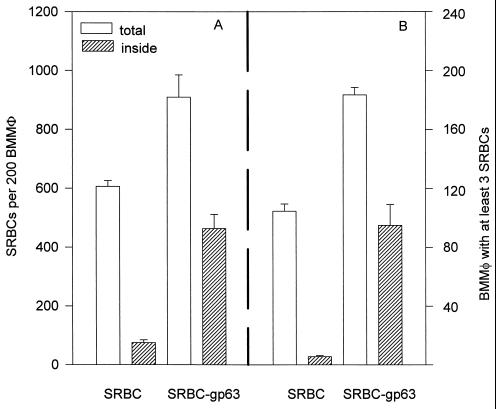
To determine whether fibronectin receptor ligation could improve not only adhesion but also phagocytosis, we used complement-opsonized sheep erythrocytes to which gp63 had been cross-linked (SRBC-gp63). As expected, normal sheep erythrocytes opsonized with complement bound to macrophages but remained peripherally attached rather than being phagocytized (Fig. 5). This observation confirms the work of several groups who have shown that on resident macrophages, complement receptors mediate particle adhesion but not internalization (36). Parallel monolayers of macrophages were exposed to SRBC-gp63. SRBC-gp63 were internalized far more efficiently than were erythrocytes opsonized with complement alone. The total number of SRBC-gp63 found inside macrophages (Fig. 5A) and the number of macrophages with three or more internalized erythrocytes (Fig. 5B) were dramatically increased relative to the data for erythrocytes opsonized with complement alone.
FIG. 5.
Binding and internalization of SRBC-gp63 by murine bone marrow-derived macrophages (BMMφ). SRBC or SRBC-gp63 were added to parallel monolayers of murine bone marrow-derived macrophages. After 1 h, all monolayers were washed to remove nonadherent cells. To determine the total number of erythrocytes associated with 200 macrophages (total), washed monolayers were fixed in 2.5% glutaraldehyde and processed for light microscopy (open bars). To determine the number of erythrocytes inside macrophages (inside), monolayers were rinsed for 15 s in water to lyse extracellularly bound erythrocytes prior to fixation and staining (hatched bars). (A) Total number of erythrocytes associated with 200 macrophages. (B) Number of macrophages, out of 200 counted, with three or more erythrocytes associated with them. This experiment is representative of two independent determinations; error bars show standard deviations.
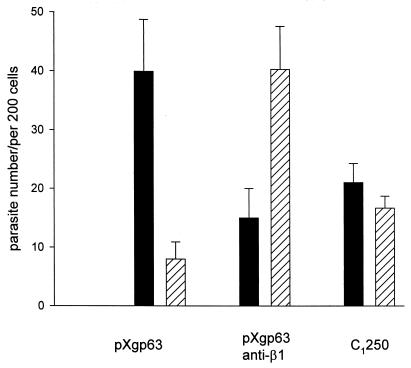
Similar phagocytosis studies were performed with Leishmania in order to correlate the efficiency of internalization with gp63 expression. Murine macrophages were chosen for these assays because a well-characterized MAb to the murine β1 integrins was available to us. Parasites expressing wild-type gp63 were added to monolayers of macrophages in the presence or absence of the MAb to the β1 integrins. In the absence of the antibody, Leishmania parasites were efficiently taken up by macrophages; by as little as 10 min, the majority of the parasites were located inside macrophages (Fig. 6), with very few remaining peripherally attached. The presence of the blocking antibody to the β1 integrins inhibited parasite internalization and increased the number of parasites that were peripherally attached to the macrophages. Parasites lacking gp63 (C1250) exhibited an intermediate rate of internalization. About half of the parasites were located inside macrophages, and a substantial percentage of parasites remained peripherally attached (Fig. 6). These data indicate that parasites expressing gp63 are internalized more efficiently than those lacking gp63 and that blocking the β1 integrins with an MAb can inhibit parasite phagocytosis.
FIG. 6.
Binding and internalization of Leishmania promastigotes by macrophages. Leishmania parasites were added to monolayers of murine bone marrow-derived macrophages in the presence of 4% C8D serum as a source of complement. After 10 min, monolayers were washed and immediately fixed in methanol. Parasites were stained by immunofluorescence, and the number of parasites inside macrophages (filled bars) or peripherally attached to macrophages (hatched bars) was determined. Parasites transfected with wild-type gp63 (pXgp63) were added to macrophages in the presence (pXgp63 anti-β1) or absence (pXgp63) of MAb to the β1 integrins (Immunotech, Westbrook, Maine). The gp63-deficient variant (C1250) was added in the absence of antibody. The number of promastigotes associated with each monolayer is expressed as the mean ± standard deviation of triplicate determinations. This experiment is representative of three experiments.
DISCUSSION
In the initial portion of these experiments, we examined the role of Leishmania gp63 in mediating parasite adhesion to mammalian cells. In vitro parasite adhesion assays were performed with both monocyte-derived macrophages and CHO cells transfected with fibronectin receptors. In both cases, when parasites were added to these cells in the absence of opsonic complement, there was a modest but significant adhesion of gp63-expressing parasites to these cells relative to the adhesion of parasites lacking gp63. Although the amount of adhesion was only modest, there were several degrees of specificity to this interaction. First, adhesion was specific for the α4/β1 fibronectin receptor, since cells expressing a different fibronectin receptor, α5/β1, failed to show parasite adhesion. Second, the adhesion of parasites to these cells required gp63, since a variant of L. amazonensis that expressed minimal amounts of endogenous gp63 failed to bind to α4/β1 receptor-expressing cells. Finally, the SRYD region of gp63 was not required for this interaction, since parasites expressing a mutated form of gp63 bound to cells as well as did those expressing wild-type gp63. This work confirms previous studies by others which have shown that gp63 may be involved in the adhesion of parasites to macrophages (22, 25, 29) and extends these studies in several ways.
First, we identify one of the fibronectin receptors capable of gp63 recognition. This is the α4/β1 fibronectin receptor. Second, we also demonstrate that the SRYD region of gp63 is not necessary for parasite adhesion to the α4/β1 receptor. Previous studies have suggested that the SRYD sequence of gp63 may mediate the adhesion of parasites to fibronectin receptors. This assumption was based on the observations that the SRYD region of gp63 is antigenically similar to the RGD sequence of fibronectin (29) and that fibroblasts plated on gp63 exhibit enhanced spreading, similar to their spreading on fibronectin or RGD substrates (22, 37). The results of these previous studies are consistent with the adhesion of fibroblasts to gp63 being dependent on the SRYD sequence. Our experiments do not refute this point. In fact, we also observed a low level of parasite adhesion to fibroblasts which depended on an intact SRYD domain of gp63 (data not shown), confirming the fibronectin-like character of this sequence. Rather than precluding a role for the SRYD region of gp63 in binding to fibronectin receptors, our present observations indicate that this region is not essential for the adhesion of parasites to macrophages. The lack of reliance on the SRYD region of gp63 for recognition by α4 integrins is consistent with previous reports that the α4/β1 integrin recognizes an EILDV sequence (12) rather than an RGD sequence. Although L. major gp63 contains no EILDV amino acid sequence, it does contain an EYLEV sequence (amino acids 318 to 322) (5), which may mimic the α4/β1 recognition domain. The putative role of this potential α4/β1 recognition domain in gp63 must be further investigated in light of previous work in which the LDV region of fibronectin was changed to LEV, with a consequent reduction in the binding of fibronectin to the α4/β1 receptor (12).
The relatively small amounts of direct binding of parasites to fibronectin receptors might initially suggest that this interaction has only marginal biological significance. Subsequent data, however, indicate that this interaction may play a significant role in parasite adhesion. We show that the binding of gp63 to the Fn receptors may cooperate with complement-dependent adhesion to augment the complement-dependent adhesion of parasites to macrophages. Parasites deficient in gp63 exhibited markedly less complement-dependent adhesion to macrophages than did parasites expressing gp63 (Fig. 2). The fact that all three strains fixed comparable amounts of complement and bound reasonably well to recombinant human complement receptors (Fig. 4) suggests that this augmentation was due to cooperation between fibronectin and complement receptors on macrophages rather than to differences in complement fixation.
The ability of gp63 to bind to fibronectin receptors not only may contribute to parasite adhesion but also may play an important role in promoting parasite internalization by macrophages. Previous studies have shown that complement-opsonized Leishmania promastigotes are rapidly and efficiently internalized by resting macrophages (18). Complement-opsonized erythrocytes, in contrast, form rosettes around resting macrophages but are generally not efficiently internalized (36). Internalization of complement-opsonized particles may depend on the ligation of a second class of receptors to cooperate with complement receptors. In other model systems, the receptors for fibronectin have been implicated in this cooperative role (21, 35). In the present work, we show that both Leishmania organisms and erythrocytes coated with gp63 are efficiently internalized by resting macrophages. This internalization is blocked by an MAb to the β1 integrins, suggesting that fibronectin receptors may participate in parasite internalization. The delayed phagocytosis of the gp63-deficient variant C1250 is consistent with gp63 being a ligand for these receptors. The fact that this variant does eventually enter macrophages suggests that gp63 may not be the only fibronectin-like molecule on Leishmania.
Our previous experiments demonstrated that gp63 could participate in the interaction of promastigotes with macrophages by influencing complement fixation (4). The present experiments indicate a second way in which gp63 may facilitate the infection of macrophages by promastigotes. By interacting with cellular receptors for fibronectin, gp63 stabilizes the complement-dependent adhesion of parasites to macrophages and allows their rapid and efficient internalization.
ACKNOWLEDGMENTS
We thank Robert McMaster for the generous gift of gp63.
This work was supported by Public Health Service grants AI24313 (to D.M.M.) and AI20486 (to K.-P.C.) from the National Institutes of Health.
Andrew Brittingham and Gang Chen contributed equally to this work.
REFERENCES
- 1.Bauer J S, Schreiner C L, Giancotti F G, Ruoslahti E, Juliano R L. Motility of fibronectin receptor deficient cells on fibronectin and vitronectin: collaborative interactions among integrins. J Cell Biol. 1992;116:477–487. doi: 10.1083/jcb.116.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohnsack J F, O’Shea J J, Takahashi T, Brown E J. Fibronectin-enhanced phagocytosis of an alternative pathway activator by human culture-derived macrophages is mediated by the C4b/C3b complement receptor (CR1) J Immunol. 1985;135:2680–2686. [PubMed] [Google Scholar]
- 3.Bouvier J, Etges R J, Bordier C. Identification and purification of membrane and soluble forms of the major surface protein of leishmania promastigotes. J Biol Chem. 1985;260:15504–15509. [PubMed] [Google Scholar]
- 4.Brittingham A, Morrison C J, McMaster W R, McGwire B S, Chang K P, Mosser D M. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J Immunol. 1995;155:3102–3111. [PubMed] [Google Scholar]
- 5.Button L L, McMaster W R. Molecular cloning of the major surface antigen of leishmania. J Exp Med. 1988;167:724–729. doi: 10.1084/jem.167.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarty R, Mukherjee S, Lu H-G, McGwire B S, Chang K P, Basu M K. Kinetics of entry of virulent and avirulent strains of Leishmania donovani into macrophages: a possible role of virulence molecules (gp63 and LPG) J Parasitol. 1996;82:632–635. [PubMed] [Google Scholar]
- 7.Chang K P, Chaudhuri G. Molecular determinants of Leishmania virulence. Annu Rev Microbiol. 1990;44:499–529. doi: 10.1146/annurev.mi.44.100190.002435. [DOI] [PubMed] [Google Scholar]
- 8.Diamond M S, Garcia-Aquilar J, Bickford J K, Corbi A L, Springer T A. The I domain is a major recognition site on leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etges R, Bouvier J, Bordier C. The major surface protein of Leishmania promastigotes is a protease. J Biol Chem. 1986;261:9098–9101. [PubMed] [Google Scholar]
- 10.Hayashi T, Rao S P, Catanzaro A. Binding of the 68-kilodalton protein of Mycobacterium avium to alpha(v)beta3 on human monocyte-derived macrophages enhances complement receptor type 3 expression. Infect Immun. 1997;65:1211–1216. doi: 10.1128/iai.65.4.1211-1216.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazenbos W L, van den Berg B M, van Furth R. Very late antigen-5 and complement receptor type 3 cooperatively mediate the interaction between Bordetella pertussis and human macrophages. J Immunol. 1993;151:6274–6282. [PubMed] [Google Scholar]
- 12.Komoriya A, Green L J, Miljenko M, Yamada K M, Humphries M J. The minimal essential sequence for a major cell type-specific adhesion site (CS-1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J Biol Chem. 1991;266:15075–15079. [PubMed] [Google Scholar]
- 13.Liu X, Chang K P. Extrachromosomal genetic complementation of surface metalloproteinase (gp63)-deficient Leishmania increases their binding to macrophages. Proc Natl Acad Sci USA. 1992;89:4991–4995. doi: 10.1073/pnas.89.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGwire B S, Chang K P. Genetic rescue of surface metalloproteinase (gp63) deficiency in Leishmania amazonensis variants increases their infection of macrophages at the early phase. Mol Biochem Parasitol. 1994;66:345–347. doi: 10.1016/0166-6851(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 15.McGwire B S, Chang K P. Posttranslational regulation of a Leishmania HEXXH metalloprotease (gp63) J Biol Chem. 1996;271:7903–7909. doi: 10.1074/jbc.271.14.7903. [DOI] [PubMed] [Google Scholar]
- 16.Medina-Acosta E, Beverley S M, Russell D G. Evolution and expression of the Leishmania surface proteinase (gp63) gene locus. Infect Agents Dis. 1993;2:25–34. [PubMed] [Google Scholar]
- 17.Mosser D M, Burke S K, Coutavas E E, Wedgwood J F, Edelson P J. Leishmania species: mechanisms of complement activation by five strains of promastigotes. Exp Parasitol. 1986;62:394–404. doi: 10.1016/0014-4894(86)90048-2. [DOI] [PubMed] [Google Scholar]
- 18.Mosser D M, Edelson P J. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of leishmania promastigotes. J Immunol. 1985;135:2785–2789. [PubMed] [Google Scholar]
- 19.Mosser D M, Springer T A, Diamond M S. Leishmania promastigotes require opsonic complement to bind to the human leukocyte integrin Mac-1 (CD11b/CD18) J Cell Biol. 1992;116:511–520. doi: 10.1083/jcb.116.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierschbacher M D, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 21.Pommier C G, Inada S, Fries L F, Takahashi T, Frang M M, Brown E J. Plasma fibronectin enhances phagocytosis of opsonized particles by human peripheral blood monocytes. J Exp Med. 1983;157:1844–1854. doi: 10.1084/jem.157.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizvi F S, Ouaissi M A, Marty B, Santoro F, Capron A. The major surface protein of Leishmania promastigotes is a fibronectin-like molecule. Eur J Immunol. 1988;18:473–476. doi: 10.1002/eji.1830180323. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal L A, Sutterwala F S, Kehrli M E, Mosser D M. Leishmania major-human macrophage interactions: cooperation between Mac-1 (CD11b/CD18) and complement receptor type 1 (CD35) in promastigote adhesion. Infect Immun. 1996;64:2206–2215. doi: 10.1128/iai.64.6.2206-2215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruoslahti E. Integrins. J Clin Investig. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell D G, Wilhelm H. The involvement of the major surface glycoprotein (gp63) of leishmania promastigotes in attachment to macrophages. J Immunol. 1986;136:2613–2620. [PubMed] [Google Scholar]
- 26.Russell D G, Wright S D. Complement receptor type 3 (CR3) binds to an Arg-Gly-Asp-containing region on the major surface glycoprotein, gp63, of leishmania promastigotes. J Exp Med. 1988;168:279–292. doi: 10.1084/jem.168.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savoia D, Biglino S, Cestaro A, Zucca M. Effect of fibronectin and interferon-gamma on the uptake of Leishmania major and Leishmania infantum promastigotes by U937 cells. Microbiologica. 1992;15:51–56. [PubMed] [Google Scholar]
- 28.Schreiner C L, Bauer J S, Danilov Y N, Hussein S, Sczkan M M, Juliano R L. Isolation and characterization of CHO cell variants deficient in the expression of fibronectin receptor. J Cell Biol. 1989;109:3157–3167. doi: 10.1083/jcb.109.6.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soteriadou K P, Remoundos M S, Katsikas M C, Tzinia A K, Tsikaris V, Sakarellos C, Tzartos S J. The Ser-Arg-Tyr-Asp region of the major surface glycoprotein of Leishmania mimics the Arg-Gly-Asp-Ser cell attachment region of fibronectin. J Biol Chem. 1992;267:13980–13985. [PubMed] [Google Scholar]
- 30.Sutterwala F S, Noel G J, Salgame P S, Mosser D M. Reversal of proinflammatory responses by ligating the macrophage FcγRI. J Exp Med. 1998;188:1–6. doi: 10.1084/jem.188.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutterwala F S, Rosenthal L A, Mosser D M. Cooperation between CR1 (CD35) and CR3 (CD11b/CD18) in the binding of complement-opsonized particles. J Leukocyte Biol. 1996;59:883–890. doi: 10.1002/jlb.59.6.883. [DOI] [PubMed] [Google Scholar]
- 32.Takada Y, Strominger J L, Hemler M E. The very late antigen family of heterodimers is part of a superfamily of molecules involved in adhesion and embryogenesis. Proc Natl Acad Sci USA. 1987;84:3239–3243. doi: 10.1073/pnas.84.10.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Strijp J A G, Russell D G, Toumanen E, Brown E J, Wright S D. Ligand specificity of purified complement receptor type three (CD11b/CD18, αmβ2, Mac-1) J Immunol. 1993;151:3324–3336. [PubMed] [Google Scholar]
- 34.Wayner E A, Garcia-Pardo A, Humphries M J, McDonald J A, Carter W G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989;109:1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright S D, Craigmyle L S, Silverstein S C. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983;158:1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright S D, Silverstein S C. Tumor-promoting phorbol esters stimulate C3b and C3b′ receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982;156:1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyler D J, Sypek J P, McDonald J A. In vitro parasite-monocyte interactions in human leishmaniasis: possible role of fibronectin in parasite attachment. Infect Immun. 1985;49:305–311. doi: 10.1128/iai.49.2.305-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]