Abstract
Acute coronary syndromes (ACSs) are classified as ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) based on the presence of guideline-recommended ST-segment elevation (STE) criteria on the electrocardiogram (ECG). STEMI is associated with acute total coronary occlusion (ATO) and transmural myocardial necrosis and is managed with emergent reperfusion therapy, and NSTEMI is supposedly synonymous with subendocardial myocardial infarction without ATO. However, coronary angiograms reveal that a significant proportion of patients with NSTEMI have ATO. Here, we review articles that studied the frequency and cardiovascular outcomes of ATO in NSTEMI patients compared with those without ATO. We discuss ECG patterns of patients with suspected acute myocardial infarction that do not fulfill STEMI criteria but are associated with ATO. Under-recognition of these atypical patterns results in delays to reperfusion therapy. We also advocate revision of the current STEMI/NSTEMI paradigm because consideration of STE, by itself, out of context of other clinical and ECG features, leads to the ECG diagnosis of STEMI when the ECG actually represents a mimic [“Pseudo-STEMI”], and suggest renaming the ACSs classification as the Occlusion Myocardial Infarction (OMI)/Non-Occlusion Myocardial Infarction (NOMI) paradigm.
Key Words: Acute coronary syndrome, coronary occlusion, myocardial infarction, myocardial reperfusion, non-ST elevated myocardial infarction
1. Introduction:
Acute coronary syndromes (ACSs) remain a leading cause of morbidity and mortality worldwide. The 12-lead electrocardiogram (ECG) is a valuable tool for early recognition of acute myocardial ischemia. The presence or absence of pathologic Q waves on the ECG formerly resulted in classification of acute myocardial infarction (AMI) as Q-wave or non-Q-wave myocardial infarction (MI). In 2000, the ACC/AHA guidelines (1) announced a paradigm shift: patients presenting with ST-segment elevation (STE) on the ECG were grouped as having ST-segment elevation acute myocardial infarction (STEMI) and those without STE as non-ST-segment elevation myocardial infarction (NSTEMI). STEMI would purportedly represent acute total coronary occlusion (ATO) myocardial infarction (Occlusion MI or OMI) and NSTEMI would purportedly represent AMI without ATO (Non-Occlusion MI or NOMI). Prompt recognition of STE in the pre-hospital setting or in the Emergency Department by the paramedics and/or physicians is of great importance because the treatment of STEMI is urgent reperfusion with thrombolytics or primary coronary intervention. However, in reality, many patients who present with NSTEMI have ATO or subtotal occlusion on the coronary angiogram. A meta-analysis of seven studies showed that 25.5% of NSTEMI patients had ATO with increased adverse short and medium to long term cardiovascular outcomes, compared with NSTEMI patients without ATO (2). Furthermore, advances in ECG interpretation have revealed numerous high-risk ECG presentations without STE that are associated with ATO for which early reperfusion therapy could be beneficial. The aim of the current narrative review is to study the frequency and cardiovascular outcomes of ATO in NSTEMI patients, provide a summary of ECG patterns without STE that are associated with ATO, and support a paradigm shift of the current STEMI/NSTEMI classification of ACS.
2. Method
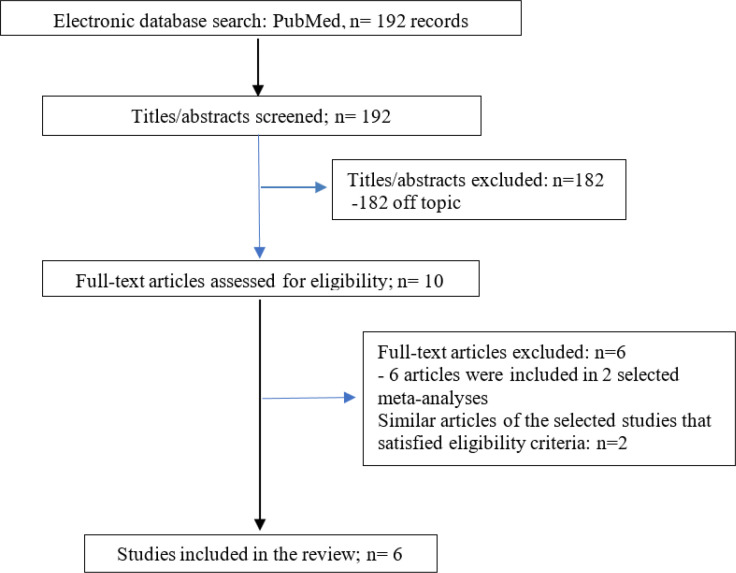
We searched the electronic database of PubMed from inception up to 22 August 2022 for articles that studied: i) patients who underwent percutaneous coronary angiogram/intervention (PCI) for NSTEMI, ii) the incidence (or prevalence) of ATO on the coronary angiogram of this population, and iii) cardiovascular outcomes of patients with ATO compared with those without ATO. Exclusion criteria included non-English manuscripts, case reports and editorials. The following terms were used: (incidence OR prevalence OR frequency) AND (impact OR outcomes) AND (total artery occlusion OR culprit lesion) AND (NSTEMI OR non-ST elevation myocardial infarction). The titles and abstracts were reviewed, independently, by 2 authors (GA, GM) and unrelated studies were excluded. Full-text evaluation of the remaining articles was performed and studies that satisfied eligibility criteria were included in the review. Disagreements between reviewers were resolved after discussion with the third author (SS). Similar articles of the selected studies were also evaluated for eligibility. Figure 1 shows the flow diagram of the study.
Figure 1.
Flow diagram of the study
3. Findings
3.1. Outcomes of NSTEMI cases with ATO
We identified 192 records. Four observational studies and 2 meta-analyses were included in the present review. In a systematic review and meta-analysis (3) data from 25 studies were analyzed, the average proportion of ATO in NSTEMI patients was 34% (95% CI 30%-37%). Death rate, recurrent MI and cardiogenic shock were significantly higher in ATO NSTEMI patients compared with those without ATO (OR: 1.72, 95% CI: 1.49-1.98, p < 0.001, OR 1.7: 95% CI: 1.06-2.75, p = 0.029 and OR: 1.66, 95% CI: 1.35-2.04, p < 0.001, respectively). Khan et al. (2) published a systematic review and meta-analysis of 7 studies and reported Thrombolysis in Myocardial Infarction (TIMI) flow 0-1 in 25.5% of NSTEMI patients. This group had increased short and medium-to long-term risk of major adverse cardiovascular events (MACE) (RR: 1.41, 95% CI: 1.17-1.70, p = 0.0003 and RR: 1.32, 95% CI: 1.11-1.56, p = 0.001, respectively) and all-cause mortality (RR: 1.67, 95% CI: 1.31-2.13, p<0.0001 and RR: 1.42, 95% CI: 1.08-1.86, p=0.01, respectively).
More recently, in a large Polish registry (4) the proportion of ATO with TIMI flow 0 was 19.9% among 81415 NSTEMI patients and was associated with higher incidence of cardiac arrest before admission (3.09% vs. 2.19%, p<0.0001), Killip class IV on admission (2.48% vs. 1.69%, p<0.0001), death during PCI (0.97% vs. 0.43%, p<0.0001), and the lower frequency of TIMI flow 3 after PCI (83.36% vs. 88.61%, p<0.0001). Morawska et al. (5) found increased in-hospital and one-year mortality in NSTEMI patients presenting with ATO compared with NSTEMI patients with patent coronary arteries (2.8 % vs. 1.1%, p=0.007 and 18.1% vs. 6.5%, p<0.001, respectively). Another observational study (6) found statistically insignificant differences between ATO and non-ATO NSTEMI patients regarding in- hospital (5.3% vs. 1%, p=0.07) and 6-month MACE (5.4% vs. 4.6%, p=0.24). Fernando et al. (7) studied the long-term cardiovascular outcomes of occluded culprit arteries in NSTEMI patients and found lower rates of mortality in ATO NSTEMI patients when compared with NSTEMI patients without ATO with an average follow up of 4.9 years (12% vs. 18%, p<0.01), despite the increased 30-day MACE observed in this group (6.7% vs. 3.8%, p<0.001). Multivariate analysis of this study showed that age, traditional cardiovascular risk factors, heart failure, renal impairment, and multivessel coronary artery disease are all independent predictors of long-term mortality, whereas ATO was not. Thus, higher rates of long-term mortality in NSTEMI patients without ATO may be attributed to the greater prevalence of comorbidities in this group. Table 1 shows published articles that studied the frequency of ATO and cardiovascular outcomes in NSTEMI patients compared with NSTEMI patients without ATO.
Table 1.
Studies showing the frequency of acute total coronary occlusion (ATO) and cardiovascular outcomes in non-ST-segment elevation myocardial infarction (NSTEMI) patients compared with NSTEMI patients without ATO
| Study | Year |
ATO definition
(TIMI flow grade) |
Frequency of ATO in NSTEMI |
Cardiovascular outcomes
(ATO vs . non-ATO) |
|---|---|---|---|---|
| Morawska et al. (5)-observational study | 2021 | 0 | 34.6% (138/399) | -in-hospital mortality: (2.8% vs. 1.1%, p=0.007) -1-year mortality: (18.1% vs. 6.5%, p<0.001) |
| Fernando et al. (7)-observational study | 2021 | 0 | 14% (954/6829) | -30-day MACE: (6.7% vs. 3.8%, p<0.001) -4.9-years mortality: (12% vs. 18%, p<0.01) |
| Terlecki et al. (4)-observational study | 2021 | 0 | 19.9% (16209/81415) | -C.A. before admission (3.09% vs. 2.19%, P<0.0001) -Killip IV on admission: (2.48% vs. 1.69%, p<0.0001) -death during PCI: (0.97% vs. 0.43%, p<0.0001) -TIMI flow 3 after PCI: (83.36% vs. 88.61%, p<0.0001) |
| Ayad et al. (6)-observational study | 2021 | 0 | 22.4% (112/500) | -in-hospital MACCE: (5.3% vs. 1%, p=0.07) -6-month MACCE: (5.4% vs. 4.6%, p=0.24) |
| Hung et al. (3)-meta-analysis | 2018 | -in 21 studies: 0-1 -in 3 studies: 0 -in 1 study: 0-2 |
34% (95% CI 30%-37%) average proportion | -death rate: (OR 1.72, 95% CI 1.49-1.98, p<0.001) -recurrent MI: (OR 1.7, 95% CI 1.06-2.75, p=0.029) -cardiogenic shock: (OR 1.66, 95% CI 1.35-2.04, p<0.001) |
| Khan et al. (2)-meta-analysis | 2017 | 0-1 | 25.5% (10415/40777) | -short-term MACE: (RR 1.41, CI 1.17-1.70, p=0.0003) -medium- to long-term MACE: (RR 1.32, CI 1.11-1.56, p=0.001) -short-term all-cause mortality: (RR 1.67, CI 1.31-2.13, p<0.0001) -medium- to long-term all-cause mortality: (RR 1.42, CI 1.08-1.86, p=0.01) |
CI: confidence interval; TIMI: Thrombolysis in Myocardial Infarction, MACE: major adverse cardiac events, C.A: cardiac arrest, PCI: percutaneous intervention, MACCE: major adverse cardiac and cerebrovascular events, MI: myocardial infarction; OR: odds ratio; RR: relative risk.
Overall, approximately 25%-30% of patients presenting with NSTEMI have ATO with increased adverse short-term cardiovascular outcomes compared with NSTEMI patients with patent coronary arteries, with some discrepancies regarding long-term cardiovascular outcomes.
3.2. High-risk ECG patterns associated with ATO
According to the fourth universal definition of MI, ECG criteria for STEMI diagnosis are STE ≥1 mm in two contiguous leads, except leads V2-V3 where the following cut-points apply: ≥2 mm in men ≥40 years; ≥2.5 mm in men <40 years or ≥1.5 mm in women regardless of age (8). These current formal criteria are based on the modification of the 2000 ACC/ESC criteria by Macfarlane et al. (9) However, some patients present with ECG patterns that are associated with ATO but do not fulfill the above-mentioned STEMI criteria. Below, we briefly discuss these ECG patterns.
Acute myocardial infarction is a dynamic phenomenon and hyperacute T waves often precede STE. There is not a clear definition of hyperacute T waves, but they are often described as symmetric, tall, with high amplitude proportional to the QRS and depend mainly on recognition rather than on criteria (10). An old study (11) found that a combination of J point/T wave amplitude >25%, T wave/QRS amplitude >75% and J point >0.30 mV in patients >45 years old predicts clinically verifiable MI with 98% specificity and 61.9% sensitivity. Many authors agree that T-wave height is not as important as T-wave “bulk,” defined as a proportion of the area under the curve of the T-wave to QRS size (10, 12, 13).
Diagnosis of ACS in the presence of left bundle branch block (LBBB) can be challenging. Sgarbossa et al. (14) proposed a score composed of 3 criteria for the diagnosis of acute MI in patients presenting with LBBB; however, it has low sensitivity. More recently, Smith’s modified Sgarbossa criteria were published: STE ≥1mm concordant with QRS in any lead, or ST-segment depression (STD) ≥ 1mm in any of leads V1-V3, or excessively discordant STE with ST/S ratio ≥ 25% in any lead predicted Occlusion MI (OMI) in patients with LBBB (15). These criteria were validated in a study by Meyers et al. (16) and showed significantly higher sensitivity and similarly high specificity compared with the original Sgarbossa criteria. Using a ST/S ratio criterion of 20% improves sensitivity to 84% and only reduces specificity to 94%.
Right ventricular paced rhythm poses another diagnostic challenge. Dodd et al. (17) compared Smith’s modified Sgarbossa criteria, which defines excessively discordant STE as an ST/S ratio of 25% (with extension of the second criterion of STD ≥1mm to leads V4-V6), with the original Sgarbossa criteria, which defines excessively discordant STE as 5 mm, and found that it has higher sensitivity with similarly high specificity in diagnosis of OMI. Interpretation of the ECG in the setting of acute chest pain and new-onset right bundle branch block (RBBB) should be done with much care, because RBBB can mask subtle STE in leads V1-V3 due to secondary repolarization changes resulting in the depression of ST-segment and T wave inversion. New RBBB, especially when combined with left anterior fascicular block, is associated with occlusion of proximal left anterior descending artery (LAD) and increased in-hospital mortality (18).
Wellens’ syndrome (19, 20) is defined by a post-anginal period and ECG findings of preserved R-waves and an isoelectric or <1 mm J point elevation and biphasic T waves in leads V2-V3 (Pattern A) or deeply inverted and symmetric T waves in leads V2-V3 (Pattern B); the T wave changes in both types may extend to V1, V4, V5 and V6. Wellens’ syndrome is related with critical stenosis of proximal LAD and impending MI, and is actually a post-reperfusion pattern: if an ECG had been recorded during pain, the ECG would have manifested acute occlusion. Other myocardial locations (inferior, lateral) manifest identical findings in the post-reperfusion state; in the posterior wall, it manifests as increased T-wave amplitude in V2 (“Posterior Reperfusion T-waves; Wellens’ syndrome of the posterior wall”) (21) . More recently, de Winter’s ECG pattern (22) was associated with proximal LAD occlusion: minimal STE in lead aVR and hyperacute T waves with upsloping STD in leads V1-V6. Left circumflex artery (LCx) is the culprit artery of isolated posterior MI. Horizontal STD of any amount, maximal in leads V1-V4 versus V5-V6, predicts acute posterior OMI (versus nonocclusive ischemia) with 97% specificity (23) . Another ECG pattern seen in LCx total occlusion is the N wave sign, which is recognized as notch or deflection ≥2mm in the terminal QRS complex in leads II, III and aVF and/or leads I, aVL with continuous change of the notch ≥2mm in ≥2 leads in 24 hours and prolonged QRS duration in these leads (24) . Aslanger’s pattern (25) is a high-risk ECG presentation connected with occlusion of LCx or right coronary artery (RCA) with at least one accompanying stable but critical stenosis in one of the non-infarct-related arteries. Diagnostic criteria are any STE in lead III, with reciprocal STD in aVL, but no STE in other inferior leads, STD in any of leads V4-V6 (but not in V2) with positive T wave and ST in V1 higher than in V2. Critical stenosis of the left main coronary artery (LM) or severe 3-vessel disease can present with diffuse STD >1mm in at least 6 leads plus STE in aVR and/or V1 (26) . Marti et al. (27) found that 18% of patients with ATO had subtle STE, defined as STE 0.1-1mm; 86% of them had TIMI flow grade 0/1. Patients with subtle STE had longer delays to reperfusion and similar rates of deaths and reinfarction compared with those who fulfilled formal STEMI criteria. Reciprocal STD ≥0.5 mm was present in 68% of patients with subtle STEMI and more specific in 75% of patients with inferior infarctions. Table 2 summarizes the high-risk ECG patterns associated with ATO.
Table 2.
High risk electrocardiogram (ECG) patterns associated with acute total coronary occlusion (ATO)
| High risk ECG pattern | Criteria for diagnosis of ATO | Sensitivity/specificity | Culprit artery |
|---|---|---|---|
| Hyperacute T waves (10, 12, 13) | - “Bulky”, fat, wide, and often also tall and symmetric T-waves, such that the area under the curve, compared to the QRS amplitude, is large -a combination of: •J point/T wave > 25% •T wave/QRS > 75% •J point > 0.30 mv and •age > 45 years old |
61.9%/98% | LAD, LCx or RCA |
| Lbbb (15, 16) | -Smith’s modified Sgarbossa criteria: •STE ≥1 mm concordant with QRS in any lead or •STD≥1 mm in any of V1-V3 or •STE/S wave ≥ 25% in any lead |
80%/99% | LAD, LCx or RCA |
| RV paced rhythm (17) | -Smith’s modified Sgarbossa criteria: •STE ≥1 mm concordant with QRS or •STD≥1mm in any of V1-V6 or •STE/S wave ≥ 25% in any lead |
86%/83% | LAD, LCx or RCA |
| New RBBB ± LAH (18) | - RBBB diagnostic criteria -Any STE is suspicious |
proximal LAD | |
| De Winter’s pattern (22) | - 1-2 mm STE in aVR and: •hyperacute T waves in V1-V6 •upsloping STD 1-3 mm in V1-V6 |
proximal LAD | |
| Wellens’ syndrome (19, 20) | -isoelectric or < 1mm elevated J point in a post-anginal period plus: •biphasic T waves in V2-V3 (Pattern A) or •deeply inverted and symmetric T waves in V2-V3 (Pattern B) •T wave changes may extend to V1, V4, V5, V6 |
69%/89% | proximal LAD |
| Diffuse STD (26) | -STD > 1mm in ≥ 6 leads and: •STE in aVR and/or V1 |
LM or severe 3 vessel disease | |
| Aslanger’s pattern (25) | -any STE in III, but not in other inferior leads, with reciprocal STD in aVL plus: •STD in any of V4-V6 but not in V2 with positive T wave •STE in V1 higher than in V2 |
LCx or RCA and 2 or 3 vessel disease | |
| N wave sign (24) | -notch or deflection in the terminal QRS complex ≥2 mm in II, III and aVF and/or I, aVL plus •continuous change of the notch ≥2 mm in ≥ 2 leads in 24 hours •prolonged QRS in these leads |
77%/89% and 53%/97% / or 64%/96% | LCx |
| Acute posterior OMI (23) | -STD of any amplitude maximal in leads V1-V4 versus V5-V6 | 37.4%/97.6% | LCx |
| Subtle STEMI (27) | -STE 0.1-1 mm combined with reciprocal STD ≥0.5 mm | LCx, RCA or LAD |
LAD: left anterior descending artery, LCx: left circumflex artery, RCA: right coronary artery, LBBB: left bundle branch block, STE: ST-segment elevation, STD: ST-segment depression, RV: right ventricular, RBBB: right bundle branch block, LAH: left anterior hemiblock, LM: left main coronary artery, OMI: occlusion myocardial infarction; STEMI: ST-segment elevation myocardial infarction; aVR: augmented Vector Right; aVL: augmented Vector Left.
3.3. Differentiation of normal, baseline STE from STE due to OMI
Very often physicians face difficulties in diagnosing STEMI because many patients have non-ischemic STE. Normal variant STE (NV-STE) is sometimes referred to as “early repolarization,” even though this term has a more specific meaning. NV-STE may be present in anterior, lateral, or inferior leads. It is crucial to differentiate anterior STEMI from NV-STE ECG pattern in anterior leads in the setting of acute chest pain. Smith et al. (28) found a formula of 3 variables (STE 60ms after J point in lead V3, QTc segment duration, and amplitude of R wave in lead V4) that predicted anterior STEMI with 86% sensitivity, 91% specificity and accuracy of 88%. After adding QRS voltage in lead V2, the accuracy of the 4-variable formula increased to 92% (29). These 2 formulas were externally validated and showed high sensitivity, specificity and diagnostic accuracy (30). Moreover, presence of terminal QRS distortion (TQRSD = absence of both an S and J wave in either V2 or V3) is a useful ECG sign to differentiate anterior STEMI from NV-STE with 100% specificity (i.e., no case of NV-STE had TQRSD) (31). ECG presentation of left ventricular aneurysm can mask acute critical occlusion of LAD. Criterion that supports the diagnosis of anterior STEMI is any V1-V4 T wave/QRS amplitude ≥0.36 (32, 33). Table 3 shows ECG patterns with normal, baseline STE and proposed criteria to differentiate STE due to OMI.
Table 3.
Electrocardiogram (ECG)patterns with normal, baseline ST-segment elevation (STE) and proposed criteria to differentiate STE due to Occlusion Myocardial Infraction (OMI)
| ECG pattern | Criteria for diagnosis of ATO | Sensitivity/specificity | Culprit artery |
|---|---|---|---|
| NV-STE (“early repolarization”) (10, 29, 31) |
-4-variable formula: •0.052xQTc – 0.151xQRSV2-0.268xRV4 + 1.062xSTE60V3 ≥ 18.2 -terminal QRS distortion: •absence of both an S and J wave in either V2 or V3 |
88.8%/94.7% 20%/100% |
LAD |
| left ventricular aneurysm (32, 33) | -any V1-V4 T /QRS ≥ 0.36 | 91.5%/81.3% | LAD |
ATO: acute total coronary occlusion; NV-STE: normal variant ST-elevation; QTc: Bazett-corrected QT segment; QRSV2: QRS voltage in lead V2, RV4: R wave amplitude in lead V4; STE60V3: ST-segment elevation in lead V3 60ms after J point; LAD: left anterior descending artery.
3.4. From Q/non-Q MI to STEMI/NSTEMI
The previous paradigm of ACS (Q/non-Q MI) reflects the natural history of MI: treatment with antithrombotics without reperfusion cannot reverse ongoing myocardial necrosis in the case of ATO and it ultimately leads to large territory complete infarction, with subsequent scarring. This phenomenon is expressed with Q wave on the ECG. The Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group, published in 1994 a landmark systematic review of 9 randomized trials, which studied the effect of fibrinolytic therapy in suspected acute MI (34). They concluded that thrombolysis was associated with reduction in mortality and this benefit was observed mainly among patients presenting with STE or bundle branch block (BBB) and when therapy was received up to 12 hours from symptom onset. However, enrollment criteria were “suspected MI”; 4/9 studies required STE but poorly defined; 5/9 had no required ECG findings; 38% of patients were enrolled after at least 6 hours of chest pain, when treatment is much less effective, and 7/9 studies used the less effective streptokinase (35-43). For example, in the second International Study of Infarct Survival (ISIS-2) (37) and the Anglo-Scandinavian Study of Early Thrombolysis (ASSET) (43) trials patients with clinically suspected acute MI and without specific ECG changes (including unspecified STE, BBB, STD or normal ECG) were included and in other studies (35, 39) patients with STE ≥1mm in limb leads or ≥2mm in chest leads were deemed eligible, these cut-offs differ from those recommended in the fourth universal definition of MI for STEMI diagnosis. STE and STD were also retrospectively classified. Based on this data, they concluded that STE is the best way to decide on thrombolytic therapy (no placebo-controlled trial of mechanical intervention has ever been done). But this is only true if ECG interpretation is crude, patients are treated after 6 hours, and streptokinase is used (not PCI). But what about less obvious STE, hyperacute T-waves, ST-T morphology, terminal QRS distortion, proportionality between QRS and ST-T, associated Q-waves, etc.? Since no coronary angiograms were performed, an unknown number of patients with ECG presentations like normal ECG, pre-existing BBB, acute pericarditis, acute myocarditis, hyperkalemia, “early repolarization”, and left ventricular hypertrophy received fibrinolytic therapy without actually having acute MI. Furthermore, according to the authors, it was unclear whether fibrinolytic therapy benefits patients presenting with STD or other ECG abnormalities but without STE or BBB and they mentioned that the number of deaths among such patients was relatively small. Due to the results of the FTT Collaborative Group systematic review, classification of ACS changed officially from Q/non-Q MI to STEMI/NSTEMI in the 2000 ACC/AHA guidelines.
3.5. STEMI/NSTEMI paradigm in the era of mechanical reperfusion
According to the European guidelines (44), therapy of STEMI is immediate reperfusion with thrombolysis or PCI, and analogous treatment (invasive therapy <2h from hospital admission) should be administered to NSTEMI patients with mechanical complications, cardiogenic shock, refractory angina, life-threatening arrhythmias, and acute heart failure (26). In spite of these NSTEMI guidelines, very few (6.4%) such patients are actually taken for angiography within 2 hours (45). Early recognition of STE in the setting of acute chest pain is a critical initial step in the management of ACS. However, not all STE are due to acute MI: acute pericarditis, “early repolarization”, left ventricular hypertrophy and aneurysm, takotsubo cardiomyopathy, and hyperkalemia are some examples that present with STE on the ECG. It was found that 15%-36% (46, 47) of catheter laboratory activations due to perceived STEMI were false positive without culprit lesion on the coronary angiogram. Major dilemmas emerge in non-PCI-capable centers and time to PCI >120 min, where thrombolysis is the only option for urgent reperfusion of STEMI and false diagnosis could be catastrophic due to possible serious hemorrhagic complications. On the other hand, as we discussed earlier in the present review, not all acute MI with proven ATO present with STE and a number of such high-risk ECG patterns can be recognized (the so-called STEMI equivalents). In addition, about 25%-30% of NSTEMI patients have ATO with increased adverse cardiovascular outcomes compared with those with patent coronary arteries. Strict application of STEMI criteria in clinical practice excludes patients with de Winter’s pattern from receiving immediate reperfusion therapy and activates catheter laboratory for patients presenting with “early repolarization” (Figure 2). A “holistic” ECG approach is needed: ECG interpretation in the clinical context of acute chest pain indicative of myocardial ischemia should not focus only on ST segment nor on whether any specific millimeter-based STE criteria are satisfied. In addition, ST and T wave should always be assessed in proportion to QRS.
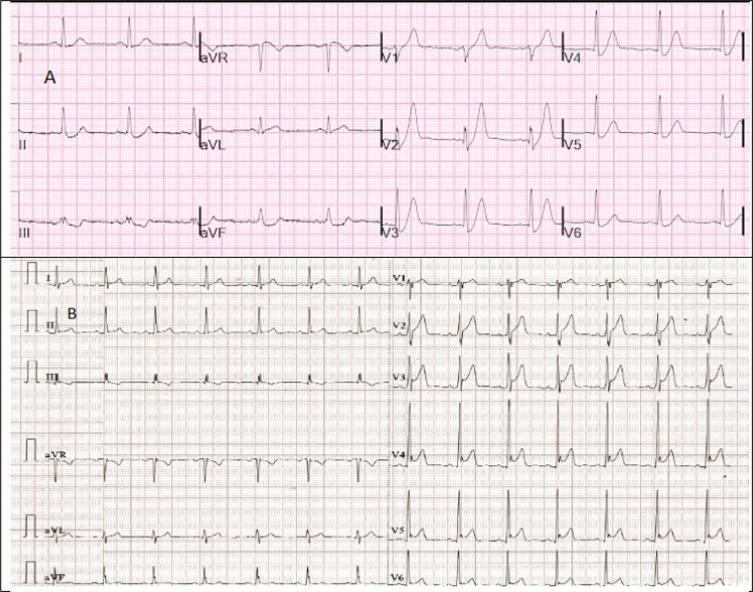
Figure 2.
Electrocardiogram (ECG) recordings from two male patients in their early 30s presenting with acute chest pain. ECG (A) is from a patient with de Winter’s pattern and ECG (B) is consistent with “early repolarization”. ECG (A) does not meet STEMI criteria but catheter laboratory was activated emergently and a total occluded left anterior descending artery was identified on the coronary angiogram and was stented successfully. ECG (B) satisfies current STEMI criteria but emergent treatment was not required. Serial unchanged ECG recordings with normal values of troponin and normal echocardiogram ruled out acute coronary syndrome. {ECG (A) is reproduced after permission from Dr. Smith’s ECG blog. Available from: https://hqmeded-ecg.blogspot.com/2021/03/de-winters-t-waves-are-not-stable-ecg.html, courtesy of Stephen W. Smith, MD}
3. 6. What’s new in the European guidelines?
In 2017, the ESC guidelines for STEMI (44) in addition to the new LBBB and the isolated posterior infarction, considered two more atypical ECG presentations as high risk, which should prompt a primary coronary intervention strategy in patients presenting with ongoing symptoms consistent with myocardial ischemia: RBBB and diffuse STD ≥1 mm in at least 8 leads coupled with STE in aVR and/or V1. The former pattern suggests proximal critical LAD occlusion with poor prognosis and the latter points to ischemia due to left main coronary artery critical stenosis or severe multivessel disease. According to the latest ESC guidelines for NSTEMI in 2020 (26), those presenting with STD>1 mm in at least 6 leads plus STE in aVR and/or V1 are also considered very high-risk patients who should be managed with immediate (<2 hours) invasive strategy. Furthermore, in the supplementary data, de Winter’s and Wellens’ patterns are mentioned as high-risk ECG presentations without STE associated with proximal LAD occlusion. There are many other subtle patterns of occlusion, which are more difficult to describe and teach. In their study (48), showing that expert ECG interpretation has equal specificity to the STEMI criteria and is more than twice sensitive in detection of OMI, Meyers et al. found the 7 patterns shown in Table 4.
Table 4.
Frequency of 7 findings among 146 patients with Occlusion Myocardial Infarction (OMI), identified by expert earlier than by criteria/angiogram
| Feature | Frequency |
|---|---|
| Hyperacute T waves | 49% |
| Pathologic Q waves, along with subtle STE | 47% |
| Terminal QRS distortion | 53% |
| Reciprocal STD and/or Reciprocal T-wave inversion | 82% |
| Subtle STE not meeting criteria, but with other features | 83% |
| Any amount of STD maximal in V1-V4 | 45% |
| Any STE in inferior leads with any STD/T-wave inversion in aVL | 50% |
STE: ST-segment elevation; STD: ST-segment depression; aVL: augmented Vector Left.
3.7. The OMI/NOMI paradigm
Many authors express concerns about the clinical performance of STEMI/NSTEMI classification of ACS (49-52). Misinterpretation of STEMI mimics as STEMI, false positive catheter laboratory activations, under-recognition of high-risk ECG patterns without STE, proper risk stratification of NSTEMI patients, delays to reperfusion therapy, and disagreements between emergency physicians and interventional cardiologists are common problems. The main goal in the management of patients presenting with suspected acute MI is to provide immediate reperfusion therapy to those with ATO. Thus, the question that should be answered is whether the patient presenting to the emergency department with chest pain has an ECG indicative of ATO and not if pre-specified STE criteria are fulfilled. Based on this principle, Meyers, Weingart and Smith proposed the OMI/NOMI research classification of ACS (53). Occlusion Myocardial Infarction (OMI) is defined as an acute culprit coronary artery and either 1) TIMI flow 0-2 or 2) TIMI flow 3 plus 4th generation troponin T ≥ 1.0 ng/ml or I ≥10.0 ng/ml (5th generation, high sensitivity troponin, would be in ng/L and multiply by 1000) (48). OMI refers to type 1 acute coronary syndrome involving acute occlusion or near occlusion of a major epicardial coronary vessel with insufficient collateral circulation, resulting in imminent necrosis of downstream myocardium without emergent reperfusion. OMI is the anatomic and pathophysiologic substrate of STEMI, but not all OMI manifest as STEMI. Non-occlusion Myocardial Infarction (NOMI) refers to acute MI without angiographic, laboratory or clinical evidence of OMI (NSTEMI without ATO). The term OMI includes STEMI patients who fulfill the current STEMI criteria [STEMI (+) OMI] and those who do not meet these criteria [STEMI (-) OMI or NSTEMI with ATO]. OMI requires emergent reperfusion therapy because of ATO. The DIagnostic accuracy oF electrocardiogram for acute coronary OCClUsion resuLTing in myocardial infarction (DIFOCCULT) study (54) found that the OMI/NOMI approach to ECG interpretation had superior diagnostic accuracy compared with the STEMI/NSTEMI approach in prediction of ATO and long-term mortality. Meyers et al. (48) showed that STEMI (-) OMI (NSTEMI with ATO) patients had significant delays to catheterization but adverse outcomes more similar to STEMI (+) OMI. More importantly, they found that expert ECG interpretation had sensitivity of 86% for diagnosis of OMI (vs. 41% for STEMI criteria) with specificity equal to STEMI criteria.
Before the era of reperfusion therapy, anti-thrombotics (mainly aspirin and heparin) were used for the treatment of ACS. In the case of a total occluded coronary artery, transmural myocardial necrosis could not be reversed and Q wave was recorded on the ECG. This Q/non-Q paradigm was replaced by the STEMI/NSTEMI dichotomy based on the reduced mortality of patients who presented with suspected acute MI and STE who received thrombolytic therapy. Since then, STE is considered as a surrogate of ATO. However, coronary angiograms revealed that not all ECG presentations with STE are due to ATO and that a number of ECG patterns without STE are associated with ATO. This relation is expressed by the OMI/NOMI paradigm. Figure 3 shows a proposed evolution of ACS classification. One final thought: what other pathology has been named for a test? STEMI is named for one very imperfect aspect of one test (STE on the ECG). The pathology should be named for what it is: Occlusion MI. Its diagnosis can usually be made by expert ECG interpretation, but it is important to know that many OMIs do not manifest on the ECG, that many which do manifest on the ECG are not accurately interpreted by providers, and that one must often use modalities other than the ECG to make the diagnosis, including emergent echocardiogram, CT coronary angiography, or angiogram itself. For acute symptoms, initial (and especially 1- or 2-hour) troponin concentration is more likely to be less than the 99th percentile and the delay to reperfusion is too long (55).
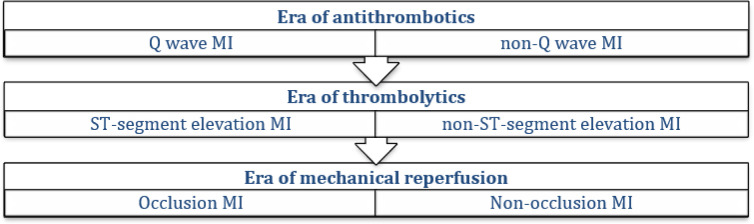
Figure 3.
Proposed evolution of acute coronary syndrome classifications. MI: Myocardial Infarction
4. Conclusion
Recent studies suggest that 25%-30% of NSTEMI patients have ATO with increased adverse cardiovascular outcomes compared with those with patent coronary arteries. Early recognition of this high-risk group of ACS patients is based on the identification of ECG patterns that are related to ATO and do not satisfy current STEMI criteria. Knowledge and continuous training in interpretation of these ECG presentations could improve management and outcomes of ACS patients. Considering STE as a hallmark of acute MI with ATO can be at times misleading and STEMI/NSTEMI classification should be revised to include more high-risk ECG patterns that signify ATO. We agree with renaming the paradigm as the Occlusion MI/Non-Occlusion MI paradigm.
5. Declarations
5.1. Acknowledgements
None.
5.2. Authors’ contributions
G.A. and G.M. wrote the manuscript, and searched and analyzed the data. S.S. wrote and revised the article. All authors read and approved the final version of manuscript.
5.3. Funding and supports
None.
5.4. Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina) Circulation. 2000;102(10):1193–209. doi: 10.1161/01.cir.102.10.1193. [DOI] [PubMed] [Google Scholar]
- 2.Khan AR, Golwala H, Tripathi A, Bin Abdulhak AA, Bavishi C, Riaz H, et al. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: a systematic review and meta-analysis. Eur Heart J. 2017;38(41):3082–9. doi: 10.1093/eurheartj/ehx418. [DOI] [PubMed] [Google Scholar]
- 3.Hung C-S, Chen Y-H, Huang C-C, Lin M-S, Yeh C-F, Li H-Y, et al. Prevalence and outcome of patients with non-ST segment elevation myocardial infarction with occluded “culprit” artery–a systemic review and meta-analysis. Crit Care. 2018;22(1):1–11. doi: 10.1186/s13054-018-1944-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terlecki M, Wojciechowska W, Dudek D, Siudak Z, Plens K, Guzik TJ, et al. Impact of acute total occlusion of the culprit artery on outcome in NSTEMI based on the results of a large national registry. BMC Cardiovasc. Disord. 2021;21(1):1–9. doi: 10.1186/s12872-021-02099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morawska I, Niemiec R, Stec M, Wrona K, Bańka P, Swinarew A, et al. Total Occlusion of the Infarct-Related Artery in Non-ST-Elevation Myocardial Infarction (NSTEMI)—How Can We Identify These Patients? Medicina. 2021;57(11):1196. doi: 10.3390/medicina57111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayad SW, El Zawawy TH, Lotfy MI, Naguib AM, El Amrawy AM. Incidence and impact of totally occluded culprit coronary artery in patients with non-ST segment elevation myocardial infarction acute coronary syndrome. Egypt Heart J. 2021;73(1):1–9. doi: 10.1186/s43044-021-00160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernando H, Duffy SJ, Low A, Dinh D, Adrianopoulos N, Sharma A, et al. Totally Occluded Culprit Coronary Artery in Patients with Non-ST-Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Am J Cardiol. 2021;156:52–7. doi: 10.1016/j.amjcard.2021.06.043. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–64. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 9.Macfarlane PW, Browne D, Devine B, Clark E, Miller E, Seyal J, et al. Modification of ACC/ESC criteria for acute myocardial infarction. J Electrocardiol. 2004;37:98–103. doi: 10.1016/j.jelectrocard.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Miranda DF, Lobo AS, Walsh B, Sandoval Y, Smith SW. New insights into the use of the 12-lead electrocardiogram for diagnosing acute myocardial infarction in the emergency department. Can J Cardiol. 2018;34(2):132–45. doi: 10.1016/j.cjca.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Collins MS, Carter JE, Dougherty JM, Majercik SM, Hodsden JE, Logue EE. Hyperacute T-wave criteria using computer ECG analysis. Ann Emerg Med. 1990;19(2):114–20. doi: 10.1016/s0196-0644(05)81792-5. [DOI] [PubMed] [Google Scholar]
- 12.Aslanger EK, Meyers HP, Smith SW. Recognizing electrocardiographically subtle occlusion myocardial infarction and differentiating it from mimics: Ten steps to or away from cath lab. Turk Kardiyol Dern Ars. 2021;49(6):488. doi: 10.5543/tkda.2021.21026. [DOI] [PubMed] [Google Scholar]
- 13.Smith SW. Dr Smith’s ECG Blog. [[cited 22 August 2022]]. available at: http://hqmeded-ecgblogspotcom [Internet]
- 14.Sgarbossa EB, Pinski SL, Barbagelata A, Underwood DA, Gates KB, Topol EJ, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. N Engl J Med. 1996;334(8):481–7. doi: 10.1056/NEJM199602223340801. [DOI] [PubMed] [Google Scholar]
- 15.Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012;60(6):766–76. doi: 10.1016/j.annemergmed.2012.07.119. [DOI] [PubMed] [Google Scholar]
- 16.Meyers HP, Limkakeng Jr AT, Jaffa EJ, Patel A, Theiling BJ, Rezaie SR, et al. Validation of the modified Sgarbossa criteria for acute coronary occlusion in the setting of left bundle branch block: A retrospective case-control study. Am Heart J. 2015;170(6):1255–64. doi: 10.1016/j.ahj.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Dodd KW, Zvosec DL, Hart MA, Glass III G, Bannister LE, Body RM, et al. Electrocardiographic diagnosis of acute coronary occlusion myocardial infarction in ventricular paced rhythm using the modified Sgarbossa criteria. Ann Emerg Med. 2021;78(4):517–29. doi: 10.1016/j.annemergmed.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Widimsky P, Roháč F, Štásek J, Kala P, Rokyta R, Kuzmanov B, et al. Primary angioplasty in acute myocardial infarction with right bundle branch block: should new onset right bundle branch block be added to future guidelines as an indication for reperfusion therapy? Eur Heart J. 2012;33(1):86–95. doi: 10.1093/eurheartj/ehr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Zwaan C, Bär FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J. 1982;103(4):730–6. doi: 10.1016/0002-8703(82)90480-x. [DOI] [PubMed] [Google Scholar]
- 20.Haines DE, Raabe DS, Gundel WD, Frans JT. Anatomic and prognostic significance of new T-wave inversion in unstable angina. Am J Cardiol. 1983;52(1):14–8. doi: 10.1016/0002-9149(83)90061-9. [DOI] [PubMed] [Google Scholar]
- 21.Driver BE, Shroff GR, Smith SW. Posterior reperfusion T-waves: Wellens' syndrome of the posterior wall. Emerg Med J. 2017;34(2):119–23. doi: 10.1136/emermed-2016-205852. [DOI] [PubMed] [Google Scholar]
- 22.de Winter RJ, Verouden NJ, Wellens HJ, Wilde AA. A new ECG sign of proximal LAD occlusion. N Engl J Med. 2008;359(19):2071–3. doi: 10.1056/NEJMc0804737. [DOI] [PubMed] [Google Scholar]
- 23.Meyers HP, Bracey A, Lee D, Lichtenheld A, Li WJ, Singer DD, et al. Ischemic ST‐Segment Depression Maximal in V1–V4 (Versus V5–V6) of Any Amplitude Is Specific for Occlusion Myocardial Infarction (Versus Nonocclusive Ischemia) J Am Heart Assoc. 2021;10(23):e022866. doi: 10.1161/JAHA.121.022866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu T, Fu P, Jia C, Dong Y, Liang C, Cao Q, et al. The delayed activation wave in non-ST-elevation myocardial infarction. Int J Cardiol. 2013;162(2):107–11. doi: 10.1016/j.ijcard.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 25.Aslanger E, Yıldırımtürk Ö, Şimşek B, Sungur A, Cabbar AT, Bozbeyoğlu E, et al. A new electrocardiographic pattern indicating inferior myocardial infarction. J Electrocardiol. 2020;61:41–6. doi: 10.1016/j.jelectrocard.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. ESC Scientific Document Group 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 27.Martí D, Mestre JL, Salido L, Esteban MJ, Casas E, Pey J, et al. Incidence, angiographic features and outcomes of patients presenting with subtle ST-elevation myocardial infarction. Am Heart J. 2014;168(6):884–90. doi: 10.1016/j.ahj.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Smith SW, Khalil A, Henry TD, Rosas M, Chang RJ, Heller K, et al. Electrocardiographic differentiation of early repolarization from subtle anterior ST-segment elevation myocardial infarction. Ann Emerg Med. 2012;60(1):45–56. doi: 10.1016/j.annemergmed.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Driver BE, Khalil A, Henry T, Kazmi F, Adil A, Smith SW. A new 4-variable formula to differentiate normal variant ST segment elevation in V2-V4 (early repolarization) from subtle left anterior descending coronary occlusion-Adding QRS amplitude of V2 improves the model. J Electrocardiol. 2017;50(5):561–9. doi: 10.1016/j.jelectrocard.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Bozbeyoğlu E, Aslanger E, Yıldırımtürk Ö, Şimşek B, Karabay CY, Şimşek MA, et al. A tale of two formulas: Differentiation of subtle anterior MI from benign ST segment elevation. Ann Noninvasive Electrocardiol. 2018;23(6):e12568. doi: 10.1111/anec.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee DH, Walsh B, Smith SW. Terminal QRS distortion is present in anterior myocardial infarction but absent in early repolarization. Am J Emerg Med. 2016;34(11):2182–5. doi: 10.1016/j.ajem.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 32.Klein LR, Shroff GR, Beeman W, Smith SW. Electrocardiographic criteria to differentiate acute anterior ST-elevation myocardial infarction from left ventricular aneurysm. Am J Emerg Med. 2015;33(6):786–90. doi: 10.1016/j.ajem.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 33.Smith SW. T/QRS ratio best distinguishes ventricular aneurysm from anterior myocardial infarction. Am J Emerg Med. 2005;23(3):279–87. doi: 10.1016/j.ajem.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Trialists FT. Indications for fibronolytic therapy in suspected acute myocardial infarction: Collaborative trials of more than 1000 patients. Lancet. 1994;343(8893):311–22. [PubMed] [Google Scholar]
- 35.ISAM Study Group. A prospective trial of intravenous streptokinase in acute myocardial infarction (ISAM) N Engl J Med. 1986;314(23):1465–71. doi: 10.1056/NEJM198606053142301. [DOI] [PubMed] [Google Scholar]
- 36.AIMS Trial Study Group. Effect of intravenous APSAC on mortality after acute myocardial infarction: preliminary report of a placebo-controlled clinical trial. Lancet. 1988;331(8585):545–9. [PubMed] [Google Scholar]
- 37.ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. J Am Coll Cardiol. 1988;12(6SA):A3–A13. doi: 10.1016/0735-1097(88)92635-6. [DOI] [PubMed] [Google Scholar]
- 38.LATE Study Group. Late Assessment of Thrombolytic Efficacy (LATE) study with alteplase 6-24 hours after onset of acute myocardial infarction. Lancet. 1993;342(8874):759–66. [PubMed] [Google Scholar]
- 39.Della DI, Miocardico SN. Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet. 1986;1(8478):397–402. [PubMed] [Google Scholar]
- 40.Hunt D, Varigos J, Dienstl F, Lechleitner P, De Backer G, KORNITZER M, et al. ISIS-3: a randomised comparison of streptokinase vs tissue plasminogen activator vs antistreplase and of aspirin plus heparin vs aspirin along among 41 299 cases of suspected acute myocardial infarction. Lancet. 1992;339(8796):753–70. [PubMed] [Google Scholar]
- 41.Paolasso E, Ravizzini G. Randomized trial of late thrombolysis in patients with suspected acute myocardial infarction. Lancet. 1993;342(8874):767–72. [PubMed] [Google Scholar]
- 42.Rossi P, Bolognese L. Comparison of intravenous urokinase plus heparin versus heparin alone in acute myocardial infarction. Am J Cardiol. 1991;68(6):585–92. doi: 10.1016/0002-9149(91)90348-o. [DOI] [PubMed] [Google Scholar]
- 43.Wilcox R, Olsson C, Skene A, Von Der Lippe G, Jensen G, Hampton J, et al. Trial of tissue plasminogen activator for mortality reduction in acute myocardial infarction: Anglo-Scandinavian Study of Early Thrombolysis (ASSET) Lancet. 1988;332(8610):525–30. doi: 10.1016/s0140-6736(88)92656-6. [DOI] [PubMed] [Google Scholar]
- 44.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 45.Lupu L, Taha L, Banai A, Shmueli H, Borohovitz A, Matetzky S, et al. Immediate and early percutaneous coronary intervention in very high‐risk and high‐risk non‐ST segment elevation myocardial infarction patients. Clin Cardiol. 2022;45(4):359–69. doi: 10.1002/clc.23781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kontos MC, Kurz MC, Roberts CS, Joyner SE, Kreisa L, Ornato JP, et al. An evaluation of the accuracy of emergency physician activation of the cardiac catheterization laboratory for patients with suspected ST-segment elevation myocardial infarction. Ann Emerg Med. 2010;55(5):423–30. doi: 10.1016/j.annemergmed.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 47.McCabe JM, Armstrong EJ, Kulkarni A, Hoffmayer KS, Bhave PD, Garg S, et al. Prevalence and factors associated with false-positive ST-segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention–capable centers: a report from the Activate-SF registry. Arch Intern Med. 2012;172(11):864–71. doi: 10.1001/archinternmed.2012.945. [DOI] [PubMed] [Google Scholar]
- 48.Meyers HP, Bracey A, Lee D, Lichtenheld A, Li WJ, Singer DD, et al. Accuracy of OMI ECG findings versus STEMI criteria for diagnosis of acute coronary occlusion myocardial infarction. Int J Cardiol Heart Vasc. 2021;33:100767. doi: 10.1016/j.ijcha.2021.100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aslanger EK, Meyers HP, Smith SW. Time for a new paradigm shift in myocardial infarction. Anatol J Cardiol. 2021;25(3) doi: 10.5152/AnatolJCardiol.2021.89304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phibbs B, Nelson W. Differential classification of acute myocardial infarction into ST‐and non‐ST segment elevation is not valid or rational. Ann Noninvasive Electrocardiol. 2010;15(3):191–9. doi: 10.1111/j.1542-474X.2010.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tziakas D, Chalikias G, Al-Lamee R, Kaski JC. Total coronary occlusion in non ST elevation myocardial infarction: Time to change our practice? Int J Cardiol. 2021;329:1–8. doi: 10.1016/j.ijcard.2020.12.082. [DOI] [PubMed] [Google Scholar]
- 52.Widimský P, Rokyta R, Št J, Bělohlávek J, Červinka P, Kala P, et al. Acute coronary syndromes with ongoing myocardial ischemia (ACS with OMI) versus acute coronary syndromes without ongoing ischemia (ACS without OMI): The new classification of acute coronary syndromes should replace old classification based on ST segment elevation presence or absence—Expert consensus statement of the Czech Society of Cardiology. Cor Vasa. 2013;55(3):e225–e7. [Google Scholar]
- 53.Meyers HP, Weingart SD, Smith SW. The OMI Manifesto Dr Smith’s ECG Blog. [[cited 22 August 2022]]. Available from: http://hqmeded-ecgblogspotcom/2018/04/the-omi-manifestohtml [Internet]
- 54.Aslanger EK, Yıldırımtürk Ö, Şimşek B, Bozbeyoğlu E, Şimşek MA, Karabay CY, et al. DIagnostic accuracy oF electrocardiogram for acute coronary OCClUsion resuLTing in myocardial infarction (DIFOCCULT Study) Int J Cardiol Heart Vasc. 2020;30:100603. doi: 10.1016/j.ijcha.2020.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wereski R, Chapman AR, Lee KK, Smith SW, Lowe DJ, Gray A, et al. High-sensitivity cardiac troponin concentrations at presentation in patients with ST-segment elevation myocardial infarction. JAMA Cardiol. 2020;5(11):1302–4. doi: 10.1001/jamacardio.2020.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]