Abstract
Despite women being disproportionally affected by cancer deaths at young ages, there are no global estimates of the resulting maternal orphans, who experience health and education disadvantages throughout their lives. We estimated the number of children who became maternal orphans in 2020 due to their mother dying from cancer in that year, for 185 countries worldwide and by cause of cancer-related death. Female cancer deaths—by country, cancer type and age (derived from GLOBOCAN estimates)—were multiplied by each woman’s estimated number of children under the age of 18 years at the time of her death (fertility data were derived from United Nations World Population Prospects for birth cohort), accounting for child mortality and parity-cancer risk associations. Globally, there were 1,047,000 such orphans. Over half of these were orphans due to maternal deaths from breast (258,000, 25%), cervix (210,000, 20%) and upper-gastrointestinal cancers (136,000, 13%), and most occurred in Asia (48%: India 15%, China 10%, rest of Asia 23%) and Africa (35%). Globally, there were 40 new maternal orphans due to cancer per 100,000 children, with a declining trend with a higher Human Development Index (range: 121 in Malawi to 15 in Malta). An estimated 7 million children were prevalent maternal orphans due to cancer in mid-2020. Accelerating the implementation of the World Health Organization’s cervical and breast cancer initiatives has the potential to avert not only millions of preventable female cancer deaths but also the associated, often-overlooked, intergenerational consequences of these deaths.
Subject terms: Cancer, Social sciences
According to 2020 estimates from 185 countries, Asia and Africa have the most maternal orphans due to cancer, with breast and cervical cancers responsible for almost half of maternal cancers.
Main
Cancer is the second-leading cause of death worldwide, causing 5.5 million deaths in men and 4.4 million in women in 20201. This mortality burden has an immense impact on patients, their families and health systems and societies, leading to over 170 million years of life lost and often catastrophic economic costs2,3. Less often considered is the extent and impact of parental deaths from cancer on their children. During the follow-up of a sub-Saharan African breast cancer cohort, we found that the number of children still under the age of 18 years at the time of their mothers’ death exceeded the number of breast cancer deaths, thus unveiling a common occurrence of orphans due to cancer in this setting4. As per the United Nations International Children’s Emergency Fund (UNICEF) definition, while these children are under the age of 18 years, they are maternal orphans, whereas children who have lost a father or both parents are referred to as paternal and double orphans respectively5.
The consequences of orphanhood from any cause of parental death can have a long-term impact on a child’s life in multiple domains, many of which are family, context and setting specific6. Maternal orphans have higher rates of mortality in childhood than their peers, both in low- and high-income settings7,8. As they grow up, orphaned children are at an increased risk of mental health disorders and suicide, as well as experiencing sexual violence9–11. Orphanhood is also associated with raised risks of teenage pregnancy, infectious diseases, including HIV/AIDS during adolescence and chronic diseases later in life12. In some settings, orphans are more likely to leave school prematurely and become entangled in a cycle of poverty13.
Overall, 71% of cancer deaths occur at the age of 60 years or older14, by which time most children of deceased adults are already aged 18 years or older; that is, at the time of death, most women last gave birth over 18 years ago. However, cancer deaths at younger ages can result in orphans. At these younger ages, both globally and in lower-income countries (LICs) in particular, women are disproportionately affected by cancer deaths compared to men14. This is primarily due to deaths from common cancers that predominantly or exclusively affect women, namely breast and cervix15. Additionally, cancer deaths in LICs are more likely to result in maternal orphans because LICs are characterized by younger populations and, hence, lower average age of cancer diagnosis16, as well as higher fertility and later maternal age at last birth17. Thus in LICs, if a women dies premanturely from cancer, her children are more likely to still be minors than in HICs.
In the present study, we aimed to fill the information gap on maternal orphans due to cancer. We focus on maternal orphans given the disproportionate burden of cancer deaths at young ages in women, the availability of high-quality fertility estimates and the central role of a mother in a child’s development, caregiving and education. Our aims were to provide country, regional and global estimates, for 2020, of the following: (i) the number of new maternal orphans due to cancer, for all cancers combined and by cancer site; (ii) risk of a child becoming a maternal orphan due to cancer; (iii) the number of new maternal orphans per 100 female cancer deaths; (iv) the number of prevalent (existing) maternal orphans due to cancer, by country; and (v) the age-distribution of new and prevalent maternal orphans due to cancer. We also examined variations of these estimates by a country’s place in the Human Development Index (HDI).
Results
Absolute burden of new maternal orphans due to cancer
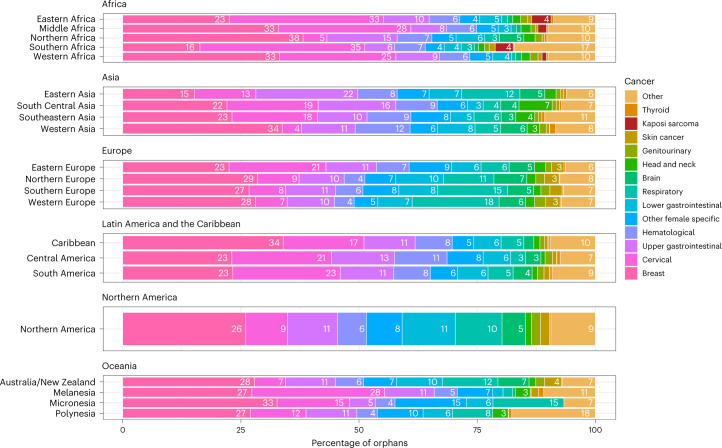
We estimated that the 4,404,000 cancer deaths in women in 2020 resulted in 1,047,000 new maternal orphans globally. Almost half (48%, 508,000) of these children were in Asia and over one-third in Africa (35%, 370,000), while Europe (60,000 orphans), Latin America and the Caribbean (76,000), Northern America (28,000) and Oceania (6,400) together comprised the remaining 16% (Table 1). The predominance of these orphans in Asia and Africa was driven by six countries, which comprised two-fifths of the worldwide total—India (157,000), China (107,000), Nigeria (53,000), Indonesia (42,000), Ethiopia (39,000) and Pakistan (38,000) (Supplementary Table 1). In terms of contributing cancer sites, deaths from breast cancer were the single largest cause of new maternal orphans globally (25%), followed by cervix (20%) and upper gastrointestinal (GI) cancers (13%, of which the majority were gastric or esophageal) (Table 2). The ranks of these top three cancers varied between regions and HDI categories, although breast cancer deaths always occupied first or second place throughout (Fig. 1 and Extended Data Table 1). In Eastern and Southern Africa, cervical cancer deaths led to more maternal orphans than breast cancer deaths. These two regions were also the only two where female deaths from Kaposi sarcoma contributed substantially (4% in each). In Eastern Asia, upper GI cancer deaths led to more maternal orphans than breast and cervical cancer deaths. In Europe, New Zealand and Australia, respiratory cancer deaths (majority lung) were the second-leading cause of maternal orphans (11–18%) after breast cancer, with the exception of Eastern Europe where cervical cancer deaths ranked second (21%). Lower GI deaths (dominated by colorectal cancer) contributed to 5% of maternal orphans globally but represented at least 10% in Australia, New Zealand, North America and Northern Europe.
Table 1.
Global and regional distribution of new and prevalent maternal orphans due to cancer in 2020 and the ages of these orphans at the time of their mothers’ death
| Cancer deaths in women in 2020 (all ages) | New maternal orphans due to cancer in 2020 | Prevalent maternal orphans due to cancer in 2020 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Average age at cancer death (years)a | No. | World’s total (%) | No. per 100 cancer deaths in women | No. per 100,000 children | Age distribution (years, row %) | No. | World’s total (%) | No. per 100,000 children | Age distribution (years, row %) | |||||
| <5 | 5–9 | 10–17 | <5 | 5–9 | 10–17 | ||||||||||
| World | 4,404,419 | 56 | 1,047,178 | 100 | 24 | 40 | 11 | 21 | 68 | 7,048,905 | 100 | 272 | 4 | 16 | 79 |
| By region | |||||||||||||||
| Africa | 387,313 | 369,547 | 35 | 95 | 54 | 2,558,332 | 36 | 377 | |||||||
| Eastern Africa | 134,037 | 51 | 154,332 | 15 | 115 | 66 | 13 | 24 | 63 | 1,082,846 | 15 | 460 | 5 | 18 | 77 |
| Middle Africa | 39,756 | 50 | 51,205 | 5 | 129 | 51 | 14 | 24 | 61 | 351,725 | 5 | 351 | 5 | 19 | 76 |
| Northern Africa | 87,046 | 54 | 39,688 | 4 | 46 | 39 | 13 | 24 | 63 | 264,460 | 4 | 262 | 5 | 19 | 76 |
| Southern Africa | 32,081 | 52 | 15,633 | 1 | 49 | 61 | 13 | 24 | 62 | 112,140 | 2 | 437 | 5 | 18 | 77 |
| Western Africa | 94,393 | 52 | 108,689 | 10 | 115 | 50 | 14 | 24 | 62 | 747,160 | 11 | 345 | 5 | 19 | 76 |
| Asia | 2,436,533 | 507,726 | 48 | 21 | 35 | 3,373,923 | 48 | 234 | |||||||
| Eastern Asia | 1,419,391 | 58 | 119,893 | 11 | 8 | 32 | 9 | 18 | 73 | 731,981 | 10 | 194 | 4 | 15 | 81 |
| South Central Asia | 598,324 | 54 | 247,322 | 24 | 41 | 33 | 10 | 19 | 70 | 1,691,687 | 24 | 229 | 4 | 15 | 81 |
| Southeastern Asia | 315,298 | 55 | 99,548 | 10 | 32 | 45 | 10 | 20 | 70 | 671,395 | 10 | 301 | 4 | 15 | 81 |
| Western Asia | 103,520 | 55 | 40,962 | 4 | 40 | 40 | 11 | 22 | 67 | 278,859 | 4 | 276 | 4 | 17 | 79 |
| Europe | 870,658 | 59,801 | 6 | 7 | 38 | 370,856 | 5.3 | 235 | |||||||
| Eastern Europe | 313,825 | 59 | 24,118 | 2 | 8 | 38 | 12 | 23 | 65 | 149,308 | 2.1 | 234 | 5 | 19 | 76 |
| Northern Europe | 128,775 | 60 | 8,065 | 1 | 6 | 33 | 9 | 20 | 71 | 50,823 | 0.7 | 209 | 4 | 16 | 80 |
| Southern Europe | 179,004 | 59 | 12,140 | 1 | 7 | 42 | 8 | 19 | 73 | 76,346 | 1.1 | 264 | 3 | 15 | 82 |
| Western Europe | 249,054 | 60 | 15,478 | 1 | 6 | 38 | 9 | 18 | 73 | 94,380 | 1.3 | 229 | 4 | 15 | 82 |
| Latin America and the Caribbean | 347,421 | 76,094 | 7 | 22 | 36 | 528,854 | 7.5 | 252 | |||||||
| Caribbean | 28,806 | 56 | 5,416 | 1 | 19 | 40 | 10 | 20 | 70 | 37,256 | 0.5 | 274 | 4 | 15 | 81 |
| Central America | 64,527 | 54 | 19,710 | 2 | 31 | 31 | 11 | 20 | 69 | 138,247 | 2.0 | 214 | 4 | 16 | 80 |
| South America | 254,088 | 56 | 50,969 | 5 | 20 | 39 | 11 | 20 | 69 | 353,351 | 5.0 | 269 | 4 | 16 | 81 |
| Northern America | 331,325 | 59 | 27,553 | 3 | 8 | 31 | 9 | 18 | 73 | 173,072 | 2.5 | 192 | 4 | 14 | 82 |
| Oceania | 31,169 | 6,457 | 1 | 21 | 50 | 43,867 | 0.6 | 340 | |||||||
| Australia and New Zealand | 25,998 | 59 | 2,394 | 0 | 9 | 31 | 10 | 20 | 70 | 14,977 | 0.2 | 195 | 4 | 16 | 80 |
| Melanesia and Micronesia | 4,867 | 54 | 3,950 | 0 | 81 | 78 | 13 | 23 | 64 | 28,157 | 0.4 | 559 | 5 | 17 | 78 |
| Polynesia | 304 | 55 | 113 | 0 | 37 | 64 | 8 | 18 | 74 | 733 | 0 | 414 | 3 | 14 | 83 |
| By HDI categoriesb | |||||||||||||||
| Very high HDI countries | 1,549,055 | 59 | 129,026 | 12 | 8 | 35 | 10 | 20 | 70 | 822,198 | 12 | 226 | 4 | 16 | 80 |
| High HDI countries | 1,922,069 | 57 | 308,072 | 29 | 16 | 36 | 10 | 20 | 70 | 2,004,701 | 28 | 236 | 4 | 16 | 80 |
| Medium HDI countries | 666,966 | 54 | 319,241 | 30 | 48 | 37 | 11 | 20 | 69 | 2,193,349 | 31 | 257 | 4 | 15 | 81 |
| Low HDI countries | 238,244 | 51 | 280,310 | 27 | 118 | 55 | 14 | 24 | 62 | 1,952,392 | 28 | 384 | 5 | 19 | 76 |
aAmong cancer deaths at ages 15–69 years. The children of women above these ages were adults in 2020. bTen of 185 countries/territories are not included as they do not have an HDI value. They represent 10,000 new maternal orphans due to cancer in 2020.
Table 2.
New and prevalent maternal orphans in 2020 due to cancer deaths by 14 major cancers and the rest of the cancers (by total number, number per 100 cancer deaths in women, number per 100,000 children and the ages of orphans)
| Cancer deaths in women in 2020 (all ages) | New maternal orphans due to cancer in 2020 | Prevalent maternal orphans due to cancer in 2020 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Average age at cancer death (years)a | No. | Percent of total | No. per 100 cancer deaths in women | No. per 100,000 children | Age distribution (years, row %) | No. | Percent of total | No. per 100,000 children | Age distribution (years, row %) | |||||
| <5 | 5–9 | 10–17 | <5 | 5–9 | 10–17 | ||||||||||
| All cancer deaths | 4,404,419 | 56 | 1,047,178 | 100 | 24 | 40 | 11 | 21 | 68 | 7,048,905 | 100 | 272 | 4 | 16 | 79 |
| By cancer-related death | |||||||||||||||
| Breast | 682,288 | 55 | 257,561 | 24.6 | 38 | 9.9 | 11 | 22 | 67 | 1,724,459 | 24.5 | 66.6 | 4.2 | 16.4 | 79.4 |
| Cervical | 340,841 | 53 | 209,857 | 20.0 | 62 | 8.1 | 11 | 21 | 68 | 1,392,226 | 19.8 | 53.7 | 4.1 | 16.1 | 79.8 |
| Upper gastrointestinal | 955,243 | 58 | 135,962 | 13.0 | 14 | 5.2 | 9 | 19 | 72 | 841,825 | 11.9 | 32.5 | 3.7 | 14.8 | 81.5 |
| Hematological | 305,860 | 53 | 81,879 | 7.8 | 27 | 3.2 | 16 | 24 | 59 | 657,219 | 9.3 | 25.4 | 5.7 | 19.2 | 75.1 |
| Other female-specific | 328,930 | 57 | 63,054 | 6.0 | 19 | 2.4 | 9 | 19 | 72 | 395,339 | 5.6 | 15.3 | 3.8 | 15.1 | 81.1 |
| Lower gastrointestinal | 415,944 | 58 | 53,172 | 5.1 | 13 | 2.1 | 11 | 21 | 69 | 347,937 | 4.9 | 13.4 | 4.1 | 16 | 79.8 |
| Respiratory | 610,696 | 60 | 50,580 | 4.8 | 8 | 2.0 | 7 | 16 | 77 | 280,060 | 4.0 | 10.8 | 3.1 | 13.1 | 83.9 |
| Brain and nervous system | 112,711 | 53 | 34,704 | 3.3 | 31 | 1.3 | 15 | 23 | 62 | 265,564 | 3.8 | 10.3 | 5.3 | 18.3 | 76.4 |
| Head and Neck | 112,849 | 56 | 34,643 | 3.3 | 31 | 1.3 | 11 | 20 | 69 | 232,844 | 3.3 | 9.0 | 4.1 | 15.6 | 80.3 |
| Genitourinary | 116,709 | 59 | 12,750 | 1.2 | 11 | 0.5 | 11 | 20 | 69 | 82,075 | 1.2 | 3.2 | 4.2 | 16.1 | 79.7 |
| Skin cancer | 50,598 | 56 | 9,355 | 0.9 | 18 | 0.4 | 11 | 21 | 69 | 78,360 | 1.1 | 3.0 | 7.1 | 22.7 | 70.2 |
| Kaposi Sarcoma | 5,149 | 37 | 9,009 | 0.9 | 175 | 0.3 | 22 | 30 | 48 | 62,205 | 0.9 | 2.4 | 4.1 | 16 | 79.8 |
| Thyroid | 27,657 | 57 | 5,171 | 0.5 | 19 | 0.2 | 12 | 21 | 67 | 35,859 | 0.5 | 1.4 | 4.6 | 16.9 | 78.4 |
| Other | 338,944 | 54 | 89,482 | 8.5 | 26.4 | 3.5 | 14 | 23 | 63 | 652,934 | 9.3 | 25.2 | 5.2 | 18.1 | 76.7 |
aAmong cancer deaths at ages 15–69 years. The children of women above these ages were adults in 2020.
Fig. 1. Percentage distribution of site-specific cancer deaths giving rise to new maternal orphans due to cancer in 2020, by region around the globe.
Cancer codes (ICD-10) included in each of the 14 cancer groups are listed in Extended Data Table 3.
Extended Data Table 1.
New maternal orphans in 2020 (number, number per 100 cancer deaths in women, number per 100,000 children) for all cancers and for 14 major cancers by level of HDI
| Low HDI countries | Medium HDI countries | High HDI countries | Very High HDI countries | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. per 100 cancer deaths in women | No. per 100,000 children | No. | % | No. per 100 cancer deaths in women | No. per 100,000 children | No. | % | No. per 100 cancer deaths in women | No. per 100,000 children | No. | % | No. per 100 cancer deaths in women | No. per 100,000 children | |
| New maternal orphans due to all cancer deaths | 280,310 | 27 | 118 | 55 | 319,241 | 30 | 48 | 37 | 308,072 | 29 | 16 | 36 | 129,026 | 12 | 8 | 35 |
| New maternal orphans by type of cancer-related death -t | ||||||||||||||||
| Breast | 79,919 | 28.5 | 147 | 15.7 | 73,593 | 23.1 | 53 | 8.6 | 65,847 | 21.4 | 26 | 7.8 | 34,866 | 27.0 | 15 | 9.6 |
| Cervical | 77,560 | 27.7 | 143 | 15.3 | 67,326 | 21.1 | 61 | 7.9 | 47,857 | 15.5 | 37 | 5.6 | 14,852 | 11.5 | 34 | 4.1 |
| Upper Gastrointestinal | 26,877 | 9.6 | 85 | 5.3 | 47,300 | 14.8 | 40 | 5.5 | 46,308 | 15.0 | 9 | 5.5 | 14,383 | 11.1 | 5 | 4.0 |
| Hematological | 18,114 | 6.5 | 106 | 3.6 | 27,116 | 8.5 | 57 | 3.2 | 27,307 | 8.9 | 24 | 3.2 | 8,627 | 6.7 | 7 | 2.4 |
| Other female specific | 12,924 | 4.6 | 84 | 2.5 | 18,872 | 5.9 | 31 | 2.2 | 21,180 | 6.9 | 18 | 2.5 | 9,442 | 7.3 | 7 | 2.6 |
| Lower Gastrointestinal | 11,632 | 4.1 | 94 | 2.3 | 11,150 | 3.5 | 43 | 1.3 | 18,490 | 6.0 | 10 | 2.2 | 11,246 | 8.7 | 6 | 3.1 |
| Respiratory | 3,397 | 1.2 | 63 | 0.7 | 10,687 | 3.3 | 32 | 1.3 | 23,671 | 7.7 | 8 | 2.8 | 12,364 | 9.6 | 5 | 3.4 |
| Brain and nervous system | 3,770 | 1.3 | 111 | 0.7 | 10,203 | 3.2 | 64 | 1.2 | 14,203 | 4.6 | 27 | 1.7 | 6,364 | 4.9 | 16 | 1.8 |
| Head and Neck | 5,596 | 2.0 | 94 | 1.1 | 18,695 | 5.9 | 42 | 2.2 | 7,782 | 2.5 | 21 | 0.9 | 2,381 | 1.8 | 10 | 0.7 |
| Genitourinary | 4,430 | 1.6 | 72 | 0.9 | 2,825 | 0.9 | 28 | 0.3 | 3,172 | 1.0 | 8 | 0.4 | 2,172 | 1.7 | 4 | 0.6 |
| Skin cancer | 2,352 | 0.8 | 62 | 0.5 | 2,025 | 0.6 | 37 | 0.2 | 2,334 | 0.8 | 14 | 0.3 | 2,546 | 2.0 | 10 | 0.7 |
| Kaposi Sarcoma | 5,863 | 2.1 | 204 | 1.2 | 2,547 | 0.8 | 182 | 0.3 | 531 | 0.2 | 85 | 0.1 | 22 | 0.0 | 10 | 0.0 |
| Thyroid | 1,378 | 0.5 | 66 | 0.3 | 1,335 | 0.4 | 31 | 0.2 | 1,850 | 0.6 | 14 | 0.2 | 503 | 0.4 | 6 | 0.1 |
| Other | 26,496 | 9.5 | 114 | 5.2 | 25,570 | 8.0 | 49 | 3.0 | 27,540 | 8.9 | 21 | 3.2 | 9,258 | 7.2 | 7 | 2.5 |
HDI: Human Development Index; No: Number of new maternal orphans due to cancer in 2020. 10 of 185 countries/territories are not included as they do not have a HDI value. They represent 10,000 new maternal orphans due to cancer in 2020.
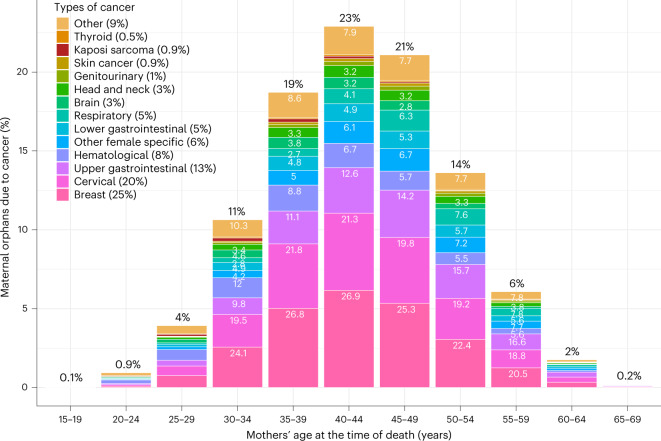
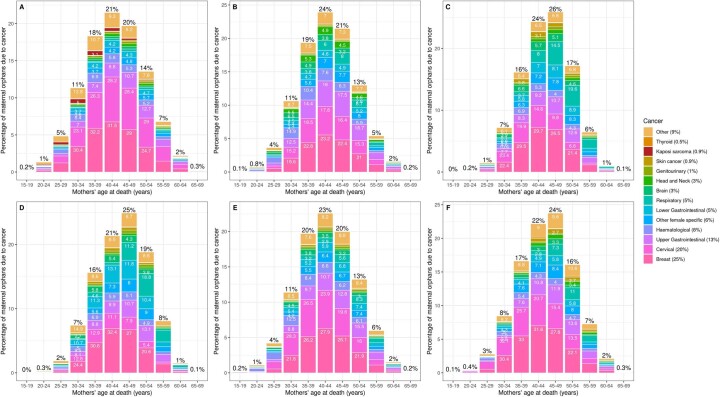
In total, 63% of new maternal orphans arose from female cancer deaths at ages 35–49 years, with a mode at ages 40–44 years (Fig. 2), while 22% of orphaned children lost their mother when she was 50+ years and 16% under the age of 35 years. The contributing cancers to maternal orphans also shifted across these maternal age-at-death groups in each region, for example, with respiratory cancer deaths contributing to more maternal orphans for maternal deaths at older ages (Fig. 2 and Extended Data Fig. 1).
Fig. 2. Percent distribution of maternal orphans due to cancer (worldwide) by mother’s age at the time of death and by type of cancer death within each category.
Cancer codes (ICD-10) included in each of the 14 cancer groups are listed in Extended Data Table 3. Percentages on top of bars are % of maternal orphans due to cancer in each age group. Percentages within bars are % of maternal orphans arising from each cause of cancer-related death within each maternal age at death group.
Extended Data Fig. 1. Distribution of maternal age at death when new maternal cancer orphans occurred, and of the type of cancer-related death within each age-at-death band.
Data for six world regions: (a) Africa, (b) Asia, (c) Europe, (d) Northern America, (e) Latin American and the Caribbean and (f) Oceania.
Risks of maternal orphanhood
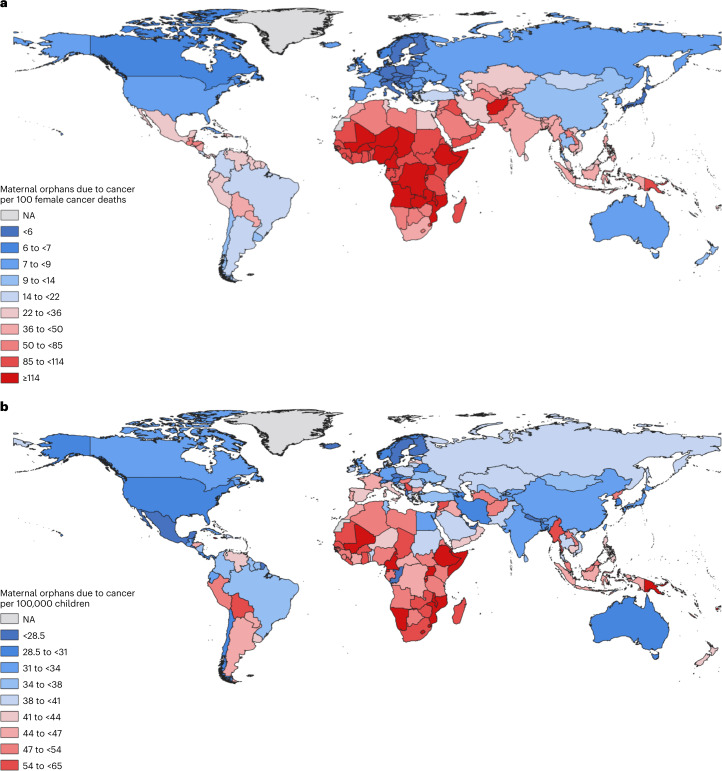
Apart from the total population size, the following two factors strongly influence a country’s total number of maternal orphans due to cancer. They are the average number of children under the age of 18 years per 100 female cancer deaths, largely reflecting past fertility (Fig. 3a), and the risk of cancer death among women at ages when most children remain under 18 years of age (i.e. deaths of women at ages 15–54 years which give rise to 92% of maternal orphans, Extended Data Fig. 2). The latter is a strong determinant of the number of maternal orphans due to cancer per 100,000 children, that is, a child’s risk of becoming a maternal cancer orphan independent of fertility rates (Fig. 3b).
Fig. 3. World map of new maternal orphans due to cancer in 2020.
a, Number of new maternal orphans due to cancer per 100 cancer deaths in women in 2020. b, Number of new maternal orphans due to cancer per 100,000 children in 2020. NA: Not available.
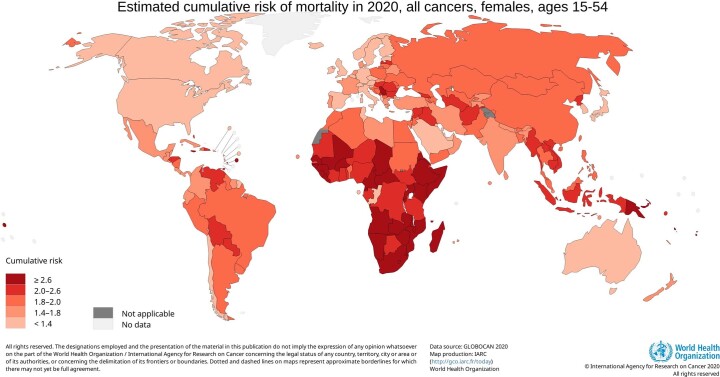
Extended Data Fig. 2.
Cumulative risk (%) of death from cancer in women at ages 15–54 years (ages when 92% of maternal orphans due to cancer arise), 2020.
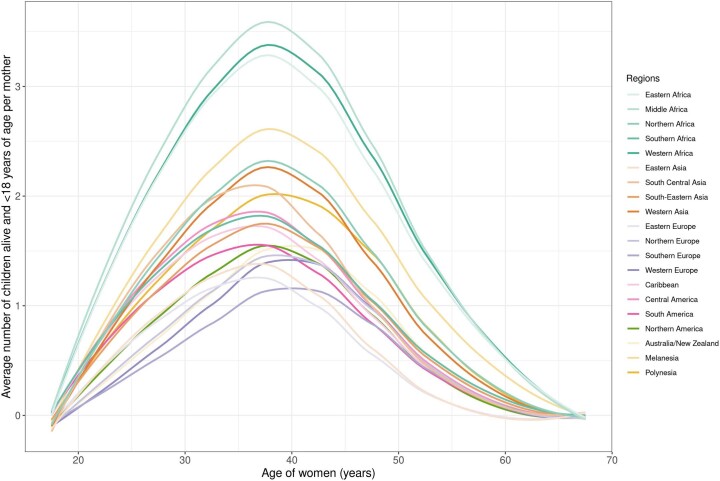
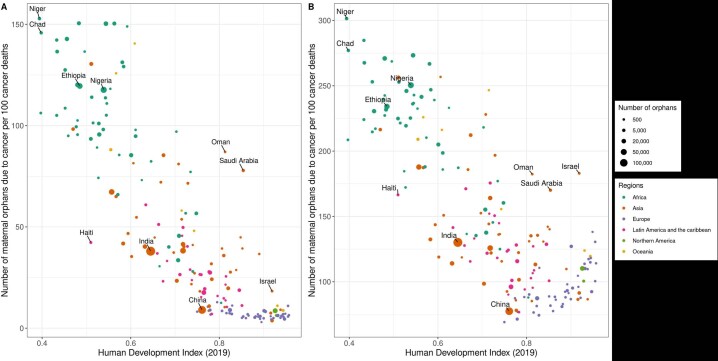
The number of new maternal orphans per 100 female cancer deaths was the highest in Africa and other low-/middle-income countries (LMICs) (Fig. 3a) due to their high fertility rates (Extended Data Fig. 3). This was particularly the case in Middle, Western and Eastern Africa (average 3−3.3 children <18 years per woman aged 40–44 years), thus the ratio of new maternal orphans per 100 female cancer deaths was over 110 in these countries. Conversely, the ratio of new maternal orphans per 100 female cancer deaths was lower than ten in regions of low fertility - and often higher HDI - such as Eastern Asia (including Japan and China), Europe and Northern America (Fig. 3a). Although these differentials were partly influenced by the younger demographic of Africa and other LMICs, a strong inverse relationship between new maternal orphans per 100 female cancer deaths and a country’s HDI remained when restricted to deaths under age 50 (Extended Data Fig. 4).
Extended Data Fig. 3.
Mean number of living children < 18 years according to women’s age in 2020, by world region.
Extended Data Fig. 4. New maternal orphans due to cancer per 100 female cancer deaths by a country’s human development index for (A) cancer deaths at any age and (B) restricted to those occuring under age 50 years.
10 of 185 countries/territories are not included as they do not have a HDI value. They represent 10,000 new maternal orphans due to cancer in 2020.
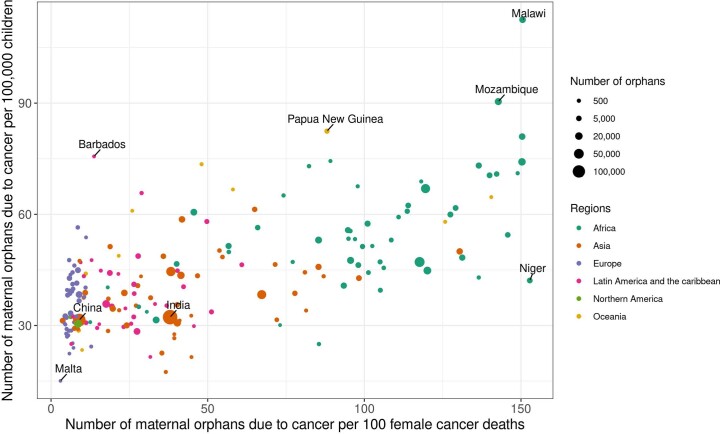
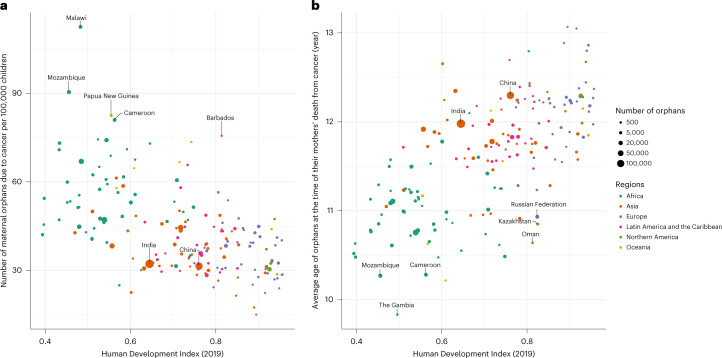
The 1,047,000 new maternal orphans globally translated to a global average of 40 orphans per 100,000 children. At a regional level, this number ranged from 15 in Malta to 113 in Malawi (Fig. 3b and Extended Data Fig. 5), but in 110 of 185 countries (59%) this number lay close to the global average, that is, between 30 and 50 orphans per 100,000 children (Extended Data Fig. 5). There was a general tendency for greater numbers of newly orphaned children per 100,000 children in countries with lower HDI (Fig. 4a), driven, for the most part, by the risk of cancer death in women at ages 15–54 years, which was highest in parts of Africa, followed by some Asian and South American countries (Extended Data Fig. 2). Indeed, of the 10% of countries with a ratio over 65 new maternal orphans per 100,000 children, 14 were in Africa (in descending order: Malawi, Mozambique, Cameroon, Djibouti, Uganda, Mali, Namibia, Equatorial Guinea, Burundi, Somalia, Comoros, Eswatini, Ethiopia and Lesotho), two in the Caribbean (Barbados and Jamaica) and three in Oceania (Papua New Guinea, Fiji and Samoa; Supplementary Table 1). In most of these listed African countries, as well as in Papua New Guinea and Fiji, cervical cancer mortality rates at ages 15–54 years were between two and eight times higher than the global average. Each of these countries also had high fertility rates. In contrast, in Jamaica (612 new maternal orphans in 2020) and Barbados (51 new maternal orphans), fertility rates and thus maternal orphans per 100 cancer deaths were not particularly high, but breast cancer mortality rates at ages 15–54 years were three to four times the global average. Outside of this top decile, other countries that had a high number of orphans per 100,000 children relative to other countries in their region were Serbia (56 new maternal orphans per 100,000 children) and Montenegro (54) in Europe—where female cancer deaths at ages 15–54 were dominated by breast, cervix and lung—Syria (61) and Myanmar (59) in Asia and Bolivia (58) and Peru (49) in South America. Conversely, there were several countries, such as Nepal, Niger and Bangladesh, where the number of new maternal orphans per 100 female cancer deaths was high, but the absolute risk of becoming a maternal orphan per 100,000 children was relatively low or moderate, due to the low/moderate cancer mortality rates in women. Finally, India and China make interesting contrasts. Both had similar numbers of new maternal orphans per 100,000 children (32), resulting from similar rates of female cancer mortality at ages 15–54 years, but India had 50% more orphans (157,0000) than China (107,000) due to its higher fertility rate and much younger population structure.
Extended Data Fig. 5.
Number of new maternal orphans due to cancer per 100,000 children vs per 100 female cancer deaths for each country.
Fig. 4. Number of new maternal orphans due to cancer per 100,000 children and the mean age at orphaning versus a country’s human development index (HDI).
a, Number of new maternal orphans due to cancer per 100,000 children, in 2020, plotted against a country’s human development index. b, Average age at orphaning at their mother’s death, in 2020, plotted against a country’s HDI. Ten of 185 countries/territories are not included as they do not have an HDI value. They represent 10,000 new maternal orphans due to cancer in 2020.
Age of children at orphaning
Most newly orphaned children were aged 10 years or over at the time of cancer bereavement (69%), 21% were aged 5–9 years and 11% were aged under 5 years (Table 1). The mean age of new maternal orphans for most countries (166 of 185 countries, 90%) varied between 10.5 and 12.5 years, with a clear positive relationship of older age at orphaning in countries with higher HDI (Fig. 4b and Table 1). Note that these differences in age at orphaning by HDI were relatively small compared to international differences in age at first/last birth because many of the children born to younger mothers will be adults before a potential maternal cancer death (for example, a child born to a 20-year-old mother is an adult when the mother is 38 years and age of cancer diagnosis and thus potential cancer death is most often over age 38 years). These differences imply that, although 35% of all maternal cancer orphans occurred in Africa, this percentage was higher (43%) among the subset of these children who were under the age of 5 years at orphaning.
Prevalent maternal orphans due to cancer
The number of prevalent maternal orphans due to cancer in mid-2020 was estimated to be 7,048,000. The geographic distribution (Table 1) was similar to that of new maternal orphans but prevalent orphans were older—approximately 4% were aged under 5 years, 16% were aged 5–9 years, and 79% were aged over 10 years (Table 1). Sensitivity analyses revealed that this estimate of 7,048,000 varied between 6,615,000 and 7,423,000 (Extended Data Table 2). The scenarios considered for the impact of maternal death on subsequent child survival only marginally altered the prevalence estimate (reducing the global prevalence by up to 90,000 (<1.5%)), whereas models with varying historic cancer mortality rates altered the estimates by up to ±375,000 (5.3%).
Extended Data Table 2.
Range of plausible estimates of global maternal orphan due to cancer point prevalence in mid-2020
| 2002–2019 GLOBOCAN cancer mortality rates (MR) in women as a function of 2020 rates | Mortality Rate Ratios in children associated with being a maternal orphan | |||||
|---|---|---|---|---|---|---|
| 1.25 | 1.5 | 2 | 2.6 | 1 | ||
| Scenario | a | b | c | d | e | |
| 1 | Stable MR=MR2020 | 7,048,905* | 7,030980 | 6,995,799 | 6,954,709 | 7,067,062 |
| 2 | Increasing MR=MR2020// 1.01^(2020-Year) | 6,701,135 | 6,684,748 | 6,652,573 | 6,614,974 | 6,717,728 |
| 3 | Decreasing MR=MR2020 x 1.01^(2020-Year) | 7,422,926 | 7,403,297 | 7,364,789 | 7,319,840 | 7,442,813 |
| 4** | Region-specific MR | 6,917,011 | 6,900,619 | 6,868,421 | 6,830,770 | 6,933,603 |
*Estimates from Scenario 1a are our best-estimates and thus are the central estimates provided in the text; **For scenario 4, we applied scenario 2 cancer mortality rates for low and middle HDI countries/territories and scenario 3 rates to high and very high HDI countries.
MR: Mortality rates.
Discussion
The global cancer burden can be evaluated from many different perspectives, which together help to inform cancer policy and resource allocation along the continuum of cancer prevention and care. To date, the intergenerational consequences of the cancer burden have been afforded scarce attention. We therefore provide global, regional and national estimates of maternal orphans due to cancer in this study. Our estimate of approximately 1 million new maternal orphans due to cancer in 2020 and a point prevalence of 7 million maternal orphans due to cancer implies that cancer deaths were responsible for approximately 15% of prevalent maternal orphans in 202018. The disaggregation of maternal orphans by cause of cancer-related death, country and HDI highlights further dimensions of global health inequities, as well as the preventability of many of the cancer deaths contributing to the vast number of maternal orphans due to cancer worldwide.
The two cancer-related deaths causing almost half of maternal cancer orphans are the female-specific cancers of breast and cervix. By no coincidence, these are the two cancers for which the World Health Organization (WHO) has launched targeted initiatives in the form of the Global Breast Cancer Initiative19 (in 2021) and the Cervical Cancer Elimination Initiative20 (in 2020). The scale of the maternal orphans resulting from these two cancers provides yet another incentive for investment into and accelerating scale-up of these programs. Action is especially needed in Asian, African and other countries where orphaning at young ages is more common. For cervical cancer, screening and treatment of cervical intra-epithelial neoplasia in adult women will greatly reduce cervical cancer mortality rates, whereas human papillomavirus (HPV) vaccination of young girls before the onset of sexual activity will protect the future generations of women from cancer and thus their children from orphanhood21. While this outlook is promising, the situation of averting breast cancer deaths is more complex. Incidence rates of breast cancer at postmenopausal ages are rising as fertility and lifestyle transitions continue22, and any subsequent deaths would lead to older maternal orphans. Reducing breast cancer-associated maternal orphans will require improvements in breast cancer survival in LMICs toward the high levels that have been achieved in high-income countries (HICs)23. The diagnosis of breast cancer at early stages coupled with timely quality treatment will be essential components herein, for which an immense investment into cancer diagnostics, pathology, surgery, chemotherapy and radiotherapy facilities, and professional training is needed. The third contributor to maternal cancer orphans globally is upper-GI cancers, mostly due to deaths in Asia and Africa. These include cancers such as those of esophagus, stomach and pancreas, for which survival has remained poor everywhere, even in HICs, and which have been granted little research funding or attention relative to their global mortality burden24,25.
From a social inequalities perspective, cancer often displays complex divergent patterns by cancer site and between incidence and mortality rates26, which are revealed once again in the multiple influences on maternal orphans. At the relevant ages (15–54 years), women living in countries with higher HDI have three to four times higher risks of cancer (all sites) than their counterparts in countries with lower HDI. Yet this relationship reverses when the risk of cancer death at the population-level is considered. Indeed, women in countries with lower HDI have a high risk of cancer deaths owing to lower patient survival rates. Thus, maternal orphans due to cancer were highest in low HDI countries, both in terms of orphans per 100,000 children and per 100 female cancer deaths. Our previous work in the African ABC-DO breast cancer cohort illustrated further within-country inequities in the risk of maternal orphans based on the real-life experience of over 2,200 women. Those with lower educational levels were diagnosed with breast cancer at later stages, were less likely to receive treatment and thus had lower survival rates than women with higher educational levels27–29. They also had more children, who thus became maternal orphans4. In that study, similar to the global estimates presented here, 17% of the maternal orphans were under age 5 at the time of maternal death. An intergenerational cycle of poverty and disadvantage is thus perpetuated as families are often left impoverished through catastrophic financial expenditure during cancer care4. Orphans are less likely to complete school, more likely to experience poverty, and more likely to have substantial health challenges across their life course than non-orphaned children7,8,11,13,30. Maternal orphans due to cancer thus need to be tackled within the realm of SDG10 to ‘reduce inequality within and among countries’.
In 2020, there were approximately 147 million prevalent orphans from any cause18 and approximately 16 million children were newly orphaned. Far more orphans have lost a father (58%) than a mother (31%) and 11% are double orphans. The major causes of orphaning include HIV, natural disasters, wars, chronic poverty, chronic diseases or disease outbreaks such as coronavirus disease 2019 (COVID-19)12,18. Cancer as a cause of orphaning has not been investigated in great detail in terms of its impact on children, but it might confer unique risks. For example, unlike sudden deaths, the catastrophic financial toxicity of a failed cancer treatment can leave families struggling to provide for the needs of children4. A parental cancer death may also be associated with a real risk in the future, or stigma-related perceived risk, of cancer in the orphan. The HIV field has long estimated orphanhood due to HIV/AIDS as a key measure of the social impact of the epidemic. In that context, the staggering figures have been a catalyst for integrating child-centered responses into the HIV response. For example, the President’s Emergency Plan for AIDS Relief (PEPFAR) allocates 10% of its budget toward programming for orphans and vulnerable children31. Although maternal orphans due to cancer and HIV have many differences—orphans due to HIV are much younger, are concentrated in sub-Saharan Africa, many were HIV-positive themselves in the early stages of the HIV epidemic and are often double orphans, which would be rare for orphans of cancer5—a lot that has been learned from the support needed for orphans due to HIV is relevant to all orphans. To avoid stigmatizing children, most programs serve those who have been orphaned by any cause, which would include those orphaned due to cancer. The current estimates indicate that many HIV-endemic countries are indeed grappling with large numbers of maternal orphans due to cancer32. These observations agree with our estimates, as the highest corresponding risks were in East and Southern Africa. Indeed, some orphans of HIV are also orphans of cancer due to the many HIV-defining malignancies33. Most orphans will and should be taken in by surviving parents or extended family within the community, as in the context of HIV34. Strengthening the family’s capacity for caregiving through cash transfers, parenting interventions and psychosocial support are promising support strategies30,35. For cancer specifically, some cancer foundations such as the Lalla Salma foundation in Morocco also support the education costs of orphans of cancer36. The age distributions of new and prevalent orphans at the time of their mothers’ death, which were remarkably similar across the different world regions, highlight the need to ensure that such strategies will cater to the varying age-related needs of the orphans, stretching from very early childhood to late adolescence.
This study has some limitations. Our estimates required several explicit assumptions and reliance on global databases. GLOBOCAN includes worldwide estimates but in some countries, and particularly in LMICs, complete coverage of cancer deaths was not available or was of limited quality, thus interpretations should be made with caution37. Analyses at a country level were aggregated to provide the totals worldwide, which meant 99.4% of global cancer deaths in women were included (as some small territories included in global totals but do not appear as individual countries). Thus uncertainty intervals around maternal cancer orphan estimates would be driven largely by uncertainties in the GLOBOCAN estimates, which are provided on the Global Cancer Observatory, ‘Cancer Today’ website (https://gco.iarc.fr/). At the 95% level, they extend ±3.6% around the best estimates for global female cancer deaths (all ages), but with regional variations in ascending order as follows: ±0.5% for Northern America, ±2.8% for Asia, ±4.0% for Europe, ±4.6% for Latin America and the Caribbean, ±10% for Oceania and ±22.6% for Africa. We also focussed the detailed results on new rather than prevalent maternal cancer orphans because the latter requires additional assumptions. Indeed, GLOBOCAN estimates are for a single year (2020), whereas historic data are only available for a small subset of countries. We thus had to make assumptions about the number of cancer deaths from 2003 to 2019. Nevertheless, we provided sensitivity analyses of prevalence estimates based on different scenarios of all-cancer mortality trends and showed that the prevalence estimate of 7 million is robust to the order of ±0.4 million. Assuming that past trends were stable may underestimate prevalent orphans because, as can be seen on IARC’s Global Cancer Observatory’s Cancer Over Time data, mortality rates have been declining in the past 15 years for the countries (mostly high-income) where data are available38. Further, the cancer-site-specific breakdown of prevalent maternal cancer orphans is likely to be less reliable than for new orphans. An additional consideration for the 2020 prevalent orphan estimates was the subtraction of children who died as a result of their mothers’ death, i.e. between their mother’s death and mid-20208,39. We illustrate that the estimates were robust to a range of relevant mortality rate ratios applied, from 1.25 to 2.6. These ratios were selected to match the ages of maternal orphans due to cancer, which are typically much older than orphans from other causes (89% of maternal orphans of cancer were orphaned aged 5 years or older). In addition, estimates of the past fertility of women incorporated the associations between reproduction and cancer risks for three cancers, but beyond this adjustment, we assumed that the fertility history of women who died from cancer (at ages when orphans are possible) did not differ from that of their birth cohort. This assumption may lead to a slight over-estimation of orphans because cancer treatment affects subsequent fertility. This effect is not likely to be as large as cancer deaths at younger ages are concentrated in LMIC settings where median survival times are short (approximately 3 years)28,40, that is too short a period to alter a woman’s average fertility. However, future estimates would benefit from estimating the time-since-diagnosis among a cross-section of women who die from cancer and incorporating a reduced or zero fertility in the postdiagnosis period, especially for cancers such as ovarian and endometrial where treatment may involve removal of the uterus and ovaries. Such modifications may affect estimates of maternal orphans in settings where survival rates are high and thus deaths may be many years after the original cancer diagnosis. Considerations of the availability of fertility-preservation treatments in some settings will also be pertinent. Overall, we note that—with some assurance—our estimates for Africa were very similar to those observed in the ABC-DO cohort study4.
This study provides partial insight into the orphans resulting from cancer deaths as we have only estimated the orphans due to cancer deaths in women and not the corresponding orphans due to cancer deaths in men. The latter estimates are more complex to make because of the need to first estimate male fertility rates, for which several methods are proposed41. The cancer deaths that give rise to paternal orphans are expected to differ greatly from those for maternal orphans, not only in terms of contributing cancers but also because of the longer time span when men can reproduce. While the female reproductive life is assumed (in national fertility data) to span from 15 to 49 years, it can extend up to 70 years of age for men, that is ages when cancer incidence and mortality rates are substantially higher. Thus, for a comprehensive assessment, it will be imperative to extend to estimates of the number of paternal orphans due to cancer and to examine the impact on children’s lives after the loss of either parent due to cancer.
In conclusion, we have revealed that children are affected on large scale by the loss of their mothers due to cancer globally. This impact reveals yet another reason for the urgency of cancer control plans, propelling programs to prevent cancer and cancer deaths in the first place. Alongside these efforts, support to families and communities caring for orphaned children is needed to ensure that these children receive the same opportunities, education and good health as other children worldwide.
Methods
Our working definition of a maternal orphan due to cancer is a child under age 18 whose mother has died from cancer. Cancer deaths were considered to be deaths from all malignancies (International Classification of Disease Tenth revision (ICD-10) C00-97). For 2020 estimates, new maternal orphans were orphaned in 2020, while prevalent maternal orphans were children under age 18 in mid-2020 who became maternal orphans at any time in the past. Central to all estimates of these orphans are the estimates of new orphans in a particular year. For a brief overview of the methodology, to estimate the absolute number of these orphans, we adopted methods similar to that for COVID-19-orphans12, that is using the number of female cancer deaths, by cancer site, at a given age at death and in a given country as the starting point. We multiplied these deaths by the estimated average number of children expected to be alive in 2020, per woman, at the age the woman died. The number of children was assumed to be a function of the age-specific fertility rates of that woman’s birth cohort in the prior 18 years, adjusted to the specific cancer death in question (details below) and additionally accounting for the probability that the child survived in 2020 (using the country-age-specific child mortality rates as the child advances from birth to 2020).
Data sources
We needed country-level data on three items: (a) the absolute number of female cancer deaths in 2020, (b) fertility rates in women during 2002–2019 and (c) mortality rates of children during 2003–2020.
-
Estimates of the number of female cancer deaths in 2020 were sourced from GLOBOCAN14,42. These data were obtained in 5-year age groups (0–4, 5–9,…, 85+) for 185 countries/territories (simply called ‘countries’ in the rest of the manuscript) and 35 exhaustive and mutually exclusive cancer sites (listed in Supplementary Table 1). In brief, the GLOBOCAN estimates were assembled at the national level using the best available sources of cancer incidence and mortality data for a given country, with priority given to short-term projections of incidence and mortality rates42. In instances where country data were not available, GLOBOCAN extrapolates from neighboring countries’ data. Of the 185 countries, 2020 mortality data are most accurate for 80 countries, being based on observed national mortality rates projected for 2020. For 21 countries, recent national or regional mortality rates were applied to the 2020 population, whereas for 81 countries, mortality rates were modeled based on incidence-to-mortality ratios derived from registries in neighboring countries (models are specific to regions: Western Asia, sub-Saharan Africa, Northern Africa, Oceania, South Central Asia and Southeastern Asia) and finally in three countries rates were estimated as an average of those from selected neighboring countries.
Mortality data were obtained for 35 topographic cancer sites, which were then categorized into 13 groups for reporting purposes. Their codes in the ICD-10 and grouping are provided in Extended Data Table 3. This grouping was exhaustive and mutually exclusive.
Fertility rates in women, that is the average number of births per 1,000 women, were extracted from the 2019 revision of the United Nations (UN) World Population Prospects, which are available for 201 countries/territories with at least 90,000 inhabitants43. The UN countries and the composition of geographical areas follow those in ‘standard country or area codes for statistical use’ WPP (ST/ESA/STAT/SER.M/49/Rev.3). Fertility rates for each country are based on a combination of birth histories from Demographic and Health Surveys, census data, reproductive and health surveys, multiple indicator cluster surveys and intercensal demographic surveys. The published fertility rates correspond to 5-year age groups from ages 15−49 years (outside of which fertility is zero) and 5-year calendar periods (2000–2019), running from 1 July to 30 June of the initial and final years. Note that because UNWPP fertility rates are zero at age 50 years and over, for all women who die at age 68 or over, these deaths cannot give rise to maternal orphans.
Mortality rates from ages 0−17 years during each of the years from 2003 to 2020 were also extracted from UNWPP for the same 201 territories43.
Extended Data Table 3.
List of the 35 topographical cancer sites and their groupings
| No. | Cancer groups | Cancer sites (ICD 10) |
|---|---|---|
| 1 | Brain | Brain, nervous system (C70–72) |
| 2 | Breast | Breast (C50) |
| 3 | Cervical | Cervix uteri (C53) |
| 4 | Genitourinary | Kidney and renal pelvis (C64–65) |
| 5 | Bladder (C67) | |
| 6 | Hematological | Hodgkin lymphoma (C81) |
| 7 | Non-Hodgkin lymphoma (C82–86, C96) | |
| 8 | Multiple myeloma and immunoproliferative diseases (C88+C90) | |
| 9 | Leukemia (C91–95) | |
| 10 | Head and Neck | Lip, oral cavity (C00–06) |
| 11 | Salivary glands (C07–08) | |
| 12 | Oropharynx (C09–10) | |
| 13 | Nasopharynx (C11) | |
| 14 | Hypopharynx (C12–13) | |
| 15 | Larynx (C32) | |
| 16 | Kaposi sarcoma | Kaposi sarcoma (C46) |
| 17 | Lower Gastrointestinal | Colon (C18) |
| 18 | Rectum (C19–20) | |
| 19 | Anus (C21) | |
| 20 | Other | Other specified sites (C17, C24, C30–31, C37-38, C40-41,C47-49,C57-58,C63,C66,C69,C74-75) |
| 21 | Unspecified sites (C76-80+C97) | |
| 22 | Other female specific | Vulva (C51) |
| 23 | Vagina (C52) | |
| 24 | Corpus uteri (C54) | |
| 25 | Ovary (C56) | |
| 26 | Respiratory | Trachea, bronchus and lung (C33-34) |
| 27 | Mesothelioma (C45) | |
| 28 | Skin cancer | Melanoma of skin (C43) |
| 29 | Non-melanoma skin cancer (C44) | |
| 30 | Thyroid | Thyroid (C73) |
| 31 | Upper Gastrointestinal | Esophagus (C15) |
| 32 | Stomach (C16) | |
| 33 | Liver and intrahepatic bile ducts (C22) | |
| 34 | Gallbladder (C23) | |
| 35 | Pancreas (C25) |
ICD-10: International Classification of Disease 10th revision
In total, 185 countries, for which cancer mortality estimates are available in GLOBOCAN, were included in the present estimates of maternal cancer orphans. This differs from the 201 territories/countries in the UN data, due to two UN listings (Hong Kong and Macau) being included within China in GLOBOCAN and 14 UN listings for which GLOBOCAN estimates are not available (Antigua and Barbuda; Aruba; Channel Islands; China, Taiwan Province of China; Curacao; Grenada; Kiribati; Mayotte; Micronesia (Fed. States of); Saint Vincent and the Grenadine; Seychelles; Tonga; United States Virgin Islands; and Western Sahara). For all regional distributions presented, Micronesia (13 new maternal orphans due to cancer) and Polynesia (114 new maternal orphans due to cancer) in Oceania were combined due to the small number of maternal orphans due to cancer. We also examined variations in the number of new maternal orphans (per 100 female cancer deaths and per 100,000 children) by a country’s 2020 HDI. HDI for a country reflects a composite measure of a nation’s longevity (life expectancy at birth), education (mean years of education of children and of adults) and income (gross national income per capita). Of the 185 countries/territories included in this analysis, 175 had an HDI value in 2020 (all except French Guyana, French Polynesia, Guadeloupe, Guam, Democratic People Republic of Korea, Martinique, New Caledonia, Puerto Rico, La Reunion and Somalia). In 2020, HDI values ranged from 0.39 in South Sudan to 0.96 in Norway. HDI categories are low (<0.55), medium (0.55–0.69), high (0.70–0.79) and very high (0.8–1.0).
Statistical analysis
New maternal orphans due to cancer in 2020
Calculations of new maternal orphans due to cancer in 2020 were made at the level of country, age at death and cause of cancer death. We denote NMC2020,c,t,md as the number of new maternal orphans due to cancer (NMC) occurring in 2020 in country c due to cancer deaths from cancer-related death type t in women who died at age md. The total number of new maternal orphans due to cancer in a country is the sum of NMC2020,c,t,md across all the ages at cancer death which can give rise to NMCs and across the 35 topographical cancer types: . NMC2020,c,t,md was estimated by multiplying the number of deaths from cancer type t in women who died at age md in country c in 2020 (D2020,c,t,md) by the average number of living children (per woman, A2020,c,md) who were aged under 18 for the corresponding cohort of women who died from cancer type t at age md in country c in 2020: NMC2020,c,t,md = D2020,c,t,md x A2020,c,md,t. D2020,c,t,md (cancer deaths for a single year of age at death) were calculated from the number of deaths in the corresponding 5-year age-at-death category, divided by five.
The average (per woman) number of children under 18 years in 2020, A2020,c,md,t is a function of the annual fertility rates between mid-2002 and mid-2019 of the woman’s birth cohort (that is with year of birth (2020-md)), multiplied by the probability that the child survived until their mothers’ death in 2020.
Here
-
(i)
FRc,y,ma is the fertility rate (per woman) in country c in calendar year y for women at age ma, where ma = md − (2020 − y) and FRc,y,ma = 0 if ma > 50;
-
(ii)Pc(alive2020|y) is the probability that a child born in year y in country c is alive in 2020. This probability was calculated using each country’s lifetables as follows:
where Mxc,y+x is the mortality rate at age x in country c in year y+x, that is year of birth + x years; -
(iii)
RRt incorporates an adjustment to account for the fact that, for women who died of cancers where parity is a known risk factor, fertility would differ from the average fertility of her birth cohort and therefore, the average fertility rates needed correcting. This adjustment was to multiply the average number of children in a woman’s birth cohort by RRt, where RR is the relative risk of the association of parity with the risk of the specific cancer t (per unit increase in parity). RRt was assumed to be 1 for all cancer types, except cervix (RR=1.1), ovary (RR=0.80) and breast cancer deaths over the age of 50 years (RR=0.93)44. These RRs were obtained from high-quality meta-analyses or large studies, which assessed the associations of parity with risk of the specific cancer type. High parity increases and decreases the risk of cervical and ovarian cancer, respectively, thus for these cancer deaths, in accordance with their parity-risk associations, the adjustments imply that the average number of children was increased by 10% for women who died from cervical cancer45 and reduced by 20% for ovarian cancer deaths46. For breast cancer risk, high parity is inversely associated with breast cancer risk at postmenopausal ages only (RR 0.93); however, at premenopausal ages, when most maternal orphans arise, it is at most weakly associated, thus we only made an adjustment for breast cancer deaths over the age of 50 years47. For other cancer sites, RRs were 1, because parity is not an established risk factor for upper GI cancers (gastric, esophageal), or lower GI cancers (colorectal), thus no adjustment of national fertility rates was needed. No adjustments were made for lung cancer as meta-analyses did not establish an association but rather observed large between-study heterogeneity48,49.
Prevalent maternal orphans due to cancer in 2020
As an overview, the point prevalence of maternal orphans (PMCc,t) in country c due to cancer death t was made for mid-year 2020, that is the number of children under the age of 18 years on 1 July 2020, who had lost their mother due to cancer at any time in the past. This prevalence was estimated as the sum of new maternal orphans due to cancer for female cancer deaths across the year of death (yd) for each year from mid-2003 to mid-2020, as described above, minus any children who were estimated to have died before mid-2020. Prior deaths were estimated using the country’s age-specific mortality rates Mx up to the maternal death, and thereafter by applying a higher mortality rate than that in national lifetables (MRRMC x Mx) because studies across diverse settings have observed increased mortality of children who had lost a parent to any cause, including to cancer. The magnitude of this effect may vary between settings. In Nordic countries, children of parents who died from cancer have 1.25 times higher mortality than their peers, while in Bangladesh, mortality rates were 2.6 times higher at ages 2−10 years7,8, that is at the ages when 95% of new maternal orphans due to cancer occur. Thus, we applied the following range of mortality rate ratios associated with being a maternal orphan due to cancer (MRRMC): (a) 1.25 (considered a minimum), (b) 1.5, (c) 2.0, (d) 2.60 (maximum) and (e) as a comparator to gauge the total effect, an MRRMC of 1.0 (that is, motherless children have the same mortality rates as their peers).
Here
-
(i)
c = country, md = woman’s age at death, yd = woman’s year of death, y = year of birth of child;
-
(ii)
(Dyd,c,t,md) is the number of deaths in year yd, in country c, from cancer type t, in women who died at age md;
-
(iii)
RRt is an adjustment factor for the association between parity and risk of cancer, for cancer types where parity is a known risk factor (see above);
-
(iv)
FRc,y,ma is the fertility rate (per woman) in country c in calendar year y for women at age ma, where ma = yd − (2020 − y);
-
(v)
Pc(U182020|y,yd) is the probability that a child born in year y whose mother died in year yd in country c is alive and under 18 years of age in 2020:
otherwise, the probability was calculated with different mortality rates before and after maternal death in year yd as follows:
For the prevalent estimates of maternal orphans due to cancer, we needed the number of cancer deaths in women during 2003–2019, which could not be taken from previous GLOBOCAN releases because the estimation methodology changes over time. Thus for compatibility with GLOBOCAN 2020, we estimated the 2002–2019 cancer deaths by applying multiples of the age-specific mortality rates for 2020 to the corresponding age-specific annual female population sizes extracted from the United Nations World Population Prospects37. We considered four scenarios (S) for the multiples of the 2020 age-specific mortality rates as follows: (S1) Stable mortality rates (λy in year y) throughout 2002–2020 that is, λy=λ2020; (S2) Mortality rates increased by 1% every year during 2002–2020, thus λy= 0.99(2020-y)λ2020; (S3) Mortality rates decreased by 1% every year during 2002–2020, thus λy=1.01(2020-y)λ2020; (S4) Mortality rate changes depended on region, with increasing mortality rates (scenario 2) for low and middle HDI countries and decreasing mortality rates (scenario 3) for high and very high HDI countries. Combined with the varying mortality rate ratios (MRR) due to being MC-orphans (MRRMC above), the main prevalence estimates are provided for scenarios S1a and S2–4a/e as sensitivity analyses. (Extended Data Table 3).
All analyses were performed in R (version 4.1.2). All estimates were rounded to the nearest thousand when reported in the text.
This analysis was not submitted for institutional ethical approval as all data sources used are publicly available aggregate-level estimates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information, details of author contributions and competing interests, and statements of data and code availability are available at 10.1038/s41591-022-02109-2.
Supplementary information
Number of new and prevalent maternal orphans due to cancer, 2020, by country.
Acknowledgements
This work was funded by the US National Cancer Institute (R01CA244559 to V.McC and I.d.S.S). The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. Where authors are identified as personnel of the International Agency for Research on Cancer and/or the World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer and/or the World Health Organization. The opinions expressed by the authors are their own and this material should not be interpreted as representing the official viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health or the National Cancer Institute.
Extended data
Author contributions
Analyses were conducted by F.G. under the supervision of V.McC. The study concept was developed by V.McC and I.d.S.S., with inputs from M.G., G.P., J.S., B.A., K.C. and A.I. J.F., F.B., S.V. and I.S. advised on cancer mortality data. R.K., B.K., O.G. and R.M.V. provided expertise on orphans due to cancer.
Peer review
Peer review information
Nature Medicine thanks Z. He, L. Pace and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: M. Yang, in collaboration with the Nature Medicine team.
Data availability
The data sources are all publicly available datasets: The number of female cancer deaths in 2020 was sourced from IARC, GLOBOCAN (https://gco.iarc.fr/today/). They are available for 185 countries worldwide. Fertility and mortality rates were extracted from the 2019 revision of the United Nations World Population Prospect (UN-WPP; https://population.un.org/wpp/), which are available for 201 countries/territories with at least 90,000 inhabitants and included the 185 countries for which cancer deaths are available. The country-specific estimates of maternal orphans due to cancer are all provided in Supplementary Table 1.
Code availability
All R codes used for data preparation and analysis are publicly available at https://code.iarc.fr/guidaf/maternalcancerorphans.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
twitter:@Flo_research.
twitter:@valmacsee.
Contributor Information
Florence Guida, Email: guidaf@iarc.who.int.
Valerie McCormack, Email: mccormackv@iarc.who.int.
Extended data
is available for this paper at 10.1038/s41591-022-02109-2.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-022-02109-2.
References
- 1.Institute for Health Metrics and Evaluation (IHME). GBD 2019 cause and risk summary. https://www.healthdata.org/results/gbd_summaries/2019 2020.
- 2.Soerjomataram I, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 3.Yabroff KR, Lund J, Kepka D, Mariotto A. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol. Biomark. Prev. 2011;20:2006–2014. doi: 10.1158/1055-9965.EPI-11-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galukande M, et al. Maternally orphaned children and intergenerational concerns associated with breast cancer deaths among women in Sub-Saharan Africa. JAMA Oncol. 2021;7:285–289. doi: 10.1001/jamaoncol.2020.6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS, UNICEF & USAID. Children on the Brink 2004: A Joint Report of New Orphan Estimates and a Framework for Action (UNICEF, 2004).
- 6.Bergman AS, Axberg U, Hanson E. When a parent dies—a systematic review of the effects of support programs for parentally bereaved children and their caregivers. BMC Palliat. Care. 2017;16:39. doi: 10.1186/s12904-017-0223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, et al. Mortality after parental death in childhood: a nationwide cohort study from three Nordic countries. PLoS Med. 2014;11:e1001679. doi: 10.1371/journal.pmed.1001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ronsmans C, Chowdhury ME, Dasgupta SK, Ahmed A, Koblinsky M. Effect of parent’s death on child survival in rural Bangladesh: a cohort study. Lancet. 2010;375:2024–2031. doi: 10.1016/S0140-6736(10)60704-0. [DOI] [PubMed] [Google Scholar]
- 9.Guldin MB, et al. Incidence of suicide among persons who had a parent who died during their childhood: a population-based cohort study. JAMA Psychiatry. 2015;72:1227–1234. doi: 10.1001/jamapsychiatry.2015.2094. [DOI] [PubMed] [Google Scholar]
- 10.Kidman R, Palermo T. The relationship between parental presence and child sexual violence: evidence from thirteen countries in sub-Saharan Africa. Child Abus. Negl. 2016;51:172–180. doi: 10.1016/j.chiabu.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kailaheimo-Lonnqvist S, Kotimaki S. Cause of parental death and child’s health and education: the role of parental resources. SSM Popul. Health. 2020;11:100632. doi: 10.1016/j.ssmph.2020.100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillis SD, et al. Global minimum estimates of children affected by COVID-19-associated orphanhood and deaths of caregivers: a modelling study. Lancet. 2021;398:391–402. doi: 10.1016/S0140-6736(21)01253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Case A, Paxson C, Ableidinger J. Orphans in Africa: parental death, poverty, and school enrollment. Demography. 2004;41:483–508. doi: 10.1353/dem.2004.0019. [DOI] [PubMed] [Google Scholar]
- 14.Ferlay, J., et al. Global Cancer Observatory: Cancer Today (International Agency for Research on Cancer, 2020).
- 15.Allemani C, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bidoli E, et al. Worldwide age at onset of female breast cancer: a 25-year population-based cancer registry study. Sci. Rep. 2019;9:14111. doi: 10.1038/s41598-019-50680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross J, Bietsch K. The nature of the open birth interval distribution. Gates Open Res. 2020;4:153. doi: 10.12688/gatesopenres.13177.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UNICEF. Orphanhood. https://data.unicef.org/topic/hivaids/orphanhood/ (2022).
- 19.Anderson BO, et al. The Global Breast Cancer Initiative: a strategic collaboration to strengthen health care for non-communicable diseases. Lancet Oncol. 2021;22:578–581. doi: 10.1016/S1470-2045(21)00071-1. [DOI] [PubMed] [Google Scholar]
- 20.Canfell K, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:591–603. doi: 10.1016/S0140-6736(20)30157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Man I, et al. Evidence-based impact projections of single-dose human papillomavirus vaccination in India: a modelling study. Lancet Oncol. 2022;23:1419–1429. doi: 10.1016/S1470-2045(22)00543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heer E, et al. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob. Health. 2020;8:e1027–e1037. doi: 10.1016/S2214-109X(20)30215-1. [DOI] [PubMed] [Google Scholar]
- 23.Arnold M, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.African Esophageal Cancer Consortium Expanding oesophageal cancer research and care in eastern Africa. Nat. Rev. Cancer. 2022;22:253–254. doi: 10.1038/s41568-022-00458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564–1571. doi: 10.1136/gutjnl-2020-321600. [DOI] [PubMed] [Google Scholar]
- 26.Fidler, M. M. et al. Reducing Social Inequalities in Cancer: Evidence and Priorities for Research (IARC, 2019). [PubMed]
- 27.Foerster M, et al. Inequities in breast cancer treatment in sub-Saharan Africa: findings from a prospective multi-country observational study. Breast Cancer Res. 2019;21:93. doi: 10.1186/s13058-019-1174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormack V, et al. Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): a prospective cohort study. Lancet Glob. Health. 2020;8:e1203–e1212. doi: 10.1016/S2214-109X(20)30261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenzie F, et al. Drivers of advanced stage at breast cancer diagnosis in the multicountry African breast cancer—disparities in outcomes (ABC-DO) study. Int. J. Cancer. 2018;142:1568–1579. doi: 10.1002/ijc.31187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hillis, S. et al. Children: The Hidden Pandemic 2021: A Joint Report of COVID-19–Associated Orphanhood and a Strategy for Action (Centers for Disease Control and Prevention, 2021).
- 31.Yates, D., Richter, L., Zingu, J., Yates, R., Wolfe, J. PEPFAR HKID Portfolio Review: Children in the HIV/AIDS Epidemic (USAID, 2011).
- 32.Mejia-Pailles G, Berrington A, McGrath N, Hosegood V. Trends in the prevalence and incidence of orphanhood in children and adolescents <20 years in rural KwaZulu-Natal South Africa, 2000-2014. PLoS ONE. 2020;15:e0238563. doi: 10.1371/journal.pone.0238563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr. Opin. HIV AIDS. 2017;12:6–11. doi: 10.1097/COH.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster G, Williamson J. A review of current literature on the impact of HIV/AIDS on children in sub-Saharan Africa. AIDS. 2000;14:S275–S284. [PubMed] [Google Scholar]
- 35.Kidman R. Use HIV’s Lessons to Help Children Orphaned by COVID-19. Nature. 2021;596:185–188. doi: 10.1038/d41586-021-02155-9. [DOI] [PubMed] [Google Scholar]
- 36.Fondation Lalla Salma. Scope of activities: social support: orphans of cancer. https://www.contrelecancer.ma/en/orphelins-du-cancer/ (2022).
- 37.United Nations, Department of Economic and Social Affairs, Population Division. World population prospects 2019, rev. 1. https://population.un.org/wpp/publications/ (2022).
- 38.IARC. Global Cancer Observatory—cancer over time. https://gco.iarc.fr/ (2022).
- 39.Mailhot Vega RB, et al. Estimating child mortality associated with maternal mortality from breast and cervical cancer. Cancer. 2019;125:109–117. doi: 10.1002/cncr.31780. [DOI] [PubMed] [Google Scholar]
- 40.Sengayi-Muchengeti M, et al. Cervical cancer survival in sub-Saharan Africa by age, stage at diagnosis and Human Development Index: a population-based registry study. Int. J. Cancer. 2020;147:3037–3048. doi: 10.1002/ijc.33120. [DOI] [PubMed] [Google Scholar]
- 41.Schoumaker B. Measuring male fertility rates in developing countries with Demographic and Health Surveys: an assessment of three methods. Demographic Res. 2017;36:803–850. doi: 10.4054/DemRes.2017.36.28. [DOI] [Google Scholar]
- 42.Ferlay J, et al. Cancer statistics for the year 2020: an overview. Int. J. Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 43.Clarke AR, Tonge BJ, Einfeld SL, Mackinnon A. Assessment of change with the developmental behaviour checklist. J. Intellect. Disabil. Res. 2003;47:210–212. doi: 10.1046/j.1365-2788.2003.00470.x. [DOI] [PubMed] [Google Scholar]
- 44.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 45.Munoz N, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 2002;359:1093–1101. doi: 10.1016/S0140-6736(02)08151-5. [DOI] [PubMed] [Google Scholar]
- 46.Adami HO, et al. Parity, age at first childbirth, and risk of ovarian cancer. Lancet. 1994;344:1250–1254. doi: 10.1016/S0140-6736(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 47.Clavel-Chapelon F, Gerber M. Reproductive factors and breast cancer risk. Do they differ according to age at diagnosis? Breast Cancer Res. Treat. 2002;72:107–115. doi: 10.1023/A:1014891216621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahabreh IJ, Trikalinos TA, Paulus JK. Parity and risk of lung cancer in women: systematic review and meta-analysis of epidemiological studies. Lung Cancer. 2012;76:150–158. doi: 10.1016/j.lungcan.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin X, Zhu Z, Hosgood HD, Lan Q, Seow WJ. Reproductive factors and lung cancer risk: a comprehensive systematic review and meta-analysis. BMC Public Health. 2020;20:1458. doi: 10.1186/s12889-020-09530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of new and prevalent maternal orphans due to cancer, 2020, by country.
Data Availability Statement
The data sources are all publicly available datasets: The number of female cancer deaths in 2020 was sourced from IARC, GLOBOCAN (https://gco.iarc.fr/today/). They are available for 185 countries worldwide. Fertility and mortality rates were extracted from the 2019 revision of the United Nations World Population Prospect (UN-WPP; https://population.un.org/wpp/), which are available for 201 countries/territories with at least 90,000 inhabitants and included the 185 countries for which cancer deaths are available. The country-specific estimates of maternal orphans due to cancer are all provided in Supplementary Table 1.
All R codes used for data preparation and analysis are publicly available at https://code.iarc.fr/guidaf/maternalcancerorphans.