Abstract
Polatuzumab vedotin (Pola) with bendamustine and rituximab (BR) is a promising option for patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). We analyzed the data of 71 R/R DLBCL patients who had been treated with Pola-BR in the named patient program from March 2018 to April 2021 from 32 centers in Turkey. All patients received up to six cycles of Pola 1.8 mg/kg, rituximab 375 mg/m2 on day 1, and bendamustine 90 mg/m2 on days 1–2 of each cycle. Median age at Pola-BR initiation was 55 (19–84). The overall response rate was 47.9%, including 32.4% CR rate when a median of 3 cycles was applied. With a median follow-up of 5 months, the median OS was 5 months. Grade 3–4 neutropenia and thrombocytopenia were the most common hematological toxicities. The real-world data from our cohort showed the Pola-BR is an effective option with a manageable toxicity profile.
Keywords: Diffuse large B-cell lymphoma, Polatuzumab vedotin, Refractory, Relapsed, Rituximab, Bendamustine
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma and accounts for approximately 25–30% of all newly diagnosed patients with non-Hodgkin lymphoma [1, 2]. Survival rates have improved in recent decades, 5-year survival rates in the first-line setting range from 60 to 70% [3]. In the chemoimmunotherapy era, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemoimmunotherapy is the standard care for first-line treatment; however, 30–40% of patients are relapsed or refractory to R-CHOP [4]. The combination of bendamustine and rituximab (BR) is administered for salvage, especially as first salvage therapy in patients with relapsed or refractory (R/R) DLBCL; therefore, the overall response (ORR) rates were 46–63% in three studies with acceptable toxicities [5–8]. For eligible patients, if they have an inadequate response to salvage chemotherapies and relapse after autologous stem cell transplantation, the prognosis is very poor [9]. The overall survival is expressed at nearly 4–6 months. There is a significant unmet treatment need for relapsed or refractory and heavily treated patients [10].
Polatuzumab vedotin (Pola) is a novel antibody–drug conjugate and consists of a monoclonal antibody against CD79b which is a B-cell receptor component located on normal B cells which is covalently conjugated to the microtubule-disrupting anti-mitotic agent monomethyl auristatin [11, 12]. A phase 1 study showed the safety of the single-agent polatuzumab vedotin in patients within heavily pretreated B-cell malignancies such as non-Hodgkin lymphoma and chronic lymphocytic leukemia [13]. Polatuzumab vedotin plus an anti-CD20 monoclonal antibody rituximab showed encouraging results in a phase 2 study with objective response and complete response rates of 54% and 21%, respectively [14]. Polatuzumab vedotin in combination with BR provided higher ORR (45% vs. 18%) and prolonged OS (12.4 vs. 4.7 months) in R/R DLBCL patients compared to patients who received BR in a phase 2 study [15]. The polatuzumab vedotin and BR combination was considered rational and decreased the risk of neurotoxicity that could occur with platinum-based regimens. The Pola-BR combination was granted accelerated approval by the Food and Drug Administration for the treatment of patients with R/R DLBCL who failed two prior therapies in June 2019. In Europe, the European Medicines Agency approved Pola-BR for the treatment of adult patients with R/R DLBCL who are ineligible for hematopoietic stem cell transplant in January 2020 [16, 17].
Although early phase studies of Pola-BR were promising, there is still an unmet need in heavily treated R/R DLBCL and, in particular, a lack of real-world data for Pola-BR. Therefore, we present a retrospective, multicenter, real-world data study consisting of 71 R/R DLBCL patients treated with Pola-BR in the named patient program in Turkey.
Material and methods
Patients and study design
We analyzed the data of 71 R/R DLBCL patients who had been treated with Pola-BR through their participation in the named patient program from March 2018 to April 2021 from 32 hematology centers in Turkey. The eligible patients were aged ≥ 18 years, had received at least two prior treatments including R-CHOP, had progression or intolerance with the last treatment protocols, and were ineligible for hematopoietic stem cell transplantation. In patients with prior BR exposure, if the BR duration of response had exceeded at least 12 months, they were allowed to be included in the study. Patients with grade ≥ 2 peripheral neuropathy were excluded.
This observational, retrospective, and multicenter study was designed to evaluate the ORR and complete response rates for the primary endpoint, and overall survival and toxicities for the secondary endpoint of Pola-BR in patients with R/R DLBCL. After obtaining approval from local authorities and the ethics committee for the study, patients’ data was collected from hospital records and patient files. We recorded patients’ demographic features such as age, gender, B symptoms, stage, extranodal involvement, histological subtype, cell of origin determined by immunohistochemical staining and Hans algorithm, international prognostic index (IPI) score, performance status (PS) assessed according to the Eastern Cooperative Oncology Group (ECOG) scale, the disease status before Pola-BR treatment, number of therapies before Pola-BR, number of Pola-BR cycles, toxicities, the responses to the treatment, date of progression, and death or last follow-up. Bulky disease was evaluated as a mass of ≥ 7.5 cm on any imaging. The response evaluation was evaluated by [18F] fluorodeoxyglucose positron emission tomography–computed tomography using Lugano Response Criteria at the end of treatment (6–8 weeks after day 1 of the cycle) [18]. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
Treatment: polatuzumab vedotin, bendamustine, rituximab
All patients received polatuzumab vedotin 1.8 mg/kg intravenously (IV) on day 1 of each cycle, rituximab 375 mg/m2 IV on day 1 of each cycle, and bendamustine 90 mg/m2 IV on days 1 and 2 of each cycle in any order. Patients were treated for up to six cycles. Each cycle was applied every 21 days. Patients received at least one cycle of Pola-BR. Polatuzumab vedotin was diluted by adding 7.2 ml of sterile water with a disposable syringe. The dose solutions were prepared in 100-ml IV infusion bags containing normal saline. The saline equivalent to the drug volume was removed before the reconstituted drug was added to the IV infusion bag. The prepared drug was infused with an infusion pump within 30–90 min.
On the first day of the treatment, 30–60 min before the start of rituximab infusion, corticosteroids, an antihistamine, and an antipyretic were administered as pre-medications for the patients. All patients had received an antihistamine and antipyretic before polatuzumab vedotin infusion. The patients were monitored for infusion-related reactions during the infusion and a minimum of 90 min following completion of the initial dose. If an infusion-related reaction occurred, the infusion was interrupted, and appropriate medical management was instituted. No pre-medication was administered before bendamustine on the second day of treatment. Antiemetic treatment was administered in case of nausea symptoms. Neutropenic patients received growth factor support during treatment.
Ethical statement
The trial was conducted following all procedures performed in studies involving human participants in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or ethical standards comparable to the guidelines of the Declaration of Helsinki. The ethical committee approved the study protocol of the coordinating center of Dr. Abdurrahman Yurtaslan, Ankara Oncology Training and Research Hospital (2020–05/626).
Statistical analysis
IBM SPSS Statistics (version 25) was used for statistical analysis. Descriptive statistics were used to present the data. Categorical data were presented as numbers and ratios, and numerical data were presented as median, minimum, and maximum. Overall survival (OS) was defined as the duration from the date of the first day of the Pola-BR to the date of death or time to the survivors’ last follow-up date. Kaplan–Meier survival analysis was applied for PFS and OS, and log-rank tests were used to examine the factors affecting survival. Cox regression analysis was applied to evaluate factors affecting survival; variables with p < 0.05 were included in the multivariate model. p values of ≤ 0.05 were considered statistically significant.
Results
Patient characteristics
Over a 3-year period, seventy-one R/R DLBCL patients that participated in the NPP in Turkey, from 32 hematology centers, were included in the study. The data were analyzed in December 2021. The characteristics of the patients are summarized in Table 1. Forty-four (62%) males and 27 (38%) females were included in the study. Thirty-five (49.3%) of the patients had primary refractory disease. Evaluating cell of origin determined by immunohistochemical staining, 27 (38%) of the patients had germinal center B-cell and 23 (32.4%) had activated B-cell. The patients were heavily pretreated with a median of three lines prior to Pola-BR treatment in our cohort. Most of the patients (n = 56, 73%) had advanced stage disease, B symptoms (n = 47, 66.2%) and a high IPI score (IPI 3–5) (n = 42, 61%) at diagnosis. Twenty-three (32.4%) patients had bulky disease and 45 (63.4%) had extranodal disease. Early-relapsed patients (defined as relapse < 12 months following most recent treatment) constituted 64.8% (n = 46) of our study population, whereas late-relapsed patients (defined as relapse > 12 months following most recent treatment) were 35.2% (n = 25). Twelve (16.9%) patients had exposure to bendamustine for relapsed disease before Pola-BR. At the time of relapse/refractory setting, 53.5% (n = 38) of patients had a high IPI (IPI 3–5). Thirty-five (49.3%) patients had received radiotherapy. Nearly half of the patients (n = 35) had undergone hematopoietic stem cell transplantation (HSCT) and 36 patients could not receive any HSCT. Twenty-two patients (31%) had only undergone autologous stem cell transplantation, three of whom underwent it after Pola-BR treatment. Seven patients (9.8%) had both autologous and allogeneic stem cell transplantation. Six patients (8.4%) received Pola-BR as a bridge to allogeneic stem cell transplantation.
Table 1.
Patient’s characteristics
| Number (%) | |
|---|---|
| Age at Pola-BR initiation, years (median) | 55 (19–84) |
| Male sex, n (%) | 44 (62%) |
| ECOG performance status | |
| 0–1, n (%) | 45 (63.4%) |
| ≥ 2, n (%) | 26 (36.6%) |
| Histological subtype (cell of origin) | |
| GCB | 27 (38%) |
| ABC | 23 (32.4%) |
| Unknown | 21 (29.6%) |
| Advanced stage (stages 3–4) | 56 (73%) |
| IPI score 3–5 at diagnosis | 42 (61%) |
| Bulky disease (≥ 7.5 cm), n (%) | 23 (32.4%) |
| B symptoms, n (%) | 47 (66.2%) |
| Extranodal disease, n (%) | 45 (63.4%) |
| Bone marrow involvement, n (%) | 18 (25.4%) |
Pola-BR, Polatuzumab vedotin, bendamustine, and rituximab; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B-cell–like subtype; ABC, activated B-cell–like subtype; IPI, international prognostic index
Polatuzumab vedotin, bendamustine, rituximab treatment points
All patients received Pola-BR. The median age at the time of Pola-BR initiation was 55 years (range, 19–84 years). The patients had undergone a median of 3 treatment lines (range, 2–5 lines) before Pola-BR treatment. Median lactate dehydrogenase was 331 U/L (128–2185), median hemoglobin was 10.4 g/dl (5.9–13.7), white blood cells were 4.5 × 103/μl (0.5–19.5), and median thrombocytes were 134 × 103/μl (12–700) before Pola-BR, while hemoglobin was 11 g/dl (8–13.6), white blood cells were 3.48 × 103/μl (6.1–12.4), and median thrombocytes were 116 × 103/μl (30–510) following Pola-BR treatment. The median number of Pola-BR cycles was 3 (range, 1–6).
The median follow-time after Pola-BR treatment was 5 months (range, 1–31 months). ORR of the entire cohort was 47.9%, including 32.4% with a complete response (n = 23) and 15.5% with a partial response (n = 11). Nearly 10% of patients (n = 7) achieved stable disease and 36.5% (n = 26) had progressive disease at the end of treatment. Four patients died before response assessment.
Survival analysis
The median OS of entire cohort was 5 months (95% CI: 3.3–6.6). The median OS in the ECOG 0–1 group was 8 months (95% CI: 8.9–16.4 months), while the median OS in the ECOG ≥ 2 group was 2 months (95% CI: 2.1–4.6 months). The median OS in the overall response group was 8 months (95% CI: 8.8–17.9 months) while the median OS in the stable disease and progressive disease group was 4 months (95% CI: 3.1–5.7 months). The survival curves are shown in Figs. 1, 2, and 3. Fifty-five (77.5%) patients died from their disease in the follow-up period.
Fig. 1.

Overall survival curve of entire cohort
Fig. 2.
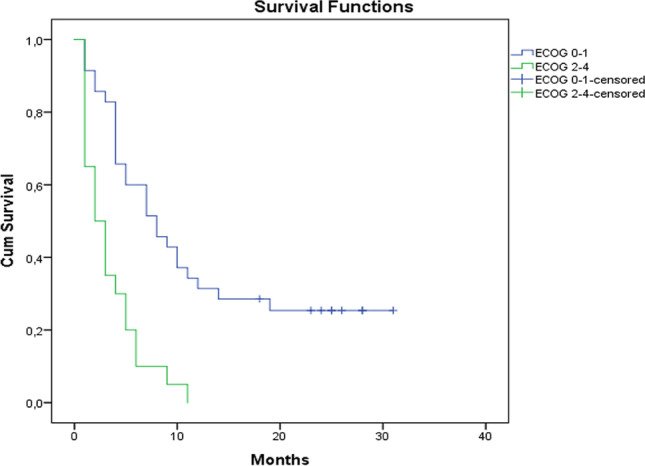
Overall survival curves according to Eastern Cooperative Oncology Group (ECOG) performance status
Fig. 3.

Overall survival curves according to response situation of polatuzumab vedotin, bendamustine, rituximab treatment
Univariate analysis did not show a significant association between survival and age, number of treatment lines before Pola-BR, gender, bulky disease, IPI score at diagnosis and relapse time, advanced stage, extranodal involvement, transplantation situation, and primary refractoriness (all p values > 0.05). Factors that yielded a p value < 0.05 after univariate analysis were then included in the multivariate analysis. Response of Pola-BR, LDH before Pola-BR, and ECOG score were analyzed in the multivariate analysis. Patients who were chemorefractory to Pola-BR and patients who had ECOG score ≥ 2 had a worse survival which was almost double in the multivariate analysis (p = 0.042 and p = 0.029, respectively). Univariate and multivariate analyses are shown in Table 2.
Table 2.
Univariate and multivariate analysis of overall survival
| Parameters | Univariate risk ratio (95% CI), p value | Multivariate risk ratio (95% CI), p value |
|---|---|---|
| Age 0.982 (0.963–1) p = 0.54 | ||
| Response of Pola-BR (CR + PR) | 2.658 (1.42–4.960) p = 0.002* | 1.987 (0.047–1.987) p = 0.042** |
| Number of lines before Pola-BR(based on < 2lines) | 1.26 (0.69–2.30) p = 0.44 | |
| LDH (before Pola-BR) | 1.001 (1–1.002) p = 0.01* | 1.001 (1–1.0001) p = 0.186 |
| Gender (male-based) | 0.601 (0.326–1.11) p = 0.105 | |
| Bulky disease (< 7.5 cm) | 0.747 (0.401–1.392) p = 0.358 | |
| ECOG score 0–1 vs ≥ 2 | 3.361 (1.789–6.314) p = 0.00* | 2.213 (1.087–4.507) p = 0.029** |
| IPI score at diagnosis (0–1 vs > 1) | 1.412 (0.762–2.615) p = 0.273 | |
| IPI score at relapse (0–1 vs > 1) | 1.718 (0.959–3.077) p = 0.69 | |
| Advanced stage | 0.929 (0.448–2.082) p = 0.929 | |
| Extranodal involvement | 1.149 (0.619–2.134) p = 0.660 | |
| Hematopoietic transplantation | 1.653 (0.919–2.973) p = 0.093 | |
| Primary refractoriness | 0.647 (0.361–1.161) p = 0.145 |
Estimation was performed by Cox regression analysis
*Variables with p < 0.05 were included in the multivariate model. Enter method was applied to assess independent variables regarded as significant
**Statistically significant
Pola-BR, polatuzumab vedotin, bendamustine, rituximab; CR, complete response; PR, partial response; ECOG, Eastern Cooperative Oncology Group; IPI, international prognostic index
Adverse events
Forty-nine (69%) patients experienced hematological adverse events (AE) and the same number of patients had non-hematological AEs in all grades. The adverse events are presented in Table 3. The most common AEs of grades 3–4 were infections and cytopenia. When cytopenia was evaluated, the most frequent grades 1–2 hematological AE was anemia observed with the rate of 45%. Neutropenia was the most common in both grades 3–4 and all grades of hematological AEs with a rate of 33.8% and 64.7%, respectively. Bacteremia and sepsis were commonly reported. One of the patients died due to mucormycosis; another one died of severe COVID-19. Grades 1–2 peripheral neuropathy occurred in 19% (n = 14) of patients, whereas grades 3–4 neuropathy were not seen. Fatigue, the most common non-hematological AE, was reported in 53.5% of patients in all grades.
Table 3.
Adverse events
| Adverse events | Grades 1–2 n (%) | Grades 3–4 n (%) | All grades n (%) |
|---|---|---|---|
| Neutropenia | 22 (30.9%) | 24 (33.8%) | 46 (64.7%) |
| Thrombocytopenia | 21 (29.5%) | 21 (29.5%) | 42 (59%) |
| Anemia | 32 (45%) | 12 (16.9%) | 44 (61.9%) |
| Proven infection | 11 (15.4%) | 15 (21.1%) | 26 (36.5%) |
| Febrile neutropenia | 12 (16.9%) | 17 (23.9%) | 29 (40.8%) |
| Nausea | 21 (29.6%) | 4 (5.6%) | 25 (35.2%) |
| Fatigue | 23 (32.4%) | 15 (21.1%) | 38 (53.5%) |
| Diarrhea | 12 (16.9%) | 3 (4.2%) | 15 (21.1%) |
| Decreased appetite | 16 (22.5%) | 9 (12.7%) | 25 (35.2%) |
| Peripheral neuropathy | 14 (19.7%) | 0 | 14 (19.7%) |
Discussion
In our series, the Pola-BR combination in relapsed or refractory DLBCL revealed the following: (i) the overall response rate (ORR) was 47.9%; (ii) with a median follow-up of 5 months, the median OS of our cohort was 5 months; (iii) chemorefractory response of Pola-BR and ECOG score ≥ 2 showed poor survival rates. As far as we know, our study presents the largest cohort in a real-world setting.
The approach of R/R DLBCL has an urgent clinical need despite novel therapies. Patients with R/R DLBCL have very poor outcomes if they experienced treatment failure with immunochemotherapy or any subsequent salvage regimens and early disease relapses after ASCT [9]. In this area, developing therapeutic options should focus on features such as fewer toxicities, better efficacy, and broad accessibility. The Pola-BR treatment modality is a promising and approved chemoimmunotherapy in patients with R/R DLBCL with a manageable safety profile [15–17].
Currently, there are few studies that evaluate the efficacy of Pola-BR treatment in patients with R/R DLBCL. In the randomized, phase 2, GO29365 trial, 40 patients with R/R DLBCL achieved ORR and CR rates of 45% and 40% with 1-year PFS and OS of approximately 40% and 55%, respectively [15]. Following the initial phase 2 trial, an updated result of the extension cohort of a total of 192 R/R DLBCL patients who were transplant ineligible was published [20]. Significant survival benefit with Pola-BR vs. BR was observed with the median OS, 12.4 vs 4.7 months, respectively, and the ORR was 41.5%.
Recently, retrospective real-world studies on DLBCL patients receiving the Pola-BR regimen have been published [8, 10, 19]. Segman et al. reported the tolerability and efficacy of Polatuzumab vedotin, with or without BR, in a real-world setting of 47 heavily pretreated R/R DLBCL patients from Israel [10]. The overall response rate was 61%, including 40% CR and the median PFS and OS were 5.6 months and 8.3 months, respectively. Dimaou et al. reported the outcomes of 61 Greek patients, who received Pola-BR mainly; bendamustine was omitted in three patients due to previous short-lived responses. The ORR was 43% with a CR rate of 25%. The median PFS and OS were 4 and 8.5 months, respectively. Wang et al. evaluated Pola-based salvage chemotherapy, including regimens other than BR, as third-line treatment or beyond for 40 patients with R/R DLBCL from Taiwan. The ORR was 52.5% and 25% of patients had CR with a median follow-up of 18.8 months. The median OS of the cohort was 8.5 months, and that of those receiving subsequent HSCT was 24 months [19]. They speculated that further consolidation therapy after transplantation could provide survival benefit in those patient groups.
Given the lower response rates of real-world studies compared to the phase 2 GO29365 trial, the overall response rate of our study was similar to the results of other retrospective real-world experiences. In the GO29365 trial, 13% of the patients were ECOG PS ≥ 2, and PS 3–4 were excluded whereas 36.6% patients were ECOG PS 2–4 in our cohort. Compared to the results reported in recent studies, the numerically inferior OS in our study might be due to the inclusion of patients with more adverse prognostic factors: advanced stage, high IPI score, bulky disease, and extranodal disease. Univariate analysis revealed no significant association between advanced and refractory disease with poor prognostic features and survival. However, chemorefractory response of Pola-BR and ECOG score ≥ 2 showed poorer survival which was almost double in the multivariate analysis.
In a recent multicenter post-marketing analysis of polatuzumab vedotin for R/R aggressive B-cell lymphoma in the USA, 66 patients with DLBCL and high-grade B-cell lymphoma were included [21]. ORR occurred in 50%, including 24% CR with a median duration of response of 5.1 months, PFS was 2.0 months, and survival was 5.3 months [21]. The results of the study were similar to our current study. We attributed this similarity to the fact that response rates and survival times were low in patients with highly resistant and refractory disease. As in this study (12%), a similar proportion of patients in our study (16.9%) had previously been exposed to bendamustine.
The adverse event profile of Pola-BR in our cohort is comparable to the published data. The most frequent grades 1–2 hematological AE was anemia. Neutropenia and thrombocytopenia were the most common grades 3–4 hematological toxicities. We did not evaluate lymphopenia in the foreground for adverse effects related to the studies conducted on R/R DLBCL lymphoma patients treated with Pola-BR [8, 10, 19, 22]. Among hematological adverse events, anemia and neutropenia were the most seen adverse effects in these studies. In most studies, lymphopenia was not examined. Only in the phase 2 GO29365 trial, while neutropenia was reported at 53.8%, and lymphopenia was reported at 12.8% in all grades in the Pola-BR cohort, lymphopenia was not observed in the BR cohort [15]. Additionally, the extended study of 27-month follow-up of the GO29365 trial in the randomized Pola-BR arm, as well as results of the single-arm extension cohort, was published. In the long-term follow-up trial, the authors did not evaluate lymphopenia [22]. More data are needed to explain the effect of polatuzumab on lymphopenia. Febrile neutropenia and proven infection were the common non-hematological toxicities of Pola-BR treatment. Unlike the literature, fatigue was the one of the common adverse events in our cohort. Grades 1–2 peripheral neuropathy was observed; however, grades 3–4 neuropathy was uncommon. The Pola-BR regimen seems to have a manageable toxicity profile.
Several trials have been conducted with polatuzumab vedotin as the treatment backbone of DLBCL. Polatuzumab vedotin has been used in combination with chemoimmunotherapies in multiple trials [12]. The efficacy and state of polatuzumab vedotin in the treatment of DLBCL will become clear as prospective trials continue. However, it has been proven that the Pola-BR treatment achieves effective and sustainable responses in heavily treated DLBCL patients especially transplant ineligible patients [15, 20]. Polatuzumab vedotin may also be an effective treatment for the intention of bridging to a cellular immunotherapy such as chimeric antigen receptor T-cell therapy. However, this is an evolving area and the use of bendamustine has been shown to compromise T-cell function and the negative effect of apheresis success prior to CAR-T-cell therapy [22]. In our study, no patient had received CAR-T cell since this treatment option is not available in Turkey. Nevertheless, nine patients had the chance to receive HSCT; six of them received allogeneic stem cell transplantation after Pola-BR treatment in our study.
Our study has some limitations including the absence of a comparison group and the retrospective design, where we depended mainly on medical records for data collection. Side effects were also collected retrospectively from medical records. The follow-up period was limited since our sample consisted of a group of high-risk patients who have exhausted all available treatment options. Most of the recruited patients had adverse prognostic factors including the presence of advanced stage, high IPI score, bulky disease, and the requirement of multiple prior lines of treatment. Another important point which may explain the short follow-up duration is the fact that a third of included patients had ECOG PS scores of 2–4. In the follow-up period, fifty-five (77.5%) patients died from their disease who received only median 3 cycles of Pola-BR.
In conclusion, the Pola-BR combination was deemed an effective agent that might provide a therapeutic option for patients with R/R DLBCL given significant response and survival rates compared with BR alone. The real-world data from our cohort of hard-to-treat and aggressive R/R DLBCL showed that the Pola-BR combination is a promising entrant to the R/R DLBCL treatment landscape with a manageable toxicity profile. Further studies are required to improve the identification of patients who will likely benefit from polatuzumab vedotin treatment.
Acknowledgements
We would like to express our gratitude to all dear researchers and centers who contributed from the data collection to the publishing stage of the study:
Tahir Darçın, Hatice Terzi, Abdülkadir Baştürk, Ayşe Nilgün Kul, Burcu Ülküden, Burcu Altındağ, Burhan Turgut, Emine Gültürk, Figen Atalay Noyan, Hakan Özdoğu, Haluk Demiroğlu, Hasan Mücahit Özbaş, Nur Hilal Bozkurt, Rahşan Yıldırım, Tuğrul Elverdi, Neslihan Andiç, Yasin Kalpakçı, Elif Eylül Dikengil, Metban Güzel Mastanzade, Osman Akıdan, Serkan Ünal, Berrin Balık Aydın, Engin Kelkitli, Memiş Hilmi Atay, Tuba Hacıbekiroğlu, Erdal Kurtoğlu, Mehmet Turgut, Semra Paydaş, Levent Ündar.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL et al (2017) WHO Classification of tumours of haematopoietic and lymphoid tissues. In: Swerdlow SH (ed) International Agency for Research on Cancer 2 (rev. ed. 4). IARC Publication, Lyon, France, pp 48–72
- 3.Sant M, Minicozzi P, Mounier M, et al. EUROCARE-5 working group. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 2014;15(9):931–942. doi: 10.1016/S1470-2045(14)70282-7. [DOI] [PubMed] [Google Scholar]
- 4.Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 5.Vacira J, Acs P, Tabbara I, Rosen P, Lee P, Lyman E. Bendamustine combined with rituximab for patients with relapsed or refractory diffuse large B-cell lymphoma. Ann Hematol. 2014;93(3):403–409. doi: 10.1007/s00277-013-1879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohmach K, Niitsu N, Uchida T, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2013;31(17):2103–2109. doi: 10.1200/JCO.2012.46.5203. [DOI] [PubMed] [Google Scholar]
- 7.Arcari A, Chiappella A, Spina M, et al. Safety and efficacy of rituximab plus bendamustine in relapsed or refractory diffuse large B-cell lymphoma patients: an Italian retrospective multicenter study. Leuk Lymphoma. 2016;57(8):1823–1830. doi: 10.3109/10428194.2015.1106536. [DOI] [PubMed] [Google Scholar]
- 8.Dimou M, Papageorgiou SG, Stavroyianni N, et al. Real-life experience with the combination of polatuzumab vedotin, rituximab, and bendamustine in aggressive B-cell lymphomas. Hematol Oncol. 2021;39(3):336–348. doi: 10.1002/hon.2842. [DOI] [PubMed] [Google Scholar]
- 9.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segman Y, Ribakovsky E, Avigdor A, Goldhecht Y, Vainstein V, Goldschmidt N, Harlev S, Horwitz N, Gutwein O, Gurion R, Itchaki G, Abadi U, Nemets A, Sofer O, Zektser M, Tadmor T, Dally N, Filanovsky K, Leiba M, Sarid N, Benyamini N, Herishanu Y, Ram R, Perry C, Avivi I. Outcome of relapsed/refractory diffuse large B-cell lymphoma patients treated with Polatuzumabvedotin-based therapy: real-life experience. Leuk Lymphoma. 2021;62(1):118–124. doi: 10.1080/10428194.2020.1824069. [DOI] [PubMed] [Google Scholar]
- 11.Dornan D, Bennett F, Chen Y, et al. Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkinlymphoma. Blood. 2009;114:2721–2729. doi: 10.1182/blood-2009-02-205500. [DOI] [PubMed] [Google Scholar]
- 12.Deeks ED. Polatuzumab vedotin: first global approval. Drugs. 2019;79:1467–1475. doi: 10.1007/s40265-019-01175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palanca-Wessels MCA, Czuczman M, Salles G, et al. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed orrefractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 2015;16:704–715. doi: 10.1016/S1470-2045(15)70128-2. [DOI] [PubMed] [Google Scholar]
- 14.Morschhauser F, Flinn IW, Advani R, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS) Lancet Haematol. 2019;6:254–265. doi: 10.1016/S2352-3026(19)30026-2. [DOI] [PubMed] [Google Scholar]
- 15.Sehn L, Herrera A, Flowers C, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155–165. doi: 10.1200/JCO.19.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polivy PrescribingInformation US (2020). https://www.gene.com/download/pdf/polivy_prescribing.pdf
- 17.European Commission approves Roche’s Polivy for people with previously treated aggressive lymphoma. Roche. Published January 21, 2020. https://bit.ly/2GcYia0. Accessed January 21, 2020.
- 18.Cheson B, Fisher R, Barrington S, et al. United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y-W, Tsai XC-H, Hou H-A, Tien F-M, Liu J-H, Chou W-C, et al. Polatuzumab vedotin–based salvage immunochemotherapy as third-line or beyond treatment for patients with diffuse large B-cell lymphoma: a real-world experience. Ann Hematol. 2022;101(2):349–58. doi: 10.1007/s00277-021-04711-9. [DOI] [PubMed] [Google Scholar]
- 20.Sehn LH, Hertzberg M, Opat S, Herrera AF, Assouline S, Flowers CR, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533–543. doi: 10.1182/bloodadvances.2021005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SD, Lopedote P, Samara Y, Mei M, Herrera AF, Winter AM, et al. Polatuzumab vedotin for relapsed/refractory aggressive B-cell lymphoma: a multicenter post-marketing analysis. Clin Lymphoma Myeloma Leuk. 2021;21(3):170–175. doi: 10.1016/j.clml.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Liebers N, Duell J, Fitzgerald D, Kerkhoff A, Noerenberg D, Kaebisch E, et al. Polatuzumab vedotin as a salvage and bridging treatment in relapsed or refractory large B-cell lymphomas. Blood Adv. 2021;5(13):2707–2716. doi: 10.1182/bloodadvances.2020004155. [DOI] [PMC free article] [PubMed] [Google Scholar]


