Abstract
Macrophages are among the most sensitive targets of bacterial endotoxin (LPS), responding to minute amounts of LPS by releasing a battery of inflammatory mediators. Transfection of macrophages with secretory leukocyte protease inhibitor (SLPI) renders these cells refractory to LPS stimulation. Here we show that uptake of LPS from soluble CD14 (sCD14)-LPS complexes by SLPI-overexpressing cells was only 50% of that seen in control cells. SLPI transfectants and mock transfectants did not differ in the surface expression of CD14 or CD18. We show, in addition, that recombinant human SLPI can bind to purified endotoxin in vitro. SLPI caused a decrease in the binding of LPS to sCD14 as assessed both by fluorescence quenching of labeled LPS and by nondenaturing polyacrylamide gel electrophoresis. These results suggest that the inhibitory effect of SLPI on macrophage responses to LPS may, in part, be due to its blockade of LPS transfer to soluble CD14 and its interference with uptake of LPS from LPS-sCD14 complexes by macrophages.
Bacterial lipopolysaccharide (LPS) is a potent stimulator of the immune system (26, 32, 38). In mammals, very low concentrations of LPS signify the threat of gram-negative bacteria and evoke a rapid innate response from the host to ensure that bacteria are properly contained and destroyed. However, the overexuberant systemic human response to LPS is a major clinical problem that has been traced in rodent models to LPS-mediated elicitation of numerous bioactive products from macrophages, including tumor necrosis factor (TNF); interleukins-1, -6, -8, and -10; eicosanoids; platelet-activating factor; H2O2; and NO (3, 27, 30). Identifying cellular products that can suppress macrophage responses to LPS and elucidating the mechanisms involved are crucial for understanding how macrophages regulate their response to LPS and for developing novel therapeutics for the treatment of septic shock.
To learn more about LPS signaling, we previously performed differential-display analysis on matched macrophage cell lines from two strains of mice (C3H/HeJ and C3H/HeN) congenic for a locus (lps) markedly affecting LPS sensitivity (33, 37). Secretory leukocyte protease inhibitor (SLPI) was identified as one of two transcripts highly abundant in LPS-hyporesponsive cells but absent in LPS-responsive cells (20, 21). SLPI appears to be an inhibitor of macrophage responses to LPS, since transfection of SLPI into an LPS-normoresponsive macrophage cell line, HeN.C2, induces an LPS-hyporesponsive phenotype as measured by LPS-induced secretion of nitric oxide and TNF (21). This inhibition likely reflects SLPI’s interference with early signaling events, since activation of transcription factor NF-κB complexes by LPS is also inhibited (21). The mechanism by which SLPI suppresses macrophage responses to LPS is unknown.
CD14 plays a crucial role in mediating cellular responses to endotoxin by facilitating uptake of LPS by macrophages in a two-step process (38). In the first step, an LPS monomer exits the bacterial outer membrane or LPS aggregate and binds to a site on CD14. LPS-binding protein (LBP) facilitates this step (15). In the second step, CD14 transfers LPS monomers into cell membranes (16, 36, 41). Monomeric LPS presented to cells as LPS-soluble CD14 (sCD14) complexes is rapidly incorporated into plasma membranes of neutrophils and macrophages (5, 10, 16, 36) and provokes strong responses. To explain the anti-inflammatory effect of SLPI observed in the above-mentioned studies, we hypothesized that SLPI may have an effect on early inflammatory responses by blocking either the binding of LPS to sCD14 or the movement of LPS from sCD14 into cell membranes. We report here that SLPI has both of these activities. Recombinant human SLPI (rhSLPI) binds to purified LPS and prevents formation of LPS-sCD14 complexes. Additional studies show that SLPI also binds LPS-sCD14 complexes and it slows the transfer of LPS from the complexes to macrophages.
MATERIALS AND METHODS
Reagents.
rhSLPI was kindly provided by Amgen Inc. (Thousand Oaks, Calif.). Recombinant human sCD14, purified from conditioned medium of Schneider-2 insect cells transfected with cDNA encoding human CD14, was a gift from R. Thieringer (Merck Research Laboratories, Rahway, N.J.). Purified rabbit immunoglobulin G (IgG) raised against human sCD14 was provided by P. Detmers (Merck Research Laboratories). Rabbit antiserum to SLPI was raised against recombinant mouse SLPI as previously described and cross-reacts with human SLPI (21). Phycoerythrin (PE)-conjugated antibodies against mouse CD14 (lot M024094) and mouse CD18 (lot M019408) and control rabbit anti-mouse IgG1 were from Pharmingen (San Diego, Calif.). The reagents ovalbumin, glycerol, bromophenol blue, EDTA, and paraformaldehyde were from Sigma.
LPS prepared by phenol extraction from Escherichia coli 0111:B4 and [3H]LPS, biosynthetically radiolabeled from E. coli K-12 LCD25 (Rb chemotype; specific activity, 2.08 × 106 dpm/μg), were from List Biological Laboratories (Campbell, Calif.). Boron dipyrromethene difluoride (BODIPY)-conjugated LPS (BODIPY-LPS) was prepared from LPS (Salmonella minnesota) by using a BODIPY FL amine labeling kit (Molecular Probes, Eugene, Oreg.) as previously described (41). To deliver LPS as a monomer, we first formed complexes of BODIPY-LPS with sCD14 by incubating 100 μg of sCD14 per ml with 5 μg of LPS per ml for 16 h at 37°C. Under these conditions, all of the LPS forms stoichiometric complexes with monomeric sCD14. These complexes efficiently stimulate cells and deliver LPS to the membrane (16, 36).
Cells.
HeN.C2 and GG2EE cells are bone marrow-derived, v-myc- and v-raf-transformed macrophage cell lines (1, 4). HeN.C2 cells stably transfected with p463-neo-SLPI and p463-neo vectors were generated as previously described (21) and maintained in 500 μg of G418 per ml. The RAW 264.7 macrophage cell line was from the American Type Culture Collection, Manassas, Va. All cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah), 2 mM l-glutamine, 200 U of penicillin per ml, and 200 μg of streptomycin per ml at 37°C in 5% CO2–95% air. Complete culture medium was routinely monitored for LPS contamination with a chromogenic Limulus amebocyte lysate test (BioWhittaker Inc., Walkersville, Md.) and found to contain <25 pg of LPS per ml.
Secretion of nitrite.
Cells were plated in 96-well plates at 105 cells per well in 150 μl of medium and treated for 48 h with various concentrations of LPS. Conditioned medium (100 μl) was mixed with an equal volume of Griess’s reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, 2.5% H3PO4). A550 was recorded in a microplate reader (MR5000; Dynatech, Chantilly, Va.) with sodium nitrite as the standard. The nitrite content of similarly incubated cell-free medium was subtracted from all samples.
Measurement of BODIPY-LPS uptake.
To measure the relative BODIPY-LPS uptake by murine macrophages, 106 cells were incubated with BODIPY-LPS-sCD14 complexes (25 ng of BODIPY-LPS per ml) for 30 min at 37°C. After washing in phosphate-buffered saline (PBS) with 0.5 U of aprotinin per ml–0.05% human serum albumin–3 mM d-glucose (HAP buffer), the cells were analyzed by flow cytometry on a FACScan (Becton-Dickinson, Mountain View, Calif.) to measure the cell-associated fluorescence intensity of BODIPY-LPS. Ten thousand events were acquired per sample. Background fluorescence was determined with cells incubated without BODIPY-LPS-sCD14 or BODIPY-LPS complexes, and this was subtracted from the mean fluorescence channel for the experimental samples.
Flow cytometric analysis of CD14 and CD18 expression in macrophages.
One million cells of each type were washed once with PBS and resuspended in PBS plus 0.1% bovine serum albumin. The following PE-conjugated antibodies were added: anti-mouse IgG1, anti-mouse CD14, and anti-mouse CD18. After 1 h of incubation on ice, the cells were washed with PBS twice and resuspended in 1 ml of 1% paraformaldehyde in PBS. Flow cytometric analysis was performed by using the Coulter EPICS-C system (Coulter Electronics, Hialeah, Fla.). Background binding was estimated by using an isotype-matched anti-mouse IgG1 antibody.
Fluorescence quenching assay of LPS binding to CD14.
The fluorescence of BODIPY-LPS is quenched when the LPS is aggregated, but quenching is reduced upon the movement of an LPS monomer into sCD14. To assay binding of BODIPY-LPS to CD14, fluorescence was measured as previously described (29). BODIPY-LPS aggregates (0.2 μg/ml) were added to PBS containing 2 mg of human serum albumin per ml and incubated for 30 min at 37°C with increasing concentrations of hrSLPI alone or with sCD14 (5 μg/ml). At the end of the incubation, the fluorescence of each sample was measured before and after addition of sodium dodecyl sulfate (SDS) in a spectrofluorometer with excitation at 485 nm and emission at 515 nm. SDS disaggregates LPS and yields maximal fluorescence efficiency. Results are expressed as relative fluorescence (percent), which represents the ratio of fluorescence measured to the total fluorescence in the sample (measured in 4% SDS).
Native polyacrylamide gel electrophoresis.
Proteins and sonicated LPS were mixed in 10 μl of PBS with 1 mM EDTA and incubated at 37°C overnight on a shaker. Two microliters of a loading buffer containing 0.015% bromophenol blue and 50% glycerol was added to each sample before electrophoresis. Electrophoresis was performed on 8% Tris-glycine polyacrylamide minigels at 100 V for 1.5 h in a running buffer containing 24 mM Tris–192 mM glycine, pH 8.3. Gels containing [3H]LPS were fixed in 30% methanol and 8% acetic acid for 1 h, soaked in En3Hance (DuPont NEN, Boston, Mass.) for 1 h, rinsed with cold water for 30 min, dried, and exposed to Kodak XAR film for 3 days. Alternatively, gels were silver stained.
RESULTS
Overexpression of SLPI decreases the sensitivity of macrophages to LPS but not their ability to respond to LPS.
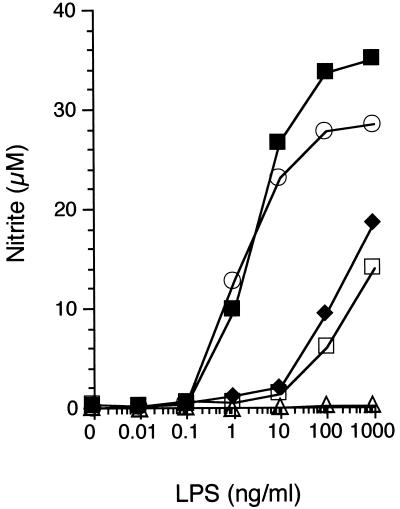
To assess whether overexpression of SLPI in HeN.C2 cells alters their sensitivity to LPS or whether it interferes with their global capacity to generate LPS-inducible products, we compared LPS-induced nitrite and TNF production in five macrophage cell lines: HeN.C2 parental cells, two independently selected SLPI-overexpressing cell lines (HeN.C2-C6C10 and HeN.C2-D4F9), one mock transfectant (HeN.C2-C2C7), and GG2EE, an LPS-hyporesponsive cell line constitutively expressing a high level of SLPI (21). Macrophages from each cell line were incubated with increasing concentrations of LPS, and the conditioned media were harvested 24 to 48 h after stimulation. As in previous work (21), both SLPI-overexpressing cell lines were unresponsive to concentrations of LPS that induced copious amounts of nitrite (Fig. 1) or TNF (data not shown). However, they generated substantial quantities of nitrite and TNF in response to high concentrations of LPS. These data indicate that SLPI decreases the cells’ sensitivity to LPS but does not abolish the ability of these cells to produce inflammatory mediators such as NO or TNF. The inability of GG2EE cells to respond to high concentrations of LPS, in contrast to the SLPI-overexpressing cells, most likely reflects a compound phenotype involving both SLPI overexpression and its lps gene defect (1, 21, 31, 34).
FIG. 1.
Overexpression of SLPI interferes with macrophage sensitivity to LPS. HeN.C2 (■, low SLPI expression), GG2EE (▵, high SLPI expression), HeN.C2-C2C7 (○, low SLPI expression), HeN.C2-C6C10 (⧫, SLPI overexpression), and HeN.C2-D4F9 (□, SLPI overexpression) cells (105 per well) were exposed to different concentrations of LPS, as indicated, for 48 h. Nitrite accumulation from the conditioned medium was determined. Results are means of triplicate determinations. Error bars indicating the standard error of the mean fall within the symbols. The results of one of five similar experiments are shown.
Inhibition of BODIPY-LPS uptake by macrophages overexpressing SLPI.
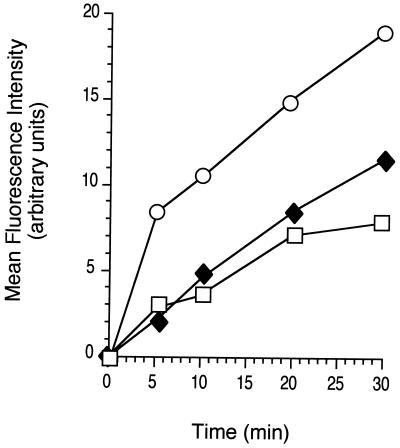
The secretory nature of SLPI prompted us to examine whether the attenuated response to LPS in SLPI-overexpressing cells is due to impairment of their ability to take up LPS. BODIPY-LPS and recombinant sCD14 were first incubated overnight to form BODIPY-LPS-sCD14 complexes. Macrophage cell lines overexpressing SLPI (HeN.C2-D4F9 and HeN.C2-C6C10) and the mock transfectant (HeN.C2-C2C7) were incubated with BODIPY-LPS-sCD14 complexes for 0, 5, 10, 20, or 30 min at 37°C. Cell-associated fluorescence was analyzed by flow cytometry on a FACScan and used as an indicator for the uptake of LPS. Both stable transfectants had approximately half as much cell-associated fluorescence as the mock transfectant at all time points, which was readily detected 2 to 5 min after incubation of cells with LPS-sCD14 complexes (Fig. 2). Thus, overexpression of SLPI appears to interfere with uptake of LPS by macrophages.
FIG. 2.
Decreased uptake of BODIPY-LPS-sCD14 complexes by SLPI-transfected macrophages. One million cells each of the mock transfectant HeN.C2-C2C7 (○) and SLPI transfectants HeN.C2-C6C10 (⧫) and HeN.C2-D4F9 (□) were incubated with BODIPY-LPS-sCD14 (25 ng of BODIPY-LPS per ml) for 30 min at 37°C and washed, and uptake of BODIPY-LPS was measured by flow cytometry. The results of one of four similar experiments are shown.
SLPI-producing and SLPI-nonproducing cells express comparable levels of surface CD14 and surface CD18.
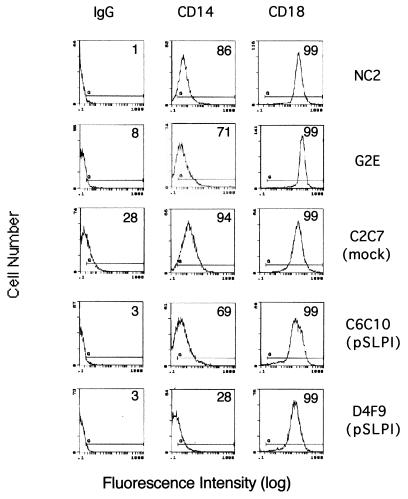
The decrease in LPS uptake by SLPI-producing cells prompted us to examine whether these cells have diminished surface expression of known LPS-binding proteins. Two LPS-binding proteins on the macrophage surface have been implicated in LPS functions to date: myeloid differentiation antigen CD14 (23, 38, 40) and β2 integrins CD11/CD18 (9, 18, 39). We compared the surface expression of CD14 and CD18 on two stable SLPI transfectants, mock-transfected cells, parental HeN.C2 cells, and GG2EE cells by flow cytometry. All five cell lines had comparable CD18 surface expression (Fig. 3). One of the SLPI-overexpressing cell lines, HeN.C2-D4F9, had slightly decreased surface expression of CD14. However, neither the second SLPI-overexpressing cell line nor GG2EE cells had diminished surface CD14 compared to HeN.C2 cells. Thus, reduced surface expression of CD14 and CD18 could not account for the LPS-hyporesponsive phenotype of GG2EE cells and SLPI transfectants, including their diminished uptake of LPS.
FIG. 3.
SLPI-producing and SLPI-nonproducing cells express comparable CD14 and CD18. The surface CD14 and surface CD18 expression of cells of different lines that express abundant or no SLPI was assessed by fluorescence-activated cell sorter analysis with direct immunofluorescence staining using purified rabbit anti-mouse IgG1-PE (isotype-matched antibody control), anti-mouse CD14-PE, and anti-mouse CD18-PE. Histograms of fluorescence versus cell population are shown. The percentage of positive cells for each condition is shown.
SLPI decreases movement of LPS to CD14.
CD14 binds LPS monomers, and the resulting LPS-sCD14 complexes play a key role in initiating cellular responses. To test whether SLPI might affect complex formation, we mixed rhSLPI with BODIPY-LPS in the absence or presence of sCD14 and measured the change in fluorescence after incubation at 37°C. rhSLPI was used since recombinant mouse SLPI was unavailable. The overall sequence homology of these two proteins is 60%, with positional conservation of all 16 cysteine residues (21).
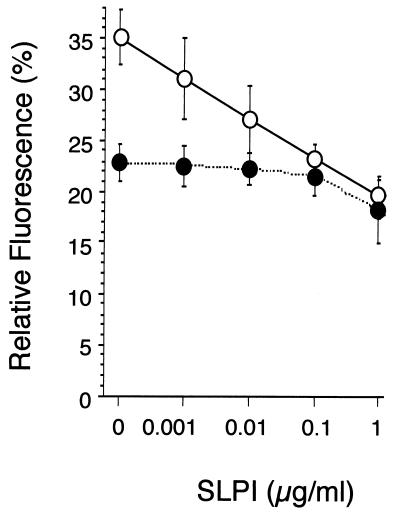
When LPS is in aqueous solution, the micelle form of LPS predominates. In its micelle form, the fluorescence of BODIPY-LPS is quenched to about 23% (Fig. 4). Addition of 1 μg of SLPI per ml enhanced the quenching of BODIPY-LPS (Fig. 4), which was readily detectable after 5 min of incubation (data not shown). This suggests that SLPI might interact with and stabilize BODIPY-LPS aggregates. Transfer of BODIPY-LPS monomers from micelles into sCD14 relieves the fluorescence quenching (41). As shown in Fig. 4, addition of sCD14 to BODIPY-LPS micelles induced an increase in relative fluorescence after 30 min of incubation at 37°C. However, increasing concentrations of SLPI coincubated with sCD14 and BODIPY-LPS prevented the increase in fluorescence. The change in fluorescence of BODIPY-LPS-sCD14 complexes caused by rhSLPI suggests that SLPI blocks movement of LPS from aggregates to sCD14.
FIG. 4.
Dose dependence of SLPI blockage of the transfer of LPS to sCD14. BODIPY-LPS (0.2 μg/ml) was incubated for 30 min at 37°C with increasing concentrations of rhSLPI in the absence (●) or presence (○) of sCD14 (5 μg/ml). Fluorescence intensity was measured before and after addition of SDS by spectrofluorometry. Results are expressed as the percentage of relative fluorescence, representing the ratio of fluorescence measured to the total fluorescence in the sample (in 4% SDS). Samples were tested in duplicate, and averages and standard deviations are shown. The experiment was repeated twice with essentially similar results.
Interference of rhSLPI with LPS-CD14 complex formation.
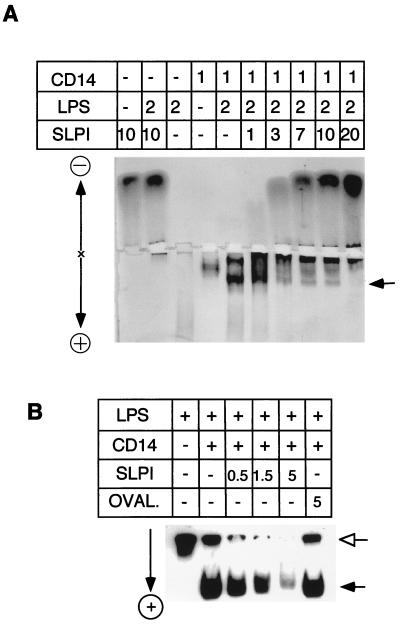
Nondenaturing polyacrylamide gel electrophoresis has been used to detect the interaction of LPS and sCD14 (14, 15), and we used this technique to further study the blockade of LPS-sCD14 formation by SLPI. To see whether SLPI can interfere with the formation of a complex, LPS and sCD14 were incubated overnight with increasing concentrations of rhSLPI and the complexes were resolved on a nondenaturing polyacrylamide gel and finally visualized by silver staining. Increasing concentrations of rhSLPI significantly decreased the staining of the LPS-sCD14 complex (Fig. 5A, arrow). Although LPS stains poorly in the gel (lane 3), the influence of SLPI on its migration was detectable (compare lane 2 with 3). To further clarify how SLPI affects the migration pattern of LPS, we repeated the experiment with tritiated LPS prepared from E. coli K-12 LCD25. Figure 5B shows that incubation with 0.5, 1.5, or 5 μg of SLPI caused a decrease in the intensity of labeled LPS at both sCD14-associated (filled arrow) and -nonassociated (open arrow) positions, indicating interactions of SLPI with both LPS-sCD14 complexes and LPS alone. As a control protein, ovalbumin had no effect on the migration of LPS-sCD14. The decrease of tritiated LPS in the gel upon addition of SLPI most likely reflects the fact that SLPI forms complexes with tritiated LPS which have little net charge and therefore migrate toward neither the anode nor the cathode.
FIG. 5.
Interaction of SLPI and LPS or CD14-LPS complexes in a cell-free system. LPS, sCD14, and rhSLPI were incubated overnight at 37°C, either alone or in combination, as indicated (quantities are expressed in micrograms), and separated on nondenaturing gels. (A) Effect of SLPI on LPS-CD14 complexes on a silver-stained polyacrylamide gel. (B) Autoradiogram of mixtures of [3H]LPS (0.05 μg) with or without 0.5 μg of sCD14 plus the indicated amounts of SLPI or ovalbumin (OVAL.) in a nondenaturing gel. Electrophoretic polarities are indicated next to the gels. The arrows indicate the positions of LPS (open) and LPS-sCD14 complexes (filled).
DISCUSSION
Discovered as an 11.7-kDa secretory product of epithelial cells (35), SLPI was best known for its inhibitory actions on leukocyte serine proteases such as elastase and cathepsin G from neutrophils (28, 35), chymase and tryptase from mast cells (6, 8), and trypsin and chymotrypsin from pancreatic acinar cells (11). It has been suggested that SLPI’s primary role is to protect the epithelia from proteolytic degradation by leukocytes during inflammation (13). Recent work in this laboratory and others has identified new sources of SLPI—macrophages and neutrophils (2, 21); new patterns of gene regulation—induction by gram-positive or gram-negative bacterial products (19); and three new functions—to protect monocytes from human immunodeficiency virus infection (24), to display defensinlike antibacterial activities against E. coli and S. aureus (17), and to suppress inflammatory mediator production by macrophages (21, 42). In addition, we have observed increased serum SLPI levels both in human volunteers given intravenous LPS and in septic patients (12).
The mechanisms by which SLPI exerts its multiple effects are largely unknown, with the exception of its antiproteinase function, which is believed to result from strong binding of SLPI to the active sites of proteases (13, 22). Interestingly, human mutant SLPIs with greatly reduced antiproteinase activity are still capable of preventing viral infection in monocytes (25) and suppressing prostaglandin H synthase-2 expression and secretion of prostaglandin E2 from human monocytes (42). Thus, SLPI may affect macrophage function independently of its antiproteinase activity.
Our data indicate that SLPI interacts directly with bacterial LPS in vitro (Fig. 4 and 5). Fath et al. recently reported that SLPI could interact in vitro with polysaccharides such as dermatan, heparan, and dextran sulfates, enhancing the antiproteinase activity of SLPI (7). Given the structural and ionic similarities between mammalian polysaccharides and bacterial LPS, it is possible that LPS may also influence the antiproteinase activity of SLPI. Likewise, by binding to polysaccharides, SLPI may affect the physiological functions of these molecules. We propose here that SLPI blocks the response to LPS by binding to endotoxin. This binding has at least two distinct effects. First, it blocks the formation of LPS-sCD14 complexes (Fig. 4 and 5), a key step in the response to LPS. Second, SLPI appears to bind not only to LPS aggregates but also to the LPS in LPS-sCD14 complexes (Fig. 5). This binding may account for the decreased transfer of LPS from LPS-sCD14 complexes to macrophages (Fig. 2). Both effects can contribute to diminished cellular responsiveness to LPS. Because cells including macrophages and neutrophils produce increased amounts of SLPI in response to inflammatory signals (19), accumulation of SLPI in the local tissue environment may thus represent an endogenous feedback inhibition mechanism.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI30165 to A.D.
We thank Amgen for providing hrSLPI, P. Detmers and R. Thieringer for reagents, and C. Nathan and M. Shiloh for critical reading of the manuscript.
REFERENCES
- 1.Blasi E, Radzioch D, Durum S K, Varesio L. A murine macrophage cell line, immortalized by v-raf and v-myc oncogenes, exhibits normal macrophage functions. Eur J Immunol. 1987;17:1491–1498. doi: 10.1002/eji.1830171016. [DOI] [PubMed] [Google Scholar]
- 2.Bohm B, Aigner T, Kinne R, Burkhardt H. The serine-protease inhibitor of cartilage matrix is not a chondrocytic gene product. Eur J Biochem. 1992;207:773–779. doi: 10.1111/j.1432-1033.1992.tb17108.x. [DOI] [PubMed] [Google Scholar]
- 3.Bone R C. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 4.Cox G W, Mathieson B J, Gandino L, Blasi E, Radzioch D, Varesio L. Heterogeneity of hematopoietic cells immortalized by v-myc/v-raf recombinant retrovirus infection of bone marrow or fetal liver. J Natl Cancer Inst. 1989;81:1492–1496. doi: 10.1093/jnci/81.19.1492. [DOI] [PubMed] [Google Scholar]
- 5.Detmers P A, Thieblemont N, Vasselon T, Pironkova R, Miller D S, Wright S D. Potential role of membrane internalization and vesicle fusion in adhesion of neutrophils in response to lipopolysaccharide and TNF. J Immunol. 1996;157:5589–5596. [PubMed] [Google Scholar]
- 6.Dietze S C, Sommerhoff C P, Fritz H. Inhibition of histamine release from human mast cells ex vivo by natural and synthetic chymase inhibitors. Biol Chem Hoppe-Seyler. 1990;371:75–79. [PubMed] [Google Scholar]
- 7.Fath M A, Wu X, Hileman R E, Linhardt R J, Kashem M A, Nelson R M, Wright C D, Abraham W M. Interaction of secretory leukocyte protease inhibitor with heparin inhibits proteases involved in asthma. J Biol Chem. 1998;273:13563–13569. doi: 10.1074/jbc.273.22.13563. [DOI] [PubMed] [Google Scholar]
- 8.Fink E, Nettelbeck R, Fritz H. Inhibition of mast cell chymase by eglin c and antileukoprotease (HUSI-I). Indications for potential biological functions of these inhibitors. Biol Chem Hoppe-Seyler. 1986;367:567–571. doi: 10.1515/bchm3.1986.367.2.567. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty S F, Golenbock D T, Milham F H, Ingalls R R. CD11/CD18 leukocyte integrins: new signaling receptors for bacterial endotoxin. J Surg Res. 1997;73:85–89. doi: 10.1006/jsre.1997.5195. [DOI] [PubMed] [Google Scholar]
- 10.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz H. Human mucus proteinase inhibitor (human MPI). Human seminal inhibitor I (HUSI-I), antileukoprotease (ALP), secretory leukocyte protease inhibitor (SLPI) Biol Chem Hoppe-Seyler. 1988;369:79–82. [PubMed] [Google Scholar]
- 12.Grobmyer, S. R., P. S. Barie, C. F. Nathan, M. Fuortes, E. Lin, S. F. Lowry, C. D. Wright, M. J. Weyant, L. Hydo, F. Reeves, M. U. Shiloh, and A. Ding. Secretory leukocyte protease inhibitor, an inhibitor of neutrophil activation, is elevated in serum in human sepsis and experimental endotoxemia. Crit. Care Med., in press. [DOI] [PubMed]
- 13.Grutter M G, Fendrich G, Huber R, Bode W. The 2.5 A X-ray crystal structure of the acid-stable proteinase inhibitor from human mucous secretions analysed in its complex with bovine alpha-chymotrypsin. EMBO J. 1988;7:345–351. doi: 10.1002/j.1460-2075.1988.tb02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hailman E, Albers J J, Wolfbauer G, Tu A Y, Wright S D. Neutralization and transfer of lipopolysaccharide by phospholipid transfer protein. J Biol Chem. 1996;271:12172–12178. doi: 10.1074/jbc.271.21.12172. [DOI] [PubMed] [Google Scholar]
- 15.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hailman E, Vasselon T, Kelley M, Busse L A, Hu M C, Lichenstein H S, Detmers P A, Wright S D. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- 17.Hiemstra P S, Maassen R J, Stolk J, Heinzel-Wieland R, Steffens G J, Dijkman J H. Antibacterial activity of antileukoprotease. Infect Immun. 1996;64:4520–4524. doi: 10.1128/iai.64.11.4520-4524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingalls R R, Golenbock D T. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med. 1995;181:1473–1479. doi: 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin F, Nathan C F, Radzioch D, Ding A. Lipopolysaccharide-related stimuli induce expression of the secretory leukocyte protease inhibitor, a macrophage-derived lipopolysaccharide inhibitor. Infect Immun. 1998;66:2447–2452. doi: 10.1128/iai.66.6.2447-2452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin F Y, Nathan C, Ding A. Paradoxical preservation of an LPS response in C3H/HeJ macrophages: induction of matrix metalloproteinase-9. J Immunol. 1999;162:3596–3600. [PubMed] [Google Scholar]
- 21.Jin F Y, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 22.Kramps J A, van Twisk C, Appelhans H, Meckelein B, Nikiforov T, Dijkman J H. Proteinase inhibitory activities of antileukoprotease are represented by its second COOH-terminal domain. Biochim Biophys Acta. 1990;1038:178–185. doi: 10.1016/0167-4838(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 23.Lee J D, Kato K, Tobias P S, Kirkland T N, Ulevitch R J. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992;175:1697–1705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeely T B, Dealy M, Dripps D J, Orenstein J M, Eisenberg S P, Wahl S M. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Investig. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeely T B, Shugars D C, Rosendahl M, Tucker C, Eisenberg S P, Wahl S M. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90:1141–1149. [PubMed] [Google Scholar]
- 26.Morrison D C, Ryan J L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- 27.Nathan C F. Secretory products of macrophages. J Clin Investig. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohlsson K, Bergenfeldt M, Bjork P. Functional studies of human secretory leukocyte protease inhibitor. Adv Exp Med Biol. 1988;240:123–131. doi: 10.1007/978-1-4613-1057-0_15. [DOI] [PubMed] [Google Scholar]
- 29.Park C T, Creasey A A, Wright S D. Tissue factor pathway inhibitor blocks cellular effects of endotoxin by binding to endotoxin and interfering with transfer to CD14. Blood. 1997;89:4268–4274. [PubMed] [Google Scholar]
- 30.Parker M M, Parrillo J E. Septic shock. Hemodynamics and pathogenesis. JAMA. 1983;250:3324–3327. [PubMed] [Google Scholar]
- 31.Qureshi S T, Larivière L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:611–614. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raetz C R, Ulevitch R J, Wright S D, Sibley C H, Ding A, Nathan C F. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J. 1991;5:2652–2660. doi: 10.1096/fasebj.5.12.1916089. [DOI] [PubMed] [Google Scholar]
- 33.Sultzer B M. Genetic control of leucocyte responses to endotoxin. Nature. 1968;219:1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- 34.Sultzer B M. Genetic factors in leucocyte responses to endotoxin: further studies in mice. J Immunol. 1969;103:32–38. [PubMed] [Google Scholar]
- 35.Thompson R C, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci USA. 1986;83:6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasselon T, Pironkova R, Detmers P A. Sensitive responses of leukocytes to lipopolysaccharide require a protein distinct from CD14 at the cell surface. J Immunol. 1997;159:4498–4505. [PubMed] [Google Scholar]
- 37.Watson J, Largen M, McAdam K P. Genetic control of endotoxic responses in mice. J Exp Med. 1978;147:39–49. doi: 10.1084/jem.147.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright S D. Innate recognition of microbial lipids. In: Gallin R S J I, Fearon D, Haynes B, Nathan C, editors. Inflammation: basic principles and clinical correlates. New York, N.Y: Raven Press; 1999. [Google Scholar]
- 39.Wright S D, Jong M T. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986;164:1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 41.Yu B, Wright S D. Catalytic properties of lipopolysaccharide (LPS) binding protein. Transfer of LPS to soluble CD14. J Biol Chem. 1996;271:4100–4105. doi: 10.1074/jbc.271.8.4100. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, DeWitt D L, McNeely T B, Wahl S M, Wahl L M. Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase-2, prostaglandin E2 and matrix metalloproteinases. J Clin Investig. 1997;99:894–900. doi: 10.1172/JCI119254. [DOI] [PMC free article] [PubMed] [Google Scholar]