Abstract
Purpose
High-flow nasal cannula (HFNC) oxygen therapy was noninferior to noninvasive ventilation (NIV) for preventing reintubation in a heterogeneous population at high-risk for extubation failure. However, outcomes might differ in certain subgroups of patients. Thus, we aimed to determine whether NIV with active humidification is superior to HFNC in preventing reintubation in patients with ≥ 4 risk factors (very high risk for extubation failure).
Methods
Randomized controlled trial in two intensive care units in Spain (June 2020‒June 2021). Patients ready for planned extubation with ≥ 4 of the following risk factors for reintubation were included: age > 65 years, Acute Physiology and Chronic Health Evaluation II score > 12 on extubation day, body mass index > 30, inadequate secretions management, difficult or prolonged weaning, ≥ 2 comorbidities, acute heart failure indicating mechanical ventilation, moderate-to-severe chronic obstructive pulmonary disease, airway patency problems, prolonged mechanical ventilation, or hypercapnia on finishing the spontaneous breathing trial. Patients were randomized to undergo NIV with active humidification or HFNC for 48 h after extubation. The primary outcome was reintubation rate within 7 days after extubation. Secondary outcomes included postextubation respiratory failure, respiratory infection, sepsis, multiorgan failure, length of stay, mortality, adverse events, and time to reintubation.
Results
Of 182 patients (mean age, 60 [standard deviation (SD), 15] years; 117 [64%] men), 92 received NIV and 90 HFNC. Reintubation was required in 21 (23.3%) patients receiving NIV vs 35 (38.8%) of those receiving HFNC (difference −15.5%; 95% confidence interval (CI) −28.3 to −1%). Hospital length of stay was lower in those patients treated with NIV (20 [12‒36.7] days vs 26.5 [15‒45] days, difference 6.5 [95%CI 0.5–21.1]). No additional differences in the other secondary outcomes were observed.
Conclusions
Among adult critically ill patients at very high-risk for extubation failure, NIV with active humidification was superior to HFNC for preventing reintubation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06919-3.
Keywords: Weaning, Reintubation, High-flow nasal cannula, Noninvasive ventilation, Active humidification, Outcome
Take-home message
| 1) Our findings support a redefinition of high risk for extubation failure, as the different response to preventive choices deserve at least two subgroups of patients at high risk. | |
| 2) The additive effect of high-risk factors for extubation failure associate a higher risk for reintubation and a better response to preventive noninvasive ventilation. | |
| 3) Noninvasive ventilation with active humidification improves patient´s comfort and increase the real time of application. There is a risk for delaying reintubation. |
Introduction
Recent clinical guidelines for liberating patients at risk for extubation failure recommend the use of preventive noninvasive ventilation (NIV) [1, 2]. NIV is recommended in patients whom clinicians would normally consider NIV beneficial (i.e. hypercapnic and obese patients) [3], however, it is not clear which subgroups of patients could benefit with HFNC or NIV. In addition, combined HFNC plus NIV was recently added to the postextubation arsental [4], further increasing the complexity of this topic.
Traditionally, the risk for extubation failure has been defined as the presence of at least one high-risk factor for reintubation [4–7]. However, this definition has important shortcomings mainly due to the fact that, in critically ill patients, various high-risk factors have been used. These risk factors included age [4–7], prolonged mechanical ventilation [7, 8], Acute Physiology and Chronic Health Evaluation (APACHE) II score [5–7], difficult weaning [5, 7], obesity [7, 9], the presence of comorbidities [5, 7], the occurrence of hypercapnia at the end of the spontaneous breathing trial [4, 10], airway patency problems [5, 7], difficulties in managing respiratory secretions [5, 7], acute [5, 7] or chronic heart failure [4], and chronic lung disease [4, 7], with different factors described for postsurgical patients [11]. Using this definition (at least one risk factor) and including ten different risk factors, HFNC was noninferior to NIV in a population of critically ill patients ventilated more than 24 h. Importantly, this cohort included a significant number of patients with acute heart disease, underlying chronic heart disease, and chronic obstructive pulmonary disease without hypercapnia [7]. However, a more recent post hoc analysis of another randomized controlled trial, showed that the response to preventive HFNC or NIV after extubation may be different in certain subgroups of patients with different risk factors (e.g., obese patients responded better to NIV) [12]. Moreover, it was also shown that the risk of extubation failure increased with the number of risk factors and suggested that patients with ≥ 4 risk factors might respond better to NIV.
Discomfort related to preventive NIV decreases the amount of time patients receive this treatment [4, 7, 13] and may limits its efficacy. Thus, different strategies to enhance comfort during treatment with NIV have been analyzed, including interface optimization [7], daily rest periods during which patients receive HFNC [4], as well as actively humidifying the inspired gases [14].
Our aim was to test the hypothesis that the prolonged use of NIV with active humidification is superior to HFNC in the specific population of patients with ≥ 4 high-risk factors for extubation failure.
Methods
The trial was conducted in two intensive care units (ICUs) in Spain (Trial registration: ClinicalTrials.gov ID: NCT04125342, which includes two separate analyses, patients at very high-risk of extubation failure (≥ 4 risk factors) and obese patients (body mass index (BMI) > 30) with less than 4 risk factors for reintubation (including BMI > 30 and excluding hypercapnia at the end of the spontaneous breathing trial) [15]. The departments of health of the regional governments to which these hospitals are affiliated (Madrid and Castilla-la Mancha) approved the study protocol (Online Supplement; eAppendix 1). The ethics committee at each center approved the trial (07/10/2019, no 427), and all patients or their relatives provided written informed consent. The study was performed according the CONSORT guidelines.
Patients
All adult patients receiving mechanical ventilation ≥ 24 h who were ready for scheduled extubation after tolerating a spontaneous breathing trial (SBT) were screened for inclusion (Online Supplement; eAppendix 1, recruitment). SBT were performed on pressure support of 7 cm H2O without expiratory pressure. Patients were considered at very high-risk of extubation failure if they fulfilled ≥ 4 of the following criteria [15]: age > 65 years, heart failure as the primary indication for mechanical ventilation, moderate-to-severe chronic obstructive pulmonary disease [7, 10], APACHE II score > 12 on extubation day [6, 7], body mass index (weight in kg/height in m2) > 30 [9], airway patency problems (including high risk of developing laryngeal edema—Online Supplement; eAppendix 2), inability to deal with respiratory secretions (inadequate cough reflex or suctioning required > 2 times within 8 h before extubation), difficult or prolonged weaning (failing ≥ 1 attempt at disconnection from mechanical ventilation), ≥ 2 comorbidities (Online Supplement; eAppendix 3), mechanical ventilation ≥ 7 days, and/or hypercapnia (PaCO2 > 45 mmHg) at the end of the SBT [10]. Exclusion criteria were do-not-resuscitate orders, tracheostomies, age < 18 years, accidental or self-extubation, do-not-reintubate orders, or any contraindication for NIV therapy (e.g., recent faciocervical surgery, active upper gastrointestinal bleeding, copious respiratory secretions). Obese patients (BMI > 30) at intermediate risk (maximum of two additional high-risk factors excluding hypercapnia) are analyzed separately. The baseline variables recorded are detailed in eAppendix 1 in the online supplement.
Weaning protocol
The weaning protocol included daily screening for weaning readiness (Online Supplement; eAppendix 1, recruitment) [16]. Patients deemed ready for weaning underwent an SBT with 7 cmH2O pressure support with zero positive end-expiratory pressure for 30 min [17, 18]. Standard criteria for failure of the SBT were used (Online Supplement; eAppendix 1). Patients who tolerated the SBT were reconnected for 1 h with the previous ventilator settings before extubation for rest and clinical evaluation of airway patency, respiratory secretions, and upper airway obstruction (Online Supplement; eAppendix 1) [7].
Randomization
Patients who passed the SBT and underwent scheduled extubation were randomized to receive NIV with active humidification or HFNC for 48 h by concealed allocation and allocation ratio 1:1 with a random number generator (simple randomization) through a telephone call center.
Interventions
In the group of patients randomized to receive HFNC (Optiflow™, Fisher and Paykel Healthcare), it was applied immediately before extubation through a specific size adjusted nasal cannula. Flow was initially set at 10 L/min; after extubation, flow was titrated upward in 5 L/min steps until patients experienced discomfort or up to 60 L/min. Temperature was initially set at 37 °C.
In the other group of patients, NIV with active humidification was started immediately after extubation. Humidified gas (F&P 950™ Respiratory Humidifier, Fisher & Paykel Healthcare) was continuously delivered by bilevel positive airway pressure (BiPAP) (V60 and V60 plus; Philips) through a size-fitted facemask (Nivairo™; Fisher and Paykel Healthcare) for 48 h. Positive end-expiratory pressure and inspiratory pressure support were adjusted to target a respiratory rate of < 26 breaths per minute with a tidal volume of 6–8 ml/kg of predicted body weight and adequate gas exchange (SpO2 ≥ 92%, with pH 7.35). Sedatives to increase tolerance to NIV were not allowed.
Fraction of inspired oxygen (FiO2) was adjusted to maintain SpO2 ≥ 92% in both groups. After 48 h, HFNC and NIV were stopped and patients received conventional oxygen therapy if necessary, irrespective of clinical condition. Rescue therapy with NIV was not allowed in the HFNC group.
Both groups were treated by the same medical, nursing, and respiratory therapy staff and received similar medical management.
Outcomes
The primary outcome was all-cause reintubation within 7 days after extubation. Predefined criteria for immediate respiratory-related, non-respiratory-related, and persistent-postextubation-respiratory-failure-related reintubations are detailed in eAppendix 1 (Online Supplement).
Secondary outcomes were postextubation respiratory failure within 7 days of extubation (Online Supplement; eAppendix 4) [4, 19], respiratory infection (ventilator-associated pneumonia or ventilator-associated tracheobronchitis) (Online Supplement; eAppendix 1), sepsis or multiple organ failure, ICU and hospital length of stay, ICU and hospital mortality, and the reason for failure of the assigned treatment, if applicable, including patient discomfort requiring withdrawal of the therapy for > 30 min and nasal septum or skin trauma. Delayed reintubation was the main safety concern and, therefore, the time to reintubation was measured as a surrogate for safety.
Statistical analysis
Based on a previous study [12], the absolute reduction in reintubation rate was estimated at 21%, from a basal rate of 45%. To achieve 80% power to detect this difference with an α level of 5% and a maximum tolerated patient loss rate of 10%, a sample of 88 patients in each arm of the study was considered adequate for two-sided tests.
All analyses were performed on an intention-to-treat basis. Kaplan–Meier curves were plotted to assess the time from extubation to reintubation and compared by means of the log-rank test.
To assess the probability of reintubation, the Cochran-Mantel–Haenszel χ2 test stratified according to recruiting hospital was used.
Analyses of secondary and exploratory outcomes used Fisher’s exact test, t test, the Mann–Whitney U test, or the Cochran–Mantel–Haenszel χ2 test (stratified for hospitals), as appropriate. The number needed to treat was calculated using the Newcombe-Wilson method. Confidence intervals (CI) for comparison of medians were calculated with the reference method. The analysis included a simple sequentially multiple test to adjust for multiple comparisons for all secondary outcomes [20]. Logistic regression analysis was performed to exclude a possible effect of some high-risk factors that have been previously described to benefit from preventive NIV on decreasing the need for reintubation [4, 6]. Additional logistic regressions analyses were performed to assess whether the need for reintubation was independently associated with hospital mortality. Finally, another multivariate analysis was also performed to determine whether the type of respiratory support was associated with the need for reintubation.
The two-sided level of significance was set at 0.05. SPSS version 13.0 (SPSS Inc) and Stata Statistical Software 14 (StataCorp 15. College Station, TX: StataCorp LP) were used for all statistical analyses.
Results
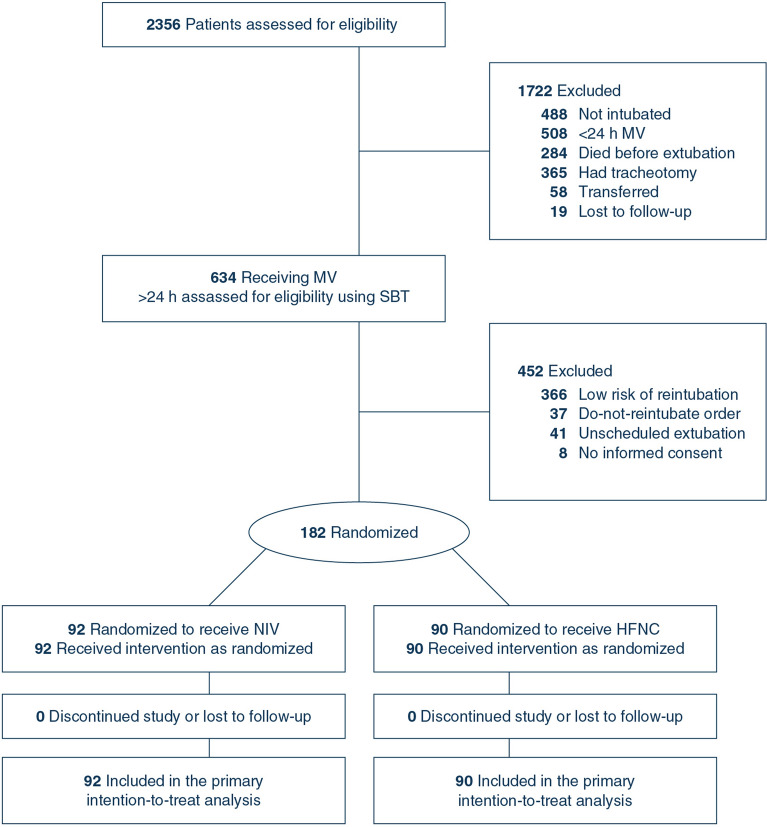
During the study period, 634 weanable patients were screened; 144 (22.7%) of these were randomized in the separate analysis of obese patients at intermediate risk and 182 (28.7%) were randomized for the current analysis: 90 to the HFNC group and 92 to the NIV group (Fig. 1). There were no dropouts in either group. Demographic and clinical characteristics of patients in the two groups are reported in Table 1. Some risk factors, more likely to benefit from positive pressure ventilation, were more prevalent in the NIV group (e.g., acute heart failure, COPD, and hypercapnia at the end of the SBT).
Fig. 1.
Flowchart of participants in a study comparing postextubation noninvasive ventilation with active humidification vs high-flow oxygen therapy for preventing reintubation in very high-risk patients. MV mechanical ventilation; SBT spontaneous breathing trial; NIV noninvasive ventilation with active humidification; HFNC high-flow nasal cannula
Table 1.
Baseline patient characteristics
| NIV (n = 92) | HFNC (n = 90) | |
|---|---|---|
| Age, mean (SD), y | 60.9 (14.3) | 59.9 (15.4) |
| Men, n (%) | 67 (72.8) | 50 (55.6) |
| APACHE II at ICU admission, median (IQR)a | 19 (16.3–24) | 19 (15–23) |
| Length of MV before extubation, median (IQR), days | 4.5 (2–9) | 5.5 (2–10) |
| Comorbiditiesb, n (%) | ||
| Heart disease | 38 (41.3) | 22 (24.4) |
| COPD | 27 (29.3) | 11 (12.2) |
| Other respiratory disease | 46 (50) | 30 (33.3) |
| High-risk factors for reintubation, no (%) | ||
| Age > 65 y | 42 (45.7) | 41 (45.6) |
| Heart failure as primary indication for MV | 25 (27.2) | 6 (6.7) |
| COPD | 28 (30.4) | 14 (15.6) |
| APACHE II > 12 on extubation daya | 53 (57.6) | 56 (62.2) |
| Body mass index > 30c | 49 (53.3) | 46 (51.1) |
| Airway patency problems | 33 (35.9) | 31 (34.4) |
| Inability to deal with respiratory secretions | 31 (33.7) | 47 (52.2) |
| Difficult or prolonged weaningd | 60 (66.7) | 59 (64.1) |
| ≥ 2 comorbidities | 75 (81.5) | 61 (67.8) |
| Prolonged MV | 36 (39.1) | 43 (47.8) |
| Hypercapnia at the end of the SBT | 47 (51.1) | 27 (30) |
| High-risk factors, median (IQR) | 5 (4–6) | 4 (4–6) |
| Diagnosis at admissione | ||
| Respiratory primary failure | 59 (64.8) | 49 (54.4) |
| SARS COVID-19f | 12 (13.1) | 15 (16.7) |
| Hemodynamic failure | 53 (57.6) | 57 (63.3) |
| Neurologic failure | 44 (47.8) | 57 (63.3) |
| Trauma | 15 (16.3) | 15 (16.7) |
| Surgical | 19 (20.7) | 28 (31.1) |
| Baseline physiologic variables at the end of the SBT prior to extubation, mean (SD) | ||
| PaO2, mm Hg | 116.2 (41.1) | 102.7 (34.8) |
| FiO2, % | 38.8 (7) | 38.9 (5.1) |
| PaCO2, mm Hg | 44.2 (8.6) | 41.6 (6.9) |
| Arterial pH | 7.39 (0.2) | 7.42 (0.06) |
| Respiratory rate, breaths/min | 15.6 (4.1) | 16.9 (3.6) |
APACHE II acute physiology and chronic health evaluation II; COPD chronic obstructive pulmonary disease; HFNC high-flow nasal cannula; ICU intensive care unit; IQR interquartile range; MV mechanical ventilation; NIV noninvasive ventilation; SARS COVID-19 severe acute respiratory syndrome secondary to coronavirus disease 2019; SBT spontaneous breathing trial; SD standard deviation
aAPACHE II score was calculated from 17 variables. Scores range from 0 to 71 points, with higher scores indicating more severe disease
bComorbidities were categorized based on the Charlson Comorbidity Index (eAppendix 3 in online supplement). Fully detailed in Table S1
cCalculated as weight in kilograms divided by height in meters squared
dDefinitions according to the Sixth International Consensus Conference on Intensive Care Medicine [18]
ePatients can have more than one diagnosis. All diagnoses detailed in Table S1
fSevere hypoxemic respiratory failure secondary to RT-PCR-confirmed COVID-19 pneumonia
Primary outcome
The reintubation rate was significantly lower in the NIV group than in the HFNC group (21 [23.3%] patients vs 35 [38.8%] patients, respectively; absolute risk difference, −15.5% [95%CI −28.3 to −1.0]) (Table 2). Figure 2 shows the Kaplan–Meier curve for the time from extubation to reintubation for any cause. The number needed to treat with NIV to avoid one reintubation was 6.2 (95%CI: 3.5–37.9). When adjusting for risk factors that were more likely to benefit from NIV (acute heart failure, COPD, and hypercapnia at the end of the SBT), patients treated with NIV were less likely to be reintubated (odds ratio (OR) 0.42 [95%CI 0.21–0.85]; p = 0.016).
Table 2.
Primary and secondary outcomes
| NIV (n = 92) | HFNC (n = 90) | Difference between groups (95%CI), p | |
|---|---|---|---|
| Primary outcome, n (%) | |||
| All-cause reintubation | 21 (22.8) | 35 (38.9) | −16.0 (−29.2 to −0.3), p = 0.019 |
| Secondary outcomes | |||
| Postextubation respiratory failure, n (%) | 40 (43.5) | 40 (44.4) | −0.9 (−15.4‒13.5), p = 0.896 |
| Ventilator-associated tracheobronchitis, n (%) | 0 (0) | 1 (1.1) | −1.1 (−3.3‒1.1), p = 0.495 |
| Ventilator-associated pneumonia, n (%) | 4 (4.3) | 7 (7.8) | −3.4 (−10.4‒3.5), p = 0.369 |
| Other infections, n (%) | 1 (1.1) | 2 (2.2) | −1.1 (−4.8‒2.6), p = 0.619 |
| Sepsis, n (%) | 4 (4.3) | 3 (3.3) | 1 (−5.5‒7.6), p = 1.000 |
| Multiorgan failure, n (%) | 3 (3.3) | 2 (2.2) | 1 (−4.5‒6.6), p = 1.000 |
| Hospital LOS, median (IQR), d | 20 (12‒36.7) | 26.5 (15‒45) | 6.5 (0.5‒21.1), p = 0.068 |
| ICU LOS, median (IQR), d | 9.5 (4‒15) | 12.5 (6.7‒19) | 3 (−0.6‒5.6), p = 0.047 |
| ICU mortality, n (%) | 12 (13) | 4 (4.4) | 9.7 (−1.1‒18.7), p = 0.356 |
| Hospital mortality, n (%) | 14 (15.2) | 6 (6.7) | 8.5 (−0.7‒18), p = 0.475 |
| Time to reintubation, median (IQR), h | 27 (6‒47) | 27 (8‒48) | 0 (−25‒25), p = 0.582 |
| Intolerance to therapy, n (%) | 19 (20.7) | 8 (8.9) | 11.7 (1.6–21.9), p = 0.026 |
| Nasal discomfort, n (%) | 18 (19.6) | 6 (6.7) | 12.9 (3.3–22.5), p = 0.010 |
| Facial skin ulcer, n (%) | 4 (4.3) | 0 (0) | 4.3 (0.1–8.5), p = 0.045 |
| Exploratory outcomes | |||
| Time on therapy 0–24 h, median (IQR), h | 22.5 (19.25‒24) | 24 (24‒24) | < 0.001 |
| Time on therapy 24‒48 h, median (IQR), h | 18 (10.5‒22) | 20 (8‒24) | 0.060 |
| Temperature, median (IQR), °C | 29 (29‒29) | 37 (37‒37) | < 0.001 |
| Reintubation rate at 5 d, n (%) | 21 (23.3) | 26 (28.8) | 0.321 |
| Time to reintubation at 5 d, median (IQR), h | 27 (6‒47) | 10 (6.5‒28) | 0.029 |
| FiO2 after 48 h, median (IQR), % | 31.3 (9) | 31.8 (8) | 0.645 |
HFNC high-flow nasal cannula; ICU intensive care unit; IQR interquartile range; LOS length of stay; NIV noninvasive ventilation
Fig. 2.
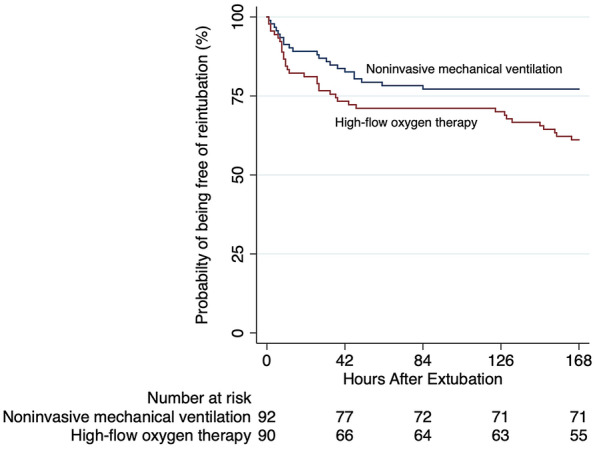
Kaplan–Meier analysis of time from extubation to reintubation
Secondary outcomes
Postextubation respiratory failure rate did not differ significantly between groups (Table 2). ICU mortality tended to be higher in the NIV group (14.1% vs 4.4% in the HFNC group; absolute risk difference 8.6% [95%CI −1.1 to 18.7]). No differences in the median time to reintubation were observed (27 [interquartile range (IQR), 6‒47] h in the NIV group vs 27 [IQR, 8‒48] h in the HFNC group; absolute difference, 0 h [95%CI −25 to 25 h]). All comfort-related outcomes, including intolerance requiring temporary discontinuation from therapy, nasal discomfort, and facial skin ulcer, occurred more frequently in the NIV group (Table 2).
Median hospital length of stay after randomization was lower in the NIV group (20 [IQR, 12‒36.7] days vs 26.5 [IQR, 15‒45] days in the HFNC group; difference between groups 6.5 [95%CI 0.5‒21.1] days). Other secondary outcomes were similar between the two groups (Table 2), including the causes of reintubation and postextubation respiratory failure (Table S2), although a trend toward an increased ICU and hospital mortality rates were observed in the NIV group (13% vs 4.4%, 95%CI −1.1 to 18.7 and 15.2% vs 6.7%, 95%CI −0.7 to 18 respectively). Time of use of NIV was 22.5 h (19.25‒24) the first day, and 18 h (10.5‒22) the second day.
Exploratory outcomes
After the fifth day, only patients in the HFNC group needed to be reintubated. Indeed, seven of the nine patients in this subgroup were reintubated after ICU discharge. The reasons for these late reintubations were nosocomial pneumonia (3 patients, including the 2 patients reintubated before ICU discharge), nosocomial infection by coronavirus disease 2019 (COVID-19) (1 patient), neurological deterioration (4 patients), and extrapulmonary sepsis (1 patient). None of the patients reintubated after the fifth day died. The reintubation rate in the first three days was not significantly different between groups (21.7% in the NIV group vs 28.9% in the HFNC group, p = 0.31), and the same for the first five days was not significantly different between groups (22.8% in the NIV group vs 28.9% in the HFNC group, p = 0.39). However, excluding those patients who were reintubated after the first five days, the median time to reintubation in the NIV group was significantly longer [27 (IQR, 6‒47) h vs 10 (IQR, 7‒28) h in the HFNC group, p = 0.029].
The need for reintubation was associated with higher mortality (table S3). The type of respiratory support used had no effect on mortality (Table S3). The use of NIV protects against the need for reintubation (table S4). Finally, prolonged mechanical ventilation was selected in the multivariate analysis for reitubation including covariates in addition to preventive therapy (Table S4).
Discussion
In this study in critically ill adult patients at very high-risk for extubation failure, prolonged use of NIV with active humidification was superior to HFNC at preventing reintubation within seven days of extubation. Moreover NIV patients had a shorter hospital length of stay. Importantly, tolerance to NIV in the current study was much better than ever reported before, with a median time of use around 20 h per day within the first 48 h after extubation [21].
To our knowledge, this is the first time that prolonged use of NIV with active humidification has been tested in critically ill patients after extubation. Indeed, only one previous study analyzed the effect of NIV with active humidification on intubation rates in patients with acute hypoxemic respiratory failure using ICU ventilators [22]. However, in this study, NIV treatment daily duration was considerably lower (i.e. only 8 h during the second day of treatment). Three important facts that may improve NIV tolerance should be also highlightened. First, temperature was adjusted to improve patient’s comfort. Indeed, the most frequently tolerated temperature in the current study was 29 °C, in line with the reported range in healthy subjects (25‒30 °C) and the use of facemasks [21]. Second, only 4.3% of patients on NIV developed skin ulcers. This rate is low, considering the very prolonged time on NIV with active humidification compared with previous studies [23]. Technological developments in facial interfaces for humidified NIV, including soft seal rolls, tube seal zones, and reduced pressure on the bridge of the nose, might have helped prevent skin complications. And third, NIV settings in the current study with low inspiratory pressures (< 5 cmH2O) can have partially contributed to the increased comfort and prolonged total time on NIV. Thus, intolerance to therapy leading to withdrawal and reduction in the total time on preventive therapy was similar in the two groups. Remarkably, 10% of patients on HFNC in the current study reported discomfort. The inclusion of patients intubated for severe acute respiratory syndrome secondary to COVID-19 could partially explain these results, as ICU-acquired delirium is more common in this population [24].
Overall, time to reintubation was similar between groups. However, after the fifth day, nine patients in the HFNC group but none of those in the NIV group were reintubated. These results are in line with those reported in patients who received preventive HFNC for a fixed 24-h period [7], suggesting that the benefit of preventive therapy decreases faster after withdrawal in patients receiving HFNC than in those receiving NIV. Other protocols setting preventive therapy according to clinical parameters with more prolonged time on therapy have not found these delayed reintubation episodes [4]. Indeed, the majority of late reintubations in the HFNC group occurred even after discharge from the ICU, and the reasons for which they were done suggest that it would be more appropriate to consider them as new intubations rather than reintubations. An exploratory analysis examining reintubation within the first three and five days showed that the reintubation rate was not significantly higher in the HFNC group. However, the time to reintubation was higher in the NIV group when defining reitubation at five days.
We observed a higher mortality rate in NIV patients (ICU mortality 13% vs 4.4%). It is worth noting that conditioning the gases in NIV decreases discomfort, thus enabling preventive therapy to be applied for longer periods and potentially leading to delayed reintubations in those patients who need it. These results are in line with those reported in a trial testing the use of NIV for treating postextubation respiratory failure [25]. We performed a sensitivity analysis to explore possible causes for this difference. Although we observed that only reintubation, age, hypercapnia at the end of the SBT and obesity were significantly associated to mortality, time to reintubation was close to significance, maybe influenced by a low statistical power due to a reduce sample size.
It should be also noted that, despite the present study included a similar population in terms of comorbidities compared with Thille et al.’s [4] study, our reintubation rate was about more than twice higher in both groups (38.8% vs 18.2% in the HFNC group and 23.3% vs 11.8% in the NIV group). The main reason for this discrepancy is probably the additive effect of the presence of more than 3 risk factors for reintubation in our 10-factor model. A recent study by Casey et al. [26] reported no significant differences with a short time prevention time based on clinical parameters, showing a linear increase in the reintubation rate in patients with more high risk factors for reintubation. This study reinforces the idea that clinical benefit is strongly related to the real time on prevention and the number of high-risk factors.
Limitations of the study
After randomization, the proportion of patients with factors that are likely to benefit from positive pressure ventilation (e.g., hypercapnia, and lung or heart disease) was higher in the NIV group. However, after adjusting for risk factors that are likely to benefit from positive pressure ventilation (e.g., chronic lung disease, especially when hypercapnia is present, and chronic heart disease), preventive therapy based on NIV seems to be more effective against the need for reintubation. Finally, attending teams could not be blinded to the study group and a possible bias was unavoidable.
Conclusions
In critically ill adult patients at very high-risk for extubation failure who underwent planned extubation, NIV with active humidification was superior to HFNC for preventing reintubation. Improved comfort due to conditioning gases in NIV might increase the risk of delaying reintubation in patients who need it.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
GH had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: GH. Acquisition, analysis, and interpretation of data: GH, IP, FM, MB, LC, MLR, AV, PR, MJP-P, FS-S, and AC. Drafting of the manuscript: GH and OR. Critical revision of the manuscript for important intellectual content: IP, FM, MB, LC, MLR, AV, PR, MJP-P, FS-S, AC, RC, LB and OR. Statistical analysis: GH, RC and OR. Administrative, technical, or material support: GH. Study supervision: GH.
Declarations
Conflicts of interest
All authors have completed and submitted the ICMJE Form for disclosure of potential conflicts of interest. GH reported travel expenses and personal fees from Fisher & Paykel Healthcare Ltd. OR reported a research grant from Hamilton Medical AG, speaker fees from Hamilton Medical AG, Fisher&Paykel Healthcare Ltd, Aerogen Ltd and Ambu, and non-financial research support from Timpel; all outside the submitted work.
Funding
Fisher & Paykel Healthcare Ltd provided humidification devices for NIV conditioning and facial masks. They had no other involvement in the study, including in its design and conduct; the collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Acknowledgements
We thank all the patients and medical and nursing staff for their cooperation. John Giba, received financial compensation for editing the English in the manuscript.
Footnotes
The original online version of this article was revised: In this article the y-axis of Fig. 2 was incorrectly given as 'Reintubation (%)' but should have been "Probability of being free of reintubation (%)".
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/27/2023
A Correction to this paper has been published: 10.1007/s00134-023-06978-0
References
- 1.Ouellette DR, Patel S, Girard TD, et al. Liberation from mechanical ventilation in critically ill adults: an official American College of Chest Physicians/American Thoracic Society clinical practice guideline. Chest. 2017;151(1):166–180. doi: 10.1016/j.chest.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 2.Rochberg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 3.Rochwerg B, Einav S, Chaudhuri D, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46(12):2226–2237. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thille AW, Muller G, Gacouin A, et al. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA. 2019;322(15):1465–1475. doi: 10.1001/jama.2019.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nava S, Gregoretti C, Fanfulla F, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med. 2005;33:2465–2470. doi: 10.1097/01.CCM.0000186416.44752.72. [DOI] [PubMed] [Google Scholar]
- 6.Ferrer M, Valencia M, Nicolas JM, et al. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006;173:164–170. doi: 10.1164/rccm.200505-718OC. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez G, Vaquero C, Colinas L, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316(15):1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 8.Vallverdu I, Calaf N, Subirana M, Net A, et al. Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Care Med. 1998;158(6):1855–1862. doi: 10.1164/ajrccm.158.6.9712135. [DOI] [PubMed] [Google Scholar]
- 9.El Solh AA, Aquilina A, Pineda L, Dhanvantri V, Grant B, Bouquin P. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J. 2006;28(3):588–595. doi: 10.1183/09031936.06.00150705. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer M, Sellarés J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet. 2009;374(9695):1082–1088. doi: 10.1016/S0140-6736(09)61038-2. [DOI] [PubMed] [Google Scholar]
- 11.Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 12.Thille AW, Coudroy R, Nay MA, et al. Beneficial effects of non-invasive ventilation after extubation in obese or overweight patients: a post-hoc analysis of a randomized clinical trial. Am J Respir Crit Care Med. 2021 doi: 10.1164/rccm.202106-1452OC. [DOI] [PubMed] [Google Scholar]
- 13.Stéphan F, Barrucand B, Petit P, et al. High-flow oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized trial. JAMA. 2015;313(23):2331–2339. doi: 10.1001/jama.2015.5213. [DOI] [PubMed] [Google Scholar]
- 14.Restrepo RD, Walsh BK. AARC clinical practice guideline: humidification during invasive and noninvasive mechanical ventilation: 2012. Respir Care. 2012;57(5):782–788. doi: 10.4187/respcare.01766. [DOI] [PubMed] [Google Scholar]
- 15.Hernández G, Vaquero C, Ortiz R, et al. Benefit with preventive noninvasive ventilation in subgroups of patients at high-risk for reintubation: a post hoc analysis. J Intensive Care. 2022;10:43. doi: 10.1186/s40560-022-00635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39(12):2612–2618. doi: 10.1097/CCM.0b013e3182282a5a. [DOI] [PubMed] [Google Scholar]
- 17.Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 18.Subira C, Hernandez G, Vazquez A, et al. Effect of pressure support vs T-piece ventilation strategies during spontaneous breathing trial son successful extubation among patients receiving mechanical ventilation. JAMA. 2019;321(22):2175–2182. doi: 10.1001/jama.2019.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouellette DR, Patel S, Girard TD, et al. Liberation from mechanical ventilation in critically ill adults: an official American college of chest physicians/American Thoracic Society Clinical Practice Guideline. Inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest. 2017;151(1):166–180. doi: 10.1016/j.chest.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 21.Lellouche F, Maggiore SM, Lyazidi A, et al. Water content of delivered gases during non-invasive ventilation in healthy subjects. Intensive Care Med. 2009;35(6):987–995. doi: 10.1007/s00134-009-1455-y. [DOI] [PubMed] [Google Scholar]
- 22.Lellouche F, L’Her E, Abroug F, Deye N, et al. Impact of the humidification device on intubation rate during noninvasive ventilation with ICU ventilators: results of a multicenter randomized controlled trial. Intensive Care Med. 2014;40(2):211–219. doi: 10.1007/s00134-013-3145-z. [DOI] [PubMed] [Google Scholar]
- 23.Alqahtani JS, Worsley P, Voegeli D. Effect of humidified noninvasive ventilation on the development of facial skin breakdown. Respir Care. 2018;63(9):1102–1110. doi: 10.4187/respcare.06087. [DOI] [PubMed] [Google Scholar]
- 24.Pun BT, Badenes R, Heras de la Calle G, Orun OM, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9(3):239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350(24):2452–2460. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- 26.Casey JD, Vaughan EM, Lloyd BD, et al. Protocolized postextubation respiratory support to prevent reintubation: a randomized clinical trial. Am J Respir Crit Care Med. 2021 doi: 10.1164/rccm.202009-3561OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.