Abstract
Deucravacitinib (SOTYKTU™) is a first-in-class, highly selective, oral tyrosine kinase 2 (TYK2) inhibitor. It acts via an allosteric mechanism, binding to the catalytically inactive pseudokinase regulatory domain of TYK2 and stabilizing an inhibitory interaction between the regulatory and catalytic domains. Deucravacitinib is being developed by Bristol Myers Squibb for the treatment of multiple immune-mediated diseases, including psoriasis, psoriatic arthritis, lupus and inflammatory bowel disease. It received its first approval (in the USA on 9 September 2022) for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. On 26 September 2022, it was subsequently approved in Japan for the treatment of plaque psoriasis, generalized pustular psoriasis and erythrodermic psoriasis. The Marketing Authorisation Application for deucravacitinib for the treatment of adults with moderate to severe plaque psoriasis has been validated in the EU, and clinical development of the drug for the treatment of multiple immune-mediated diseases is underway in numerous countries worldwide. This article summarizes the milestones in the development of deucravacitinib leading to this first approval for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-022-01796-y.
| Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.21350856. |
Deucravacitinib (SOTYKTU™): Key Points
| A TYK2 inhibitor being developed by Bristol Myers Squibb for the treatment of multiple immune-mediated diseases, including psoriasis (e.g. plaque psoriasis), psoriatic arthritis, lupus and inflammatory bowel disease |
| Received its first approval on 9 September 2022 in the USA |
| Approved for use in adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy |
Introduction
The Janus kinase (JAK) family comprises JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2), with each member involved in cytokine signalling [1]. TYK2 mediates the intracellular signalling initiated by cytokines involved in adaptive [interleukin (IL)-12, IL-23) and innate [type I interferons (IFNs)] immune responses, and is considered a viable therapeutic target for psoriasis following the identification of the IL-23/IL-17 axis as the main pathogenic pathway for this disease [2–4].
Deucravacitinib (SOTYKTU™) is a first-in-class oral inhibitor of TYK2 [5, 6]. Unlike other tyrosine kinase inhibitors (e.g. JAK1–3 inhibitors), which act through competitive inhibition (by binding to the active kinase domain), deucravacitinib acts via allosteric inhibition: it binds to the catalytically inactive pseudokinase regulatory domain (JH2) [2, 7]. Such binding stabilizes an inhibitory interaction between the regulatory and catalytic domains of TYK2, resulting in the (allosteric) inhibition of the receptor-mediated activation of TYK2, thereby preventing downstream signalling [4, 5] (Sect. 2.1). However, the precise mechanism linking the inhibition of TYK2 to the therapeutic effectiveness of deucravacitinib in the treatment of adults with moderate to severe plaque psoriasis is not yet known [5].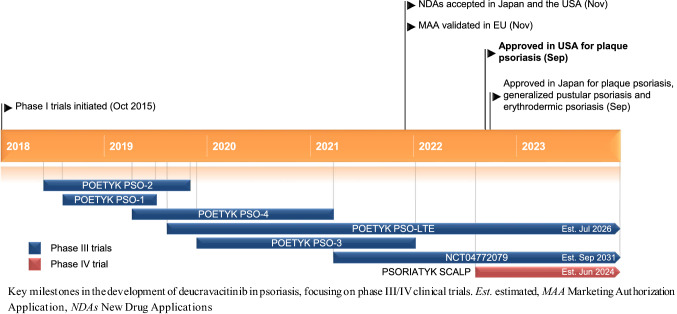
Deucravacitinib is being developed by Bristol Myers Squibb for the treatment of multiple immune-mediated diseases, including psoriasis, psoriatic arthritis, lupus and inflammatory bowel disease [5, 8]. It received its first approval (in the USA on 9 September 2022) for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy [5, 6]. On 26 September 2022, it was subsequently approved in Japan for the treatment of plaque psoriasis, generalized pustular psoriasis and erythrodermic psoriasis [9]. The recommended dosage of deucravacitinib is 6 mg (one 6 mg tablet), administered orally, once daily with or without food [5] (Sect. 2.2). Deucravacitinib is not recommended for use in combination with other potent immunosuppressants nor in patients with severe hepatic impairment (Child–Pugh C), and patients should be evaluated for active and latent tuberculosis infection prior to treatment initiation, with the treatment of TB for those who are positive started prior to the initiation of deucravacitinib therapy. There are insufficient data regarding the use of deucravacitinib in pregnant women; no effects on embryo-foetal development were seen in rat and rabbit reproduction studies. There are no data on the presence of deucravacitinib in human milk, or on the effects on the breastfed infant or milk production. However, the drug and its metabolites have been detected in the milk of lactating rats. The benefits of breastfeeding for the infant and the clinical benefits of deucravacitinib for the mother should be considered [5].
The Marketing Authorisation Application for deucravacitinib for the treatment of adults with moderate to severe plaque psoriasis has been validated in the EU [8]. Clinical development for the treatment of multiple immune-mediated diseases, including Crohn’s disease, cutaneous lupus erythematosus, discoid lupus erythematosus, lupus nephritis, plaque psoriasis, psoriatic arthritis, systemic lupus erythematosus (SLE) and ulcerative colitis, is underway in numerous countries worldwide.
Scientific Summary
Pharmacodynamics
Deucravacitinib binds to the pseudokinase domain of TYK2 with high potency (mean inhibitory constant 0.02 nmol/L) [10] and high selectivity [11]. Simulations indicate that daily average inhibition (based on whole blood assay results) by deucravacitinib (at the recommended dosage of 6 mg once daily) is 50% for TYK2, < 1% for JAK1/3 and < 1% for JAK 2/2 [11]. Projected steady-state plasma concentrations of deucravacitinib 6 mg once daily were above the whole blood half-maximal inhibitory concentration (IC50) of TYK2 for 9 h of the daily dosing interval, but above the whole blood IC50 of JAK1/3 and JAK 2/2 for 0 and 0 h. In addition, maximum plasma concentrations (Cmax) of deucravacitinib (at the recommended dosage) were 17-fold lower than the whole blood JAK1/3 IC50 value and > 102-fold lower than the whole blood JAK2/2 IC50 value [11].
In vitro, deucravacitinib potently (IC50 2–14 nmol/L) inhibited IL-12-, IL-23- and type 1 IFN-driven cellular signalling and transcriptional responses, and was ≈ 200-fold more selective against JAK1/3-dependent signalling in IL-2-stimulated T cells and > 3000-fold more selective over JAK2-dependent erythropoietin-induced signalling in TF-1 cells [10, 12]. Deucravacitinib also inhibited IFNα-induced signalling (IC50 13 nmol/L) in human whole blood [10, 12]. In vivo, deucravacitinib dose-dependently inhibited IL-12-dependent weight loss and prevented histologically-evident colitis in a mouse colitis model [12] and demonstrated dose-dependent nephritis and other disease endpoint protection (which correlated with the inhibition of type I IFN-dependent gene expression) in a mouse lupus model [10].
In a skin biopsy substudy [7] of a phase II trial (NCT02931838) [13] in adults with moderate to severe plaque psoriasis, deucravacitinib was associated with reductions in IL-23/TH17 and IFN pathway biomarkers, but had no effect on select laboratory parameters affected by JAK1–3 inhibition. Moreover, in a phase II study (NCT03881059) in adults with psoriatic arthritis (Sect. 2.3.2), deucravacitinib suppressed IL-23/IL-17 and IFN pathway biomarkers [14]. It is worth noting, however, that the relationship between pharmacodynamic markers and the mechanism(s) by which deucravacitinib exerts its clinical effects is unknown [5].
At a supratherapeutic dose (i.e. 6-fold the recommended dose), deucravacitinib did not prolong the corrected QT interval to any clinically relevant extent in patients with psoriasis [5].
In rat and transgenic mouse carcinogenicity studies, there was no evidence of tumourigenicity following exposure to oral deucravacitinib doses of up to 15 mg/kg/day (51-fold the maximum recommended human dose) and 60 mg/kg/day, respectively [5].
Pharmacokinetics
The pharmacokinetics of deucravacitinib and its active metabolite (BMT-153261) were comparable between healthy subjects and patients with psoriasis [5]. Administering deucravacitinib with a high-fat, high-calorie meal did not affect its pharmacokinetics to a clinically relevant extent; thus, deucravacitinib can be administered with or without food. In healthy subjects, oral deucravacitinib Cmax and exposure values were dose proportional over a 3–36 mg (0.5–6-fold the recommended dosage) dose range. The absolute oral bioavailability of deucravacitinib was 99% and the median time to Cmax was 2–3 h. Although BMT-153261, the major metabolite of deucravacitinib, has a comparable potency to the parent drug, it accounts for ≈ 20% of the systemic exposure of all drug-related components. Deucravacitinib is 82–90% bound to proteins and has a blood:plasma concentration of 1.26; at steady state, its volume of distribution is 140 L. Following once-daily dosing in healthy subjects, the accumulation of deucravacitinib was < 1.4-fold [5].
Features and properties of deucravacitinib
| Alternative names | BMS-986165; BMS-986165-01; SOTYKTU |
| Class | Amides; aniline compounds; anti-inflammatories; antipsoriatics; antirheumatics; cyclopropanes; ethers; hepatoprotectants; organic deuterium compounds; pyridazines; skin disorder therapies; small molecules; triazoles; urologics |
| Mechanism of action | Allosteric TYK2 kinase inhibitor |
| Route of administration | Oral |
| Pharmacodynamics | Binds to the pseudokinase regulatory domain of TYK2 with high potency and high functional selectivity; associated with reductions in IL-23/TH17 and IFN pathway biomarkers in adults with moderate to severe plaque psoriasis and in IL-23/IL-17 and IFN pathway biomarkers in adults with psoriatic arthritis |
| Pharmacokinetics | Cmax and exposure values were dose proportional over a 3–36 mg dose range; absolute oral bioavailability of 99%; median time to Cmax of 2–3 h; terminal half-life of 10 h |
| Most frequent adverse events | Upper respiratory tract infection, increased blood creatinine phosphokinase levels, herpes simplex, mouth ulcers, folliculitis and acne |
| ATC codes | |
| WHO ATC code | L04A-A56 (Deucravacitinib) |
| EphMRA ATC code | D11 (Other Dermatological Preparations); L4 (Immunosuppressants) |
| Chemical name | 6-(cyclopropanecarbonylamino)-4-[2-methoxy-3-(1-methyl-1,2,4-triazol-3-yl)anilino]-N-(trideuteriomethyl)pyridazine-3-carboxamide |
Cmax maximum concentration, IFN interferon, IL interleukin, TYK2 tyrosine kinase 2
Deucravacitinib is metabolized by CYP1A2 (to form BMT-153261) and by CYP2B6, CYP2D6, carboxylesterase 2 and uridine glucuronyl transferase (UGT) 1A9 [5]. Following the administration of a single dose of radiolabeled deucravacitinib, ≈ 26% and ≈ 13% of the dose was recovered unchanged in the faeces and urine, with ≈ 12% and ≈ 6% of the dose detected as BMT-153261 in the faeces and urine. Deucravacitinib has a terminal half-life of 10 h [5].
Deucravacitinib exposure is not affected to a clinically meaningful extent by age, race, gender or body weight [5]. There are no dose adjustment recommendations for patients with mild, moderate or severe renal impairment, those with end-stage renal disease on dialysis and those with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. Deucravacitinib is not recommended for use in patients with severe hepatic impairment (Child–Pugh C) [5]. Moreover, results from healthy volunteer studies showed that deucravacitinib can be administered regardless of relevant concomitant medications that inhibit or induce various drug-metabolizing enzymes and transporters, including ciclosporin (BCRP/OCT1/P-gp inhibitor), diflunisal (UGT1A9 inhibitor), fluvoxamine (CYP1A2 inhibitor), ritonavir (CYP1A2 inducer) and oral contraceptives [15].
Therapeutic Trials
Plaque Psoriasis
Phase III Studies
Therapy with oral deucravacitinib 6 mg once daily was effective in reducing the severity and extent of moderate to severe plaque psoriasis in adults participating in two 52-week, randomized, double-blind, placebo- and active comparator-controlled, multinational phase III studies [POETYK PSO-1 (NCT03624127) [16] and POETYK PSO-2 (NCT03611751) [17]]. Eligible patients had a Psoriasis Area and Severity Index (PASI) score of ≥ 12, a static Physician’s Global Assessment (sPGA) score of ≥ 3 (i.e. moderate or severe) and ≥ 10% body surface a (BSinvolvement, and were randomized 2:1:1 to receive deucravacitinib (n = 332 and 511 in POETYK PSO-1 and POETYK PSO-2), placebo (n = 166 and 255) or apremilast (30 mg twice daily) [n = 168 and 254]. Patients receiving placebo were switched to deucravacitinib at week 16 in both studies [16, 17], while those randomized to deucravacitinib in POETYK PSO-1 received it continuously for 52 weeks [16]. POETYK PSO-2 included a randomized withdrawal period: patients originally randomized to deucravacitinib who achieved a ≥ 75% improvement from baseline in the PASI score (i.e. a PASI 75 response) at week 24 were re-randomized 1:1 to deucravacitinib or placebo, while those who did not achieve a PASI 75 response at this timepoint continued to receive deucravacitinib [17]. In each study, the key secondary endpoints were evaluated in a split hierarchical manner (i.e. deucravacitinib versus placebo and deucravacitinib versus apremilast) only if the between-group differences in both co-primary endpoints were significant [16, 17].
Key clinical trials of deucravacitinib (sponsored by Bristol Myers Squibb)
| Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
| Deucravacitinib, apremilast, placebo | Plaque psoriasis | III | Completed | Multinational | NCT03624127 (POETYK PSO-1) |
| Deucravacitinib, apremilast, placebo | Plaque psoriasis | III | Completed | Multinational | NCT03611751 (POETYK PSO-2) |
| Deucravacitinib, placebo | Plaque psoriasis | III | Completed | Multinational | NCT04167462 (POETYK PSO-3) |
| Deucravacitinib | Plaque psoriasis | III | Completed | Japan | NCT03924427 (POETYK PSO-4) |
| Deucravacitinib | Plaque psoriasis | III | Active, not recruiting | Multinational | NCT04036435 (POETYK PSO-LTE) |
| Deucravacitinib, placebo | Plaque psoriasis | III | Recruiting | Multinational | NCT04772079 |
| Deucravacitinib, placebo | Psoriatic arthritis | III | Recruiting | Multinational | NCT04908202 (POETYK PsA-1) |
| Deucravacitinib, apremilast, placebo | Psoriatic arthritis | III | Recruiting | Multinational | NCT04908189 (POETYK PsA-2) |
| Deucravacitinib, placebo | Scalp psoriasis | IV | Not yet recruiting | Multinational | NCT05478499 (PSORIATYK SCALP) |
| Deucravacitinib | Plaque psoriasis | II | Completed | Multinational | NCT02931838 |
| Deucravacitinib, ustekinumab, placebo | Psoriatic arthritis | II | Completed | Multinational | NCT03881059 |
| Deucravacitinib, placebo | Systemic lupus erythematosus | II | Completed | Multinational | NCT03252587 (PAISLEY) |
| Deucravacitinib | Systemic lupus erythematosus | II | Active, not recruiting | Multinational | NCT03920267 |
| Deucravacitinib, placebo | Ulcerative colitis | II | Active, not recruiting | Multinational | NCT03934216 (LATTICE-UC) |
| Deucravacitinib, placebo | Discoid lupus erythematosus, subacute cutaneous lupus erythematosus | II | Recruiting | Multinational | NCT04857034 |
| Deucravacitinib, placebo | Crohn's disease | II | Recruiting | Multinational | NCT03599622 (LATTICE-CD) |
| Deucravacitinib | Crohn's disease, ulcerative colitis | II | Recruiting | Multinational | NCT04877990 |
| Deucravacitinib, placebo | Ulcerative colitis | II | Recruiting | Multinational | NCT04613518 |
At week 16, significantly (p < 0.0001) more deucravacitinib recipients than placebo recipients had achieved each of the co-primary endpoints: an sPGA score of 0 or 1 (i.e. clear or almost clear) with a ≥ 2-point improvement from baseline (53.6% vs 7.2% in POETYK PSO-1 [16] and 49.5% vs 8.6% in POETYK PSO-2 [17]) and a PASI 75 response (58.4% vs 12.7% [16] and 53.0% vs 9.4% [17]). Deucravacitinib was also significantly (p ≤ 0.025) more effective than placebo and apremilast across multiple key secondary endpoints at weeks 16 and/or 24, demonstrating improvements in skin clearance, scalp psoriasis, psoriasis symptom and health-related quality of life measures [16, 17]. For instance, at weeks 16 and 24, respectively, the proportion of patients achieving an sPGA score of 0 or 1 with a ≥ 2-point improvement from baseline (53.6% vs 32.1% and 58.7% vs 31.0% in POETYK PSO-1 [16]; 49.5% vs 33.9% and 49.8% vs 29.5% in POETYK PSO-2 [17]) and a PASI 75 response (58.4% vs 35.1% and 69.3% vs 38.1% [16]; 53.0% vs 39.8% and 58.7% vs 37.8% [17]) was significantly (p ≤ 0.0004) higher with deucravacitinib than apremilast.
The efficacy results achieved at weeks 16 and 24 with deucravacitinib were maintained through to week 52 with continuous therapy in both studies [16, 17]. In POETYK PSO-1, 52.7% and 65.1% of 332 patients had achieved an sPGA score of 0 or 1 with a ≥ 2-point improvement from baseline and a PASI 75 response at week 52 [16, 18]. In this study, improvements in these respective endpoints were also seen in 53.8% and 68.3% of 145 patients who switched from placebo to deucravacitinib at week 16 [16, 18]. In POETYK PSO-2, 70.3% of 118 and 80.4% of 148 deucravacitinib recipients who had achieved a PASI 75 response at week 24 and continued treatment with deucravacitinib maintained an sPGA score of 0 or 1 with a ≥ 2-point improvement from baseline and a PASI 75 response at week 52 [17, 18]. Durable responses were also noted in this study, with 31.3% of 150 patients who had achieved a PASI 75 response at week 24 and were re-randomised to placebo maintaining a PASI 75 response at week 52 (i.e. 28 weeks’ following treatment cessation). The median time to a loss of PASI 75 in these patients was 85 days [17].
At baseline in POETYK PSO-1 and POETYK PSO-2, patients had a median PASI score of 19 and a median affected BSA of 20%; 80% and 20% of patients had an sPGA score of 3 and 4, respectively, and ≈ 18% had a history of psoriatic arthritis [5]. Following completion of POETYK PSO-1 and POETYK PSO-2, eligible patients were permitted to enter an open-label phase III extension study [POETYK PSO-LTE (NCT04036435)] [16, 17].
The benefits of deucravacitinib on the severity and extent of moderate to severe plaque psoriasis appear to persist over the longer term (up to 2 years), according to preliminary data from POETYK PSO-LTE (n = 1221; cumulative exposure of 2166.9 person–years) [19]. An sPGA score of 0 or 1 with a ≥ 2-point improvement from baseline and a PASI 75 response was achieved by 56.4% and 75.7% of patients at week 48 (vs 50.9% and 65.1% at enrolment) [19] and by 58.7% and 77.7% at week 60 [20]. Moreover, in 262 deucravacitinib recipients from POETYK PSO-1 who transitioned into POETYK PSO-LTE, PASI 75, PASI 90 and sPGA score of 0 or 1 response rates (modified non-responder imputation analysis) were achieved in 82.4%, 55.2% and 66.5% of patients, respectively, following 112 weeks’ treatment [21].
Phase II Study
The findings of the POETYK PSO-1 and -2 studies are supported by efficacy data from 267 adults with moderate to severe plaque psoriasis participating in a 12-week, randomized, double-blind, placebo-controlled, multinational, phase II, dose-ranging study (NCT02931838) [13]. At week 12, oral deucravacitinib (at doses of 3 mg once daily and higher) was significantly (p < 0.001) more effective that placebo with regard to the proportion of patients achieving a PASI 75 response (primary endpoint) [9%, 39%, 69%, 67% and 75% in the deucravacitinib 3 mg every other day, 3 mg once daily, 3 mg twice daily, 6 mg twice daily or 12 mg once daily groups (n = 44, 44, 45, 45, and 44), respectively, vs 7% in the placebo group (n = 45). Patients that have previously not responded to drugs targeting cytokine signalling through the same tyrosine kinase pathway were excluded from NCT02931838 [13].
Psoriatic Arthritis
Treatment with oral deucravacitinib 6 mg and 12 mg once daily improved multiple psoriatic arthritis domains, including arthritis, enthesitis, dactylitis and skin inflammation, in adults with active disease participating in a 16-week, randomized, double-blind, placebo-controlled, multinational phase II study (NCT03881059) [22]. A ≥ 20% improvement in the American College of Rheumatology response criteria (ACR20; primary endpoint) at week 16 was achieved by 52.9% of 70 deucravacitinib 6 mg once daily recipients and 62.7% of 67 deucravacitinib 12 mg once daily recipients compared with 31.8% of 66 placebo recipients; the adjusted odds ratios for deucravacitinib 6 mg and 12 mg once daily versus placebo were 2.4 (95% CI 1.2–4.8; p = 0.0134) and 3.6 (95% CI 1.8–7.4; p = 0.0004). Significant (p < 0.05) improvements with both dosages of deucravacitinib over placebo were also seen in the multiplicity controlled secondary endpoints [changes from baseline in the Health Assessment Questionnaire–Disability Index (HAQ–DI) and Short Form-36 Physical Component Summary score, and in PASI 75 response) [22].
Eligible patients in NCT03881059 had a ≥ 6-month psoriatic arthritis diagnosis, active joint disease (≥ 3 tender and ≥ 3 swollen joints), a high-sensitivity C-reactive protein (hs-CRP) level of ≥ 3 mg/L and ≥ 1 plaque psoriasis lesion (≥ 2 cm), and had failed to respond or were intolerant to ≥ 1 prior therapy [22]. Concomitant conventional synthetic disease modifying anti-rheumatic drug (csDMARD) [e.g. hydroxychloroquine, leflunomide, methotrexate, sulfasalazine] treatment was permitted if used for ≥ 3 months and with a stable dose for ≥ 28 days prior to the study. At baseline, the median duration of psoriatic arthritis from diagnosis was 4.5 years, the mean swollen joint count was 11.3, the mean tender joint count was 18.1, and the mean PASI score was 8.5 in those with BSA involvement of ≥ 3%. Enthesitis and dactylitis were present in 47.3% and 38.9% of patients. The ACR20 response was defined as meeting the following criteria: a ≥ 20% improvement from baseline in the number of tender joints (total joint count of 68); a ≥ 20% improvement from baseline in the number of swollen joints (total joint count of 66); and a ≥ 20% improvement from baseline in ≥ 3 of the following 5 domains: Patient Global Assessment of Pain, Patient Global Assessment of Disease Activity, Physician Global Assessment of Disease Activity, HAQ–DI and hs-CRP level [22].
Systemic Lupus Erythematosus
Oral deucravacitinib showed promise as a treatment for SLE in adults with active disease participating in a 48-week, randomized, double-blind, placebo-controlled, phase II study [PAISLEY (NCT03252587)] [23]. In this study, patients on standard background medications received deucravacitinib 3 mg twice daily, 6 mg twice daily or 12 mg once daily, or placebo. The tapering of oral corticosteroids to 7.5 mg/day was required from weeks 8-20, with further tapering optional from weeks 32-40. At week 32, the proportion of patients achieving an SLE Responder Index [SRI(4)] response (primary endpoint) was significantly (p = 0.0006 and 0.021) higher with deucravacitinib 3 mg twice daily and 6 mg twice daily than placebo (58.2% and 49.5% vs 34.4%) [n = 91, 93 and 90, respectively]. In the deucravacitinib 12 mg once daily group, 44.9% of 89 patients met the primary endpoint. The SRI(4) response was sustained across all of the deucravacitinib groups up to week 48, with 57.1%, 47.3% and 47.2%, respectively, of deucravacitinib 3 mg twice daily, 6 mg twice daily and 12 mg once daily recipients (vs 34.4% of placebo recipients) achieving this endpoint. Of note, the dose-response relationship for this parameter appears to be flat above the 3 mg twice daily dosage. Improvements versus placebo were also seen in the deucravacitinib groups across multiple composite and organ-specific measures at week 48 [23].
Eligible patients in PAISLEY met Systemic Lupus International Collaborating Clinics criteria, were seropositive and had a Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) score of ≥ 6, and ≥ 1 British Isles Lupus Assessment Group (BILAG) index A or > 2 BILAG B manifestations from the musculoskeletal or mucocutaneous domain [23]. An SRI(4) response was defined as a patient whose disease course fulfills all of the following: a ≥ 4-point reduction from baseline in the SLEDAI-2K score; no new BILAG A (severe disease activity) and ≤ 1 new BILAG B (moderate disease activity) organ domain grade; and no worsening from baseline in the Physician's Global Assessment of Disease Activity Scale score of > 0.3 points on a 3-point visual analogue scale from no disease activity to severe disease activity [24].
Ulcerative Colitis
In adults with moderately-to-severely active ulcerative colitis participating in a randomized, double-blind, placebo-controlled, multinational, phase II study [LATTICE-UC (NCT03934216)] [25], deucravacitinib 6 mg twice daily did not meet its primary endpoint (clinical remission). At week 12, the proportion of patients achieving clinical remission was 14.8% in the deucravacitinib group (n = 88) and 16.3% in the placebo group (n = 43). In the respective treatment groups, clinical remission rates were 14.0% and 25.9% in biologically-naïve patients (n = 57 and 27) and 16.1% and 0.0% in biologically-experienced patients (n = 31 and 16). An endoscopic response (secondary endpoint; defined as an Mayo endoscopic subscore of ≤ 1) at week 12 was achieved by 19.3% and 27.9% of patients in the deucravacitinib and placebo groups (overall population), 15.8% and 37.0% (biologically-naïve population) and 25.8% and 12.5% (biologically-experienced population) [25].
Eligible patients in LATTICE-UC had moderate to severe ulcerative colitis [defined as a modified Mayo score of 5–9 (an endoscopic subscore of ≥ 2, a rectal bleeding subscore of ≥ 1 and a stool frequency subscore of ≥ 2)] and an inadequate response, loss of response or intolerance to ≥ 1 conventional or biological the[25]. At baseline, 63.4% of patients were biologically naïve and 40.5% were receiving concomitant oral corticosteroids. Clinical remission was defined as a modified (excluding friability) Mayo score with an endoscopic subscore of ≤ 1, a rectal bleeding subscore of 0 and a stool frequency subscore of ≤ 1 with a ≥ 1-point reduction from baseline at week 12 [25].
Adverse Events
Therapy with oral deucravacitinib 6 mg once daily was well tolerated in adults with moderate to severe plaque psoriasis participating in POETYK PSO-1 [16] and POETYK PSO-2 [17]. In both studies, the incidence of adverse events (AEs) was similar between the deucravacitinib 6 mg once daily, placebo and apremilast 30 mg twice daily groups in both the 16-week placebo-controlled period and the entire (52 weeks) treatment period [16, 17]. Of note, the exposure adjusted incidence rate of adverse reactions in patients who received deucravacitinib continuously for 52 weeks did not increase compared to the rate seen during the first 16 weeks of therapy [5].
In pooled data [5] from the placebo-controlled periods of POETYK PSO-1 [16] and POETYK PSO-2 [17], the most frequently reported (occurring in ≥ 1% of patients in the deucravacitinib group and with a numerically higher incidence in the deucravacitinib group than the placebo group) adverse reactions in patients receiving deucravacitinib (n = 840) and those receiving placebo (n = 419) were upper respiratory tract infection (URTI; grouped term) [19.2% vs 14.8%], increased blood creatinine phosphokinase (CPK) levels (2.7% vs 1.2%), herpes simplex (grouped term) [2.0% vs 1.2%], mouth ulcers (grouped term) [1.9% vs 0.0%]), folliculitis (1.7% vs 0.0%) and acne (grouped term) [1.4% vs 0.2%]. Treatment discontinuation due to AEs in the 16-week placebo-controlled period occurred in 2.4% of 842 deucravacitinib recipients, 3.8% of 419 placebo recipients and 5.2% of 422 apremilast recipients (pooled data) [26].
Deucravacitinib may increase the risk of infections [5]. In pooled data from the placebo-controlled periods, infections were reported in 29% of deucravacitinib recipients (vs 22% of placebo recipients) [116 vs 83.7 events per 100 person–years], with the majority being mild to moderate in severity and non-serious, and not resulting in deucravacitinib discontinuation [5]. Serious infections occurred in five and two patients receiving deucravacitinib and placebo (2.0 vs 1.6 per 100 patient–years) during this period, with pneumonia and COVID-19 being the most common serious infections during the entire treatment period. In pooled data from the entire treatment periods (total deucravacitinib exposure of 986 patient–years), malignancies (excluding non-melanoma skin cancer) were reported in three deucravacitinib recipients (one case each of breast cancer, hepatocellular carcinoma and lymphoma) [0.3 per 100 patient–years] [5].
In terms of pooled laboratory abnormality data from the placebo-controlled periods, increased CPK levels (including grade 4) occurred in 23 deucravacitinib recipients and 5 placebo recipients (9.3 vs 4.1 per 100 patient–years), elevations in alanine aminotransferase levels ≥ 3 × the upper limit of normal (ULN) were reported in 9 and 2 patients (3.6 vs 1.6 per 100 patient–years), elevations in aspartate aminotransferase levels ≥ 3 × ULN were observed in 13 and 2 patients (5.2 vs 1.6 per 100 patient–years), and glomerular filtration rate (GFR) reductions occurred in 4 and 1 patient (1.6 vs 0.8 per 100 patient–years) with moderate renal impairment (estimated GFR 30–59 mL/min) at baseline [5]. Two deucravacitinib recipients demonstrated a worsening of baseline proteinuria. Increases in mean triglyceride levels of 10.3 mg/dL during the 16-week treatment periods and 9.1 mg/dL during the 52-week treatment periods were seen in patients receiving deucravacitinib [5].
Deucravacitinib demonstrated a consistent safety profile for up to 2 years, according to preliminary data from POETYK PSO-LTE (n = 1221; cumulative exposure of 2482.0 person–years) [19]. Similar exposure adjusted incidence rates per 100 person–years were seen between the entire (52 weeks) treatment periods of POETYK PSO-1 and -2 and the cumulative POETYK PSO-1, -2 and -LTE treatment periods for AEs (229.2 vs 154.4), serious AEs (5.7 vs 6.1), discontinuations (4.4 vs 2.8), malignancies (1.0 vs 0.9), herpes zoster (0.9 vs 0.7), major adverse cardiovascular events (0.3 vs 0.4), deaths (0.2 vs 0.4) and venous thromboembolism (0.1 vs 0.1) [20].
The results in POETYK PSO-1 [16] and POETYK PSO-2 [17] are consistent with those in the phase II study (NCT02931838) in the same patient population [13].
Various dosages of oral deucravacitinib (3 mg twice daily [23], 6 mg once daily [22], 6 mg twice daily [23] and 12 mg once daily [22, 23]), administered in combination with or without a csDMARD [22], and with standard background medications [23], were well tolerated in adults with psoriatic arthritis [22] and SLE [23]. Moreover, the safety and laboratory parameter profiles of deucravacitinib in patients with psoriatic arthritis [22] and those with ulcerative colitis [25] were consistent with those seen in phase III and II studies in patients with plaque psoriasis, and with the selective mechanism of action of the agent [22].
In adults with SLE participating in PAISLEY [23], the incidence of AEs, AEs of interest and serious AEs were similar between the various deucravacitinib groups and the placebo group, with the most common (incidence ≥ 10%) AEs in the deucravacitinib groups being URTI, nasopharyngitis, headache, and urinary tract infection. There were no systemic opportunistic infections, active tuberculosis, thrombotic events, major cardiac events or deaths in PAISLEY, and no meaningful abnormalities in the mean levels of haematology and chemistry laboratory parameters occurred [23].
Ongoing Clinical Trials
In patients with plaque psoriasis, the multinational phase III extension study POETYK PSO-LTE (NCT04036435) is currently ongoing, while recruitment [of adolescents (aged 12 to < 18 years)] is underway for another multinational phase III study (NCT04772079). Three multinational phase III studies, in patients with psoriatic arthritis [POETYK PsA-1 (NCT04908202) and POETYK PsA-2 (NCT04908189)] and scalp psoriasis [PSORIATYK SCALP (NCT05478499)], are likewise recruiting.
Two multinational phase II studies are currently assessing deucravacitinib in patients with SLE (NCT03920267; an extension of PAISLEY) and ulcerative colitis [LATTICE-UC (NCT03934216)], while the recruitment of participants with discoid lupus erythematosus and subacute cutaneous lupus erythematosus (NCT04857034); Crohn’s disease [LATTICE-CD (NCT03599622)]; Crohn’s disease and ulcerative colitis (NCT04877990; an extension trial) and ulcerative colitis (NCT04613518) is occurring for several other multinational phase II studies.
Current Status
Deucravacitinib received its first approval on 9 September 2022 in the USA for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy [5, 6]. Subsequently, it was approved on 26 September 2022 in Japan for the treatment of plaque psoriasis, generalized pustular psoriasis and erythrodermic psoriasis [9].
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sheridan M. Hoy is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Footnotes
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
References
- 1.Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57(12):5023–5038. doi: 10.1021/jm401490p. [DOI] [PubMed] [Google Scholar]
- 2.Le AM, Puig L, Torres T. Deucravacitinib for the treatment of psoriatic disease. Am J Clin Dermatol. 2022 doi: 10.1007/s40257-022-00720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogueira M, Puig L, Torres T. JAK Inhibitors for treatment of psoriasis: focus on selective TYK2 inhibitors. Drugs. 2020;80(4):341–352. doi: 10.1007/s40265-020-01261-8. [DOI] [PubMed] [Google Scholar]
- 4.Kim LS, Wu JJ, Han G. Deucravacitinib for psoriasis. Current Dermatology Reports. 2021;10(1):1–5. doi: 10.1007/s13671-020-00326-x. [DOI] [Google Scholar]
- 5.Bristol Myers Squibb. SOTYKTU™ (deucravacitinib) tablets, for oral use: US prescribing information. 2022. https://packageinserts.bms.com/pi/pi_sotyktu.pdf. Accessed 12 Sep 2022.
- 6.Bristol Myers Squibb. U.S. Food and Drug Administration approves Sotyktu™ (deucravacitinib), oral treatment for adults with moderate-to-severe plaque psoriasis [media release]. 9 Sept 2022. https://www.bms.com/
- 7.Catlett IM, Hu Y, Gao L, et al. Molecular and clinical effects of selective tyrosine kinase 2 inhibition with deucravacitinib in psoriasis. J Allergy Clin Immunol. 2022;149(6):2010–20.e8. doi: 10.1016/j.jaci.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Bristol Myers Squibb. Bristol Myers Squibbs applications for deucravacitinib for the treatment of moderate to severe plaque psoriasis accepted by U.S. Food and Drug Administration and validated by European Medicines Agency [media release]. 29 Nov 2021. https://www.bms.com/
- 9.Bristol-Myers Squibb K.K. Bristol-Myers Squibb K.K. received manufacturing and marketing approval for TYK2 inhibitor SOTYKTU® tablets 6 mg [media release]. 26 Sept 2022. https://www.bms.com/assets/bms/japan/pressrelease/20220926.pdf
- 10.Gillooly K, Zhang Y, Yang X, et al. BMS-986165 is a highly potent and selective allosteric inhibitor of Tyk2, blocks IL-12, IL-23 and type I interferon signaling and provides for robust efficacy in preclinical models of systemic lupus erythematosus and inflammatory bowel disease [abstract no. 11L]. Arthritis Rheumatol. 2016;68(Suppl 10).
- 11.Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with Janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11(5):1763–1776. doi: 10.1007/s13555-021-00596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie JH, Gillooly K, Zhang Y, et al. BMS-986165 is a highly potent and selective allosteric inhibitor of Tyk2, blocks Il-12, Il-23 and type I interferon signaling and provides for robust efficacy in preclinical models of inflammatory bowel disease [abstract no. 349]. In: Digestive Disease Week. 2018.
- 13.Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379(14):1313–1321. doi: 10.1056/NEJMoa1806382. [DOI] [PubMed] [Google Scholar]
- 14.FitzGerald O, Gladman D, Mease P, et al. Biomarker changes with selective tyrosine kinase 2 inhibitor, deucravacitinib, in PsA: effects on disease markers and tyrosine kinase 2-versus Janus kinase 1/2/3-mediated pathways [abstract no. 0490]. Arthritis Rheumatol. 2021;73(Suppl 9):1013–5.
- 15.Chimalakonda A, Singhal S, Dockens R, et al. Deucravacitinib, a selective tyrosine kinase 2 (TYK2) inhibitor: overview of clinical pharmacology including ADME, food and pH effects, pharmacokinetics in special populations, and drug-drug interactions [poster no. P1336]. In: EADV 30th Congress. 2021.
- 16.Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2022 doi: 10.1016/j.jaad.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 POETYK PSO-2 trial. J Am Acad Dermatol. 2022 doi: 10.1016/j.jaad.2022.08.061. [DOI] [PubMed] [Google Scholar]
- 18.Warren RB, Armstrong A, Gooderham M, et al. Deucravacitinib, an oral, selective tyrosine kinase 2 inhibitor, in moderate to severe plaque psoriasis: 52-week efficacy results from the phase 3 POETYK PSO-1 and POETYK PSO-2 trials [abstract no. AB0890]. Ann Rheum Dis. 2022;81(Suppl 1):1570.
- 19.Warren RB, Sofen H, Imafuku S, et al. Deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract no. POS1046]. Ann Rheum Dis. 2022;81(Suppl 1).
- 20.Bristol Myers Squibb. New two-year deucravacitinib data reinforce durable efficacy and consistent safety profile in treatment of moderate to severe plaque psoriasis [media release]. 12 May 2022. http://www.bms.com
- 21.Bristol Myers Squibb. Bristol Myers Squibb announces new SotyktuTM (deucravacitinib) long-term data showing clinical efficacy maintained for up to two years with continuous treatment in moderate-to-severe plaque psoriasis [media release]. 10 Sep 2022. http://www.bms.com
- 22.Mease PJ, Deodhar AA, Van Der Heijde D, et al. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis. 2022;81(6):815–822. doi: 10.1136/annrheumdis-2021-221664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morand E, Pike M, Merrill JT, et al. Efficacy and safety of deucravacitinib, an oral, selective, allosteric Tyk2 inhibitor, in patients with active systemic lupus erythematosus: a phase 2, randomized, double-blind, placebo-controlled study [abstract no. LB0004]. Ann Rheum Dis. 2022;81(Suppl 1):209.
- 24.US National Institutes of Health. ClinicalTrials.gov identifier NCT0325258. 2022. https://clinicaltrials.gov/. Accessed 7 Oct 2022.
- 25.Danese S, Panaccione R, D'Haens G, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 inhibitor, in patients with moderately-to-severely active ulcerative colitis: 12-week results from the phase 2 LATTICE-UC study [abstract no. DOP42] J Crohns Colitis. 2022;16:i091–i92. doi: 10.1093/ecco-jcc/jjab232.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong A, Gooderham M, Warren RB, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the phase 3 POETYK PSO-1 study [abstract no. POS1042 plus poster]. Ann Rheum Dis. 2021;80(Suppl 1):795–6.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


